- 1Medical Oncology Unit, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, ASST Spedali Civili, Brescia, Italy
- 2Otorhinolaryngology-Head and Neck Surgery Unit, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, ASST Spedali Civili, Brescia, Italy
- 3Department of Pathology, ASST Spedali Civili, Brescia, Italy
- 4Angelo Nocivelli Institute of Molecular Medicine, University of Brescia and ASST Spedali Civili, Brescia, Italy
- 5Department or Radiology, University of Brescia, ASST Spedali Civili;, Brescia, Italy
- 6Dental Clinic, Oral Medicine Unit, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, ASST Spedali Civili, Brescia, Italy
- 7Biostatistics and Bioinformatics Unit, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy
- 8BDbiomed, Big and Open Data Innovation Laboratory, University of Brescia, Brescia, Italy
Background: Oral potentially malignant disorders (OPMDs) represent a heterogeneous set of different histological lesions, characterized by the capacity to transform in oral squamous cell carcinoma (OSCC). Despite optimal surgical treatment, approximately 20%–30% of OPMDs may evolve into OSCC. No clear clinical/histological factors are able to identify OPMDs at higher risk of malignant transformation.
Materials and Methods: We considered surgically treated patients with a diagnosis of OPMDs, enrolled from 1996 to 2019 at ASST Spedali Civili of Brescia without a diagnosis of OSCC within the previous 2 years. Clinical and histological characteristics were recorded. Outcomes of interest were recurrence-free survival (RFS), defined as the time from surgery for primary OPMD to any relapse of OPMD or malignant transformation, whichever occurred first, and carcinoma-free survival (CFS), defined as the time from surgery for OPMD to malignant transformation.
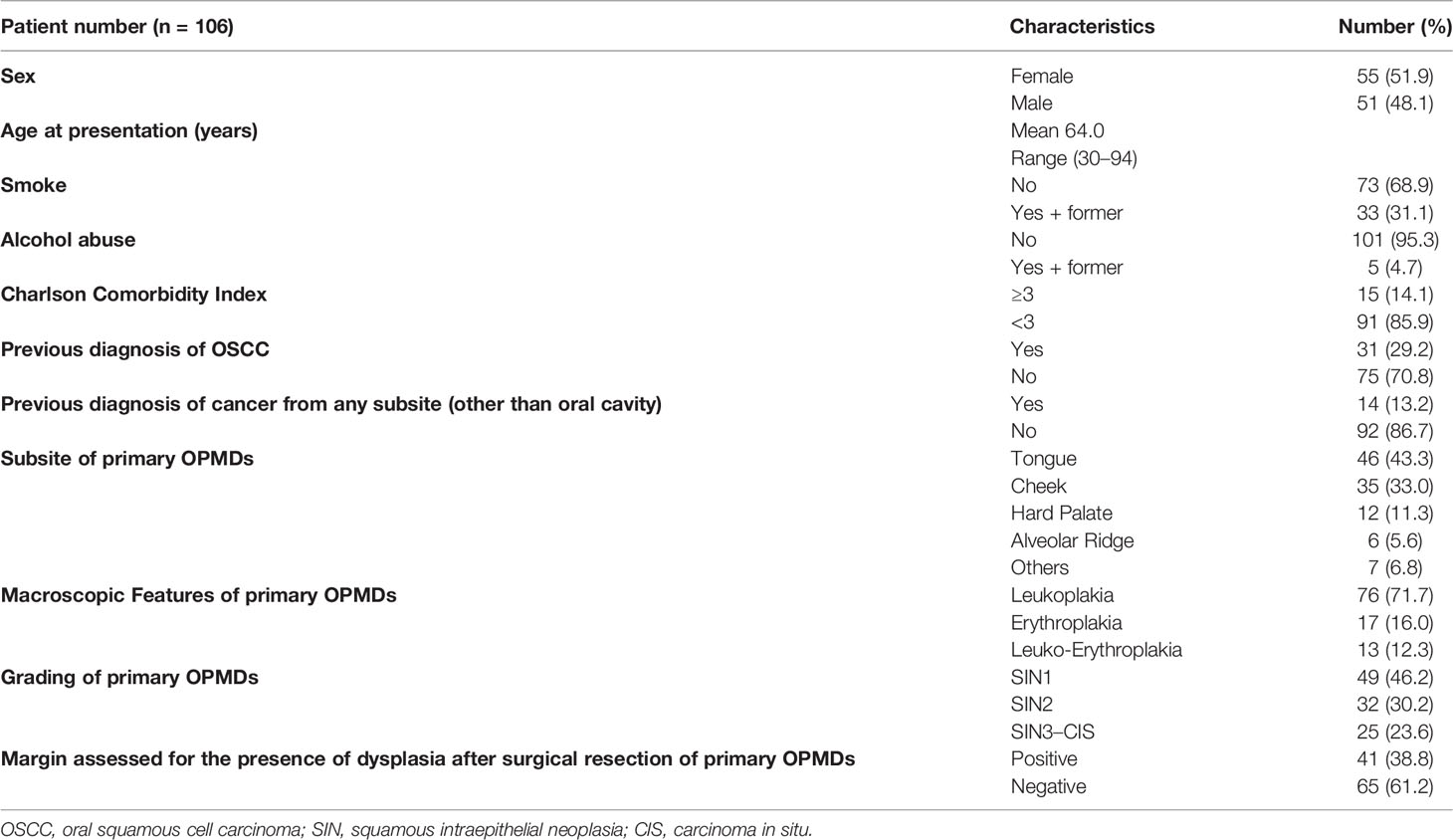
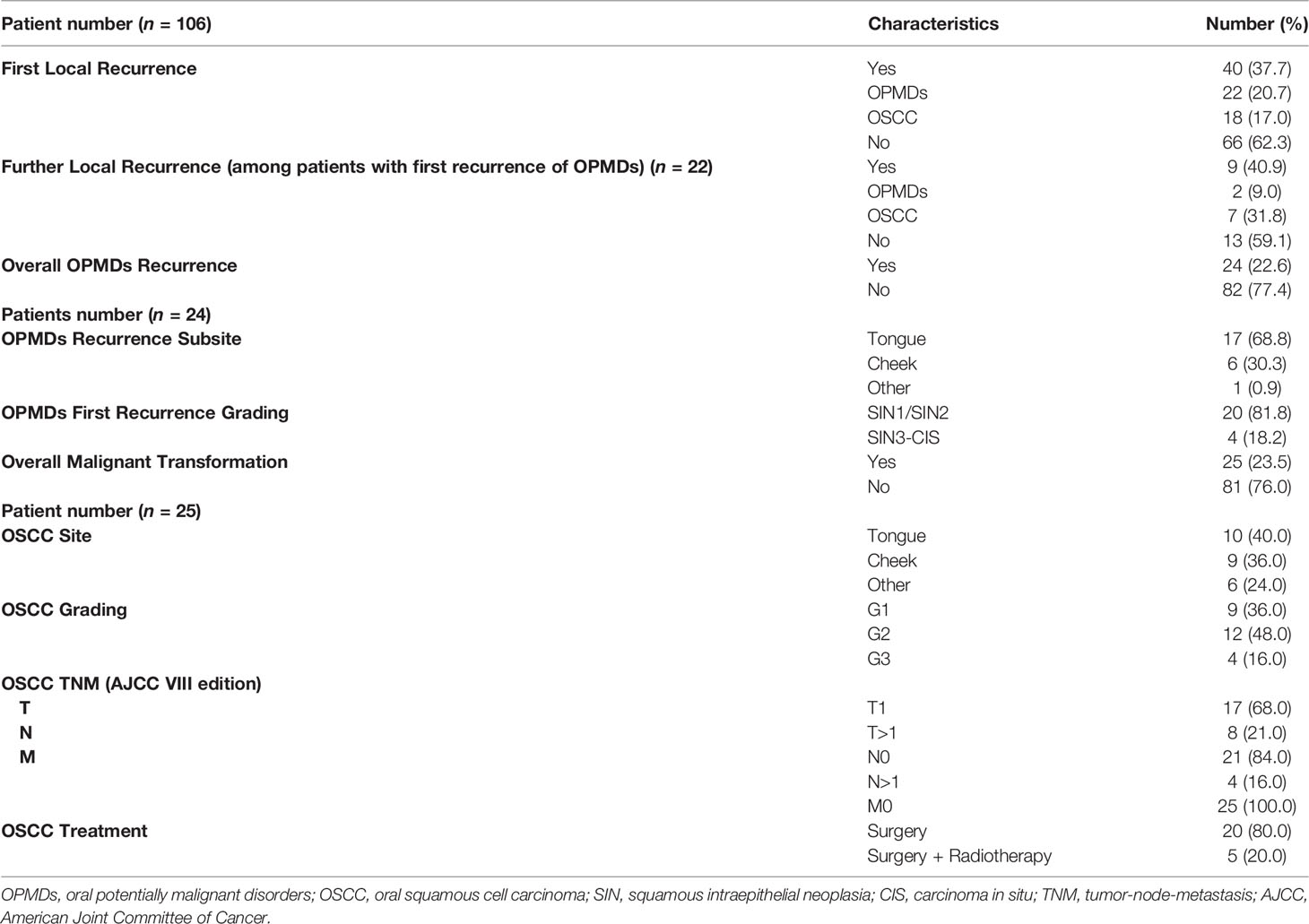
Results: We retrospectively reviewed 106 OPMDs cases. Median age at first diagnosis was 64 years old (IQR = 18.75); female patients comprise 51.9% of the cases. During a median follow-up of 30.5 months (IQR = 44), in 23.5% of patients, malignant transformation occurred. RFS at 1, 5, and 10 years was 92.4%, 60.9%, and 43.2%, respectively. Female sex and history of previous OSCC were independent risk factors for RFS. CFS at 1, 5, and 10 years of follow-up was 97.1%, 75.9%, and 64.4%, respectively. Previous OSCC was an independent risk factor for CFS.
Conclusions: In this large series of OPMDs, only previous diagnosis of OSCC was a prognostic factor for further OSCC occurrence. Given the lack of additional clinical/pathological prognostic factors, we advocate further studies into molecular characterization of OPMDs to better stratify the risk of malignant transformation.
Introduction
More than 90% of oral cancers are represented by OSCCs. OSCCs are among the most frequent neoplasms worldwide, with an increasing incidence, especially in developing countries (1). Prognosis of oral cancer is poor, with an overall 5-year overall survival (5-year OS) ranging from 40%, if diagnosed in advanced stage (III–IV), to 80% if diagnosed in early stage (I–II) (2). Unfortunately, more than half of oral cancers are diagnosed when already in stage III–IV (3). Given the high mortality rate of advanced oral cancers, their early detection and anticipation in diagnosis can result in a significant gain in patient survival (4). Oral potentially malignant disorders (OPMDs), a group of oral mucosal lesions at increased risk of malignant transformation, represent the most common oral precancerous condition with a prevalence worldwide of approximately 4.5% (5). OPMDs comprise various entities such as leukoplakia, erythroplakia, erythroleukoplakia, proliferative verrucous leukoplakia, oral lichen planus, oral submucous fibrosis, oral lichenoid lesion, oral graft versus host disease, and oral dysplasia (6, 7). Currently, the staging proposed by the World Health Organization (WHO) is the most widely accepted, dividing OPMDs based on the grade of dysplasia (low, moderate, high/carcinoma in situ) and assigning them a different risk of malignant transformation (6%, 18%, and 39%, respectively) (8). The main risk factors for the development of OPMDs are shared with OSCC and are represented by smoking and alcoholic abuse, derivates of betel nut and areca nut, human papillomavirus infection (HPV) mainly type 16, oral mucosal trauma, chronic mucosal inflammation, and genetic diseases such as the Fanconi Anemia or Xeroderma pigmentosum (9). None of these risk factors, generally considered in the etiopathogenesis of OPMDs, have consistent data, across studies, as a prognosticator of malignant transformation. Identification of patients with OPMDs at higher risk of malignant transformation is fundamental to improve preventive strategies and to consequently prevent malignant transformation. The aim of this retrospective, observational, monocentric study is therefore the evaluation of the clinic/pathological prognostic factors associated with malignant transformation and recurrence of OPMDs after their primary surgical treatment.
Materials and Methods
The database of patients affected by OPMDs and treated at the Department of Otolaryngology-Head and Neck Surgery of the University of Brescia, Italy, from January 1996 to June 2019 was reviewed. Data concerning survival were retrieved from mortality registries. Inclusion criteria were (a) first histopathological diagnosis of OPMD, treated with curative intent surgery (i.e., resection of the whole lesion in healthy margins), (b) availability of histological information regarding grade of dysplasia and margins of resection, and (c) availability of follow-up information regarding local recurrence either as dysplasia or carcinoma. Exclusion criteria were (a) previous surgery for OPMD performed elsewhere, (b) concurrent diagnosis of head and neck squamous cell carcinoma, and (c) previous history of OSCC within 2 years before the first diagnosis of OPMD.
Data management and study accomplishment are in accordance with principles stated in the Declaration of Helsinki; the study was approved by the local ethics committee (NP4713).
Demographics, and Clinical and Pathological Data
Data concerning demographics, smoking, and alcohol habits (alcohol abuse has been considered as more than 4 alcohol units per day), Charlson Comorbidity Index (CCI) (10), and previous diagnosis of OSCC were collected. The first diagnosis of OPMDs has been recorded with the following characteristics: site; macroscopical aspect (leukoplakia or erythroplakia); grade of dysplasia according to the WHO classification into squamous intraepithelial neoplasia (SIN) 1, SIN2, SIN3; carcinoma in situ (CIS) (8); and margin status after surgery (resections with histological diagnosis of at least 1 mm of normal tissue from surgical margins were considered as negative margins; the presence of any grade of dysplasia at less than 1 mm from positive surgical margins was considered as positive).
Statistical Analysis
Descriptive analysis was conducted expressing data in terms of median, inter-quartile range (IQR), range of values, and percentages. Differences in sex, age (cutoff: 65 years), high grade of dysplasia, and surgical margins of OPMD resection between patients with and without history of previous OSCC and according to the site of origin were assessed through Fisher’s exact test. Outcomes of interest of the survival analysis were (a) recurrence-free survival (RFS), defined as the time from surgery for OPMD to the diagnosis of OPMD relapse or carcinoma, whichever occurred first; (b) OPMD-specific RFS, defined as the time from surgery for OPMD to the diagnosis of OPMD relapse (recurrence in the form of carcinoma censored; new-onset OPMD distant from the site of primary OPMD surgically resected has been considered as OPMD relapse if within the same subsite of the primary); and (c) carcinoma-free survival (CFS), defined as the time from surgery for OPMD to malignant transformation (distant onset of OSCC from previous OPMDs has been considered as OPMD underwent malignant transformation if within the same subsite of the primary). A sub-analysis of CFS of patients without a previous history of OSCC was conducted as well. Survival curves with relative 95% confidence intervals (CIs) and the number of patients at risk by the time were plotted using the Kaplan–Meier method and compared with the log-rank test, reporting the 5-year survival estimates with relative 95% CI. Median follow-up time and corresponding IQR was estimated using the Kaplan–Meier estimator. Hazard ratio (HR) and 95% CI were derived using the Cox proportional hazard model. All the variables considered for univariate analysis were included in a multivariable model for RFS. Because of the low number of events (N = 26), multivariable analysis for CFS was limited to variables with univariate p-values <0.1.
Schoenfeld test was performed to assess the proportional hazards assumption. Statistical analysis was performed using R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria); p-values < 0.05 (two-tailed) were considered statistically significant.
Results
Demographics and Clinical–Pathological Features
One-hundred-six patients with OPMDs met the inclusion criteria. The median age at first diagnosis was 64 years (IQR = 18.75). Sex was almost equally distributed (51.9% female patients). Alcohol abuse and current or previous smoking were reported by 4.7% and 31.1% of patients, respectively. A previous diagnosis of OSCC more than 2 years before index OPMD evaluation and treatment was documented in 31 cases (29.2%). A CCI > 3 was found in 14.1% of patients. Patients were primarily affected by OPMDs located on the tongue (43.3%). OPMDs have been classified as SIN1, SIN2, and SIN3-CIS in 46.2%, 30.2%, and 23.6% of cases, respectively. The main macroscopic aspect of OPMDs was leukoplakia (71.7%) followed by erythroplakia (16.0%) and leuko-erythroplakia (12.3%). All patients have been treated by surgical resection; margins were positive for the presence of dysplasia in 38.8% of cases. Further details are available in Table 1. Patients previously treated for OSCC showed a significantly higher risk of presenting with high-grade dysplasia (41.9%) than patients without previous OSCC (16%) (p = 0.009). No significant differences in sex (p = 0.859), age (cutoff: 65 years, p = 0.901) and OPMD surgical margins (p = 0.924) emerged according to history of previous OSCC. No differences in sex (p = 0.990), age (cutoff: 65 years, p = 0.843), high grade of dysplasia (p = 0.168), and margins of OPMD resection (p = 0.980) were observed according to subsite of origin.
Recurrence-Free Survival Analysis
During a median follow-up of 30.5 months (IQR = 44), 40 (37.7%) patients experienced a first local recurrence either as OPMD (22 cases, 20.7%) or OSCC (18 cases, 17.0%). Among the 22 patients with a first recurrence of OPMD, 9 (40.9%) experienced a further recurrence, and the rate of OSCC almost doubled (7, 31.8%). In total, malignant transformation has been assessed in 23.5% of cases with a median time to malignant transformation of 40 months (IQR = 57.5).
When disease recurred as OPMD, dysplasia was mostly of low grade (SIN1-2, 81.8%), with only 7.5% showing an increase in grade. Most of the relapse were in the same subsite of primary OPMDs (68.8% on tongue, 30.3% on cheek). All cases of dysplasia relapse were treated with surgery.
On the other side, when disease recurred as OSCC, 50% of patients had a previous diagnosis of OSCC. Tumor grading was low to intermediate in 52.0% of cases, staged as T1 in 39.1% and N0 in 47.8%. Surgical treatment of OSCC was performed in more than 90% of cases. Further data are presented in Table 2.
RFS at 1, 5, and 10 years of follow-up was 92.4% (95% CI, 87.5–97.6%), 60.9% (95% CI, 50.5–73.4%), and 43.2% (95% CI, 29.8–62.5%), respectively (Figure 1A). Univariable analysis proved that female sex (HR = 2.24, 95% CI 1.15–4.38, p = 0.018) (Figure 2A) and a history of previous OSCC (HR = 2.60, 95% CI 1.33–5.07, p = 0.005) (Figure 2B), but not age at diagnosis (p = 0.403), site of origin (p = 0.584), grade of OPMD (p = 0.121), and margins of resection (p = 0.219), were associated with worse outcome. Both female (HR = 2.07, 95% CI 1.02–4.20, p = 0.044) and a history of previous OSCC (HR = 2.54, 95% CI 1.26–5.13, p = 0.009) were independently associated with relapse at the multivariable analysis (Table 3).
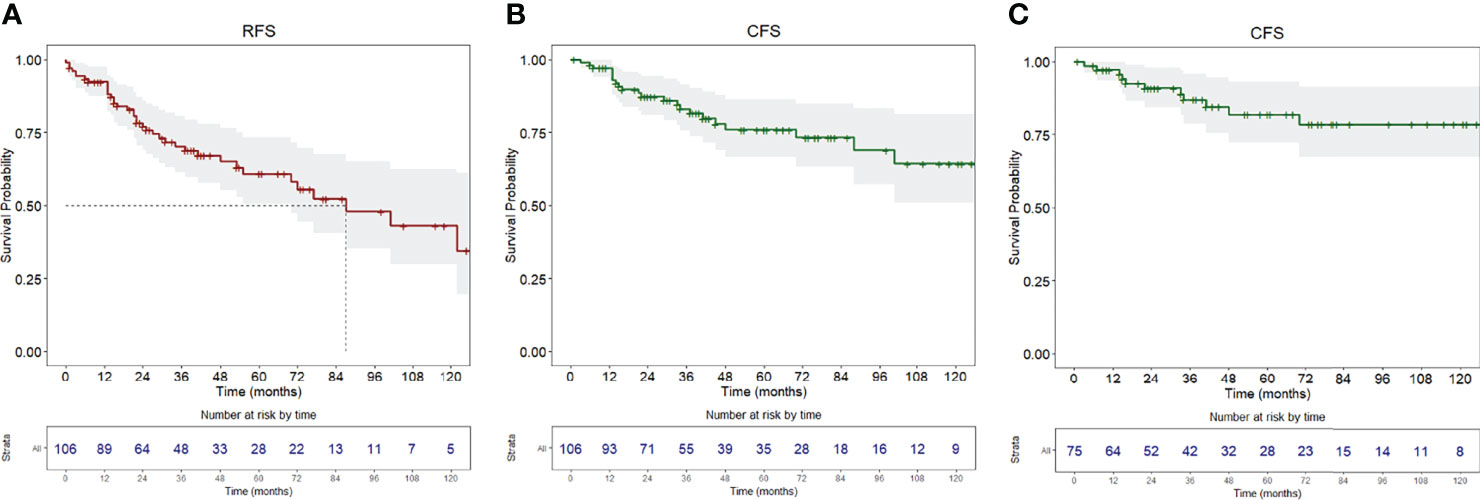
Figure 1 (A) Recurrence-free survival, (B) carcinoma-free survival of the whole population, (C) carcinoma-free survival of the population without a history of previous OSCC. Survival curves are reported with relative tables of patients at risk.
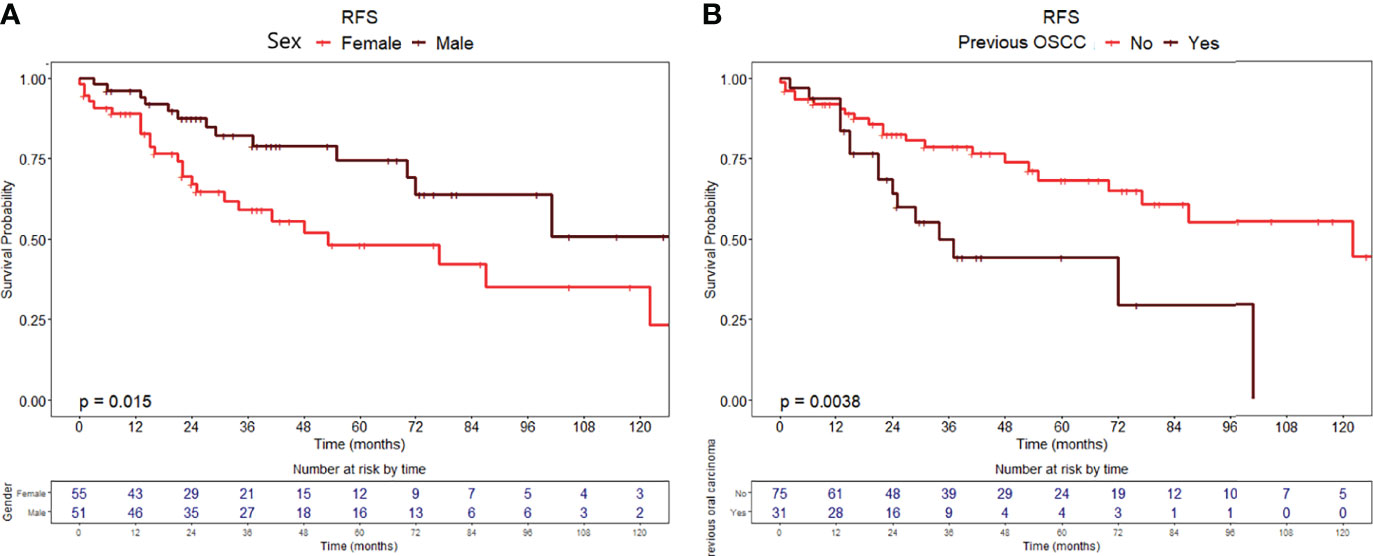
Figure 2 Recurrence-free survival curves with relative tables of patients at risk according to (A) sex and (B) history of previous OSCC.
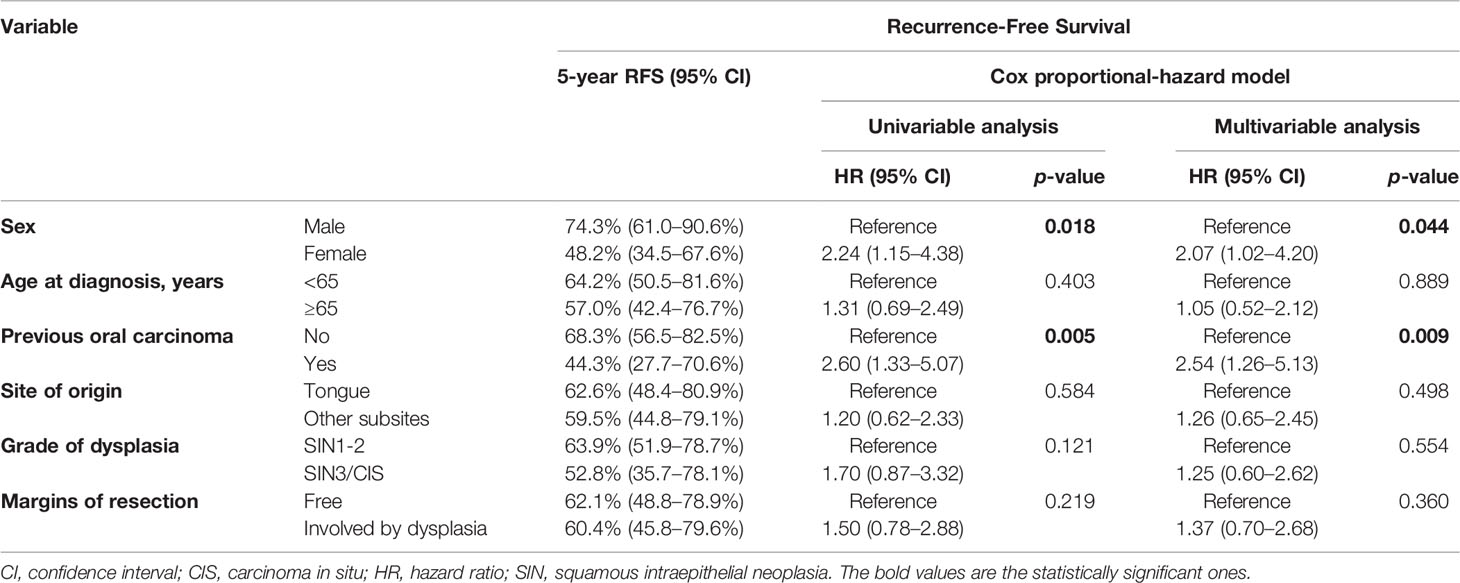
Table 3 Uni- and multi-variable analysis of the most relevant demographics and clinical–pathological factors according to RFS (recurrence-free survival) and CFS (carcinoma-free survival).
When considering OPMD-specific RFS, the only factor associated with higher risk was female sex (HR = 3.02, 95% CI 11.8–7.74, p = 0.021), whereas older age (p = 0.538), previous OSCC (p = 0.102), subsite (p = 0.408), grade of dysplasia (p = 0.947), and margins (p = 0.131) were not associated.
Carcinoma-Free Survival Analysis
Twenty-six patients (24.5%) were diagnosed with OSCC during follow-up, with a median time from diagnosis of OPMD to OSCC of 31 months (IQR = 51.2). In the whole population, CFS at 1, 5, and 10 years of follow-up was 97.1% (95% CI, 93.9–100%), 75.9% (95% CI, 66.7–86.5%), and 64.4% (95% CI, 51.0–81.3%), respectively (Figure 1B). At univariable analysis, age (p = 0.136), site of origin (p = 0.663), and positive margins (p = 0.479) did not influence CFS, whereas female sex (HR = 2.10, 95% CI 0.90–4.80, p = 0.085) (Figure 3A), grade of dysplasia (HR = 2.11, 95% CI 0.92–4.84, p = 0.077), and history of previous OSCC (HR = 2.65, 95% CI 1.18–5.96, p = 0.019) (Figure 3B) showed a significant or close-to-significant association. Multivariable analysis revealed the independent effect of previous OSCC (HR = 2.73, 95% CI 1.18–6.31, p = 0.019) (Table 4).
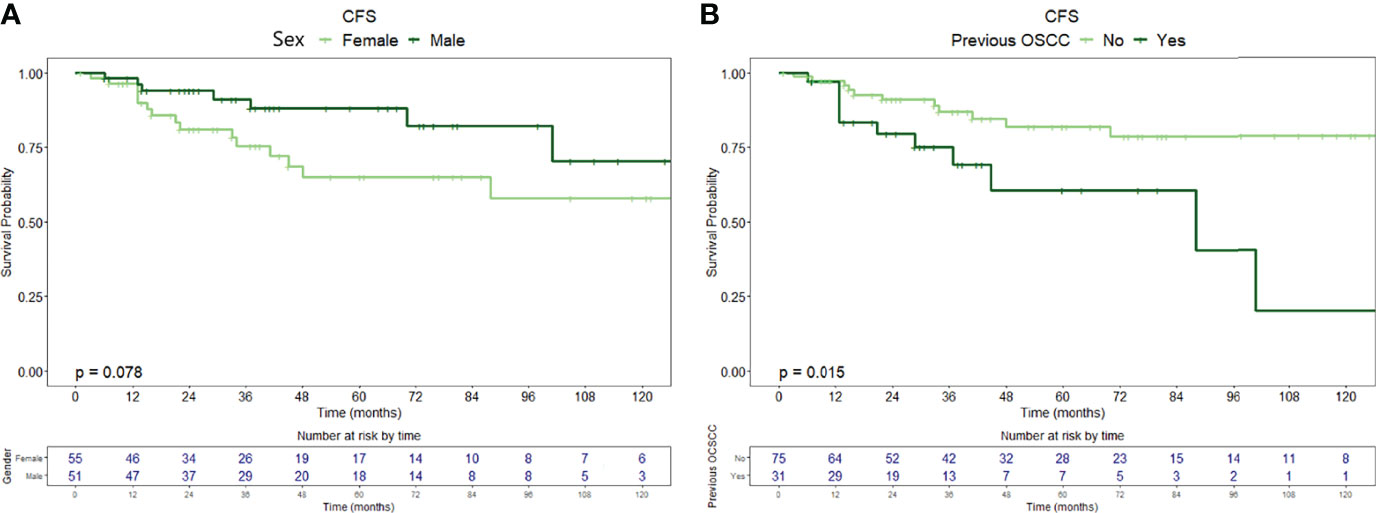
Figure 3 Carcinoma-free survival curves with relative tables of patients (whole population) at risk according to (A) sex and (B) history of previous OSCC.
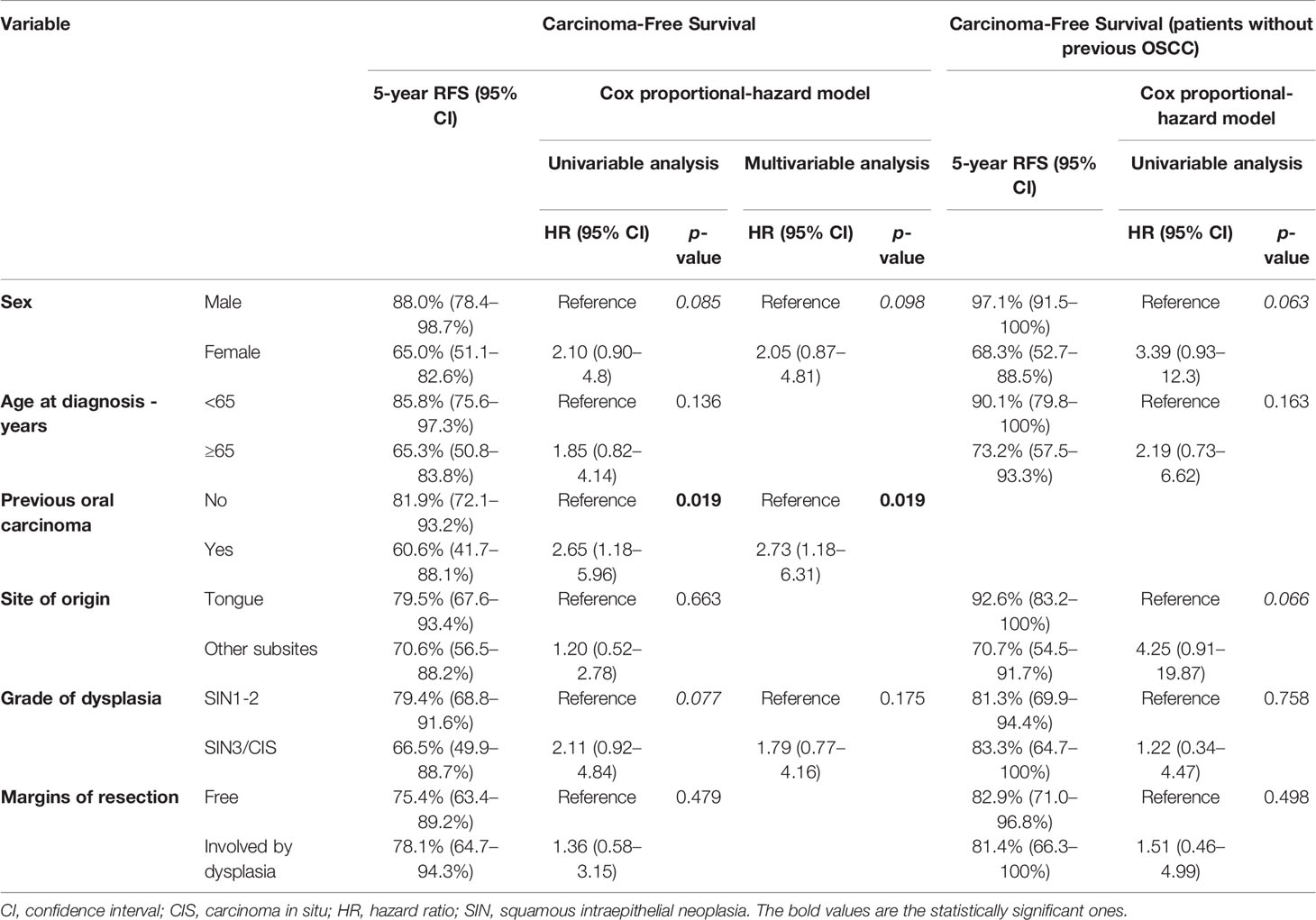
Table 4 Uni- and multi-variable analysis of the most relevant demographics and clinical–pathological factors according to CFS (carcinoma-free survival).
When restricting the analysis to patients with an OPMD without previous diagnosis of OSCC, we identified 75 patients. No demographic differences were found compared to the whole cohort (52% of female patients, median age at diagnosis of 64 years). OPMDs were evenly distributed throughout the oral cavity subsite (42.5% on the tongue, 60.3% on other subsites). SIN1, SIN2, and SIN3-CIS were diagnosed in 42 (56.0%), 21 (28.0%), and 12 cases (16.0%), respectively. Margins of resection were involved by dysplasia in 29 specimens (38.6%).
In this group of patients, a diagnosis of OSCC during follow-up was registered in 15 (20%), with a median time from diagnosis of OPMD to OSCC of 40 months (IQR = 57.5). CFS at 1, 5, and 10 years of follow-up was 97.2% (95% CI, 93.5–100%), 81.9% (95% CI, 72.1–93.2%), and 78.5% (95% CI, 67.4–91.5%), respectively (Figure 1C).
In univariable analysis, sex (HR = 3.39, 95% CI 0.93–12.30, p = 0.063) and OPMD location (HR = 4.25, 95% CI 0.91–19.87, p = 0.066) were close-to-significant. On the contrary, age at diagnosis (p = 0.150), grade of dysplasia (p = 0.760), and margin of resection (p = 0.500) were not significantly associated with an increased risk of malignant transformation (Table 4).
Considering the scarce number of events (N = 15) we did not perform a multivariable analysis.
Discussion
Identification of high-risk OPMDs is an important unmet medical need (11). The main aim of our study was to evaluate the impact of clinical/histological factors related to patients primarily affected by OPMDs and the risk of recurrence and malignant transformation. In our cohort of patients surgically treated for OPMDs, we identified a malignant transformation rate of 24.5% with a median interval of 31 months (IQR = 51.2). When considering patients without a history of previous OSCC, malignant transformation rate was slightly lower (20%), with a longer median time from diagnosis of OPMD to OSCC (40 months, IQR = 57.5) and higher 5-year CFS (81.9% vs 75.9%).
Female sex and a previous diagnosis of OSCC were independent negative prognosticators for both RFS and CFS. We then analyzed the CFS in the subgroup of patients with an OPMD without any previous diagnosis of OSCC and we found that female sex and primarily location of OPMD outside the tongue were associated with shorter CFS.
There is no high consensus on risk factors in the natural history of OPMD. Other series reported that heavy tobacco smoking, alcohol consumption, immunosuppression status, and histological characteristics such as non-homogenous leukoplakia, OPMDs size >200 mm2, moderate or greater than moderate dysplasia, and lesions displaying a progression towards worse grades are at higher risk of malignant transformation (12, 13). Recently, a series evaluating high-grade OPMDs showed that factors significantly associated with higher risk of malignant transformation are age and positive margins after surgical excision (14). Differences in these factors could be explained by heterogeneity of inclusion criteria and possibly geographic risk factors. Moreover, it should be stressed that some series also considered patients with a previous diagnosis of OSCC within 2 years before diagnosis of OPMDs, when the risk of OSCC recurrence after curative treatment is higher (15), increasing the possibility to confound an OSCC recurrence with OPMD malignant transformation.
A higher incidence of malignant transformation for female patients is generally accepted in most of the series (16, 17). In this regard, a role of the sexual hormones could be hypothesized to contribute to malignant transformation. Oral carcinogenesis is a slowly multistep process, in which some genetic deregulations have been related to estrogen deficiency; a longer menopause period could pose women at higher risk of oral cancer, as some papers suggested (18, 19).
We have classified OPMDs according to the World Health Organization (WHO) classification (8), as SIN1, SIN2, SIN3, and CIS (20, 21). Coherently, we found an indication of a shorter CFS related to higher grade of dysplasia, though not statistically significant. The cutoff between SIN1, SIN2, or SIN3 is poorly defined and comparison between different series is difficult due to suboptimal inter-observer reliability and reproducibility. In this regard, data in the literature are not homogeneous. To overcome this, recently the WHO Classification of Head and Neck Tumors also tabled a binary system (high- versus low-grade dysplasia) and suggested cutoff criteria between the grades (22). Despite literature data showing higher risk of malignant transformation for lesions with high grade of dysplasia (2, 6, 12, 23), we could not confirm this in our series, indicating that classification of OPMDs dysplasia remains conflicting likely due to “subjective” definition.
According to recent review data (6), we found that OPMDs were mainly located on the tongue (43.3% of cases). The relationship between the site of OPMD and risk of malignant transformation is controversial (16, 24–27). In our study, we found that patients without a previous diagnosis of OSCC with lesions located outside the tongue had shorter CFS (HR = 4.25, p = 0.066). Lesions in the tongue are more easily identified by the patients themselves, with a consequent earlier diagnosis and less time to accumulate carcinogenetic alterations; this is more evident in patients without previous OSCC diagnosis and therefore undergoing less frequent follow-up visits.
A previous diagnosis of OSCC was an independent prognosticator of shorter RFS (HR = 2.54, p = 0.009) and CFS (HR = 2.73, 95% CI 1.18–6.31, p = 0.019). These data overlap with literature that showed how pre-existent molecular alteration could lead to an increased risk of malignant transformation. The loss of heterozygosity (LOH) has a central role on OPMDs’ malignant transformation. LOH on certain chromosomal loci such as 9p21 or 3p14 led to a higher risk of malignant transformation, due to implication on the encoding of various tumor suppressor genes, critical in malignant transformation, such as tumor protein 53 (TP53), and cyclin-dependent kinase inhibitor 2A (CDKN2A). Additional LOH on 17p or 8p or 11p or 4q or 13q could increase the risk of malignant transformation (28–30). An increased risk of OPMD recurrence and malignant transformation in patients with previous OSCC could be explained also by the theory of field cancerization referred to as the occurrence of molecular abnormalities in the tumor adjacent mucosal field, driven by the activities of local cancer stem cells (31).
Overall, the most relevant finding of our analysis is that clinical–pathological factors cannot thoroughly describe the risk of malignant transformation of OPMDs. Malignant transformation seems to be the sum of various risk factors, in this regard, the application of validated nomogram and the assistance of Deep Learning models could help in the identification of OPMDs at higher risk of malignant transformation (32, 33). With a window on the near future, we aim to get more precise molecular OPMD characterization to better define their malignant transformation risk and potentially tailor both therapeutic approach and the follow-up; moreover, a better characterization could allow patient enrichment for prevention trials.
Intriguingly, it is possible to describe, from a transcriptomic point of view, six distinct clusters of disease based on main biological features and deregulated pathways, mirroring the pathways present in head and neck squamous cell carcinomas: defense response, immunoreactive, human papillomavirus-related, classical, hypoxia, and mesenchymal clusters, where mesenchymal, hypoxia, and classical clusters have a higher risk of malignant transformation if compared with immunoreactive clusters (34). Even immune infiltration seems to play an important role in malignant transformation for OPMDs (35); consequently, ongoing clinical trials are exploring the activity of ICIs such as avelumab (NCT04504552) and sintilimab (NCT04065737) in high-risk OPMDs defined based on molecular criteria (i.e., LOH 3p14 and/or 9p21), and pembrolizumab (NCT03603223) in OPMDs.
The main limitations of our cohort were the retrospective nature of the study, the lack of information on previous OPMD before OSCC, and the absence of more in-depth histological (e.g., immune infiltration and HPV status) analysis.
Conclusions
In our study, only a previous OSCC was a prognosticator of further OSCC recurrence. Molecular characterization of OPMDs should be included in future studies, better stratify the risk of malignant transformation, and consequently define an enriched population for preventative strategies within clinical trials.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: bHVpZ2lsb3Jpbmk5MUBnbWFpbC5jb20=.
Author Contributions
LL, PB, and MT conceived the study. LL, MT, PB, and CM contributed to manuscript writing. PB, CP, DM, AD, FF, AP, AG, AM, EB, CR, DL, and MR revised the manuscript. SC and MT contributed to the statistical analysis. CM, LL, MT, MF, SB, AO, AB, and CG collected data. All authors contributed to the article and approved the submitted version.
Funding
This work has been funded by Associazione Italiana Ricerca sul Cancro (AIRC), thanks to an Investigator Grant (IG 21740 to PB).
Conflict of Interest
PB declares advisory board participation or conference honoraria from Merck, Sanofi-Regeneron, Merck Sharp & Dohme, Sun Pharma, Angelini, Molteni, Bristol-Myers Squibb, GSK, and Nestlè.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abati S, Bramati C, Bondi S, Lissoni A, Trimarchi M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int J Environ Res Public Health (2020) 17(24):9160. doi: 10.3390/ijerph17249160
2. Speight PM, Khurram SA, Kujan O. Oral Potentially Malignant Disorders: Risk of Progression to Malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol (2018) 125(6):612–27. doi: 10.1016/j.oooo.2017.12.011
3. McCullough M, Prasad G, Farah C. Oral Mucosal Malignancy and Potentially Malignant Lesions: An Update on the Epidemiology, Risk Factors, Diagnosis and Management: Oral Mucosal Malignancy. Aust Dent J (2010) 55:61–5. doi: 10.1111/j.1834-7819.2010.01200.x
4. Dost F, Lê Cao K, Ford PJ, Ades C, Farah CS. Malignant Transformation of Oral Epithelial Dysplasia: A Real-World Evaluation of Histopathologic Grading. Oral Surg Oral Med Oral Pathol Oral Radiol (2014) 117(3):343–52. doi: 10.1016/j.oooo.2013.09.017
5. Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, et al. Prevalence of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. J Oral Pathol Med (2018) 47(7):633–40. doi: 10.1111/jop.12726
6. Aguirre-Urizar JM, Lafuente-Ibáñez de Mendoza I, Warnakulasuriya S. Malignant Transformation of Oral Leukoplakia: Systematic Review and Meta-Analysis of the Last 5 Years. Oral Dis (2021) 27(8):1881–95. doi: 10.1111/odi.13810
7. Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles MÁ, Kerr AR, et al. Oral Potentially Malignant Disorders: A Consensus Report From an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis (2021) 27(8):1862–80. doi: 10.1111/odi.13704
8. Reibel J, Gale N, Hille M, Williams J, et al. Oral Potentially Malignant Disorders and Oral Epithelial Dysplasia. In: el-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al, editors. WHO Classification of Tumours of the Head and Neck, 4th ed. Lyon: IARC Press (2017).
9. Porter S, Gueiros LA, Leão JC, Fedele S. Risk Factors and Etiopathogenesis of Potentially Premalignant Oral Epithelial Lesions. Oral Surg Oral Med Oral Pathol Oral Radiol (2018) 125(6):603–11. doi: 10.1016/j.oooo.2018.03.008
10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
11. Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, et al. Interventions for Treating Oral Leukoplakia to Prevent Oral Cancer, in: Cochrane Database Syst Rev (2016). Available at: http://doi.wiley.com/10.1002/14651858.CD001829.pub4 (Accessed 2022 Feb 19).
12. Cerqueira JM, Pontes FS, Santos-Silva AR, Almeida OP, Costa RF, Fonseca FP, et al. Malignant Transformation of Oral Leukoplakia: A Multicentric Retrospective Study in Brazilian Population. Med Oral Patol Oral Cirugia Bucal (2021) 26(3):e292–8. doi: 10.4317/medoral.24175
13. Kierce J, Shi Y, Klieb H, Blanas N, Xu W, Magalhaes M. Identification of Specific Clinical Risk Factors Associated With the Malignant Transformation of Oral Epithelial Dysplasia. Head Neck (2021) 43(11):3552–61. doi: 10.1002/hed.26851
14. Gilvetti C, Soneji C, Bisase B, Barrett AW. Recurrence and Malignant Transformation Rates of High Grade Oral Epithelial Dysplasia Over a 10 Year Follow Up Period and the Influence of Surgical Intervention, Size of Excision Biopsy and Marginal Clearance in a UK Regional Maxillofacial Surgery Unit. Oral Oncol (2021) 121:105462. doi: 10.1016/j.oraloncology.2021.105462
15. Borsetto D, Sethi M, Polesel J, Tomasoni M, Deganello A, Nicolai P, et al. The Risk of Recurrence in Surgically Treated Head and Neck Squamous Cell Carcinomas: A Conditional Probability Approach. Acta Oncol (2021) 60(7):942–7. doi: 10.1080/0284186X.2021.1925343
16. Warnakulasuriya S, Ariyawardana A. Malignant Transformation of Oral Leukoplakia: A Systematic Review of Observational Studies. J Oral Pathol Med (2016) 45(3):155–66. doi: 10.1111/jop.12339
17. Napier SS, Speight PM. Natural History of Potentially Malignant Oral Lesions and Conditions: An Overview of the Literature: Potentially Malignant Oral Lesions and Conditions. J Oral Pathol Med (2007) 37(1):1–10. doi: 10.1111/j.1600-0714.2007.00579.x
18. Huber JC, Schneeberger C, Tempfer CB. Genetic Modelling of the Estrogen Metabolism as a Risk Factor of Hormone-Dependent Disorders. Maturitas (2002) 42(1):1–12. doi: 10.1016/S0378-5122(02)00021-X
19. Suba Z. Gender-Related Hormonal Risk Factors for Oral Cancer. Pathol Oncol Res (2007) 13(3):195–202. doi: 10.1007/BF02893499
20. Barnes L, Eveson JW, Reichart P, Sidransky D eds. Pathology and Genetics of Head and Neck Tumours. World Health Organization, IARC Lyon. (2005).
21. Müller S. Oral Epithelial Dysplasia, Atypical Verrucous Lesions and Oral Potentially Malignant Disorders: Focus on Histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol (2018) 125(6):591–602. doi: 10.1016/j.oooo.2018.02.012
22. Nankivell P, Williams H, Matthews P, Suortamo S, Snead D, McConkey C, et al. The Binary Oral Dysplasia Grading System: Validity Testing and Suggested Improvement. Oral Surg Oral Med Oral Pathol Oral Radiol (2013) 115(1):87–94. doi: 10.1016/j.oooo.2012.10.015
23. Chaturvedi AK, Udaltsova N, Engels EA, Katzel JA, Yanik EL, Katki HA, et al. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. JNCI J Natl Cancer Inst (2020) 112(10):1047–54. doi: 10.1093/jnci/djz238
24. Wu W, Wang Z, Zhou Z. Risk Factors Associated With Malignant Transformation in Patients With Oral Leukoplakia in a Chinese Population: A Retrospective Study. J Oral Maxillofac Surg (2019) 77(12):2483–93. doi: 10.1016/j.joms.2019.08.002
25. Evren I, Brouns ER, Wils LJ, Poell JB, Peeters CFW, Brakenhoff RH, et al. Annual Malignant Transformation Rate of Oral Leukoplakia Remains Consistent: A Long-Term Follow-Up Study. Oral Oncol (2020) 110:105014. doi: 10.1016/j.oraloncology.2020.105014
26. Shearston K, Fateh B, Tai S, Hove D, Farah CS. Malignant Transformation Rate of Oral Leukoplakia in an Australian Population. J Oral Pathol Med (2019) 48(7):530–7. doi: 10.1111/jop.12899
27. Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-Term Treatment Outcome of Oral Premalignant Lesions. Oral Oncol (2006) 42(5):461–74. doi: 10.1016/j.oraloncology.2005.08.011
28. Mao L, Papadimitrakopoulou V, Shin DM, Fan Y, Zhou X, Lee JS, et al. Phenotype and Genotype of Advanced Premalignant Head and Neck Lesions After Chemopreventive Therapy. JNCI J Natl Cancer Inst (1998) 90(20):1545–51. doi: 10.1093/jnci/90.20.1545
29. Zhang L, Poh CF, Williams M, Laronde DM, Berean K, Gardner PJ, et al. Loss of Heterozygosity (LOH) Profiles—Validated Risk Predictors for Progression to Oral Cancer. Cancer Prev Res (Phila Pa) (2012) 5(9):1081–9. doi: 10.1158/1940-6207.CAPR-12-0173
30. William WN, Papadimitrakopoulou V, Lee JJ, Mao L, Cohen EEW, Lin HY, et al. Erlotinib and the Risk of Oral Cancer: The Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA Oncol (2016) 2(2):209. doi: 10.1001/jamaoncol.2015.4364
31. Simple M, Suresh A, Das D, Kuriakose MA. Cancer Stem Cells and Field Cancerization of Oral Squamous Cell Carcinoma. Oral Oncol (2015) 51(7):643–51. doi: 10.1016/j.oraloncology.2015.04.006
32. Cai X, Zhang J, Han Y, Tang Q, Zhang H, Li T. Development and Validation of a Nomogram Prediction Model for Malignant Transformation of Oral Potentially Malignant Disorders. Oral Oncol (2021) 123:105619. doi: 10.1016/j.oraloncology.2021.105619
33. Adeoye J, Koohi-Moghadam M, Lo AWI, Tsang RK-Y, Chow VLY, Zheng L-W, et al. Deep Learning Predicts the Malignant-Transformation-Free Survival of Oral Potentially Malignant Disorders. Cancers (2021) 13(23):6054. doi: 10.3390/cancers13236054
34. Carenzo A, Serafini MS, Roca E, Paderno A, Mattavelli D, Romani C, et al. Gene Expression Clustering and Selected Head and Neck Cancer Gene Signatures Highlight Risk Probability Differences in Oral Premalignant Lesions. Cells (2020) 9(8):1828. doi: 10.3390/cells9081828
Keywords: oral potentially malignant disease, oral squamous cell carcinoma (OSCC), head and neck squamous cell carcinoma (HNSCC), oral carcinoma risk factors, prevention of malignant transformation
Citation: Lorini L, Tomasoni M, Gurizzan C, Magri C, Facchetti M, Battocchio S, Romani C, Ravanelli M, Oberti A, Bozzola A, Bardellini E, Paderno A, Mattavelli D, Lombardi D, Grammatica A, Deganello A, Facchetti F, Calza S, Majorana A, Piazza C and Bossi P (2022) Clinical and Histological Prognostic Factors of Recurrence and Malignant Transformation in a Large Series of Oral Potentially Malignant Disorders. Front. Oncol. 12:886404. doi: 10.3389/fonc.2022.886404
Received: 28 February 2022; Accepted: 22 March 2022;
Published: 21 April 2022.
Edited by:
Nerina Denaro, Azienda Sanitaria Ospedaliera S.Croce e Carle Cuneo, ItalyReviewed by:
Linjun Shi, Shanghai Jiao Tong University, ChinaVito Carlo Alberto Caponio, University of Foggia, Italy
Copyright © 2022 Lorini, Tomasoni, Gurizzan, Magri, Facchetti, Battocchio, Romani, Ravanelli, Oberti, Bozzola, Bardellini, Paderno, Mattavelli, Lombardi, Grammatica, Deganello, Facchetti, Calza, Majorana, Piazza and Bossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Lorini, bHVpZ2lsb3Jpbmk5MUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Luigi Lorini
Luigi Lorini Michele Tomasoni
Michele Tomasoni Cristina Gurizzan
Cristina Gurizzan Chiara Magri1
Chiara Magri1 Alberto Paderno
Alberto Paderno Davide Mattavelli
Davide Mattavelli Davide Lombardi
Davide Lombardi Alberto Grammatica
Alberto Grammatica Alberto Deganello
Alberto Deganello Stefano Calza
Stefano Calza Alessandra Majorana
Alessandra Majorana Cesare Piazza
Cesare Piazza Paolo Bossi
Paolo Bossi