
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 11 May 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.881346
 Hailing Liu1,2,3†
Hailing Liu1,2,3† Xiao Shi1,2,3†
Xiao Shi1,2,3† Huizi Fang2†
Huizi Fang2† Lei Cao1,2,3
Lei Cao1,2,3 Yi Miao1,2,3
Yi Miao1,2,3 Xiaoli Zhao1,2,3
Xiaoli Zhao1,2,3 Wei Wu1,2,3
Wei Wu1,2,3 Wei Xu1,2,3
Wei Xu1,2,3 Jianyong Li1,2,3*
Jianyong Li1,2,3* Lei Fan1,2,3*
Lei Fan1,2,3*Background: In the era of immunotherapy, autologous stem cell transplantation (ASCT) in first-line therapy in patients with mantle cell lymphoma (MCL) has been a controversial topic. This report aimed to explore the association between ASCT and MCL survival through a systematic review with meta-analysis.
Methods: We performed a systematic search of original articles published from inception to September 2021 using PubMed, MEDLINE, Embase, and Cochrane Library databases.
Results: We included studies that compared ASCT with non-ASCT consolidation in newly diagnosed transplant-eligible MCL. The endpoints were progression-free survival (PFS) and overall survival (OS). There were seven eligible studies (one randomized clinical trial, one prospective cohort study, and five observational studies) published between 2012 and 2021, in which the total number of participants was 3,271. In the non-intensive induction subgroup, patients with ASCT experienced a significant PFS but no OS benefit compared with those without ASCT. In the intensive induction subgroup, the PFS benefit from ASCT still existed but largely attenuated; no OS benefit was observed though only one study was suitable for evaluation. When compared to the rituximab maintenance arm, ASCT had a worse PFS and OS.
Conclusions: In the rituximab plus HiDAC era, the benefit of ASCT as a component of first-line treatment has been weakened. First-line maintenance strategy instead of ASCT seems worth exploring.
Mantle cell lymphoma (MCL) is an aggressive and incurable non-Hodgkin lymphoma, with an increasing annual incidence of 0.8 per 100,000 population in Western countries (1). Survival for MCL patients (pts) has historically been very dismal, with median overall survival (OS) in the range of 3–5 years (2, 3). Front-line consolidative ASCT was first recommended to treat young MCL pts to prolong survival, whose support was from evidence in the pre-rituximab era (4). However, some scholars showed the benefit of ASCT as a component of first-line treatment was weakened in the rituximab era (5–7). Moreover, several studies demonstrated the survival superiority of intensive induction regimens such as the Nordic regimen (8), DHAP alternating with R-CHOP (9), and R-hyper-CVAD with methotrexate and high-dose cytarabine (HiDAC) (10, 11) compared with historical controls. Chemo-immunotherapy containing rituximab and HiDAC followed by ASCT started to become a widely accepted standard for newly diagnosed transplant-eligible MCL (12), but notably, ASCT has once more not been proven to be a necessary protocol with survival benefit in the rituximab plus HiDAC era. In the study by LaCasce et al. (13), R-hyper-CVAD with or without ASCT had similar disease-specific outcomes, firstly raising the question about the consolidation role of ASCT under the premise of using rituximab and HiDAC. Even the newest follow-up data from the only randomized controlled trial (RCT) supporting the value of ASCT consolidation reported that ASCT failed to prolong progression-free survival (PFS) and OS after a median follow-up of 14 years (14). This controversy is evident in the current practice, but no well-designed prospective clinical trials have resolved it. It would be helpful to combine data from available studies to get a clearer view of the therapeutic value of ASCT in MCL. We therefore conducted a systematic review and meta-analysis on the treatment efficacy of ASCT for newly diagnosed transplant-eligible MCL pts.
We systematically searched PubMed, Medline, Embase, and Cochrane Library databases from inception to August 2021. We combined subject words with free words for retrieval and adjusted according to the characteristics of different databases without any restriction on gender, ethnicity, and languages to reduce bias. Supplemental File 1 shows this in more detail. Two investigators independently assessed RCTs using the Jadad scale and non-RCTs using the Newcastle-Ottawa Scale (NOS). The populations, interventions, comparisons, outcomes, and study designs (PICOS) considered for review are listed in Table 1. The co-primary variables were OS, defined as the time from random assignment to death from any cause, and PFS, defined as the time from randomization to documented disease progression or death. Three investigators independently screened the studies and solved any disagreements regarding trial selection through discussion. The search process is depicted in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart, outlining the numbers of identified and excluded records and the final number of included trials.
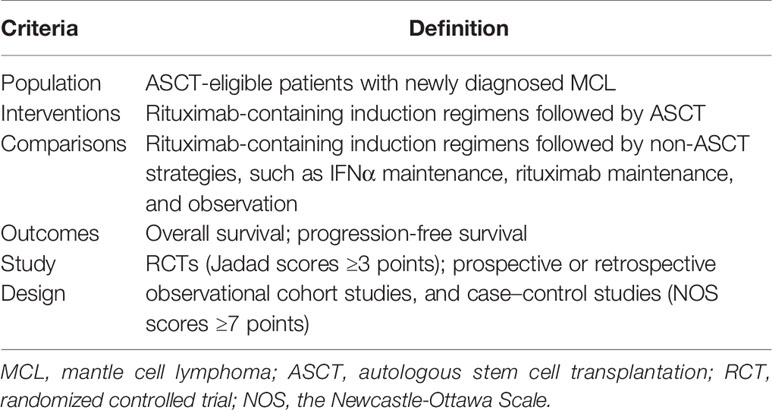
Table 1 Population, interventions, comparisons, outcomes, and study design (PICOS) criteria for study.
Study characteristics, including first author, year of publication, data sources, study type, recruitment interval, the number of ASCT-eligible pts, and median follow-up, were extracted. The principal data for analysis derived from included studies were the log hazard ratio (HR) and its standard error or the information to estimate them, such as an HR and its 95% confidence interval (CI) or Kaplan–Meier (KM) plots. When raw data were not available, we used Adobe Photoshop to process the KM curve pictures and Engauge Digitizer 11.1 software (http://digitizer.sourceforge.net) to extract survival data, then pooled the effect estimates together using the fixed and random-effects model. Statistical heterogeneity was tested by Cochran’s Q (χ2) (p < 0.10) and quantified by I2, where I2 of 0%–25% indicates no or mild heterogeneity, 25%–50% indicates moderate heterogeneity, 50%–75% indicates high heterogeneity, and 75%–100% indicates extreme heterogeneity. Furthermore, we conducted subgroup analyses and sensitivity analyses to identify the possible causes of substantial heterogeneity. A risk-of-bias assessment was also planned but was not applicable due to the small number of included trials.
Through the initial search of four databases (PubMed, Medline, Embase, and Cochrane Library databases) and hand-searching relevant bibliographies, we identified 3,517 records and then excluded 1,053 duplicates through literature manager software. Based on title/abstract content, three authors independently reviewed and excluded 2,434 references that did not satisfy the selection criteria. After reviewing the full text of the remaining 30 records, we excluded 23 for the following reasons: same data source (n = 6), no available data on results (n = 11), and low quality (n = 6). Finally, seven studies published between 2012 and 2021 met our inclusion criteria, including one RCT, one prospective cohort study, and five observational studies. Figure 1 shows the PRISMA diagram.
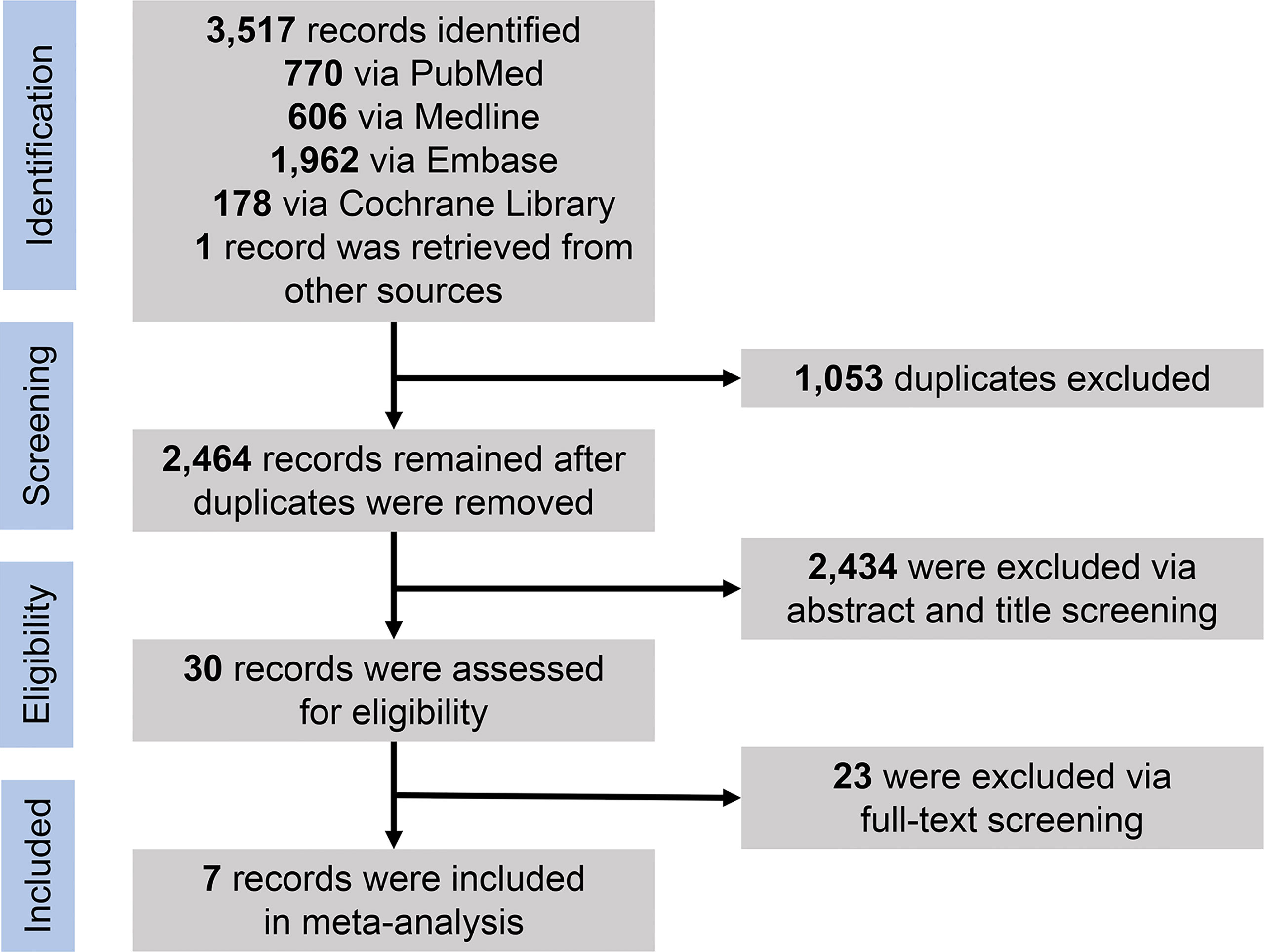
Figure 1 PRISMA flowchart for selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 2 shows the general characteristics of included studies (5, 13–18). The final datasets contained 3,271 pts, with a recruitment interval of 1996 to 2019 and a median age ranged from 55 to 62 years. The shortest median follow-up was 33 months, and the longest was 168 months. All induction regimens included rituximab and were classified into intensive and non-intensive induction groups. The former involved HiDAC-containing regimens, R-hyper-CVAD, and R-maxi-CHOP, while the latter include R-CHOP, BR, VcR-CVAD (5), and so on. Post-induction strategies consisted of ASCT consolidation, IFNα maintenance, rituximab maintenance (RM), and observation.
As in Figure 2, all studies were of high quality and low bias risk (one RCT scored 3 points using the Jadad scale, and six non-RCTs scored 7–9 points using the NOS scale). Because the number of included studies was less than 10, we did not perform the funnel plot.
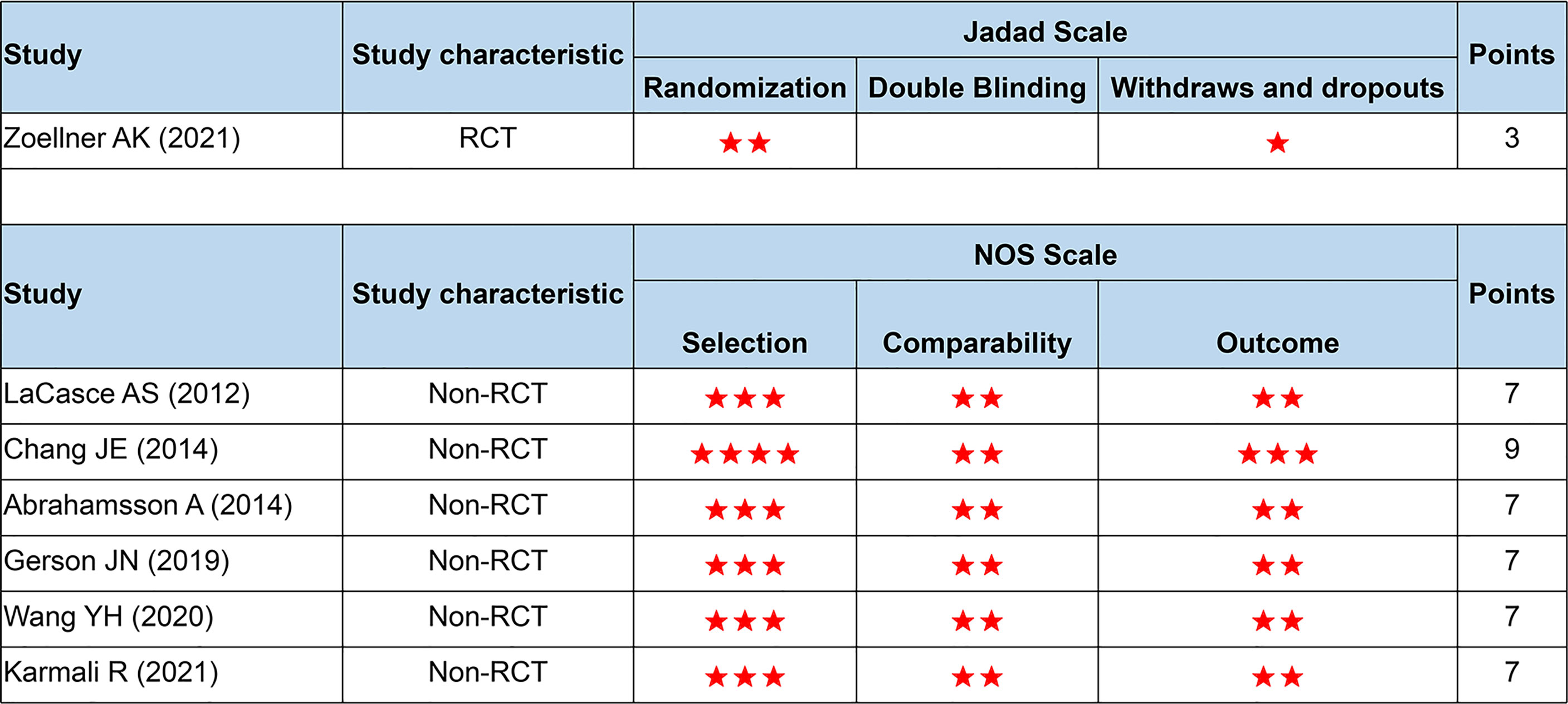
Figure 2 Quality assessment based on the Jadad and NOS scale. NOS, the Newcastle-Ottawa Scale; RCT, randomized controlled trials. Number of asterisks denotes scores.
Five of the seven studies were available for HR calculation of PFS between ASCT and non-ASCT arms. Due to the lack of the comparison of overall data in the study by LaCasce et al. (13), we exhibited two data subsets according to the original article. Compared with the non-ASCT arm, pts undergoing ASCT experienced a significant PFS improvement (HR = 0.64, 95% CI 0.47 to 0.87). However, moderate heterogeneity existed among these trial results (I2 = 56%, p = 0.04). Among those, two studies (rows 1 and 4) showed minimal 95% CI overlap. After eliminating the above data, there was little heterogeneity between the remaining studies (I2 = 11%, p = 0.34), whose sensitivity analysis demonstrated stable outcomes across the I2 value (range, 0% to 40%). These results led us to postulate that this heterogeneity could originate from the intensity of first-line induction protocols and the choice of post-induction strategies. See Figure 3 for details.
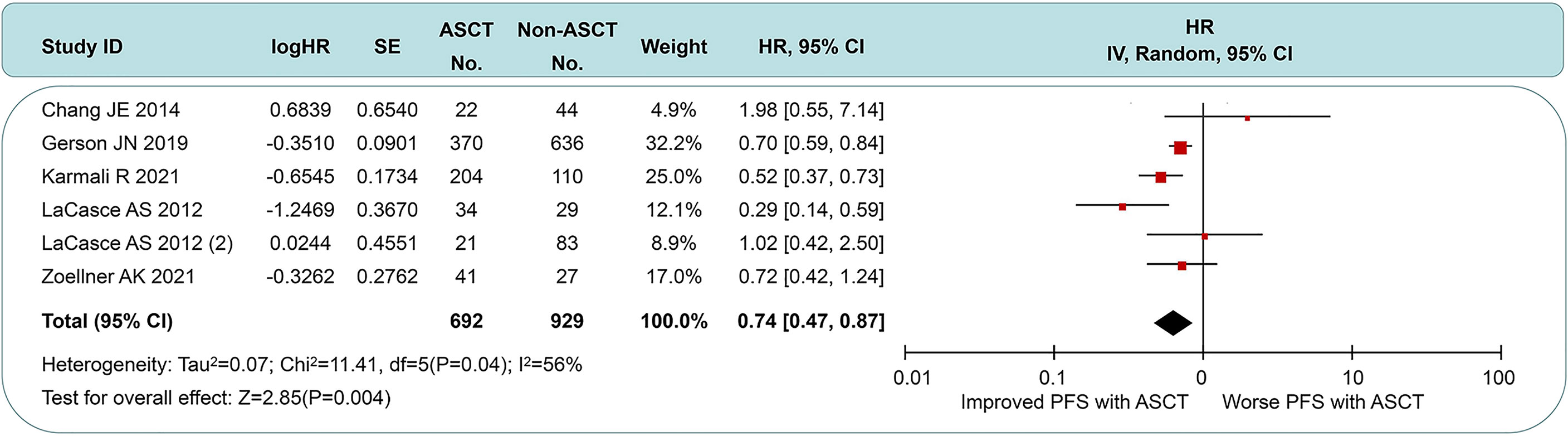
Figure 3 Forest plot and meta-analysis of progression-free survival between ASCT and non-ASCT group in MCL patients. ASCT, autologous stem cell transplantation; MCL, mantle cell lymphoma.
In the non-intensive induction subgroup, pts undergoing ASCT yield a better PFS (HR = 0.43, 95% CI 0.29 to 0.63) than the non-ASCT arm, but the long-term outcomes of Zoellner et al. displayed marked heterogeneity (Figure 4A). If we had chosen to use the short-interval follow-up data (logHR = −0.499, SE = 0.3291, extracted by software) in Dreyling et al.’s paper (4), the I2 value decreased from 56% to 46%. We speculate that this heterogeneity may stem from the length of follow-up and the addition of rituximab. In the intensive induction subgroup, a PFS benefit was still found in MCL pts treated with ASCT compared to pts without ASCT (HR = 0.58, 95% CI 0.51 to 0.89), but this was less significant than the non-intensive induction subgroup (Figure 4B). There was no evidence of heterogeneity (I2 = 0%, p = 0.61). As in Figure 4C, the ASCT arm exhibited a worse PFS than the rituximab arm (HR 1.54, 95% CI 1.09 to 2.18). Despite the smaller sample size, there was high homogeneity between the groups (I2 = 0%, p = 0.69).
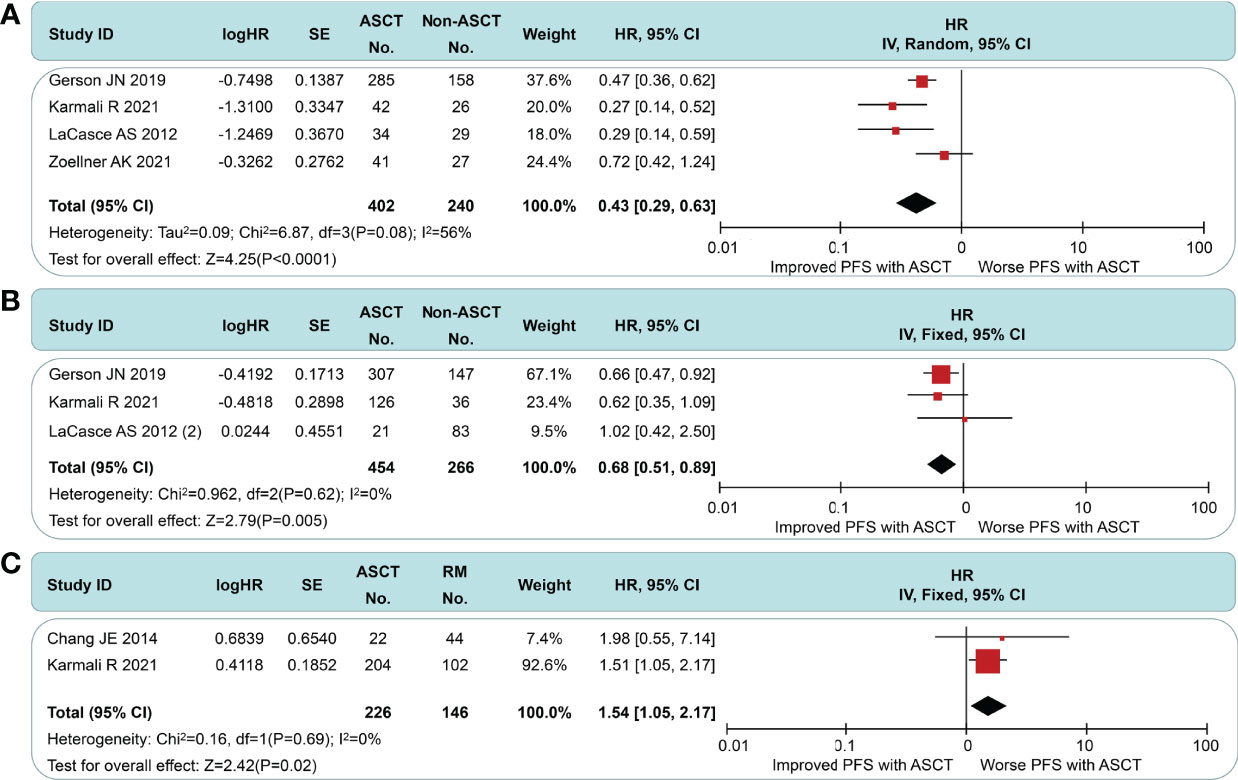
Figure 4 Meta-analysis results of progression-free survival: (A) Subgroup analysis for patients with non-intensive induction. (B) Subgroup analysis for patients with intensive induction. (C) Survival analysis between patients undergoing autologous stem cell transplantation and rituximab.
All of the included studies had available OS data (Figure 5), and a pooled analysis showed a significant OS benefit with the ASCT approach (HR = 0.77, 95% CI 0.65 to 0.92). Among-group dissimilarities differed only weakly (I2 = 29%, p = 0.21). In the subgroup comparison of non-intensive induction therapy (Figure 6A), three studies were pooled, and no OS differences were observed (HR = 0.75, 95% CI 0.56 to 1.00). I2 value was 0 (p = 0.21), which suggested a low degree of heterogeneity. In the intensive induction subgroup analysis, only one article was for eligibility. The analytic sample consisted of 454 individuals: 307 (67.6%) in the ASCT group and 147 (32.4%) in the control group. The OS of both groups did not exhibit a significant difference (HR = 0.92, 95% CI 0.61 to 1.40). Similarly, we performed the subgroup analyses of OS between ASCT and rituximab arms (Figure 6B). The ASCT group had poorer OS than the rituximab group (HR = 1.99, 95% CI 1.11 to 3.56), with acceptable heterogeneity (I2 = 45%, p = 0.18).
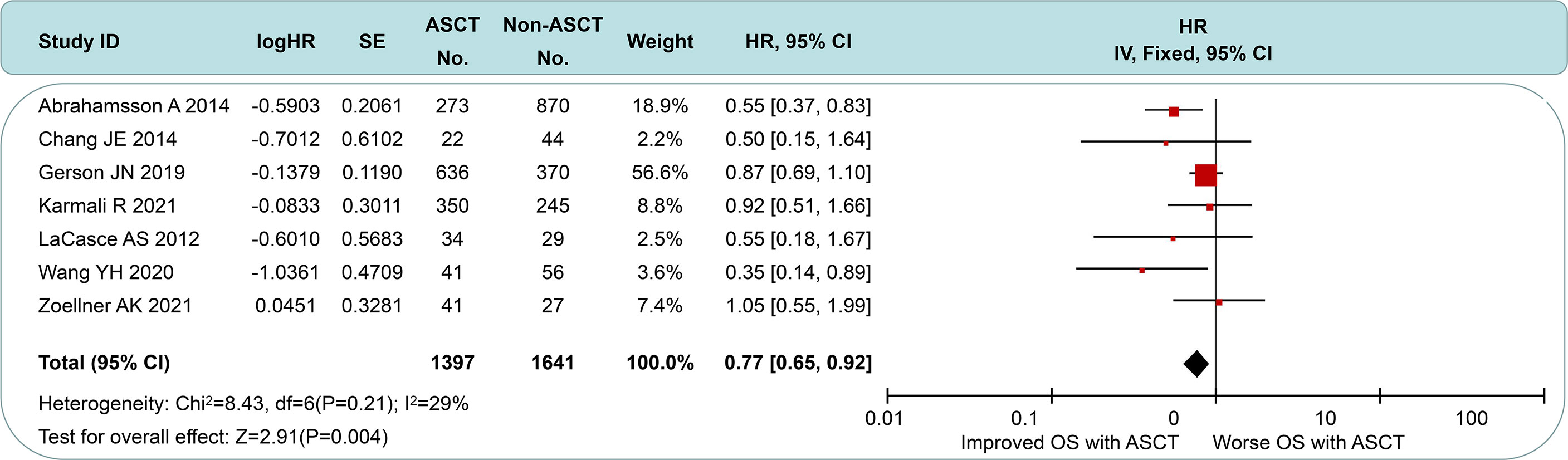
Figure 5 Forest plot and meta-analysis of overall survival between the ASCT and non-ASCT group in MCL patients. ASCT, autologous stem cell transplantation; MCL, mantle cell lymphoma.
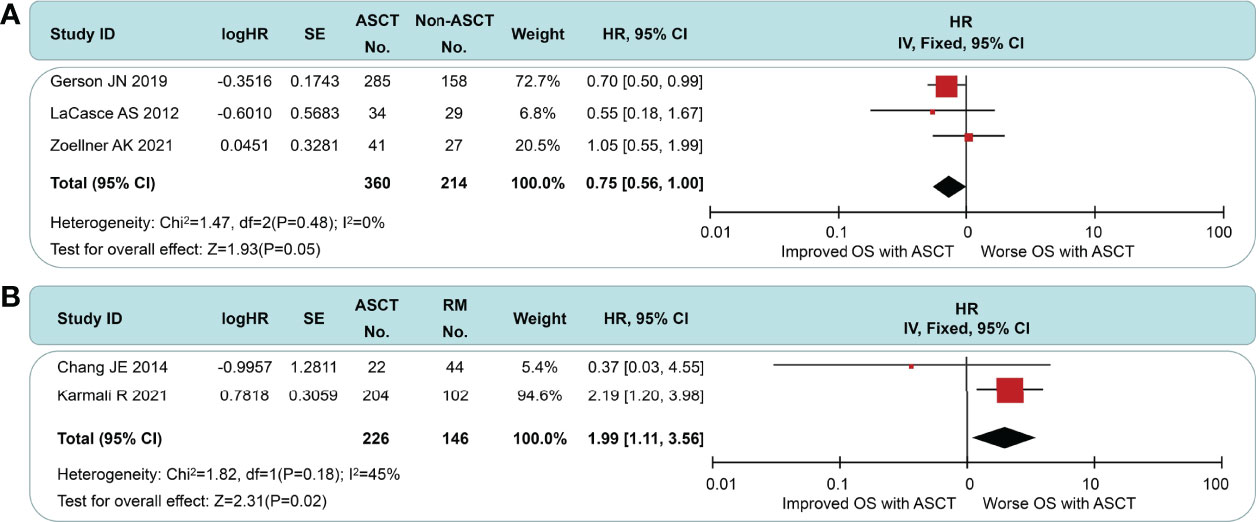
Figure 6 Meta-analysis results of overall survival: (A) Subgroup analysis for patients with non-intensive induction. (B) Survival analysis between patients undergoing autologous stem cell transplantation and rituximab.
Current first-line treatments were chemo-immunotherapy regimens containing rituximab and HiDAC followed by ASCT for young and fit MCL pts. However, the only evidence supporting ASCT is from the pre-rituximab and non-HiDAC era. Recent data of 14-year follow-up of this RCT showed a lack of significant PFS and OS benefit for pts who received rituximab plus ASCT (14). Due to the lack of new RCTs, whether ASCT is still the modality of choice in MCL becomes a matter of debate in the rituximab plus HiDAC era. To our knowledge, this was the first meta-analysis assessing the therapeutic value of early ASCT in newly diagnosed transplant-eligible MCL pts across studies worldwide.
We must emphasize three critical aspects of this study design. First, we excluded pts without rituximab induction. A real-world observational study from the Nordic Lymphoma Group proposed that rituximab can significantly improve the prognosis of MCL (15). More retrospective and prospective data have confirmed this view (19–21). To avoid the impact of the absence of rituximab on survival, we specified a limit for induction strategy. Second, we carried out subgroup analyses based on the intensity of induction regimens. Due to the small sample size, augmented intensity induction treatments are not restricted to HiDAC-containing regimens. So far, no prospective studies were using intensive induction to compare ASCT consolidation with other post-induction strategies. Last, we compared early ASCT consolidation with post-induction RM instead of ASCT. Previous studies looked toward RM after ASCT in young and fit pts or RM without ASCT in elder and unfit pts (19, 22, 23). There was no attempt to systematically analyze the possibility of ablating ASCT for transplant-eligible MCL in the front-line setting.
In this study, we observed that the consolidation efficacy of ASCT was related to the intensity of induction regimens. ASCT consolidation could provide a significant PFS benefit for pts with non-intensive therapy but an attenuated PFS benefit for pts using intensive induction regimens. For OS, ASCT had no benefit regardless of induction intensity. A likely explanation is that ASCT is a distinct form of high-intensity chemotherapy, which could delay disease progression to some extent but not improve long-term survival. Based on the study by Zoellner et al. (14), we know that relapse after ASCT is inevitable. Increased risk of long-term adverse events and secondary malignancies associated with ASCT were also concerns in clinical practice. A study by LaCasce et al. (13) showed that rates of febrile neutropenia (44%) and hospitalization caused by complications of therapy (75%) were numerically highest in the R-hyper-CVAD+ASCT therapy group compared to the non-ASCT group. In the study by Gerson et al. (16), at a median follow-up of 76.8 months, 2.5% (n = 16) of pts who underwent consolidative ASCT developed secondary myelodysplastic syndrome or acute myeloid leukemia, although no statistical differences were found when compared with those treated without ASCT (2.5% vs. 1.3%, p = 0.36). Despite a lack of sufficient comparable data, we still expect alternative ways with more promising efficacy and lower toxicity eagerly. Subgroup analysis showed both PFS and OS benefits in favor of RM than ASCT, similar to Vidal et al.’s conclusions (24). Hilal et al. (23) demonstrated that for MCL pts who responded to induction chemotherapy and underwent ASCT consolidation, RM therapy after transplantation improved PFS and OS. Despite some variability in pts’ characteristics, RM is beneficial in most trials. Kluin-Nelemans et al. (19) found that RM obtained benefits after R-CHOP but not after FCR. In the present analysis, we cannot explore the relationship between the effect of RM and induction regimens because of the small sample size.
The specific scheme of RM therapy may be another concern. In the trial by Chang et al. (5), RM was administered at 375 mg/m2 weekly × 4 every 6 months for 16 doses, to be initiated 4 to 8 weeks after completion of chemotherapy. In the trial by Tessoulin et al. (22), RM was administered as a single dose of 375 mg/m2/day every 2 months for 3 years. In one meta-analysis of 7 such studies, there are many other similar protocols for RM therapy. In short, regarding the optimal RM strategy, the study is inconclusive. There is still much work to be done in the future.
Besides RM, other maintenance strategies such as bortezomib, lenalidomide, and ibrutinib are under investigation as well (25–29). The comparison between studies 50403 and 59909 with long-term follow-up suggests a PFS benefit from the addition of bortezomib post-transplant (25). The SWOG S0601 trial of R-CHOP with concurrent and maintenance bortezomib suggested a PFS benefit in 65 non-transplant MCL pts (26). Another randomized phase III trial of transplant-ineligible MCL pts showed that VR-CAP improved PFS and OS than R-CHOP, whose benefits may be due to the use of bortezomib during induction or maintenance or both (27). The Italian Lymphoma Foundation recently showed that lenalidomide maintenance after ASCT can improve PFS in MCL pts (28). Triangle, an ongoing randomized phase III trial (EudraCT-no. 2014-001363-12), randomly allocated young, fit pts newly diagnosed with MCL into three arms: (i) an alternating R-CHOP/R-DHAP induction followed by myeloablative consolidation (arm A); (ii) ibrutinib is added to the R-CHOP cycles and applied as maintenance for 2 years (arm A+I); and (iii) the same induction and maintenance are applied, but high-dose consolidation and ASCT are skipped (arm I). As of July 30, 511 of up to 870 pts have been randomized from 12 different European countries (29). We look forward to the results of Triangle, especially for arm I. Long-term combined results of two trials implementing R-MACLO-IVAM induction followed by thalidomide or RM in 44 untreated MCL pts indicated that R-MACLO-IVAM followed by maintenance therapy is an effective regimen to induce long-term remission in MCL without the need for consolidation with ASCT (30). Accumulative evidence suggests that a paradigm shift is occurring in the treatment of newly diagnosed MCL pts in the era of the new drug, suggesting the possibility of using new drug maintenance as an alternative treatment. The optimal maintenance agent type, dose, and duration of MCL should be explored in future clinical investigations.
Minimal residual disease (MRD) has shown the prognosis value in MCL (21, 31). One meta-analysis indicated that MRD positivity after induction and consolidation treatments was associated with worse PFS and OS for MCL. Tan et al. (32) reported that MCL pts achieving MRD negativity after induction therapy with R-hyper-CVAD have excellent long-term outcomes and may reasonably avoid consolidative ASCT despite its small size. In the MRD analysis by Callanan et al. (33), RM provides longer PFS and OS regardless of MRD status pre- and post-ASCT in MCL pts who completed R-DHAP induction therapy. Sequential MRD monitoring during treatment offers strong potential for early clinical outcome prediction, as a surrogate clinical endpoint, and for MRD-guided, risk-adapted treatment in MCL.
Some limitations are worth mentioning. First, the number of included studies was too small to assess the potential publication bias. Second, only one RCT met the inclusion studies due to the low incidence of MCL. In the future, our conclusions still need to be verified by large-sample, multi-center, and more rigorously designed RCTs.
In the rituximab plus HiDAC era, the benefit of ASCT as a component of first-line treatment has been weakened. RM therapy may have a potential to be used as an alternative to conventional ASCT. These are, of course, speculative based on the current dataset; thus, our meta-analysis cannot provide firm advice on this matter. As more and more clinical trials are ongoing, the challenge in the treatment of MCL will be how to use the best treatment combinations to reach the following goals: low recurrence rate, fewer adverse events, and long-term survival.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
HL: Conceptualization, data curation, validation, writing—original draft, and visualization. XS: Methodology, data curation, and writing—original draft. HF: Software, investigation, data curation, and writing—original draft. LC: Resources, methodology, and writing—review and editing. YM: Methodology, validation, and writing—review and editing. XZ: Investigation, resources, and writing—review and editing. WW: Validation, visualization, and writing—review and editing. WX: Conceptualization, methodology, and writing—review and editing. JL: Conceptualization, writing—review and editing, and project administration. LF: Conceptualization, writing—review and editing, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (81720108002), the National Science and Technology Major Project (2018ZX09734-007), the Translational Research Grant of NCRCH (2020ZKZB01), and the CSCO Research Foundation (Y-Roche2019/2-0090).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.881346/full#supplementary-material
1. Epperla N, Hamadani M, Fenske TS, Costa LJ. Incidence and Survival Trends in Mantle Cell Lymphoma. Br J Haematol (2018) 181:703–6. doi: 10.1111/bjh.14699
2. Campo E, Rule S. Mantle Cell Lymphoma: Evolving Management Strategies. Blood (2015) 125:48–55. doi: 10.1182/blood-2014-05-521898
3. Cheah CY, Seymour JF, Wang ML. Mantle Cell Lymphoma. J Clin Oncol (2016) 34:1256–69. doi: 10.1200/JCO.2015.63.5904
4. Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early Consolidation by Myeloablative Radiochemotherapy Followed by Autologous Stem Cell Transplantation in First Remission Significantly Prolongs Progression-Free Survival in Mantle-Cell Lymphoma: Results of a Prospective Randomized Trial of the European MCL Network. Blood (2005) 105:2677–84. doi: 10.1182/blood-2004-10-3883
5. Chang JE, Li H, Smith MR, Gascoyne RD, Paietta EM, Yang DT, et al. Phase 2 Study of VcR-CVAD With Maintenance Rituximab for Untreated Mantle Cell Lymphoma: An Eastern Cooperative Oncology Group Study (E1405). Blood (2014) 123:1665–73. doi: 10.1182/blood-2013-08-523845
6. Widmer F, Balabanov S, Soldini D, Samaras P, Gerber B, Manz MG, et al. R-Hyper-CVAD Versus R-CHOP/cytarabine With High-Dose Therapy and Autologous Haematopoietic Stem Cell Support in Fit Patients With Mantle Cell Lymphoma: 20 Years of Single-Center Experience. Ann Hematol (2018) 97:277–87. doi: 10.1007/s00277-017-3180-x
7. Romaguera JE, Fayad LE, Feng L, Hartig K, Weaver P, Rodriguez MA, et al. Ten-Year Follow-Up After Intense Chemoimmunotherapy With Rituximab-Hyper CVAD Alternating With Rituximab-High Dose Methotrexate/Cytarabine (R-MA) and Without Stem Cell Transplantation in Patients With Untreated Aggressive Mantle Cell Lymphoma. Br J Haematol (2010) 150:200–8. doi: 10.1111/j.1365-2141.2010.08228.x
8. Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-Term Progression-Free Survival of Mantle Cell Lymphoma After Intensive Front-Line Immunochemotherapy With In Vivo-Purged Stem Cell Rescue: A Nonrandomized Phase 2 Multicenter Study by the Nordic Lymphoma Group. Blood (2008) 112:2687–93. doi: 10.1182/blood-2008-03-147025
9. Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of High-Dose Cytarabine to Immunochemotherapy Before Autologous Stem-Cell Transplantation in Patients Aged 65 Years or Younger With Mantle Cell Lymphoma (MCL Younger): A Randomised, Open-Label, Phase 3 Trial of the European Mantle Cell Lymphoma Network. Lancet (2016) 388:565–75. doi: 10.1016/S0140-6736(16)00739-X
10. Bernstein SH, Epner E, Unger JM, Leblanc M, Cebula E, Burack R, et al. A Phase II Multicenter Trial of hyperCVAD MTX/Ara-C and Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma; SWOG 0213. Ann Oncol (2013) 24:1587–93. doi: 10.1093/annonc/mdt070
11. Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High Rate of Durable Remissions After Treatment of Newly Diagnosed Aggressive Mantle-Cell Lymphoma With Rituximab Plus Hyper-CVAD Alternating With Rituximab Plus High-Dose Methotrexate and Cytarabine. J Clin Oncol (2005) 23:7013–23. doi: 10.1200/JCO.2005.01.1825
12. Bhella S, Varela NP, Aw A, Bredeson C, Cheung M, Crump M, et al. First-Line Therapy, Autologous Stem-Cell Transplantation, and Post-Transplantation Maintenance in the Management of Newly Diagnosed Mantle Cell Lymphoma. Curr Oncol (2020) 27:e632–44. doi: 10.3747/co.27.7053
13. LaCasce AS, Vandergrift JL, Rodriguez MA, Abel GA, Crosby AL, Czuczman MS, et al. Comparative Outcome of Initial Therapy for Younger Patients With Mantle Cell Lymphoma: An Analysis From the NCCN NHL Database. Blood (2012) 119:2093–9. doi: 10.1182/blood-2011-07-369629
14. Zoellner AK, Unterhalt M, Stilgenbauer S, Hübel K, Thieblemont C, Metzner B, et al. Long-Term Survival of Patients With Mantle Cell Lymphoma After Autologous Haematopoietic Stem-Cell Transplantation in First Remission: A Post-Hoc Analysis of an Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Haematol (2021) 8:e648–57. doi: 10.1016/S2352-3026(21)00195-2
15. Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D'Amore F, et al. Real World Data on Primary Treatment for Mantle Cell Lymphoma: A Nordic Lymphoma Group Observational Study. Blood (2014) 124:1288–95. doi: 10.1182/blood-2014-03-559930
16. Gerson JN, Handorf E, Villa D, Gerrie AS, Chapani P, Li S, et al. Survival Outcomes of Younger Patients With Mantle Cell Lymphoma Treated in the Rituximab Era. J Clin Oncol (2019) 37:471–80. doi: 10.1200/JCO.18.00690
17. Wang YH, Yu SC, Ko BS, Yang YT, Yao M, Tang JL, et al. Correlative Analysis of Overall Survival With Clinical Characteristics in 127 Patients With Mantle Cell Lymphoma: A Multi-Institutional Cohort in Taiwan. Int J Hematol (2020) 112:385–94. doi: 10.1007/s12185-020-02903-z
18. Karmali R, Switchenko JM, Goyal S, Shanmugasundaram K, Churnetski MC, Kolla B, et al. Multi-Center Analysis of Practice Patterns and Outcomes of Younger and Older Patients With Mantle Cell Lymphoma in the Rituximab Era. Am J Hematol (2021) 96:1374–84. doi: 10.1002/ajh.26306
19. Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Geisler CH, Trneny M, et al. Treatment of Older Patients With Mantle Cell Lymphoma (MCL): Long-Term Follow-Up of the Randomized European MCL Elderly Trial. J Clin Oncol (2020) 38:248–56. doi: 10.1200/JCO.19.01294
20. Graf SA, Stevenson PA, Holmberg LA, Till BG, Press OW, Chauncey TR, et al. Maintenance Rituximab After Autologous Stem Cell Transplantation in Patients With Mantle Cell Lymphoma. Ann Oncol (2015) 26:2323–8. doi: 10.1093/annonc/mdv364
21. Andersen NS, Pedersen LB, Laurell A, Elonen E, Kolstad A, Boesen AM, et al. Pre-Emptive Treatment With Rituximab of Molecular Relapse After Autologous Stem Cell Transplantation in Mantle Cell Lymphoma. J Clin Oncol (2009) 27:4365–70. doi: 10.1200/JCO.2008.21.3116
22. Tessoulin B, Chiron D, Thieblemont C, Oberic L, Bouadballah K, Gyan E, et al. Oxaliplatin Before Autologous Transplantation in Combination With High-Dose Cytarabine and Rituximab Provides Longer Disease Control Than Cisplatin or Carboplatin in Patients With Mantle-Cell Lymphoma: Results From the LyMA Prospective Trial. Bone Marrow Transplant (2021) 56:1700–9. doi: 10.1038/s41409-020-01198-2
23. Hilal T, Wang Z, Almader-Douglas D, Rosenthal A, Reeder CB, Jain T. Rituximab Maintenance Therapy for Mantle Cell Lymphoma: A Systematic Review and Meta-Analysis. Am J Hematol (2018) 93:1220–6. doi: 10.1002/ajh.25226
24. Vidal L, Gafter-Gvili A, Dreyling M, Ghielmini M, Witzens-Harig M, Shpilberg O, et al. Maintenance Treatment for Patients With Mantle Cell Lymphoma: A Systematic Review and Meta-Analysis of Randomized Trials. Hemasphere (2018) 2:e136. doi: 10.1097/HS9.0000000000000136
25. Kaplan LD, Maurer MJ, Stock W, Bartlett NL, Fulton N, Pettinger A, et al. Bortezomib Consolidation or Maintenance Following Immunochemotherapy and Autologous Stem Cell Transplantation for Mantle Cell Lymphoma: CALGB/Alliance 50403. Am J Hematol (2020) 95:583–93. doi: 10.1002/ajh.25783
26. Till BG, Li H, Bernstein SH, Fisher RI, Burack WR, Rimsza LM, et al. Phase II Trial of R-CHOP Plus Bortezomib Induction Therapy Followed by Bortezomib Maintenance for Newly Diagnosed Mantle Cell Lymphoma: SWOG S0601. Br J Haematol (2016) 172:208–18. doi: 10.1111/bjh.13818
27. Robak T, Jin J, Pylypenko H, Verhoef G, Siritanaratkul N, Drach J, et al. Frontline Bortezomib, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (VR-CAP) Versus Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Transplantation-Ineligible Patients With Newly Diagnosed Mantle Cell Lymphoma: Final Overall Survival Results of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2018) 19:1449–58. doi: 10.1016/S1470-2045(18)30685-5
28. Ladetto M, Cortelazzo S, Ferrero S, Evangelista A, Mian M, Tavarozzi R, et al. Lenalidomide Maintenance After Autologous Haematopoietic Stem-Cell Transplantation in Mantle Cell Lymphoma: Results of a Fondazione Italiana Linfomi (FIL) Multicentre, Randomised, Phase 3 Trial. Lancet Haematol (2021) 8:e34–44. doi: 10.1016/S2352-3026(20)30358-6
29. Dreyling M, Ladetto M, Doorduijn JK, Gine E, Jerkeman M, Mey U, et al. Triangle: Autologous Transplantation After a Rituximab/Ibrutinib/Ara-C Containing Induction in Generalized Mantle Cell Lymphoma - A Randomized European MCL Network Trial. Blood (2019) 134 Suppl1:2816. doi: 10.1182/blood-2019-127863
30. Alderuccio JP, Saul EE, Iyer SG, Reis IM, Alencar AJ, Rosenblatt JD, et al. R-MACLO-IVAM Regimen Followed by Maintenance Therapy Induces Durable Remissions in Untreated Mantle Cell Lymphoma - Long Term Follow Up Results. Am J Hamatol (2021) 96:680–9. doi: 10.1002/ajh.26163
31. Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Bottcher S, Asnafi V, et al. Molecular Remission is an Independent Predictor of Clinical Outcome in Patients With Mantle Cell Lymphoma After Combined Immunochemotherapy: A European MCL Intergroup Study. Blood (2010) 115:3215–23. doi: 10.1182/blood-2009-06-230250
32. Tan J, Ng M, Kipp D, Routledge D, Rose H, Ratnasingam S, et al. Adapted Approach in Mantle Cell Lymphoma (CT/PET and MRD Assessment) Enables CR Patients to Avoid ASCT Following Intensive Chemo-Immunotherapy. EHA Library (2020) 293685:EP1195.
33. Callanan MB, Macintyre E, Delfau-Larue MH, Thieblemont C, Oberic L, Gyan E, et al. Predictive Power of Early, Sequential MRD Monitoring in Peripheral Blood and Bone Marrow in Patients With Mantle Cell Lymphoma Following Autologous Stem Cell Transplantation With or Without Rituximab Maintenance; Final Results From the LyMa-MRD Project, Conducted on Behalf of the Lysa Group. Blood (2020) 136 Suppl1:12–3. doi: 10.1182/blood-2020-140457
Keywords: mantle cell lymphoma, autologous stem cell transplantation, rituximab, meta-analysis, treatment
Citation: Liu H, Shi X, Fang H, Cao L, Miao Y, Zhao X, Wu W, Xu W, Li J and Fan L (2022) First-Line Autologous Stem Cell Transplantation for Mantle Cell Lymphoma: A Systematic Analysis and Treatment Recommendation. Front. Oncol. 12:881346. doi: 10.3389/fonc.2022.881346
Received: 22 February 2022; Accepted: 11 April 2022;
Published: 11 May 2022.
Edited by:
Carlo Visco, University of Verona, ItalyReviewed by:
Monica Balzarotti, Humanitas Research Hospital, ItalyCopyright © 2022 Liu, Shi, Fang, Cao, Miao, Zhao, Wu, Xu, Li and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Fan, ZmFubGVpMzAxNEAxMjYuY29t; Jianyong Li, bGlqaWFueW9uZ2xtQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.