- Department and Institute of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Numerous studies have reported the role of statins on biochemical recurrence (BCR) among patients with prostate cancer (PCa) after definite treatment. However, the conclusions of these studies are contradictory. We aimed to determine the effect of statins on BCR of PCa using a systematic review and meta-analysis.
Methods: We searched PubMed (Medline) and other databases for cohort studies evaluating the effect of statins on the BCR of patients with PCa between January 1, 2000, and December 31, 2021. The random effects (RE) model and quality effects (QE) model were used to calculate the pooled hazard ratio (pHR) and pooled risk ratio (pRR) and their 95% confidence interval (95% CI).
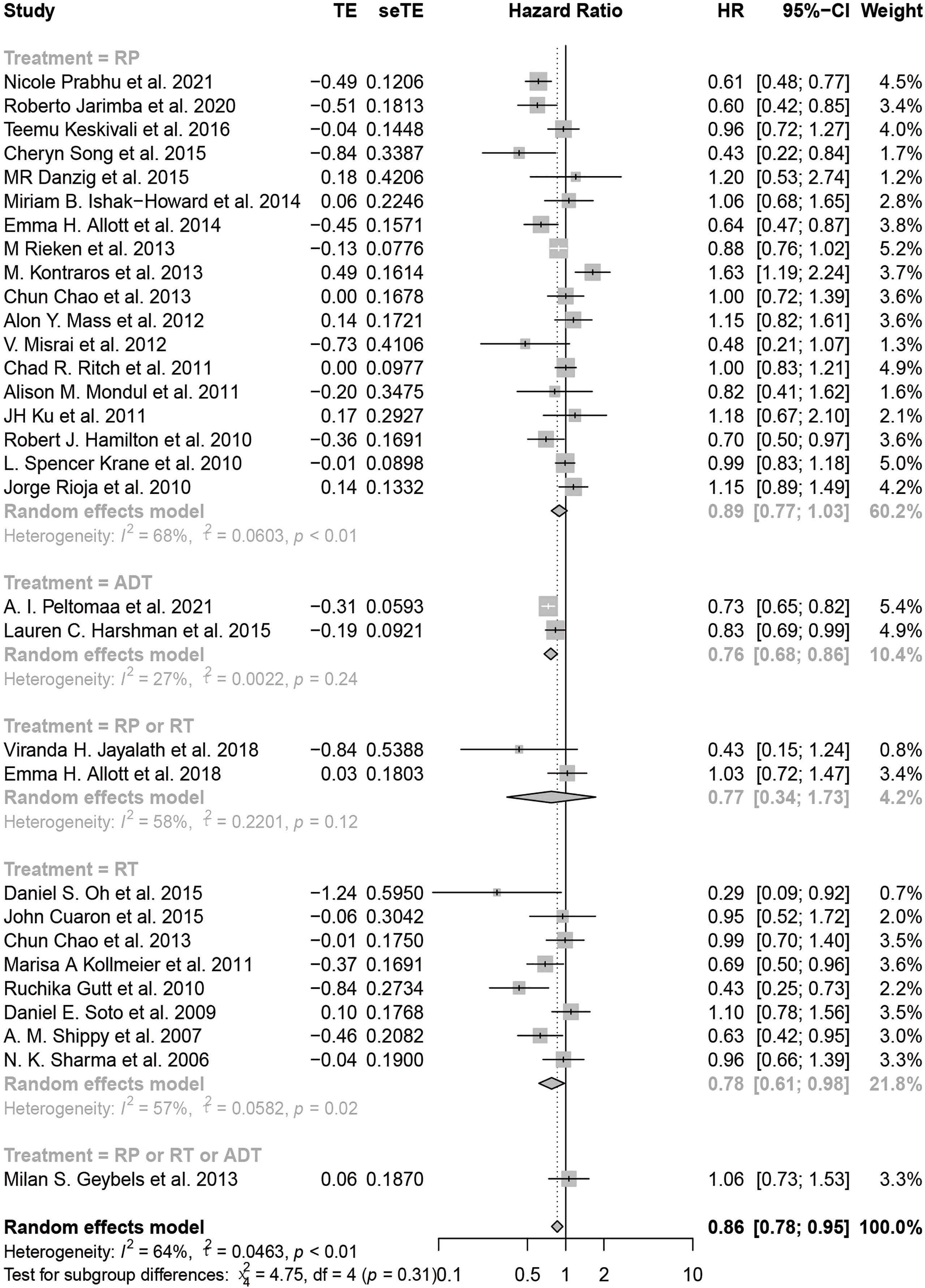
Results: A total of 33 cohort studies were finally selected and included in this systematic review and meta-analysis. Statin use was significantly associated with a 14% reduction in the HR of BCR (pHR: 0.86, 95% CI: 0.78 to 0.95, I2 = 64%, random effects model, 31 studies) and a 26% reduction in the RR of BCR (pRR: 0.74, 95% CI: 0.57 to 0.94, 24,591 patients, I2 = 88%, random effects model, 15 studies) among patients with PCa. The subgroup analyses showed that statins could result in 22% reduction in the HR of BCR (pHR: 0.78, 95% CI: 0.61 to 0.98, I2 = 57%, random effects model) among patients accepting radiotherapy (RT).
Conclusions: Our study suggests that statins have a unique role in the reduction of BCR in patients with PCa after definite treatment, especially RT. In the future, more clinical trials and in vitro and animal experiments are needed to further verify the effects of statins in PCa and the potential mechanisms.
Introduction
Prostate cancer (PCa) has the second highest incidence and the fifth highest mortality among all the malignant tumors in men around the world, causing more than 1,600,000 new cases and approximately 366,000 deaths annually (1). According to the data provided by the Global Burden of Disease Database, in 2017, there were 144,887 newly diagnosed PCa and 51,718 deaths in China, and the incidence of PCa is increasing year by year, which brought a heavy burden to public health and the national economy (2). Despite the high incidence, patients with non-metastatic PCa could choose various treatments such as active surveillance, radical prostatectomy (RP) and pelvic lymph node dissection (PLND), radiotherapy (RT), and androgen deprivation therapy (ADT) according to the stage of disease and the prognosis is good for those with low risk PCa (3). After treatment with curative intent, the measurement of prostate-specific antigen (PSA) becomes the most validated and sensitive method to monitor relapse (4). Biochemical recurrence (BCR) is defined as the return of detectable PSA, and nearly 20%–40% men treated with RP (5) or 30%–50% of those treated with RT will develop BCR (6), which indicates a nearly 30% probability of clinical recurrence after RP (7) and approximately 16.4% probability of death (8). Since BCR is one of the strongest evidences for clinical recurrence and progression of PCa, it is urgent for us to find effective treatment and protective factors to decrease the risk of BCR and improve the survival of patients after primary treatments.
Statins are 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors, which could inhibit the cholesterol synthesis by suppressing the activity of the rate-limiting enzyme in the liver. As commonly used drugs for secondary prevention of cardiovascular disease, statins are widely used worldwide. A cross-sectional study based on a total of 2,613,035 participants in 31 provinces in China showed that about 19.3% of them had ever used or were using statins (9). Although the role of statins in preventing cardiovascular disease by improving hypercholesterolemia is indisputable, in recent years, increasing evidence has suggested that statins also play a non-negligible role in chemoprevention and treatment of other diseases such as erectile dysfunction possibly by improving hyperhomocysteinemia (10, 11), and advanced tumors, including colon cancer, pancreatic cancer, and PCa (12–17). Existing studies have shown that the effects of statins on PCa are mainly achieved through two kinds of mechanisms: cholesterol-mediated and non-cholesterol-mediated pathways (18). Statins could influence the growth and progression of PCa mainly by cholesterol-mediated pathways. A positive correlation between cholesterol accumulation in prostate tissue and PCa incidence was reported as early as 1981 (19). Several mechanisms have demonstrated that dysregulation of cholesterol homeostasis in prostate cells contributes to the development of PCa. One study found that hypermethylation of the ABCA1 promoter resulted in a decreased expression of cholesterol efflux transporters, resulting in lower cholesterol efflux rates and increased cholesterol levels in prostate cancer cells. The presence of this epigenetic alteration is associated with high-grade prostate cancer (20). In addition, the mTOR pathway is also important in the regulation of sterol regulatory element-binding proteins (SREBPs), which are important transcription factors that control lipid and cholesterol homeostasis (21). A study reported that the intracellular accumulation of cholesterol lipid droplets is driven by loss of expression of the tumor-suppressor PTEN and subsequent activation of the PI3K–AKT–mTOR signaling pathway, which is also connected with high-grade prostate cancer in humans (22). The areas of cholesterol accumulation on the cell membrane are called lipid rafts, which could initiate downstream signaling pathways and lead to the growth and development of PCa. Statins could reduce the level of cholesterol and affect the formation of lipid rafts on the cell membrane, thereby affecting the androgen receptor (AR) pathway, epidermal growth factor receptor (EGFR) pathway, luteinizing hormone receptor pathway, and others (23–25), thus inhibiting downstream signaling pathways such as AKT and JAK-STAT3 (26), and then suppressing tumor cell growth and promoting cell apoptosis. Therefore, statins could affect the accumulation of cholesterol and block the necessary survival signals needed by tumors.
Additionally, cholesterol is the precursor of androgen, so statins can affect the synthesis of intracellular androgen by reducing the level of serum cholesterol, thereby affecting the growth of prostate cancer cells. An randomized controlled trial (RCT) showed that 80 mg/day of atorvastatin was associated with a reduction in serum androgen levels in PCa patients, but whether androgen levels in prostate tissue were also significantly reduced remains to be studied (27). Besides, statins could also suppress cancer cell proliferation by reducing the levels of mevalonate (MVA) and isoprenoids derived from it, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which were essential for posttranslational modifications of a variety of proteins called protein prenylation. Protein prenylation was important for the localization, membrane anchoring, and function of numerous signaling proteins, including Rho-GTPase family members such as Ras and the Rho GTPases, which could function as intermediators between extra- and intracellular signaling and regulate the activity of several kinases to regulate different physiological processes (28). Rho GTPases were tightly connected with growth-promoting pathways like mTOR and MAPK signaling pathways and contributed to tumorigenesis, metastasis, and drug resistance (29). Besides, the mevalonate pathway could influence Hippo/YAP signaling, which was important in tissue proliferation and tumorigenesis (30). Furthermore, statins could possibly induce apoptosis in cancer cells independent of their effect on cholesterol levels by suppressing cyclin−dependent kinase 2 (CDK2) or activating caspases and promoting cell-cycle arrest in PCa (31, 32).
More than 60 studies have reported the interaction between statin use and the prognosis of patients with PCa after definite treatment, including BCR, prostate cancer-specific mortality (PCSM), and overall survival (OS). The results of most literatures are encouraging but contradictory at the same time, which indicates that the effect of statins on the prognosis of PCa patients remains controversial. A meta-analysis of 34 observational studies published in 2016 showed that statin use could significantly reduce the risk of biochemical recurrence (BCR) in patients receiving RT (HR: 0.79, 95% CI: 0.65, 0.95, p = 0.01), but there was no statistically significant reduction in BCR risk in patients treated with RP (HR: 0.94, 95% CI: 0.81, 1.09, p = 0.43). Meanwhile, statins have a significant effect on the reduction of tumor metastasis, all-cause mortality, and PCSM after treatment (33). The investigators also observed a significant heterogeneity in the included studies. Since many new studies have been published since 2016, we decided to conduct this systematic review and meta-analysis to reevaluate the association between statin use and the risk of BCR among patients with prostate cancer after definite treatments.
Materials and Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 reporting guideline (34).
Criteria for Study Selection
All the studies were included into this systematic review and meta-analysis if they met the following criteria: (1) the exposure of interest was statin use; (2) the study design was cohort; (3) the outcome of interest was BCR of prostate cancer; (4) the follow-up ≥ 6 months; and (5) risk estimates and 95% confidence intervals (CIs) were reported (or information to calculate them). The animal studies, in vitro studies, RCTs, and case–control studies were excluded. No language or publication status limits were applied.
Literature Search and Search Procedure
We searched PubMed (Medline), EMBASE, and Cochrane Library for cohort studies evaluating the effects of statins on the BCR of patients with prostate cancer between January 1, 2000, and December 31, 2021. We also searched Google Scholar to retrieve gray literatures such as meeting abstracts. We searched these databases using key words such as “statins,” “HMG-CoA inhibitor,” “prostate cancer,” and “prostatic neoplasms.” The detailed search strategy for each database is reported in Supplementary Table 1 with keywords and the number of retrieved citations per string. During the screening procedure, two reviewers (J-XS and X-YZ) independently searched abstracts and selected them according to the search criteria. The inter-rater kappa statistic was calculated to evaluate the consistency between the two authors for using the inclusion and exclusion criteria. Discrepancies about the inclusion or exclusion were resolved by consensus of the third author (Q-DX). The EndNote application (version X9) was used to remove the duplicates and apply the inclusion criteria. We utilized a PRISMA flowchart to depict the literature search procedure (Figure 1).
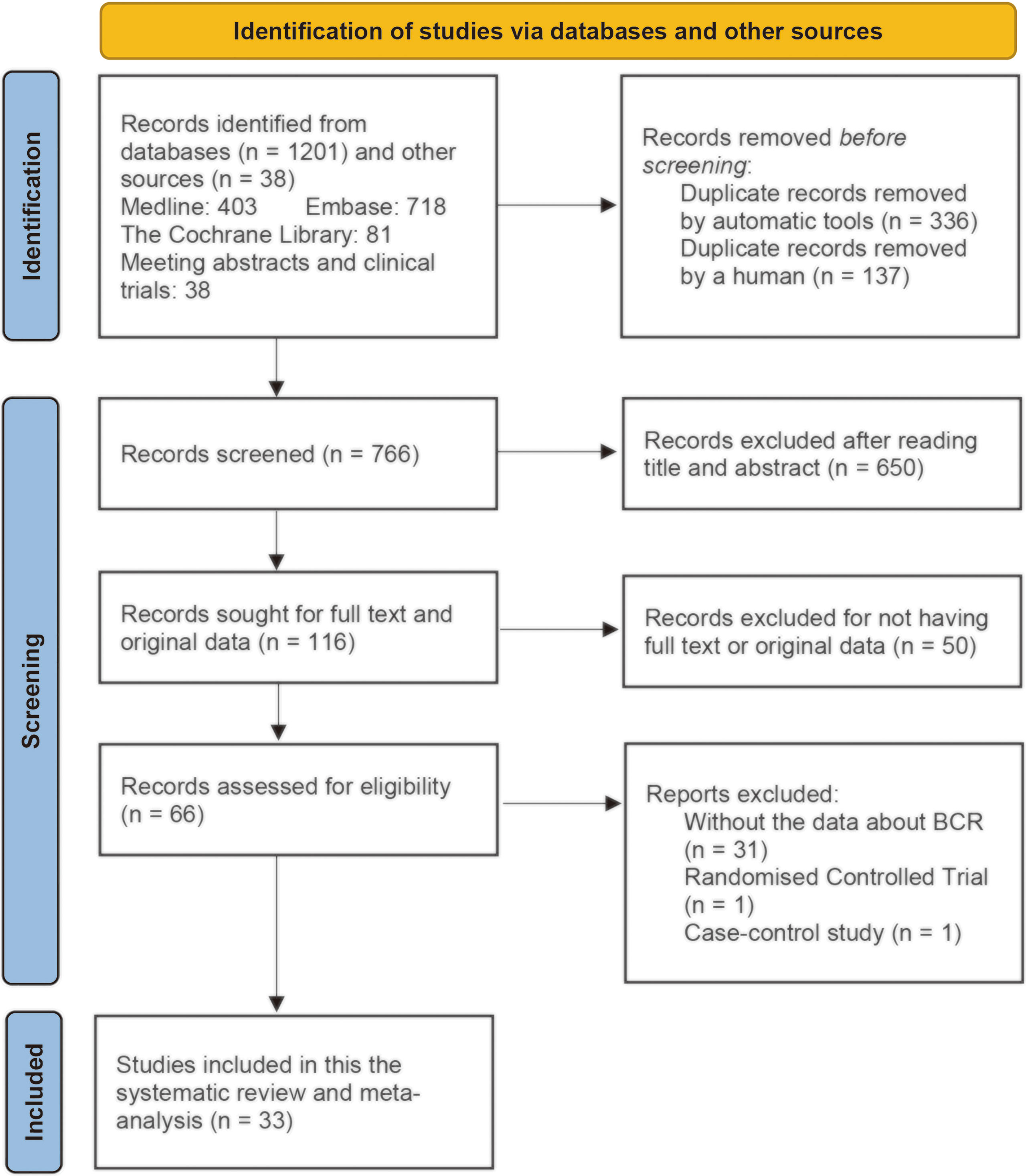
Figure 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart for study selection for the systematic review on statins and clinical outcomes among patients with prostate cancer following definitive therapy.
Data Extraction
Three authors (J-XS, C-QL, and Q-DX) independently extracted information from the included studies using a designed data extraction sheet. The data extraction sheet consisted of bibliographic information and background information. Bibliographic information included author name, year of publication, and journal name and title. Background information included the inclusion and exclusion criteria for patients, age, follow-up period, body mass index (BMI), the level of serum cholesterol, race, the level of PSA, Gleason score (GS), tumor stage, primary treatment, the definition of statin use, the dose and median duration of usage of statins, definition of BCR, the number of patients, the number of statin users, and the number of patients with BCR. Moreover, we also extracted the data about the outcomes. The primary outcome of interest for this study was BCR. The adjusted multivariate hazard ratio (HR) and risk ratio (RR) with corresponding 95% confidence intervals (CIs) were used to assess the potential association between statin use and BCR after primary treatment.
Literature Quality Assessment
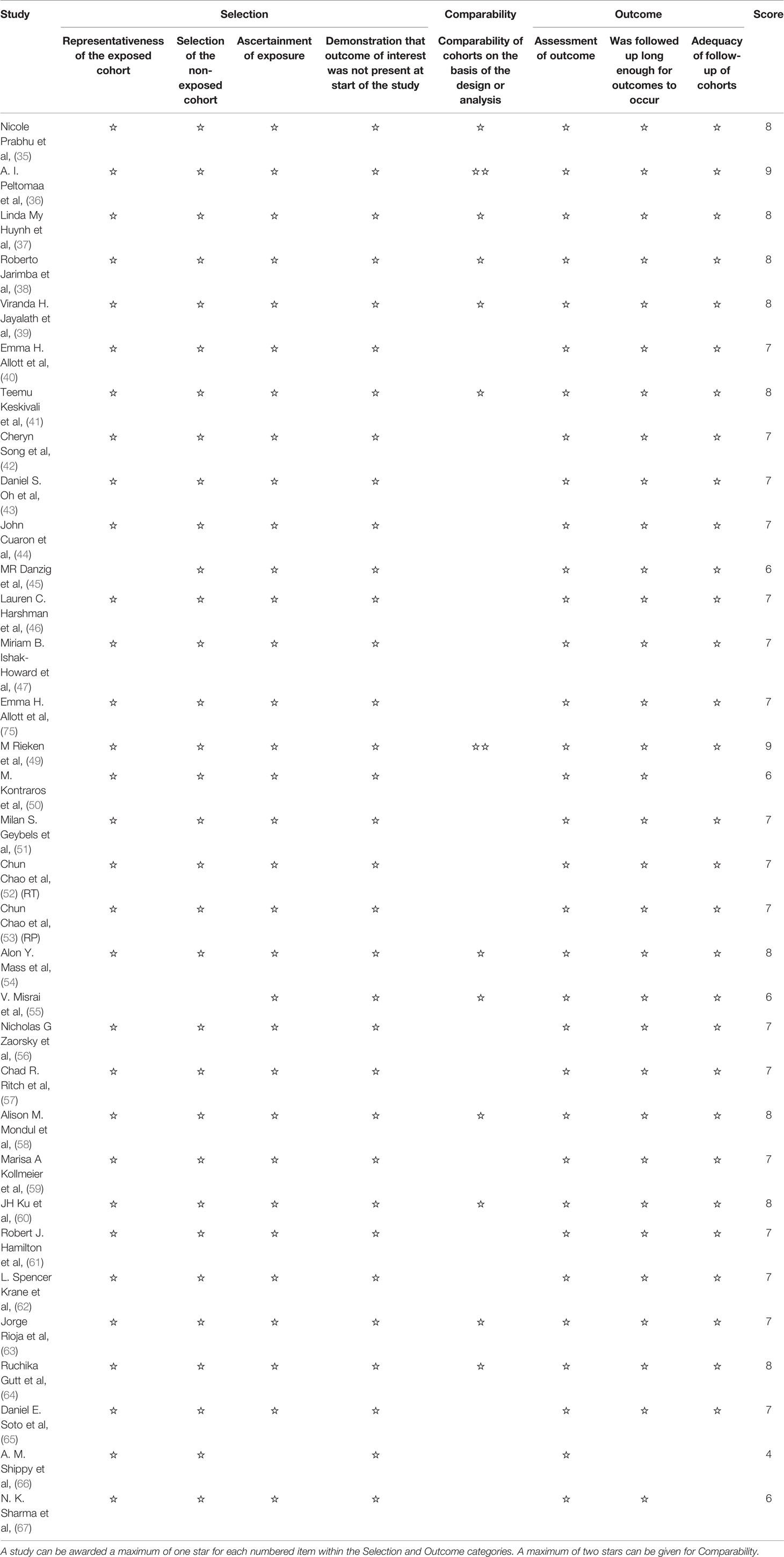
We adapted the Newcastle–Ottawa scale (NOS) tool to assess the risk of bias of the included cohort studies. The NOS consists of three categories (Selection, Comparability, and Outcome) and a total of eight items (Table 2). A study can be awarded a maximum of one point for each numbered item within the Selection and Outcome categories, and a maximum of two points can be given for Comparability (68). Therefore, a study can be awarded at most nine points in total. The quality of the studies was considered as good, fair, or poor based on the Agency of Healthcare Research and Quality (AHRQ) standards using the scores obtained from the NOS (69).
Data Synthesis and Analysis
We calculated a pooled hazard ratio (pHR) and a pooled risk ratio (pRR) with 95% confidence interval (CI) for BCR reported in the included studies using random effects (RE) models and quality effects (QE) models, respectively. We analyzed the heterogeneity between studies using the standard Cochrane chi-square χ2 (Cochrane’s Q) test with a significance level of α = 0.10 and the I2 test. An I2 statistic ≥50% indicates a considerable level of heterogeneity. The L’Abbé plot and Galbraith plot were used to visually display the heterogeneity of included studies. We performed subgroup analyses stratified by parameters such as primary treatment and country to find out the potential source of heterogeneity. We also performed meta-regression using parameters such as age, follow-up duration, publication year, PSA level, BMI (value or the percentage of BMI < 30 kg/m2), serum cholesterol level, percentage of patients in tumor stage ⩾T3, percentage of patients with Gleason score ⩾7, and percentage of patients with black race, which could be responsible for the differences in the outcomes observed among the studies. We determined the presence of publication bias in observational studies using both the Begg’s (70) and Egger’s (71) tests. A contour-enhanced funnel plot was utilized to determine other causes of publication bias by examining the symmetry of the plot. Further, we did sensitivity analyses and cumulative meta-analysis by stepwise adding or omitting included studies. We also applied the trim-and-fill method to evaluate the effect of publication bias (72), and a filled forest plot was constructed to preclude the publication bias on pHR and pRR. The meta-analyses using a QE model were performed using the MetaXL software to estimate the pHR and pRR. All the other data processing and statistical analysis were conducted by R software version 4.1.1. All the p-values were on two sides, and p-value <0.05 was considered with statistical significance.
Results
A total of 1,239 publications were retrieved from electronic databases and gray literatures, and a total of 33 studies were selected and included in this systematic review and meta-analysis after employing exclusion criteria (Figure 1). A total of 473 duplicates were removed by automatic tools and artificial identification successively. A total of 650 records were excluded after reading the title and abstract, and 50 records were excluded for not having full-text or original data. After reading the full text, 31 records were excluded due to lack of data about BCR and two records were excluded because they did not belong to cohort studies (one RCT (73) and one case–control (74) study). Finally, 33 studies met the inclusion criteria for the current review. The inter-rater reliability between the two authors during the selection process was good (κ = 0.87).
Characteristics of Included Studies
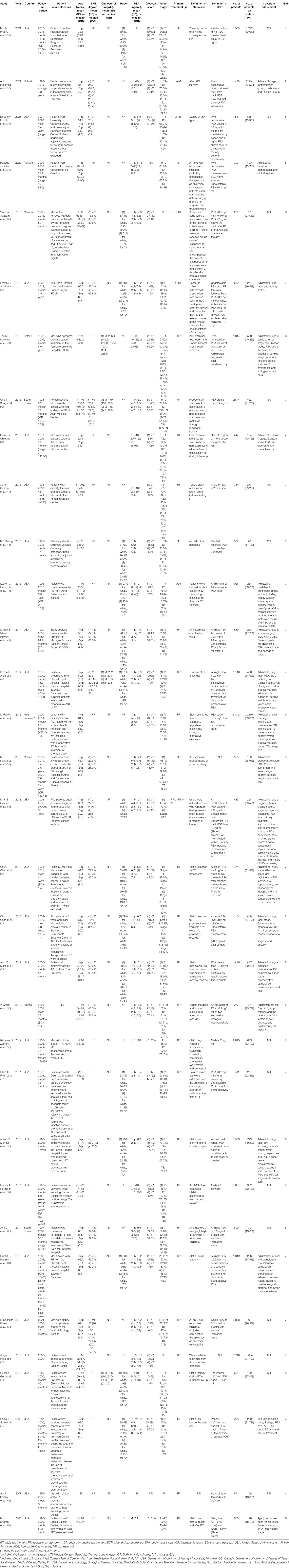
The characteristics of all the 33 studies are presented in Table 1. All the studies were observational cohort studies published between 2006 and 2021. Twenty-four studies were conducted in the United States (35, 37, 40, 43–48, 51–54, 56–59, 61–67), two in South Korea (42, 60), one in Portugal (38), one in Greece (50), one in France (55), two in Finland (36, 41), and one in Canada (39), and one study collected data from six centers located in the North America and Europe (49). The study cohort size ranged from 247 (43) to 6,842 (49) among the included studies. The percentage of statin users ranged from 11.4% (45) to 70.4% (43). All the included studies had at least 2 years of median or mean follow-up duration. The primary treatment of patients for 18 studies was RP, including open, laparoscopic, or robot-assisted RP. Nine studies used RT (either external beam, brachytherapy, or a combination of them) as primary treatment. Three studies included patients treated with RP or RT. Two studies chose ADT as their primary treatment, and one study brought into patients treated with RP or RT or ADT. In some studies, patients accepting adjuvant treatment before RP or RT were excluded (37, 45, 52, 53, 57, 60, 65). The definition of the BCR in most studies were the same: a posttreatment PSA value of 0.2 ng/ml or greater in men who underwent RP; nadir PSA level +2 ng/ml (Phoenix criteria), for men treated with RT; any PSA increase in men treated with primary ADT; and no evidence of clinical and/or radiographically detected disease. However, the definition of statin use was various in different studies. Patients were considered as statin users if they had ever used statins of any type or any dose at any time recorded in the medication database in many studies. However, the type, dose, or duration of statin use were strictly defined in some studies. For example, five studies recorded the dose of statin use (36, 40, 41, 50, 61) and four studies recorded the type of statins (42, 50, 61, 65). Three studies restricted the duration of statin use such as statin use longer than 10 years (35, 47, 51).
Characteristics of Patients With Prostate Cancer
The mean or median age of the patients in the included studies ranged from 55.2 to 72.8 years. Seventeen studies collected the data about BMI, in the form of either BMI value or the percentage of patients with BMI < 30 kg/m2. The mean or median BMI of patients ranged from 24.4 to 28.4, and the percentage of patients with BMI < 30 kg/m2 ranged from 43.4% to 99.2%, which indicated that most patients included were overweight or obese. Only four studies provided the data about the level of serum cholesterol (41, 42, 48, 64). The median serum cholesterol level ranged from 166 to 206.4 mg/dl. The majority of studies consist of mainly patients of white race with black race less than 20%. However, the patients with black race constituted >30% in five studies (40, 45, 48, 61, 64). There were no significant differences in race and statin use. Thirty-one studies reported the level of PSA before primary treatment. The median or mean PSA level for statin users ranged from 3.1 to 9.6 ng/ml, and the percentage of patients with PSA > 10 ng/ml ranged from 5.7% to 30%. Statin users had a significant lower baseline PSA level than non-users. Thirty-one studies reported the Gleason score, and the percentage of patients with Gleason score ≥7 in the majority of studies was between 50% and 70%. However, all the patients had a Gleason score <7 in two studies (37, 39). There were no significant differences in Gleason score and statin use. Twenty-nine studies recorded the clinical tumor stage of prostate cancer. The tumor stage varies dramatically in different studies, and there existed a significant difference between tumor stage among statin users and non-users in some studies.
Quality Assessment of the Included Studies
We applied the NOS tool to assess the quality of included studies (Table 1). The majority of the included studies had a good or fair quality except two meeting abstracts (66, 67) for lack of complete data.
Statin Use and the HR and RR of BCR
The HR of BCR was reported in 31 including studies, and the RR of BCR was reported in 15 including studies. As shown in the forest plots, statin users were significantly less likely to experience the BCR of prostate cancer after primary treatment, with a pHR of 0.86 (95% CI: 0.78 to 0.95, I2 = 64%, random effects model, Figure 2A) and a pRR of 0.74 (95% CI: 0.57 to 0.94, 24591 patients, I2 = 88%, random effects model, Figure 2B). Subgroup analyses according to primary treatment for HR showed that there still existed significant BCR reduction among patients accepting RT (pHR: 0.78, 95% CI: 0.61 to 0.98, I2 = 57%, random effects model, Figure 3) or ADT (pHR: 0.76, 95% CI: 0.68 to 0.86, I2 = 27%, random effects model, Figure 3) as their primary treatment, which was consistent with previously published articles (33, 76). However, as for RR, the subgroup analyses according to primary treatment exhibited that there just existed a significant difference in patients accepting ADT but only one study was divided into this group (Figure S1A). Subgroup analyses according to country for both RR (Figure S1B) and HR (Figure S1C) showed that statin use was significantly associated with BCR reduction in patients from USA.
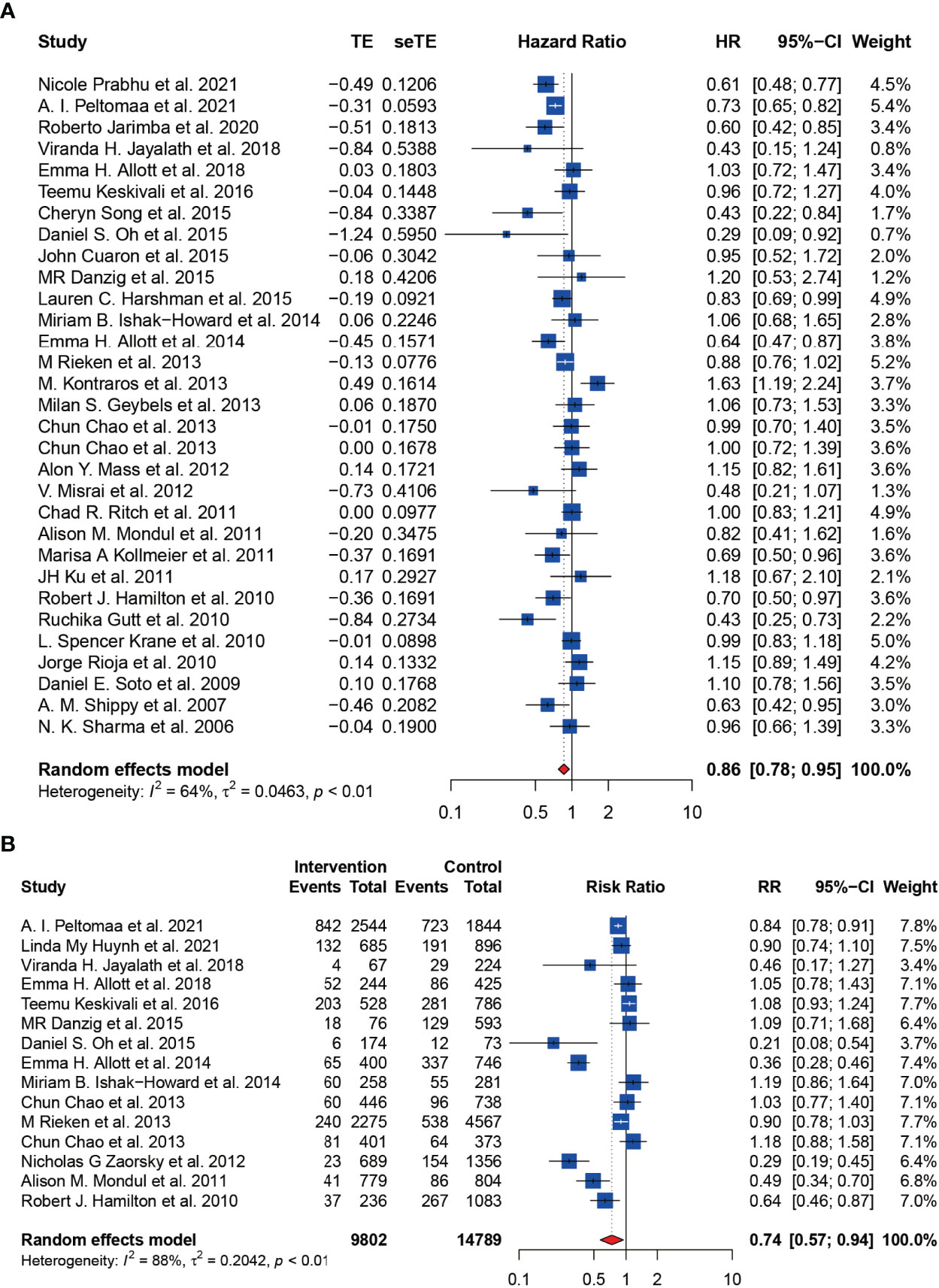
Figure 2 The effect of statins on BCR risk of prostate cancer among men following definitive therapy using the random effects model. (A) Forest plot for the HR of BCR. (B) Forest plot for the RR of BCR.
As the heterogeneity was high in both the main analysis and subgroup analyses, we then performed meta-regression to find out the covariates causing this variability. We used publication year, follow-up duration, age, BMI value, the percentage of BMI <30 kg/m2, serum cholesterol level, the percentage of AA, serum PSA level, GS, and stage to construct the univariate meta-regression model. For HR, we found that the serum cholesterol level was significantly associated with BCR (p = 0.0074, Figure 4A). As for RR, there existed a remarkable connection between GS and BCR (p = 0.0448, Figure 4B). However, we did not find a significant association between publication year, follow-up duration, age, BMI value, the percentage of BMI <30 kg/m2, the percentage of AA, serum PSA level, GS or stage, and BCR in HR (Figures S2A–I, Table S2), and publication year, follow-up duration, age, BMI value, the percentage of BMI <30 kg/m2, the percentage of AA, serum PSA level or stage, and BCR in RR (Figures S3A–H, Table S3).
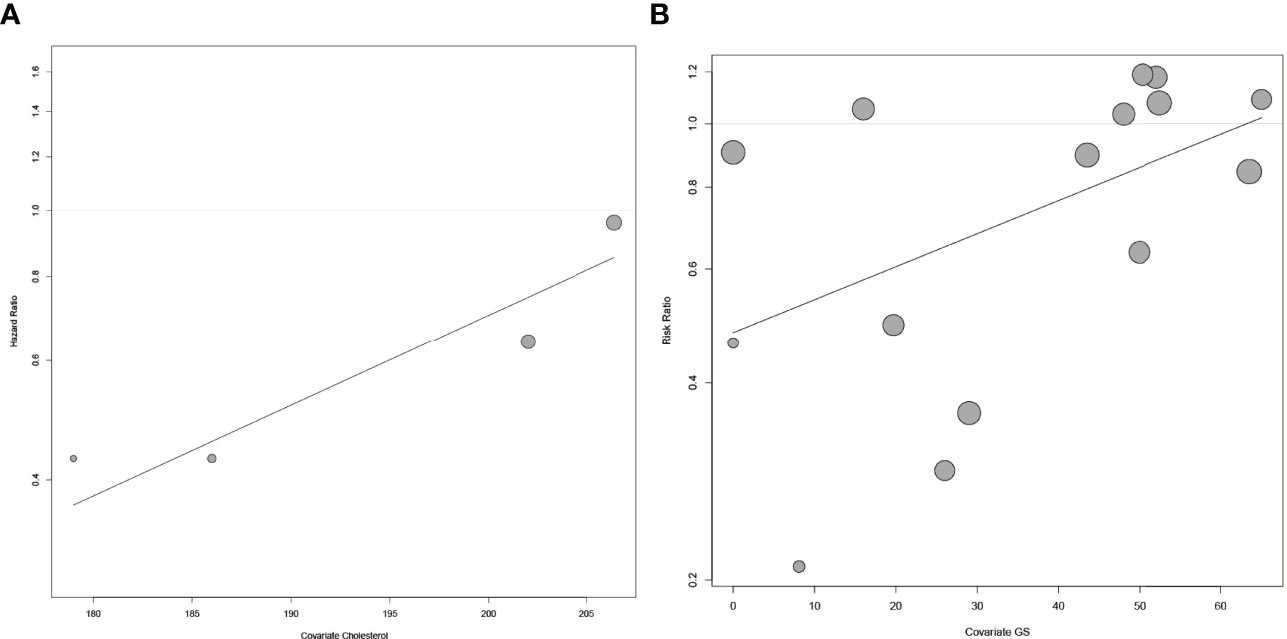
Figure 4 The meta-regression for risk of BCR and covariates. (A) The meta-regression for HR of BCR and the level of serum cholesterol. (B) The meta-regression for RR of BCR and GS. Each dot represents an individual study. Symbol size represents sample size.
Then we conducted sensitivity analysis and cumulative meta-analysis by sequentially omitting or adding each study in turn to evaluate its effect on the pHR or pRR. For HR, we could observe that the overall estimates remained stable after omitting (Figure 5A) and adding (Figure 5B) each study. Moreover, we did not detect a statistically significant publication bias based on Begg’s test (z = -1.22, p = 0.2210) and Egger’s test (t = -0.27, p = 0.7897). Besides, the contour-enhanced funnel plot also showed good symmetry of the plot (Figure 5C). The trim-and-fill method suggested little evidence of publication bias (Figure 5D) and estimated that two studies were missing resulting from publication bias (Figure 5E). After filling the two missing studies, the filled forest plot also showed a significant reduction in BCR among statin users with a pHR of 0.88 (95% CI: 0.79 to 0.97, I2 = 66%, random effects model), which was in accordance with the original model. As shown in Figure 5F, the Galbraith plot also exhibited a low publication bias with most studies located between the dashed lines. As for RR, we could observe a significant change in the pooled effect when omitting or adding three studies including Oh et al., Allott et al., and Zaorsky et al. (43, 48, 56) (Figure S4A, B). The contour-enhanced funnel plot also did not show good symmetry of the plot visually with most studies lying outside of the dashed lines (Figure S4C). However, we did not identify a statistically significant publication bias based on Begg’s test (z = -1.14, p = 0.2550) and Egger’s test (t = -1.17, p = 0.2639). In contrast, the trim-and-fill method supported the result of the contour-enhanced funnel plot (Figure S4D) and estimated that two studies were missing resulting from publication bias (Figure S4E). After filling the two missing studies, the pooled effect lost statistical significance with a pHR of 0.83 (95% CI: 0.62 to 1.11, I2 = 90%, random effects model), which indicated that the publication bias might have influenced the original outcome. The Galbraith plot also exhibited a relatively high publication bias with almost half of the studies outside between the dashed lines (Figure S4F). Nevertheless, the L’Abbé plot showed that included studies generally agreed on the positive effect of statins in reducing the RR of BCR (Figure S4G).
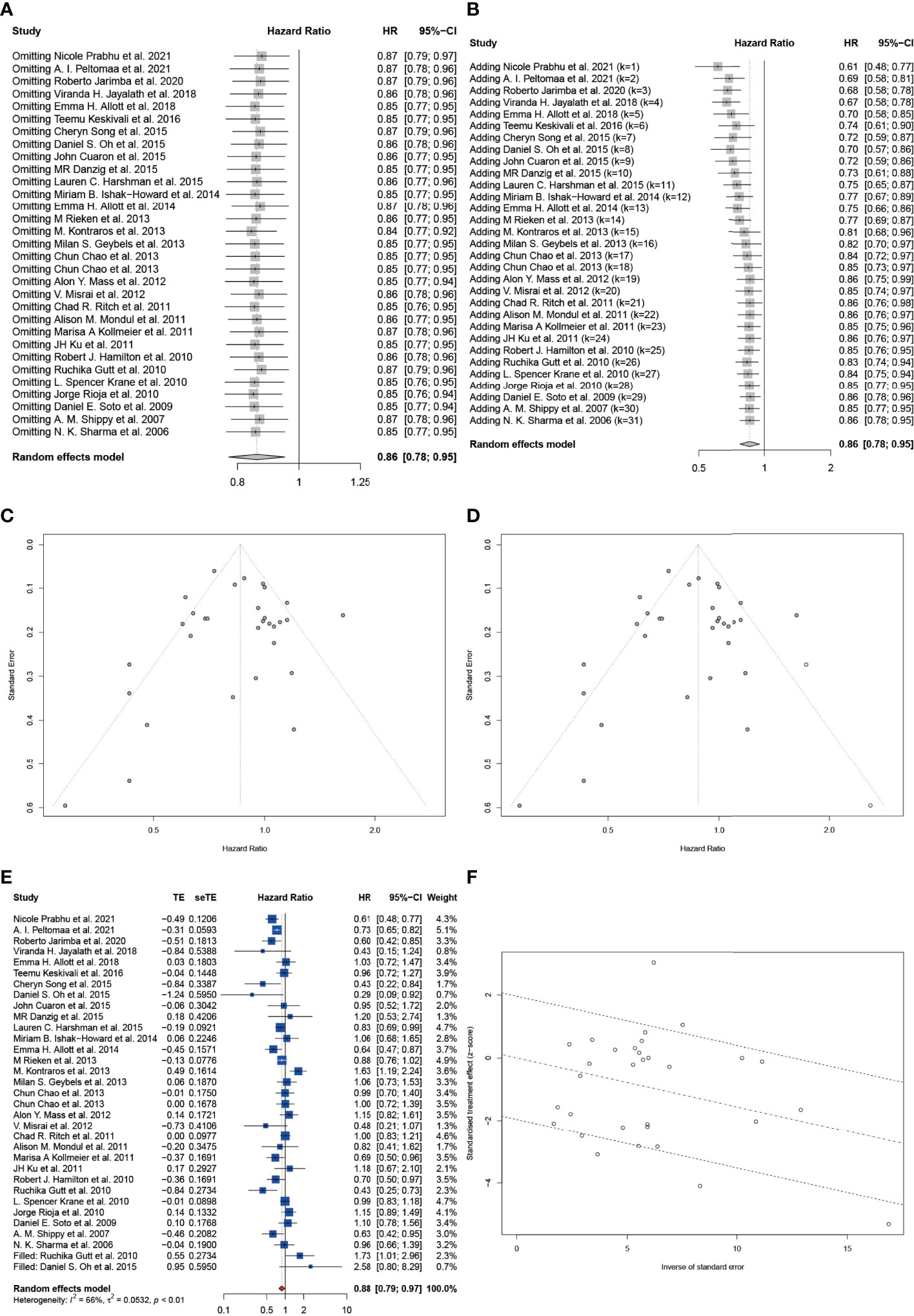
Figure 5 Sensitivity analysis and the detection of publication bias for included studies on HR of BCR. (A) Sensitivity analysis by stepwise omitting the included studies. (B) Cumulative meta-analysis by stepwise adding the included studies. (C) The funnel plot. (D) The trim and fill funnel plot. (E) The filled forest plot. (F) The Galbraith plot. Effect size as z-scores plotted as a function of the inverse standard error for each study reported in the present study. The middle line is the line of best fit, while the upper and lower dashed lines represent the upper and lower 95% confidence limits.
Considering the quality of the included studies, we also performed meta-analyses using a QE model using the MetaXL software. As shown in Figure 6A, for HR, there still existed a significant reduction in BCR among statin users with study quality taken into consideration (pHR: 0.84, 95% CI: 0.75 to 0.95, I2 = 64%, quality effects model). However, as for RR, the result lost statistical significance when using the QE model (pHR: 0.83, 95% CI: 0.62 to 1.12, I2 = 88%, quality effects model, Figure 6B), which revealed that there existed a remarkable heterogeneity in the quality of included studies on the RR of BCR.
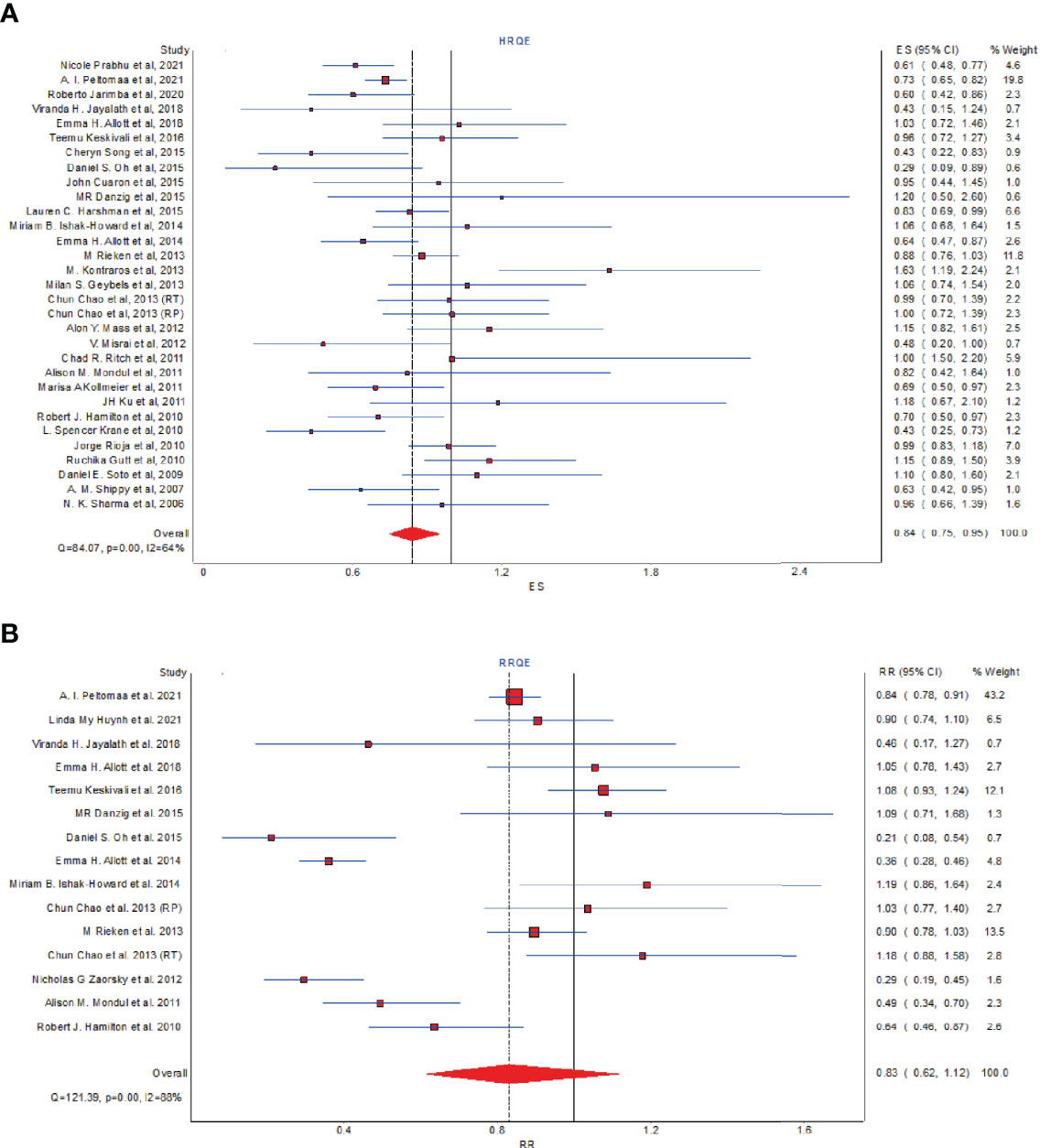
Figure 6 The effect of statins on BCR risk of prostate cancer among men following definitive therapy using the quality effects model. (A) Forest plot for the HR of BCR. (B) Forest plot for the RR of BCR.
Discussion
The aim of our study was to reevaluate the association between statin use and the risk of BCR among patients with PCa after definite treatment. This review comprised 33 cohort studies, including 31 studies reporting the HR of BCR and 15 studies reporting the RR of BCR. We found that statin use was tightly connected with the reduction of BCR among patients with PCa, especially for those accepting RT as their primary treatment, which was consistent with previously published meta-analyses (33, 76). Although patients in the subgroup accepting ADT also showed a significant reduction in HR of BCR, the number of studies in this subgroup was too limited and part of the patients have also accepted other treatments except ADT in these studies, which could bring bias into the final results. Although five studies have reported a remarkable effect of statins on the reduction of BCR after RP, the pooled effect showed no statistical significance due to the heterogeneity of included studies. An RCT study published by Jeong et al. in 2021 showed that 20 mg/day of atorvastatin use for 24 months had no significant effect on the risk of BCR in patients with high-grade prostate cancer after RP (HR, 1.00; 95% CI, 0.71–1.41), which was in accordance with our conclusion. The reason why statins could reduce the risk of BCR could be that statin might improve the radiosensitivity of prostate cancer by causing cell-cycle arrest in the late G1 phase (77). Statins could induce late G1 arrest and apoptosis by inhibition of cdk2, E2F1, p21, and/or p27 (78). A recent study showed that statins could also enhance the effects of RT by triggering the interaction between Bcl-2 and MSH2 (79) and compromising DNA double-strand breaks repair (80). The development and progression of PCa were dependent on androgens, and cholesterol is a precursor for androgen synthesis. Therefore, cholesterol lowering by statins could suppress androgen synthesis and enhance the efficacy of ADT treatment. It was also reported that statins could compete with androgens for influx by the SLCO2B1 transporter, thus decreasing tumor’s androgen supply (81). In vitro studies have also discovered that statins could increase the therapeutic effect of abiraterone acetate and enzalutamide (82). Further studies are needed, and more clinical trials should be carried out to verify the hypotheses.
Previous epidemiological observations and preclinical models suggested that hypercholesterolemia might play a crucial role in the incidence and progression of PCa, especially in increasing the risk of high-grade, aggressive disease and castration resistance (83, 84). It was also reported that elevated cholesterol was associated with increased risk of recurrence among men with dyslipidemia after RP (75). In our study, using meta-regression, we found that serum cholesterol level was a significant confounder which could neutralize the protective effect of statins on BCR, which indicated that statins might reduce the risk of BCR by mediating hypercholesterolemia. A recent study has found that sterol-O-acyl transferases (SOAT) 1, an enzyme involved in cholesteryl ester synthesis, was remarkably connected to earlier BCR in high-risk prostate cancer (85). However, Lefebvre et al. observed that there existed no significant association between metabolic syndrome including hypercholesterolemia and the risk of BCR in Afro-Caribbean men with PCa after RP (86). Allott et al. also found that high cholesterol was not associated with progression of PCa after RP or RT (40). Therefore, it was still controversial and more studies were needed.
We also observed that serum PSA level was significantly lower in statin users compared with non-users in many included studies (46, 57, 59, 62, 65). It was reported that PSA level could be influenced by smoking status, Gleason score, and 5α-reductase inhibitor for benign prostate hyperplasia treatment, but not associated with other clinical factors including hypercholesterolemia in a retrospective study (87, 88). An RCT study published by Murtola et al. in 2018 showed that 80 mg/day of atorvastatin could not significantly reduce the tumor proliferation index (Ki-67) and PSA level, but in subgroup analyses, atorvastatin use over 28 days exhibited a significant reduction in Ki-67 and PSA (89). Therefore, it was possible that statin use could only cover the truth of BCR and disease progression through decreasing the PSA level instead of preventing BCR. However, if this hypothesis was true, the detection of BCR would be delayed and the prognosis would be worse. However, the previously published meta-analysis found that statins have a significant effect on the reduction of tumor metastasis, all-cause mortality, and PCSM after treatment (33), which indicated a better prognosis and was contradictory to this hypothesis.
In this review, we have also used various methods to detect, evaluate, and diminish the probable heterogeneity and publication bias of included studies. For studies about the HR of BCR, the sensitivity analysis and cumulative meta-analysis all showed a stable pooled result. The funnel plot, Begg’s test, and Egger’s test all exhibited little publication bias from qualitative and quantitative perspectives, respectively. After the trim-and-fill method, the pooled result also had a statistical significance. However, as for studies about the RR of BCR, there did exist a relatively high heterogeneity and publication bias. This could result from the limited number of included studies, and RR did not take time into consideration, which could lead into bias in the methodology. We also used the QE model, which took the quality of studies into account, to reevaluate the pooled results of RR and HR. Not surprisingly, the pHR remained stable with a statistical significance, which further proved that our results were religious and authentic.
Nevertheless, there still existed many limitations in our review. First, the definitions of statin use were various in the included studies. Information about the types of statins, the duration of statin use, the dose of statins, and the initiation of statin use (before or after primary treatment) was not complete and detailed in the included studies. Therefore, we could not take this into consideration, which will definitely contribute to the heterogeneity of studies. Second, there existed great heterogeneity in the characteristics of the studying cohort. Many patients in the statin group had preexisting comorbidities such as cardiovascular diseases and metabolic syndrome, which could influence the progression of PCa. Third, the characteristics of PCa could also be a potential confounder of the results. Although tumor stage did not show a statistical significance in the meta-regression, the GS, metastasis status, PCa volume, and surgical margin status could all be connected with BCR and contribute to the heterogeneity of studies. Fourth, although we have performed subgroup analyses according to the primary treatment, many patients did not accept only one kind of treatment and part of patients also accepted ADT after RT or RP, which could interfere with the result of subgroup analyses. Fifth, although many studies have provided the results adjusted for important covariates, some unadjusted results might influence the final pooled effect. Finally, although the pHR showed that statins lowered the BCR of PCa, the upper confidence interval was close to 1.00. Thus, the result needs to be deliberately explained.
In conclusion, despite some limitations, our study suggests that statin, a widely used and relatively cheap drug, has a unique role in the reduction of BCR in patients with PCa after definite treatment, especially RT. In the future, more clinical trials and in vitro and animal experiments were needed to further verify the effects of statins in PCa and the mechanisms behind this phenomenon.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
J-XS, X-YZ, Z-BZ, Q-DX, and S-GW contributed to developing the main research question, carrying out the literature search, collecting the included studies’ information, and describing the results. J-XS performed the meta-analysis and wrote the first draft of the manuscript. C-QL and J-ZX contributed to developing the main research question and revised the manuscript. YA, M-YX, and JH revised the manuscript. All authors contributed to the article and approved the submitted version. J-XS, Z-BZ, Q-DX, and S-GW contributed equally to this work.
Funding
This work was supported by the Natural Science Foundation of China (81772729) and Undergraduate Training Program for Innovation and Entrepreneurship (DELC2022010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the R programming package developers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.887854/full#supplementary-material
Supplementary Figure 1 | The forest plot for subgroup analyses. (A) The forest plot for the RR of BCR with subgroup analyses by primary treatment. (B) The forest plot for the RR of BCR with subgroup analyses by country. (C) The forest plot for the HR of BCR with subgroup analyses by country.
Supplementary Figure 2 | The meta-regression for HR of BCR and covariates. (A) Tumor stage. (B) GS. (C) PSA. (D) The percentage of AA. (E) BMI<30. (F) BMI value. (G) Age. (H) Follow-up duration. (I) Publication year. Each dot represents an individual study. Symbol size represents sample size.
Supplementary Figure 3 | The meta-regression for RR of BCR and covariates. (A) BMI<30. (B) BMI value. (C) PSA. (D) Tumor stage. (E) Publication year. (F) Age. (G) Follow-up duration. (H) The percentage of AA. Each dot represents an individual study. Symbol size represents sample size.
Supplementary Figure 4 | Sensitivity analysis and the detection of publication bias for included studies on RR of BCR. (A) Sensitivity analysis by stepwise omitting the included studies. (B) Cumulative meta-analysis by stepwise adding the included studies. (C) The funnel plot. (D) The trim and fill funnel plot. (E) The filled forest plot. (F). The Galbraith plot. Effect size as z-scores plotted as a function of the inverse standard error for each study reported in the present study. The middle line is the line of best fit, while upper and lower dashed lines represent the upper and lower 95% confidence limits. (G) The L’Abbé plot for incidence of BCR. Each dot represents an individual study. Symbol size represents sample size.
Supplementary Table 3 | Univariable meta-regression for the RR of biochemical recurrence.
References
1. Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med (2018) 8(12):030361. doi: 10.1101/cshperspect.a030361
2. Liu X, Yu C, Bi Y, Zhang ZJ. Trends and Age-Period-Cohort Effect on Incidence and Mortality of Prostate Cancer From 1990 to 2017 in China. Public Health (2019) 172:70–80. doi: 10.1016/j.puhe.2019.04.016
3. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent. Eur Urol (2021) 79(2):243–62. doi: 10.1016/j.eururo.2020.09.042
4. Amaro A, Esposito AI, Gallina A, Nees M, Angelini G, Albini A, et al. Validation of Proposed Prostate Cancer Biomarkers With Gene Expression Data: A Long Road to Travel. Cancer Metastasis Rev (2014) 33(2):657–71. doi: 10.1007/s10555-013-9470-4
5. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer Progression and Survival Rates Following Anatomical Radical Retropubic Prostatectomy in 3,478 Consecutive Patients: Long-Term Results. J Urol (2004) 172(3):910–4. doi: 10.1097/01.ju.0000134888.22332.bb
6. Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of Different Definitions of Biochemical Failure After External Beam Radiotherapy Changes Conclusions About Relative Treatment Efficacy for Localized Prostate Cancer. Urology (2006) 68(3):593–8. doi: 10.1016/j.urology.2006.03.075
7. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. J Am Med Assoc (1999) 281(17):1591–7. doi: 10.1001/jama.281.17.1591
8. Boorjian SA, Thompson RH, Tollefson MK, Rangel LJ, Bergstralh EJ, Blute ML, et al. Long-Term Risk of Clinical Progression After Biochemical Recurrence Following Radical Prostatectomy: The Impact of Time From Surgery to Recurrence. Eur Urol (2011) 59(6):893–9. doi: 10.1016/j.eururo.2011.02.026
9. Lu J, Zhang L, Lu Y, Su M, Li X, Liu J, et al. Secondary Prevention of Cardiovascular Disease in China. Heart (2020) 106(17):1349–56. doi: 10.1136/heartjnl-2019-315884
10. Giovannone R, Busetto GM, Antonini G, De Cobelli O, Ferro M, Tricarico S, et al. Hyperhomocysteinemia as an Early Predictor of Erectile Dysfunction: International Index of Erectile Function (IIEF) and Penile Doppler Ultrasound Correlation With Plasma Levels of Homocysteine. Med (Baltimore) (2015) 94(39):e1556. doi: 10.1097/MD.0000000000001556
11. Kostis JB, Dobrzynski JM. Statins and Erectile Dysfunction. World J Mens Health (2019) 37(1):1–3. doi: 10.5534/wjmh.180015
12. Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the Risk of Colorectal Cancer. N Engl J Med (2005) 352(21):2184–92. doi: 10.1056/NEJMoa043792
13. Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin Drugs and Risk of Advanced Prostate Cancer. J Natl Cancer Inst (2006) 98(24):1819–25. doi: 10.1093/jnci/djj499
14. Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, et al. Statins and Prostate Cancer Risk: A Case-Control Study. Am J Epidemiol (2005) 162(4):318–25. doi: 10.1093/aje/kwi203
15. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The Risk of Cancer in Users of Statins. J Clin Oncol (2004) 22(12):2388–94. doi: 10.1200/JCO.2004.02.027
16. Zhang P, Liu B. Statin Use and the Risk of Multiple Myeloma: A PRISMA-Compliant Meta-Analysis. Ann Hematol (2020) 99(8):1805–12. doi: 10.1007/s00277-020-04157-5
17. Wang Y, Li Q, Yuan Z, Ma S, Shao S, Wu Y, et al. Statin Use and Benefits of Thyroid Function: A Retrospective Cohort Study. Front Endocrinol (Lausanne) (2021) 12:578909. doi: 10.3389/fendo.2021.578909
18. Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The Current Evidence on Statin Use and Prostate Cancer Prevention: Are We There Yet? Nat Rev Urol (2017) 14(2):107–19. doi: 10.1038/nrurol.2016.199
19. Schaffner CP. Prostatic Cholesterol Metabolism: Regulation and Alteration. Prog Clin Biol Res (1981) 75a:279–324.
20. Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, et al. Dysregulation of Cholesterol Homeostasis in Human Prostate Cancer Through Loss of ABCA1. Cancer Res (2013) 73(3):1211–8. doi: 10.1158/0008-5472.CAN-12-3128
21. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP Activity is Regulated by Mtorc1 and Contributes to Akt-Dependent Cell Growth. Cell Metab (2008) 8(3):224–36. doi: 10.1016/j.cmet.2008.07.007
22. Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab (2014) 19(3):393–406. doi: 10.1016/j.cmet.2014.01.019
23. Freeman MR, Cinar B, Lu ML. Membrane Rafts as Potential Sites of Nongenomic Hormonal Signaling in Prostate Cancer. Trends Endocrinol Metab (2005) 16(6):273–9. doi: 10.1016/j.tem.2005.06.002
24. Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-Rich Lipid Rafts Mediate Akt-Regulated Survival in Prostate Cancer Cells. Cancer Res (2002) 62(8):2227–31.
25. Smith SM, Lei Y, Liu J, Cahill ME, Hagen GM, Barisas BG, et al. Luteinizing Hormone Receptors Translocate to Plasma Membrane Microdomains After Binding of Human Chorionic Gonadotropin. Endocrinology (2006) 147(4):1789–95. doi: 10.1210/en.2005-1046
26. Jung YY, Ko JH, Um JY, Chinnathambi A, Alharbi SA, Sethi G, et al. LDL Cholesterol Promotes the Proliferation of Prostate and Pancreatic Cancer Cells by Activating the STAT3 Pathway. J Cell Physiol (2021) 236(7):5253–64. doi: 10.1002/jcp.30229
27. Raittinen PVH, Syvälä H, Tammela TLJ, Häkkinen MR, Ilmonen P, Auriola S, et al. Atorvastatin Induces Adrenal Androgen Downshift in Men With Prostate Cancer: A Post Hoc Analysis of a Pilot Adaptive Randomised Clinical Trial. EBioMedicine (2021) 68:103432. doi: 10.1016/j.ebiom.2021.103432
28. Göbel A, Rauner M, Hofbauer LC, Rachner TD. Cholesterol and Beyond - The Role of the Mevalonate Pathway in Cancer Biology. Biochim Biophys Acta Rev Cancer (2020) 1873(2):188351. doi: 10.1016/j.bbcan.2020.188351
29. Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-Translational Modifications and Regulation of the RAS Superfamily of GTPases as Anticancer Targets. Nat Rev Drug Discov (2007) 6(7):541–55. doi: 10.1038/nrd2221
30. Ingallina E, Sorrentino G, Bertolio R, Lisek K, Zannini A, Azzolin L, et al. Mechanical Cues Control Mutant P53 Stability Through a Mevalonate-RhoA Axis. Nat Cell Biol (2018) 20(1):28–35. doi: 10.1038/s41556-017-0009-8
31. Lee SJ, Ha MJ, Lee J, Nguyen P, Choi YH, Pirnia F, et al. Inhibition of the 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Pathway Induces P53-Independent Transcriptional Regulation of P21(WAF1/CIP1) in Human Prostate Carcinoma Cells. J Biol Chem (1998) 273(17):10618–23. doi: 10.1074/jbc.273.17.10618
32. Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, et al. Caspase-7 is Activated During Lovastatin-Induced Apoptosis of the Prostate Cancer Cell Line LNCaP. Cancer Res (1998) 58(1):76–83.
33. Raval AD, Thakker D, Negi H, Vyas A, Kaur H, Salkini MW. Association Between Statins and Clinical Outcomes Among Men With Prostate Cancer: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis (2016) 19(2):151–62. doi: 10.1038/pcan.2015.58
34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj (2021) 372:n71. doi: 10.1186/s13643-021-01626-4
35. Prabhu N, Kapur N, Catalona W, Leikin R, Helenowski I, Jovanovich B, et al. Statin Use and Risk of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy. Urol Oncol (2021) 39(2):130.e9–.e15. doi: 10.1016/j.urolonc.2020.09.027
36. Peltomaa AI, Raittinen P, Talala K, Taari K, Tammela TLJ, Auvinen A, et al. Prostate Cancer Prognosis After Initiation of Androgen Deprivation Therapy Among Statin Users. A Population-Based Cohort Study. Prostate Cancer Prostatic Dis (2021) 24(3):917–24. doi: 10.1038/s41391-021-00351-2
37. Huynh LM, Keit E, Schuller AA, Carrillo RC, Huang E, Ahlering TE, et al. Impact of Statin Use on Overall and Time to Biochemical Failure Following Radical Prostatectomy or Radiation Therapy. World J Urol (2021) 39(9):3287–93. doi: 10.1007/s00345-021-03600-0
38. Jarimba R, Lima JP, Eliseu M, Carvalho J, Antunes H, da Silva ET, et al. Statins Prevent Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Single-Center Retrospective Study With a Median Follow-Up of 51.20 Months. Res Rep Urol (2020) 12:439–46. doi: 10.2147/RRU.S258267
39. Jayalath VH, Nayan M, Finelli A, Komisarenki M, Timilshina N, Kulkarni GS, et al. Statin Use and Time to Progression in Men on Active Surveillance for Prostate Cancer. Prostate Cancer Prostatic Dis (2018) 21(4):509–15. doi: 10.1038/s41391-018-0053-x
40. Allott EH, Farnan L, Steck SE, Song L, Arab L, Su LJ, et al. Statin Use, High Cholesterol and Prostate Cancer Progression; Results From HCaP-NC. Prostate (2018) 78(11):857–64. doi: 10.1002/pros.23644
41. Keskivali T, Kujala P, Visakorpi T, Tammela TLJ, Murtola TJ. Statin Use and Risk of Disease Recurrence and Death After Radical Prostatectomy. Prostate (2016) 76(5):469–78. doi: 10.1002/pros.23138
42. Song C, Park S, Park J, Shim M, Kim A, Jeong IG, et al. Statin Use After Radical Prostatectomy Reduces Biochemical Recurrence in Men With Prostate Cancer. Prostate (2015) 75(2):211–7. doi: 10.1002/pros.22907
43. Oh DS, Koontz B, Freedland SJ, Gerber L, Patel P, Lewis S, et al. Statin Use is Associated With Decreased Prostate Cancer Recurrence in Men Treated With Brachytherapy. World J Urol (2015) 33(1):93–7. doi: 10.1007/s00345-014-1281-x
44. Cuaron J, Pei X, Cohen GN, Cox BW, Yamada Y, Zelefsky MJ, et al. Statin Use Not Associated With Improved Outcomes in Patients Treated With Brachytherapy for Prostate Cancer. Brachytherapy (2015) 14(2):179–84. doi: 10.1016/j.brachy.2014.05.019
45. Danzig MR, Kotamarti S, Ghandour RA, Rothberg MB, Dubow BP, Benson MC, et al. Synergism Between Metformin and Statins in Modifying the Risk of Biochemical Recurrence Following Radical Prostatectomy in Men With Diabetes. Prostate Cancer Prostatic Dis (2015) 18(1):63–8. doi: 10.1038/pcan.2014.47
46. Harshman LC, Wang X, Nakabayashi M, Xie W, Valenca L, Werner L, et al. Statin Use at the Time of Initiation of Androgen Deprivation Therapy and Time to Progression in Patients With Hormone-Sensitive Prostate Cancer. JAMA Oncol (2015) 1(4):495–504. doi: 10.1001/jamaoncol.2015.0829
47. Ishak-Howard MB, Okoth LA, Cooney KA. Statin Use and the Risk of Recurrence After Radical Prostatectomy in a Cohort of Men With Inherited and/or Early-Onset Forms of Prostate Cancer. Urology (2014) 83(6):1356–61. doi: 10.1016/j.urology.2014.02.015
48. Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Postoperative Statin Use and Risk of Biochemical Recurrence Following Radical Prostatectomy: Results From the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. BJU Int (2014) 114(5):661–6. doi: 10.1111/bju.12720
49. Rieken M, Kluth LA, Xylinas E, Seitz C, Fajkovic H, Karakiewicz PI, et al. Impact of Statin Use on Biochemical Recurrence in Patients Treated With Radical Prostatectomy. Prostate Cancer Prostatic Dis (2013) 16(4):367–71. doi: 10.1038/pcan.2013.31
50. Kontraros M, Varkarakis I, Ntoumas K, Deliveliotis C. Pathological Characteristics, Biochemical Recurrence and Functional Outcome in Radical Prostatectomy Patients on Statin Therapy. Urologia Internationalis (2013) 90(3):263–9. doi: 10.1159/000346751
51. Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. Statin Use in Relation to Prostate Cancer Outcomes in a Population-Based Patient Cohort Study. Prostate (2013) 73(11):1214–22. doi: 10.1002/pros.22671
52. Chao C, Williams SG, Xu L, Chen J, Wallner LP, Porter KR, et al. Statin Therapy is Not Associated With Prostate Cancer Recurrence Among Patients Who Underwent Radiation Therapy. Cancer Lett (2013) 335(1):214–8. doi: 10.1016/j.canlet.2013.02.017
53. Chao C, Jacobsen SJ, Xu L, Wallner LP, Porter KR, Williams SG. Use of Statins and Prostate Cancer Recurrence Among Patients Treated With Radical Prostatectomy. BJU Int (2013) 111(6):954–62. doi: 10.1111/j.1464-410X.2012.11639.x
54. Mass AY, Agalliu I, Laze J, Lepor H. Preoperative Statin Therapy is Not Associated With Biochemical Recurrence After Radical Prostatectomy: Our Experience and Meta-Analysis. J Urol (2012) 188(3):786–91. doi: 10.1016/j.juro.2012.05.011
55. Misrai V, Do C, Lhez JM, Elman B, Latorzeff I, Portalez D, et al. Is Statin Use Associated With D'Amico Risk Groups and Biochemical Recurrence After Radical Prostatectomy? Prog Urol (2012) 22(5):273–8. doi: 10.1016/j.purol.2011.11.001
56. Zaorsky NG, Buyyounouski MK, Li T, Horwitz EM. Aspirin and Statin Nonuse Associated With Early Biochemical Failure After Prostate Radiation Therapy. Int J Radiat Oncol Biol Phys (2012) 84(1):e13–7. doi: 10.1016/j.ijrobp.2012.02.050
57. Ritch CR, Hruby G, Badani KK, Benson MC, McKiernan JM. Effect of Statin Use on Biochemical Outcome Following Radical Prostatectomy. BJU Int (2011) 108(8 Pt 2):E211–6. doi: 10.1111/j.1464-410X.2011.10159.x
58. Mondul AM, Han M, Humphreys EB, Meinhold CL, Walsh PC, Platz EA. Association of Statin Use With Pathological Tumor Characteristics and Prostate Cancer Recurrence After Surgery. J Urol (2011) 185(4):1268–73. doi: 10.1016/j.juro.2010.11.089
59. Kollmeier MA, Katz MS, Mak K, Yamada Y, Feder DJ, Zhang Z, et al. Improved Biochemical Outcomes With Statin Use in Patients With High-Risk Localized Prostate Cancer Treated With Radiotherapy. Int J Radiat Oncol Biol Phys (2011) 79(3):713–8. doi: 10.1016/j.ijrobp.2009.12.006
60. Ku JH, Jeong CW, Park YH, Cho MC, Kwak C, Kim HH. Relationship of Statins to Clinical Presentation and Biochemical Outcomes After Radical Prostatectomy in Korean Patients. Prostate Cancer Prostatic Dis (2011) 14(1):63–8. doi: 10.1038/pcan.2010.39
61. Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, et al. Statin Medication Use and the Risk of Biochemical Recurrence After Radical Prostatectomy: Results From the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer (2010) 116(14):3389–98. doi: 10.1002/cncr.25308
62. Krane LS, Kaul SA, Stricker HJ, Peabody JO, Menon M, Agarwal PK. Men Presenting for Radical Prostatectomy on Preoperative Statin Therapy Have Reduced Serum Prostate Specific Antigen. J Urol (2010) 183(1):118–24. doi: 10.1016/j.juro.2009.08.151
63. Rioja J, Pinochet R, Savage CJ, Guillonneau BD, Scardino PT, Eastham JA, et al. 125 Impact of Statin Use on Pathologic Features in Men Treated With Radical Prostatectomy. J Urol Postate Cancer: Epidemiol Natural History (2010) 183(4S):e51. doi: 10.1016/j.juro.2010.02.176
64. Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin Use and Risk of Prostate Cancer Recurrence in Men Treated With Radiation Therapy. J Clin Oncol (2010) 28(16):2653–9. doi: 10.1200/JCO.2009.27.3003
65. Soto DE, Daignault S, Sandler HM, Ray ME. No Effect of Statins on Biochemical Outcomes After Radiotherapy for Localized Prostate Cancer. Urology (2009) 73(1):158–62. doi: 10.1016/j.urology.2008.02.055
66. Shippy A, Katz M, Yamada Y, Feder D, Zelefsky MJ. Statin Use and Clinical Outcomes After High Dose Radiotherapy for Prostate Cancer. J Radiat Oncol Biol Phys (2007) 69(3):S113. doi: 10.1016/j.ijrobp.2007.07.209
67. Sharma N, Ruth K, Horwitz E, Buyyounouski M, Pollack A. 2281: Statin Use Prior to Radiation Therapy for Prostate Cancer Does Not Improve Outcome: The Fox Chase Experience. J Radiat Oncol Biol Phys (2006) 66(3):S366.
68. Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. AHRQ Methods for Effective Health Care. In: Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments. Rockville (MD: Agency for Healthcare Research and Quality (US (2012).
69. Zakhari A, Delpero E, McKeown S, Tomlinson G, Bougie O, Murji A. Endometriosis Recurrence Following Post-Operative Hormonal Suppression: A Systematic Review and Meta-Analysis. Hum Reprod Update (2021) 27(1):96–107. doi: 10.1093/humupd/dmaa033
70. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
71. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
72. Duval S, Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
73. Jeong IG, Lim B, Yun SC, Lim JH, Hong JH, Kim CS. Adjuvant Low-Dose Statin Use After Radical Prostatectomy: The PRO-STAT Randomized Clinical Trial. Clin Cancer Res (2021) 27(18):5004–11. doi: 10.1158/1078-0432.CCR-21-0480
74. Meijer D, van Moorselaar RJA, Vis AN, Bijnsdorp IV. Prostate Cancer Development Is Not Affected by Statin Use in Patients With Elevated PSA Levels. Cancers (Basel) (2019) 11(7):953. doi: 10.3390/cancers11070953
75. Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum Lipid Profile and Risk of Prostate Cancer Recurrence: Results From the SEARCH Database. Cancer Epidemiol Biomarkers Prev (2014) 23(11):2349–56. doi: 10.1158/1055-9965.EPI-14-0458
76. Tan P, Wei S, Yang L, Tang Z, Cao D, Liu L, et al. The Effect of Statins on Prostate Cancer Recurrence and Mortality After Definitive Therapy: A Systematic Review and Meta-Analysis. Sci Rep (2016) 6:29106. doi: 10.1038/srep29106
78. Hutchinson J, Marignol L. Clinical Potential of Statins in Prostate Cancer Radiation Therapy. Anticancer Res (2017) 37(10):5363–72. doi: 10.21873/anticanres.11962
79. He Z, Yuan J, Shen F, Zeng F, Qi P, Wang Z, et al. Atorvastatin Enhances Effects of Radiotherapy on Prostate Cancer Cells and Xenograft Tumor Mice Through Triggering Interaction Between Bcl-2 and MSH2. Med Sci Monit (2020) 26:e923560. doi: 10.12659/MSM.923560
80. Chen YA, Shih HW, Lin YC, Hsu HY, Wu TF, Tsai CH, et al. Simvastatin Sensitizes Radioresistant Prostate Cancer Cells by Compromising DNA Double-Strand Break Repair. Front Pharmacol (2018) 9:600. doi: 10.3389/fphar.2018.00600
81. Harshman LC, Werner L, Tripathi A, Wang X, Maughan BL, Antonarakis ES, et al. The Impact of Statin Use on the Efficacy of Abiraterone Acetate in Patients With Castration-Resistant Prostate Cancer. Prostate (2017) 77(13):1303–11. doi: 10.1002/pros.23390
82. Syvälä H, Pennanen P, Bläuer M, Tammela TL, Murtola TJ. Additive Inhibitory Effects of Simvastatin and Enzalutamide on Androgen-Sensitive LNCaP and VCaP Prostate Cancer Cells. Biochem Biophys Res Commun (2016) 481(1-2):46–50. doi: 10.1016/j.bbrc.2016.11.021
83. Pelton K, Freeman MR, Solomon KR. Cholesterol and Prostate Cancer. Curr Opin Pharmacol (2012) 12(6):751–9. doi: 10.1016/j.coph.2012.07.006
84. Dickerman B, Mucci L. Metabolic Factors and Prostate Cancer Risk. Clin Chem (2019) 65(1):42–4. doi: 10.1373/clinchem.2018.287243
85. Eckhardt C, Sbiera I, Krebs M, Sbiera S, Spahn M, Kneitz B, et al. High Expression of Sterol-O-Acyl Transferase 1 (SOAT1), an Enzyme Involved in Cholesterol Metabolism, is Associated With Earlier Biochemical Recurrence in High Risk Prostate Cancer. Prostate Cancer Prostatic Dis (2021). doi: 10.1038/s41391-021-00431-3
86. Lefebvre F, Blanchet-Deverly A, Michineau L, Blanchet P, Multigner L, Brureau L. Metabolic Syndrome and Prostate Cancer in Afro-Caribbean Men. Prostate (2022) 82(3):359–65. doi: 10.1002/pros.24281
87. Tarantino G, Crocetto F, Vito CD, Martino R, Pandolfo SD, Creta M, et al. Clinical Factors Affecting Prostate-Specific Antigen Levels in Prostate Cancer Patients Undergoing Radical Prostatectomy: A Retrospective Study. Future Sci OA (2021) 7(3):Fso643. doi: 10.2144/fsoa-2020-0154
88. Busetto GM, Giovannone R, Antonini G, Rossi A, Del Giudice F, Tricarico S, et al. Short-Term Pretreatment With a Dual 5α-Reductase Inhibitor Before Bipolar Transurethral Resection of the Prostate (B-TURP): Evaluation of Prostate Vascularity and Decreased Surgical Blood Loss in Large Prostates. BJU Int (2015) 116(1):117–23. doi: 10.1111/bju.12917
Keywords: statins, prostate cancer, biochemical recurrence, meta‐analysis, radical prostatectomy, radiotherapy
Citation: Sun J-X, Liu C-Q, Zhong X-Y, Xu J-Z, An Y, Xu M-Y, Hu J, Zhang Z-B, Xia Q-D and Wang S-G (2022) Statin Use and the Risk of Prostate Cancer Biochemical Recurrence Following Definitive Therapy: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Oncol. 12:887854. doi: 10.3389/fonc.2022.887854
Received: 03 March 2022; Accepted: 11 April 2022;
Published: 09 May 2022.
Edited by:
Matteo Ferro, European Institute of Oncology (IEO), ItalyReviewed by:
Felice Crocetto, Federico II University Hospital, ItalyEttore De Berardinis, Sapienza University of Rome, Italy
Copyright © 2022 Sun, Liu, Zhong, Xu, An, Xu, Hu, Zhang, Xia and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zong-Biao Zhang, enpiMDcwQDEyNi5jb20=; Qi-Dong Xia, cWlkb25neGlhX21kQDE2My5jb20=; Shao-Gang Wang, c2d3YW5ndGptQDE2My5jb20=
†These authors have contributed equally to this work
 Jian-Xuan Sun
Jian-Xuan Sun Chen-Qian Liu
Chen-Qian Liu Xing-Yu Zhong
Xing-Yu Zhong Jin-Zhou Xu
Jin-Zhou Xu Ye An
Ye An Jia Hu
Jia Hu Qi-Dong Xia
Qi-Dong Xia Shao-Gang Wang
Shao-Gang Wang