- 1Department of Anesthesiology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Anesthesiology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Objectives: To investigate the relationship between obesity status and perioperative outcomes in elderly patients undergoing thoracoscopic anatomic lung cancer surgery.
Methods: From January 2016 to December 2018, we performed a monocentric retrospective cohort study among 4164 consecutive patients aged 65 years or older who underwent thoracoscopic anatomic lung cancer surgery at Shanghai Chest Hospital. Two groups were stratified by body mass index (BMI): nonobese (BMI<28kg/m2) and obese status (BMI≥28kg/m2). Using a 1:1 propensity score matching (PSM) analysis to compare perioperative outcomes between two groups.
Results: 4035 older patients were eventually enrolled, with a mean age of 69.8 years (range: 65-87), and 305 patients were eligible for obese status, with a mean BMI of 29.8 ± 1.7kg/m2. Compared with nonobese patients, obese patients were more likely to have higher rates of intraoperative hypoxemia (1.2% vs 3.9%, P=0.001) and new-onset arrhythmia (2.3% vs 4.3%, P=0.034). The difference in intraoperative transfusion and conversion rates and postoperative outcomes regarding pulmonary complications, new-onset arrhythmia, transfusion, length of hospital stay, 30-day readmission and hospitalization costs between two groups were not significant (P>0.05). After a 1:1 PSM analysis, the difference in both intraoperative and postoperative complications among two groups were not significant (P>0.05). In subgroup analysis, patients with BMI≥30kg/m2 had a similar incidence of perioperative complications compared to patients with BMI between 28 and 30 kg/m2 (P>0.05).
Conclusions: Our research data support evidence for “obesity paradox” and also contribute the growing body of evidence that obesity in older patients should not exclude candidates for thoracoscopic anatomic lung cancer surgery.
Introduction
Over the last few decades, the prevalence of obesity has shifted dramatically (1, 2), and obesity-related metabolic diseases such as hypertension, diabetes and hyperlipidemia have also gradually increased (3–5), becoming a worldwide health problem. Currently, obesity-related cancer rates have become a focus of concern, although the effect varies widely depending on the types of cancer (4, 6–9). Among these types, an inverse relationship between obesity and lung cancer risk has been reported in previous studies (6–9). And the biological mechanisms underlying the major role of obesity in chronic inflammation and carcinogenesis have also been extensively evaluated (4, 10–12).
Indeed, the proportion of overweight and obese lung cancer patients undergoing lung surgery has increased during the last decades (13, 14). Besides, obesity-related preoperative comorbidities increase the risk of perioperative surgical complications, mutually reinforcing (15–17). Additionally, obesity characterized by substantial girth and excess mediastinal fat has a profound impact on the technical issues during the procedure, especially in minimally invasive surgery (MIS) (18, 19). Moreover, with the aging of society, the influence of obesity on perioperative outcomes is further complicated by the specific attributes of elderly surgical patients, such as frailty, cognitive decline, impaired preoperative pulmonary function and tissue fragility (20, 21).
Recently, a retrospective study conducted by Tabatabai and colleagues showed that obesity class II-III had prognostic value in predicting increased rates of postoperative complications, prolonged length of hospital stay and no-home discharge locations in elderly patients undergoing spine, hip, and knee procedures (22). Up to now, there was no literature about the influence of obesity on perioperative outcomes in elderly patients during thoracoscopic anatomic lung cancer surgery. In the present study, by reviewing a large sample of clinical data, we aimed to evaluate the relationship of obesity to perioperative outcomes in thoracoscopic anatomic lung cancer surgery.
Materials and Methods
Study Design and Patients
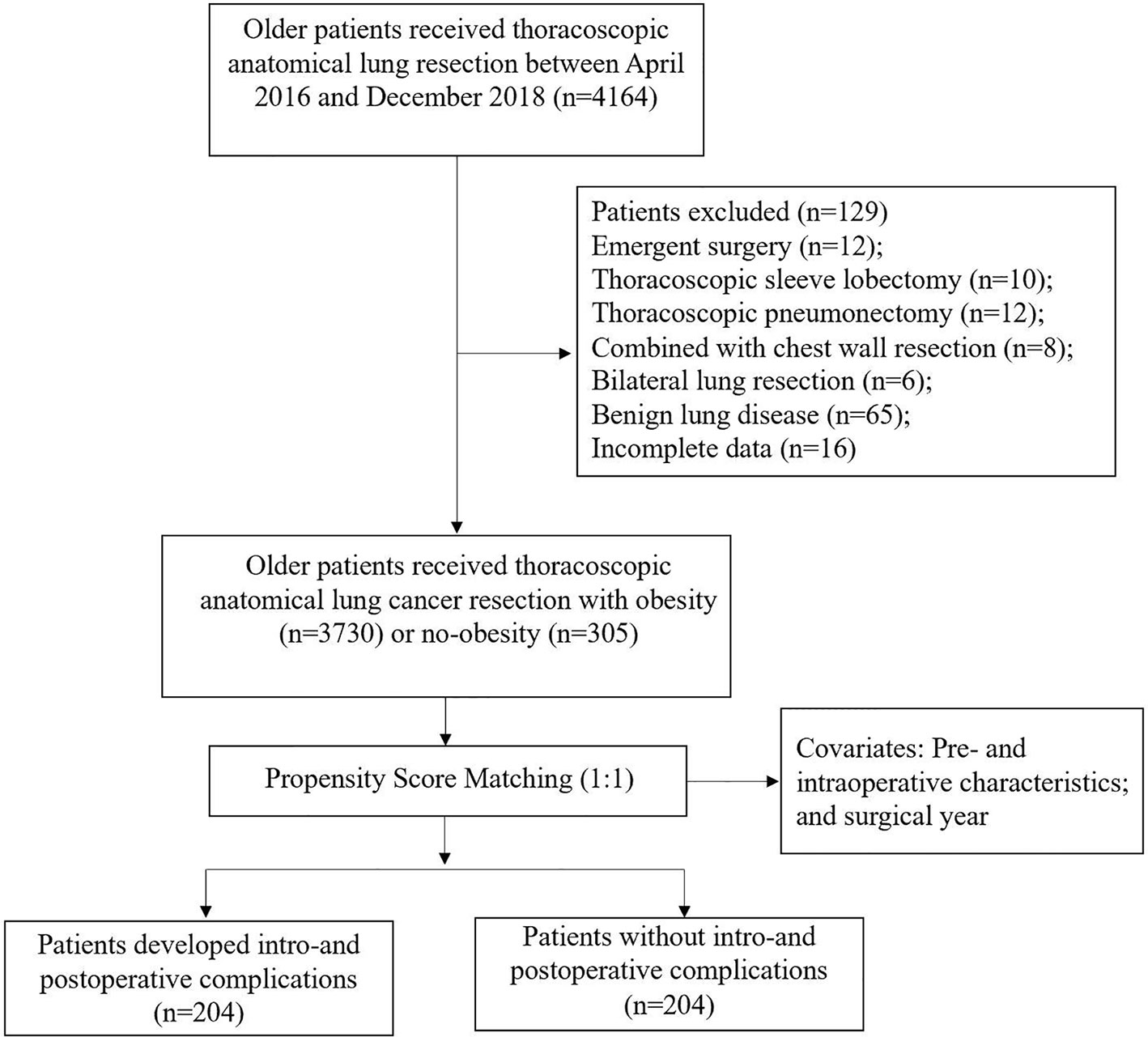
From January 2016 to December 2018, we performed a monocentric retrospective cohort study based on a prospectively collected database, including 4164 consecutive patients aged 65 years or older who underwent thoracoscopic anatomic lung cancer surgery. Excluded patients were described in the flow diagram (Figure 1). 4035 elderly patients were enrolled in the final analysis. The Institutional Review Board (IRB) of Shanghai Jiao tong University, Shanghai Chest Hospital (IS21119) approved this study and waived the need for informed consent.
Anesthesia Protocol
All patients were routinely monitored by electrocardiogram, pulse oximetry, non-invasive blood pressure (NIBP) and capnography. Radial artery intubation and right internal jugular central venous catheterization (CVP) were used to monitor invasive blood pressure (IBP). For thoracic paravertebral blockade (TPVB), 20 mL 0.5% ropivacaine was injected to the T4-T5 by the experienced anesthesiologist under the guidance of ultrasound before surgery. Intraoperative lung protective ventilation (LPV) strategies consisted of low-tide ventilation based on ideal body weight (≤8mL/kg), PEEP=5cmH2O, lung recruitment and maintenance of airway pressure <30cmH2O. Extubation was performed in the operating room or post anesthesia care unit (PACU) for all suitable patients. All patients treated with patient-controlled analgesia (PCA) pump, including sufentanil1.0 μg/kg + desoxocin 0.4mg/kg.
Technique of Operation
Since 2016, the high-volume center of Shanghai Chest Hospital has performed nearly 10,000 lung operations annually, of which thoracoscopic surgery accounts for more than 80 percent. All possible thoracoscopic surgery was determined by the participating surgeons based on the individual patient’s preoperative evaluation. For all patients, thoracoscopic anatomic lung resection plus systematic lymph node dissection were considered as the best treatment for primary lung cancer.
Data Collection and Definition
Perioperative clinical data were prospectively pooled from our institution’s electronic medical system, enrolling patient’s baseline and intraoperative characteristics, and perioperative outcomes including hypoxemia, transfusion, new-onset arrhythmia, conversion to thoracotomy, pulmonary complications, length of hospital stay (LOS), 30-day readmission and hospitalization costs. Hypoxemia was defined as SpO2<90% for more than 10 consecutive minutes. Postoperative pulmonary complications (PPCs) refer to the European Perioperative Clinical Outcome (EPCO) (23). According to the 2014 Guidelines of the American Association of Thoracic Surgeons (AATS) (24), perioperative new-onset arrhythmia included incidents of atrial fibrillation (AF) and atrial flutter. The 30-day readmission included in the analysis was an unplanned return to the hospital due to various postoperative complications.
We classified the obesity status according to the body mass index (BMI, weight in kilograms divided by height in meters squared) of the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults (25). Two groups were stratified by BMI: nonobese (BMI<28kg/m2) and obese status (BMI≥28kg/m2). In subgroup analysis, the perioperative outcomes between patients with obesity class I (28kg/m2 ≤BMI<30kg/m2) and obesity class II (BMI≥30kg/m2) were investigated.
Statistical Analysis
Data was tested for normal distribution with Q-Q plot. Continuous variables were compared between nonobese and obese patients using Two independent sample t-test or Mann-Whitney U test. Categorical variables were compared with Chi-square test or Fisher exact test, depending on the sample size. To reduce the selection bias and other potential confounding effects, we performed a 1:1 propensity score matching (PSM) analysis using a caliper size of 0.01 to compare perioperative outcomes between two cohorts. All pre-, intraoperative variables and surgical year were included in the PSM analysis. Standardized mean difference (SMD) between two cohorts on all covariables after matching was calculated, with differences of <10% indicating adequate balance in the matched cohort. Statistical analysis was performed using the SPSS 26.0 software (IBM Corp., Armonk, NY, USA). R version 4.1.2 was used with the tableone, ggplot2, reshape2, survey and Matching packages. P-value<0.05 was considered statistically significant.
Results
Study Cohort
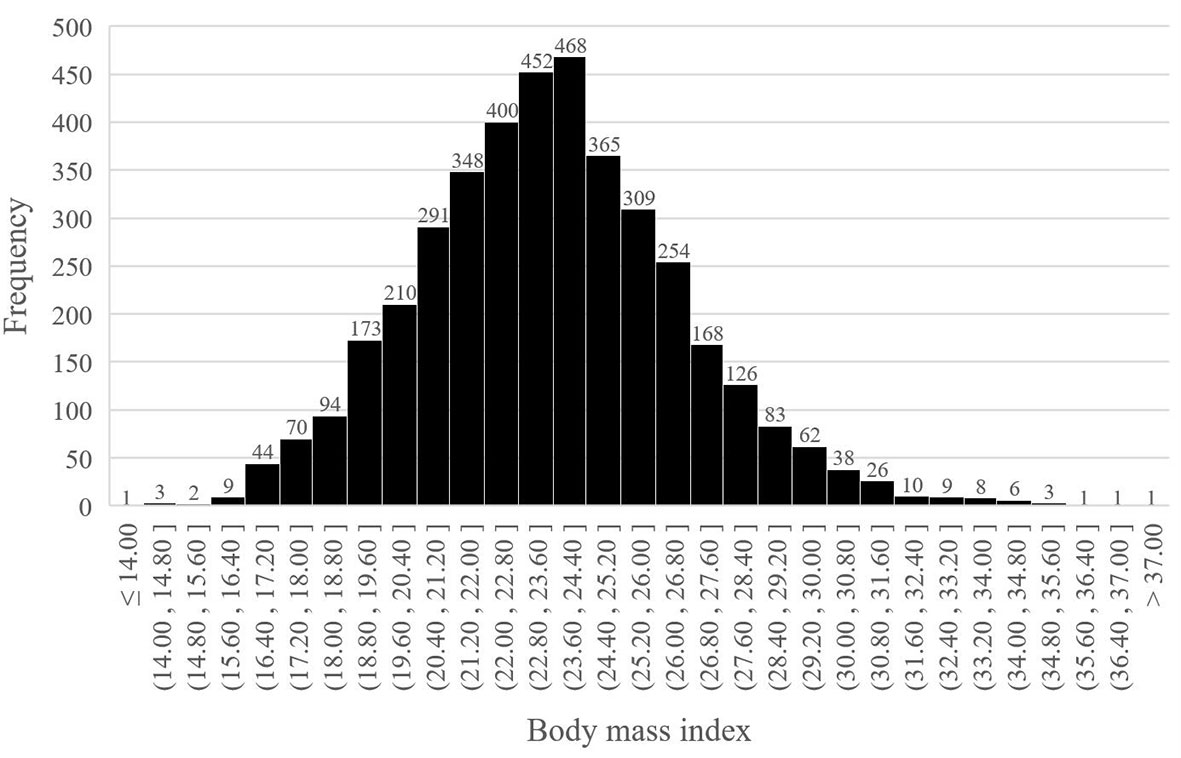
From January 2016 to December 2018, 4035 older patients with a mean age of 69.8 years (range: 65-87) underwent thoracoscopic anatomical lung cancer surgery, of which 17.6% (712 out of 4035) underwent segmentectomy resection and 82.4% (3323 out of 4035) underwent lobectomy resection, and 17.6% (305 out of 4035) were eligible for obese status, with a mean BMI of 29.8 ± 1.7kg/m2 (Figure 1). The BMI distribution of all enrolled patients were depicted in Figure 2.
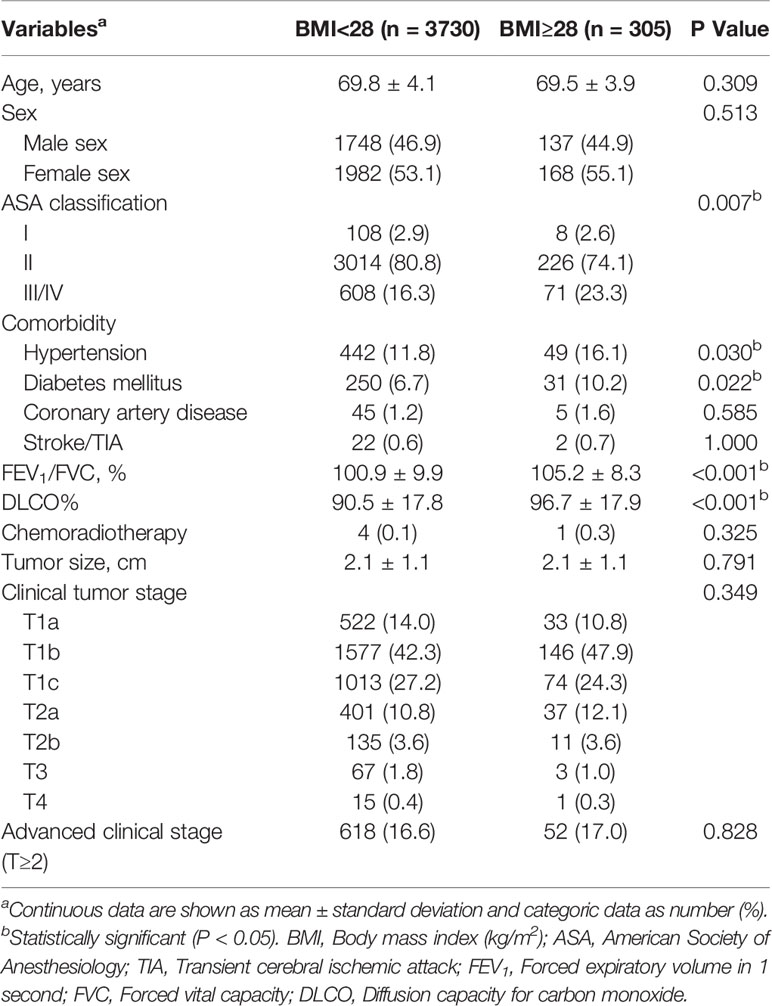
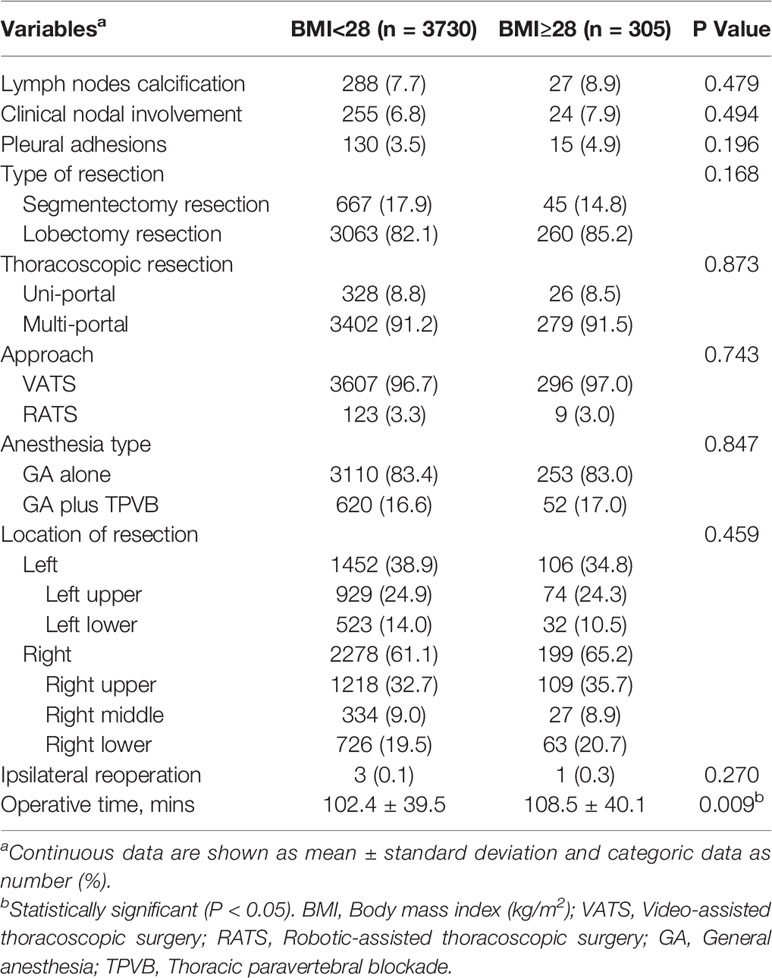
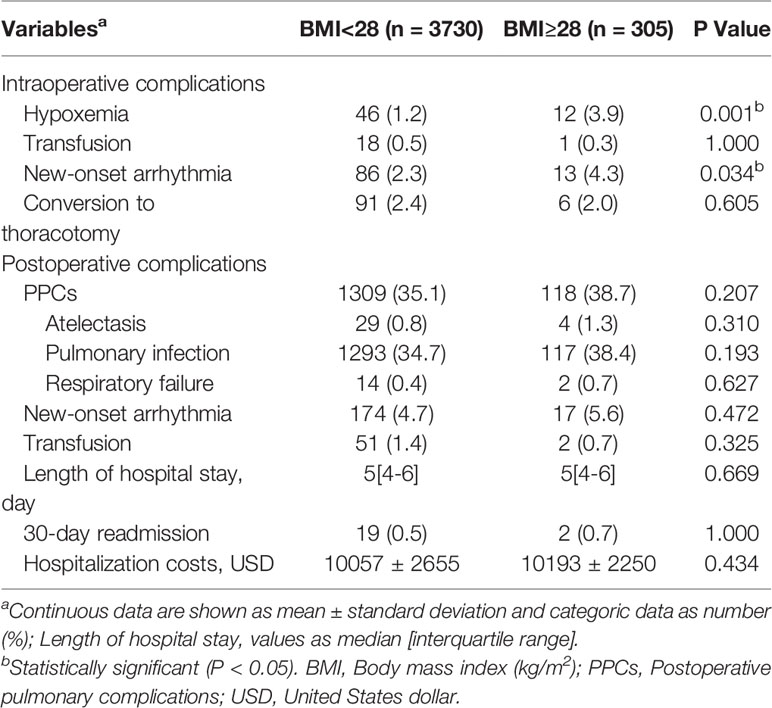
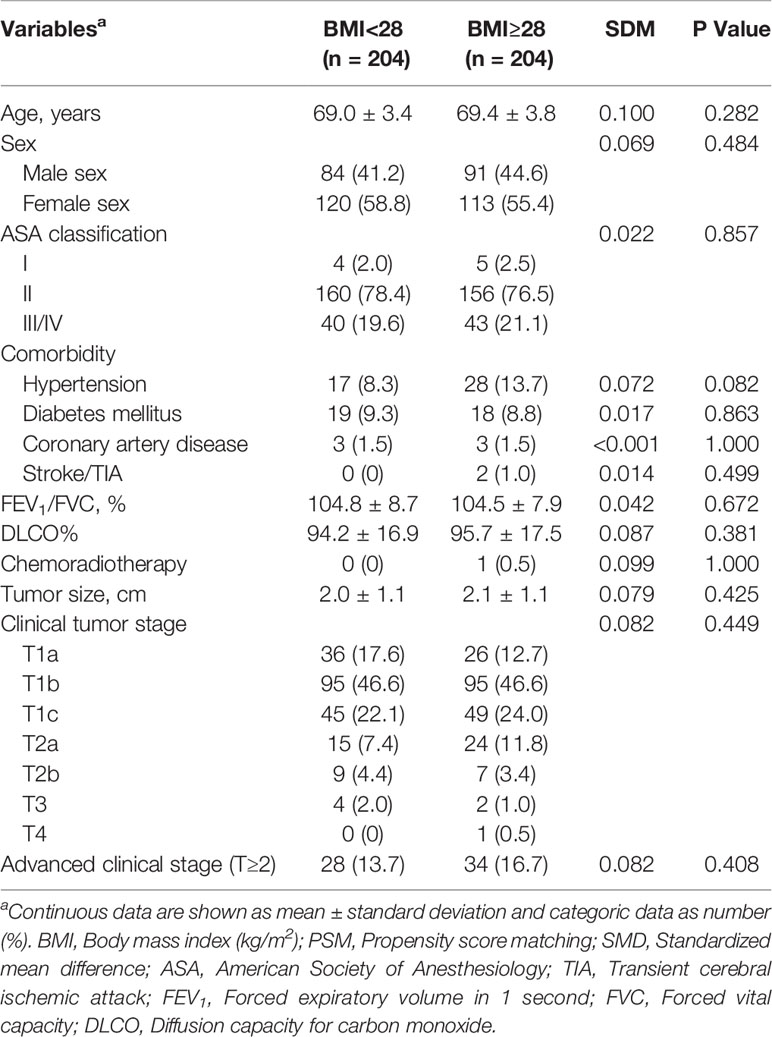
Patients with obese status had a higher American Society of Anesthesiologists (ASA) classification (III/IV, 23.3% vs 16.3%, P=0.007), and increased rates of hypertension (16.1% vs 11.8%, P=0.03) and diabetes mellitus (10.2% vs 6.7%, P=0.022), and better preoperative lung function (FEV1/FVC, 105.2 ± 8.3 vs 100.9 ± 9.9, P<0.001; DLCO%, 96.7 ± 17.9 vs 90.5 ± 17.8, P<0.001) when compared with their counterparts. Additionally, patients developed with obese status required longer operative time (108.5 ± 40.1 vs 102.4 ± 39.5mins, P=0.009) compared with nonobese patients (Tables 1, 2).
Perioperative Outcomes Between Two Cohorts Before and After a 1:1 PSM
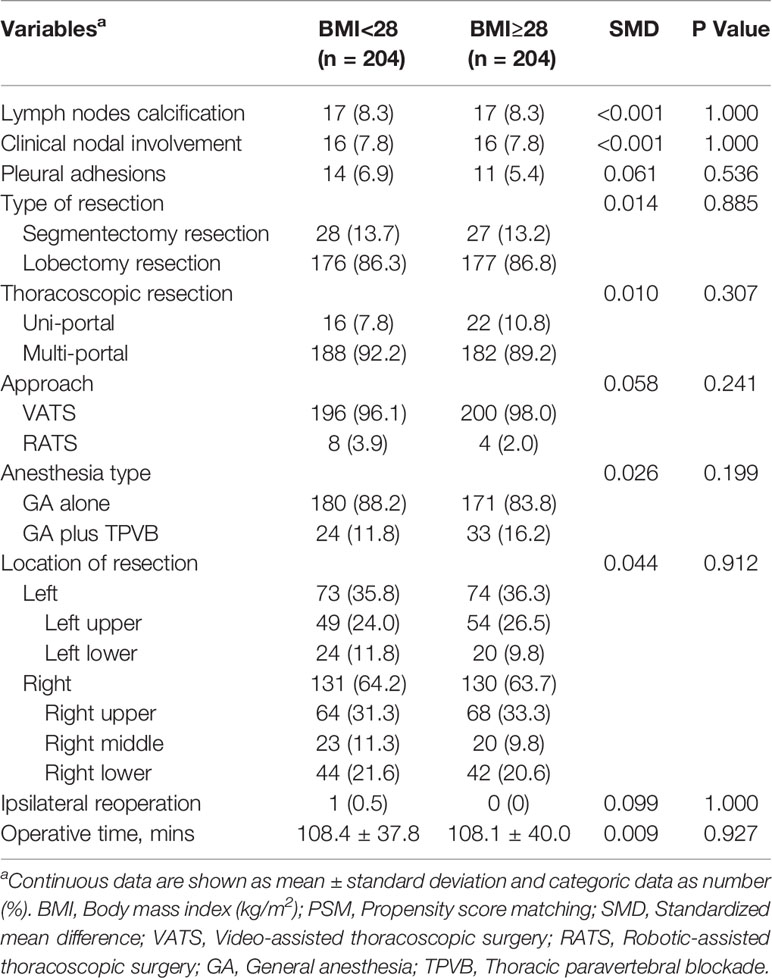
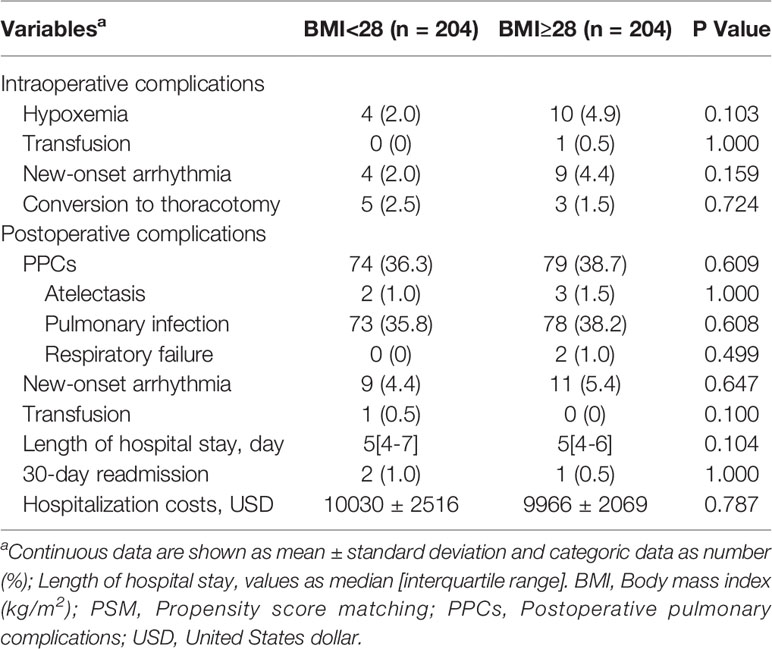
Compared with nonobese patients, the rates of intraoperative hypoxemia (1.2% vs 3.9%, P=0.001) and new-onset arrhythmia (2.3% vs 4.3%, P=0.034) were higher in obese patients (Table 3). The differences among intraoperative transfusion, conversion rates and postoperative outcomes including pulmonary complications, new-onset arrhythmia, transfusion, LOS, 30-day readmission and hospitalization costs were not significant (Table 3). After a 1:1 PSM analysis, all patient characteristics were comparable in two cohorts (Tables 4, 5). We investigated perioperative outcomes in 408 patients (204 pairs), the differences in both intra- and postoperative complications were not significant (Table 6). In subgroup analysis, the perioperative outcomes between patients with obesity class I and obesity class II were explored. When all perioperative data were comparable (Supplementary Tables S1, S2), we found that higher severity of obesity did not increase the incidence of perioperative complications (Supplementary Table S3).
Discussion
305 older patients who underwent thoracoscopic anatomical lung cancer surgery were eligible for obese status. Our study found that elderly obese patients had similar rates of perioperative complications compared to nonobese patients, and that the severity of obesity was unlikely to increase the incidence of adverse outcomes. Therefore, obesity status among elderly patients should not be a hindrance to preoperative evaluation and surgical planning during thoracoscopic anatomic lung cancer surgery.
The World Health Organization (WHO) (26) has recommended BMI cutoff points for underweight(<18.5kg/m2), normal weight (18.5 to 24.9kg/m2), overweight (25 to 29.9 kg/m2) and obesity (>30kg/m2) to predict health risks, including risk of all cancer types and non-cancer diseases. However, whether the criteria applied to Asian populations remains controversial (25, 27, 28). The BMI classification criteria for obesity in this investigation by referring to the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults, which may be more suitable for clinical studies in Chinese population.
MIS has been established to improve perioperative adverse outcomes, especially in patients at high risk such as elderly and obese patients, with regard to the standard open approaches (13, 29). Presumably, the proportion of elderly patients with elevated BMI undergoing thoracic surgery will constantly increase in the future (13, 14, 19). Intuitively, it seems that longer operative time, reduced mobility, impaired diaphragm movement, and obesity-related comorbidities should be associated with an increased risk of lung resection. Therefore, it is mandatory to fully understand the influence of obesity on perioperative outcomes of elderly patients.
In terms of intraoperative complications, although before a 1:1 PSM, obese patients developed higher rates of hypoxemia and new-onset arrhythmia, but had comparable transfusion, conversion rates and operative time compared with nonobese patients, and none of these differences were significant after PSM. Our previous published literature echoed these results and did not find that elevated BMI was associated with high rates of intraoperative conversion and new-onset arrhythmia (30, 31). Similarly, Guerrera has evaluated the impact of morbidly obesity on perioperative clinical outcomes after thoracoscopic lobectomy and found that obese patients did not increase conversion rates, blood loss and surgical time (32). Conversely, a few studies have shown that obesity was associated with increased operative time (33).
Our research results may also provide the evidence for the “obesity paradox” in elderly patients undergoing thoracoscopic anatomic lung cancer surgery, which has been widely reported in published documents (14–17). The postoperative complications regarding pulmonary complications, new-onset arrhythmia, transfusion, LOS, 30-day readmission and hospitalization costs were not significant between obese and nonobese patients. Ferguson et al. showed that being overweight or obese did not increase the risk of postoperative complications in any category after major lung resection (34). Also, Thomas et al. conducted a retrospective cohort study of 19,635 patients undergoing lobectomy for primary lung cancer and concluded that obesity was not associated with increased incidence of postoperative complications, except for cardiovascular complications and had a statistical protective effect regarding surgical complications (35). Moreover, a systematic review with meta-analysis demonstrated that overall morbidity and in-hospital mortality were significantly decreased in obese patients (36).
Our investigation also assessed the effect of obesity severity on perioperative outcomes in older patients and found that the difference was not significant between the obesity class I and obesity class II. And the results were consistent with other published researches (14, 32). A retrospective study conducted by Williams using Society of Thoracic Surgeons General Thoracic Surgery Database indicated that overweight and obese class I to II patients had a lower risk of pulmonary complications and any postoperative events (14). In contrast to the results of our present study, Zogg (37) and De Oliveira’s (38) research showed that obese class II and III patients experienced marginally increased odds of morbidity and an increased risk of pulmonary complications, respectively. The variability of results may be related to the fact that high BMI does not distinguish between body composition phenotypes that have important effects on surgical risk and outcomes (39). Relying on BMI augmentation categories alone to assess the surgical risk of major lung resection is fraught with challenges.
Potential shortcomings of this study include as follows. First, as a retrospective study based on a prospectively collected database, it has the inherent design biases. Besides, single-institute study has specific generalization limitations. Second, due to the limited granularity of postoperative care data, some poor outcomes, such as pain control (40), other surgical complications and mortality, could not be pooled in this study. Third, the relationship between obesity and long-term outcomes needs further investigation (41, 42).
Conclusions
By conducting a monocentric retrospective cohort study of 4035 elderly patients receiving thoracoscopic anatomic lung cancer surgery, our study found that obesity status and obesity severity dose not increase perioperative adverse outcomes. Even among older patients, these data support evidence for “obesity paradox”. These data also contribute the growing body of evidence that obesity in older patients should not exclude candidates for thoracoscopic anatomic lung cancer surgery.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was approved by the Institutional Review Board at Shanghai Chest Hospital (IS21119), and the informed consent was waived because of the retrospective nature of the study.
Author Contributions
CT, TL and YS: study conception, design, statistic analysis and drafting of the manuscript. CT, YS and HZ: acquisition of data. CT, JZ and JW: analysis and interpretation of data. JWand JZ: critical revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82071233) and Shanghai Shen Kang Hospital Development Center Project (SHDC2020CR4063).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Our research team would like to thank Professor Liu for statistical analysis and Professor Luo for study design in Shanghai Chest Hospital, Shanghai, China, for their involvement and support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.881467/full#supplementary-material
References
2. Hurt RT, Frazier TH, McClave SA, Kaplan LM. Obesity Epidemic: Overview, Pathophysiology, and the Intensive Care Unit Conundrum. JPEN J Parenter Enteral Nutr (2011) 35(5 Suppl):4S–13S. doi: 10.1177/0148607111415110
3. Apovian CM. Obesity: Definition, Comorbidities, Causes, and Burden. Am J Manag Care (2016) 22(7 Suppl):s176–85.
4. Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med (2017) 376(3):254–66. doi: 10.1056/NEJMra1514009
5. Stefan N. Causes, Consequences, and Treatment of Metabolically Unhealthy Fat Distribution. Lancet Diabetes Endocrinol (2020) 8(7):616–27. doi: 10.1016/S2213-8587(20)30110-8
6. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-Mass Index and Risk of 22 Specific Cancers: A Population-Based Cohort Study of 5.24 Million UK Adults. Lancet (2014) 384(9945):755–65. doi: 10.1016/S0140-6736(14)60892-8
7. Gupta A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, et al. Premorbid Body Mass Index and Mortality in Patients With Lung Cancer: A Systematic Review and Meta-Analysis. Lung Cancer (2016) 102:49–59. doi: 10.1016/j.lungcan.2016.10.017
8. Zhou W, Liu G, Hung RJ, Haycock PC, Aldrich MC, Andrew AS, et al. Causal Relationships Between Body Mass Index, Smoking and Lung Cancer: Univariable and Multivariable Mendelian Randomization. Int J Cancer (2021) 148(5):1077–86. doi: 10.1002/ijc.33292
9. Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open (2021) 4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520
10. Saltiel AR, Olefsky JM. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J Clin Invest (2017) 127(1):1–4. doi: 10.1172/JCI92035
11. Sarode P, Schaefer MB, Grimminger F, Seeger W, Savai R. Macrophage and Tumor Cell Cross-Talk Is Fundamental for Lung Tumor Progression: We Need to Talk. Front Oncol (2020) 10:324. doi: 10.3389/fonc.2020.00324
12. Hillers-Ziemer LE, Williams AE, Janquart A, Grogan C, Thompson V, Sanchez A, et al. Obesity-Activated Lung Stromal Cells Promote Myeloid Lineage Cell Accumulation and Breast Cancer Metastasis. Cancers (Basel) (2021) 13(5):1005. doi: 10.3390/cancers13051005
13. Ferguson MK, Vigneswaran WT. Changes in Patient Presentation and Outcomes for Major Lung Resection Over Three Decades. Eur J Cardiothorac Surg (2008) 33(3):497–501. doi: 10.1016/j.ejcts.2007.12.023
14. Williams T, Gulack BC, Kim S, Fernandez FG, Ferguson MK. Operative Risk for Major Lung Resection Increases at Extremes of Body Mass Index. Ann Thorac Surg (2017) 103(1):296–302. doi: 10.1016/j.athoracsur.2016.05.057
15. Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of Obesity on Postoperative and Long-Term Outcomes in a General Surgery Population: A Retrospective Cohort Study. World J Surg (2013) 37(11):2561–8. doi: 10.1007/s00268-013-2162-y
16. Mungo B, Zogg CK, Hooker CM, Yang SC, Battafarano RJ, Brock MV, et al. Does Obesity Affect the Outcomes of Pulmonary Resections for Lung Cancer? A National Surgical Quality Improvement Program Analysis. Surgery (2015) 157:792–800. doi: 10.1016/j.surg.2014.10.016
17. Dotan I, Shochat T, Shimon I, Akirov A. The Association Between BMI and Mortality in Surgical Patients. World J Surg (2021) 45(5):1390–9. doi: 10.1007/s00268-021-05961-4
18. Lang LH, Parekh K, Tsui BYK, Maze M. Perioperative Management of the Obese Surgical Patient. Br Med Bull (2017) 124(1):135–55. doi: 10.1093/bmb/ldx041
19. Liou DZ, Berry MF. Thoracic Surgery Considerations in Obese Patients. Thorac Surg Clin (2018) 28(1):27–41. doi: 10.1016/j.thorsurg.2017.09.004
20. Billmeier SE, Jaklitsch MT. Pulmonary Surgery for Malignant Disease in the Elderly. In: Rosenthal RA, Zenilman ME, Katlic MR, editors. Principles and Practice of Geriatric Surgery, 2nd ed. New York, NY: Springer (2011). p. 605–16. doi: 10.1007/978-1-4419-6999-6_48
21. Alvarez-Nebreda ML, Bentov N, Urman RD, Setia S, Huang JC, Pfeifer K, et al. Recommendations for Preoperative Management of Frailty From the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth (2018) 47:33–42. doi: 10.1016/j.jclinane.2018.02.011
22. Tabatabai S, Do Q, Min J, Tang CJ, Pleasants D, Sands LP, et al. Obesity and Perioperative Outcomes in Older Surgical Patients Undergoing Elective Spine and Major Arthroplasty Surgery. J Clin Anesth (2021) 75:110475. doi: 10.1016/j.jclinane.2021.110475
23. Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for Definitions and Use of Outcome Measures for Clinical Effectiveness Research in Perioperative Medicine: European Perioperative Clinical Outcome (EPCO) Definitions: A Statement From the ESA-ESICM Joint Taskforce on Perioperative Outcome Measures. Eur J Anaesthesiol (2015) 32:88–105. doi: 10.1097/EJA.0000000000000118
24. Frendl G, Sodickson AC, Chung MK, Waldo AL, Gersh BJ, Tisdale JE, et al. 2014 AATS Guidelines for the Prevention and Management of Perioperative Atrial Fibrillation and Flutter for Thoracic Surgical Procedures. Executive Summary J Thorac Cardiovasc Surg (2014) 148(3):772–91. doi: 10.1016/j.jtcvs.2014.06.037
25. Department of Disease Control of the Ministry of Health of the People’s Republic of China. Guidelines for the Prevention and Control of Overweight and Obesity of Adult in China. Beijing: People’s Medical Publishing House (2006) p. 34–6.
26. Organization WH. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation.World Health Organ Tech Rep Ser (2020) 894:i-xii, 1–253.
27. Yang L, Yang G, Zhou M, Smith M, Ge H, Boreham J, et al. Body Mass Index and Mortality From Lung Cancer in Smokers and Nonsmokers: A Nationally Representative Prospective Study of 220,000 Men in China. Int J Cancer (2009) 125(9):2136–43. doi: 10.1002/ijc.24527
28. Koh WP, Yuan JM, Wang R, Lee HP, Yu MC. Body Mass Index and Smoking− Related Lung Cancer Risk in the Singapore Chinese Health Study. Br J Cancer (2010) 102(3):610–4. doi: 10.1038/sj.bjc.6605496
29. Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hürtgen M, Petersen RH, et al. Video-Assisted Thoracoscopic Surgery Versus Open Lobectomy for Primary Non-Small-Cell Lung Cancer: A Propensity-Matched Analysis of Outcome From the European Society of Thoracic Surgeon Database. Eur J Cardiothorac Surg (2015) 49:602–9. doi: 10.1093/ejcts/ezv154
30. Tong C, Li T, Huang C, Ji C, Liu Y, Wu J, et al. Risk Factors and Impact of Conversion to Thoracotomy From 20,565 Cases of Thoracoscopic Lung Surgery. Ann Thorac Surg (2020) 109(5):1522–9. doi: 10.1016/j.athoracsur.2019.12.009
31. Tong C, Zhang Q, Liu Y, Xu M, Wu J, Cao H. Risk Factors and Outcomes of Intraoperative Atrial Fibrillation in Patients Undergoing Thoracoscopic Anatomic Lung Surgery. Ann Transl Med (2021) 9(7):543. doi: 10.21037/atm-20-5035
32. Guerrera F, Lyberis P, Lausi PO, Cristofori RC, Giobbe R, Molinatti M, et al; On the behalf of the Italian VATS Group. Does Morbid Obesity Influence Perioperative Outcomes After Video-Assisted Thoracic Surgery (VATS) Lobectomy for Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Registry. Surg Endosc (2021) 36(5):3567–73. doi: 10.1007/s00464-021-08680-y
33. St Julien JB, Aldrich MC, Sheng S, Deppen SA, Burfeind Jr WR, Putnam JB, et al. Obesity Increases Operating Room Time for Lobectomy in the Society of Thoracic Surgeons Database. Ann Thorac Surg (2012) 94:1841–7. doi: 10.1016/j.athoracsur.2012.08.006
34. Ferguson MK, Im HK, Watson S, Johnson E, Wigfield CH, Vigneswaran WT. Association of Body Mass Index and Outcomes After Major Lung Resection. Eur J Cardiothorac Surg (2014) 45(4):e94–9; discussion e99. doi: 10.1093/ejcts/ezu008
35. Thomas PA, Berbis J, Falcoz PE, Le Pimpec-Barthes F, Bernard A, Jougon J, et al; EPITHOR Group. National Perioperative Outcomes of Pulmonary Lobectomy for Cancer: The Influence of Nutritional Status. Eur J Cardiothorac Surg (2014) 45(4):652–9; discussion 659. doi: 10.1093/ejcts/ezt452
36. Li S, Wang Z, Huang J, Fan J, Du H, Liu L, et al. Systematic Review of Prognostic Roles of Body Mass Index for Patients Undergoing Lung Cancer Surgery: Does the 'Obesity Paradox' Really Exist? Eur J Cardiothorac Surg (2017) 51(5):817–28. doi: 10.1093/ejcts/ezw386
37. Zogg CK, Mungo B, Lidor AO, Stem M, Rios Diaz AJ, Haider AH, et al. Influence of Body Mass Index on Outcomes After Major Resection for Cancer. Surgery (2015) 158(2):472–85. doi: 10.1016/j.surg.2015.02.023
38. De Oliveira GS Jr, McCarthy RJ, Davignon K, Chen H, Panaro H, Cioffi WG. Predictors of 30-Day Pulmonary Complications After Outpatient Surgery: Relative Importance of Body Mass Index Weight Classifications in Risk Assessment. J Am Coll Surg (2017) 225(2):312–23.e7. doi: 10.1016/j.jamcollsurg.2017.04.013
39. Prado CM, Gonzalez MC, Heymsfield SB. Body Composition Phenotypes and Obesity Paradox. Curr Opin Clin Nutr Metab Care (2015) 18(6):535–51. doi: 10.1097/MCO.0000000000000216
40. Majchrzak M, Brzecka A, Daroszewski C, Błasiak P, Rzechonek A, Tarasov VV, et al. Increased Pain Sensitivity in Obese Patients After Lung Cancer Surgery. Front Pharmacol (2019) 10:626. doi: 10.3389/fphar.2019.00626
41. Sepesi B, Gold KA, Correa AM, Heymach JV, Vaporciyan AA, Roszik J, et al. The Influence of Body Mass Index on Overall Survival Following Surgical Resection of Non-Small Cell Lung Cancer. J Thorac Oncol (2017) 12(8):1280–7. doi: 10.1016/j.jtho.2017.05.010
42. Alifano M, Daffré E, Iannelli A, Brouchet L, Falcoz PE, Le Pimpec, et al. The Reality of Lung Cancer Paradox: The Impact of Body Mass Index on Long-Term Survival of Resected Lung Cancer. A French Nationwide Analysis From the Epithor Database. Cancers (Basel) (2021) 13(18):4574. doi: 10.3390/cancers13184574
Keywords: elderly, obesity, thoracoscopic surgery, lung cancer, outcomes
Citation: Tong C, Li T, Shen Y, Zhu H, Zheng J and Wu J (2022) Obesity Does Not Increase Perioperative Outcomes in Older Patients Undergoing Thoracoscopic Anatomic Lung Cancer Surgery. Front. Oncol. 12:881467. doi: 10.3389/fonc.2022.881467
Received: 22 February 2022; Accepted: 07 April 2022;
Published: 06 May 2022.
Edited by:
Vamsi Velcheti, New York University, United StatesReviewed by:
Massimo Baudo, Spedali Civili Brescia, ItalyMichael Ried, University Hospital Regensburg, Germany
Copyright © 2022 Tong, Li, Shen, Zhu, Zheng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxiang Wu, d2p4MTEzMnhrQDE2My5jb20=; Jijian Zheng, emhlbmdqaWppYW42MjZAc2luYS5jb20=
†These authors share first authorship
 Chaoyang Tong1,2†
Chaoyang Tong1,2† Jingxiang Wu
Jingxiang Wu