- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Geneseeq Research Institute, Nanjing Geneseeq Technology Inc., Nanjing, China
- 3Medical Department, Nanjing Geneseeq Technology Inc., Nanjing, China
The histological transformation from adenocarcinoma (ADC) to squamous cell carcinoma (SCC) is rare but recurrently occurs post TKI treatment in EGFR-mutated non-small cell lung cancer patients with a very limited number of clinical cases published. The outcome of patients after SCC onset is poor as no established treatment guidelines were available. Here we report a case who was initially diagnosed with lung ADC with EGFR L858R driver mutation and demonstrated a partial response (PR) to gefitinib for 27 months before disease progression. The rapidly progressive lung metastatic lesions were determined as an SCC histology with positive PD-L1 expression. Besides EGFR L858R, the metastatic SCC harbored the amplification of CD274 and PDCD1LG2 detected by targeted next-generation sequencing (NGS), which encode PD-L1 and PD-L2, respectively. The disease remained stable on the combination therapy of pembrolizumab plus chemotherapy for eight months until the primary ADC lesion progressed. After the failure of progressed primary ADC lesion with radiotherapy and immunotherapy, systemic ADC metastases were developed in multiple locations including kidney, liver, and chest wall with EGFR L858R mutation but negative PD-L1 expression. The patient then received the combination therapy of bevacizumab plus chemotherapy and the disease remained stable for five months. Since August 2021, afatinib has been administrated which led to a PR and the disease has remained stable up till present. This study demonstrated a primary lung ADC who developed systemic ADC metastases and local SCC transformation with distinct molecular features. The patient has achieved long-term clinical benefit upon multiple lines of chemotherapy and immunotherapy, which provided valuable insight into the treatment of advanced SCC-transformed lung ADC patients.
Introduction
The most important driver gene in non-small cell lung cancer (NSCLC) is EGFR and approximately 50% of Chinese NSCLC patients carry EGFR mutations, among which, L858R and exon 19 deletion are the two most common alterations (1). Patients with activating EGFR mutations usually showed optimal responses to EGFR tyrosine kinase inhibitors (TKIs). However, the acquired mutations during TKI therapy would eventually develop resistance, such as EGFR T790M and MET amplification (2).
Besides the acquired secondary alterations, the change of histologic phenotype could also lead to the TKI resistance. Adenocarcinoma (ADC) and squamous cell carcinoma (SCC) are the two major subtypes of NSCLC and due to the heterogenicity, about 4% to 9% of tumors are mixed with both ADC and SCC, even in the same lesion (3). Immunochemistry (IHC) examination is a clinical approach to distinguish the two histology with specific biomarkers such as thyroid transcription factor (TTF-1), NapsinA, CK5/6, and P63. In addition, EGFR oncogenic mutations and ALK/RET fusions are mostly detected in ADCs (4). The transdifferentiation from ADC to SCC was supported by both in vivo and clinical evidence. In the genetically engineered mouse model, the loss of LKB1 was proven to be a mechanism of triggering the ADC-SCC transformation (5). In real-world cases, the prognosis of ADC-to-SCC transformed NSCLC patients was extremely poor and a 3.5-month median overall survival (OS) after SCC onset was reported in a systematic literature review (6).
Herein we presented a rare case of lung ADC with EGFR L858R driver mutation developing local SCC transformation after first-line gefitinib treatment followed by systemic ADC metastases. Based on repeated pathological examinations and targeted next-generation sequencing (NGS), the rapidly progressive disease was under control and long-term clinical benefits were achieved. We present the following case in accordance with the CARE reporting checklist.
Case Presentation
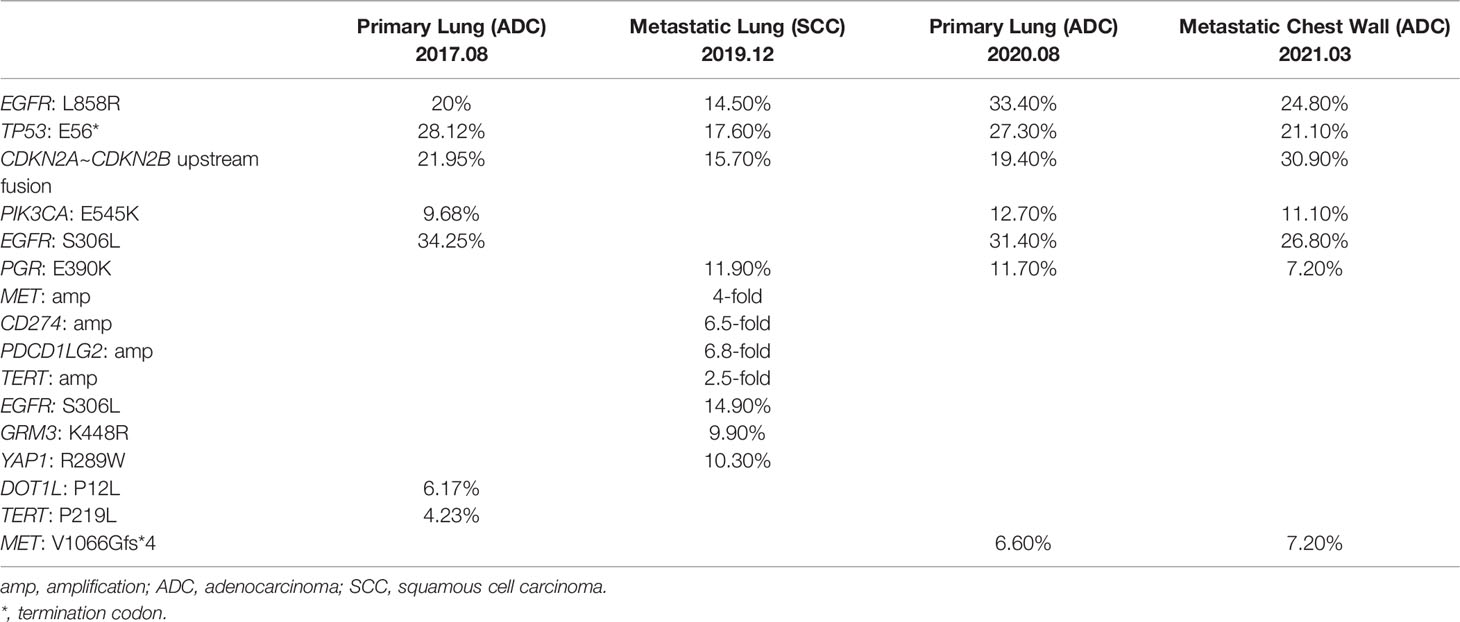
A 61-year-old non-smoking Chinese male visited our hospital in August 2017, because of a persistent cough for over one month. He had hypertension for two years, taking lercanidipine tablets for blood pressure control, without any medical history of diabetes, pulmonary tuberculosis, or drug allergy. Positron emission tomography (PET) and computed tomography (CT) examinations revealed the presence of a lesion in the right upper lobe (Figure 1A) and multiple lymph nodes metastases at the right hilum of the lung, right mediastinum, the right-side clavicle, and pleural effusion in the right side. The percutaneous biopsy and immunohistochemistry (IHC) analysis revealed a poorly differentiated adenocarcinoma (ADC, Figure 1K) with positive expression of Napsin A, thyroid transcription factor-1 (TTF-1), and negative CK5/6. The EGFR L858R mutation was detected (Table 1) by the capture-based hybrid next-generation sequencing (NGS, GeneseeqPrime™) targeting 425 cancer-related genes (Supplementary Table S1). He was primarily diagnosed with a stage IV ADC of the right lung (T2N3M1a) and received gefitinib as the first-line treatment. Partial response (PR) was achieved after one month (Figure 1B) and progression-free survival (PFS) was 27 months for the first-line gefitinib treatment. During the treatment of gefitinib, the patient experienced grade 2 rash, which was symptomatically treated.
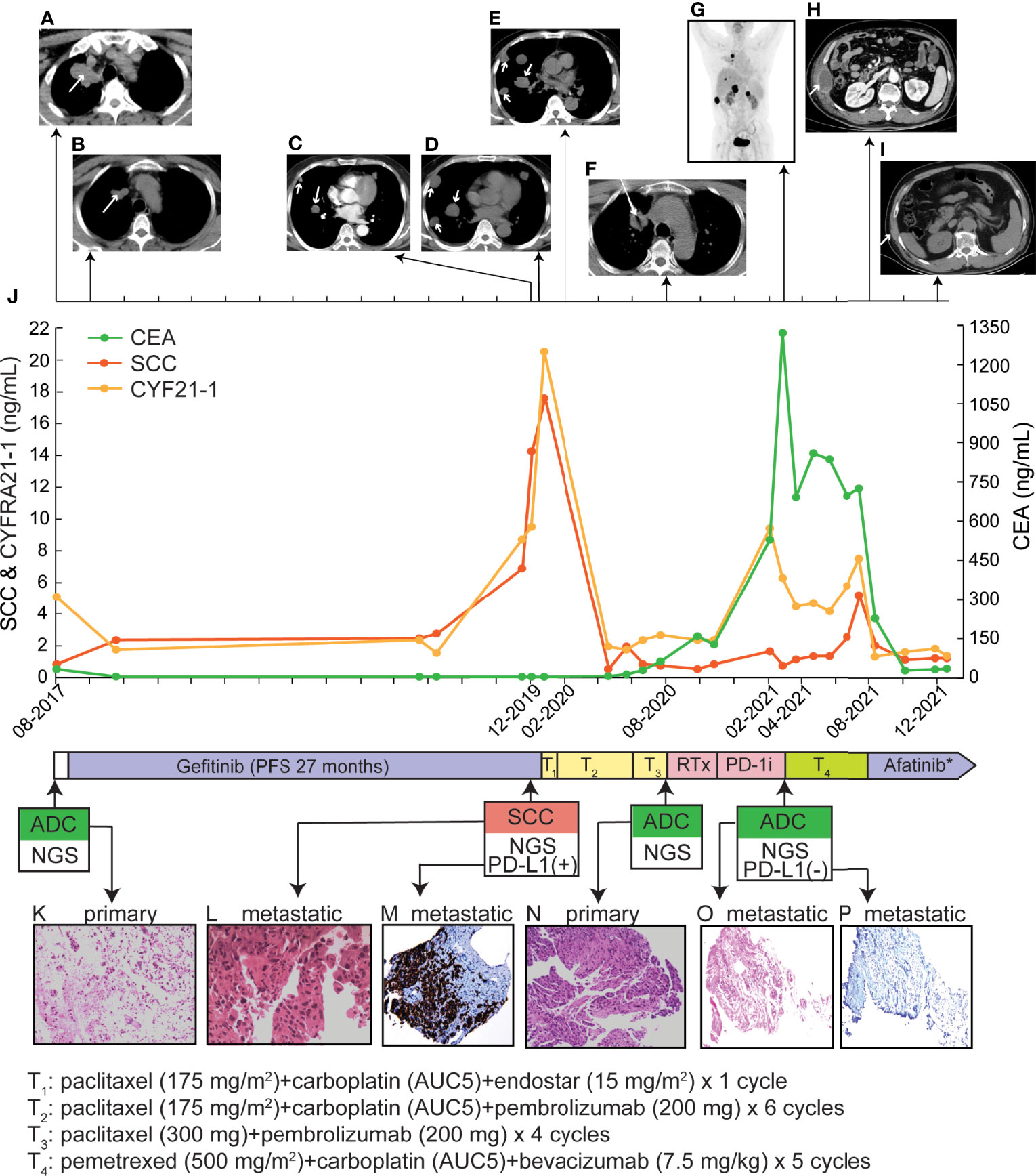
Figure 1 Treatment history and clinical information of the presented case. (A–I) PET/CT images of primary and metastatic lesions were found at different time points as shown by the time scale. (J) Multiple serum tests of SCC antigen, CYFRA21-1, and CEA were performed. (K, L, N, O) Pathological examination of primary and metastatic lesion biopsy. The magnificence of the H&E staining images was 100x. (M, P) The IHC examination of PD-L1 expression. The tumor proportion score of the metastatic SCC lesion and the chest wall lesion were > 50% and < 1%, respectively. RTx, radiotherapy; *, during the first month of afatinib treatment, anlotinib was co-administrated, which was discontinued due to side effects.
In December 2019, multiple metastatic lesions were found in both lungs and rapidly progressed. As shown in Figures 1C, D, the two CT images taken with an interval of 19 days (2019.12.06 and 2019.12.25) showed over two times size increase on multiple metastatic sites. As the primary tumor remained stable, a re-biopsy of the metastatic lesion was performed. However, the pathological examinations suggested a histology change to squamous cell carcinoma (SCC, Figure 1L) with positive expression of CK5/6 and P63 but negative for NapsinA and TTF-1. The SCC antigen and CYFRA21-1 levels in serum were significantly increased compared to the baseline tests (Figure 1J). He firstly received one cycle of paclitaxel (175 mg/m2), carboplatin (AUC5) plus endostar (15 mg/m2). Then, the IHC test showed a positive PD-L1 expression with a tumor proportion score (TPS) of over 50% (Figure 1M). The L858R mutation of EGFR was maintained in the metastatic SCC and companied by the amplification of MET, CD274, and PDCD1LG2 (Table 1). Thus, a combination of paclitaxel (175 mg/m2), carboplatin (AUC5) plus pembrolizumab (200 mg) was administrated. The follow-up examination after one cycle of combined treatment showed that the rapidly progressed metastatic lesions had been under control (Figure 1E). Then, partial response was achieved after an additional 5 cycles of this combined therapy and another four cycles of paclitaxel (300 mg) plus pembrolizumab (200 mg) were performed as maintained treatment. The disease remained stable until August 2020 and the patient tolerated well with current treatment.
Due to the progression of the primary tumor in August 2020, a second biopsy for the primary site was performed (Figure 1F), whose pathologic histology was still ADC (Figure 1N) with dramatic decreases in the levels of SCC antigen and CYFRA21-1 in serum (Figure 1J). Compared to the mutational profile of the metastatic SCC lesion, the progressed ADC maintained the oncogenic EGFR (L858R) mutation. However, the amplification of MET, CD274, and PDCD1LG2 was not detected (Table 1). Whereas no genetic alterations were detected in the circulating tumor DNA by targeted NGS which suggested a very low level of tumor mutational burden. After a total of 3600cGy radiotherapy targeting the progressed primary lesion (12 times in 4 weeks), the patient continued pembrolizumab (200 mg) treatment until the March of 2021.
Multiple metastatic sites were found by repeat PET-CT including kidney, liver, and chest wall (Figure 1G) with a dramatic increase in carcinoembryonic antigen (CEA) level (Figure 1J). The metastatic chest wall lesion was pathologically identified as ADC (Figure 1O) with the same genetic alterations as the progressed ADC (Table 1). Due to the clearance of the gene amplification of CD274 and PDCD1LG2, the IHC test of PD-L1 was negative for the chest wall lesion (Figure 1P). A combination treatment of pemetrexed (500 mg/m2), carboplatin (AUC5), and bevacizumab (7.5 mg/kg) led to a stable disease for five months until August 2021. With the progression on the metastatic chest wall lesion (Figure 1H), afatinib (40 mg/d) and anlotinib (10 mg/d) were administrated to the patient, but anolotinib was discontinued after one month because of severe side effects, including hemoptysis and tooth loss. Afatinib monotherapy relieved chest pain and led to a PR of the metastatic chest wall lesion as shown by the CT scan in December 2021 (Figure 1I). Until the last follow-up in January 2022, the PFS upon afatinib treatment reached five months and the treatment is ongoing.
Discussion
In this case report, we presented an NSCLC patient experiencing a histologic transformation of ADC to SCC in the local metastatic lesions. The primary tumor was diagnosed as ADC with EGFR L858R driver mutation and the efficacy of first-line gefitinib treatment was excellent with a 27-month PFS. While a histological transformation to SCC occurred in the local metastatic lesion. The histological transformation is a known resistant mechanism of EGFR-TKI treatment in NSCLC patients. About 3% to 14% of patients trans-differentiated to small-cell lung cancer (SCLC) which mediated the resistance to EGFR-TKI treatment (7, 8). In addition, EGFR-mutant patients with co-alterations of RB1 and TP53 experienced a higher risk of SCLC transformation (9). However, we didn’t detect any RB1 mutations during multiple sampling, though RB1 gene was covered by the targeted NGS panel used in this case (Supplementary Table S1). Thus, the molecular traits of patients experiencing ADC-to-SCC transformation might be different from those transformed to SCLC, which remained to be well characterized (10). In the engineered mouse model (KrasG12D/Lkb1L/L), LKB1 was proven to play a critical role in ADC-to-SCC transdifferentiation (5). Due to the limited number of reported cases, the clinical evidence was poorly investigated.
As the transformation from EGFR-mutated ADC to SCC is relatively rare, no established treatment guidelines were available in this scenario. According to the published cases, the transformation from ADC to SCC could occur post first-, second-, and third-generation TKIs including gefitinib, erlotinib, afatinib, and osimertinib (6). EGFR T790M is a recurrent mutation detected in transformed SCC tumors and osimertinib was often chosen as the subsequent therapy but the clinical benefits were not durable (11–14). Roca et al. reported a 3.5-month median OS of EGFR-mutant NSCLC patients after SCC transformation in a pooled analysis (6). In the presented case, the combined chemotherapy and immune checkpoint inhibitor (ICI) slowed down the rapid progression of the metastatic SCC tumors with an eight-month stable disease. The amplification of CD274 and PDCD1LG2 were detected by targeted NGS in the metastatic SCC tumors which encode PD-L1 and PD-L2, respectively. Thus, the positive expression of PD-L1/2 could explain the good response to pembrolizumab. In addition, previous studies have demonstrated the diverse responses to ICI in NSCLC patients with different EGFR alterations by affecting tumor immune microenvironments (15–17). While preclinical experiments showed that EGFR TKIs could improve ICI efficacy by triggering the changes in tumor immune microenvironments, which implicated the combination with ICI in clinical practice (18, 19). However, in this case, the progressed primary ADC lesion and the systemic metastatic tumors lost the amplification of CD274 and PDCD1LG2, which might trigger the resistance to immunotherapy. Besides, other resistant mechanisms have been reported, including the selection and accumulation of tumor subclones that escaped from the immune system, the activation of other compensatory inhibitory signaling pathways, and the exhaustion of T cells (20–22).
With the application of large-panel NGS analysis, we were not only able to identify oncogenic driver mutations, EGFR L858R in this case, but also other concurrent alterations that might assist treatment decision-making and predict therapeutic responses. For instance, a TP53 nonsense mutation was carried in both primary and all metastatic tumors. Concurrent TP53 mutation in EGFR-mutant lung cancer patients was associated with a poor prognosis with TKI treatments (23) but in this case, the patient derived benefit from gefitinib for 27 months. Notably, a four-fold amplification of MET gene was detected in the metastatic SCC tumor, which was also a known resistant mechanism to EGFR TKIs. Thus, beyond the histological transformation, MET amplification might also trigger the development of local metastases. Another genomic alteration maintained in all samples was a gene fusion where exon 1 of CDKN2A was translocated to the upstream intergenic region of CDKN2B. But whether this DNA-level alteration eventually cause transcription- and protein-level changes was unknown. Previous studies reported the association between CDKN2A deletion and poor clinical prognosis in lung cancer (24, 25).
This study has several limitations. First, whether the primary tumor underwent SCC transformation remained unclear because a histology examination was lacking when SCC was identified but the primary remained stable. Secondly, the original pathological examinations were performed based on percutaneous biopsies. Hence, the de novo primary tumor might be a mix of ADC and SCC due to intratumor heterogeneity and the SCC subclone became dominant and migrated during the first-line gefitinib treatment. However, as the NGS results revealed that the molecular profile of the SCC was quite different from that of the primary ADC, and the metastatic ADC lesions at chest wall maintained the majority of alterations detected in the primary ADC, we preferred the hypothesis that the trans-differentiation occurred during the TKI treatment and migrated to local sites. Thirdly, from this single case, we cannot conclude whether the chemotherapy plus immune checkpoint inhibitors could be widely used in other SCC-transformed EGFR-mutant NSCLC cases. Comprehensive studies of the mechanisms underlying ADC to SCC in large cohorts are warranted.
In summary, we presented a lung cancer patient experiencing an ADC-to-SCC transformation in the local metastatic lesions post 27-month gefitinib treatment. The metastatic SCC responded well to the combined chemotherapy and pembrolizumab with positive PD-L1 expression. As the outcomes of patients with SCC-transformed advanced lung cancer are usually poor, the strategies of the multiple lines of treatments in the presented case are of great value in clinical practice, which successfully stopped the rapid progression of SCC tumors and achieved durable benefits after the development of systemic ADC metastases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the committee of the first affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JYe: Conceptualization, Data curation, Writing-original writing; YM: Formal analysis, Visualization, Writing-original writing; QO: Validation, Writing-review and editing; JYa: Formal analysis, Resources; BY: Methodology, Validation; YL: Project administration; Supervision, Resources. All authors contributed to the article and approved the submitted version.
Conflict of Interest
YM, QO, JYa, and BY are current employees of Nanjing Geneseeq Technology Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the patient and family members who gave their consent to present the data in this study, as well as the investigators and research staff involved in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.883367/full#supplementary-material
Abbreviations
ADC, adenocarcinoma; SCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor; IHC, immunochemistry; TTF-1, thyroid transcription factor-1; OS, overall survival; PET, positron emission tomography; NGS, next-generation sequencing; PR, partial response; PFS, progression-free survival; TPS, tumor proportion score; SCLC, small-cell lung cancer; CEA, carcinoembryonic antigen; ICI, immune checkpoint inhibitor. Note: RTx, radiotherapy; *, during the first month of afatinib treatment, anlotinib was co-administrated, which was discontinued due to side effects.
References
1. Pi C, Xu CR, Zhang MF, Peng XX, Wei XW, Gao X, et al. EGFR Mutations in Early-Stage and Advanced-Stage Lung Adenocarcinoma: Analysis Based on Large-Scale Data From China. Thorac Cancer (2018) 9(7):814–9. doi: 10.1111/1759-7714.12651
2. Ma C, Wei S, Song Y. T790M and Acquired Resistance of EGFR TKI: A Literature Review of Clinical Reports. J Thorac Dis (2011) 3(1):10–8. doi: 10.3978/j.issn.2072-1439.2010.12.02
3. Hou S, Zhou S, Qin Z, Yang L, Han X, Yao S, et al. Evidence, Mechanism, and Clinical Relevance of the Transdifferentiation From Lung Adenocarcinoma to Squamous Cell Carcinoma. Am J Pathol (2017) 187(5):954–62. doi: 10.1016/j.ajpath.2017.01.009
4. Cancer Genome Atlas Research N. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature (2014) 511(7511):543–50. doi: 10.1038/nature13385
5. Han X, Li F, Fang Z, Gao Y, Li F, Fang R, et al. Transdifferentiation of Lung Adenocarcinoma in Mice With Lkb1 Deficiency to Squamous Cell Carcinoma. Nat Commun (2014) 5:3261. doi: 10.1038/ncomms4261
6. Roca E, Pozzari M, Vermi W, Tovazzi V, Baggi A, Amoroso V, et al. Outcome of EGFR-Mutated Adenocarcinoma NSCLC Patients With Changed Phenotype to Squamous Cell Carcinoma After Tyrosine Kinase Inhibitors: A Pooled Analysis With an Additional Case. Lung Cancer (2019) 127:12–8. doi: 10.1016/j.lungcan.2018.11.016
7. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients With EGFR-Mutant Lung Cancers. Clin Cancer Res (2013) 19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246
8. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med (2011) 3(75):75ra26. doi: 10.1126/scitranslmed.3002003
9. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at Risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol (2019) 14(10):1784–93. doi: 10.1016/j.jtho.2019.06.002
10. Shaurova T, Zhang L, Goodrich DW, Hershberger PA. Understanding Lineage Plasticity as a Path to Targeted Therapy Failure in EGFR-Mutant Non-Small Cell Lung Cancer. Front Genet (2020) 11:281. doi: 10.3389/fgene.2020.00281
11. Izumi H, Yamasaki A, Ueda Y, Sumikawa T, Maeta H, Nakamoto S, et al. Squamous Cell Carcinoma Transformation From EGFR-Mutated Lung Adenocarcinoma: A Case Report and Literature Review. Clin Lung Cancer (2018) 19(1):e63–e6. doi: 10.1016/j.cllc.2017.10.005
12. Bugano DDG, Kalhor N, Zhang J, Neskey M, William WN. Squamous-Cell Transformation in a Patient With Lung Adenocarcinoma Receiving Erlotinib: Co-Occurrence With T790M Mutation. Cancer Treat Commun (2015) 4:34–6. doi: 10.1016/j.ctrc.2015.03.007
13. Bruno R, Proietti A, Alì G, Puppo G, Ribechini A, Chella A, et al. Squamous Cell Transformation and EGFR T790M Mutation as Acquired Resistance Mechanisms in a Patient With Lung Adenocarcinoma Treated With a Tyrosine Kinase Inhibitor: A Case Report. Oncol Lett (2017) 14(5):5947–51. doi: 10.3892/ol.2017.6913
14. Okabe N, Takagi H, Mine H, Fukai S, Minemura H, Suzuki H. Osimertinib for Epidermal Growth Factor Receptor Mutation-Positive Lung Adenocarcinoma That Transformed to T790M-Positive Squamous Cell Carcinoma. J Thorac Oncol (2017) 12(10):e167–e9. doi: 10.1016/j.jtho.2017.06.071
15. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR Mutation Subtypes and Response to Immune Checkpoint Blockade Treatment in non-Small-Cell Lung Cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
16. Chen K, Cheng G, Zhang F, Zhu G, Xu Y, Yu X, et al. PD-L1 Expression and T Cells Infiltration in Patients With Uncommon EGFR-Mutant Non-Small Cell Lung Cancer and the Response to Immunotherapy. Lung Cancer (2020) 142:98–105. doi: 10.1016/j.lungcan.2020.02.010
17. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor Immune Microenvironment and Nivolumab Efficacy in EGFR Mutation-Positive Non-Small-Cell Lung Cancer Based on T790M Status After Disease Progression During EGFR-TKI Treatment. Ann Oncol (2017) 28(7):1532–9. doi: 10.1093/annonc/mdx183
18. Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L, et al. EGFR-Targeted Therapy Alters the Tumor Microenvironment in EGFR-Driven Lung Tumors: Implications for Combination Therapies. Int J Cancer (2019) 145(5):1432–44. doi: 10.1002/ijc.32191
19. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR Improves Responsiveness to PD-1 Blockade in EGFR-Mutated Non-Small Cell Lung Cancer. Sci Immunol (2020) 5(43):eaav3937. doi: 10.1126/sciimmunol.aav3937
20. Johnson DB, Nixon MJ, Wang Y, Wang DY, Castellanos E, Estrada MV, et al. Tumor-Specific MHC-II Expression Drives a Unique Pattern of Resistance to Immunotherapy via LAG-3/FCRL6 Engagement. JCI Insight (2018) 3(24):e120360. doi: 10.1172/jci.insight.120360
21. Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, et al. Epigenetic Stability of Exhausted T Cells Limits Durability of Reinvigoration by PD-1 Blockade. Science (2016) 354(6316):1160–5. doi: 10.1126/science.aaf2807
22. Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity's Roles in Cancer Suppression and Promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
23. Christopoulos P, Kirchner M, Roeper J, Saalfeld F, Janning M, Bozorgmehr F, et al. Risk Stratification of EGFR(+) Lung Cancer Diagnosed With Panel-Based Next-Generation Sequencing. Lung Cancer (2020) 148:105–12. doi: 10.1016/j.lungcan.2020.08.007
24. Liu W, Zhuang C, Huang T, Yang S, Zhang M, Lin B, et al. Loss of CDKN2A at Chromosome 9 Has a Poor Clinical Prognosis and Promotes Lung Cancer Progression. Mol Genet Genom Med (2020) 8(12):e1521. doi: 10.1002/mgg3.1521
Keywords: adenocarcinoma, squamous cell carcinoma, PD-L1, EGFR TKI, case report
Citation: Ye J, Ma Y, Ou Q, Yan J, Ye B and Li Y (2022) Long-Term Clinical Benefit in EGFR-Mutant Lung Adenocarcinoma With Local Squamous Cell Carcinoma Transformation After EGFR TKI Resistance: A Case Report. Front. Oncol. 12:883367. doi: 10.3389/fonc.2022.883367
Received: 28 March 2022; Accepted: 02 May 2022;
Published: 26 May 2022.
Edited by:
Petros Christopoulos, Heidelberg University Hospital, GermanyReviewed by:
Li Ling, Shanghai Jiao Tong University, ChinaQin Wenxing, Shanghai Changzheng Hospital, China
Copyright © 2022 Ye, Ma, Ou, Yan, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuping Li, d3psaXlwQDE2My5jb20=
 Junru Ye
Junru Ye Yutong Ma
Yutong Ma Qiuxiang Ou
Qiuxiang Ou Junrong Yan
Junrong Yan Bin Ye
Bin Ye Yuping Li
Yuping Li