
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 22 June 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.878635
Giant cell tumor of tendon sheath (GCTTS) is a benign tumor. It occurs predominantly in the hands, ankles, and knees. A 39-year-old female presented with GCTTS in the right breast after breast augmentation. There was a clear borderline between the tumor and breast tissue. In terms of morphological appearance, synovial metaplasia could be observed in part of the collagenous capsule. The tumor was moderately cellular and was composed of synovium-like monocytes. The main part of the tumor was blended with nested and scattered xanthomatous cells, lymphocytes, and osteoclast-like giant cells. Hemosiderin granules were distributed in the lesion. Immunohistochemical staining and fluorescence in situ hybridization (FISH) analyses were performed. CD68 staining was positive in osteoclast-like giant cells. In addition, neither significant USP6 translocation nor CSF1 translocation was detected by FISH. We hypothesized that the pathogenesis of this rare GCT-TS was based on synovial metaplasia and did not depend on the translocation of classical CSF1.
Giant cell tumor of tendon sheath (GCTTS), also known as tenosynovial giant cell tumor, is a benign tumor. Similar to giant cell tumors of bone (GCTB), GCTTS presents with multinucleated osteoclast-like cells, frequent recurrences, rare metastases, and occurrence of bone erosion in advanced cases (1–3). Differently from GCTB, GCTTS arises from the synovium of joints, bursae, or tendon sheaths (4). Based on the growth pattern and clinical characteristics, GCTTS is classified into localized GCTTS and diffuse GCTTS (5). Although GCTTS is regarded as benign tumors, the recurrence rates of localized GCTTS range from 9 to 17%, and those of diffuse GCTTS range from 33 to 50% (6, 7). Moreover, distant metastasis was reported to occur in a few diffuse GCTTS (6).
Despite differences in the growth pattern between localized GCTTS and diffuse GCTTS, the translocation and the expression of CSF1, which is caused by the fusion of CSF1 and COL6A3, are crucial to tumorigenesis in both subtypes of GCTTS (8, 9). CSF1 secretion attracts macrophages that express CSF1R and may induce the formation of multinucleated giant cells (9).
Currently, surgery is the preferred treatment for GCTTS, and local excision with a negative margin of normal tissue is usually considered adequate therapy (10). In some aggressive cases, extensive resection and radiation therapy are needed (11). Targeted agents can block the CSF1/CSF1R pathway, which is an attractive therapeutic strategy for patients with symptomatic GCTTS (12). Currently, targeted agents that directly block CSF1/CSF1R are in clinical development (12). Moreover, GCTTS patients could benefit from CSF1R inhibitors, even patients with absent CSF1 translocation (13, 14).
In this report, we describe a case of GCTTS in breast with a history of prosthesis implantation and studied the relationship between tumorigenesis and the molecular changes involved in this rare tumor.
A 39-year-old female was admitted to the hospital with a palpable lump in the right breast for 3 months. A year earlier, the patient underwent bilateral breast augmentation. The physical examination revealed a rubbery, well-circumscribed, solitary mass in the 6 o’clock position of the right breast. An ultrasonography of the breast revealed a regular hypoechoic mass measuring 3.0 cm × 2.4 cm ×2.8 cm in size and 4 cm distant from the nipple (Figure 1). There were no palpable axillary nodes. Considering the risk of rupture of prosthesis by biopsy and the well-circumscribed boundary of the tumor, wide excision of the lesion was performed for diagnostic and therapeutic purposes. The timeline is shown in Figure 2.
The gross appearance presented as a well-circumscribed mass of 3 cm in diameter. The cut surface was grayish yellow and solitary. Histologically, there was a regular collagenous capsule around the mass. Most areas of the mass had a flat surface covered with finger-like projections or villi. These sheets of cells were composed of many rounded macrophage-like synovial cells and fibroblasts synovial cells and represented as synovial metaplasia (Figure 3A). Hemosiderin granules were distributed in the lesion (Figure 3B). The tumor was composed of round and polygonal synovium-like monocytes with abundant eosinophilic cytoplasm and rounded and reniform nuclei. The main part of tumor was blended with nested and scattered xanthomatous cells and osteoclast-like giant cells (Figure 3C). The osteoclast-like giant cells contained an abundant cytoplasm and many evenly distributed and usually centrally located oval nuclei, some of which contained small nucleoli. Only a few lymphocytes were scattered in the monocytes. Neither seepage of material from prosthesis nor from foreign refractile material was identified in this nodule. Epithelial cells in spindle cell elements were not observed. Cleft-like spaces, rare mitotic figures, and hyalinized stroma could be seen.
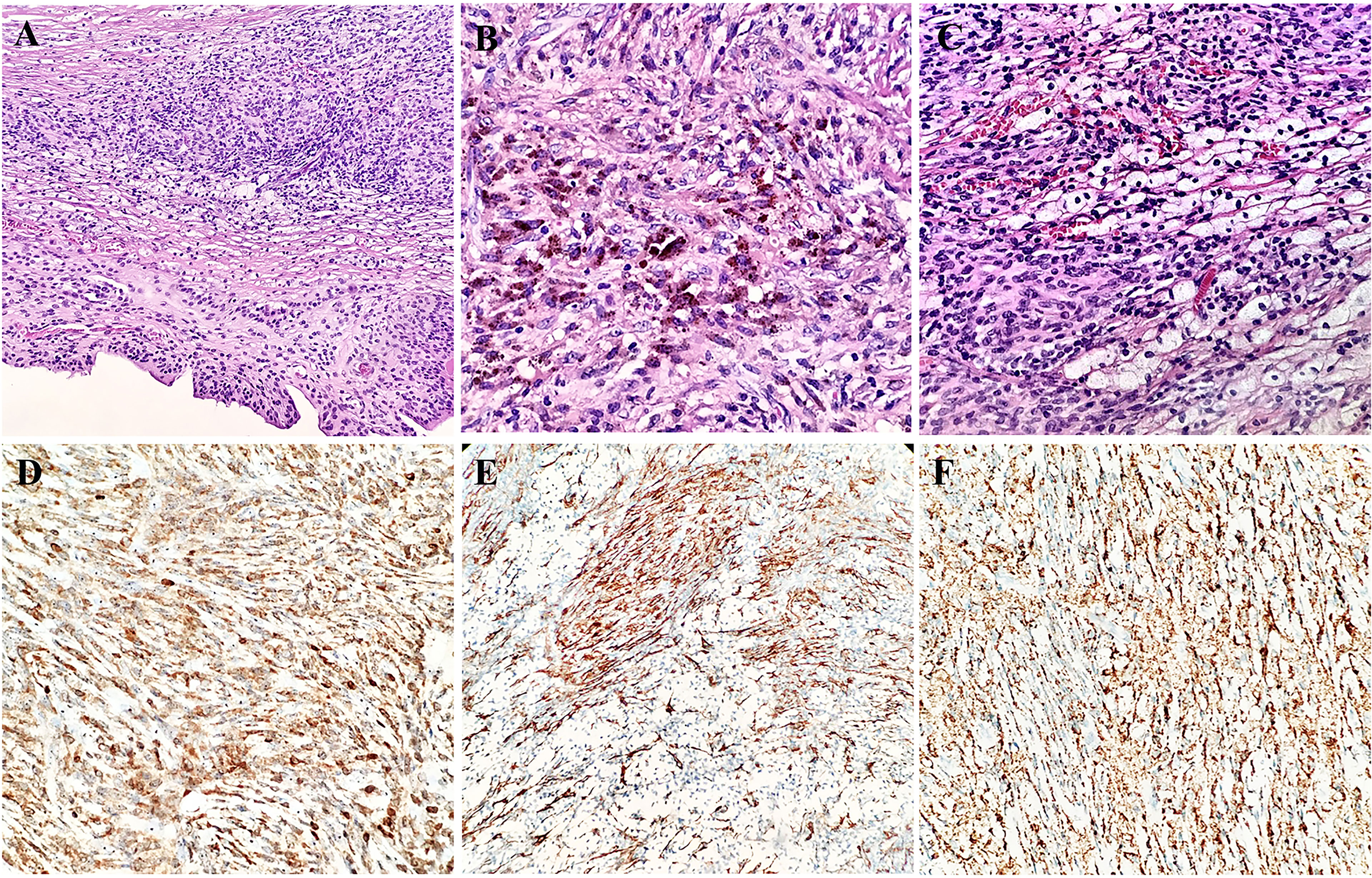
Figure 3 Morphology and immunohistochemistry features of the synovial metaplasia and giant cell tumor of tendon sheath (A). Hemosiderin granule deposition in the lesion (B). Nest-like distribution of xanthomatous cells among monocytes (C). Immunoreactivity for Bcl-2 (D), SMA (E), and CD68 (F) in synovium-like cells, mononuclear cells, and osteoclast-like giant cells.
The immunohistochemical analysis revealed that the synovium-like monocytes and xanthomatous cells were positive for Bcl-2 (Figure 3D), SMA (Figure 3E), CD68 (Figure 3F), and CD10. P63 was focally positive in mononuclear cells. Ki67 was positive in about 10%. The tumor cells were negative for desmin, CD34, S-100, EMA, PCK, TLE-1, CK5/6, HMB45, ALK, and CD30. In terms of fluorescence in situ hybridization (FISH) detection for the translocation of CSF1 gene and USP6 gene, the results were negative (Figure 4).
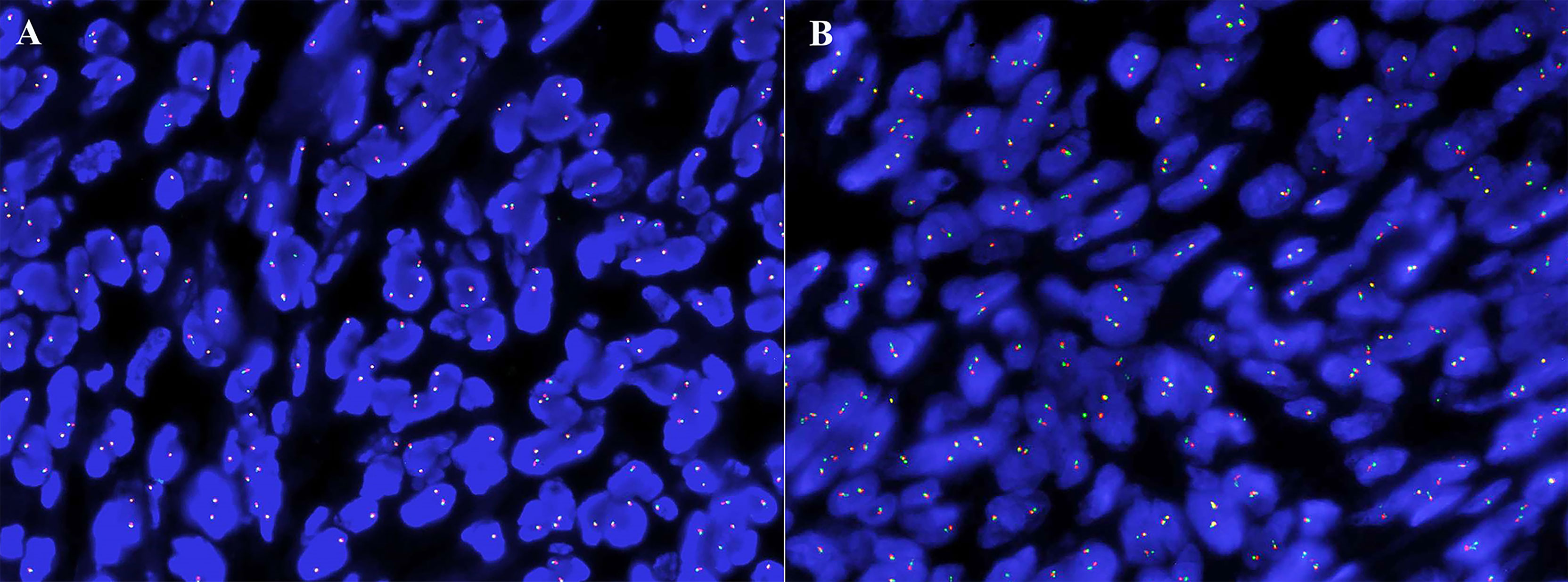
Figure 4 The absence of CSF1 (A) and USP6 (B) translocation in fluorescence in situ hybridization analysis.
A diagnosis of GCTTS was made based on morphological and immunohistochemical characteristics and the results of FISH. As of this writing, the patient is still alive, without local recurrence or distant metastasis detected at 15 months into follow-up.
Primary giant cell tumors in the breast are not common. Nine studies had reported primary giant cell tumors of soft tissue (GCT-ST) arising in the breast (15–23). One case occurred in an elderly male, and the other 8 cases occurred in females. The age of the patients ranged from 36 to 74 years old (the median age was 59 years). The tumor sizes ranged from 2.5 to 13 cm (the median size was 3 cm). The physical examinations or image examinations showed well-circumscribed masses in 7 cases (17–21) and irregular-shaped masses in 2 cases (22, 23). Of the 8 cases with follow-up results, 1 patient died of lung metastasis after the initial presentation, and in the other 7 patients, local recurrence or distant metastasis did not occur (18). In all cases of GCT-ST arising in the breast, the use of FISH to detect CSF1 translocation was not performed. GCTTS is rarer compared with GCT-ST. In our case, a 39-year-old female presented with a well-circumscribed 3-cm mass, and the patient is still alive without local recurrence or distant metastasis after 15 months of follow-up. There was no difference in the growth pattern or clinical characteristics between breast primary GCT-ST and GCTTS.
The differential diagnoses of GCTTS include metaplastic carcinoma (MC), carcinoma with osteoclast-like stromal giant cells (OGCs), phyllodes tumor (PT), breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) as well as soft tissue tumor such as GCT-ST, fibroma of tendon sheath (FTS), and synovial sarcoma (SS). The presence of ductal carcinoma in situ within the lesion and the evidence of epithelial differentiation could be observed by histopathological morphology and immunohistochemical results in both MC and carcinoma with OGCs. Although spindle stromal cells could be the main component in PT, the presence of leafy with epithelial-lined clefts and cellular fibroblasts could prompt the proper diagnosis. BIA-ALCL was characterized by a monotonous proliferation of malignant and large cells with positivity for CD30 and ALK. GCT-ST contains round/oval mononuclear cells and multinucleated osteoclast-like giant cells with similar nuclei. The stroma is vascularized. The tumor interstitium of GCT-ST contains calcification, interlaced metaplastic bone, and abundant blood vessels, which may lead to a blood lake with cystic hemorrhage. In some cases, mitoses ranged from 1 to 30/10 HPF. Additionally, the lesion in GCTTS shows more foamy-like histiocytes, Touton giant cells, and inflammatory cells mixed up with interstitial collagenization and accompanied by hemosiderin reaction or cholesterol crystallization than that of GCT-ST, and metaplastic bone is uncommon.
The most important and difficult thing is to differentiate GCTTS from cellular FTS and SS. Cellular FTS is composed of bland fibroblasts with elongated nuclei and dense eosinophilic collagen fibers. Slit-like vascular spaces are common in FTS. Compared with GCTTS, cellular FTS has less giant cells, xanthomatous cells, and hemosiderin. It is reported that USP6 translocation by FISH could serve as useful criteria for cellular FTS (24). Combining the histological characteristics and USP6 gene result, we excluded the diagnosis of cellular FTS. SS is a malignant soft tissue tumor most often found in the extremities of young adults. Spindle-shaped cells are arranged in herringbone and whorled patterns. Epithelial cells usually can be observed in spindle cell elements. Mitotic figures are visible. Most SS display immunoreactivity for cytokeratins, EMA, and TLE-1. The histological characteristics and immunohistochemical results helped us to exclude the diagnosis of SS. SS18 gene rearrangement by FISH could serve as useful criteria for SS, respectively, when the diagnosis is difficult (25).
Synovial metaplasia is a particular histological feature of this case. As previously reported, synovial metaplasia is one of the complications after breast augmentation (26). These metaplasia components have a similar morphology and transport capacity as with the synovial membrane (27). The mechanisms underlying the formation of synovial membranes might be a unique repair process (28). Some studies have described pigmented villonodular synovitis (PVNS) after knee arthroplasty (TKA), and the exact etiology of PVNS after TKA was considered relevant to polyethylene, trauma, and hemarthrosis (29, 30). In our case, GCTTS was diagnosed within 12 months after breast augmentation, which was similar to the case reported by Bunting et al. (29), supporting that operative procedure initiated the proliferative process. Combined with breast augmentation history, we concluded that the existence of synovial metaplasia of this patient was initiated by the proliferative repair process.
The pathogenesis of GCTTS in joint was thought to be related to the overexpression of CSF1, although it was first considered to be a reactive or hyperplastic process caused by inflammation (31). The translocation involved COL6A3 and CSF1, resulting in an overexpression of CSF1 (9). The secreted CSF1 fosters the accumulation of non-neoplastic mononuclear and multinucleated cells that form masses (8, 9). Detecting CSF1 translocation could assist in the diagnosis of GCTTS, and a total of 61–76% GCTTS displayed CSF1 translocations involving the 1p13 locus (8, 32). The CSF1 rearrangements in mononuclear cells create a landscaping effect in which most of the GCTTS contain non-neoplastic cells, which accumulate abnormally, and a smaller proportion of neoplastic cells (9). To explore the underlying molecular mechanism, CSF1 translocation was also assessed in this study. However, a significant CSF1 translocation was not detected in this rare case. Similarly, liposarcoma is the most common soft tissue sarcoma, but malignant PT and MC with heterologous liposarcoma are more common than primary liposarcoma in the breast (33). MDM2 amplification is a diagnostic criterion for liposarcoma in soft tissue (34), but at least 80% liposarcomatous differentiation in malignant PT and MC are unassociated with MDM2/CDK4 amplification (33). The studies showed that liposarcomatous differentiation within malignant PT and MC lacked the characteristic molecular alterations of soft tissue liposarcoma (35–37). In the same way, GCT-ST is a primary soft tissue neoplasm that is histologically similar to the GCT of bone (GCT-B) (38). It has been reported that >90% of GCT-Bs have a driver mutation in the H3F3A gene (39), but in a series of 15 GCT-ST, we found no H3F3A mutation in any case (38). According to the results, Lee et al. (38) believed that GCT-ST was probably genetically distinct from GCT-B. We hypothesized that, despite the histological similarity to GCTTS in arthrosis, the synovial metaplasia of capsules after implantation could cause GCTTS in the breast with the absence of CSF1 translocation. Despite the lack of CSF1 translocation, there may be other rearrangements leading to CSF1 upregulation in GCTTS. This CSF1/CSF1R signaling pathway needs to be further explored.
CSF1/CSF1R targeted therapy is an attractive therapeutic strategy for patients with symptomatic GCTTS associated with severe morbidity or functional limitations and not amenable to improvement with surgery (40). Pexidartinib is an orally available CSF1R inhibitor, which is the first systemic treatment approved by the US Food and Drug Administration in August 2019 (40). In the ENLIVEEN trial (NCT02371369), a randomized, double-blind phase III clinical trial showed that pexidartinib could improve the symptoms and function outcomes of patients with symptomatic GCTTS (41). The overall response of the pexidartinib group was higher than the placebo group at week 25 ENLIVEEN, and pexidartinib also significantly increased the relative range of motion and significantly improved the physical outcomes (41). ENLIVEEN showed that pexidartinib could induce serious and potentially fatal liver injury; mixed or cholestatic hepatotoxicity was an identified risk (41). A prospective observational study (NCT02948088) involving GCTTS patients receiving pexidartinib is now in progress (42). Moreover, several CSF1R inhibitors have been reported to act against GCTTS, including nilotinib, imatinib, and emactuzumab, and have shown encouraging results (43–45).
We presented a case of GCTTS in the breast after augmentation and concluded that the pathogenesis of this tumor based on synovial metaplasia was independent of classical CSF1 translocation. It was reported that a lower recurrence of GCTTS could be achieved by complete excision (46). Moreover, GCTTS should be included in the differential diagnosis of the lesion after breast augmentation.
The main limitation of this study is that only one patient was included.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
ZZ and HB conceived and reviewed this case. YZ and YF collected the case details, wrote the article, and contributed equally to this manuscript. HZ provided and reviewed the cases. MC and JY were involved in providing technical support for molecular pathology. All authors contributed to the article and approved the submitted version.
This research received funding from the Sichuan Science and Technology Program (no. 2022YFS0376). The funder subsidized the publishing expenses of this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GCTTS, giant cell tumor of tendon sheath; FISH, fluorescence in situ hybridization; GCT-ST, giant cell tumors of soft tissue; MC, metaplastic carcinoma; OGCs, osteoclast-like stromal giant cells; PT, phyllodes tumor; FTS, fibroma of tendon sheath; SS, synovial sarcoma; DCIS, ductal carcinoma in situ; PVNS, pigmented villonodular synovitis.
1. Zheng S, Lee PY, Huang Y, Wang A, Li T. Giant Cell Tumor of Tendon Sheath and Tendinopathy as Early Features of Early Onset Sarcoidosis. Front Pediatr (2019) 7:480. doi: 10.3389/fped.2019.00480
2. De Vita A, Vanni S, Miserocchi G, Fausti V, Pieri F, Spadazzi C, et al. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines. (2022) 10(2):372. doi: 10.3390/biomedicines10020372
3. Anazawa U, Hanaoka H, Shiraishi T, Morioka H, Morii T, Toyama Y. Similarities Between Giant Cell Tumor of Bone, Giant Cell Tumor of Tendon Sheath, and Pigmented Villonodular Synovitis Concerning Ultrastructural Cytochemical Features of Multinucleated Giant Cells and Mononuclear Stromal Cells. Ultrastruct Pathol (2006) 30(3):151–8. doi: 10.1080/01913120600689707
4. Llauger J, Palmer J, Rosón N, Cremades R, Bagué S. Pigmented Villonodular Synovitis and Giant Cell Tumors of the Tendon Sheath: Radiologic and Pathologic Features. AJR Am J Roentgenol. (1999) 172:1087–91. doi: 10.2214/ajr.172.4.10587152
5. Rubin BP. Tenosynovial Giant Cell Tumor and Pigmented Villonodular Synovitis: A Proposal for Unification of These Clinically Distinct But Histologically and Genetically Identical Lesions. Skeletal Radiol (2007) 36:267–8. doi: 10.1007/s00256-006-0249-3
6. Somerhausen NS, Fletcher CD. Diffuse-Type Giant Cell Tumor: Clinicopathologic and Immunohistochemical Analysis of 50 Cases With Extraarticular Disease. Am J Surg Pathol (2000) 24:479–92. doi: 10.1097/00000478-200004000-00002
7. Bedir R, Balik MS, Sehitoglu I, Güçer H, Yurdakul C. Giant Cell Tumour of the Tendon Sheath: Analysis of 35 Cases and Their Ki-67 Proliferation Indexes. J Clin Diagn Res (2014) 8:FC12–5. doi: 10.7860/JCDR/2014/10553.5311
8. Cupp JS, Miller MA, Montgomery KD, Nielsen TO, O'Connell JX, Huntsman D, et al. Translocation and Expression of CSF1 in Pigmented Villonodular Synovitis, Tenosynovial Giant Cell Tumor, Rheumatoid Arthritis and Other Reactive Synovitides. Am J Surg Pathol (2007) 31:970–6. doi: 10.1097/PAS.0b013e31802b86f8
9. West RB, Rubin BP, Miller MA, Subramanian S, Kaygusuz G, Montgomery K, et al. A Landscape Effect in Tenosynovial Giant-Cell Tumor From Activation of CSF1 Expression by a Translocation in a Minority of Tumor Cells. Proc Natl Acad Sci U S A. (2006) 103:690–5. doi: 10.1073/pnas.0507321103
10. Granowitz SP, D'Antonio J, Mankin HL. The Pathogenesis and Long-Term End Results of Pigmented Villonodular Synovitis. Clin Orthop Relat Res (1976) 114:335–51.
11. Ravi V, Wang WL, Lewis VO. Treatment of Tenosynovial Giant Cell Tumor and Pigmented Villonodular Synovitis. Curr Opin Oncol (2011) 23:361–6. doi: 10.1097/CCO.0b013e328347e1e3
12. Benner B, Good L, Quiroga D, Schultz TE, Kassem M, Carson WE, et al. Pexidartinib, A Novel Small Molecule CSF-1r Inhibitor in Use for Tenosynovial Giant Cell Tumor: A Systematic Review of Pre-Clinical and Clinical Development. Drug Des Devel Ther (2020) 14:1693–704. doi: 10.2147/DDDT.S253232
13. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting Tumor-Associated Macrophages With Anti-CSF-1R Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell (2014) 25:846–59. doi: 10.1016/j.ccr.2014.05.016
14. Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, et al. SU11248 Inhibits Tumor Growth and CSF-1R-Dependent Osteolysis in an Experimental Breast Cancer Bone Metastasis Model. Clin Exp Metastasis. (2003) 20:757–66. doi: 10.1023/b:clin.0000006873.65590.68
15. Lucas JG, Sharma HM, O'Toole RV. Unusual Giant Cell Tumor Arising in a Male Breast. Hum Pathol (1981) 12:840–4. doi: 10.1016/s0046-8177(81)80088-3
16. Fukunaga M. Giant Cell Tumor of the Breast. Virchows Arch (2002) 441:93–5. doi: 10.1007/s00428-002-0630-0
17. Shousha S, Sinnett HD. Chest Wall Tumors Presenting as Breast Lumps. Breast J (2004) 10:150–3. doi: 10.1111/j.1075-122x.2004.21383.x
18. May SA, Deavers MT, Resetkova E, Johnson D, Albarracin CT. Giant Cell Tumor of Soft Tissue Arising in Breast. Ann Diagn Pathol (2007) 11:345–9. doi: 10.1016/j.anndiagpath.2006.03.013
19. Romics L Jr, Mallon EA, Reid R, Cordiner CM, Doughty JC. Osteoclast-Like Giant Cell Tumor Arising in the Soft Tissue of the Breast: Report of a Case. Surg Today (2009) 39:48–51. doi: 10.1007/s00595-008-3774-y
20. Gaspar BL, Sharma S, Singh R, Vasishta RK. Primary Giant Cell Tumor of the Female Breast: A Diagnostic Red Herring With Therapeutic Implications. APMIS. (2017) 125:32–7. doi: 10.1111/apm.12634
21. Sawa A, Ikeda T, Ichioka E, Tsushima Y, Iguchi-Manaka A, Bando H, et al. Preoperative Diagnosis of a Giant Cell Tumor of Soft Tissue Arising From the Breast by Ultrasound-Guided Core Needle Biopsy. J Med Ultrason (20192001) . 46:257–61. doi: 10.1007/s10396-018-0891-0
22. Terada M, Gondo N, Sawaki M, Hattori M, Yoshimura A, Kotani H, et al. A Case of Giant Cell Tumor of the Breast, Clinically Suspected as Malignant Breast Tumor. Surg Case Rep (2019) 5:77. doi: 10.1186/s40792-019-0635-4
23. Luangxay T, Osako T, Yonekura R, Sugiura Y, Kikuchi M, Gomi N, et al. Giant Cell Tumor of Soft Tissue of the Breast: Case Report With H3F3A Mutation Analysis and Review of the Literature. Pathol Res Pract (2020) 216:152750. doi: 10.1016/j.prp.2019.152750
24. Carter JM, Wang X, Dong J, Westendorf J, Chou MM, Oliveira AM. USP6 Genetic Rearrangements in Cellular Fibroma of Tendon Sheath. Mod Pathol (2016) 29:865–9. doi: 10.1038/modpathol.2016.83
25. Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX Gene Fusion as a Determinant of Morphology and Prognosis in Synovial Sarcoma. N Engl J Med (1998) 338:153–60. doi: 10.1056/NEJM199801153380303
26. Ko CY, Ahn CY, Ko J, Chopra W, Shaw WW. Capsular Synovial Metaplasia as a Common Response to Both Textured and Smooth Implants. Plast Reconstr Surg (1996) 97:1427–33. doi: 10.1097/00006534-199606000-00017
27. Emery J, Hardt N, Caffee H. Breast Implant Capsules Share Synovial Transporting Capabilities. In: Laboratory Investigation, Baltimore: Williams & Wilkins (1994). 15:21201–2436.
28. Fowler MR, Nathan CO, Abreo F. Synovial Metaplasia, a Specialized Form of Repair. Arch Pathol Lab Med (2002) 126:727–30. doi: 10.5858/2002-126-0727-SMASFO
29. Bunting D, Kampa R, Pattison R. An Unusual Case of Pigmented Villonodular Synovitis After Total Knee Arthroplasty. J Arthroplasty. (2007) 22:1229–31. doi: 10.1016/j.arth.2006.11.022
30. Kia C, O'Brien DF, Ziegler C, Pacheco R, Forouhar F, Williams V. An Unusual Case of Pigmented Villonodular Synovitis After Total Knee Arthroplasty Presenting With Recurrent Hemarthrosis. Arthroplast Today (2018) 4:426–30. doi: 10.1016/j.artd.2018.06.006
31. Cavaliere A, Sidoni A, Bucciarelli E. Giant Cell Tumor of Tendon Sheath: Immunohistochemical Study of 20 Cases. Tumori. (1997) 83(5):841–6.
32. Mastboom MJL, Hoek DM, Bovée JVMG, van de Sande MAJ, Szuhai K. Does CSF1 Overexpression or Rearrangement Influence Biological Behaviour in Tenosynovial Giant Cell Tumours of the Knee? Histopathology (2019) 74:332–40. doi: 10.1111/his.13744
33. Briski LM, Jorns JM. Primary Breast Atypical Lipomatous Tumor/ Well-Differentiated Liposarcoma and Dedifferentiated Liposarcoma. Arch Pathol Lab Med (2018) 142:268–74. doi: 10.5858/arpa.2016-0380-RSR2
34. De Vita A, Mercatali L, Recine F, Pieri F, Riva N, Bongiovanni A, et al. Current Classification, Treatment Options, and New Perspectives in the Management of Adipocytic Sarcomas. Onco Targets Ther (2016) 9:6233–46. doi: 10.2147/OTT.S112580
35. Lyle PL, Bridge JA, Simpson JF, Cates JM, Sanders ME. Liposarcomatous Differentiation in Malignant Phyllodes Tumours is Unassociated With MDM2 or CDK4 Amplification. Histopathology (2016) 68:1040–5. doi: 10.1111/his.12898
36. Bacchi CE, Wludarski SC, Lamovec J, Ben Dor D, Ober E, Salviato T, et al. Lipophyllodes of the Breast. A Reappraisal of Fat-Rich Tumors of the Breast Based on 22 Cases Integrated by Immunohistochemical Study, Molecular Pathology Insights, and Clinical Follow-Up. Ann Diagn Pathol (2016) 21:1–6. doi: 10.1016/j.anndiagpath.2015.12.001
37. Ross JS, Badve S, Wang K, Sheehan CE, Boguniewicz AB, Otto GA, et al. Genomic Profiling of Advanced-Stage, Metaplastic Breast Carcinoma by Next-Generation Sequencing Reveals Frequent, Targetable Genomic Abnormalities and Potential New Treatment Options. Arch Pathol Lab Med (2015) 139:642–9. doi: 10.5858/arpa.2014-0200-OA
38. Lee JC, Liang CW, Fletcher CD. Giant Cell Tumor of Soft Tissue is Genetically Distinct From Its Bone Counterpart. Mod Pathol (2017) 30:728–33. doi: 10.1038/modpathol.2016.236
39. Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P, et al. Distinct H3F3A and H3F3B Driver Mutations Define Chondroblastoma and Giant Cell Tumor of Bone. Nat Genet (2013) 45:1479–82. doi: 10.1038/ng.2814
40. Food and Drug Administration. FDA Approves First Therapy for Rare Joint Tumor. (2019). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-rare-joint-tumor
41. Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, et al. Pexidartinib Versus Placebo for Advanced Tenosynovial Giant Cell Tumour (ENLIVEN): A Randomised Phase 3 Trial. Lancet. (2019) 394:478–87. doi: 10.1016/S0140-6736(19)30764-0
42. Mastboom M PE, Stacchiotti S. First Prospective Observational Study in Diffuse-Type Tenosynovial Giant Cell Tumors. J Clin Oncol (2018) 36:11560.
43. Gelderblom H, Cropet C, Chevreau C, Boyle R, Tattersall M, Stacchiotti S, et al. Nilotinib in Locally Advanced Pigmented Villonodular Synovitis: A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol (2018) 19:639–48. doi: 10.1016/S1470-2045(18)30143-8
44. Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, et al. Efficacy of Imatinib Mesylate for the Treatment of Locally Advanced and/or Metastatic Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis. Cancer (2012) 118:1649–55. doi: 10.1002/cncr.26409
45. Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R Inhibition With Emactuzumab in Locally Advanced Diffuse-Type Tenosynovial Giant Cell Tumours of the Soft Tissue: A Dose-Escalation and Dose-Expansion Phase 1 Study. Lancet Oncol (2015) 16:949–56. doi: 10.1016/S1470-2045(15)00132-1
Keywords: breast, giant cell tumor of tendon sheath, breast augmentation, synovial metaplasia, CSF1
Citation: Zhang Y, Fan Y, Zhang H, Bu H, Chen M, Yang J and Zhang Z (2022) Case Report: Giant Cell Tumor of Tendon Sheath After Breast Augmentation. Front. Oncol. 12:878635. doi: 10.3389/fonc.2022.878635
Received: 18 February 2022; Accepted: 20 May 2022;
Published: 22 June 2022.
Edited by:
Cristian Scatena, University of Pisa, ItalyReviewed by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyCopyright © 2022 Zhang, Fan, Zhang, Bu, Chen, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Zhang, emhhbmd6aGFuZzcxNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.