- 1Department of Urology, Asklepios Klinik Altona, Hamburg, Germany
- 2Department of Urology, Albertinen Krankenhaus, Hamburg, Germany
- 3Faculty of Biology & Chemistry, University of Bremen, Bremen, Germany
- 4mirdetect GmbH, Bremerhaven, Germany
Background: Radiological evaluation of postchemotherapy residual masses of metastatic seminoma is characterized by poor diagnostic accuracy. Serum levels of microRNA-371a-3p (M371) involve high specificity and sensitivity for the primary diagnosis of seminoma. We evaluated if M371 levels can indicate the presence of vital disease in postchemotherapy residual masses in patients with metastatic seminoma.
Methods: Twenty-three seminoma patients (median age 52 years) with residual masses had posttreatment measurements of serum M371 levels (group A), fourteen of whom had measurements also beforehand. The posttreatment results were compared with the clinical outcome during follow-up. Eleven patients with complete remission after treatment of metastatic seminoma (group B) and 33 men with non-malignant testicular diseases (group C) served as controls. M371 serum levels were measured by quantitative real-time PCR using miR-30b-5p as endogenous control. An evaluation was performed with descriptive statistical methods.
Results: Twenty-two patients of Group A had uneventful follow-up so far, twenty-one of whom had M371 level <5, and one other had a mildly elevated level below relative quantity (RQ) = 10. One patient with a level of RQ = 26.2 rapidly progressed. The median posttreatment M371 level of the non-progressing patients of group A is not significantly different from the median level of the control group with complete remission (B). Before treatment, the median M371 levels in groups A and B were 507.6 and 143.9, respectively. In both groups, significant drops in M371 levels resulted from treatment.
Conclusion: Normal M371 serum levels at the time of completion of treatment of metastatic seminoma indicate the absence of vital seminoma in residual masses, while elevated levels >RQ = 10 predict the presence of disease. The optimal timing of M371 measurement after chemotherapy and the appropriate cutoff level still need to be determined. Based on the present results, measuring serum M371 levels involves the potential of a novel tool for assessing postchemotherapy residual masses of metastatic seminoma.
Introduction
Cisplatin-based chemotherapy can provide a cure in the majority of patients with metastatic seminoma (1–3). Yet in about 73% of cases, radiological restaging will reveal only partial remission (PR) at the completion of treatment, which denotes a persisting mass of reduced size at the metastatic site (4). Most of these masses consist of necrosis or fibrosis, and only a few cases will still harbor vital seminoma (5, 6). CT and MRI cannot accurately identify those with vital seminoma (Figure 1). Likewise, serum tumor markers beta human chorionic gonadotropin (bHCG) and lactate dehydrogenase (LDH) are usually not useful in assessing residual masses of seminoma. Currently, clinical decision-making is based on the size of the residual mass. Surgical experience with residual tumors of seminoma had revealed that masses larger than 3 cm may contain vital disease in a substantial number of cases, while masses <3 cm usually consist of necrosis/fibrosis only (7, 8). Accordingly, current guidelines recommend surveillance of residual masses <3 cm (9–12). In larger masses, the traditional management is surgical resection. However, postchemotherapy resections of seminoma involved considerable morbidity, first, because seminoma patients are for the most part somewhat older than non-seminoma patients who usually tolerate retroperitoneal lymph node dissections (RPLNDs) without major problems. Second, postchemotherapy surgery in seminoma had been shown to be particularly tedious due to desmoplastic reactions around the great retroperitoneal vessels resulting in a substantial number of vascular complications (13–15). Therefore, surgery is no longer the option of choice in residual masses of seminoma sized >3 cm. Instead, guidelines currently recommend performing fluorodeoxyglucose PET/CT (FDG-PET/CT) to identify vital seminoma in the residual mass (16). However, a number of disadvantages have become obvious with this imaging modality. First, PET/CT scan involves more radiation dosage than normal CT. Second, it is rather cost-intensive, precluding widespread use. Third, PET/CT is only informative when performed not earlier than 6–8 weeks after completion of chemotherapy (17). Fourth and importantly, PET/CT scans involve a significant number of false-negative and false-positive readings (18–20). Above all, it remained unclear what kind of therapy should be instituted in the case of a positive PET/CT scan. Currently, a repeat scan is advocated in these cases with individual decisions to be taken in case of a positive repeat scan (12). Clearly, there is a need for better tools for assessing postchemotherapy residual masses of seminoma. Serum levels of microRNA-371a-3p (M371) have been shown to have a sensitivity of 90.1% and a specificity of 94.1% in the primary diagnosis of germ cell tumors (GCTs) (21–24). Moreover, M371 is still sensitive in small tumors. In residual masses of metastatic non-seminomatous tumors, the new marker has been shown to identify those with residual vital cancer (25, 26). However, the problem was that teratoma, which is present in about 30% of non-seminoma residual masses, does not express M371 (27–29). Thus, a negative M371 test in these cases will not obviate the need for postchemotherapy RPLND. In seminoma, there is no teratoma problem, and thus, the new marker could be particularly valuable in this histologic group. Therefore, the goal of the present study is to analyze the utility of serum levels of M371 for assessing the kind of postchemotherapy residual masses of seminoma. Our hypothesis is that M371 levels in the normal range will indicate the absence of vital seminoma in the residual mass, which will be proven by an uneventful later course. Conversely, an elevated M371 level will indicate the presence of vital seminoma, which will be evidenced by progressive disease.
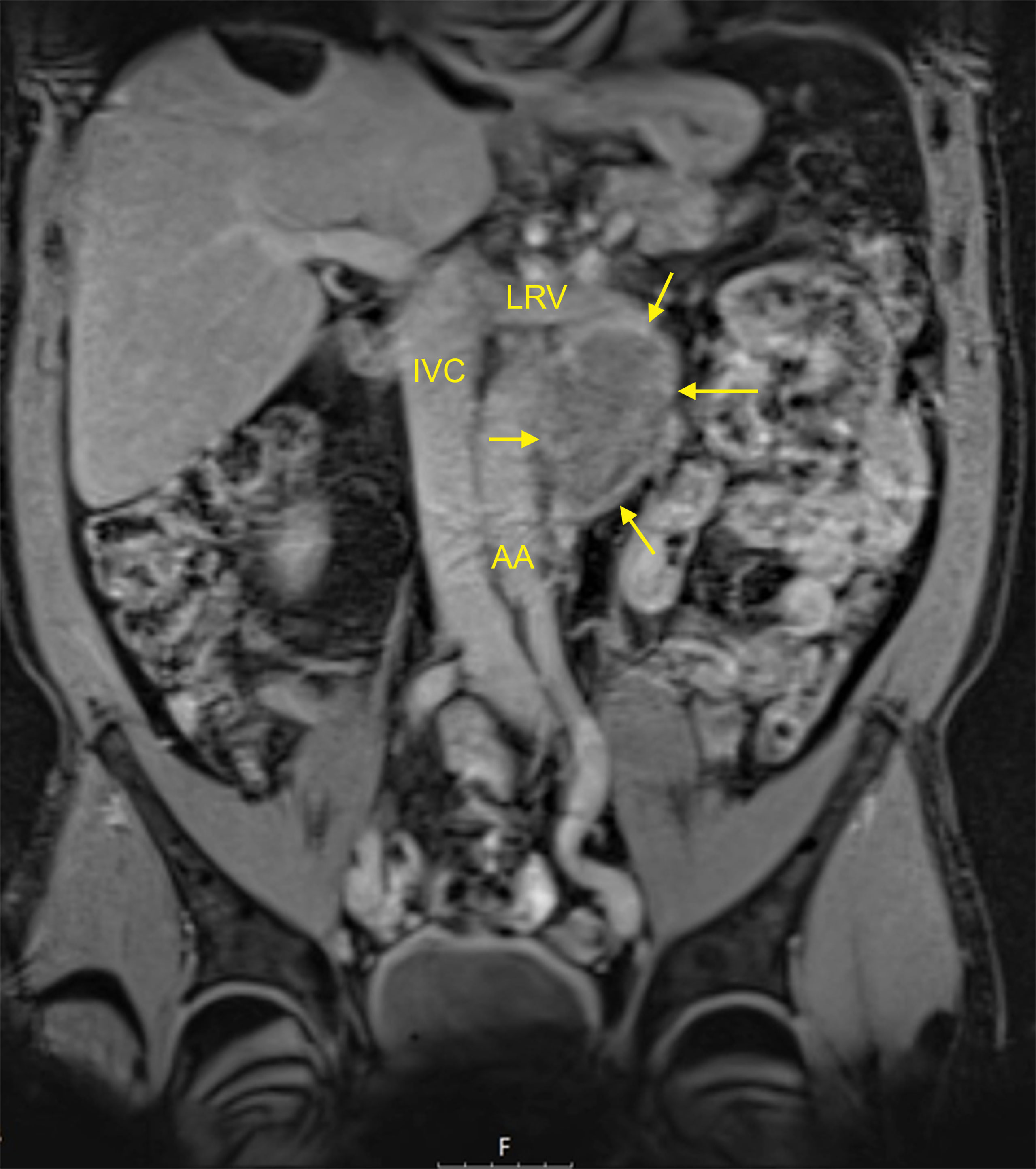
Figure 1 MRI of a typical residual mass resulting from chemotherapy of left-sided metastatic seminoma: 5 cm para-aortic mass (patient R8 in Table 1). T1-weighted imaging, fat-suppressed mode, coronal section. AA, abdominal aorta; IVC, inferior vena cava; LRV, left renal vein. Arrows denote the residual mass. This patient had an M371 level of relative quantity (RQ) = 1 and is continuously disease-free for 24 months. This figure illustrates that neither the imaging technique (i.e., MRI) nor the size of the residual mass (5 cm in this case) can safely predict the histology of the residual mass.
Patients and Methods
Twenty-three patients with residual masses subsequent to chemotherapy for metastatic seminoma (“group A”) underwent measurement of serum M371 levels at the time of completion of treatment. The median age of the patients is 52 years. Clinical stage (CS) at diagnosis according to the Lugano Classification was CS2b, CS2c, and CS3 in 5, 13, and 5 cases, respectively. CS3 was characterized by mediastinal and/or cervical lymph node metastases, and none had pulmonary metastases. With one exception, all of the patients belonged to the good prognosis group of the International Germ Cell Cancer Collaborative Group (IGCCCG) classification system. After orchiectomy with histological confirmation of seminoma, and serological confirmation of normal alpha-fetoprotein (AFP) levels, all patients underwent cisplatin-based chemotherapy according to current guideline recommendations. Modified management was applied to two patients. One received short-course radiotherapy subsequent to 2 cycles of PEB (cisplatin, etoposide, and bleomycin), because of severe gastrointestinal toxicity of PEB. The other patient received two courses of carboplatin monotherapy subsequent to two courses of PE due to critical myelodepression in the first two courses. After completion of chemotherapy, all patients had a measurement of serum levels of M371 and of classical tumor markers AFP, bHCG, and LDH after a median interval of 4 weeks (range 1–12 weeks) after the last application of chemotherapeutic drugs. Radiological re-staging revealed retroperitoneal residual masses sized 11–110 mm (Figure 1). One patient underwent postchemotherapy RPLND because of a positive PET/CT scan. None of the others received further therapy after the completion of primary treatment. Follow-up consisted of abdominal imaging with CT or MRI, along with chest X-ray, clinical examination, and tumor marker measurements according to national guidelines (9). Eleven patients had repeat measurements of M371 upon follow-up visits. The median time of follow-up is 18 months (2–51 months). We registered the results of follow-up examinations (no evidence of disease (NED) or progressive disease (PD)) and compared the clinical course with the result of the M371 measurement at the time of treatment completion.
Eleven seminoma patients served as controls, all of whom had achieved complete remission (CR) after treatment of metastatic disease; i.e., none had a residual mass larger than 1 cm (“group B”). The median age of these patients is 44 years. At diagnosis, CSs 2a, 2b, 2c, and CS3 were present in 1, 7, 1, and 2 patients, respectively. Seven of the controls underwent full-dose cisplatin-based chemotherapy, one patient received curative radiotherapy, and three had combined chemotherapy and radiotherapy. All had measurements of M371 at the completion of therapy, and all were followed up for a median interval of 18 months.
A second control group consisted of 33 patients with benign testicular diseases with a median age of 40 years (“group C”; details in Supplementary Table).
Pretreatment M371 serum levels (i.e., before chemotherapy) were measured in 14 patients of group A and in all of group B. Median pretreatment levels were compared to postchemotherapy levels in both groups. The median M371 levels of the CSs (CS2a/b, 2c, and 3) of combined groups (A and B) were compared to each other.
Serum samples for measurement of M371 were kept deep-frozen at −80°C until processing. Measurement results did not influence the clinical management of patients. Total RNA was isolated from 200 μl of serum using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Measurement of M371 was performed by quantitative real-time PCR as previously reported (22, 30) using the M371 test (mirdetect, Bremerhaven, Germany). Briefly, miR-30b-5p was used as endogenous control, and quantification of M371 was given as relative quantity (RQ) in relation to miR-30b-5p. The relative expression of miR-371a-3p was calculated according to the comparative ΔCT method (31). RQ ≤ 5 was considered a normal range or non-expression of the miR. The classical tumor markers were measured in the routine hospital laboratory according to institutional standard operating procedures.
Descriptive statistical methods were used for the analysis of data. The M371 expression of the various groups is presented as median with interquartile ranges (IQRs). The statistical evaluation was carried out with SPSS version 26 (IBM, Armonk, NY, USA). For the analysis of differences between two independent variables, the Mann–Whitney U test was used. Significance was assumed at p < 0.05.
Ethical approval was provided by the Ethical committee of Ärztekammer Hamburg (MC 152/19, July 15, 2019). All study activities were conducted according to the Declaration of Helsinki of the World Medical Association as amended by the 64th General Assembly, October 2013.
Results
At the completion of therapy, all patients (groups A and B) had normal serum levels of bHCG and LDH. Individual results of posttreatment M371 measurements along with individual time intervals from the end of treatment to blood sampling are listed in Table 1. One patient with clearly elevated M371 level after completion of chemotherapy (RQ = 26.2) developed progression 3 months later as evidenced by positive PET/CT finding and rising bHCG level. During the same time interval, the M371 level increased to RQ = 1834.9 in this patient. He was cured with high-dose chemotherapy and subsequent surgery. One patient had a marginally elevated level (RQ = 7.4) above the cutoff of RQ = 5, which is the level that was found to separate expression from non-expression of the marker in our previous studies. However, he fared well so far without progression. One patient with a postchemotherapy M371 level of zero (RQ = 0) underwent RPLND of the residual tumor. Histology of the surgical specimen revealed necrosis and fibrosis only. All other patients had M371 levels below RQ = 5, and all are well with no evidence of disease after a median time of follow-up of 18 months. Ten patients had repeat M371 measurements during follow-up; all were below the cutoff level, mirroring the disease-free course of these patients. Table 2 lists the patients with CR after treatment, one of whom had an M371 level of 8.1 at the completion of therapy. He is well with no evidence of disease at 6 months of follow-up. All others had RQ levels below the cutoff of 5, and all of them are well, with no evidence of disease after a median time of follow-up of 18 months. Figure 2 shows the decreases of M371 levels in individual patients with residual masses after chemotherapy (group A). The decreases in M371 levels in individual patients with CR are shown in Figure 3 (group B). Figure 4 shows the median M371 levels before and after treatment along with the median M371 level of controls (group C). Expectedly, there were clearly elevated pretreatment M371 levels in both seminoma groups (group A, RQ = 507.6, IQR = 158.9–46,172.3; group B, RQ = 143.9, IQR = 79.7–600.5), and in both groups, there was a significant drop of median levels after completion of treatment (group A, RQ = 0.3, IQR = 0.0–1.2; group B, RQ = 0.2, IQR = 0.0–2.8). The pretreatment levels in groups A and B were significantly higher than those of controls (group C) (p = 2.93 × 10−12 and p = 1.30 × 10−10, respectively) (Supplementary Table). Posttreatment levels of groups A and B are not significantly different from each other (p = 0.581), whereas the difference between the posttreatment levels of group B and the non-malignant controls (group C) is slightly significant (p = 0.025). However, all median values of groups A (posttreatment), B (posttreatment), and C (non-malignant controls) are below the cutoff of RQ = 5. Figure 5 shows the median pretreatment M371 levels in the various CSs of combined groups A and B. Median expression levels in the CS2a/b, CS2c, and CS3 were RQ = 97.6 (IQR = 50.6–330.3), RQ = 600.5 (IQR = 237.5–7,107.1), and RQ = 122,868.6 (IQR = 1,103.0–184,888.9), respectively. Significant differences were observed between CS2a/b and CS3 (p = 0.003) and between CS2a/b and CS2c (p = 0.002), while the difference between CS2c and CS3 was not significant (p = 0.149).
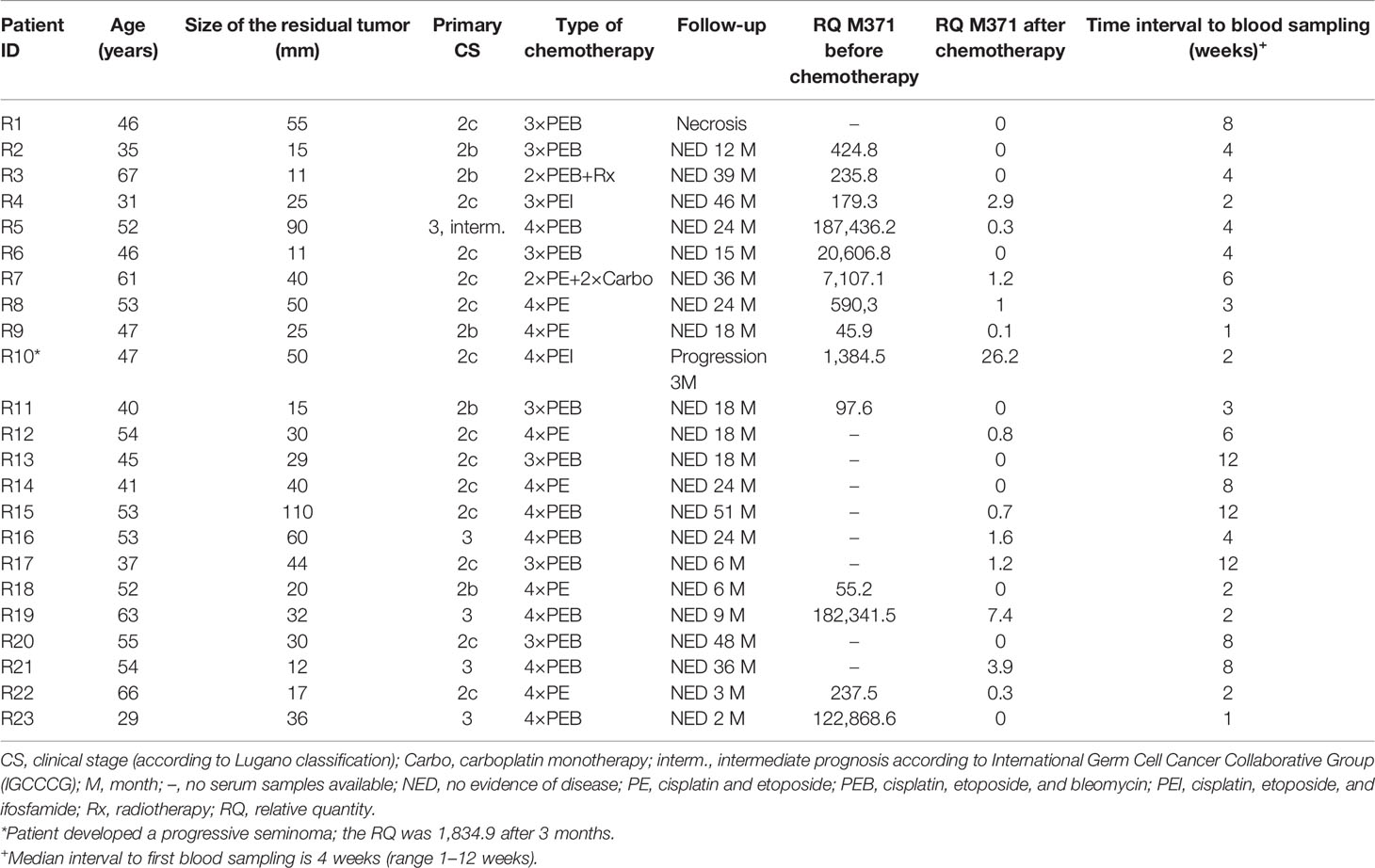
Table 1 Clinical data and relative M371 expression in serum of seminoma patients with residual tumors after chemotherapy or radiotherapy (group A).
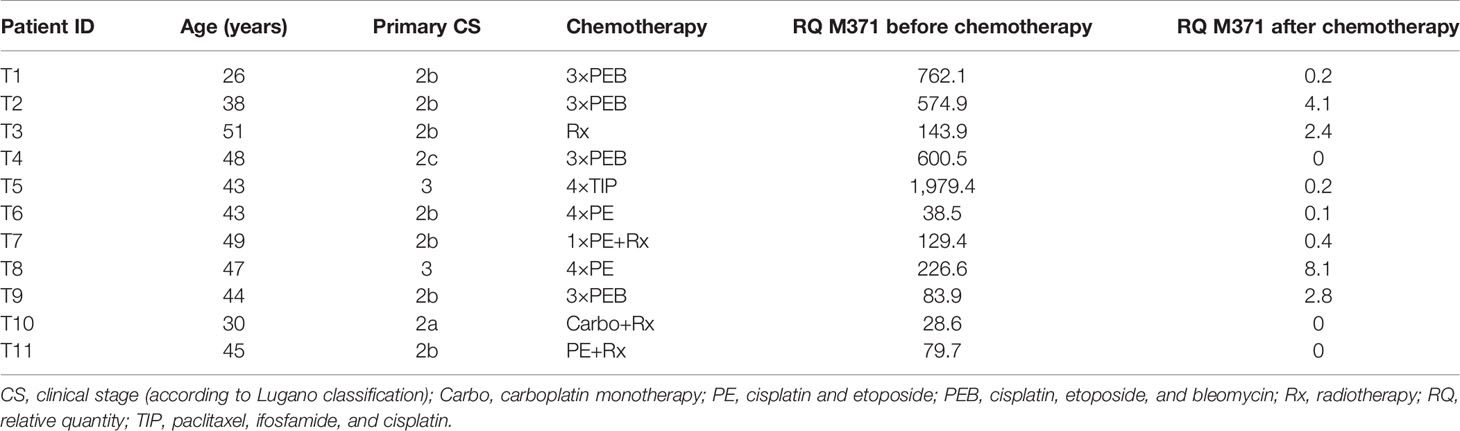
Table 2 Clinical data and relative M371 expression in serum of seminoma patients with complete remission after chemotherapy or radiotherapy (no residual tumors, group B).
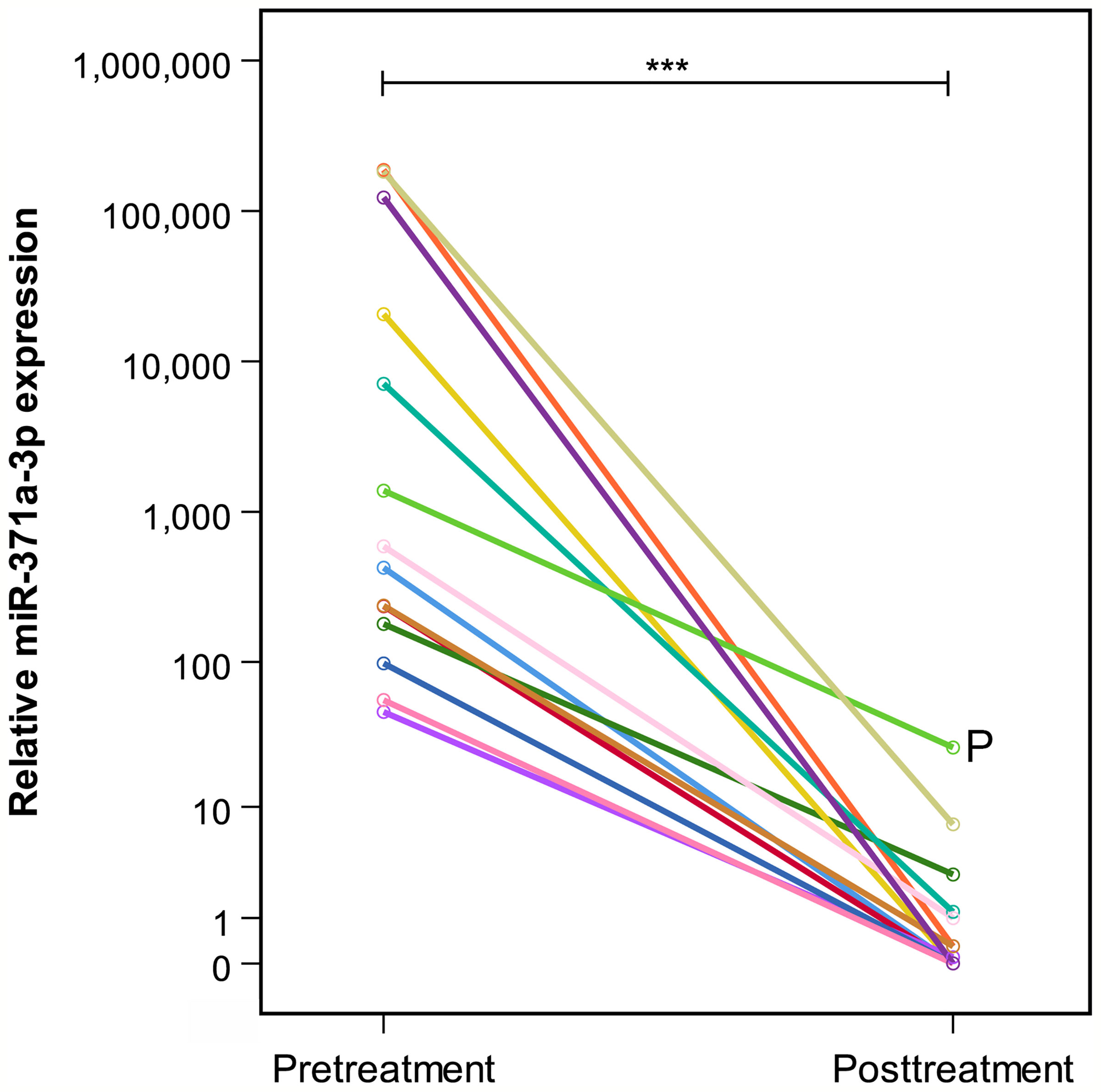
Figure 2 Comparison of individual prechemotherapy and postchemotherapy M371 expression levels in 14 patients with residual tumors (group A). All patients but one had decreased to levels below relative quantity (RQ) = 10. P indicates patient who developed progressive seminoma. He failed to have a decrease of below RQ = 10. The y-axis is plotted on a logarithmic scale. ***p ≤ 0.001.
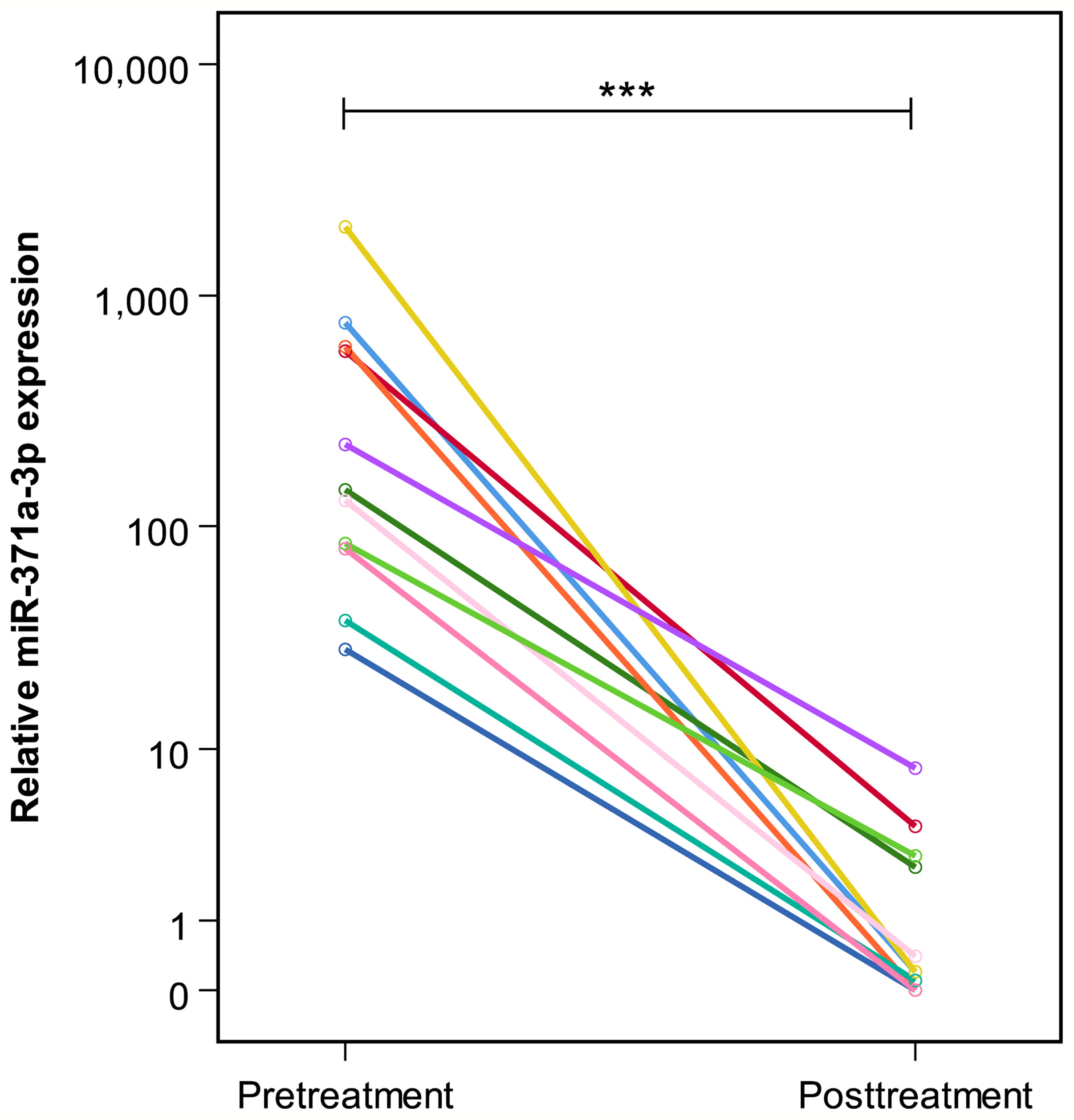
Figure 3 Pre- and posttreatment M371 levels in 11 patients with complete remission (group B). The y-axis is plotted on a logarithmic scale. ***p ≤ 0.001.
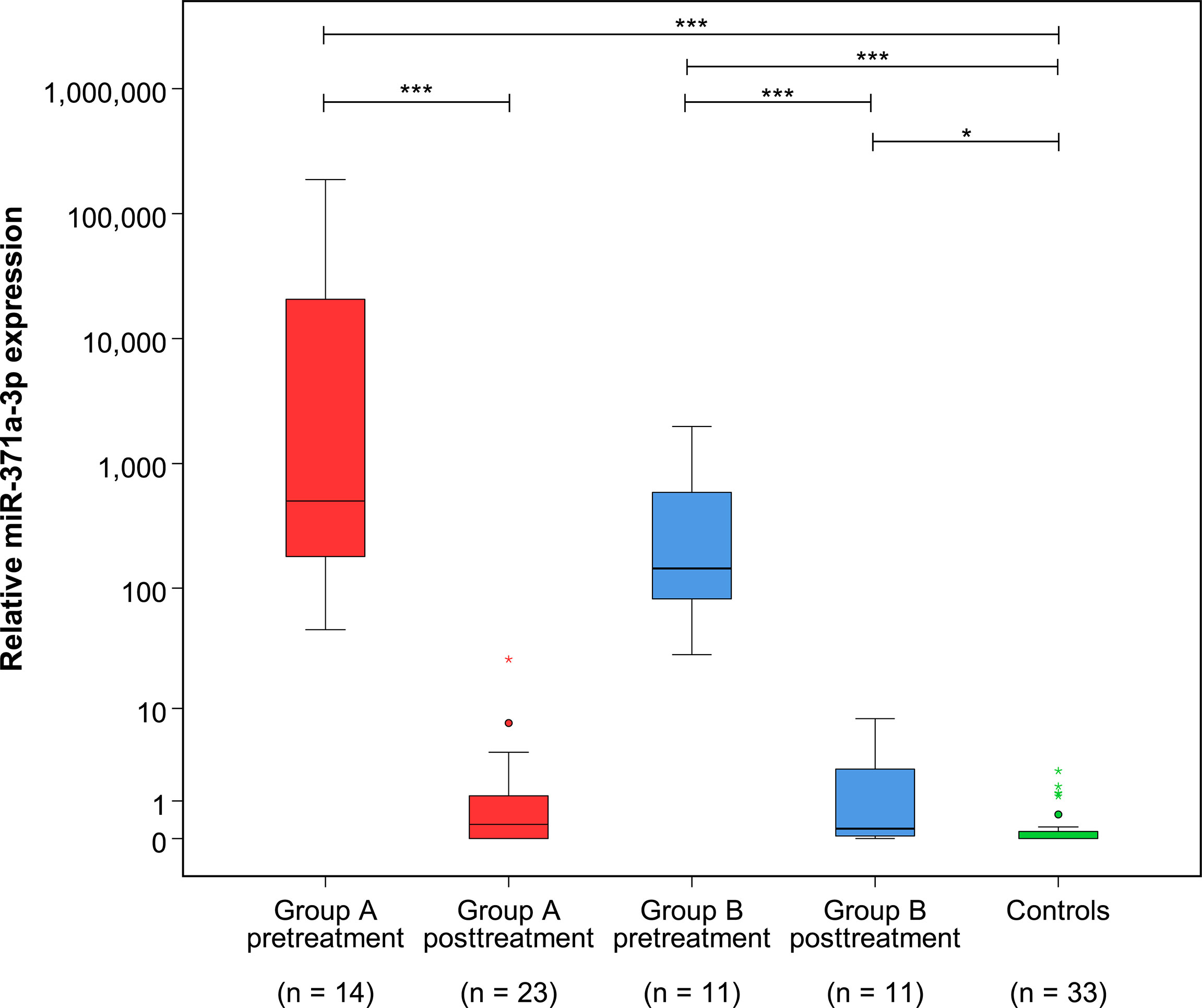
Figure 4 Relative M371 expression in serum of patients with residual tumors (group A) and complete remission (group B) pre- and posttreatment as well as in controls (group C). Boxplots indicate the median relative M371 expressions with interquartile ranges in the five groups. The median M371 expressions in group A and B pretreatment are significantly higher than posttreatment levels and the expression in controls (***p ≤ 0.001). The median posttreatment M371 expressions in group B are significantly different from those of the non-malignant control group (*p ≤ 0.05), but there is no significant difference between the posttreatment levels of groups A and B. The outlier with the highest expression in group A posttreatment is the case that developed progressive seminoma. The y-axis is plotted on a logarithmic scale.
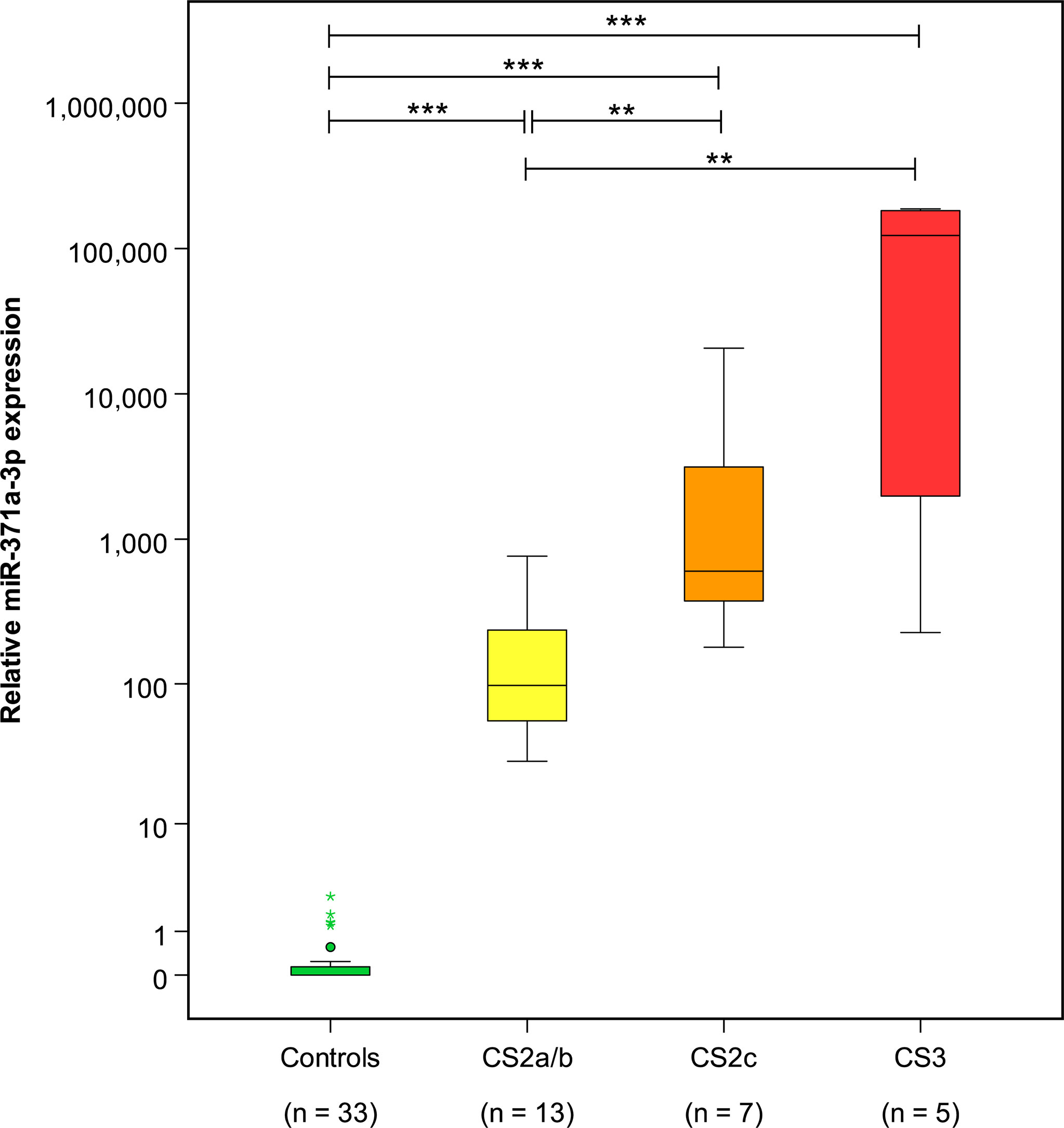
Figure 5 Pretreatment M371 levels in the various clinical stages of seminoma and the control group. Boxplots indicate the median relative M371 expression with interquartile ranges in the clinical stages CS2a/b, CS2c, and CS3. The median M371 expression in the clinical stage CS2a/b is significantly lower than the expressions in CS2c and CS3 (**p ≤ 0.01). Pretreatment median levels of all three clinical stages are significantly different from those of controls (***p ≤ 0.001). The y-axis is plotted on a logarithmic scale.
Discussion
The central result of the present study is that normal or slightly elevated M371 serum levels (RQ < 10) at the time of completion of treatment are associated with disease-free status in patients undergoing chemotherapy for metastatic seminoma. Importantly, the one patient who developed progressive disease was shown to have a distinctly elevated M371 level at the end of chemotherapy (RQ = 26.2). Clearly, as this latter observation is still a single finding, interpretations must be drawn cautiously. The median M371 serum level of the patients who remained disease-free despite residual masses after treatment of metastatic seminoma was not significantly different from the median level of seminoma patients achieving CR after treatment of metastatic seminoma, and both median levels were below the cutoff of RQ = 5. Thus, the present study provides the first piece of evidence for the understanding that normal M371 levels at the end of chemotherapy for metastatic seminoma do likely indicate the absence of viable residual seminoma. Noteworthy, the median M371 level of the healthy controls is significantly lower than the postchemotherapy median level of group B. However, this statistical finding is probably not relevant, clinically, because first, both of the two median levels are clearly below RQ = 5, and generally, M371 levels below that cutoff are considered as non-expression of the marker. Second, the differences are truly minor and may represent biological chance scatterings of M371 expressions as well as technical chance events upon miR measurements.
Our results suggest that M371 levels could in fact be used as a diagnostic tool for assessing residual masses after chemotherapy of seminoma. Presently, the cutoff value is not clear, since one patient with residual tumor and one of those with CR had slightly elevated levels in the RQ range of 5–10. As both patients experienced an uneventful clinical course, one could speculate that the cutoff level for assessing residual tumors of seminoma should be set somewhat higher than the cutoff of RQ = 5 for primary diagnosis of GCTs, probably in the range of 8–10. Noteworthy, as the two patients with slightly elevated levels were examined only shortly after completion of chemotherapy, it appears conceivable that further decreases of the M371 levels might occur during the following weeks after cessation of treatment. Moreover, both patients had CS3 disease with huge tumor masses at the time of diagnosis. It is known from previous studies on monitoring M371 levels during the courses of chemotherapy that CS3 patients had much slower decays of serum levels than patients with lower stages (22, 32, 33). It is thus conceivable that M371 levels could have further dropped during the later course. Unfortunately, repeat measurements are not available in these two patients. The hypothesis that further decreases in M371 level may occur during the weeks after chemotherapy mirrors the experience with PET/CT scans that should be performed not earlier than 6 weeks after completion of treatment because early scans may give rise to false-positive results. Accordingly, repeat PET/CT scans have shown that originally positive scans can turn negative after an interval of 6–8 weeks (20, 34).
Another noteworthy result of this study is the association of median M371 levels with CSs. Significant differences in median M371 levels between seminoma stages CS1, CS2, and CS3 had been reported earlier (22, 33, 35, 36). However, the present evaluation involves a more granular analysis of the CS2 substages. The results are in accord with the view that there is an association between serum M371 levels and tumor bulk, which in turn underscores the usefulness of this microRNA as a valuable biomarker for GCTs.
The limitations of the present study relate to the small sample size, and it must be emphasized that only one case of the present series had disease progression. One other limitation is probably the lack of postchemotherapy PET/CT examinations in the majority of cases. However, the rather unequivocal results in the patients without progression may still lend credit to the conclusions drawn. Some uncertainty may result from the shorter than 1 year of follow-up interval in five patients. Finally, the lack of repeat measurements at least in the two patients with slightly elevated levels must be considered a limitation.
In residual masses resulting from chemotherapy of non-seminoma, elevated M371 levels had been shown to denote the presence of vital germ cell cancer (25). However, a normal M371 test does not justify the omission of postchemotherapy surgery, because teratoma could still be present. This particular subtype of GCT is found in about 30% of non-seminomatous residual tumors, and it definitely requires excision (37, 38). Unfortunately, this subtype does not express M371. As seminoma does not contain teratoma elements, seminomatous residual masses can be assessed much more accurately by measurement of M371 than their non-seminomatous counterparts. In conclusion, measuring M371 levels could significantly simplify clinical decision-making in seminoma patients with residual masses after treatment of metastatic disease. If the present data are confirmed in a larger patient population, M371 measurements will probably obviate the use of PET/CT scans for assessing residual masses after treatment of metastatic seminoma. Also, adjunctive treatment measures could be tailored to patients with persisting elevated M371 levels.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical committee of Ärztekammer Hamburg (MC 152/19, July 15, 2019). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
K-PD and GB conceived the study. K-PD and CW supervised the whole project. MK, ID-F, and FG performed the data curation. AR, MK, FG, and GB performed the statistical analysis. K-PD and GB wrote the manuscript. CW, MK, and AR participated in the manuscript editing and discussion. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This investigation was supported by prize money awarded to K-PD with the Maximilian Nitze Preis der Deutschen Gesellschaft für Urologie in 2019.
Author Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Conflict of Interest
K-PD and GB declare ownership shares of each 9.71% of mirdetect GmbH, Bremerhaven, Germany. AR is an employee of mirdetect, GmbH, Bremerhaven, Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful for the technical assistance of the laboratory staff of Medilys Laboratory at Asklepios Klinik Altona, Hamburg, Germany.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.889624/full#supplementary-material
References
1. Beyer J, Collette L, Sauvé N, Daugaard G, Feldman DR, Tandstad T, et al. International Germ Cell Cancer Classification Update Consortium. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J Clin Oncol (2021) 39(14):1553–62. doi: 10.1200/JCO.20.03292
2. Heinzelbecker J, Schmidt S, Lackner J, Busch J, Bokemeyer C, Classen J, et al. Therapy of Clinical Stage IIA and IIB Seminoma: A Systematic Review. World J Urol (2021). doi: 10.1007/s00345-021-03873-5
3. Fizazi K, Delva R, Caty A, Chevreau C, Kerbrat P, Rolland F, et al. A Risk-Adapted Study of Cisplatin and Etoposide, With or Without Ifosfamide, in Patients With Metastatic Seminoma: Results of the GETUG S99 Multicenter Prospective Study. Eur Urol (2014) 65(2):381–6. doi: 10.1016/j.eururo.2013.09.004
4. Horwich A, Paluchowska B, Norman A, Huddart R, Nicholls J, Fisher C, et al. Residual Mass Following Chemotherapy of Seminoma. Ann Oncol (1997) 8(1):37–40. doi: 10.1023/A:1008241904019
5. Murthy V, Karmakar S, Carlton J, Joshi A, Krishnatry R, Prabhash K, et al. Radiotherapy for Post-Chemotherapy Residual Mass in Advanced Seminoma: A Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography-Based Risk-Adapted Approach. Clin Oncol (R Coll Radiol) (2021) 33(7):e315–e21. doi: 10.1016/j.clon.2021.01.009
6. Puc HS, Heelan R, Mazumdar M, Herr H, Scheinfeld J, Vlamis V, et al. Management of Residual Mass in Advanced Seminoma: Results and Recommendations From the Memorial Sloan-Kettering Cancer Center. J Clin Oncol (1996) 14(2):454–60. doi: 10.1200/JCO.1996.14.2.454
7. Flechon A, Bompas E, Biron P, Droz JP. Management of Post-Chemotherapy Residual Masses in Advanced Seminoma. J Urol (2002) 168(5):1975–9. doi: 10.1016/S0022-5347(05)64275-9
8. Rice KR, Beck SD, Bihrle R, Cary KC, Einhorn LH, Foster RS. Survival Analysis of Pure Seminoma at Postchemotherapy Retroperitoneal Lymph Node Dissection. J Urol (2014) 192:1397–402. doi: 10.1016/j.juro.2014.04.097
9. Kliesch S, Schmidt S, Wilborn D, Aigner C, Albrecht W, Bedke J, et al. Management of Germ Cell Tumours of the Testes in Adult Patients: German Clinical Practice Guideline, PART II - Recommendations for the Treatment of Advanced, Recurrent, and Refractory Disease and Extragonadal and Sex Cord/Stromal Tumours and for the Management of Follow-Up, Toxicity, Quality of Life, Palliative Care, and Supportive Therapy. Urol Int (2021) 105(3-4):181–91. doi: 10.1159/000511245
10. Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, et al. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(12):1529–54. doi: 10.6004/jnccn.2019.0058
11. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. Guidelines on Testicular Cancer: 2015 Update. Eur Urol (2015) 68:1054–68. doi: 10.1016/j.eururo.2015.07.044
12. Honecker F, Aparicio J, Berney D, Beyer J, Bokemeyer C, Cathomas R, et al. ESMO Consensus Conference on Testicular Germ Cell Cancer: Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29(8):1658–86. doi: 10.1093/annonc/mdy217
13. Mosharafa AA, Foster RS, Leibovich BC, Bihrle R, Johnson C, Donohue JP. Is Post-Chemotherapy Resection of Seminomatous Elements Associated With Higher Acute Morbidity? J Urol (2003) 169(6):2126–8. doi: 10.1097/01.ju.0000060121.33899.4b
14. Quek ML, Simma-Chiang V, Stein JP, Pinski J, Quinn DI, Skinner DG. Postchemotherapy Residual Masses in Advanced Seminoma: Current Management and Outcomes. Expert Rev Anticancer Ther (2005) 5(5):869–74. doi: 10.1586/14737140.5.5.869
15. Heidenreich A, Thuer D, Polyakov S. Postchemotherapy Retroperitoneal Lymph Node Dissection in Advanced Germ Cell Tumours of the Testis. Eur Urol (2008) 53(2):260–72. doi: 10.1016/j.eururo.2007.10.033
16. De Santis M, Bokemeyer C, Becherer A, Stoiber F, Oechsle K, Kletter K, et al. Predictive Impact of 2-18fluoro-2-Deoxy-D-Glucose Positron Emission Tomography for Residual Postchemotherapy Masses in Patients With Bulky Seminoma. J Clin Oncol (2001) 19(17):3740–4. doi: 10.1200/JCO.2001.19.17.3740
17. Bachner M, Loriot Y, Gross-Goupil M, Zucali PA, Horwich A, Germa-Lluch JR, et al. 2-¹8 Fluoro-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) for Postchemotherapy Seminoma Residual Lesions: A Retrospective Validation of the SEMPET Trial. Ann Oncol (2012) 23(1):59–64. doi: 10.1093/annonc/mdr052
18. Wagner M, Bellmunt J, Boutros C, Bonardel G, Loriot Y, Albiges L, et al. False Positive 2-(18)Fluroro-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) in Patients With Disseminated Seminoma and Post-Chemotherapy Residual Masses. Clin Genitourin Cancer (2013) 11(1):66–9. doi: 10.1016/j.clgc.2012.06.006
19. Siekiera J, Małkowski B, Jóźwicki W, Jasiński M, Wronczewski A, Pietrzak T, et al. Can We Rely on PET in the Follow-Up of Advanced Seminoma Patients? Urol Int (2012) 88(4):405–9. doi: 10.1159/000337056
20. Cathomas R, Klingbiel D, Bernard B, Lorch A, Garcia Del Muro X, Morelli F, et al. Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol (2018) 36:3381–7. doi: 10.1200/JCO.18.00210
21. van Agthoven T, Looijenga LH. Accurate Primary Germ Cell Cancer Diagnosis Using Serum Based microRNA Detection (Amptsmir Test). Oncotarget (2017) 8:58037–49. doi: 10.18632/oncotarget.10867
22. Dieckmann KP, Radtke A, Geczi L, Matthies C, Anheuser P, Eckardt U, et al. Serum Levels of microRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell-Tumors: Results of a Prospective Multicentric Study. J Clin Oncol (2019) 37(16):1412–23. doi: 10.1200/JCO.18.01480
23. Lafin JT, Singla N, Woldu SL, Lotan Y, Lewis CM, Majmudar K, et al. Serum MicroRNA-371a-3p Levels Predict Viable Germ Cell Tumor in Chemotherapy-Naïve Patients Undergoing Retroperitoneal Lymph Node Dissection. Eur Urol (2020) 77(2):290–2. doi: 10.1016/j.eururo.2019.10.005
24. Syring I, Bartels J, Holdenrieder S, Kristiansen G, Müller SC, Ellinger J. Circulating Serum microRNA (miR-367-3p, miR-371a-3p, miR-372-3p, miR-373-3p) as Biomarkers for Patients With Testicular Germ Cell Cancers. J Urol (2015) 193(1):331–7. doi: 10.1016/j.juro.2014.07.010
25. Leão R, van Agthoven T, Figueiredo A, Jewett MAS, Fadaak K, Sweet J, et al. Serum miRNA Predicts Viable Disease Post-Chemotherapy in Testicular non-Seminoma Germ Cell Tumor Patients. J Urol (2018) 200(1):126–35. doi: 10.1016/j.juro.2018.02.068
26. Lobo J, Gillis AJM, van den Berg A, Dorssers LCJ, Belge G, Dieckmann KP, et al. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights From Germ Cell Tumor In Vitro, In Vivo and Patient-Derived Data. Cells (2019) 8(12):1637. doi: 10.3390/cells8121637
27. Belge G, Grobelny F, Matthies C, Radtke A, Dieckmann KP. Serum Level of microRNA-375-3p Is Not a Reliable Biomarker of Teratoma. In Vivo (2020) 34(1):163–8. doi: 10.21873/invivo.11757
28. Myklebust MP, Søviknes AM, Halvorsen OJ, Thor A, Dahl O, Ræder H. MicroRNAs in Differentiation of Embryoid Bodies and the Teratoma Subtype of Testicular Cancer. Cancer Genomics Proteomics (2022) 19(2):178–93. doi: 10.21873/cgp.0313
29. Lafin JT, Kenigsberg AP, Meng X, Abe D, Savelyeva A, Singla N, et al. Serum Small RNA Sequencing and miR-375 Assay Do Not Identify the Presence of Pure Teratoma at Postchemotherapy Retroperitoneal Lymph Node Dissection. Eur Urol Open Sci (2021) 26:83–7. doi: 10.1016/j.euros.2021.02.003
30. Belge G, Grobelny F, Radtke A, Bodes J, Matthies C, Wülfing C, et al. Serum Levels of microRNA-371a-3p Are Not Elevated in Testicular Tumours of non-Germ Cell Origin. J Cancer Res Clin Oncol (2020) 147(2):435–43. doi: 10.1007/s00432-020-03429-x
31. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (2001) 25(4):402–8. doi: 10.1006/meth.2001.1262
32. Dieckmann KP, Radke A, Spiekermann M, Balks T, Matthies C, Becker P, et al. Serum Levels of MicroRNA 371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumors. Eur Urol (2017) 71(2):213–20. doi: 10.1016/j.eururo.2016.07.029
33. Rosas Plaza X, van Agthoven T, Meijer C, van Vugt MATM, de Jong S, Gietema JA, et al. miR-371a-3p, miR-373-3p and miR-367-3p as Serum Biomarkers in Metastatic Testicular Germ Cell Cancers Before, During and After Chemotherapy. Cells (2019) 8(10):1221. doi: 10.3390/cells8101221
34. Decoene J, Winter C, Albers P. False-Positive Fluorodeoxyglucose Positron Emission Tomography Results After Chemotherapy in Patients With Metastatic Seminoma. Urol Oncol (2015) 33(1):23.e15–23.e1. doi: 10.1016/j.urolonc.2014.09.019
35. Leão R, Albersen M, Looijenga LHJ, Tandstad T, Kollmannsberger C, Murray MJ, et al. Circulating MicroRNAs, the Next-Generation Serum Biomarkers in Testicular Germ Cell Tumours: A Systematic Review. Eur Urol (2021) 80(4):456–66. doi: 10.1016/j.eururo.2021.06.006
36. Murray MJ, Huddart RA, Coleman N. The Present and Future of Serum Diagnostic Tests for Testicular Germ Cell Tumours. Nat Rev Urol (2016) 3(12):715–25. doi: 10.1038/nrurol.2016.170
37. Vergouwe Y, Steyerberg EW, Foster RS, Sleijfer DT, Fosså SD, Gerl A, et al. Predicting Retroperitoneal Histology in Postchemotherapy Testicular Germ Cell Cancer: A Model Update and Multicentre Validation With More Than 1000 Patients. Eur Urol (2007) 51(2):424–32. doi: 10.1016/j.eururo.2006.06.047
Keywords: seminoma, metastases, chemotherapy, microRNA, M371, residual tumor, biomarker
Citation: Dieckmann K-P, Klemke M, Grobelny F, Radtke A, Dralle-Filiz I, Wülfing C and Belge G (2022) Serum Levels of MicroRNA-371a-3p (M371) Can Predict Absence or Presence of Vital Disease in Residual Masses After Chemotherapy of Metastatic Seminoma. Front. Oncol. 12:889624. doi: 10.3389/fonc.2022.889624
Received: 04 March 2022; Accepted: 11 April 2022;
Published: 06 May 2022.
Edited by:
João Lobo, Portuguese Oncology Institute, PortugalReviewed by:
Aditya Bagrodia, University of California, San Diego, United StatesMette Pernille Myklebust, Haukeland University Hospital, Norway
Michal Mego, Campus Bio-Medico University, Italy
Copyright © 2022 Dieckmann, Klemke, Grobelny, Radtke, Dralle-Filiz, Wülfing and Belge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gazanfer Belge, YmVsZ2VAdW5pLWJyZW1lbi5kZQ==
 Klaus-Peter Dieckmann
Klaus-Peter Dieckmann Markus Klemke3
Markus Klemke3 Gazanfer Belge
Gazanfer Belge