- 1Department of Clinical Haematology, Oxford Cancer and Haematology Centre, Churchill Hospital, Oxford University Hospitals NHS Trust, Oxford, United Kingdom
- 2Clinical Trials Unit, Department of Oncology, Churchill Hospital, Oxford University Hospitals NHS Trust, University of Oxford, Oxford, United Kingdom
Despite the development of highly effective, targeted inhibitors of B-cell proliferation and anti-apoptotic pathways in chronic lymphocytic leukemia (CLL), these treatments are not curative, and many patients will develop either intolerance or resistance to these treatments. Transformation of CLL to high-grade lymphoma—the so-called Richter syndrome (RS)—remains a highly chemoimmunotherapy-resistant disease, with the transformation occurring following targeted inhibitors for CLL treatment being particularly adverse. In light of this, cellular therapy in the form of allogenic stem cell transplantation and chimeric antigen receptor T-cell therapy continues to be explored in these entities. We reviewed the current literature assessing these treatment modalities in both high-risk CLL and RS. We also discussed their current limitations and place in treatment algorithms.
Introduction
Substantial progress in understanding the pathobiology of chronic lymphocytic leukemia (CLL) has led to the development of drugs targeting key mechanisms of tumor proliferation and survival. Agents targeting the B-cell receptor (BCR) signaling cascade and B-cell lymphoma-2 (BCL2) expand treatment options for high-risk CLL including TP53-disrupted and relapsed/refractory (R/R) disease. While combination therapy can achieve deep and durable remissions, CLL remains incurable. High-grade transformation of CLL into aggressive B-cell lymphoma called Richter syndrome (RS) complicates CLL in 2%–15% (1–4). The wide range in incidence may be explained by the heterogeneous mutational status of CLL patients from different studies. In fact, specific biomarkers (e.g., NOTCH1, TP53 abnormalities, and trisomy 21) coupled with definite microenvironmental interactions associate to a higher risk of RS transformation (5, 6). Disease progression and high-grade transformation are a frequent cause of targeted therapy discontinuation in trial (3, 7) and non-trial (8–11) populations. Infrequently, RS presents de novo in untreated CLL patients.
Most RS cases represent transformation to a clonally related activated B-cell-type diffuse large B-cell lymphoma (DLBCL) (90%–95%), with a small proportion transforming to Hodgkin lymphoma (12). RS shares morphological characteristics with DLBCL, but its molecular profile is distinct. RS is enriched for mutations in poor-risk CLL drivers and the DNA damage response pathway (13).
Therapy for RS typically mirrors DLCBL, but outcomes are considerably worse (5, 14, 15) with median overall survival (OS) of 6–12 months (16–19). Intensification with hyper-CVXD (fractionated cyclophosphamide, vincristine, liposomal daunorubicin, dexamethasone with or without methotrexate) or OFAR (oxaliplatin, fludarabine, cytarabine, and rituximab) protocols may deliver improved responses, but responses are not sustained and OS remained <12 months (14, 20–23). Novel therapies targeting the BCR pathway continue to be explored in RS. Ibrutinib (24), acalabrutinib (25), or venetoclax monotherapy experience is reported in small series, with a short progression-free survival (PFS). Acalabrutinib plus R-CHOP is being examined in the STELLAR trial (26). Venetoclax with dose-adjusted R-EPOCH has shown promise, albeit in a selected cohort (27).
History of Allogenic Stem Cell Transplantation in CLL
Given the evidence for graft-versus-leukemia (GVL) effect (28–31), there is continuing interest in defining the exact role of allogenic hematopoietic stem-cell transplantation (alloSCT) in CLL. Prospective data demonstrated a promising 2- to 6-year event-free survival (EFS) and OS rates ranging 30%–70% following reduced intensity conditioning (RIC) (32–34), demonstrating curative potential for R/R CLL patients. However, owing to significant inherent risks (33), alloSCT has historically been reserved for patients with sufficiently high-risk disease in the context of conventional chemoimmunotherapy induction.
Based on the 2007 EBMT consensus paper, high risk was defined in younger/fit patients as non-response or relapse within 24 months of having achieved a response to purine-analogue-based induction or post-autologous transplantation and the presence of deletion of 17p13 [del(17p)] by fluorescence in situ hybridization (FISH) or TP53 mutation by sequencing (28). Based primarily on retrospective data, guidelines advocated for the early consideration of related or unrelated donor alloSCT during CLL therapy in high-risk individuals (28). Complex karyotype (CK) defined as ≥3 distinct chromosomal abnormalities, in more than one metaphase, is increasingly recognized as heralding an adverse clinical course and informs selection of patients for cellular therapies (35–38).
In the pre-novel therapy era, these recommendations represented pragmatic guidance for the management of high-risk chemoimmunotherapy refractory patients and were accordingly widely adopted. However, the subsequent introduction and demonstration of long-term efficacy and safety of targeted inhibitors in CLL has unsurprisingly resulted in a marked reduction in transplantation (34, 39). The precise role of alloSCT within the current CLL treatment paradigm remains undefined.
Outcomes in Dual Targeted (BTK and BCL2) Inhibitor-Exposed CLL Patients
With the advent of highly effective targeted inhibitors of Bruton tyrosine kinase (BTKi) (40–42) and BCL2 (43, 44) as treatment at frontline or relapse, the importance of adverse factors described in the immunochemotherapy era is challenged, e.g., 11q deletion and survival outcomes continue to markedly improve. Where access allows, most CLL patients will now cycle through time-limited venetoclax-based therapy (including potentially re-treatment) and continuous covalent BTK inhibition (cBTKi) with or without anti-CD20 monoclonal antibody. Accumulating evidence suggests that the order of such therapy is of relatively little importance with evidence of cross-resistance of drug classes lacking (45–47). Although high-risk patients are still often defined as those with IGVH-unmutated disease, TP53 mutations and/or 17p deletion, and CK, outcomes are demonstrably poor in the relatively small published patient series who develop resistance or intolerance to both major classes of targeted inhibitors, namely, cBTKi and BCL2i (11, 45, 48, 49).
A series of 17 patients who developed progressive disease (PD) after both cBTKi and BCL2i classes were recently reported (49). The cohort was heavily pre-treated with a median of four prior lines of therapy and displayed high-risk genomic features (CK in 12/12 tested, del17p/TP53 mutations in 15/17). Median time to progression on prior venetoclax was 24 months and that on prior cBTKi was 25 months. Progression following both agents was with CLL in 11 patients and RS in 6. Median OS at this juncture was only 3.6 months.
Phosphoinositide 3-kinase (PI3K) inhibition is a licensed option in this space; however, data are both limited and disappointing. Seventeen cBTKi/BCL2i-exposed patients observed an overall response rate (ORR) of 47% with a median PFS (mPFS) of only 5 months (45).
Non-covalent BTKis (ncBTKi) hold great promise in this dual-exposed space. Pirtobrutinib is a reversible ncBTKi active in C481S mutated and wild-type CLL (50, 51). Accumulating data from the BRUIN trial demonstrated an ORR of ~70% in 108 dual-exposed patients (median of five prior lines) and an mPFS of 18 months (52). Other ncBTKis such as MK1026 are in development and demonstrate efficacy in dual-exposed patient, but to date, data are less mature, and small patient numbers are reported (53). Despite clear promise, ncBTKis are not licensed to date.
The largest series describes outcomes in 125 “dual-exposed” CLL patients to cBTKi and venetoclax (54). Most common subsequent strategies included ncBTKi (n=45), cBTKi (n=43), immunochemotherapy (n=23), PI3Ki (n=24), alloSCT (n=17), chimeric antigen receptor (CAR) T-cell therapy (n=9), venetoclax re-treatment (n=6), and others (n=44). ORR and PFS estimates were as follows: CAR T-cell therapy (85.7%; mPFS, 4 months), alloSCT (76.5%; mPFS, 11 months), ncBTKi (75.0%; mPFS, not reached), PI3Ki (40.9%; mPFS, 5 months), CIT (31.8%; mPFS, 3 months), and venetoclax re-treatment (ORR, 40%; mPFS, 14 months), demonstrating the lack of clear standard approach in this setting.
In summary, this so-called “dual-exposed” patient cohort now represents the area of greatest and rapidly growing unmet medical need in CLL (54, 55).
AlloSCT for CLL in the Targeted Inhibitor Era
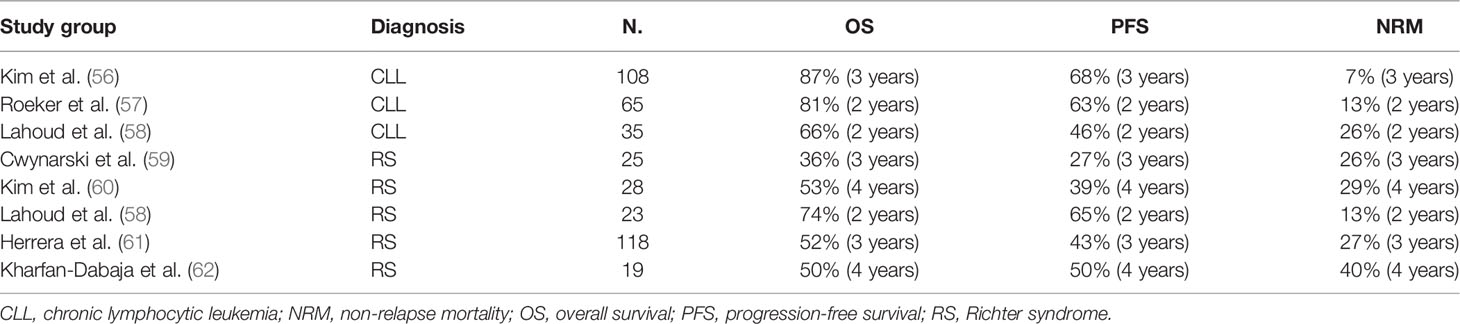
Given the limited prognosis faced by multiply R/R patients in the current targeted inhibitor era, there is renewed interest in the curative potential of alloSCT. Several recent series highlight the efficacy of alloSCT in dual-exposed patients (Table 1).
The Dana–Farber Cancer Institute (DFCI) reported outcomes of 108 RIC alloSCT for high-risk CLL, defined as any of the following: del(17p); ≥3 prior therapies; CK (≥3 abnormalities); IGHV unmutated; R/R to fludarabine, cyclophosphamide, and rituximab (FCR) prior to targeted therapy; poor response to prior chemoimmunotherapy; and poor response to targeted therapy. Thirty patients received prior targeted inhibitors, and 93% were refractory to ≥2 agents. The median age was 60 years, median prior therapies was 4, 76% had del(17p), 46.2% had ≥5 cytogenetic abnormalities, and 78.9% were IGHV unmutated. Median time to transplant from first-line therapy was 39 months. Remission status at alloSCT was CR in 20% and partial response (PR) in 73%. The 3-year OS and PFS were 87% and 69%, respectively. The cumulative incidence of relapse and non-relapse mortality (NRM) was 24% and 7%, respectively. The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) was the only baseline clinical features (including HLA matching status, number and type of prior targeted inhibitors, and adverse genetic features) associated with an increased risk of death [hazard ratio (HR), 1.4; p=0.032] on univariable analysis (56).
The above data supported by a US/European collaboration where outcomes of 65 patients treated predominantly with RIC alloSCT following exposure to ≥1 targeted therapy are reported. Most patients had adverse genetic features including TP53 mutation (51%), del(17p) (44%), and CK (50%). Two-year OS, PFS, NRM, and relapse incidence was 81%, 63%, 13%, and 27%, respectively. Grade ≥3 graft-versus-host disease (GVHD) developed in 27%, with a day+100 cumulative incidence of moderate–severe acute GVHD of 24%. Critically, adverse genetics features, prior number/type of targeted inhibitor exposure, remission status (CR vs. PR), and transplant characteristics were not independently associated with PFS/OS (57).
The most recent small (n=35) US series also analyzed the efficacy of RIC alloSCT in high-risk CLL patients (n=35), including a subset with RS. Of the CLL cohort without RS, 85% had adverse genetic features, and 65% were in PR at alloSCT. The 5-year PFS and OS was 40% and 58%, respectively. There was no statistically significant difference between RS and non-RS patients. Outcomes were again agnostic to adverse baseline genetic characteristics and prior targeted inhibitor exposure. The key clinical features associated with an improved PFS/OS following RIC alloSCT were treatment-sensitive response and ≤3 lines of prior therapy at alloSCT. Use of total body irradiation (TBI) containing RIC regimens was associated with an inferior PFS, OS, and relapse-free survival (58).
Taken together, these retrospective series highlight the efficacy and safety of RIC alloSCT in patients with high-risk R/R CLL following targeted inhibitor exposure and provide evidence for a durable graft-versus-leukemia effect. Critically, they advocate for the early identification of eligible patients and prioritization of alloSCT in those with treatment-sensitive disease.
Within the current CLL treatment paradigm, the curative potential of alloSCT must be balanced against novel agents accessible within trials and the well-described risks, which preclude a significant proportion of patients by virtue of their age, frailty, or comorbidities. For this subset of high-risk patients, alternative novel strategies, including cellular therapies and ncBTKi should be explored.
Chimeric Antigen Receptor T cell Therapy in CLL
Over the past decade, CAR T-cell therapy has revolutionized the treatment of non-Hodgkin lymphoma (NHL). Three pivotal trials in multiply R/R-aggressive NHL patients demonstrated ORR rates of 52%–74% with 1-year OS rates of 48%–59% (63–65), resulting in the incorporation of this option in clinical practice (66–68). CAR T-cell therapy has been explored in CLL, first as monotherapy and recently in combination with ibrutinib (Table 2).
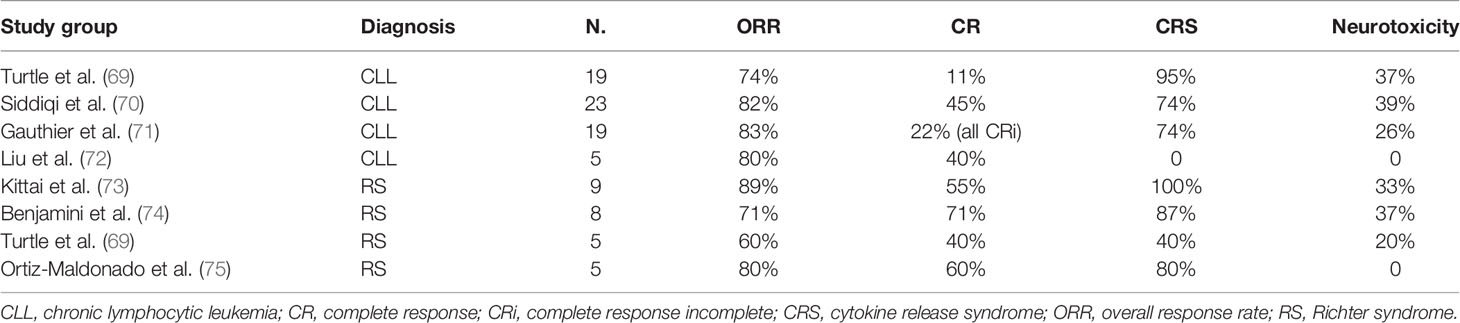
Turtle and colleague enrolled 24 R/R CLL patients in a phase I/II trial where a defined composition of autologous CD4+- and CD8+ CD19-specific CAR T cells were infused following lymphodepletion. Eighty-three percent developed cytokine release syndrome (CRS), but only 25% (n=6) required tocilizumab and corticosteroids. Thirty-three percent had concomitant neurotoxicity, with 5/8 reaching grade 3 and one fatal event (69). These data are in line with relapsed/refractory DLBCL data, where CRS and neurotoxicity were reported in 42%–92% and 21%–67% of patients, respectively (63–65, 76). Notably, the ORR was 71% with an mPFS of 12.3 months (69).
The recent TRANSCEND CLL004 study enrolled 23 R/R CLL/SLL patients to receive Lisocabtagene maraleucel (Liso-cel). ORR and CR were achieved in 82% and 45%, respectively, with 75% and 65% of patients (n=20) achieving MRD negativity in peripheral blood and bone marrow, respectively. mPFS was 18 months but was significantly longer in those who achieved MRD negativity in blood and/or marrow. CRS complicated the course in 74% (9% grade 3), and 39% had neurological immune-related toxicity (22% grade 3–4) (70).
In both studies, patients had received at least two prior lines of therapy (100% had ibrutinib; 25%–65% had venetoclax), and the majority presented high-risk features including mutated TP53 and del(17p) (69, 70).
Recent data have demonstrated the persistence of CAR T cells at more than 10 years follow-up in two CLL patients who remained in complete remission. This study suggested the presence of distinct CAR T-cell populations possibly contributing to different phases of the anti-leukemia response. In one patient, an expansion of CD8+ or CD4−CD8− Helioshi γδ CAR T cells in the first months after the infusion was seen, whereas later time points showed that a predominance of CD4+ CAR T cells was observed. In addition, both phenotype and antigenic signaling pathway analysis suggest that CAR T-cell proliferation was likely maintained through ongoing antigenic signaling through the transduced CAR (77).
Multiple groups are investigating strategies to improve CAR T-cell function in CLL patients. Several studies showed that ibrutinib is capable of modulating the immune dysfunction characterizing CLL patients by increasing Th1 and Th17 subsets (78, 79) and possibly reversing the exhausted T-cell phenotype associated to the expression of PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) on lymphocytes (79, 80). Altogether, these findings suggest that BTKi could enhance CAR T-cell expansion and effector function.
An in vitro study from Fan and colleagues investigated the effect of ibrutinib on CLL patient-derived CAR T-cell production and found that the viability and expansion were increased. In addition, the CAR T-cell pool displayed a decreased expression of exhaustion markers (PD-1, TIM-3, and LAG-3) and was enriched with less-differentiated cells (81), which are thought to have the greatest capacity of engraftment and long-term persistence in vivo (82–84).
Following these promising data, a recent study on R/R CLL patients (n=19) investigated the effect of ibrutinib administered from 2 weeks before leukapheresis to 3 months after CAR T-cell infusion. The combined treatment showed good tolerability and efficacy with an ORR of 83% and 61% of patients achieved marrow MRD negativity by IGH sequencing. Ibrutinib appeared to mitigate the CRS severity despite equivalent CAR T-cell expansion (71).
The inhibition of PI3K signaling during manufacturing has been proposed as an alternative strategy to produce less differentiated and exhausted CAR T cells. Funk and colleagues showed that the in vitro addition of duvelisib (PI3Kδ/γ inhibitor) can decrease the expression of exhaustion markers; increase the number of T-stem cell memory, naive, and memory cells; and normalize the CD4/CD8 ratio (85).
Finally, Liu and colleagues conducted a phase 1/2 study using HLA-mismatched anti-CD19 CAR-NK cells derived from cord blood in 11 patients (5 CLL, 1 concomitant RS). Notably, no patients experienced CRS or neurotoxicity. At a median follow-up of 13.8 months, three CLL patients obtained a CR, which was maintained at last follow-up although with the use of post-remission therapy. Despite some limitations, this proof of concept may ultimately lead to the possibility of well-tolerated NK-based off-the-shelf product (72).
AlloSCT for Richter Syndrome
While the outcomes for RS are dismal with conventional chemoimmunotherapy, the role of cellular therapy remains somewhat uncertain. Published data supporting alloSCT in RS are predominantly retrospective, single-center studies and report a selected RS cohort enriched for younger, fit, chemosensitive patients. Four of the 204 patients proceeded to alloSCT in one large single-institution publication of biopsy-proven RS (86), underlying the unmet need for effective induction therapies and the rarity of transplant-eligible RS patients.
EBMT reported on 25 RS patients who underwent alloSCT between 1997 and 2007 in the pre-targeted inhibitor era. One-third were chemo-refractory. The 3-year NRM was 26%, and OS was only 36%. The authors concluded that alloSCT is a viable therapeutic option for chemosensitive RS (59). Outcomes from the pre-BTKi era may not be applicable to contemporary practice.
Many recent reports are single center but describe similarly unsatisfactory outcomes in the targeted inhibitor era. One-year NRM ranges 24%–40% and 4-year OS at 49%–53% (60, 62). A systematic review and pooled meta-analysis of studies reporting ≥10 RS alloSCTs report an NRM of 24% and OS of 49% (87).
Two recent retrospective studies describe the experience of alloSCT for RS in the BCR inhibitor era. The 1-year NRM remains significant at 12%–23%. In both groups, one-third of the patients relapsed within 3–5 years (58, 61).
Comparable outcomes for RIC alloSCT for 35 R/R CLL and 23 RS were observed (58). All RS patients were considered for alloSCT at first remission. In univariate analysis, R/R CLL and treatment-responsive RS had comparable NRM, PFS, and OS following allograft.
Disease response status pre-alloSCT is predictive of outcome in the EBMT, Memorial Sloan Kettering and CIBMTR series (58, 59, 61). Thrombocytopenia, high LDH, and HCT-CI ≥2 identified patients at increased risk (60). While ≥3 lines of therapy were associated with adverse outcomes, there was no significant difference in outcomes between patients exposed and naive to BTKi, BCL2i, or PI3K inhibitors (58, 60, 61).
There remains a paucity of data to guide decisions regarding the source of stem cells, conditioning regimes, and GVHD prophylaxis. The MSK series observed inferior outcomes with TBI-containing conditioning and recommended against its use in this population. As most RS patients are older adults, non-myeloablative regimes using mobilized peripheral blood stem cells (PBSCs) are frequently employed.
Data on cellular therapy for Hodgkin-like transformation of CLL is even scarcer. One Hodgkin-like RS was reported in the Dana–Farber series; Hodgkin-like RS was excluded from the CIBMTR publication (60, 61).
The literature on alloSCT for RS is confounded by heterogeneous RS populations, variable approaches to alloSCT, and a rapidly changing therapeutic landscape for CLL and RS. In both high-risk CLL and RS, prior exposure to BTKi or BCL2i does not appear to confer an adverse prognosis in those receiving an alloSCT. Both NRM and relapse remain significant challenges in this population.
Chimeric Antigen Receptor T Cell Therapy in Richter Syndrome
The promising results of CAR T-cell therapy in aggressive NHL prompted studies in RS. Data on CAR T-cells efficacy in this setting are limited and conflicting to date.
A recent retrospective study reviewed nine RS patients who were heavily pre-treated (median, four lines for CLL and/or RS). All patients had high-risk features including del(17p) (n=3), CK (n=6), and TP53 mutation (n=2). Two patients received a BTKi as bridging before Axicabtagene ciloleucel (Axi-cel) infusion, while five other patients continued the BTKi for ≥30 days after the infusion. CRS occurred in all patients (grade ≤2, n=8; grade 4, n=1), whereas grade ≥3 neurotoxicity occurred in three patients. Five patients achieved CR, and three patients obtained a PR. One patient died of bacterial pneumonia. At a median follow-up of 6 months, only one patient had progressed, whereas all the others showed sustained responses (73).
Another cohort of eight patients with similar baseline characteristics was enrolled in a single-center phase 2 trial conducted in Israel exploring the use of CAR T cells after targeted therapies. At a median follow-up of 6 months, five patients achieved CR, while three patients progressed. Seven patients developed CRS (grade 3–4, n=3) and three developed neurotoxicity (grade 3, n=2) (74).
Heterogeneous responses were observed in the five RS patients enrolled in the study conducted by Turtle and colleagues. After CAR T-cell product JCAR017 infusion, CR was observed in two patients, PR in one patient, and PD in two other cases (69).
Interestingly, a Spanish phase I study infused ARI-0001, a novel CAR T-cell construct, to five RS patients using a fractionated dose scheme. The CRS rate was 80%, whereas neurotoxicity was not observed. One patient received only 10%–40% of the expected cell dose due to CRS. Four patients responded to treatment (CR, n=3), while one remained with SD according to iwCLL/Lugano criteria. However, MRD negativity was achieved in all patients both in peripheral blood and bone marrow (75). ARI-0001 has recently been approved by the Spanish Medicines Agency (AEMPS) for patients with R/R acute lymphoblastic leukemia (ALL) >25 years of age.
Other small studies suggested lack of response to CAR T-cell therapy or non-sustained response in the context of RS (88, 89).
Published reports examining the role of CAR T-cell therapy for RS are limited by small numbers, variable approaches to concurrent therapies, and short follow-up. Despite these restrictions, disease responses are observed at least for a minority. However, the above reports highlight manageable toxicity and promising outcomes for heavily pre-treated and high-risk patients with few therapeutic options left. Further work is needed to determine the precise role of CAR T-cell therapy in the treatment of RS.
Discussion
High-risk R/R CLL—particularly patients now “dual exposed” to BTKi and BCL2i—and R/R RS remain areas of ongoing clinical need and investigation. Cellular therapy in the form of alloSCT represents an ongoing option for fit, younger CLL patients achieving disease control in these settings and has demonstrable utility in the targeted inhibitor era. While CAR T-cell therapy provides cause for optimism, the clinical data supporting this therapeutic modality at present are limited. Ongoing investigation into improving T-cell function and further prospective clinical data are needed before this treatment becomes a de facto standard of care approach across a wider range of R/R CLL and RS patients. Despite this, the limited data in R/R RS are promising, and, where available, this modality could be considered in R/R RS patients who can be bridged to reinfusion with reasonable performance status and disease control.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported in part by the Oxford Biomedical Research Centre.
Conflict of Interest
NA received speakers fees from Gilead and research funding from Janssen. TE received education honorarium, advisory board honorarium, and travel support from Roche; received honorarium, research support, and travel to scientific conferences from Gilead; received advisory board honorarium from KITE; received honorarium from Janssen; received honorarium and travel to scientific conferences from Abbvie; received honorarium and research funding from AstraZeneca; received advisory board honorarium and a member of trial steering committee from Loxo Oncology; received advisory board honorarium and research funding from Beigene; advisory board honorarium from Incyte; and received Advisory Board Honorarium Secura Bio.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JR declared a past co-authorship with the author TE.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The Genetics of Richter Syndrome Reveals Disease Heterogeneity and Predicts Survival After Transformation. Blood (2011) 117(12):3391–401. doi: 10.1182/blood-2010-09-302174
2. Tsimberidou AM, O'Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical Outcomes and Prognostic Factors in Patients With Richter's Syndrome Treated With Chemotherapy or Chemoimmunotherapy With or Without Stem-Cell Transplantation. J Clin Oncol (2006) 24(15):2343–51. doi: 10.1200/JCO.2005.05.0187
3. Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao WQ, Abruzzo L, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol (2015) 1(1):80–7. doi: 10.1001/jamaoncol.2014.218
4. Al-Sawaf O, Robrecht S, Bahlo J, Fink AM, Cramer P, Tresckow JV, et al. Richter Transformation in Chronic Lymphocytic Leukemia (Cll)-A Pooled Analysis of German Cll Study Group (Gcllsg) Front Line Treatment Trials. Leukemia (2021) 35(1):169–76. doi: 10.1038/s41375-020-0797-x. .
5. Rossi D, Spina V, Gaidano G. Biology and Treatment of Richter Syndrome. Blood (2018) 131(25):2761–72. doi: 10.1182/blood-2018-01-791376
6. Kwok M, Wu CJ. Clonal Evolution of High-Risk Chronic Lymphocytic Leukemia: A Contemporary Perspective. Front Oncol (2021) 11:790004. doi: 10.3389/fonc.2021.790004
7. Woyach JA, Ruppert AS, Guinn D, Lehman A, Blachly JS, Lozanski A, et al. Btk(C481s)-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J Clin Oncol (2017) 35(13):1437–43. doi: 10.1200/JCO.2016.70.2282
8. Jain P, Thompson PA, Keating M, Estrov Z, Ferrajoli A, Jain N, et al. Long-Term Outcomes for Patients With Chronic Lymphocytic Leukemia Who Discontinue Ibrutinib. Cancer (2017) 123(12):2268–73. doi: 10.1002/cncr.30596
9. Winqvist M, Andersson PO, Asklid A, Karlsson K, Karlsson C, Lauri B, et al. Long-Term Real-World Results of Ibrutinib Therapy in Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia: 30-Month Follow Up of the Swedish Compassionate Use Cohort. Haematologica (2019) 104(5):e208–e10. doi: 10.3324/haematol.2018.198820
10. Bouclet F, Calleja A, Dilhuydy MS, Veronese L, Pereira B, Amorim S, et al. Real-World Outcomes Following Venetoclax Therapy in Patients With Chronic Lymphocytic Leukemia or Richter Syndrome: A Filo Study of the French Compassionate Use Cohort. Ann Hematol (2021) 100(4):987–93. doi: 10.1007/s00277-021-04419-w
11. Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-World Outcomes and Management Strategies for Venetoclax-Treated Chronic Lymphocytic Leukemia Patients in the United States. Haematologica (2018) 103(9):1511–7. doi: 10.3324/haematol.2018.193615
12. Soilleux EJ, Wotherspoon A, Eyre TA, Clifford R, Cabes M, Schuh AH. Diagnostic Dilemmas of High-Grade Transformation (Richter's Syndrome) of Chronic Lymphocytic Leukaemia: Results of the Phase Ii National Cancer Research Institute Chop-Or Clinical Trial Specialist Haemato-Pathology Central Review. Histopathology (2016) 69(6):1066–76. doi: 10.1111/his.13024
13. Klintman J, Appleby N, Stamatopoulos B, Ridout K, Eyre TA, Robbe P, et al. Genomic and Transcriptomic Correlates of Richter Transformation in Chronic Lymphocytic Leukemia. Blood (2021) 137(20):2800–16. doi: 10.1182/blood.2020005650
14. Eyre TA, Schuh A. An Update for Richter Syndrome - New Directions and Developments. Br J Haematol (2017) 178(4):508–20. doi: 10.1111/bjh.14700
15. Eyre TA, Riches JC, Patten PEM, Walewska R, Marr H, Follows G, et al. Richter Transformation of Chronic Lymphocytic Leukaemia: A British Society for Haematology Good Practice Paper. Br J Haematol (2022) 196(4):864–70. doi: 10.1111/bjh.17882
16. Langerbeins P, Busch R, Anheier N, Durig J, Bergmann M, Goebeler ME, et al. Poor Efficacy and Tolerability of R-Chop in Relapsed/Refractory Chronic Lymphocytic Leukemia and Richter Transformation. Am J Hematol (2014) 89(12):E239-43. doi: 10.1002/ajh.23841
17. Eyre TA, Clifford R, Bloor A, Boyle L, Roberts C, Cabes M, et al. Ncri Phase Ii Study of Chop in Combination With Ofatumumab in Induction and Maintenance in Newly Diagnosed Richter Syndrome. Br J Haematol (2016) 175(1):43–54. doi: 10.1111/bjh.14177
18. Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in Patients With Cll and Richter Transformation or With Relapsed Cll. Blood (2017) 129(26):3419–27. doi: 10.1182/blood-2017-02-765685
19. Rogers KA, Huang Y, Ruppert AS, Salem G, Stephens DM, Heerema NA, et al. A Single-Institution Retrospective Cohort Study of First-Line R-Epoch Chemoimmunotherapy for Richter Syndrome Demonstrating Complex Chronic Lymphocytic Leukaemia Karyotype as an Adverse Prognostic Factor. Br J Haematol (2018) 180(2):259–66. doi: 10.1111/bjh.15035
20. Tsimberidou AM, Kantarjian HM, Cortes J, Thomas DA, Faderl S, Garcia-Manero G, et al. Fractionated Cyclophosphamide, Vincristine, Liposomal Daunorubicin, and Dexamethasone Plus Rituximab and Granulocyte-Macrophage-Colony Stimulating Factor (Gm-Csf) Alternating With Methotrexate and Cytarabine Plus Rituximab and Gm-Csf in Patients With Richter Syndrome or Fludarabine-Refractory Chronic Lymphocytic Leukemia. Cancer (2003) 97(7):1711–20. doi: 10.1002/cncr.11238
21. Dabaja BS, O'Brien SM, Kantarjian HM, Cortes JE, Thomas DA, Albitar M, et al. Fractionated Cyclophosphamide, Vincristine, Liposomal Daunorubicin (Daunoxome), and Dexamethasone (Hypercvxd) Regimen in Richter's Syndrome. Leuk Lymphoma (2001) 42(3):329–37. doi: 10.3109/10428190109064589
22. Tsimberidou AM, Wierda WG, Plunkett W, Kurzrock R, O'Brien S, Wen S, et al. Phase I-Ii Study of Oxaliplatin, Fludarabine, Cytarabine, and Rituximab Combination Therapy in Patients With Richter's Syndrome or Fludarabine-Refractory Chronic Lymphocytic Leukemia. J Clin Oncol (2008) 26(2):196–203. doi: 10.1200/JCO.2007.11.8513
23. Tsimberidou AM, Wierda WG, Wen S, Plunkett W, O'Brien S, Kipps TJ, et al. Phase I-Ii Clinical Trial of Oxaliplatin, Fludarabine, Cytarabine, and Rituximab Therapy in Aggressive Relapsed/Refractory Chronic Lymphocytic Leukemia or Richter Syndrome. Clin Lymphoma Myeloma Leuk (2013) 13(5):568–74. doi: 10.1016/j.clml.2013.03.012
24. Tsang M, Shanafelt TD, Call TG, Ding W, Chanan-Khan A, Leis JF, et al. The Efficacy of Ibrutinib in the Treatment of Richter Syndrome. Blood (2015) 125(10):1676–8. doi: 10.1182/blood-2014-12-610782
25. Eyre TA, Schuh A, Wierda WG, Brown JR, Ghia P, Pagel JM, et al. Acalabrutinib Monotherapy for Treatment of Chronic Lymphocytic Leukaemia (Ace-Cl-001): Analysis of the Richter Transformation Cohort of an Open-Label, Single-Arm, Phase 1-2 Study. Lancet Haematol (2021) 8(12):e912–e21. doi: 10.1016/S2352-3026(21)00305-7
26. Appleby N, Eyre TA, Cabes M, Jackson A, Boucher R, Yates F, et al. The Stellar Trial Protocol: A Prospective Multicentre Trial for Richter's Syndrome Consisting of a Randomised Trial Investigation Chop-R With or Without Acalabrutinib for Newly Diagnosed Rs and a Single-Arm Platform Study for Evaluation of Novel Agents in Relapsed Disease. BMC Cancer (2019) 19(1):471. doi: 10.1186/s12885-019-5717-y
27. Davids MS, Rogers KA, Tyekucheva S, Wang Z, Pazienza S, Renner SK, et al. Venetoclax Plus Dose-Adjusted R-Epoch (Vr-Epoch) for Richter's Syndrome. Blood (2022) 139(5):686–9. doi: 10.1182/blood.2021011386
28. Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. Indications for Allogeneic Stem Cell Transplantation in Chronic Lymphocytic Leukemia: The Ebmt Transplant Consensus. Leukemia (2007) 21(1):12–7. doi: 10.1038/sj.leu.2404441
29. Dreger P, Ritgen M, Bottcher S, Schmitz N, Kneba M. The Prognostic Impact of Minimal Residual Disease Assessment After Stem Cell Transplantation for Chronic Lymphocytic Leukemia: Is Achievement of Molecular Remission Worthwhile? Leukemia (2005) 19(7):1135–8. doi: 10.1038/sj.leu.2403800
30. Moreno C, Villamor N, Colomer D, Esteve J, Gine E, Muntanola A, et al. Clinical Significance of Minimal Residual Disease, as Assessed by Different Techniques, After Stem Cell Transplantation for Chronic Lymphocytic Leukemia. Blood (2006) 107(11):4563–9. doi: 10.1182/blood-2005-09-3634
31. Mattsson J, Uzunel M, Remberger M, Ljungman P, Kimby E, Ringden O, et al. Minimal Residual Disease Is Common After Allogeneic Stem Cell Transplantation in Patients With B Cell Chronic Lymphocytic Leukemia and May Be Controlled by Graft-Versus-Host Disease. Leukemia (2000) 14(2):247–54. doi: 10.1038/sj.leu.2401669
32. Schetelig J, Thiede C, Bornhauser M, Schwerdtfeger R, Kiehl M, Beyer J, et al. Evidence of a Graft-Versus-Leukemia Effect in Chronic Lymphocytic Leukemia After Reduced-Intensity Conditioning and Allogeneic Stem-Cell Transplantation: The Cooperative German Transplant Study Group. J Clin Oncol (2003) 21(14):2747–53. doi: 10.1200/JCO.2003.12.011
33. Kramer I, Stilgenbauer S, Dietrich S, Bottcher S, Zeis M, Stadler M, et al. Allogeneic Hematopoietic Cell Transplantation for High-Risk Cll: 10-Year Follow-Up of the Gcllsg Cll3x Trial. Blood (2017) 130(12):1477–80. doi: 10.1182/blood-2017-04-775841
34. van Gelder M, de Wreede LC, Bornhauser M, Niederwieser D, Karas M, Anderson NS, et al. Long-Term Survival of Patients With Cll After Allogeneic Transplantation: A Report From the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant (2017) 52(3):372–80. doi: 10.1038/bmt.2016.282
35. Le Bris Y, Struski S, Guieze R, Rouvellat C, Prade N, Troussard X, et al. Major Prognostic Value of Complex Karyotype in Addition to Tp53 and Ighv Mutational Status in First-Line Chronic Lymphocytic Leukemia. Hematol Oncol (2017) 35(4):664–70. doi: 10.1002/hon.2349
36. Thompson PA, O'Brien SM, Wierda WG, Ferrajoli A, Stingo F, Smith SC, et al. Complex Karyotype Is a Stronger Predictor Than Del(17p) for an Inferior Outcome in Relapsed or Refractory Chronic Lymphocytic Leukemia Patients Treated With Ibrutinib-Based Regimens. Cancer (2015) 121(20):3612–21. doi: 10.1002/cncr.29566
37. Rigolin GM, Cavallari M, Quaglia FM, Formigaro L, Lista E, Urso A, et al. In Cll, Comorbidities and the Complex Karyotype Are Associated With an Inferior Outcome Independently of Cll-Ipi. Blood (2017) 129(26):3495–8. doi: 10.1182/blood-2017-03-772285
38. Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive Genetic Characterization of Cll: A Study on 506 Cases Analysed With Chromosome Banding Analysis, Interphase Fish, Igv(H) Status and Immunophenotyping. Leukemia (2007) 21(12):2442–51. doi: 10.1038/sj.leu.2404935
39. Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, et al. Managing High-Risk Cll During Transition to a New Treatment Era: Stem Cell Transplantation or Novel Agents? Blood (2014) 124(26):3841–9. doi: 10.1182/blood-2014-07-586826
40. Munir T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final Analysis From Resonate: Up to Six Years of Follow-Up on Ibrutinib in Patients With Previously Treated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Am J Hematol (2019) 94(12):1353–63. doi: 10.1002/ajh.25638
41. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Efficacy and Safety in a 4-Year Follow-Up of the Elevate-Tn Study Comparing Acalabrutinib With or Without Obinutuzumab Versus Obinutuzumab Plus Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia. Leukemia (2022) 36(4):1171–5. doi: 10.1038/s41375-021-01485-x
42. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 Study of the Selective Btk Inhibitor Zanubrutinib in B-Cell Malignancies and Safety and Efficacy Evaluation in Cll. Blood (2019) 134(11):851–9. doi: 10.1182/blood.2019001160
43. Al-Sawaf O, Zhang C, Lu T, Liao MZ, Panchal A, Robrecht S, et al. Minimal Residual Disease Dynamics After Venetoclax-Obinutuzumab Treatment: Extended Off-Treatment Follow-Up From the Randomized Cll14 Study. J Clin Oncol (2021) 39(36):4049–60. doi: 10.1200/JCO.21.01181
44. Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the Murano Phase Iii Study. J Clin Oncol (2020) 38(34):4042–54. doi: 10.1200/JCO.20.00948
45. Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the Efficacy of Therapies Following Venetoclax Discontinuation in Cll Reveals Btk Inhibition as an Effective Strategy. Clin Cancer Res (2020) 26(14):3589–96. doi: 10.1158/1078-0432.CCR-19-3815
46. Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, et al. Venetoclax for Chronic Lymphocytic Leukaemia Progressing After Ibrutinib: An Interim Analysis of a Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2018) 19(1):65–75. doi: 10.1016/S1470-2045(17)30909-9
47. Lin VS, Lew TE, Handunnetti SM, Blombery P, Nguyen T, Westerman DA, et al. Btk Inhibitor Therapy Is Effective in Patients With Cll Resistant to Venetoclax. Blood (2020) 135(25):2266–70. doi: 10.1182/blood.2020004782
48. Eyre TA, Kirkwood AA, Gohill S, Follows G, Walewska R, Walter H, et al. Efficacy of Venetoclax Monotherapy in Patients With Relapsed Chronic Lymphocytic Leukaemia in the Post-Bcr Inhibitor Setting: A Uk Wide Analysis. Br J Haematol (2019) 185(4):656–69. doi: 10.1111/bjh.15802
49. Lew TE, Lin VS, Cliff ER, Blombery P, Thompson ER, Handunnetti SM, et al. Outcomes of Patients With Cll Sequentially Resistant to Both Bcl2 and Btk Inhibition. Blood Adv (2021) 5(20):4054–8. doi: 10.1182/bloodadvances.2021005083
50. Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in Relapsed or Refractory B-Cell Malignancies (Bruin): A Phase 1/2 Study. Lancet (2021) 397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5
51. Mato AR, Pagel JM, Coombs CC, Shah NN, Lamanna N, Lech-Marańda E, et al. Loxo-305, a Next Generation, Highly Selective, Non-Covalent Btk Inhibitor in Previously Treated Cll/Sll: Results From the Phase 1/2 Bruin Study. Blood (2020) 136(Issue Supplement 1):35–37. doi: 10.1182/blood-2020-134970
52. Mato AR, Pagel JM, Coombs CC, Shah NN, Lamanna N, Munir T, et al. Pirtobrutinib, a Next Generation, Highly Selective, Non-Covalent Btk Inhibitor in Previously Treated Cll/Sll: Updated Results From the Phase 1/2 Bruin Study. Blood (2021) 138:391. doi: 10.1182/blood-2021-147599
53. Woyach JA, Flinn IW, Awan FT, Eradat H, Brander DM, Tees M, et al. Preliminary Efficacy and Safety of Mk-1026, a Non-Covalent Inhibitor of Wild-Type and C481s Mutated Bruton Tyrosine Kinase, in B-Cell Malignancies: A Phase 2 Dose Expansion Study. Blood (2021) 138(Supplement 1):392. doi: 10.1182/blood-2021-148672
54. Thompson MC, Roeker LE, Coombs CC, Jensen JL, Kamdar M, Skarbnik A, et al. Addressing a New Challenge in Chronic Lymphocytic Leukemia: Outcomes of Therapies After Exposure to Both a Covalent Bruton's Tyrosine Kinase Inhibitor and Venetoclax. Blood (2021) 138:2628. doi: 10.1182/blood-2021-150751
55. Mato AR, Davids MS, Sharman J, Roeker LE, Kay N, Kater AP, et al. Recognizing Unmet Need in the Era of Targeted Therapy for Cll/Sll: "What's Past Is Prologue" (Shakespeare). Clin Cancer Res (2021) 28(4):603–8. doi: 10.1158/1078-0432.CCR-21-1237
56. Kim HT, Shaughnessy CJ, Rai SC, Reynolds C, Ho VT, Cutler C, et al. Allogeneic Hematopoietic Cell Transplantation After Prior Targeted Therapy for High-Risk Chronic Lymphocytic Leukemia. Blood Adv (2020) 4(17):4113–23. doi: 10.1182/bloodadvances.2020002184
57. Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic Stem Cell Transplantation for Chronic Lymphocytic Leukemia in the Era of Novel Agents. Blood Adv (2020) 4(16):3977–89. doi: 10.1182/bloodadvances.2020001956
58. Lahoud OB, Devlin SM, Maloy MA, Roeker LE, Dahi PB, Ponce DM, et al. Reduced-Intensity Conditioning Hematopoietic Stem Cell Transplantation for Chronic Lymphocytic Leukemia and Richter's Transformation. Blood Adv (2021) 5(14):2879–89. doi: 10.1182/bloodadvances.2020003726
59. Cwynarski K, van Biezen A, de Wreede L, Stilgenbauer S, Bunjes D, Metzner B, et al. Autologous and Allogeneic Stem-Cell Transplantation for Transformed Chronic Lymphocytic Leukemia (Richter's Syndrome): A Retrospective Analysis From the Chronic Lymphocytic Leukemia Subcommittee of the Chronic Leukemia Working Party and Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol (2012) 30(18):2211–7. doi: 10.1200/JCO.2011.37.4108
60. Kim HT, Baker PO, Parry E, Davids M, Alyea EP, Ho VT, et al. Allogeneic Hematopoietic Cell Transplantation Outcomes in Patients With Richter's Transformation. Haematologica (2021) 106(12):3219–22. doi: 10.3324/haematol.2021.279033
61. Herrera AF, Ahn KW, Litovich C, Chen Y, Assal A, Bashir Q, et al. Autologous and Allogeneic Hematopoietic Cell Transplantation for Diffuse Large B-Cell Lymphoma-Type Richter Syndrome. Blood Adv (2021) 5(18):3528–39. doi: 10.1182/bloodadvances.2021004865
62. Kharfan-Dabaja MA, Kumar A, Stingo FE, Khimani F, Hussaini M, Ayala E, et al. Allogeneic Hematopoietic Cell Transplantation for Richter Syndrome: A Single-Center Experience. Clin Lymphoma Myeloma Leuk (2018) 18(1):e35–e9. doi: 10.1016/j.clml.2017.10.002
63. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (Zuma-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
64. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980
65. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene Maraleucel for Patients With Relapsed or Refractory Large B-Cell Lymphomas (Transcend Nhl 001): A Multicentre Seamless Design Study. Lancet (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0
66. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-Of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the Us Lymphoma Car T Consortium. J Clin Oncol (2020) 38(27):3119–28. doi: 10.1200/JCO.19.02104
67. Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol (2020) 38(27):3095–106. doi: 10.1200/JCO.19.02103
68. Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and Safety of Cd19-Directed Car-T Cell Therapies in Patients With Relapsed/Refractory Aggressive B-Cell Lymphomas: Observations From the Juliet, Zuma-1, and Transcend Trials. Am J Hematol (2021) 96(10):1295–312. doi: 10.1002/ajh.26301
69. Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With Cd19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol (2017) 35(26):3010–20. doi: 10.1200/JCO.2017.72.8519
70. Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason JE, et al. Phase 1 Transcend Cll 004 Study of Lisocabtagene Maraleucel in Patients With Relapsed/Refractory Cll or Sll. Blood (2021) 139(12):1794–806. doi: 10.1182/blood.2021011895
71. Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and Efficacy of Cd19-Targeted Car T Cells With Concurrent Ibrutinib for Cll After Ibrutinib Failure. Blood (2020) 135(19):1650–60. doi: 10.1182/blood.2019002936
72. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of Car-Transduced Natural Killer Cells in Cd19-Positive Lymphoid Tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607
73. Kittai AS, Bond DA, William B, Saad A, Penza S, Efebera Y, et al. Clinical Activity of Axicabtagene Ciloleucel in Adult Patients With Richter Syndrome. Blood Adv (2020) 4(19):4648–52. doi: 10.1182/bloodadvances.2020002783
74. Benjamini O, Shimoni A, Besser M, Shem-Tov N, Danylesko I, Yerushalmi R, et al. Safety and Efficacy of Cd19-Car T Cells in Richter's Transformation After Targeted Therapy for Chronic Lymphocytic Leukemia. Blood (2020) 136, Supplement 1:40. doi: 10.1182/blood-2020-138904
75. Ortiz-Maldonado V, Frigola G, Espanol-Rego M, Balague O, Martinez-Cibrian N, Magnano L, et al. Results of Ari-0001 Cart19 Cells in Patients With Chronic Lymphocytic Leukemia and Richter's Transformation. Front Oncol (2022) 12:828471. doi: 10.3389/fonc.2022.828471
76. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel Car T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
77. Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-Long Leukaemia Remissions With Persistence of Cd4(+) Car T Cells. Nature (2022) 602(7897):503–9. doi: 10.1038/s41586-021-04390-6
78. Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib Is an Irreversible Molecular Inhibitor of Itk Driving a Th1-Selective Pressure in T Lymphocytes. Blood (2013) 122(15):2539–49. doi: 10.1182/blood-2013-06-507947
79. Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib Treatment Improves T Cell Number and Function in Cll Patients. J Clin Invest (2017) 127(8):3052–64. doi: 10.1172/JCI89756
80. Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib Enhances Chimeric Antigen Receptor T-Cell Engraftment and Efficacy in Leukemia. Blood (2016) 127(9):1117–27. doi: 10.1182/blood-2015-11-679134
81. Fan F, Yoo HJ, Stock S, Wang L, Liu Y, Schubert ML, et al. Ibrutinib for Improved Chimeric Antigen Receptor T-Cell Production for Chronic Lymphocytic Leukemia Patients. Int J Cancer (2021) 148(2):419–28. doi: 10.1002/ijc.33212
82. Klaver Y, van Steenbergen SC, Sleijfer S, Debets R, Lamers CH. T Cell Maturation Stage Prior to and During Gmp Processing Informs on Car T Cell Expansion in Patients. Front Immunol (2016) 7:648. doi: 10.3389/fimmu.2016.00648
83. Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of Clinical-Grade Cd19-Specific Car-Modified Cd8+ Memory Stem Cells for the Treatment of Human B-Cell Malignancies. Blood (2016) 128(4):519–28. doi: 10.1182/blood-2015-11-683847
84. Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric Antigen Receptor-Modified T Cells Derived From Defined Cd8+ and Cd4+ Subsets Confer Superior Antitumor Reactivity in Vivo. Leukemia (2016) 30(2):492–500. doi: 10.1038/leu.2015.247
85. Funk CR, Wang S, Chen KZ, Waller A, Sharma A, Edgar CL, et al. Pi3kdelta/Gamma Inhibition Promotes Human Cart Cell Epigenetic and Metabolic Reprogramming to Enhance Antitumor Cytotoxicity. Blood (2022) 139(4):523–37. doi: 10.1182/blood.2021011597
86. Wang Y, Tschautscher MA, Rabe KG, Call TG, Leis JF, Kenderian SS, et al. Clinical Characteristics and Outcomes of Richter Transformation: Experience of 204 Patients From a Single Center. Haematologica (2020) 105(3):765–73. doi: 10.3324/haematol.2019.224121
87. Aulakh S, Reljic T, Yassine F, Ayala E, Chavez JC, Chanan-Khan A, et al. Allogeneic Hematopoietic Cell Transplantation Is an Effective Treatment for Patients With Richter Syndrome: A Systematic Review and Meta-Analysis. Hematol Oncol Stem Cell Ther (2021) 14(1):33–40. doi: 10.1016/j.hemonc.2020.05.002
88. Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of Donor-Derived Cd19-Redirected Virus-Specific T Cells for B-Cell Malignancies Relapsed After Allogeneic Stem Cell Transplant: A Phase 1 Study. Blood (2013) 122(17):2965–73. doi: 10.1182/blood-2013-06-506741
89. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-Cd19 Chimeric Antigen Receptor. J Clin Oncol (2015) 33(6):540–9. doi: 10.1200/JCO.2014.56.2025
Keywords: CLL (chronic lymphocytic leukemia), CAR (chimeric antigen receptor) T-cell therapy, richter syndrome, cellular therapy, allogenic stem cell transplantation
Citation: Barbanti MC, Appleby N, Kesavan M and Eyre TA (2022) Cellular Therapy in High-Risk Relapsed/Refractory Chronic Lymphocytic Leukemia and Richter Syndrome. Front. Oncol. 12:888109. doi: 10.3389/fonc.2022.888109
Received: 02 March 2022; Accepted: 04 April 2022;
Published: 28 April 2022.
Edited by:
John Riches, Queen Mary University of London, United KingdomReviewed by:
Adalgisa Condoluci, Oncology Institute of Southern Switzerland (IOSI), SwitzerlandJoanna Rhodes, Northwell Health, United States
Copyright © 2022 Barbanti, Appleby, Kesavan and Eyre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Chiara Barbanti, bWFyaWEuYmFyYmFudGlAb3VoLm5ocy51aw==; Toby Andrew Eyre, dG9ieS5leXJlQG91aC5uaHMudWs=
 Maria Chiara Barbanti
Maria Chiara Barbanti Niamh Appleby1,2
Niamh Appleby1,2 Toby Andrew Eyre
Toby Andrew Eyre