- 1University of Patras Medical School, Hematology Division, Patras, Greece
- 2University General Hospital Attikon, Athens, Greece
- 3Hematology Division, General University Hospital of Patras, Rion of Patras, Greece
- 4Department of Medicine, School of Health Sciences, National and Kapodistrian University of Athens, Athens, Greece
Chronic myelomonocytic leukemia (CMML) and the remaining, less frequent hybrid, mixed, or overlap myelodysplastic syndromes/myeloproliferative neoplasms (MDSs/MPNs) are difficult to treat neoplastic hematological disorders, exhibiting substantial clinical and prognostic heterogeneity, for which clear therapeutic guidelines or effective treatment options are still missing. CMML has an overall survival ranging from a few months to several years. Although patients with proliferative or dysplastic features may benefit from hydroxyurea and hypomethylating agent treatment, respectively, none of these treatments can establish long-term remission and prevent the inevitable transformation to acute leukemia. Novel targeted treatment approaches are emerging but are still under investigation. Therefore, currently, allogeneic stem cell transplantation (allo-SCT) remains the only treatment modality with a curative potential, but its widespread application is limited, due to significant morbidity and mortality associated with the procedure, especially in the elderly and in patients with comorbidities. Recognition of patient eligibility for allo-SCT is crucial, and the procedure should be addressed to patients with a good performance status without severe comorbidities and mainly to those in intermediate- to high-risk category, with a suitable stem cell donor available. The issues of best timing for performing transplantation, patient and donor eligibility, the type of conditioning regimen, and the outcomes after various allo-SCT procedures are the topics of this review.
Introduction
Myelodysplastic syndromes/myeloproliferative neoplasms (MDSs/MPNs) represent a difficult-to-treat group of clonal hematopoietic stem cell disorders, without specific molecular signatures, exhibiting both, myelodysplastic and myeloproliferative features. According to the most recent revision of the World Health Organization (WHO) classification, entities classified in this category include chronic myelomonocytic leukemia (CMML), atypical bcr/abl-negative chronic myeloid leukemia (aCML), juvenile myelomonocytic leukemia (JMML), and myelodysplastic syndrome/myeloproliferative neoplasm unclassifiable (MDS/MPN-U) (1, 2). Chronic neutrophilic leukemia (CNL), although currently classified among Myeloproliferative Neoplasms (MPNs), sometimes shares several dysplastic features, and it has been postulated that this disease might stand closer to MDS/MPN (3, 4).
For the most common entity, CMML, several prognostic systems have been proposed to best stratify patient life expectation, according to disease aggressiveness. French-American-British (FAB) Classification has defined the threshold of 13 × 109/l white blood cells (WBCs) to distinguish the dysplastic from the proliferative subtype, but this cutoff value cannot reflect the biological differences of the two subtypes. Cytogenetic risk, appears not to be comparable to that of classical MDS (5). Prognostically relevant is the WHO 2016 classification, based on the percentage of peripheral blood (PB) and/or bone marrow (BM) blasts, as CMML-0 (BM blasts 0%–4%), CMML-1 (5%–9%), and CMML-2 (10%–19%), although often clear differences between CMML-0 and CMML-1 may not be found. However, difficulties might emerge in the correct characterization of marrow blasts, since several immature monocytoid cells could be considered blasts, as also promonocytes should be considered as blasts, together with myeloblasts and monoblasts. Thus, this concept should always be kept in mind when assessing patient risk according to the WHO subclassification (CMML-0 vs. CMML-1 vs. CMML-2) and/or according to scoring systems such as the CMML Prognostic Scoring System(CPSS) including BM blast percentage among prognostic factors to be considered (6). Other systems, such as the MD Anderson Prognostic Score (MDAPS) and the Mayo prognostic model, rely on clinical, morphological, and laboratory parameters (7, 8) because either they did not test the importance of genetic markers (7) or the tested markers were not proven to be prognostically important (8). Molecular information has been incorporated within the Group Francophone (GFM) (9), the Mayo, and the CMML Prognostic Scoring System molecular (CPSS-mol) (10) prognostic systems, in the latter together with cytogenetics as genetic risk grouping. In a comparative study between the CPSS, the MDAPS, and the Mayo prognostic system, CPSS was found superior, and the authors further improved it by adding platelet count, thus creating the CPSS-P (11). However, all of these tools are applicable at baseline, and not to patients proceeding to allogeneic stem cell transplantation (allo-SCT). BM fibrosis may occur in some CMML patients, who more frequently exhibit Janus Kinase 2 (JAK2) gene mutations. Patients with fibrotic CMML have a dismal outcome and should be distinguished from patients with primary myelofibrosis and monocytosis (12). Finally, therapy-related CMML appears to be pathogenetically distinct, has worse prognosis than primary disease, and has also been suggested to be distinguished (13, 14).
Overview of Treatment Approaches for Chronic Myelomonocytic Leukemia and the Other Myelodysplastic Syndromes/Myeloproliferative Neoplasms
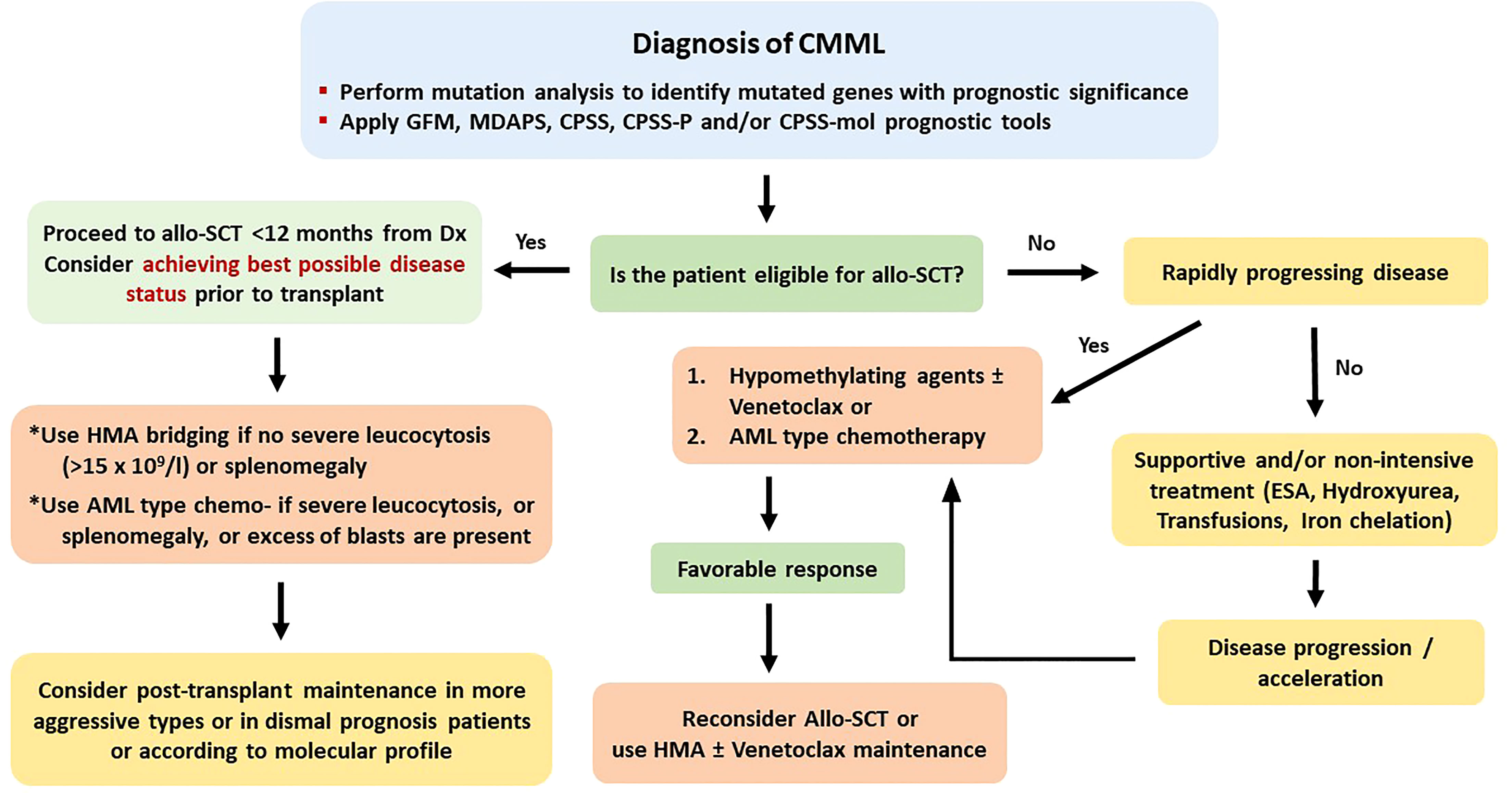
Treatment options for these diseases vary from supportive care to allo-SCT. This variability clearly reflects the extreme heterogeneity of prognosis, according to disease and patient characteristics at diagnosis. Until recently, clear treatment guidelines were lacking, although excellent reviews and treatment recommendations have been published (15–18). The lack of evidence-based recommendations is mainly attributed to the absence of large, multicenter, prospective, randomized trials investigating prespecified treatment outcomes. A potential explanation is the high degree of clinical, laboratory, molecular, and prognostic heterogeneity of these diseases. The use of hypomethylating agents (HMAs) as initial treatment becomes more and more popular; however, results are less favorable than those achieved by patients with classical MDS, fewer patients achieve complete remission (CR), and responses are shorter (19, 20). Recent molecular analyses have shown that ASXL1 and RAS mutations are associated with poorer response to HMA treatment, whereas TET2 mutations represent a favorable factor for response (21).
Oral cytoreductive treatment, usually hydroxyurea, is temporarily effective and is administered to patients with the proliferative subtype or with extramedullary organ involvement. Single-agent chemotherapy, most commonly low-dose subcutaneous or intravenous cytarabine, may be given to patients with uncontrolled monocytosis and/or increased marrow blasts. However, responses are short, and the majority of patients soon become refractory, developing multidrug resistance. Combination chemotherapy, consisting of cytarabine plus an anthracycline or a topoisomerase inhibitor, may be more effective, induces longer remissions, but is poorly tolerated by the usually advanced-aged or unfit patients. Combination chemotherapy-induced CR is usually of short duration, as compared to remissions induced in patients with de novo AML.
The only treatment with curative potential remains allo-SCT, which should be provided to all patients with prognostically unfavorable, rapidly evolving, or symptomatic CMML, or other MDSs/MPNs, who have an available stem cell donor. Ideally, this should be performed early, before disease progression, because in the latter case, non-relapse mortality (NRM) and relapse rate (RR) are higher and worse than those in AML evolved from classical MDS (22). There are several barriers, preventing the broad application of allo-SCT, including patient-related issues (advanced age, poor performance status, comorbidities) and disease-related issues (unstable disease, delayed postchemotherapy marrow reconstitution, infectious complications, and organ impairment).
Several studies aim to rationalize the therapeutic decision for allo-SCT in these diseases. According to the Mayo Clinic experience, among 406 patients, 70 underwent allo-SCT, and median leukemia-free survival (LFS) by the application of propensity score-matched analysis was clearly better in the transplanted than that in the non-transplanted group (40 vs. 20 months) as was overall survival (OS; 40 vs. 21 months) (23). Furthermore, in a multicenter analysis of 261 patients, of whom 119 underwent allo-SCT, after a prolonged median follow-up of 6.1 years, transplanted patients had significantly better median OS (4.3 vs. 2.3 years) (24).
Experience From Allogeneic Stem Cell Transplantation in Patients With MYELODYSPLASTIC SYNDROMES / MYELOPROLIFERATIVE NEOPLASMS
A. Chronic Myelomonocytic Leukemia
The existing experience is restricted to retrospective analyses from various transplant groups. However, in many retrospective studies, results of allo-SCT in patients with CMML are pooled together with the results of patients with other myeloid malignancies (other MDSs/MPNs, MDS, AML, MPN, etc.), and only exceptionally the outcomes of CMML patients are mentioned separately. Most recently, however, some retrospective studies have focused on the outcomes of CMML and occasionally on other MDSs/MPNs.
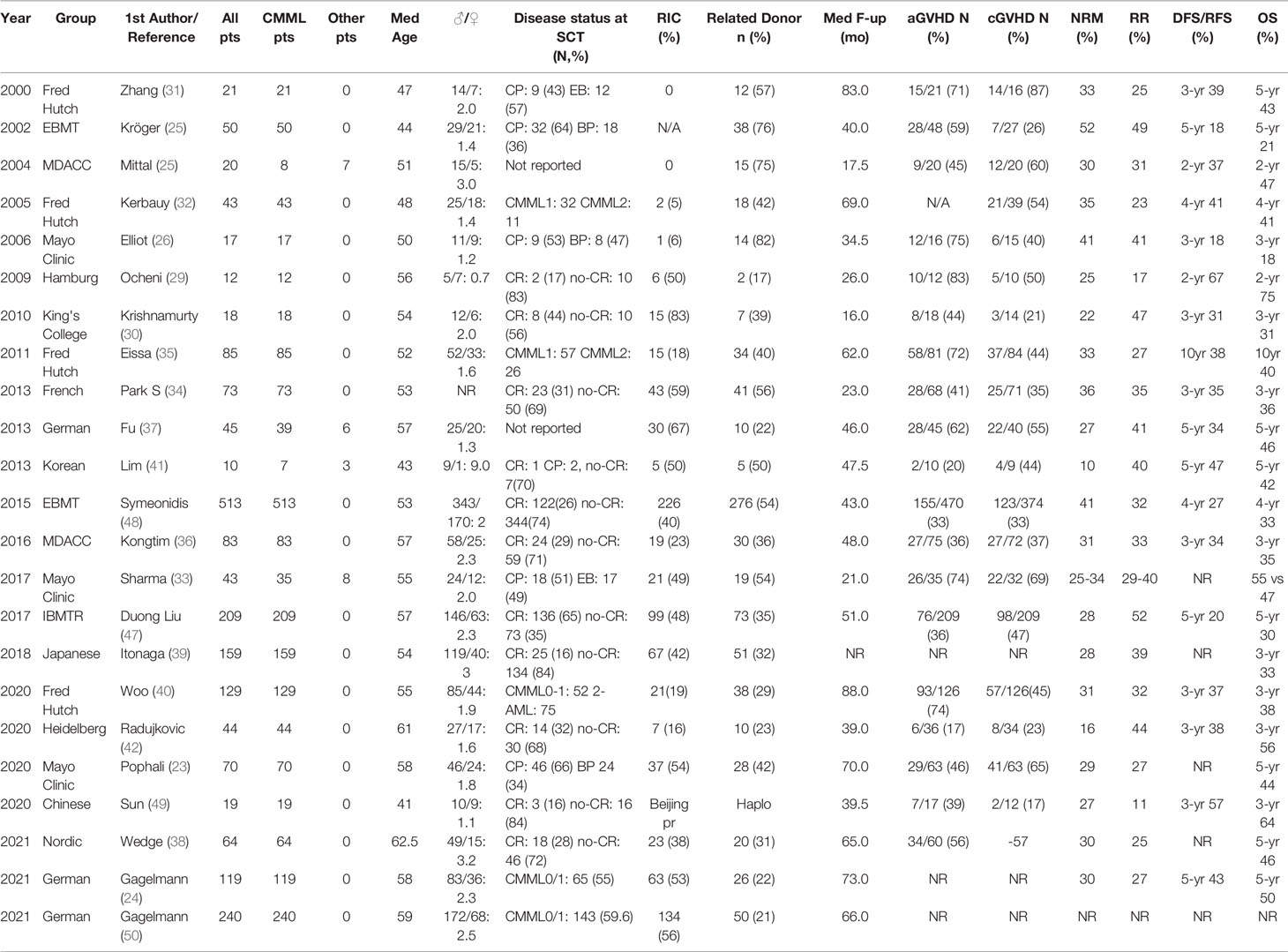
In early reports from the MD Anderson Cancer Center (MDACC) on 20 patients (8 with CMML), probabilities for disease-free survival (DFS) and OS at 2 years were 37% and 47%, respectively (25), while from the Mayo Clinic, among 17 transplanted patients <60 years, 7 were relapsed and, in 6 of them, 1–3 courses of donor leukocyte infusions (DLIs) were offered. Despite the high treatment-related mortality (TRM) of 41% at 3 years, 3 patients remained alive, indicating a graft-versus-leukemia (GVL) effect (26). In support of a GVL effect was the first analysis of the European Blood and Marrow Transplantation Registry (EBMT) on 50 patients, who, despite a 52% TRM at 5 years, demonstrated a lower probability of relapse when grade II–IV acute graft-versus-host disease (GVHD) developed (24% vs. 54%) and higher RR when patients received T cell-depleted allografts (27). Among 148 patients, who received a reduced-intensity conditioning (RIC), after a median of 47 months, relapse-free-survival (RFS), OS, and RR at 3 years were 27%, 27%, and 41%, respectively. For 7 CMML patients, RFS and OS were both 43% (28). Initial experience from Hamburg on 12 patients was also positive, with a low TRM, 50% DFS, and 75% OS at 4 years (29). At the King’s College, 18 CMML patients received an RIC and T cell-depleted allografts. The probabilities of OS, RR, and TRM at 3 years were 31%, 47%, and 31%, respectively (30). Among 21 CMML patients transplanted in the Fred Hutchinson before 2000, 9 achieved sustained Disease-Free Survival (DFS) after a median of 7 years (31), and in a later analysis of 43 patients, NRM, RR, RFS, and OS at 4 years were 35%, 23%, 41%, and 41%, respectively (32). In the Mayo Clinic, out of 43 patients (35 with CMML, 17 on AML status), after a median of 21 months, NRM, RR, and OS were 25%, 29%, and 55%, respectively, for patients transplanted in the chronic phase and 34%, 40%, and 47%, respectively, for those transplanted following AML transformation (33). Similar results were confirmed on 70 patients, of whom 46 were transplanted in the chronic phase and 24 after AML transformation. Median OS was better for patients transplanted in the chronic phase (67 vs. 16 months), and Kaplan-Meier (K-M) estimates for OS at 5 years was 51%—one of the best ever reported (23).
In the French retrospective study of 73 CMML patients, OS, NRM, Event-Free Survival (EFS), and RR at 3 years were 32%, 36%, 29%, and 35%, respectively. NRM was lower in female patients, those transplanted after 2004, and in patients without palpable splenomegaly or pretransplant infections (34). Another collaborative analysis of 85 CMML patients, including 14 with therapy-related disease, reported 25% RR at 3 years, and the use of myeloablative conditioning was associated with better outcomes, compared to RIC (35). In the analysis from MDACC on 83 patients, the 12-month TRM was 31%, and patients who were bridged with HMA had lower RR at 3 years, compared to those receiving AML-type induction chemotherapy (22% vs. 35%), resulting in significantly longer PFS (43% vs. 27%) (36). A German group report on 45 patients, mainly with CMML, observed a low 3-year NRM of 26.7%, while OS at 5 years was 51%. The presence of mutations was used as a marker of minimal residual disease, and their persistence 6 months posttransplant was associated with significantly higher RR (37). The Nordic group applied a post-hoc analysis on 51 CMML patients, with a median follow-up of 5.5 years, and identified clonal mutations in 48 of them. ASXL1, TET2, RUNX1, SRSF2, and RAS were the most frequently mutated genes. Transplantation outcomes were better than those previously reported, with a 5-year OS of 46.5%, NRM of 30%, and RR of 25% (38).
The impact of the donor was investigated on 159 Japanese patients. OS, NRM, and RR at 3 years were 33%, 28%, and 39%, respectively, and the best OS was obtained by [(MRDs), 50.4%], followed by matched-unrelated donors (MUDs, 31.4%), umbilical cord blood (UCB, 15.4%; TRM >75%), and mismatched-unrelated donors (MMUDs, 16.7%) (39). The Fred Hutchinson group described outcomes on 129 patients with the longest median follow-up (9.3 years). Estimated probabilities for relapse, DFS, and OS at 10 years were 32%, 29%, and 30%, respectively, whereas NRM was 32% (40).
Many studies have focused on the conditioning regimen, and majority of them do not report any impact, with the exception of one small study on 10 patients, in which myeloablative conditioning was associated with longer EFS (41). The same is reported by the Heidelberg group on 44 patients, in whom intermediate total body irradiation (TBI) dose (6–8 Gy) combined with mofetil mycophenolate posttransplant immunosuppression was associated with longer LFS in the elderly and less fit patients, compared to alkylator-based conditioning (42). A treosulfan-fludarabine regimen, although administered to older patients with comorbidities, was accompanied by better OS than standard myeloablative or RIC regimens (43). The addition of 2 Gy TBI over a standard treosulfan-fludarabine regimen was investigated on 51 patients with MDS and 49 with CMML. The TBI regimen showed superiority and was associated with longer PFS, whereas NRM was only 9% (44). In another prospective study on 77 patients (13 with CMML), a three-level dose-escalation TBI at non-myeloablative doses (300–450 cGy) was tested. RR, NRM, PFS, and OS at 5 years were 31%, 43%, 35%, and 38%, respectively (45). Total lymphoid irradiation (TLI) and anti-thymocyte globulin (ATG) were used in Stanford for 61 patients, and NRM at 3 years was only 11%, whereas PFS and OS were 35% and 41%, respectively. The authors recommend this regimen for patients with more advanced age (46).
The International Blood and Marrow Transplantation Registry (IBMTR) and the EBMT have published the largest retrospective studies. In the first, 209 patients were transplanted between 2001 and 2012, 35% of them receiving a graft from MRD and 27% exhibiting >5% marrow blasts. The type of bridging treatment (HMA vs. chemotherapy) and the type of conditioning (myeloablative vs. RIC) had no impact on outcome. TRM, RR, DFS, and OS at 5 years were 28%, 52%, 20%, and 30%, respectively (47). In the second, 513 patients who received a related (55.5%) or an unrelated graft (44.5%) following myeloablative conditioning (48.5%) or RIC (44%) were transplanted until December 31, 2009. Disease status at transplantation was CR in 24% and no CR in 67%. NRM, RR, DFS, and OS at 4 years were 41%, 32%, 27%, and 33%, respectively (48).
There is only one study describing encouraging outcomes with haploidentical transplantation on 19 CMML patients. The incidence of acute and chronic GVHD was acceptable, the 3-year TRM was 27%, and RR was only 11%, whereas LFS and OS were 57% and 64%, respectively. The authors suggest that this type of allo-SCT might exert a stronger GVL effect, and hence, RR may be low (49). A summary of the published reports on CMML, with the main patient and transplantation features and outcomes, is presented in Table 1.
B. Juvenile Myelomonocytic Leukemia
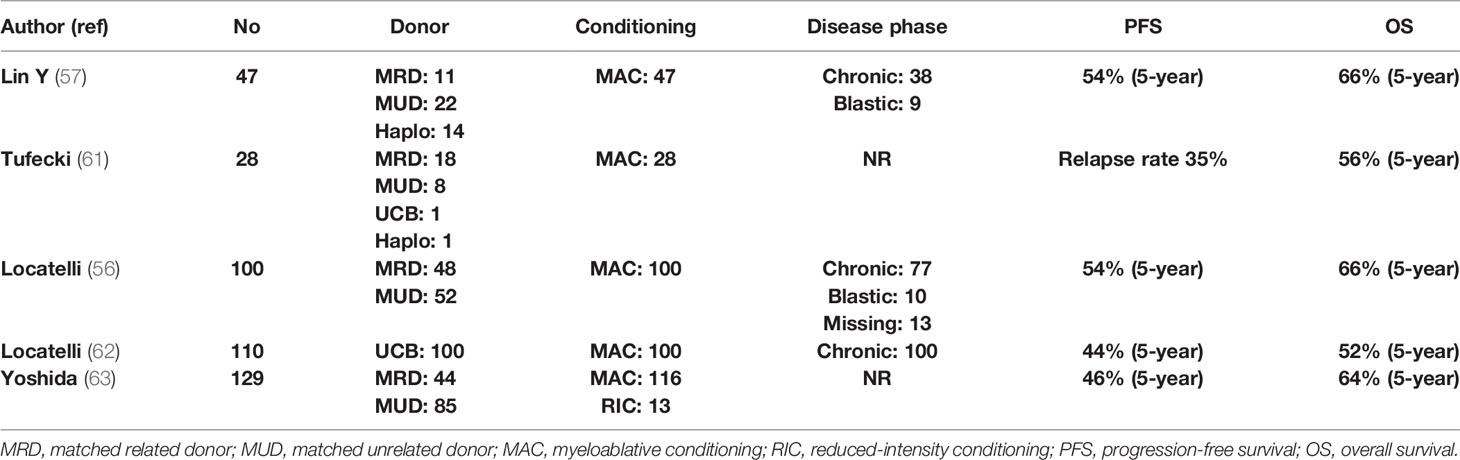
JMML is a rare pediatric leukemia affecting 1.2 children per million annually, with a median age at diagnosis of 2 years and a clear male predominance. It has an aggressive clinical course with a median OS of 10–12 months (51). Main features include splenomegaly, lung and gastrointestinal system monocytic infiltration, a leukoerythroblastic peripheral smear with absolute monocytosis, elevated fetal hemoglobin (HbF), and a hypercellular BM with increased blast percentage but <20% (52). Nearly all JMML cases (90%–95%) harbor either somatic mutations of the RAS pathway genes (PTPN11, KRAS, NRAS) or germline mutations of NF1 and CBL, which are involved in two congenital development disorders, namely, neurofibromatosis and Cbl protooncogene – E3 ubiquitin protein kinase (CBL) syndrome (53). Noonan syndrome caused by germline mutations of PTPN11, NRAS, KRAS, BRAF, SOS1, and RAF1 may exhibit a JMML-like disorder that is usually self-limited (54). Age >2 years at diagnosis, platelets <33 x 109/L, and HbF >10% have been identified as main predictors of poor survival (55).
In essentially all cases of JMML, allo-SCT is strongly indicated and ideally should be performed immediately after diagnosis. Cytoreductive strategies usually involve azacytidine or AML-type chemotherapy, while occasionally, splenectomy is performed for symptom alleviation. Since the patient population is composed of children, TBI is not usually included in the conditioning, but busulfan-based regimens are used. The European Working Group on MDS (EWOG-MDS) provides a recommendation for a three-alkylator regimen consisting of busulfan, cyclophosphamide, and melphalan (56). MRD or MUD should be the first option, while one-locus MMUD or UCB transplantation is a reasonable alternative. Recently, a large study from China compared 27 patients transplanted with an MRD or MUD (Cohort-1), with 20 patients who underwent allo-SCT by using an haploidentical or an MMUD with 2 or 3 HLA disparities (Cohort-2). With a median follow-up of 26 months, OS, DFS, and NRM were 66%, 55%, and 11%, respectively, in the entire group, but the cumulative RR was significantly increased in Cohort-1 as compared with Cohort-2 (56% vs. 5%, P ≤ 0.001). Nevertheless, haploidentical allo-SCT might represent a solution for patients with a rapidly evolving disease for whom an MRD or an MUD is not available (57).
Age at diagnosis >2 years, NF1 or PTPN11 mutation, and high DNA methylation define a patient group with an RR of >50%, raising the issue of immunosuppression intensity and posttransplant prophylaxis (58). Thus, EWOG-MDS recommends keeping immunosuppression with cyclosporine-A at low levels (~80 g/L) and tapering early (from day +40 in the absence of grade II–IV GVHD). Donor chimerism should be tested at very short intervals (even weekly in high-risk patients), since the reappearance of small autologous cell populations mandates immediate withdrawal of immunosuppression (59). Prevention of relapse by preemptive administration of azacytidine or DLI is a frequently applied strategy. Novel approaches such as MAP kinase–ERK kinase (MEK) inhibitors (trametinib) or bcl2 inhibitors (venetoclax) in combination with azacytidine and anti-CD47 monoclonal antibodies are currently evaluated in the context of clinical trials (60). Table 2 presents the results of allo-SCT in patients with JMML (56, 57, 61–63).
C. Atypical Chronic Myelogenous Leukemia and Unclassified Myelodysplastic Syndrome/Myeloproliferative Neoplasm
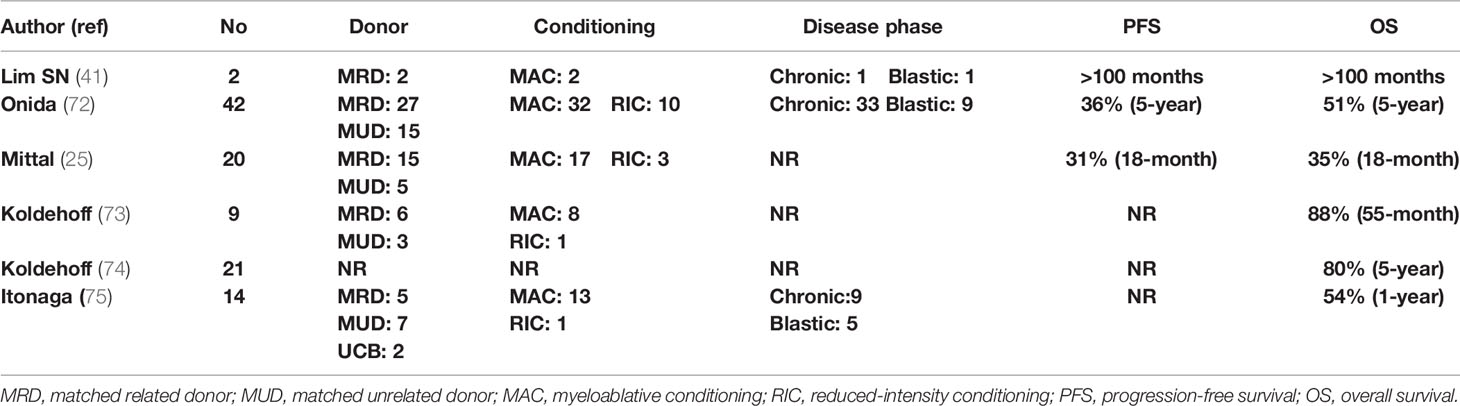
aCML mainly affects elderly patients of male predominance and is characterized by inherent propensity for AML transformation. This is a difficult-to-treat disease with the available conventional treatment options with a median OS of about 2 years from initial diagnosis. In a group of 73 patients, age >65 years, anemia (<10 g/dl) and severe leukocytosis (>50 × 109/l) at diagnosis were recognized as significant adverse prognostic factors and have been used to construct a simple prognostic system, greatly affecting survival (64). In two cohorts of 55 and 65 patients from Italy and the United States, prognosis was generally poor and median OS was 25 and 12 months, respectively. AML transformation occurred in >30% of patients between 12 and 18 months from initial diagnosis [65, 66]. In a retrospective analysis of 65 patients from MDACC, intensive chemotherapy was poorly tolerated and was associated with significantly decreased OS, as compared to HMA, hydroxyurea, or ruxolitinib treatment. Recently, in a new multivariate analysis on 65 patients, older age, thrombocytopenia, increased BM blasts, and abnormal serum lactate dehydrogenase (LDH) were parameters independently associated with decreased OS. Based on these parameters, a new scoring system was generated for a more accurate prediction of survival (67). The mutational landscape of aCML mainly involves SETBP1 and ETNK1 genes, while other commonly identified mutations include ASXL1, N/K-RAS, SRSF2, and TET2 and less frequently (<10%) CBL, CSFR3, and EZH2. JAK2, CARL, and MPL mutations are extremely uncommon (68). SETBP1 mutations have been associated with severe anemia and thrombocytopenia, increased LDH, and worse OS (69, 70). Regarding allo-SCT, many questions related to the timing of transplantation, bridging therapy, donor type, and intensity of preparative regimen still remain unanswered. In a retrospective study of 60 patients with MPN or MDS/MPN in blastic phase from the French Registry, with many of them receiving intensive cytoreduction as bridging treatment, 26 were in CR before allo-SCT, while 34 underwent transplantation with active AML. Not surprisingly, the outcome was extremely poor, with OS and LFS at 3 years of 18% and 9%, respectively. Patients with active disease before transplant had only 3% probability of 3-year OS (71).
Results of allo-SCT were also analyzed by the EBMT on 42 patients, of whom 69% were in first chronic phase, 76% received myeloablative conditioning, and 64% weretransplanted from an MRD. T-cell depletion was applied in 26% and 87% of MRD and MUD, respectively. According to the EBMT risk score (taking into account the patient’s age, disease status, time interval from diagnosis to transplant, donor type, and recipient–donor sex match), 45%, 31%, and 24% of the patients had low, intermediate, and high risk, respectively. This study confirmed the curative potential of allo-SCT in patients with aCML. RFS at 5 years was 36%, NRM was 24%, RR was 40%, and OS was 51%. Age and the EBMT score were significant predictors of OS (72).
Koldehoff et al. reported on 9 patients, of whom 4 were transplanted from MRDs, 4 from MUDs, and 1 from a syngeneic donor. Eight patients received myeloablative conditioning and 8 remain alive, with one relapse of the patient who underwent syngeneic allo-SCT (73). A subsequent follow-up from the same team including 21 patients reported a 5-year OS of 80% with a median survival of 48 months (74). In the early report from MDACC, among 7 patients with aCML, after a median follow-up of 17.5 months, OS and DFS were 35% and 31%, respectively, but five patients died (25). The Japanese group reported outcomes on 19 patients, 15 with aCML and 4 with CNL, who mainly received myeloablative conditioning. One-year OS was >58% and was higher in patients with better performance status and <5% BM blasts (75). Studies reporting results of allo-SCT in patients with aCML are presented in Table 3.
D. Chronic Neutrophilic Leukemia
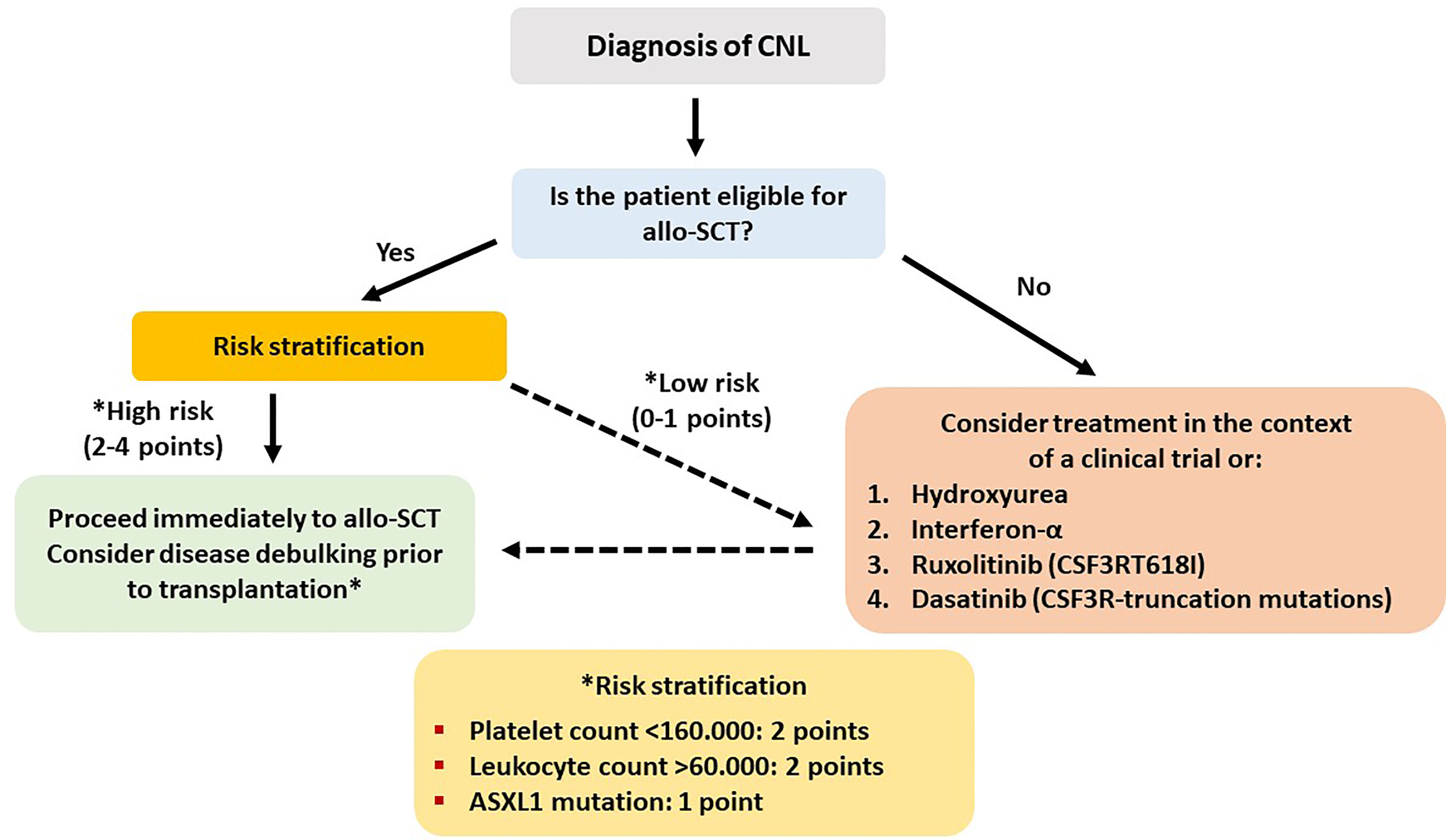
CNL is an extremely rare but aggressive disease, with an estimated annual incidence of 1 case per 10,000,000 individuals, has as a diagnostic hallmark various CSF3R mutations and a median life expectancy of about 1.8 years (76). A prognostic model has been developed in the Mayo Clinic based on data from 19 patients. Retrospective analysis from archival material revealed ASXL1 and SETBP1 gene mutations (besides CSF3R) in 47% and 32% of the patients, respectively. Median OS of the whole group was 22.4 months, and CSF3RT618I mutation (present in 14 patients or 74%) was associated with significantly inferior OS compared to truncation mutations (17.2 vs. 42.7 months). On multivariate analysis, ASXL1 mutation, thrombocytopenia (<160 × 109/L), and hyperleukocytosis (>60 × 109/L) were associated with decreased OS, and these 3 parameters created a risk model for prognostic stratification of the patients in high- and low-risk groups (77).
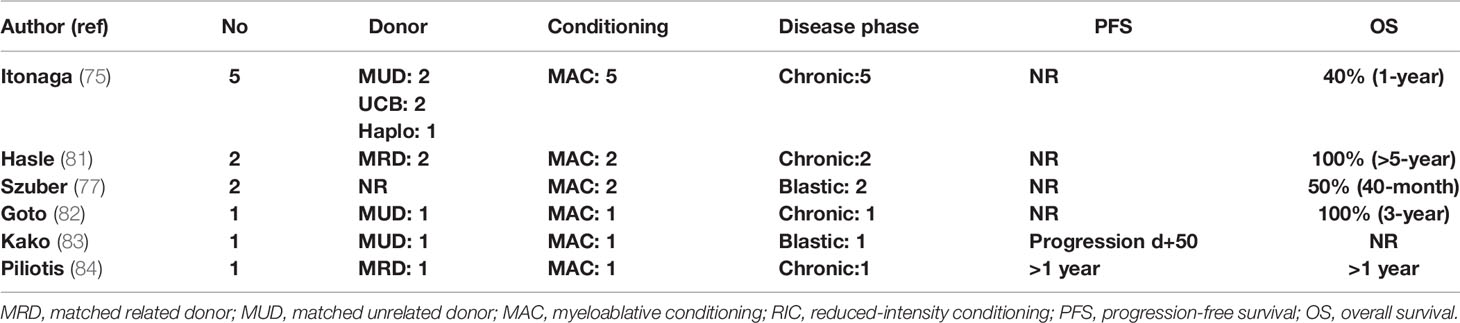
In CNL, AML progression is almost inevitable and occurs at a median of 21 months from initial diagnosis. No standard treatment recommendations exist, and current treatments, mainly consisting of hydroxyurea and interferon-alpha, do not exert any disease-modifying benefit. A recent phase II trial investigating the safety and efficacy of ruxolitinib reported an overall response rate of 35%, making ruxolitinib a promising agent that should be tested in larger patient cohorts (78). Intensive AML-type chemotherapy is usually ineffective when administered after disease progression. Therefore, allo-SCT remains the only potentially curative therapeutic option, and evidence supports early referral as an important factor for better outcome (75, 79). In the largest case series from a nationwide survey in Japan, 5 patients were transplanted between 2003 and 2014. Intention to transplant was based on either disease progression or leukocytosis and splenomegaly uncontrolled by cytoreductive treatment. All patients received myeloablative conditioning, and graft source was an MUD (2), UCB (2), and haploidentical sibling (1). One-year OS was 40%, with one patient dying from sinusoidal obstruction syndrome (d+56), one from bleeding (d+19), and one from disease progression (d+76) (75).
Hydroxyurea or ruxolitinib should be administered to all symptomatic patients or those with significant splenomegaly. Myeloablative conditioning should be administered to fit patients <65 years, while an RIC should be preferred for older or less fit patients. An MRD or MUD should be the preferred option. However, taking into account the recent progress in haplo-SCT in double cord transplants in adults and the experience on the treatment of related disorders, an alternative donor can be used in the absence of a fully matched donor. CSF3R mutation can be used as a marker of an minimal residual disease in the posttransplant period, and persistent detection can alter the adoptive immunotherapy strategy in order to prevent relapse (cyclosporine withdrawal, DLI) (80). Schematically, the treatment algorithm of CNL is shown in Figure 4. Studies reporting results on allo-SCT in patients with CNL are presented in Table 4 (75, 77, 83, 84).
Factors With Prognostic Importance for Allogeneic Stem Cell Transplantation in Myelodysplastic Syndromes/Myeloproliferative Neoplasms
Many studies, particularly when including several decades of patients, have investigated predictors of outcomes, either simple factors or prognostic tools, evaluating prognosis in the non-transplant setting of the disease. The first EBMT study found that manifestation of grade II–IV acute GVHD and the use of non-T cell-depleted allografts were associated with longer DFS (27). The importance of early transplantation was initially pointed out by the Fred Hutchinson group (31), and when more patients were analyzed, the only variable associated with a higher NRM and shorter OS was the Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) (32). The use of posttransplant DLI as an early manipulation of graft failure and of chimerism loss has been applied in at least three studies, two from the Mayo Clinic and one from the King’s College with some successful results reported (26, 30, 33). The latter group has found as important prognostic indicators the percentage of BM blasts (≥5% vs. <5%) and pretransplant cytogenetics (33). Similar results are reported by the more recent analysis from Fred Hutchinson on 85 patients, in which the importance of the HCT-CI was also confirmed. Additional important factors for survival were pretransplant hematocrit and age, whereas the MDAPS (7) and a female donor to female recipient were affecting RR (36).
The group of Milwaukee analyzing 86 transplanted patients with various myeloid malignancies, but none with CMML, found no impact of patient’s obesity on any outcome, although obese patients (Body Mass Index (BMI) >30) had longer hospitalization periods (85). The significance of the chronologic period in which transplantation is performed is easily realizable, since outcomes are improving over time and supportive treatment becomes more effective. Thus, in the French study, major determinants for higher NRM and lower EFS and OS were transplantation before 2004 and the presence of palpable splenomegaly. In the same analysis, female patients exhibited significantly higher RR and NRM, and higher NRM was associated with proliferative CMML and with pretransplant infections (34). The significance of splenomegaly was also stressed by a Chinese study on 25 patients of whom, those with splenomegaly had delayed neutrophil recovery and higher RR and incidence of chronic GVHD (86). In a later report from the Mayo Clinic, splenomegaly, lower HCT-CI, and allo-SCT performed ≤12 months from diagnosis were associated with a more favorable outcome. The small group of MDS/MPN-U, which was analyzed separately, exhibited somewhat better outcomes compared to the outcomes of CMML patients (33). In the EBMT study of 42 aCML patients, patient’s age and the EBMT prognostic score affected OS, whether RFS was higher in MUD, compared to MRD allo-SCT (72).
Increased BM fibrosis has also been recognized as an adverse prognostic factor for DFS and OS, attributed to delayed engraftment, more common cytogenetic abnormalities, and unfavorable driver mutations according to a Chinese retrospective analysis of 239 MDS patients (87). Poor risk cytogenetics and comorbidities were predictors of worse outcome in the retrospective analysis of MDACC on a patient group with MDS and various MDSs/MPNs, exhibiting dismal prognostic factors, for whom RIC was used (88). In the most recent report of the same group focusing on CMML, the outcomes of 83 patients are described, and in multivariate analysis, <5% BM blasts before allo-SCT, the manifestation of chronic GVHD, and initial treatment with HMA were independent predictors of a favorable outcome. In particular, previous treatment with HMA was associated with lower RR and longer PFS (36).
The significance of the donor was investigated on a Japanese group of 159 CMML patients. HLA-matched sibling donor allo-SCT was associated with longer OS and lower NRM, which was highest in the recipients of umbilical cord blood grafts, attributed to delayed neutrophil engraftment (39). In the study from Heidelberg, unrelated donor allo-SCT and TBI-included conditioning were associated with better OS, whereas CPSS could nicely stratify the probability of OS. In this analysis, age was not a significant factor for OS and no clear benefit was proven for transplanted patients with lower-risk CPSS over those not transplanted. However, as in other studies, CPSS could not predict NRM (42). The impact of GVHD was investigated by two Japanese studies of 115 and 141 patients, respectively. In the first, CMML and Refractory anemia with Excess of Blasts (RAEB) patients were pooled together (44 with CMML). An RIC regimen was given to 70% of the patients, and although many of them were older with poor cytogenetics, exhibited similar 4-year OS. Factors associated with poorer survival were poor cytogenetics, BM blasts ≥20% at transplantation, and absence of chronic GVHD, whereas for high-risk patients, the manifestation of chronic GVHD was associated with longer survival (89). In the second study, analysis has been focused on successfully engrafted CMML patients. Grade I acute GVHD was related to better OS and lower leukemia-related death in univariate analysis, whereas in multivariate analysis, extensive chronic GVHD was associated with significantly better OS and lower leukemia-related death in patients who were not transplanted in CR (90). In the IBMTR study, CPSS could only predict OS, which was better in the Low/Intermediate-1 group, and patients with higher CPSS had about twice as high risk for post-relapse death. On multivariate analysis, performance status, CPSS, and the type of graft were independent predictors of DFS and OS (47). In the EBMT study, besides the impact of the period of transplantation, factors associated with a longer PFS and OS were disease status at transplantation (CR vs. no CR) and shorter interval from diagnosis to allo-SCT. Patients transplanted in CR had lower probability for NRM, and disease status at transplantation was the only significant factor for RFS and OS in multivariate analysis (48).
The significance of specific mutations was initially investigated by a German group on 45 patients who were screened for ASXL1, CBL, NRAS, and TET2 gene mutations. ASXL1 and TET2 were the most commonly mutated genes, but the type and the number of mutations had no impact on any outcome. The presence of mutations was used as a marker of minimal residual disease, and their persistence at 6 months posttransplant was associated with higher RR (37). Similar results were obtained by a Chinese study on 59 CMML patients in which the significance of post-allo-SCT minimal residual disease detected by both, flow cytometry and by PCR of the WT1 gene was investigated. Both techniques demonstrated a high level of prognostic value and could predict posttransplant relapse. The significance of the presence of WT1 mutations was not investigated (91).
The Mayo Clinic group compared 4 different prognostic scores and various clinical and genetic factors, including common mutations, on 70 transplanted and in 336 non-transplanted patients. Allo-SCT in other than chronic phase, abnormal cytogenetics, and neutropenia <1.5 × 109/l were predictors of worse outcome. No prognostic score or any mutation had any impact on transplantation outcome. Patients with proliferative type had significantly longer survival when transplanted compared to those who were not transplanted (50 vs. 19 months), whereas a similar difference was not observed among patients with the dysplastic type (23, 24).
In contrast, the Nordic group found that TET2 mutations were associated with a favorable outcome, whereas ASXL1, RUNX1, and RAS mutations were associated with worse OS. Transfusion dependency and higher WBC count before transplant were also associated with earlier relapse, and NRAS mutations were linked to poorer survival due to increased TRM (43). In the more recent analysis of Fred Hutchinson, relapse was associated with poor cytogenetics, higher CPSS and MDACC score, and the presence of pretransplant residual disease, whereas death was associated with poor cytogenetics, pretransplant residual disease, and high HCT-CI. Clonal mutations were identified in 40.3% of the patients, and WT1 and ATRX mutations were associated with a higher RR and a shorter OS. NRAS and a high number of mutations (>10 in general or >4 epigenetic mutations) were also associated with higher RR (40).
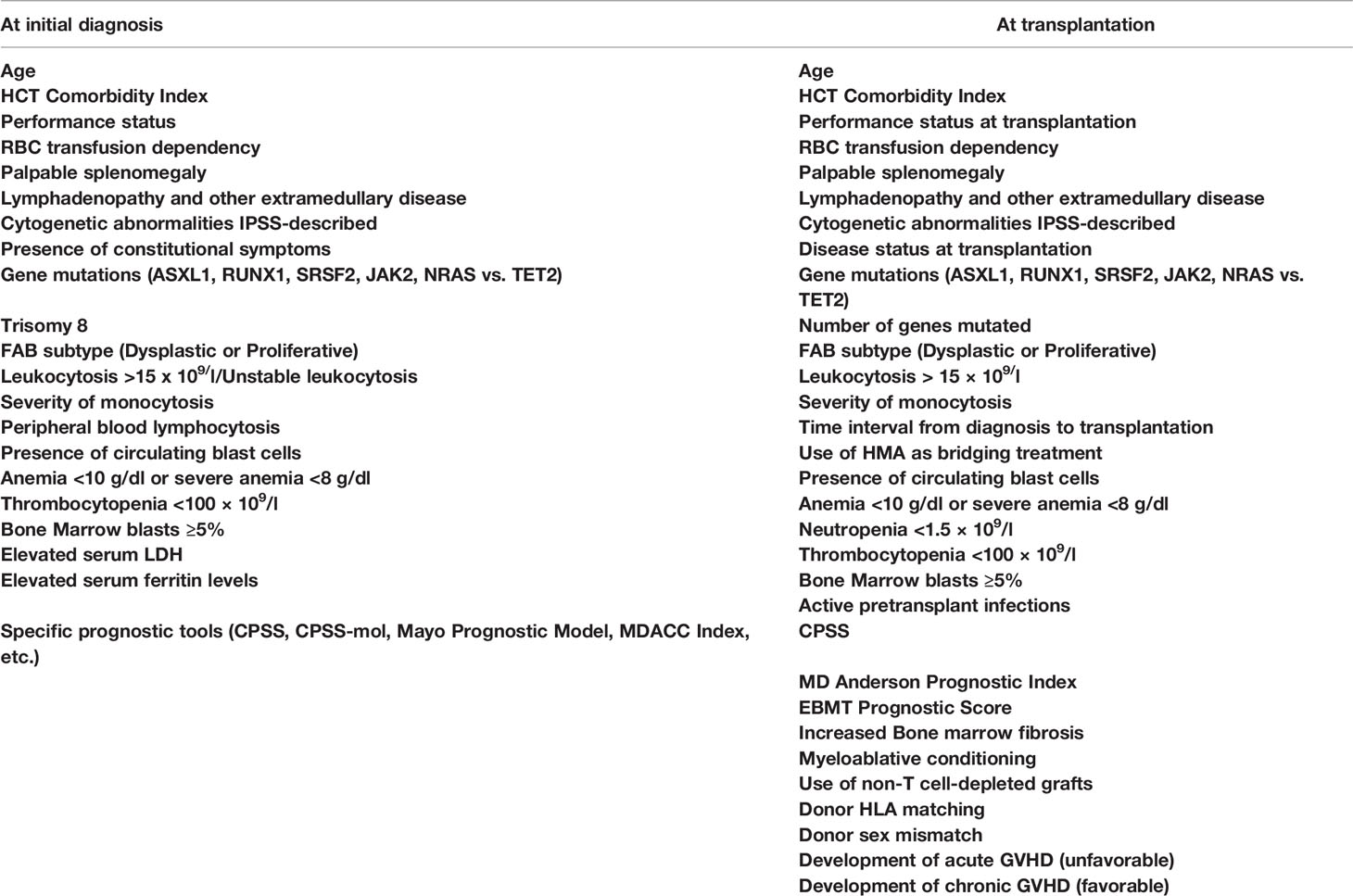
Finally, in a recently published cooperative study on 240 CMML patients with a long median follow-up, increased percentage of BM blasts (>2%), the HCT-CI, and mutations of the ASXL1 or the KRAS genes were found to retain independent prognostic significance for OS and RFS. Collecting these factors, the authors have introduced the first prognostic tool that addresses specifically CMML patients to be transplanted (CMML-specific transplantation-specific prognostic score). The score ranges between 0 and 20 and assigns 1 point to each of the 8 comorbid conditions, described by the HCT-CI, and 4 points to each of the following three factors: pretransplant BM blasts >2 and the presence of ASXL1 or NRAS mutations. This score was superior to any previously reported, nicely predicted NRM and OS by stratifying the patients in 5 groups, with 5-year OS ranging between 19% and 81%, but has not yet been prospectively validated (50). Table 5 summarizes the factors that have been shown to impact prognosis in CMML patients either following a non-transplant approach or undergoing allo-SCT.
Discussion—Conclusions and Current Recommendations
CMML and other overlap MDSs/MPNs are challenging therapeutic problems for the treating physician. As a result of the substantial disease heterogeneity, he or she has to correctly identify the profile of the risk factors in each individual patient, evaluate his or her health background, and appropriately design the interventional treatment approach (15, 16, 92). For patients younger than 60 and for those older than 70 years, such approaches are rather easy to be designed, since by now the only curative intervention remains allo-SCT, which cannot be applied to very elderly and frail patients (16, 18, 92, 93). The most challenging decision for the treating physician concerns patients between 60 and 70 years and few fit patients older than 70 years. For this age range, the physician needs to discriminate higher-risk features that have been well characterized and described (10, 11). A disease mutational profile can greatly help in any case but particularly for patients of the seventh decade of their life (20). Ideally, all patients with CMML and adverse prognostic features and all patients with other MDSs/MPNs, securing an available stem cell donor should proceed to allo-SCT. Among the adverse prognostic features, WHO classification-defined CMML-1 or CMML-2, proliferative type of disease with difficult control of leukocytosis, presence of splenomegaly, extramedullary disease, constitutional symptoms, elevated serum LDH and an otherwise unexplained proinflammatory profile, manifestation of transfusion dependency, thrombocytopenia and increased marrow fibrosis, adverse cytogenetics and/or mutation profile, and a high CPSS or other relevant prognostic score are included (8–11). When eligible patients have been identified, they should thoroughly be informed as early as possible and consent for the recommended approach should be obtained.
When the basic plan has been organized, there are some “technical issues” that should be resolved. Regarding the best timing, patients exhibiting the previously mentioned profile should rapidly undergo allo-SCT after a few months of bridging treatment. The kind of this treatment (AML-type chemotherapy or HMA) appears not to play a core role, although there is emerging evidence that HMA should be preferred in CMML patients of more advanced age and cytoreductive treatment should be the option for younger CMML patients and for those with other MDSs/MPNs (36, 92, 93). For patients with an excess (>5%) of marrow blasts, achieving a CR before transplant with the bridging treatment appears to favor a better posttransplant outcome (18, 22, 40, 43, 49). Another important “technical” issue is identification of the appropriate donor. Although data analysis from several studies has not found any significant impact of the type of donor on the outcome, it appears that this may be valid for relatively younger patients. For patients with more advanced age, the identification of a fully matched donor secures a clearly better outcome (40). A third “technical” issue concerns the use of the appropriate conditioning regimen.
For the abovementioned issues, the following basic principles can be applied. 1) Myeloablative conditioning should be preferred in younger and fit patients, while for older patients above the age of 65 years or for those with significant comorbidities, an RIC regimen appears to be more suitable. 2) A matched related or unrelated donor should be used, but in the absence of an available matched donor, haploidentical or cord blood transplantation should be considered at least for patients younger than 60 years. 3) Cytoreductive or HMA treatment should be administered in symptomatic patients and in those with splenomegaly or with CMML-1/2 before allo-SCT. For patients without an excess of marrow blasts, HMA bridging treatment might suffice. Evaluating patient risk category contributes to better predict several outcomes. To this endpoint, some of the described adverse prognostic factors might indeed reflect other already known prognostic factors. For example, in CMML, splenomegaly and leukocytosis apparently reflect a proliferative type of the disease, whereas circulating blasts and BM blasts >5% apparently fit with WHO-defined CMML-1 or CMML-2. The prognostic ability of CPSS in CMML is a debate. Some authors found it to be predictable, whereas other did not (23, 24, 35, 47). However, newer prognostic tools have been proposed, such as the CPSS-mol, the CPSS-P, and the CPSS transplant-specific, which have incorporated the prognostic importance of mutations that can predict outcome in transplanted patients, as this has been shown in several retrospective studies (23, 50, 74, 91, 93). Using these tools might help to better distinguish the transplantation risk group. The potentially ideal diagnostic and therapeutic recommendations for the four different types of myeloid neoplasia, for which this review is dedicated (CMML, aCML, JMML, and CNL), according to the authors’ opinion are shown in Figures 1–4, respectively.
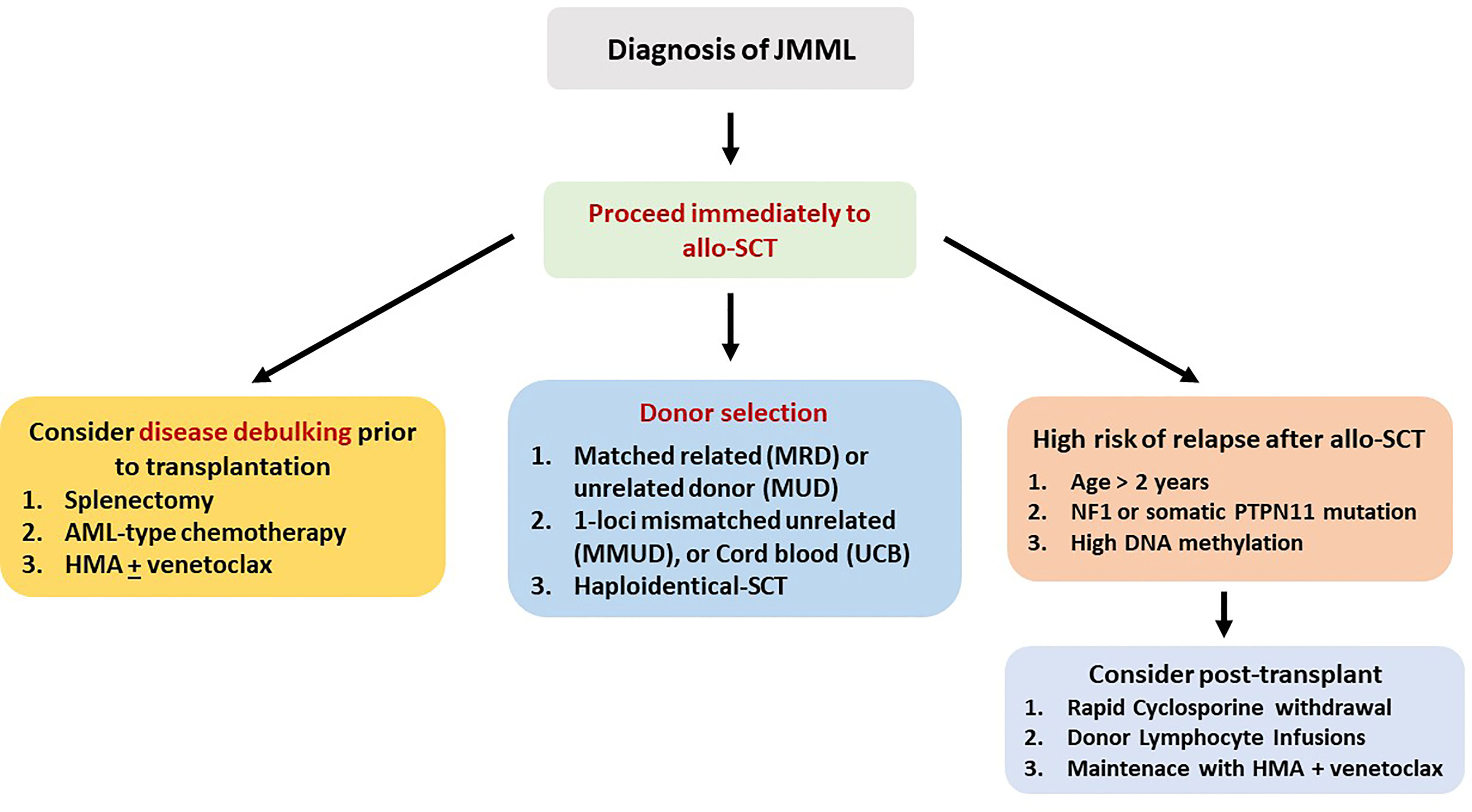
Figure 2 Recommended treatment algorithm for patients with juvenile myelomonocytic leukemia. All patients should be considered candidates for allo-SCT.
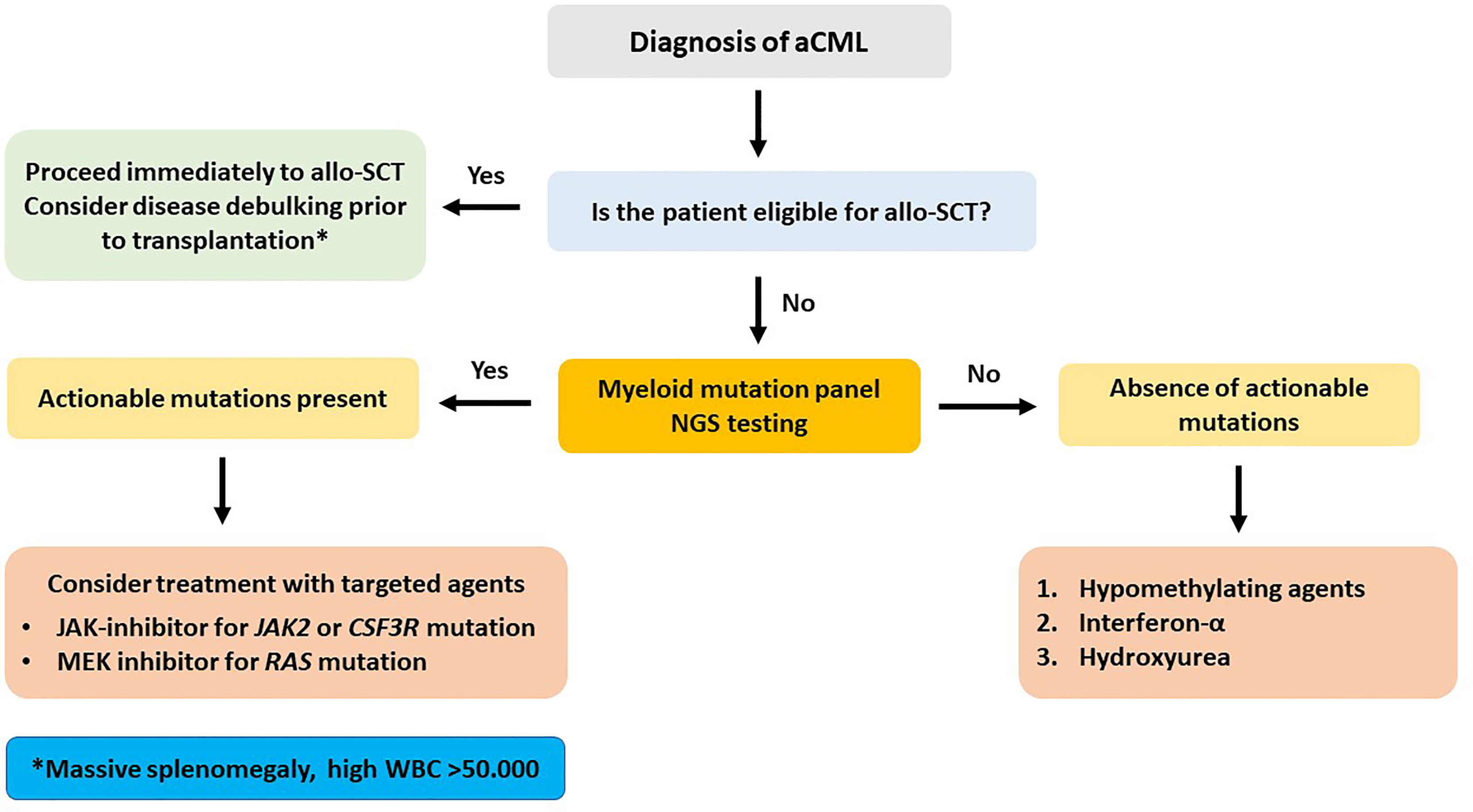
Figure 3 Recommended treatment algorithm for patients with atypical chronic myelogenous leukemia. Massive splenomegaly and extreme leukocytosis at the time of initial diagnosis bear a severely dismal prognosis.
Probably the most important parameter for a successful transplantation is to help the patients achieve the best possible disease status before transplantation. To this point, there are newer targeted treatments besides HMA, which have not yet been tested as treatment tools. These include ruxolitinib and other JAK inhibitors, the CPX-351 complex, RAS and Hedgehog pathway inhibitors, the newer approved oral combination decitabine/cedazuridine, venetoclax, IDH1/IDH2 inhibitors, and other agents. The application of these agents might induce a better remission status before transplantation, thus rendering allo-SCT more effective. However, establishing the most appropriate drug combinations in each individual patient is a long way, which could be delineated through the use of these combinations either as a bridging treatment before transplantation or by incorporating appropriate drugs in the preparatory conditioning regimens. All of these potential new directions could only be substantiated through prospective multicenter randomized trials.
Author Contributions
AS guided the article. AS and PT designed the article, wrote the main parts of the article, and critically reviewed the relevant literature. SC, VL, EV, and AK performed literature search, wrote parts of the article, and contributed to the design of the Tables and Figures. All of the authors reviewed and approved the final version of the article.
Funding
The University of Patras, through a personal account of the first author has covered the publication fees of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aCML, atypical bcr/abl-negative chronic myeloid leukemia; allo-SCT, allogeneic hematopoietic stem cell transplantation; AML, acute myelogenous leukemia; ATG, anti-thymocyte globulin; BM, bone marrow; CMML, chronic myelomonocytic leukemia; CNL, chronic neutrophilic leukemia; DFS, disease-free survival; DLI, donor lymphocyte infusion; EBMT, European Blood and Marrow Transplantation Registry; GVHD, graft-versus-host disease; GVL, graft-versus-leukemia; HbF, fetal hemoglobin; HCT-CI, Hematopoietic Cell Transplantation-Specific Comorbidity Index; HMA, hypomethylating agent; HLA, human leukocyte antigen; JMML, juvenile myelomonocytic leukemia; IBMTR, International Blood and Marrow Transplantation Registry; LDH, lactate dehydrogenase; LFS, leukemia-free survival; MDACC, MD Anderson Cancer Center; MDAPS, MD Anderson Prognostic Score; MDS/MPN, mixed or hybrid or overlap myelodysplastic syndrome/myeloproliferative neoplasm; MDS/MPN-U, myelodysplastic syndrome/myeloproliferative neoplasm unclassifiable; MMUD, mismatched unrelated donor; MPN, myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor; NRM, non-relapse mortality; OS, overall survival; PB, peripheral blood; PFS, progression-free survival; RAB, refractory anemia with an excess of blasts; RR, relapse rate; TBI, total body irradiation; TLI, total lymphoid irradiation; TRM, treatment-related mortality; UCB, umbilical cord blood; WBC, white blood cell; WHO, World Health Organization.
Glossary

References
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
2. Deininger M, Tuner JW, Solary E. Turning the Tide in Myelodysplastic/Myeloproliferative Neoplasms. Nat Rev Cancer (2017) 17(7):425–40. doi: 10.1038/nrc.2017.40
3. Zoumbos NC, Symeonidis A, Kourakli-Symeonidis A. Chronic Neutrophilic Leukemia With Dysplastic Features: A New Variant of Myelodysplastic Syndromes? Acta Hematol (1989) 82(3):156–60. doi: 10.1159/000205367
4. Bornemann L, Schuster M, Schmitz S, Sobczak C, Bessen C, Merz SF, et al. Defective Migration and Dysmorphology of Neutrophil Granulocytes in Atypical Chronic Myeloid Leukemia Treated With Ruxolitinib. BMC Cancer (2020) 20(1):650. doi: 10.1186/s12885-020-07130-7
5. Such E, Cervera J, Costa D, Solé F, Vallespí T, Luño E, et al. Cytogenetic Risk Stratification in Chronic Myelomonocytic Leukemia. Haematologica (2011) 96(3):375–83. doi: 10.3324/haematol.2010.030957
6. Such E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, et al. Development and Validation of a Prognostic Scoring System for Patients With Chronic Myelomonocytic Leukemia. Blood (2013) 121(15):3005–15. doi: 10.1182/blood-2012-08-452938
7. Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, et al. Prognostic Factors and Scoring Systems in Chronic Myelomonocytic Leukemia: A Retrospective Analysis of 213 Patients. Blood (2002) 99(3):840–9. doi: 10.1182/blood.v99.3.840
8. Patnaik MM, Padron E, LaBorde RR, Lasho TL, Finke CM, Hanson CA, et al. Mayo Prognostic Model for WHO-Defined Chronic Myelomonocytic Leukemia: ASXL1 and Spliceosome Component Mutations and Outcomes. Leukemia (2013) 27(7):1504–10. doi: 10.1038/leu.2013.88
9. Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic Score Including Gene Mutations in Chronic Myelomonocytic Leukemia. J Clin Oncol (2013) 31(19):2428–36. doi: 10.1200/JCO.2012.47.3314
10. Elena C, Gallì A, Such E, Meggendorfer M, Germing U, Rizzo E, et al. Integrating Clinical Features and Genetic Lesions in the Risk Assessment of Patients With Chronic Myelomonocytic Leukemia. Blood (2016) 128(10):1408–17. doi: 10.1182/blood-2016-05-714030
11. Calvo X, Nomdedeu M, Santacruz R, Martínez N, Costa D, Pereira A, et al. Comparison of Three Prognostic Scoring Systems in a Series of 146 Cases of Chronic Myelomonocytic Leukemia (CMML): MD Anderson Prognostic Score (MDAPS), CMML-Specific Prognostic Scoring System (CPSS) and Mayo Prognostic Model. A Detailed Review of Prognostic Factors in CMML. Leuk Res (2015) 39(11):1146–53. doi: 10.1016/j.leukres.2015.05.017. S0145-2126(15)30324-6.
12. Gur HD, Loghavi S, Garcia-Manero G, Routbort M, Kanagal-Shamanna R, Quesada A, et al. Chronic Myelomonocytic Leukemia With Fibrosis Is a Distinct Disease Subset With Myeloproliferative Features and Frequent JAK2 P.V617F Mutations. Am J Surg Pathol (2018) 42(6):799–806. doi: 10.1097/PAS.0000000000001058
13. Subari S, Patnaik M, Alfakara D, Gangat N, Elliott M, Hogan W, et al. Patients With Therapy-Related CMML Have Shorter Median Overall Survival Than Those With De Novo CMML: Mayo Clinic Long-Term Follow-Up Experience. Clin Lymphoma Myeloma Leuk (2015) 15(9):546–9. doi: 10.1016/j.clml.2015.06.002
14. Patnaik MM, Vallapureddy R, Yalniz FF, Hanson CA, Ketterling RP, Lasho TL, et al. Therapy Related-Chronic Myelomonocytic Leukemia (CMML): Molecular, Cytogenetic, and Clinical Distinctions From De Novo CMML. Am J Hematol (2018) 93(1):65–73. doi: 10.1002/ajh.24939
15. Solary E, Itzykson R. How I Treat Chronic Myelomonocytic Leukemia. Blood (2017) 130(2):126–36. doi: 10.1182/blood-2017-04-736421
16. Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, et al. Diagnosis and Treatment of Chronic Myelomonocytic Leukemias in Adults. Recommendations From the European Hematology Association and the European LeukemiaNet. Hemasphere (2018) 2(6):e150. doi: 10.1097/HS9.0000000000000150
17. de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic Hematopoietic Stem Cell Transplantation for MDS and CMML: Recommendations From an International Expert Panel. Blood (2017) 129(13):1753–62. doi: 10.1182/blood-2016-06-724500
18. Gotlib J. How I Treat Atypical Chronic Myeloid Leukemia. Blood (2017) 129(7):838–45. doi: 10.1182/blood-2016-08-693630
19. Coston T, Pophali P, Vallapureddy R, Lasho TL, Finke CM, Ketterling RP, et al. Suboptimal Response Rates to Hypomethylating Agent Therapy in Chronic Myelomonocytic Leukemia; a Single Institutional Study of 121 Patients. Am J Hematol (2019) 94(7):767–79. doi: 10.1002/ajh.25488
20. Triguero A, Xicoy B, Zamora L, Jiménez MJ, García O, Calabuig M, et al. Response to Azacitidine in Patients With Chronic Myelomonocytic Leukemia According to Overlap Myelodysplastic/ Myeloproliferative Neoplasms Criteria. Leuk Res (2022) 26:116. doi: 10.1016/j.leukres.2022.106836
21. Duchmann M, Yalniz FF, Sanna A, Sallman D, Coombs CC, Renneville A, et al. Prognostic Role of Gene Mutations in Chronic Myelomonocytic Leukemia Patients Treated With Hypomethylating Agents. EBioMedicine (2018) 31:174–81. doi: 10.1016/j.ebiom.2018.04.018
22. Kröger N, Eikema D-J, Köster L, Beelen D, de Wreede LC, Finke J, et al. Impact of Primary Disease on Outcome After Allogeneic Stem Cell Transplantation for Transformed Secondary Acute Leukaemia. Brit J Haematol (2019) 185(4):725–32. doi: 10.1111/bjh.15819
23. Pophali P, Matin A, Mangaonkar AA, Carr R, Binder M, Al-Kali A, et al. Prognostic Impact and Timing Considerations for Allogeneic Hematopoietic Stem Cell Transplantation in Chronic Myelomonocytic Leukemia. Blood Cancer J (2020) 10(11):121. doi: 10.1038/s41408-020-00387-y
24. Gagelmann N, Bogdanov R, Stölzel F, Rautenberg C, Panagiota V, Becker H, et al. Long-Term Survival Benefit After Allogeneic Hematopoietic Cell Transplantation for Chronic Myelomonocytic Leukemia. Transplant Cell Ther (2021) 27(1):95.e1–4. doi: 10.1016/j.bbmt.2020.10.007
25. Mittal P, Saliba RM, Giralt SA, Shahjahan M, Cohen AI, Karandish S, et al. Allogeneic Transplantation: A Therapeutic Option for Myelofibrosis, Chronic Myelomonocytic Leukemia and Philadelphia-Negative/BCR-ABL-Negative Chronic Myelogenous Leukemia. Bone Marrow Transplant (2004) 33(10):1005–9. doi: 10.1038/sj.bmt.1704472
26. Elliott MA, Tefferi A, Hogan WJ, Letendre L, Gastineau DA, Ansell SM, et al. Allogeneic Stem Cell Transplantation and Donor Lymphocyte Infusions for Chronic Myelomonocytic Leukemia. Bone Marrow Transplant (2006) 37(11):1003–8. doi: 10.1038/sj.bmt.1705369
27. Kröger N, Zabelina T, Guardiola P, Runde V, Sierra J, van Biezen A, et al. Allogeneic Stem Cell Transplantation of Adult Chronic Myelomonocytic Leukaemia. A Report on Behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). . Br J Haematol (2002) 118(1):67–73. doi: 10.1046/j.1365-2141.2002.03552.x
28. Laport GG, Sandmaier BM, Storer BE, Scott BL, Stuart MJ, Lange T, et al. Reduced-Intensity Conditioning Followed by Allogeneic Hematopoietic Cell Transplantation for Adult Patients With Myelodysplastic Syndrome and Myeloproliferative Disorders. Biol Blood Mar Transplant (2008) 14(2):246–55. doi: 10.1016/j.bbmt.2007.11.012
29. Ocheni S, Kröger N, Zabelina T, Zander AR, Bacher U. Outcome of Allo-SCT for Chronic Myelomonocytic Leukemia. Bone Marrow Transplant (2009) 43(8):659–61. doi: 10.1038/bmt.2008.366
30. Krishnamurthy P, Lim ZY, Nagi W, Kenyon M, Mijovic A, Ireland R, et al. Allogeneic Haematopoietic SCT for Chronic Myelomonocytic Leukaemia: A Single-Centre Experience. Bone Marrow Transplant (2010) 45(10):1502–7. doi: 10.1038/bmt.2009.375
31. Zang Y-D, Deeg HJ, Gooley D, Anderson JE, Anasetti C, Sanders J, et al. Treatment of Chronic Myelomonocytic Leukaemia by Allogeneic Bone Marrow Transplantation. Br J Haematol (2000) 110(1):217–22. doi: 10.1046/j.1365-2141.2000.02133.x
32. Kerbauy DMB, Chyou F, Gooley T, Sorror ML, Scott B, Pagel JM, et al. Allogeneic Hematopoietic Cell Transplantation for Chronic Myelomonocytic Leukemia. Biol Blood Marrow Transplant (2005) 11(9):713–20. doi: 10.1016/j.bbmt.2005.05.008
33. Sharma P, Shinde SS, Damlaj M, Hefazi Rorghabeh M, Hashmi SK, Litzow MR, et al. Allogeneic Hematopoietic Stem Cell Transplant in Adult Patients With Myelodysplastic Syndrome/Myeloproliferative Neoplasm (MDS/MPN) Overlap Syndromes. Leuk Lymphoma (2017) 58(4):872–81. doi: 10.1080/10428194.2016.1217529
34. Park S, Labopin M, Yakoub-Agha I, Delaunay J, Dhedin N, Deconinck E, et al. Allogeneic Stem Cell Transplantation for Chronic Myelomonocytic Leukemia: A Report From the Societe Francaise De Greffe De Moelle Et De Therapie Cellulaire. Eur J Haematol (2013) 90(5):355–64. doi: 10.1111/ejh.12073
35. Eissa H, Gooley TA, Sorror ML, Nguyen F, Scott BL, Doney K, et al. Allogeneic Hematopoietic Cell Transplantation for Chronic Myelomonocytic Leukemia: Relapse-Free Survival Is Determined by Karyotype and Comorbidities. Biol Blood Marrow Transplant (2011) 17(6):908–15. doi: 10.1016/j.bbmt.2010.09.018
36. Kongtim P, Popat U, Jimenez A, Gaballa S, El Fakih R, Rondon G, et al. Treatment With Hypomethylating Agents Before Allogeneic Stem Cell Transplant Improves Progression-Free Survival for Patients With Chronic Myelomonocytic Leukemia. Biol Blood Marrow Transplant (2016) 22(1):47–53. doi: 10.1016/j.bbmt.2015.08.031
37. Fu Y, Schroeder T, Zabelina T, Badbaran A, Bacher U, Kobbe G, et al. Post Allogeneic Monitoring With Molecular Markers Detected by Pretransplant Next-Generation or Sanger Sequencing Predicts Clinical Relapse in Patients With Myelodysplastic/Myeloproliferative Neoplasms. Eur J Hematol (2013) 92(3):189–94. doi: 10.1111/ejh.12223
38. Wedge E, Werner Hansen J, Dybedal I, Creignou M, Ejerblad E, Lorenz F, et al. Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myelomonocytic Leukemia: Clinical and Molecular Genetic Prognostic Factors in a Nordic Population. Transplant Cell Ther (2021) 27(12):991. doi: 10.1016/j.jtct.2021.08.028
39. Itonaga H, Aoki K, Aoki J, Ishikawa T, Ishiyama K, Uchida N, et al. Prognostic Impact of Donor Source on Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Adults With Chronic Myelomonocytic Leukemia: A Nationwide Retrospective Analysis in Japan. Biol Blood Marrow Transplant (2018) 24(4):840–8. doi: 10.1016/j.bbmt.2017.11.016
40. Woo J, Choi DR, Storer BE, Yeung C, Halpern AB, Salit RB, et al. Impact of Clinical, Cytogenetic, and Molecular Profiles on Long-Term Survival After Transplantation in Patients With Chronic Myelomonocytic Leukemia. Haematologica (2020) 105(3):652–60. doi: 10.3324/haematol.2019.218677
41. Lim SN, Lee JH, Lee JH, Kim DY, Kim SD, Kang YA, et al. Allogeneic Hematopoietic Cell Transplantation in Adult Patients With Myelodysplastic/Myeloproliferative Neoplasms. Blood Res (2013) 48(3):178–84. doi: 10.5045/br.2013.48.3.178
42. Radujkovic A, Hegenbart U, Müller-Tidow C, Herfarth K, Dreger P, Luft T. High Leukemia-Free Survival After TBI-Based Conditioning and Mycophenolate Mofetil-Containing Immunosuppression in Patients Allografted for Chronic Myelomonocytic Leukemia: A Single-Center Experience. Ann Hematol (2020) 99(4):855–66. doi: 10.1007/s00277-020-03952-4
43. Wedge E, Sengeløv H, Werner Hansen J, Smedegaard Andersen N, Schjødt I, Petersen SL, et al. Improved Outcomes After Allogenic Hematopoietic Stem Cell Transplantation With Fludarabine/Treosulfan for Patients With Myelodysplastic Syndromes. Biol Blood Marrow Transplant (2020) 26(6):1091–8. doi: 10.1016/j.bbmt.2020.02.010
44. Deeg HJ, Stevens EA, Salit BR, Ermoian RP, Fang M, Gyurkocza B, et al. Transplant Conditioning With Treosulfan/Fludarabine With or Without Total Body Irradiation: A Randomized Phase II Trial in Patients With Myelodysplastic Syndrome and Acute Myeloid Leukemia. Biol Blood Marrow Transplant (2018) 24(5):956–63. doi: 10.1016/j.bbmt.2017.12.785
45. Monaco F, Scott BL, Chauncey TR, Petersen FB, Storer BE, Baron F, et al. Total Body Irradiation Dose Escalation Decreases Risk of Progression and Graft Rejection After Hematopoietic Cell Transplantation for Myelodysplastic Syndromes or Myeloproliferative Neoplasms. Haematologica (2019) 104(6):1221–9. doi: 10.3324/haematol.2018.199398
46. Benjamin J, Chhabra S, Kohrt HE, Lavori P, Laport GG, Arai S, et al. Total Lymphoid Irradiation - Antithymocyte Globulin Conditioning and Allogeneic Transplantation for Patients With Myelodysplastic Syndromes and Myeloproliferative Neoplasms. Biol Blood Marrow Transplant (2014) 20(6):837–43. doi: 10.1016/j.bbmt.2014.02.023
47. Liu HD, Ahn KW, Hu ZH, Hamadani M, Nishihori T, Wirk B, et al. Allogeneic Hematopoietic Cell Transplantation for Adult Chronic Myelomonocytic Leukemia. Biol Blood Marrow Transplant (2017) 23(5):767–75. doi: 10.1016/j.bbmt.2017.01.078
48. Symeonidis A, van Biezen A, de Wreede L, Piciocchi A, Finke J, Beelen D, et al. Achievement of Complete Remission Predicts Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients With Chronic Myelomonocytic Leukemia (CMML). A Study of the Chronic Malignancies Working Party of the EBMT. Br J Haematol (2015) 171(2):239–46. doi: 10.1111/bjh.13576
49. Sun YQ, Zhao C, Wang Y, Yan CH, Zhang XH, Xu LP, et al. Haploidentical Stem Cell Transplantation in Patients With Chronic Myelomonocytic Leukemia. Sci China Life Sci (2020) 63(8):1261–4. doi: 10.1007/s11427-019-1606-3
50. Gagelmann N, Badbaran A, Beelen DW, Salit RB, Stölzel F, Rautenberg C, et al. A Prognostic Score Including Mutation Profile and Clinical Features for Patients With CMML Undergoing Stem Cell Transplantation. Blood Adv (2021) 5(6):1760–9. doi: 10.1182/bloodadvances.2020003600
51. Niemeyer CM, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, et al. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS). Chronic Myelomonocytic Leukemia in Childhood: A Retrospective Analysis of 110 Cases. Blood (1997) 89(10):3534–43. doi: 10.1182/blood.V89.10.3534
52. Weinberg RS, Leibowitz D, Weinblatt ME, Kochen J, Alter BP. Juvenile Chronic Myelogenous Leukemia: The Only Example of Truly Fetal (Not Fetal-Like) Erythropoiesis. Br J Haematol (1990) 76(2):307–10. doi: 10.1111/j.1365-2141.1990.tb07891.x
53. Niemeyer CM. RAS Diseases in Children. Haematologica (2014) 99(11):1653–62. doi: 10.3324/haematol.2014.114595
54. Kratz CP, Niemeyer CM, Castleberry RP, Cetin M, Bergsträsser E, Emanuel PD, et al. The Mutational Spectrum of PTPN11 in Juvenile Myelomonocytic Leukemia and Noonan Syndrome/Myeloproliferative Disease. Blood (2005) 106(6):2183–5. doi: 10.1182/blood-2005-02-0531
55. Passmore SJ, Hann IM, Stiller CA, Ramani P, Swansbury GJ, Gibbons B, et al. Pediatric Myelodysplasia: A Study of 68 Children and a New Prognostic Scoring System. Blood (1995) 85(7):1742–50. doi: 10.1182/blood.V85.7.1742.bloodjournal8571742
56. Locatelli F, Nöllke P, Zecca M, Korthof E, Lanino E, Peters C, et al. European Working Group on Childhood MDS; European Blood and Marrow Transplantation Group. Hematopoietic Stem Cell Transplantation (HSCT) in Children With Juvenile Myelomonocytic Leukemia (JMML): Results of the EWOG-MDS/EBMT Trial. Blood (2005) 105(1):410–9. doi: 10.1182/blood-2004-05-1944
57. Lin Y-C, Luo C-J, Miao Y, Wang J-M, Luo C-Y, Qin X, et al. Human Leukocyte Antigen Disparities Reduce Relapse After Hematopoietic Stem Cell Transplantation in Children With Juvenile Myelomonocytic Leukemia: A Single-Center Retrospective Study From China. Pediatr Transplant (2020) 25:e13825. doi: 10.1111/petr.13825
58. Schönung M, Meyer J, Nöllke P, Olshen AB, Hartmann M, Murakami N, et al. International Consensus Definition of DNA Methylation Subgroups in Juvenile Myelomonocytic Leukemia. Clin Cancer Res (2021) 27(1):158–68. doi: 10.1158/1078-0432.CCR-20-3184
59. Inagaki J, Fukano R, Nishikawa T, Nakashima K, Sawa D, Ito N, et al. Outcomes of Immunological Interventions for Mixed Chimerism Following Allogeneic Stem Cell Transplantation in Children With Juvenile Myelomonocytic Leukemia. Pediatr Blood Cancer (2013) 60(1):116–20. doi: 10.1002/pbc.24259
60. Tasian SK, Casas JA, Posocco D, Gandre-Babbe S, Gagne AL, Liang G, et al. Mutation-Specific Signaling Profiles and Kinase Inhibitor Sensitivities of Juvenile Myelomonocytic Leukemia Revealed by Induced Pluripotent Stem Cells. Leukemia (2019) 33(1):181–90. doi: 10.1038/s41375-018-0169-y
61. Tufecki O, Kocak U, Kaya Z, Yenicesu I, Albayrak C, Albayrak D, et al. Juvenile Myelomonocytic Leukemia in Turkey: A Retrospective Analysis of 65 Patients. Turk J Haematol (2018) 35(1):27–34. doi: 10.4274/tjh.2017.0021
62. Locatelli F, Crotta A, Ruggeri A, Eapen M, Wagner J, Macmillan M, et al. Analysis of Risk Factors Influencing Outcomes After Cord Blood Transplantation in Children With Juvenile Myelomonocytic Leukemia: A Eurocord, EBMT, EWOGMDS, CIBMTR Study. Blood (2013) 122(12):2135–41. doi: 10.1182/blood-2013-03-491589
63. Yoshida N, Sakaguchi H, Yabe M, Hasegawa D, Hama A, Hasegawa D, et al. Clinical Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Children With Juvenile Myelomonocytic Leukemia: A Report From the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(5):902–10. doi: 10.1016/j.bbmt.2019.11.029
64. Onida F, Ball G, Kantarjian HM, Smith TL, Glassman A, Albitar M, et al. Characteristics and Outcome of Patients With Philadelphia Chromosome Negative, Bcr/Abl Negative Chronic Myelogenous Leukemia. Cancer. (2002) 95(8):1673–84. doi: 10.1002/cncr.10832
65. Breccia M, Biondo F, Latagliata R, Carmosino I, Mandelli F, Alimena G. Identification of Risk Factors in Atypical Chronic Myeloid Leukemia. Haematologica (2006) 91(11):1566–8.
66. Wang SA, Hasserjian RP, Fox PS, Rogers H, Geyer J, Chabot-Richards D, et al. A Typical Chronic Myeloid Leukemia is Clinically Distinct From Unclassifiable Myelodysplastic/Myeloproliferative Neoplasms. Blood (2014) 123(17):2645–51. doi: 10.1182/blood-2014-02-553800
67. Montalban-Bravo G, Kanagal-Shamanna R, Sasaki K, Masarova L, Naqvi K, Jabbour E, et al. Clinicopathologic Correlates and Natural History of Atypical Chronic Myeloid Leukemia. Cancer (2021) 127(17):3113–24. doi: 10.1002/cncr.33622
68. Crisà E, Nicolosi M, Ferri V, Favini C, Gaidano G, Patriarca A. Atypical Chronic Myeloid Leukemia: Where Are We Now? Int J Mol Sci (2020) 21(18):6862. doi: 10.3390/ijms21186862
69. Piazza R, Valletta S, Winkelmann N, Redaelli S, Spinelli R, Pirola A, et al. Recurrent SETBP1 Mutations in Atypical Chronic Myeloid Leukemia. Nat Genet (2013) 45(1):18–24. doi: 10.1038/ng.2495
70. Meggendorfer M, Bacher U, Alpermann T, Haferlach C, Kern W, Gambacorti-Passerini C, et al. SETBP1 Mutations Occur in 9% of MDS/MPN and in 4% of MPN Cases and are Strongly Associated With Atypical CML, Monosomy 7, Isochromosome I(17)(Q10), ASXL1 and CBL Mutations. Leukemia (2013) 27(9):1852–60. doi: 10.1038/leu.2013.133
71. Cahu X, Chevallier P, Clavert A, Suarez F, Michallet M, Vincent L, et al. Allo-SCT for Philadelphia-Negative Myeloproliferative Neoplasms in Blast Phase: A Study From the Societe Française De Greffe De Moelle Et De Therapie Cellulaire (SFGM-Tc). Bone Marrow Transplant (2014) 49(6):756–60. doi: 10.1038/bmt.2014.31
72. Onida F, de Wreede L, van Biezen A, Eikema DJ, Byrne JL, Iori AP, et al. Allogeneic Stem Cell Transplantation in Patients With Atypical Chronic Myeloid Leukaemia: A Retrospective Study From the Chronic Malignancies Working Party of the EBMT. Br J Haematol (2017) 177:759–65. doi: 10.1111/bjh.14619
73. Koldehoff M, Beelen DW, Trenschel R, Steckel NK, Peceny R, Ditschkowski M, et al. Outcome of Hematopoietic Stem Cell Transplantation in Patients With Atypical Chronic Myeloid Leukemia. Bone Marrow Transplant (2004) 34:1047–50. doi: 10.1038/sj.bmt.1704686
74. Koldehoff M, Steckel NK, Hegerfeldt Y, Ditschkowski M, Beelen DW, Elmaagacli AH. Clinical Course and Molecular Features in 21 Patients With Atypical Chronic Myeloid Leukemia. Int J Lab Hematol (2012) 34(1):e3–5. doi: 10.1111/j.1751-553X.2011.01351.x
75. Itonaga H, Ota S, Ikeda T, Taji H, Amano I, Hasegawa Y, et al. Allogeneic Hematopoietic Stem Cell Transplantation for the Treatment of BCRABL1-Negative Atypical Chronic Myeloid Leukemia and Chronic Neutrophilic Leukemia: A Retrospective Nationwide Study in Japan. Leuk Res (2018) 75:50–7. doi: 10.1016/j.leukres.2018.11.003
76. Ruan GJ, Smith CJ, Day C, Harmsen WS, Zblewski DL, Alkhateeb H, et al. A Population-Based Study of Chronic Neutrophilic Leukemia in the United States. Blood Cancer J (2020) 10(6):68. doi: 10.1038/s41408-020-0334-1
77. Szuber N, Finke CM, Lasho TL, Elliott MA, Hanson CA, Pardanani A, et al. CSF3R-Mutated Chronic Neutrophilic Leukemia: Long-Term Outcome in 19 Consecutive Patients and Risk Model for Survival. Blood Cancer J (2018) 8(2):21. doi: 10.1038/s41408-018-0058-7
78. Dao KT, Gotlib J, Deininger MMN, Oh ST, Cortes JE, Collins RH Jr, et al. Efficacy of Ruxolitinib in Patients With Chronic Neutrophilic Leukemia and Atypical Chronic Myeloid Leukemia. J Clin Oncol (2020) 38(10):1006–18. doi: 10.1200/JCO.19.00895
79. Menezes J, Cigudosa JC. Chronic Neutrophilic Leukemia: A Clinical Perspective. OncoTargets Ther (2015) 8:2383–90. doi: 10.2147/OTT.S49688
80. Langabeer SE, McCarron SL, Haslam K, O'Donovan MT, Conneally E. The CSF3R T618I Mutation as a Disease-Specific Marker of Atypical CML Post Allo-SCT. Bone Marrow Transplant (2014) 49(6):843–4. doi: 10.1038/bmt.2014.35
81. Hasle H, Olesen G, Kerndrup G, Philip P, Jacobsen N. Chronic Neutrophil Leukaemia in Adolescenceand Young Adulthood. Br J Haematol (1996) 94(4):628–30. doi: 10.1046/j.1365-2141.1996.7082329.x
82. Goto H, Hara T, Tsurumi H, Tanabashi S, Moriwaki H. Chronic Neutrophilic Leukemia With Congenital Robertsonian Translocation Successfully Treated With Allogeneic Bone Marrow Transplantation in a Young Man. Inter Med (2009) 48(7):563–7. doi: 10.2169/internalmedicine.48.1334
83. Kako S, Kanda Y, Sato T, Goyama S, Noda N, Shoda E, et al. Early Relapse of JAK2 V617F-Positive Chronic Neutrophilic Leukemia With Central Nervous System Infiltration After Unrelated Bone Marrow Transplantation. Am J Hematol (2007) 82(5):386–90. doi: 10.1002/ajh.20805
84. Piliotis E, Kutas G, Lipton JH. Allogeneic Bone Marrow Transplantation in the Management of Chronic Neutrophilic Leukemia. Leuk Lymphoma (2002) 43(10):2051–4. doi: 10.1080/1042819021000016087
85. Voshtina E, Szabo A, Hamadani M, Fenske TS, D’Souza A, Chhabra S, et al. Impact of Obesity on Clinical Outcomes of Elderly Patients Undergoing Allogeneic Hematopoietic Cell Transplantation for Myeloid Malignancies. Biol Blood Mar Transplant (2019) 25(1):e33–8. doi: 10.1016/j.bbmt.2018.08.031
86. Zhao C, Huang XS, Zhao XS, Wang Y, Yan CH, Xu LP, et al. Impact of Splenomegaly on Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation in Patients With Chronic Myelomonocytic Leukemia. Zhonghua Xue Ye Xue Za Zhi (2020) 41(4):308–12. doi: 10.3760/cma.j.issn.0253-2727.2020.04.008
87. Wang J, Wang Q, Zhang H, He Y, Huang Y, Zhang R, et al. Moderate to Severe Marrow Fibrosis As a More Advanced Risk Factor for MDS and MDS-AML Patients With Excess of Blasts Receiving Allogeneic Hematopoietic Stem Cell Transplantation. Transplant Cel Ther (2021) 27(8):666. doi: 10.1016/j.jtct.2021.05.006
88. Yucel OK, Saliba RM, Rondon G, Ahmed S, Alousi A, Bashir Q, et al. Cytogenetics and Comorbidity Predict Outcomes in Older Myelodysplastic Syndrome Patients After Allogeneic Stem Cell Transplantation Using Reduced Intensity Conditioning. Cancer (2017) 123(14):2661–70. doi: 10.1002/cncr.30632
89. Hiramoto N, Kurosawa S, Tajima K, Okinaka K, Tada K, Kobayashi Y, et al. Positive Impact of Chronic Graft-Versus-Host Disease on the Outcome of Patients With De Novo Myelodysplastic Syndrome After Allogeneic Hematopoietic Cell Transplantation: A Single-Center Analysis of 115 Patients. Eur J Haematol (2013) 92:137–46. doi: 10.1111/ejh.12214
90. Itonaga H, Iwanaga M, Aoki K, Aoki J, Ishiyama K, Ishikawa T, et al. Impacts of Graft-Versus-Host Disease on Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myelomonocytic Leukemia: A Nationwide Retrospective Study. Leuk Res (2016) 41:48–55. doi: 10.1016/j.leukres.2015.12.009
91. Pan X, Gao M, Sun Y, Zhou Y, Wang K, Wang Y, et al. Significance of WT1 and Multiparameter Flow Cytometry Assessment in Patients With Chronic Myelomonocytic Leukemia Receiving Allogeneic Hematopoietic Stem Cell Transplantation. Int J Lab Hematol (2022) 44(3):510–7. doi: 10.1111/ijlh.13788
92. Liapis K, Kotsianidis I. Approaching First-Line Treatment in Patients With Advanced CMML: Hypomethylating Agents or Cytotoxic Treatment? Front Oncol (2021) 11:801524. doi: 10.3389/fonc.2021.801524
Keywords: allogeneic stem cell transplantation, chronic myelomonocytic leukemia (CMML), atypical chronic myelogenous leukemia (aCML), Juvenile Myelomonocytic Leukemia (JMML), chronic neutrophilic leukemia (CNL), outcome, prognosis
Citation: Symeonidis A, Chondropoulos S, Verigou E, Lazaris V, Kourakli A and Tsirigotis P (2022) Allogeneic Hematopoietic Stem Cell Transplantation for Mixed or Overlap Myelodysplastic/Myeloproliferative Disorders. Front. Oncol. 12:884723. doi: 10.3389/fonc.2022.884723
Received: 26 February 2022; Accepted: 23 May 2022;
Published: 05 August 2022.
Edited by:
Marcos De Lima, The Ohio State University, United StatesReviewed by:
Francesco Onida, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyDepei Wu, The First Affiliated Hospital of Soochow University, China
Copyright © 2022 Symeonidis, Chondropoulos, Verigou, Lazaris, Kourakli and Tsirigotis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Argiris Symeonidis, YXJnaXJpcy5zeW1lb25pZGlzQHlhaG9vLmdy; orcid.org/0000-0002-0543-046X
 Argiris Symeonidis
Argiris Symeonidis Spiros Chondropoulos
Spiros Chondropoulos Evgenia Verigou3
Evgenia Verigou3 Vasileios Lazaris
Vasileios Lazaris Panagiotis Tsirigotis
Panagiotis Tsirigotis