- 1Senior Department of Oncology, The Fifth Medical Center of People’s Liberation Army (PLA) General Hospital, Beijing, China
- 2Senior Department of Obstetrics and Gynecology, The Seventh Medical Center of People' s Liberation Army General Hospital, Beijing, China
- 3Senior Department of Hepato-Pancreato-Biliary Surgery, The First Medical Center of People' s Liberation Army General Hospital, Beijing, China
- 4Department of Obstetrics and Gynecology, Hainan Hospital of People' s Liberation Army General Hospital, Sanya, China
Objective: The relationship between serum lipids and prognosis of gastric cancer has not been confirmed. Our purpose in the study was to investigate the associations between preoperative and postoperative serum lipids level and prognosis in patients with gastric cancer.
Methods: A retrospective study was performed on 431 patients who received radical (R0) gastrectomy from 2011 to 2013. Preoperative and postoperative serum lipids level were recorded. Clinical-pathological characteristics, oncologic outcomes, disease-free survival (DFS) and overall survival (OS) were collected. The prognostic significance was determined by Kaplan-Meier analysis and Cox proportional hazards regression model.
Results: There was no significant difference in DFS and OS according to preoperative serum lipids level. Regarding postoperative serum lipids level, compared to normal high-density lipoprotein cholesterol (HDL-C), low postoperative HDL-C level indicated a shorter OS (hazard ratio: 1.76, 99% confidence interval: 1.31–2.38; P=0.000) and a shorter DFS (hazard ratio: 2.06, 99% confidence interval: 1.55–2.73; P=0.000). However, other postoperative serum lipid molecules were not associated with DFS and OS.
Conclusion: Postoperative HDL-C might be an independent prognostic factor of gastric cancer.
Introduction
Gastric cancer is the fifth most diagnosed malignancy worldwide (1) and ranks as the third most common cause of cancer related deaths worldwide (2). Although the popularization of early cancer screening has significantly improved the diagnosis rate of early gastric cancer, GC is still frequently advanced stage at diagnosis. With the progress of medicine, the treatment of GC has been improved, but the prognosis of GC is still poor, and the five-year survival rate is about 53% (3). In addition to the stage of cancer at presentation, many other factors of the patient will also affect the prognosis of gastric cancer. Thus, it is of great significance to explore new potential predictors of long-term prognosis. Serum lipid components, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and apo lipoprotein. So far, the influence of serum lipids on cancer is unclear. It has been reported that preoperative lipid level is associated with prognosis of non-metastatic colorectal cancer (4). Currently, researchers have found that TC may play an important role in the development of gastric cancer (5); low HDL-C and high LDL-C levels may increase the risk of GC, but no lipid components was associated with OS of GC (6). One study reported that preoperative serum ApoB/ApoA1 ratio as a novel prognostic indicator of GC, and no association of lipid markers with gastric cancer was shown (7). However, another study found a different conclusion, which suggested that HDL-C is closely related to the prognosis of gastric cancer (8). Therefore, the impact of serum lipids level on outcomes after surgery is less well confirmed, especially few studies have focused on the relationships of postoperative serum lipid components with the prognosis of gastric cancer. In this study, we aim to comprehensively investigate the relationships between preoperative and postoperative serum lipids and prognosis of gastric cancer.
Materials and methods
Patients who received gastrectomy for gastric cancer in Chinese PLA General Hospital from January 1, 2011 to December 31, 2013 were included in this study. The study was approved by the research ethics committee of Chinese PLA General Hospital. Preoperative histological confirmation of the tumor was determined by endoscopic biopsy. Subtotal or total gastrectomy was performed according to tumor location, histopathology and the possibility of obtaining negative resection margins. All postoperative patients were followed up according to guidelines of gastric cancer from surgery to January 1, 2021. The date of tumor recurrence and death was recorded. The endpoint was defined as death or last follow-up. The overall survival was based on the period from histological confirmation of GC to the end of follow-up or endpoint, and the disease-free survival was the period from surgery to the gastric cancer recurrence or metastasis. The following clinical information was recorded in detail: the sex and age of the patient; preoperative serum lipids, body mass index (BMI) and fasting blood sugar; BMI and fasting blood sugar of 6 months after operation; operative site; operative procedure; postoperative pathological results; cancer stage (the pTNM classification was updated to the 8th edition); diet intake volume. Patients with metastatic stage IV disease, serious cardiovascular and cerebrovascular diseases, diabetes, serious thyroid disease, oral lipid-lowering drugs, a non-R0 resection, overall survival of less than 6 months and incomplete information were excluded from the analysis.
The serum lipids and fasting blood sugar were measured in early morning samples obtained before breakfast (at least 8 hours of fasting) within 1 week before surgery and 6 months after surgery by a Cobas c 701 chemistry analyzer (Roche). The low and high reference values were 5.7 mmol/L for TC, 1.7 mmol/L for TG, 1.15 mmol/L for HDL-C, and 3.37 mmol/L for LDL-C. Fasting blood sugar is classified as low group (<6.1 mmol/L), normal group (6.1-7.0 mmol/L) and high group (≥7.0 mmol/L). BMI calculate as weight [kg]/height [m2]. In this study, patients with BMI less than 18.5 kg/m2 were defined as low group, over 25 kg/m2 were defined as high group, and others were normal group. The diet intake volume will reduce after surgery of most patients. Considering the preoperative diet intake volume as 100%, the intake volume increased to 75% of patients at 6 months after surgery was defined as normal group and less than 75% was defined as low group.
Statistical analyses were performed with SPSS version 22.0 software. Continuous variables were presented as the mean ± standard deviation (x ± s). Chi-square test was used to compare categorical variables between groups and the results were described as the percentage (%). The OS and DFS after surgery were calculated using Kaplan-Meier’s method. The potential prognostic factors of GC were explored in univariate and multivariable analysis using a Cox proportional hazards regression model. A P value < 0.05 was considered statistically significant in all statistical analyses.
Results
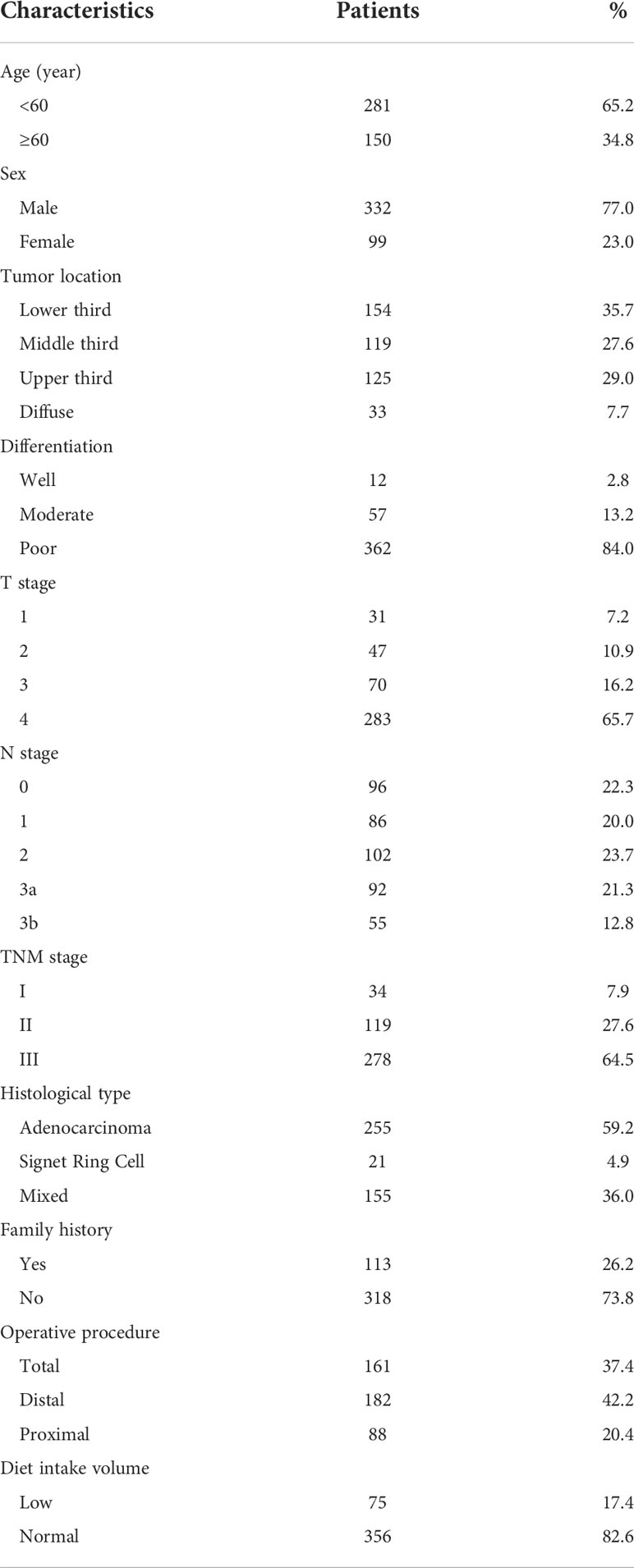
A total of 431 patients were enrolled in this study. The mean duration of follow-up was 107.1 ± 10.8months (range 85.4-133.6months). The general characteristics of the patients are summarized in Table 1. Males were the majority in this study, the male to female ratio was 332:99. The mean age was 55 years. In respect to tumor location, 125 cases (29.0%) were in the upper third, 119 cases (27.6%) were in the middle third, 154 cases (35.7%) were in the lower third, and 33 cases (7.7%) were in the diffuse tumor site. Most of the tumors progressed locally and penetrated the serosa (283 cases of T4 tumors, 65.7%). Lymph node metastasis was common (n = 335, 77.7%). There were 34 patients (7.9%) in stage I, 119 (27.6%) in stage II, and 278 (64.5%) in stage III. On pathology, only 21 cases were signet ring cell carcinoma (4.9%), most of the pathological types were adenocarcinoma (255 cases, 59.2%) and mixed type (155 cases, 36.0%). The degree of pathological differentiation is recorded in detail as follows:362 cases (84.0%) were poorly differentiated; only 12cases (2.8%) were well differentiated, and the others were moderately differentiated (n= 57, 13.2%). In addition, 113 (26.2%) of the GC cases had a positive family history of cancer, either in first- or second-degree relatives. Regarding the operative procedure, total resection was performed in 37.4% (n=161) of the patients, distal resection was performed in 42.2% (n=182) of the patients and other patients (n=88, 20.4%) were performed proximal resection. The diet intake volume of most patients (n=356, 82.6%) increased to 75% after 6 months of surgery. Lipid profile, fasting blood sugar and BMI information are summarized in Table 2. As regards lipid profile (before surgery), the distributions were as follows: TG <1.7 mmol/L (170, 39.4%) versus TG≥1.7mmol/L (261, 60.6%); TC<5.7mmol/L(318, 73.8%) versus TC≥5.7mmol/L (113, 26.2%); HDL-C <1.15mmol/L (193, 44.8%) versus HDL-C≥1.15mmol/L (238, 55.2%); LDL-C<3.37 mmol/L (295, 68.4%) versus LDL-C ≥3.37mmol/L (136, 31.6%). Six months after operation, the proportions of TC <5.7 mmol/L, TG <1.7 mmol/L, LDL<3.37 mmol/L and low BMI were significantly increased.
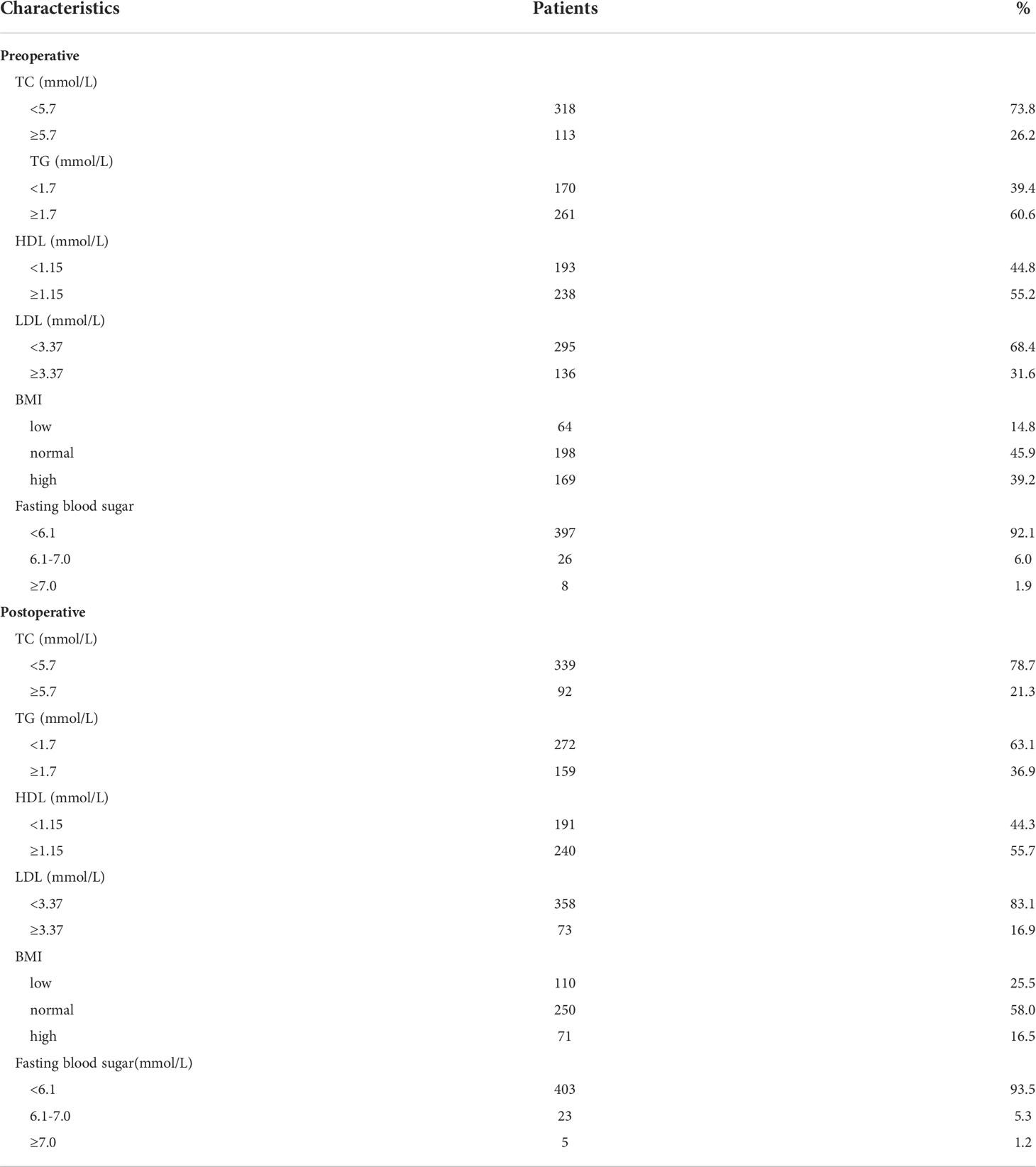
Table 2 characteristics of lipid profile, BMI and fasting blood sugar in the 431 gastric cancer patients.
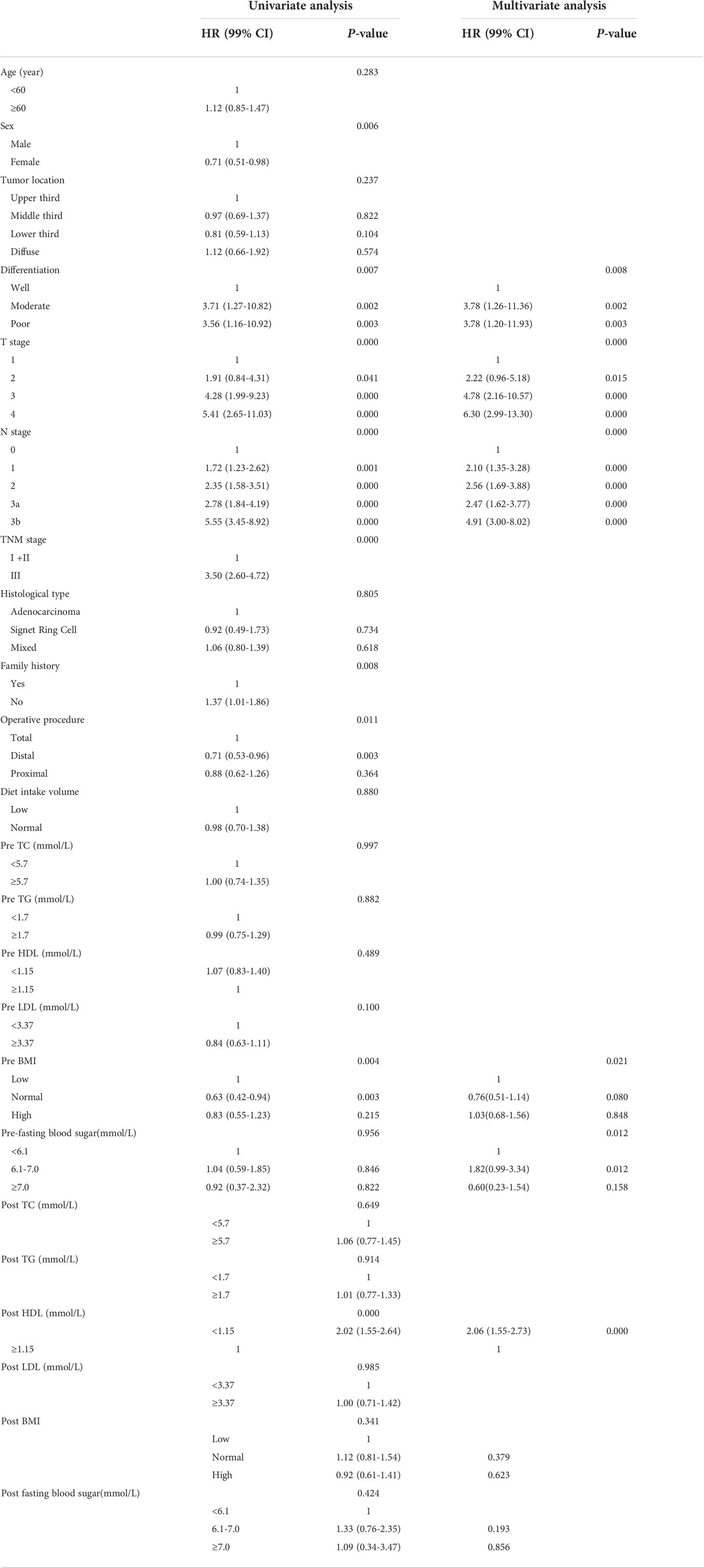
In this study, the overall median DFS was 52.3 ± 34.4 months. The 3- and 5-year DFS rates were 61.3% and 43.9%, respectively. In a univariate Cox proportional hazards model of GC with DFS (Table 3, left panel), the factors associated with the gastric cancer DFS were the sex, T stage, N stage, TNM stage, differentiation, family history, operative procedure, preoperative BMI and postoperative HDL-C levels. To determine which factors may affect the prognosis of gastric cancer, all factors were further subjected to multivariate regression analyses. As shown in Table 3 (right panel), moderate and poor differentiations of tumor (HR: 3.78, 99% CI: 1.26–11.36, P = 0.002; HR: 3.78, 99% CI: 1.20–11.93, P = 0.003, respectively), T2, T3 and T4 of tumor (HR: 2.22, 99% CI: 0.96–5.18, P = 0.015; HR: 4.78, 99% CI: 2.16–10.57, P = 0.000; HR: 6.30, 99% CI: 2.99–13.30, P = 0.000, respectively). N1, N2, N3a and N3b of tumor (HR: 2.10, 99% CI: 1.35–3.28, P = 0.000; HR: 2.56, 99% CI: 1.69–3.88, P = 0.000; HR: 2.47, 99% CI: 1.62–3.77, P = 0.000, HR: 4.91, 99% CI: 3.00–8.02, P = 0.000, respectively); preoperative fasting blood sugar in 6.1-7.0mmol/(HR:1.82, 99% CI: 0.99-3.34, P = 0.012); postoperative HDL-C<1.15mmol/L (HR: 2.06, 99% CI: 1.55–2.73, P = 0.000) were related with poor DFS.
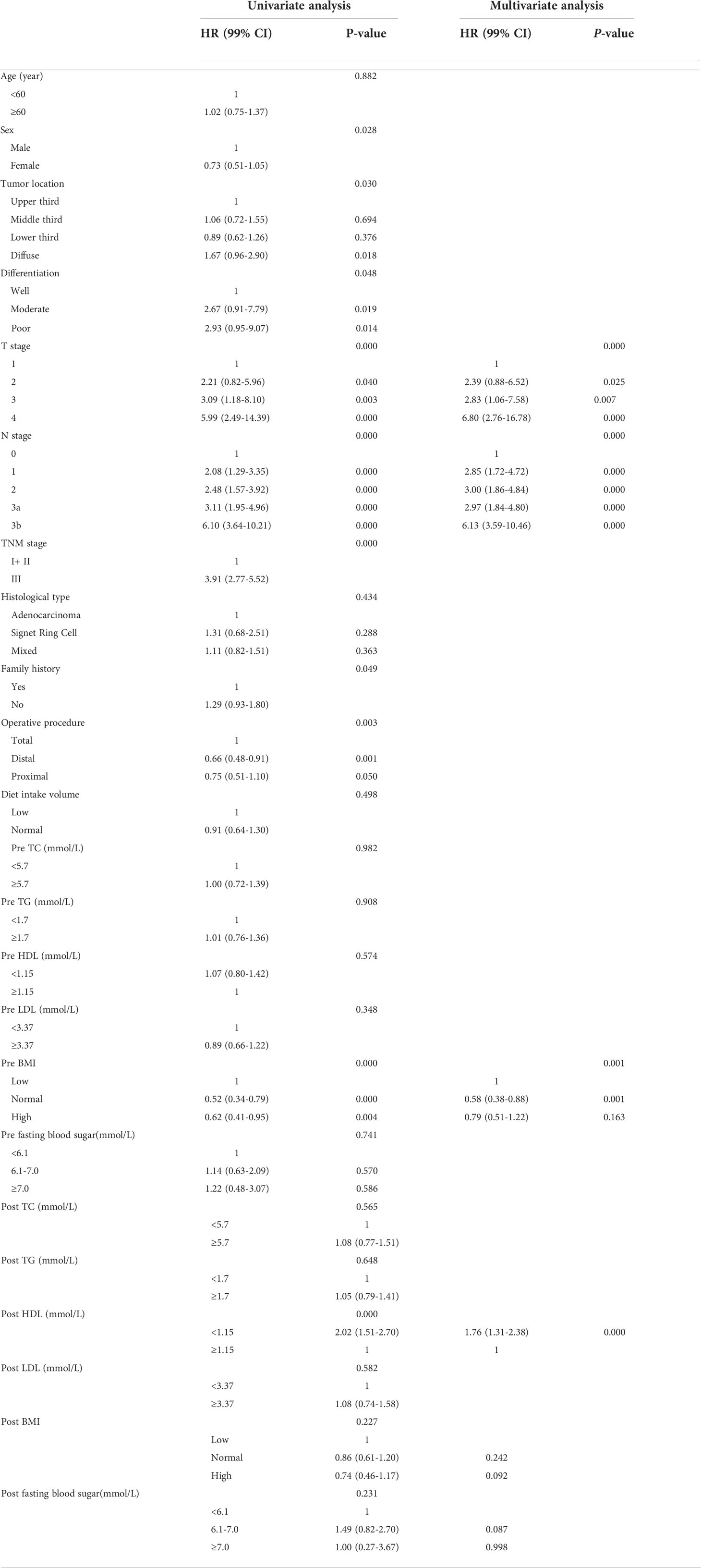
The OS curves are shown in Figures 1A–D for preoperative lipids groups; No significant differences were observed in those stratified analyses. Figure 2 show the postoperative OS curves for the lipids groups. In the Kaplan-Meier curve for HDL-C after surgery, the 5-year OS rate was greater in the normal group than in the low group (80.0% versus 55.5%) (Figure 2C), there were significant differences in the two groups (P=0.000). Regarding the TC, TG and LDL-C after operation (Figures 2A, B, D), no statistically significant differences were observed in those stratified analyses (P = 0.564, P = 0.647, P = 0.582). Furthermore, Cox proportional hazard model was used to analyze which factors could predict OS of gastric cancer. According to the univariate analysis, the factors related with the gastric cancer overall survival were the sex, tumor location, differentiation, T stage, N stage, TNM stage, family history, operative procedure, preoperative BMI and postoperative HDL-C (Table 4 left panel). The results of the multivariate analysis of factors influencing the gastric cancer OS are presented in Table 4 (right panel), increasing T stage, increasing lymph node stage, unnormal preoperative BMI and low postoperative HDL-C all indicated a low mortality rate.
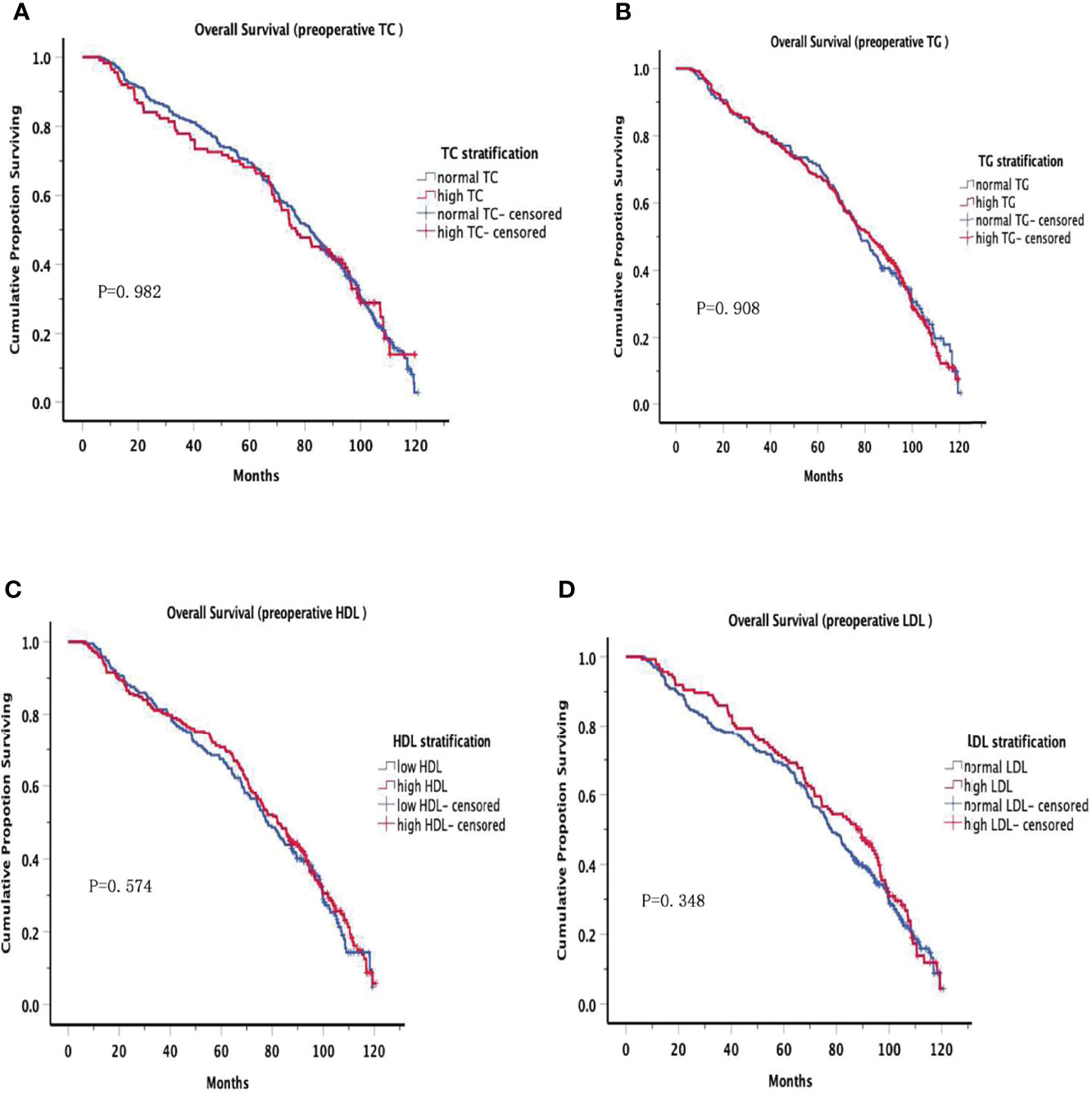
Figure 1 Overall Survival in preoperative serum lipid. Overall Survival of patients grouped by serum lipid levels before surgery, no significant differences were observed at all levels.
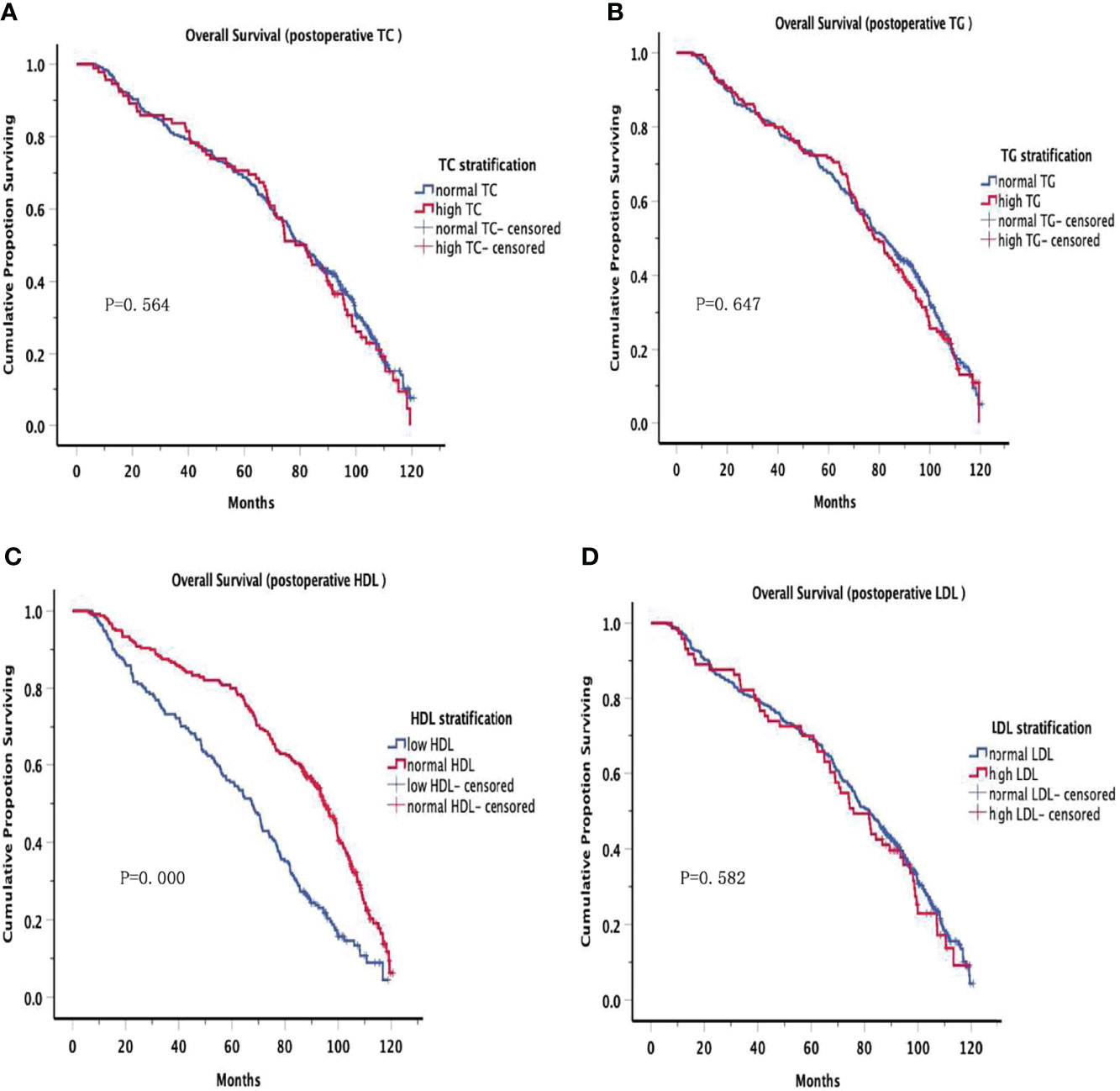
Figure 2 Overall Survival in postoperative serum lipid. There were significant differences in the low HDL and normal HDL groups (P=0.000). However, no significant differences were observed in other groups.
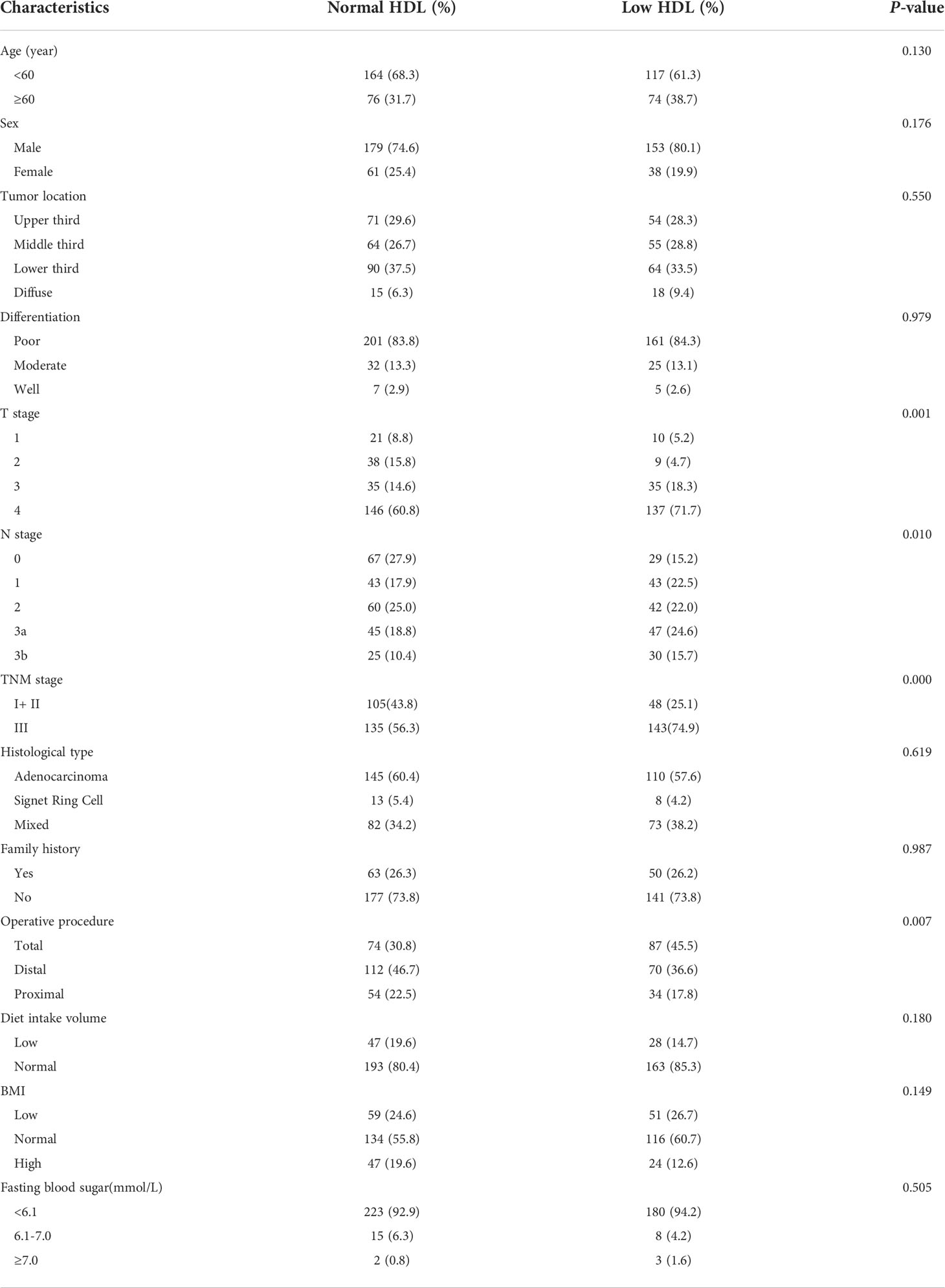
The association between postoperative HDL-C level and clinicopathological characteristics is summarized in Table 5. Of note, HDL-C level was significantly correlated with T stage (P=0.001), lymph node stage (P=0.010), TNM stage (P=0.000) and operative procedure (P=0.007). However, there was no significant relationships between postoperative HDL-C level and age, sex, tumor location, differentiation, histological type, postoperative BMI, postoperative fasting blood sugar, diet intake volume and family history.
Discussion
With the improvements in people’s living conditions and the prolongation of average life expectancy, there is an increasing number of people who are suffered from dyslipidemia. It is well known that LDL-C is called “bad” cholesterol, and positively correlated with cardiovascular and cerebrovascular diseases. On the contrary, HDL-C is called “good” cholesterol, and has a protective effect on cardiovascular and cerebrovascular system. So far, the roles played by lipids in cancer prognosis is a controversial area as there are studies reporting positive, negative or no influence of lipids on the advancement of cancer (6–9). Therefore, this study examined the impact of preoperative and postoperative serum lipids level on DFS and OS after resection for gastric.
HDL-C is known as an antioxidant and anti-inflammatory factor, which is one of the types of cholesterol (10). The major function of HDL-C is to maintain normal cell cholesterol homeostasis by removing excess cholesterol from an intracellular pool (11). Many researchers have previously investigated whether there is a relationship between serum HDL-C and tumorigenesis. Patients with gastrointestinal cancer have lower HDL-C level, when compared with normal controls (12). Low level of serum HDL-C may increase risk of gastric cancer (6, 13, 14), the possible reason is that H. pylori infection reduces HDL-C, which is an important risk factor for gastric cancer (15). As for the prognosis, some authors reported that HDL-C did not affect the OS and PFS of gastric cancer (6, 7). However, another study showed that low level of serum HDL-C is one of the factors of poor prognosis in gastric cancer (8). In this study, we found that postoperative HDL-C level no significant change compared to preoperative level. The positive correlation between postoperative HDL-C and T stage, N stage, and TNM stage indicates a more advanced tumor in patients with lower HDL-C level. In our study, the preoperative HDL-C showed negative association with the DFS and OS in gastric cancer. In addition, we also focused on the relationship between postoperative HDL-C level and prognosis of gastric cancer, which has rarely been reported. We found that low postoperative HDL-C level is associated with poor prognosis of gastric cancer. Therefore, the results of our study suggest that postoperative HDL-C level is an independent risk factor for predicting prognosis of gastric cancer.
Some studies have confirmed that high level of LDL-C and low level of HDL-C were risk factors for gastric cancer (6, 16, 17). Higher LDL-C have been reported to relate to pro-inflammatory activity and affect the suppression of the host immune system (18, 19). The results are inconsistent whether TC or TG is risk factor of gastric cancer (14, 20–22). As for the relationship between serum lipids and prognosis of gastric cancer, similar to our study which no association of preoperative TG, TC and LDL-C with gastric cancer was shown (6, 7). However, another study showed LDL-C as an independent prognosis of gastric cancer (23). Similarly, this study investigated the relationship between postoperative LDL-C, TG, TC and prognosis of gastric cancer. Eventually, no correlation between them and prognosis of gastric cancer was be found.
To the best of our knowledge, few studies that focus on the prognostic role of the postoperative lipids level in patients with gastric cancer. Thus, in our research, we incorporated both preoperative and postoperative TG, TC, LDL-C, HDL-C for analysis. Eventually, we found that postoperative HDL-C could act as an independent prognostic marker in GC among all lipid molecules. Although the underlying mechanism is unclear, stomach as one of the most important organs of the digestive system, patients will experience changes in dietary habits, poorer nutritional status, weight loss, and lower serum lipids after surgery, which requires further investigation.
It should be noted that our study has several potential limitations. First, it is a retrospective, single-center study, so the representativeness of patients is less than those in a prospective and multi-center study, and the results may be biased. Second, the results of this study are necessary to further validate by a mechanistic detail in vivo and in vitro. In spite of these limitations in this study, our analysis firstly tried to elucidate the impact of postoperative serum lipids on the prognosis in gastric cancer patients.
Conclusions
This study suggests that low serum HDL-C level of 6 months after operation is associated with poor prognosis in gastric cancer. Thus, we recommend measurement of serum HDL-C level after 6 months of gastrectomy to predict the prognosis of gastric cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CL, YF, QL, and XY have contributed equally to this work and share first authorship. LP and ND have contributed equally to this work and share last authorship. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Wang W, Zheng C, Fang C, Li P, Xie J, Lin J, et al. Time trends of clinicopathologic features and surgical treatment for gastric cancer: Results from 2 high-volume institutions in southern China. Surgery (2015) 158(6):1590–7. doi: 10.1016/j.surg.2015.04.038
4. Hong TT, Shen D, Chen XP, Wu XH, Hua D. Preoperative serum lipid profile and outcome in nonmetastatic colorectal cancer. Chronic Dis Transl Med (2016) 2(4):241–9. doi: 10.1016/j.cdtm.2016.11.015
5. Huang YK, Kang WM, Ma ZQ, Liu YQ, Zhou L, Yu JC. Body mass index, serum total cholesterol, and risk of gastric high-grade dysplasia: A case-control study among Chinese adults. Med (Baltimore) (2016) 95:e4730. doi: 10.1097/MD.0000000000004730
6. Pih GY, Gong EJ, Choi JY, Kim MJ, Ahn JY, Choe J, et al. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Res Treat (2021) 53(2):445–56. doi: 10.4143/crt.2020.599
7. Ma MZ, Yuan SQ, Chen YM, Zhou ZW. Preoperative apolipoprotein b/apolipoprotein A1 ratio: a novel prognostic factor for gastric cancer. Onco Targets Ther (2018) 11:2169–76. doi: 10.2147/OTT.S156690
8. Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol (2012) 27(10):1635–40. doi: 10.1111/j.1440-1746.2012.07189.x
9. Boretti A. Nutrition, lipidic parameters, and cancer risk and progress. Nutrition (2020) 69:110538. doi: 10.1016/j.nut.2019.06.019
10. Soran H, Schofield JD, Durrington PN. Antioxidant properties of HDL. Front Pharmacol (2015) 6:222. doi: 10.3389/fphar.2015.00222
11. Eisenberg S. High density lipoprotein metabolism. J Lipid Res (1984) 25(10):1017–58. doi: 10.1016/S0022-2275(20)37713-0
12. Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. a comparison with non-cancer subjects. Int J Clin Lab Res (2000) 30(3):141–5. doi: 10.1007/s005990070013
13. Nam SY, Park BJ, Nam JH, Kook MC. Effect of helicobacter pylori eradication and high-density lipoprotein on the risk of de novo gastric cancer development. Gastrointest Endosc (2019) 90:448–56. doi: 10.1016/j.gie.2019.04.232
14. Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, Walldius G, et al. Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol (2012) 2012:792034. doi: 10.1155/2012/792034
15. Laurila A, Bloigu A, Nayha S, Hassi J, Leinonen M, Saikku P. Association of helicobacter pylori infection with elevated serum lipids. Atherosclerosis (1999) 142:207–10. doi: 10.1016/s0021-9150(98)00194-4
16. Kim HY. Metabolic syndrome is associated with gastric dysplasia. Eur J Gastroenterol Hepatol (2011) 23:871–5. doi: 10.1097/MEG.0b013e328349aa18
17. Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, et al. Hyperglycaemia, hypercholesterolaemia and the risk for developing gastric dysplasia. Dig Liver Dis (2008) 40:361–5. doi: 10.1016/j.dld.2007.12.002
18. Caruso MG, Notarnicola M, Cavallini A, Di Leo A. 3-hyd- roxy-3-methylglutaryl coenzyme a reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J Gastroenterol (2002) 37:504–8. doi: 10.1007/s005350200078
19. Bigler RD, Khoo M, Lund-Katz S, Scerbo L, Esfahani M. Identification of low density lipoprotein as a regulator of fc receptor-mediated phagocytosis. Proc Natl Acad (1990) 87:4981–5. doi: 10.1073/pnas.87.13.4981
20. Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjorge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-can) collaborative study. Cancer Causes Control (2011) 22:291–9. doi: 10.1007/s10552-010-9697-0
21. Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer (2009) 101:1202–6. doi: 10.1038/sj.bjc.6605264
22. Iso H, Ikeda A, Inoue M, Sato S, Tsugane S, JPHC Study Group. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer (2009) 125:2679–86. doi: 10.1002/ijc
Keywords: preoperative serum lipids, postoperative serum lipids, gastric cancer, prognosis, overall survival, disease-free survival
Citation: Li C, Fu Y, Li Q, Yang X, Wang W, Jin X, Bian L, Zhao H, Li D, Gao J, Du N and Peng L (2022) Postoperative high-density lipoprotein cholesterol level: an independent prognostic factor for gastric cancer. Front. Oncol. 12:884371. doi: 10.3389/fonc.2022.884371
Received: 26 February 2022; Accepted: 29 June 2022;
Published: 18 July 2022.
Edited by:
Yuming Jiang, Stanford University, United StatesReviewed by:
Sunaryo Hadi Warsito, Airlangga University, IndonesiaSudabeh Alatab, Tehran University of Medical Sciences, Iran
Jianhui Chen, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2022 Li, Fu, Li, Yang, Wang, Jin, Bian, Zhao, Li, Gao, Du and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Peng, cGVuZ2xpYW5nXzMwMUAxNjMuY29t; Nan Du, ZHVuYW4wNUBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Chenxi Li
Chenxi Li Yan Fu1†
Yan Fu1† Qiuwen Li
Qiuwen Li Nan Du
Nan Du Liang Peng
Liang Peng