- 1Wuxi Medical College, Jiangnan University, Wuxi, China
- 2Department of Urology, Affiliated Hospital of Jiangnan University, Wuxi, China
- 3Wuxi School of Medicine, Jiangnan University, Wuxi, China
- 4School of Food Science and Technology, Jiangnan University, Wuxi, China
In our previous studies, we found that the rs231775 polymorphism of cytotoxic T-lymphocyte antigen 4 (CTLA-4) is associated with risks of different cancer types; however, the association remains controversial and ambiguous, so we conducted an in-depth meta-analysis to verify the association. A complete search of the PubMed, Google Scholar, Embase, Chinese databases, and Web of Science was conducted without regard to language limitations, covering all publications since November 20, 2021. The search criteria for cancer susceptibility associated with the polymorphism in the CTLA-4 gene rs231775 resulted in 87 case-control studies with 29,464 cases and 35,858 controls. The association strength was analyzed using odds ratios and 95% confidence intervals. Overall, we found that the CTLA-4 rs231775 polymorphism may reduce cancer risk. A stratified cancer type analysis showed that CTLA-4 rs231775 polymorphism was a risk factor for colorectal cancer and thyroid cancer; on the other hand, it was a protective factor for breast cancer, liver cancer, cervical cancer, bone cancer, head and neck, and pancreatic cancer. We also classified cancer into five systems and observed an increased association with digestive tract cancer, decreased associations with orthopedic tumors, tumors of the urinary system, and gynecological tumors. In the subgroup based on race, decreased relationships were observed in both Asians and Caucasians. The same decreased association was also shown in the analysis of the source of control analysis. Our present study indicates that the CTLA-4 rs231775 polymorphism contributes to cancer development and aggression.
Introduction
A major obstacle to increasing life expectancy is cancer, which is the primary cause of death worldwide. Cancer, in 112 of 183 countries, is also estimated to be the first or second leading cause of death before the age of 70 and third or fourth in 23 other countries (1), according to the World Health Organization analyses in 2019 (2). Across the globe, the incidence and mortality of cancer are rising rapidly; this is a result of both increasing longevity and population growth as well as changing patterns in the prevalence and distribution of cancer-causing factors, some of which are associated with social and economic development (1). The development of cancer involves multiple factors, including environmental and genetic factors (3).
One of the most common types of germline variants, the SNPs (single nucleotide polymorphisms), play a key role in human diseases, including cancer (2). Many SNPs associated with human cancer were identified through GWAS (genome-wide association studies) in the past decade (4, 5). Recent studies have noted that the expression levels of nearby genes may be influenced by these cancer risk-associated SNPs (4). Cancer treatment includes traditional surgery, chemotherapy, radiotherapy, and so on. In recent years, immunotherapy has gained more attention (6). The CTLA-4 (cytotoxic T-lymphocyte antigen 4) gene is located on chromosome 2q33 and has four exons (7). Cancer cells can acquire immune regulatory surface proteins like CTLA-4, which suppress the activation of immune cells, such as T cells (3, 8). In the early stages of tumorigenesis, it is possible that CTLA-4 may elevate the threshold of activation of T-cells as it inhibits T cell activation and proliferation. Furthermore, the CTLA-4 competitive binding to B7.1 inhibits IL-2 production and proliferation, both of which are essential in down-regulating T cell activity; in turn, this reduces anti-tumor responses and increases cancer susceptibility (5). Several SNPs in the CTLA-4 gene have been widely reported in tumors and non-tumors, such as rs4553808A/G, rs3087243G/A, rs5742909C/T, rs231726A/a, rs17268364, and rs231775A/G (9–13). The Rs231775 (+49) A/G polymorphism is one of the common SNPs in the CTLA-4 gene (4) and has been extensively reported in many types of cancers. Pavkovic et al. first reported a functional SNP in the CTLA-4 gene (rs231775), indicating that the G-allele frequency was highest among chronic lymphocytic leukemia patients who had developed autoimmune hemolytic anemia (14). Since then, the associations among rs231775 polymorphism and other types of cancer have been reported. In addition, Gouda et al. reported that the genotype (GG) was associated with relatively lower CTLA-4 expression levels than the other genotypes (like GC or CC) (11). To evaluate the effects of the functional SNP and cancer susceptibility, we carried out genotyping analyses among rs231775 A/G in 29,464 cases and 35,858 controls. Here, it would be helpful to explain the role of CTLA-4 in immune response control subsequent to completing its function. This is followed by how the polymorphism affects the function as to whether it increases or decreases the affinity of CTLA-4 to its ligand. The variability in the effect of the polymorphism on susceptibility to cancer warrants more in-depth discussions. Finally, we try to find a few potential explanations, which would add value in this regard.
Materials and Methods
Identifying and Evaluating Appropriate Studies
Searches were performed on the Embase, PubMed, Chinese database, Google Scholar, and Web of Science last updated November 20, 2021, using a keyword search that included ‘polymorphism’ or ‘carcinoma’ or ‘CTLA-4’ or ‘cytotoxic T-lymphocyte antigen 4’, or ‘variant’ and ‘cancer’ or ‘tumor’, regardless of language or publication year. These terms led to the retrieval of 592 articles, of which 87 matched the criteria for inclusion. Additionally, we manually searched references of the retrieved or review articles.
Criteria for Inclusion and Exclusion
The following criteria were required to be included in the review: (a) measured cancer risk in relation to CTLA-4 rs231775 polymorphism; (b) case-control studies; and (c) cases and controls have sufficient genotype numbers. Therefore, we also used the following exclusion criteria: (a) no population was used as control, (b) genotype frequency was not available, and (c) previous publications were duplicated.
Extraction of Data
Using the selection criteria, the data were extracted independently by two authors. The following data were collected: last name of the first author, publication year, ethnicity, country of origin, cancer type, the total number of cases and controls, source of controls, Hardy-Weinberg equilibrium (HWE) of controls, and genotyping methods.
Statistical Analysis
The first step was to stratify the subgroups based on cancer type. When a cancer type was reported in only one study, it is classified under the ‘others’ subgroup. In addition, we classified cancer into five systems: digestive tract cancer, orthopedic tumor, tumor of the urinary system, gynecological tumor, and hematological tumor. The ethnicity of the participants was categorized as Asian, Caucasian, and African using two different modes of classification, wherein the source of the control subgroup was analyzed: hospital-based (HB) and population-based (PB). On the basis of genotype frequencies in cases and controls, we calculated OR (odds ratios) with 95% CI (confidence intervals) of the association between CTLA-4 rs231775 polymorphism and the risk for cancer. The overall OR was analyzed using the Z-test (15). Heterogeneity was assessed using chi-square-based Q-tests. The Q-test showed no evidence of heterogeneity among the studies with a P-value greater than 0.05. We used the random-effects model when significant heterogeneity was detected (16); otherwise, the fixed-effects model was applied (16, 17). Using allelic contrast (G-allele vs. A-allele), homozygote comparison (GG vs. AA), dominant genetic model (GG+GA vs. AA), heterozygote comparison (GA vs. AA), and recessive genetic model (GG vs. GA+AA), we investigated the relationship between CTLA-4 rs231775 genetic variants and cancer risk. The Pearson chi-square test was used to calculate HWE in controls at P< 0.05. To estimate the likelihood of publication bias, Egger’s regression test and Begg’s funnel plots were used (18). All statistical assessments for this meta-analysis were conducted using Stata software V 11.0 (StataCorp LP, College Station, TX). We calculated the power and sample size of our meta-analysis using PS: Power and Sample Size Calculation (http://www.powerandsamplesize.com/) (19).
Meta-Regression
The source of publication bias was defined based on a random-effect meta-regression analysis using the publication bias, with publication year as subgroups, ethnicity, source of control, and methods of genotype set as independent variables and the log values regarded as dependent variables (20).
Bioinformatics Analysis
The expression of CTLA-4 between most types of tumors and para-cancerous tissue is shown from the GEPIA website (http://gepia.cancer-pku.cn/). On the same above-mentioned website, you can also find data about CTLA-4 expression levels in each tumor, which includes overall survival and disease-free survival.
Results
Meta-Analysis Study Selection and Characteristics
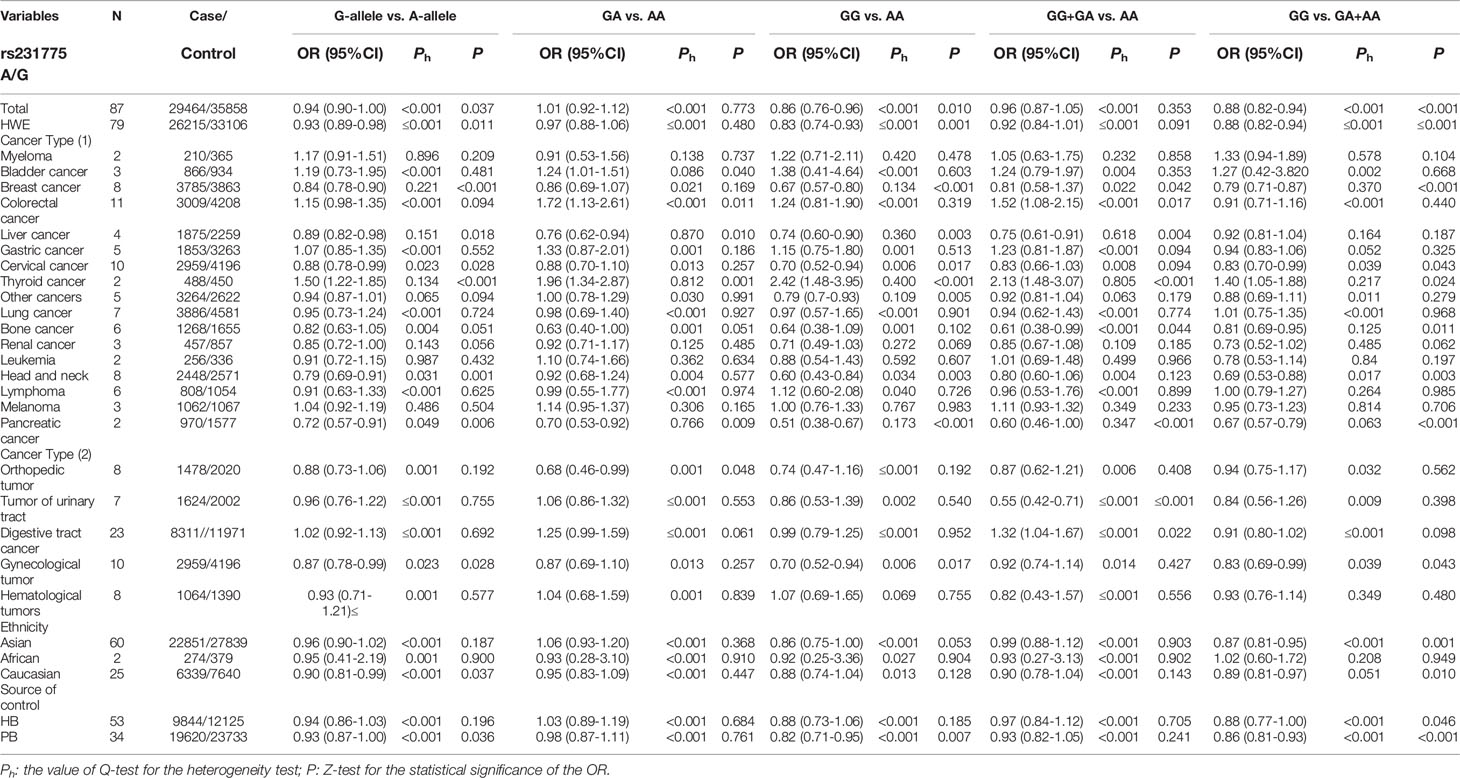
Throughout different databases, 592 articles were identified, and after a meticulous review, we included 87 varying case-control studies for this study (Figure 1). All essential information about included studies is shown in Table 1. Table 1 provides information on the first author, ethnicity, year of publication, cancer type, the numbers of controls and cases, genotyping methods and HWE, and control sources. According to the whole cancer susceptibility search criteria associated with the CTLA-4 rs231775 polymorphism, 87 case-control studies with 35,858 controls and 29,464 cases were retrieved. The controls mainly consisted of healthy populations. Therefore, we have compiled 25 Caucasian, 60 Asian, and 2 African case-control studies for our analyses. The controls in 53 studies came from the source of HB and 34 of PB. We examined the MAF (minor allele frequency) reported for the six major populations globally in the 1000 Genomes Browser (https://www.ncbi.nlm.nih.gov/snp/rs231775) (Figure 2A). Moreover, Asians exhibited significantly higher G-allele frequencies than Caucasian individuals both in cases (59.63% vs. 38.19%, P < 0.001) and controls (62.18% vs. 40.36%, P < 0.001) (Figure 2B). Third, we used the TCGA (The Cancer Genome Atlas) database to search for trends in the frequency of rs231775 polymorphism; our results indicated that the frequency of AA was relatively high compared to other genotypes, as shown in Figure 2C. The polymorphism is associated with prostate, artery, adipose-visceral, heart, nerve, pituitary, testis, and esophagus cancer (https://www.gtexportal.org/home/) (Figure 2D). All the controls except for eight studies were genotyped according to HWE. There is significantly more expression of CTLA-4 in tumor tissues than in normal tissue from four kinds of tumors (melanoma of the skin, head and neck squamous cell carcinoma, lymphoid neoplasm diffuse large B-cell lymphoma, pancreatic adenocarcinoma, P< 0.05, Figures 3A, B). Furthermore, CTLA-4 high expression contributes to a poor overall survival rate in patients with head and neck squamous cell carcinoma (P< 0.01) (Figure 3C).
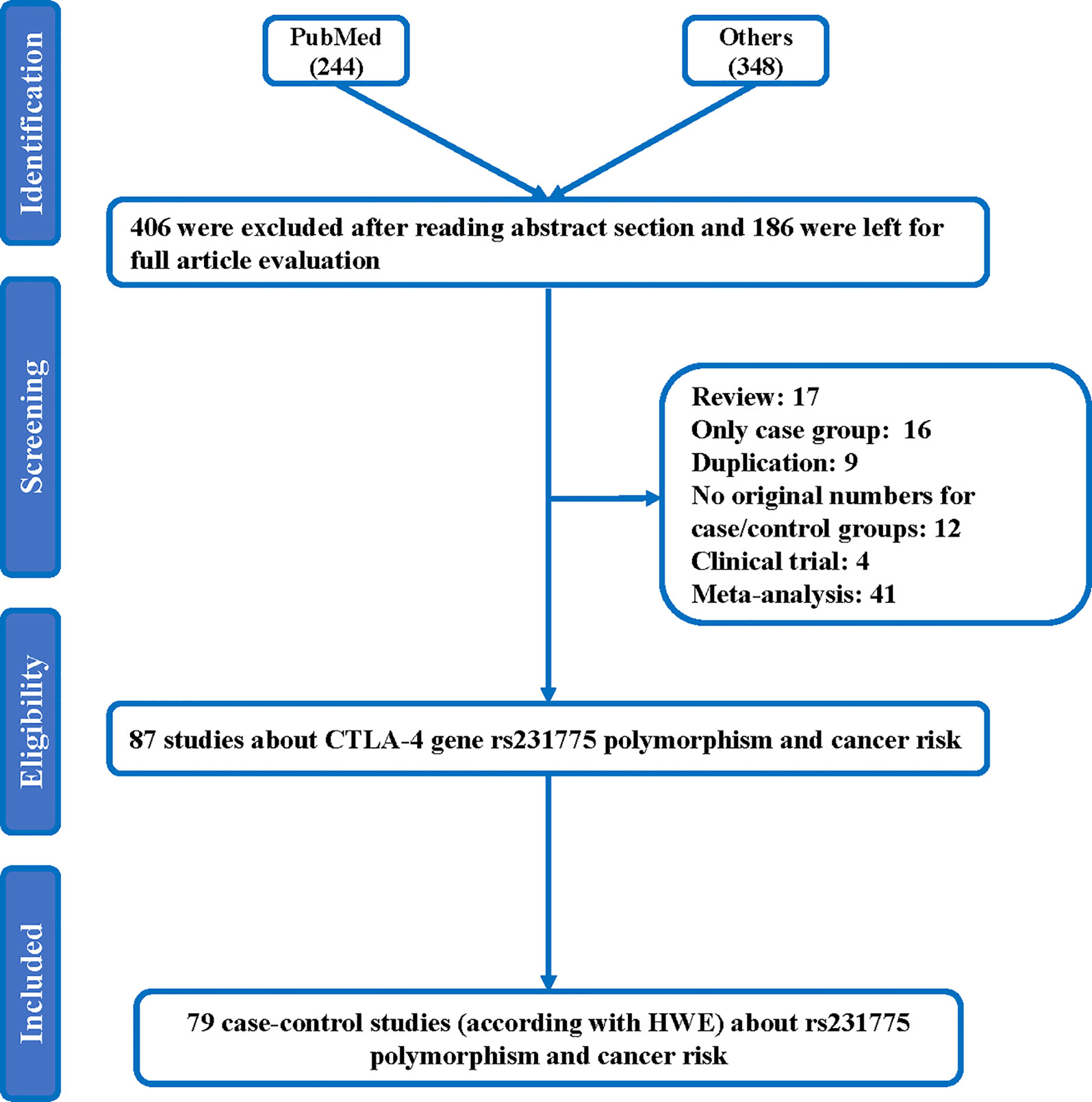
Figure 1 Flowchart illustrating the search strategy used to identify association studies for CTLA-4 rs231775 polymorphism and the total cancer risk.
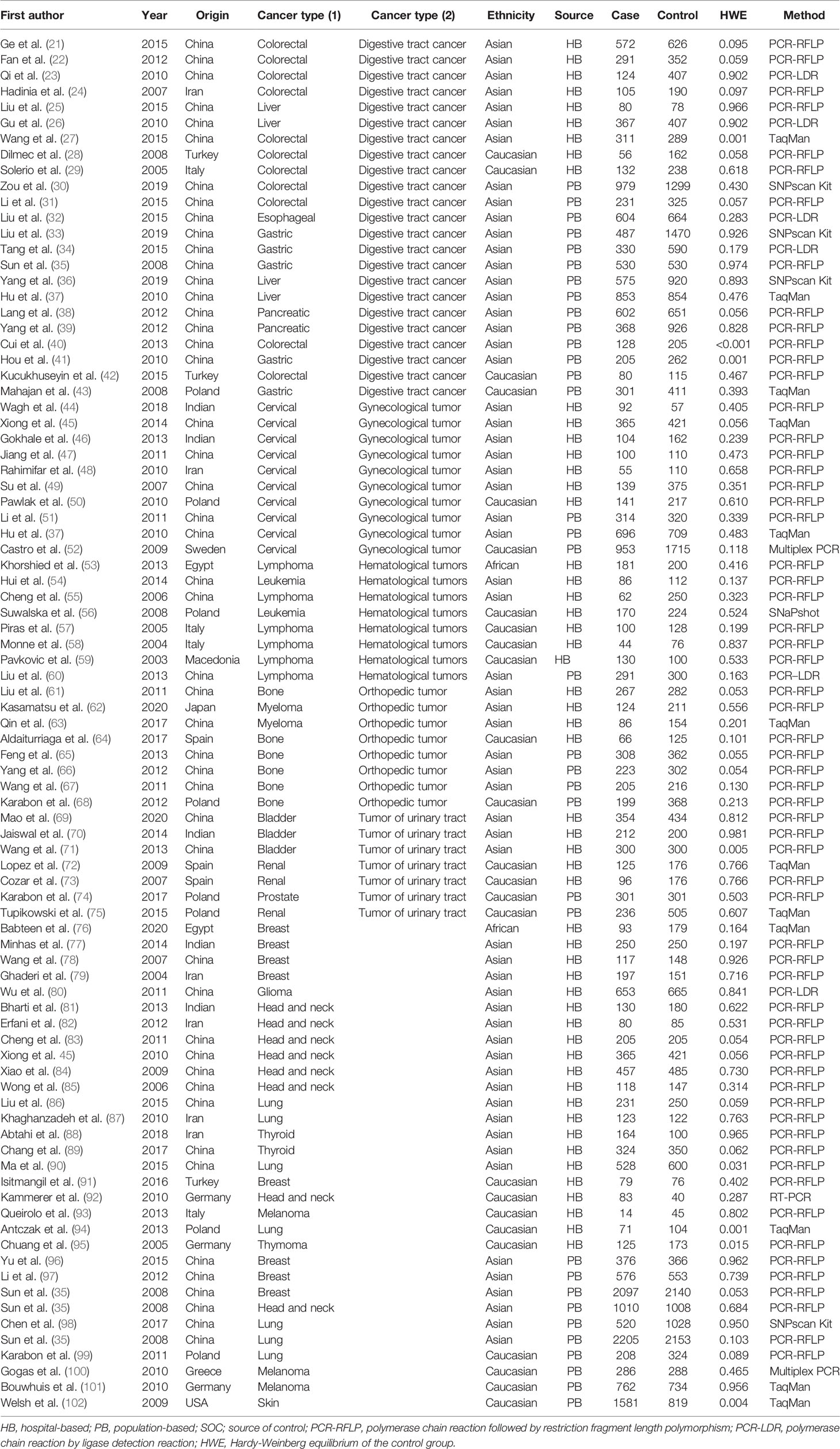
Table 1 Characteristics of studies of the CTLA-4 gene rs231775 A/G polymorphism and cancer risk included in our meta-analysis.
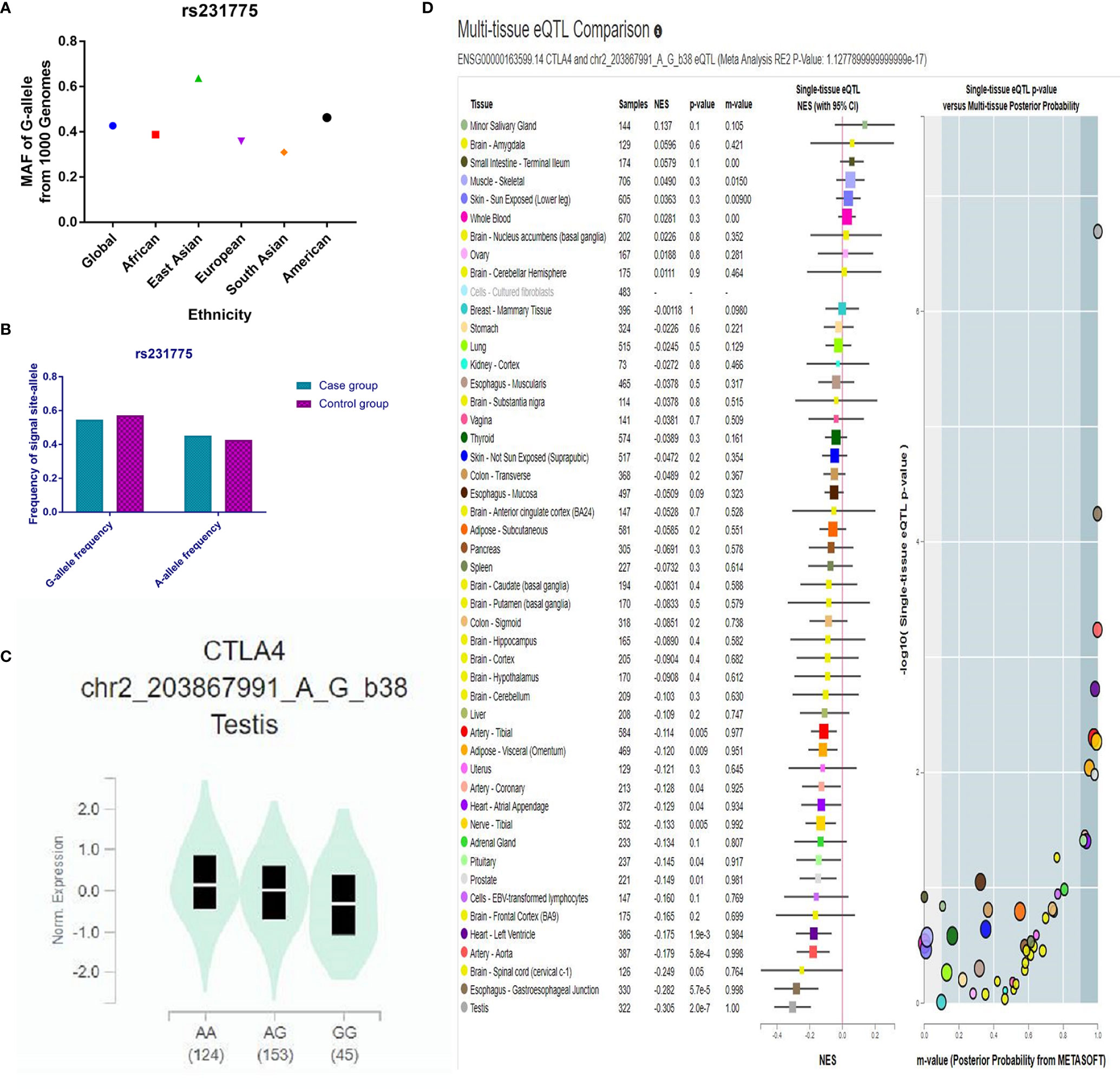
Figure 2 (A) The MAF of minor-allele (mutant-allele) for CTLA-4 rs231775 polymorphism from the 1000 Genomes online database. (B) The frequency about G-allele or A-allele both in case and control groups. (C) The distribution of each genotype from online GTEx Portal (https://www.gtexportal.org/home/). (D) The risk frequency of rs231775 polymorphism in several diseases from the TCGA database.
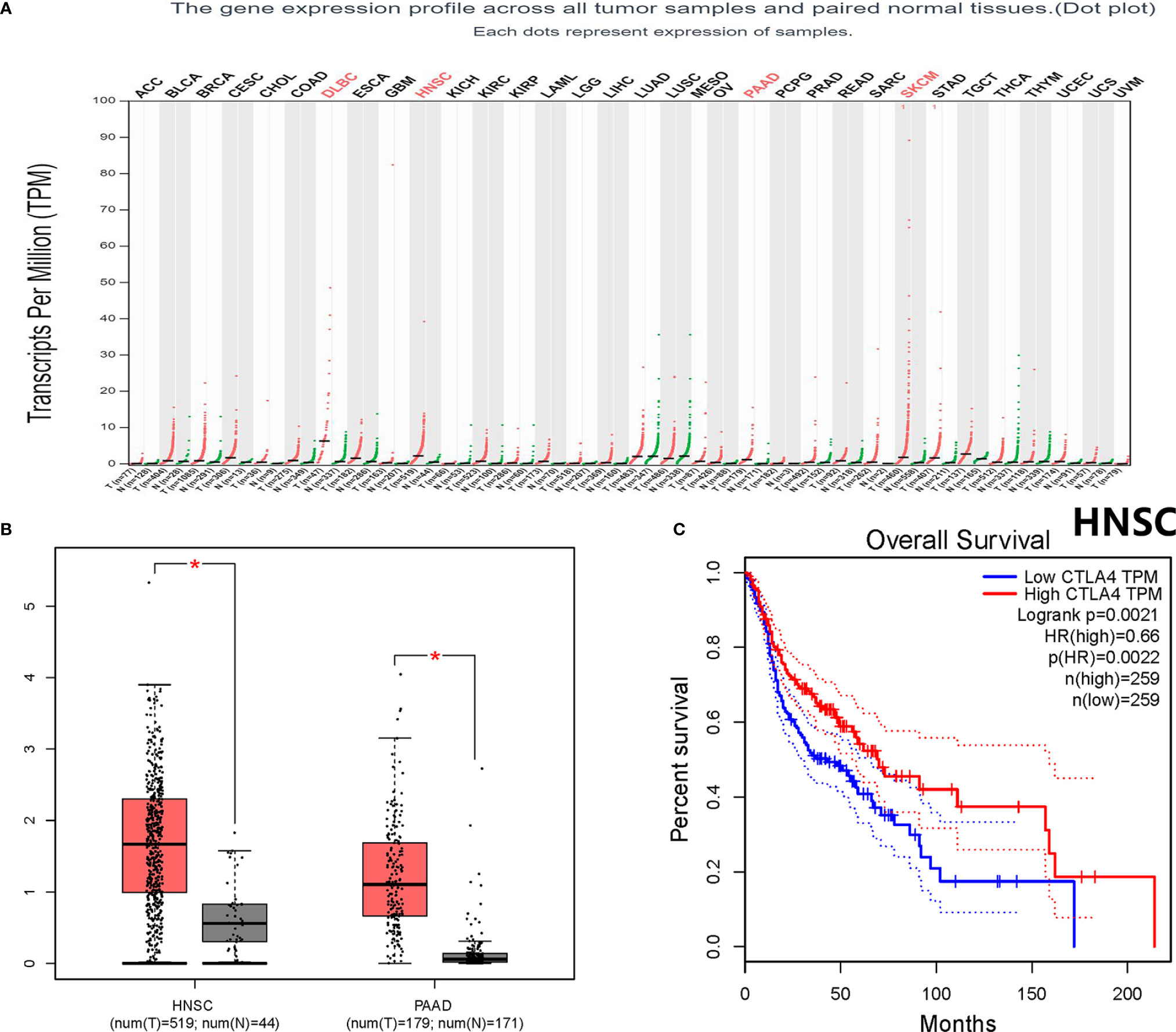
Figure 3 Bioinformatics analysis about the CTLA-4 gene. (A) The CTLA-4 gene expression profile across all tumor samples and paired normal tissues. (B) CTLA-4 gene expression both in HNSC and PAAD. *P < 0.05. (C) Overall survival analysis for HNSC. HR, hazard ratio; ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
Meta-Analysis
Using 29,464 cases and 35,858 controls, the overall risk of CTLA-4 rs231775 is summarized in Table 2. CTLA-4 rs231775 polymorphism appears to decrease cancer risk in overall genetic models (G-allele vs. A-allele, OR = 0.94, 95%CI = 0.90-1.00, Pheterogeneity< 0.001, P = 0.037; GG vs. AA, OR = 0.86, 95%CI = 0.76-0.96, Pheterogeneity< 0.001, P = 0.010; GG vs. GA+AA, OR = 0.88, 95%CI = 0.82-0.94, Pheterogeneity< 0.001, P < 0.001). There were significant associations between CTLA-4 polymorphisms and two types of cancers (colorectal cancer: GA vs. AA, OR = 1.72, 95%CI = 1.13-2.60, Pheterogeneity< 0.001, P = 0.011; GG+GA vs. AA, OR = 1.52, 95%CI = 1.08-2.15, Pheterogeneity< 0.001, P = 0.017, Figure 4; thyroid cancer: G-allele vs. A-allele, OR = 1.50, 95%CI = 1.22-1.85, Pheterogeneity= 0.134, P< 0.001). On the other hand, significantly decreased associations were detected in six kinds of cancer (breast cancer: G-allele vs. A-allele, OR = 0.84, 95%CI = 0.78-0.90, Pheterogeneity= 0.221, P< 0.001, Figure 5; liver cancer: G-allele vs. A-allele, OR = 0.89, 95%CI = 0.82-0.98, Pheterogeneity= 0.151, P = 0.018; cervical cancer: G-allele vs. A-allele, OR = 0.88, 95%CI = 0.78-0.99, Pheterogeneity= 0.023, P = 0.028, Figure 6; bone cancer: GG+GA vs. AA, OR = 0.61, 95%CI = 0.38-0.99, Pheterogeneity< 0.001, P = 0.044, Figure 7; head and neck: G-allele vs. A-allele, OR = 0.79, 95%CI = 0.69-0.91, Pheterogeneity= 0.031, P =0.001, Figure 8; pancreatic cancer: G-allele vs. A-allele, OR = 0.72, 95%CI = 0.57-0.91, Pheterogeneity= 0.049, P = 0.006).
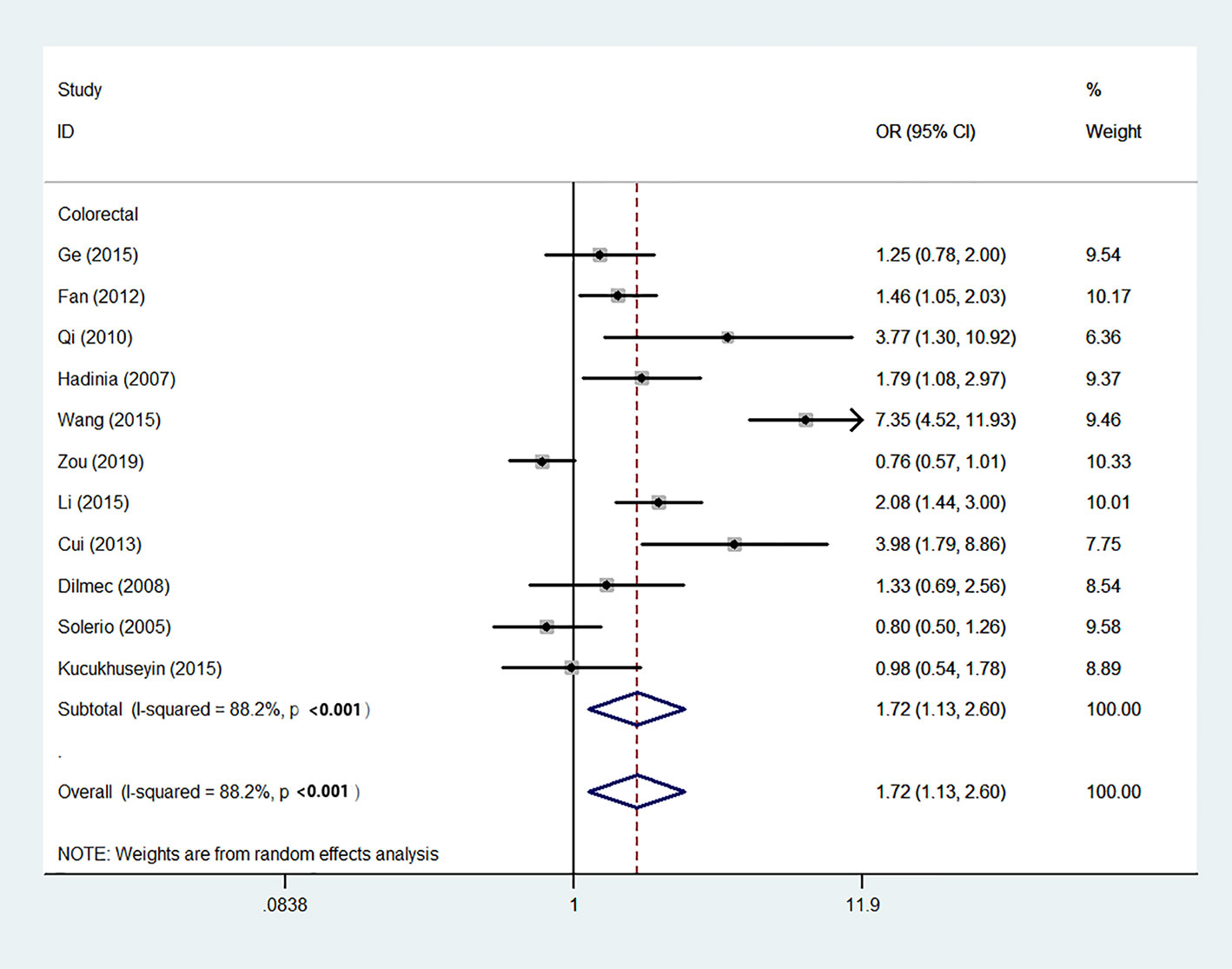
Figure 4 Forest plot of the association between the CTLA-4 gene rs231775 polymorphism and colorectal cancer risk (G-allele vs. A-allele).
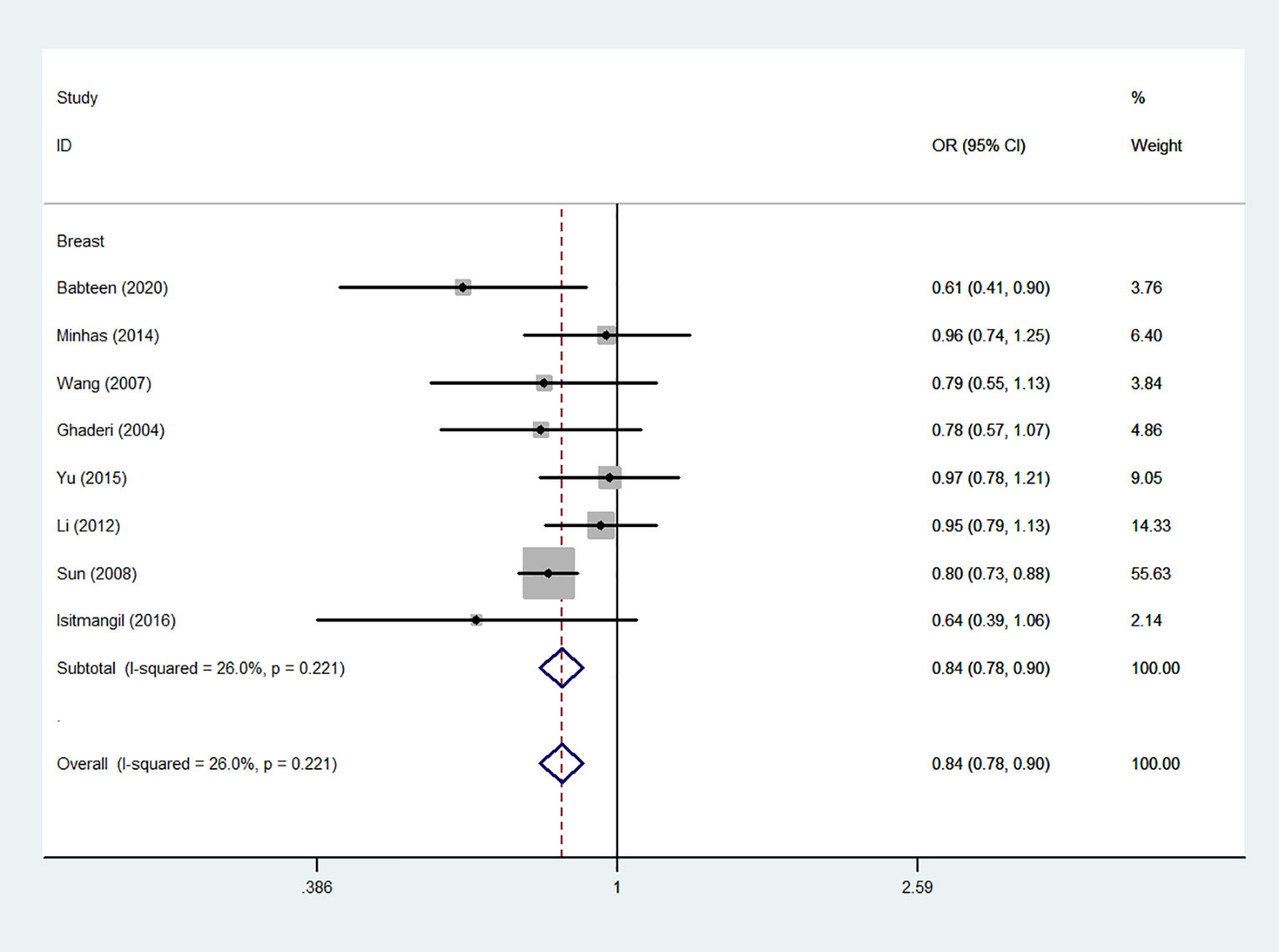
Figure 5 Forest plot of the association between the CTLA-4 gene rs231775 polymorphism and breast cancer risk (G-allele vs. A-allele).
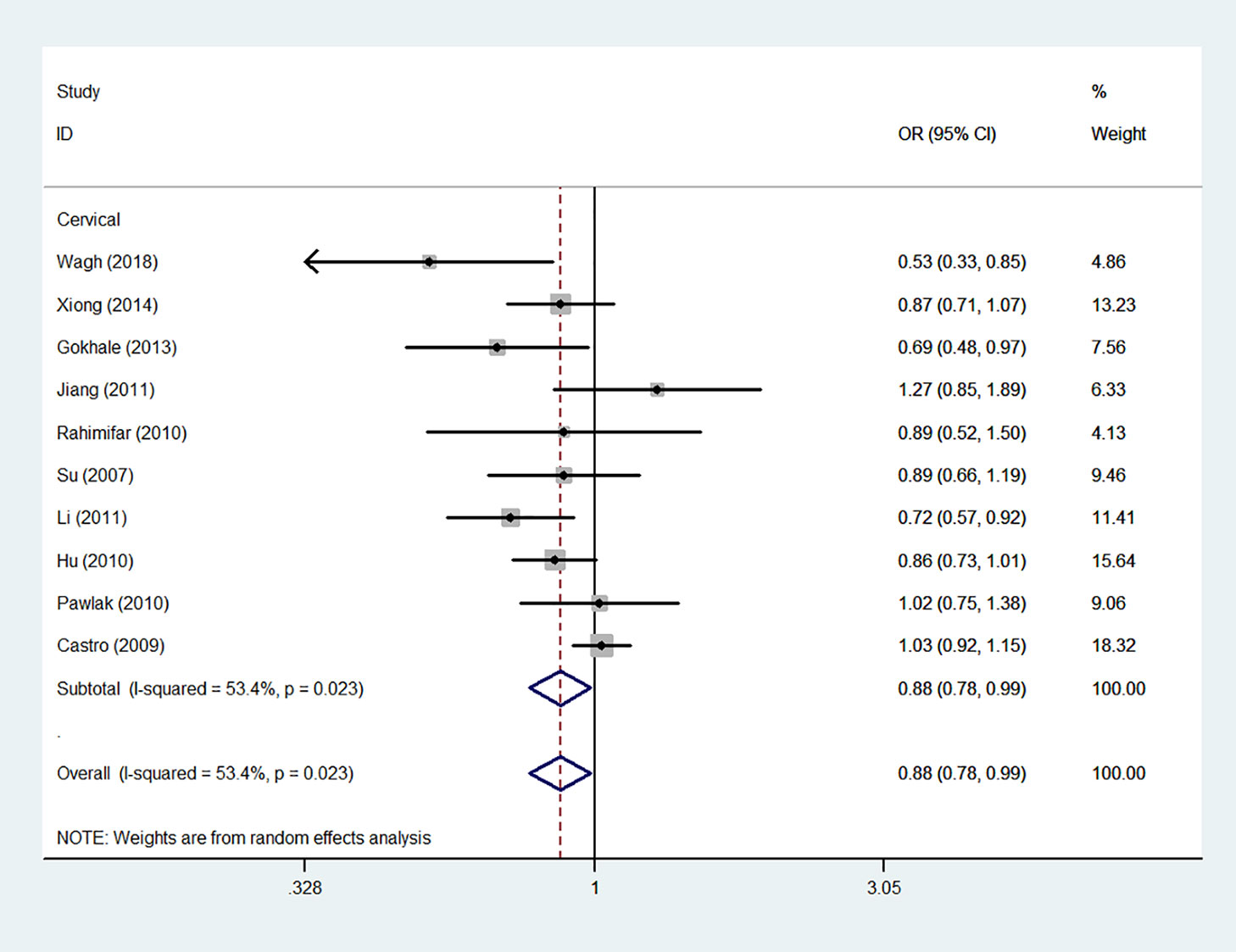
Figure 6 Forest plot of the association between the CTLA-4 gene rs231775 polymorphism and cervical cancer risk (G-allele vs. A-allele).
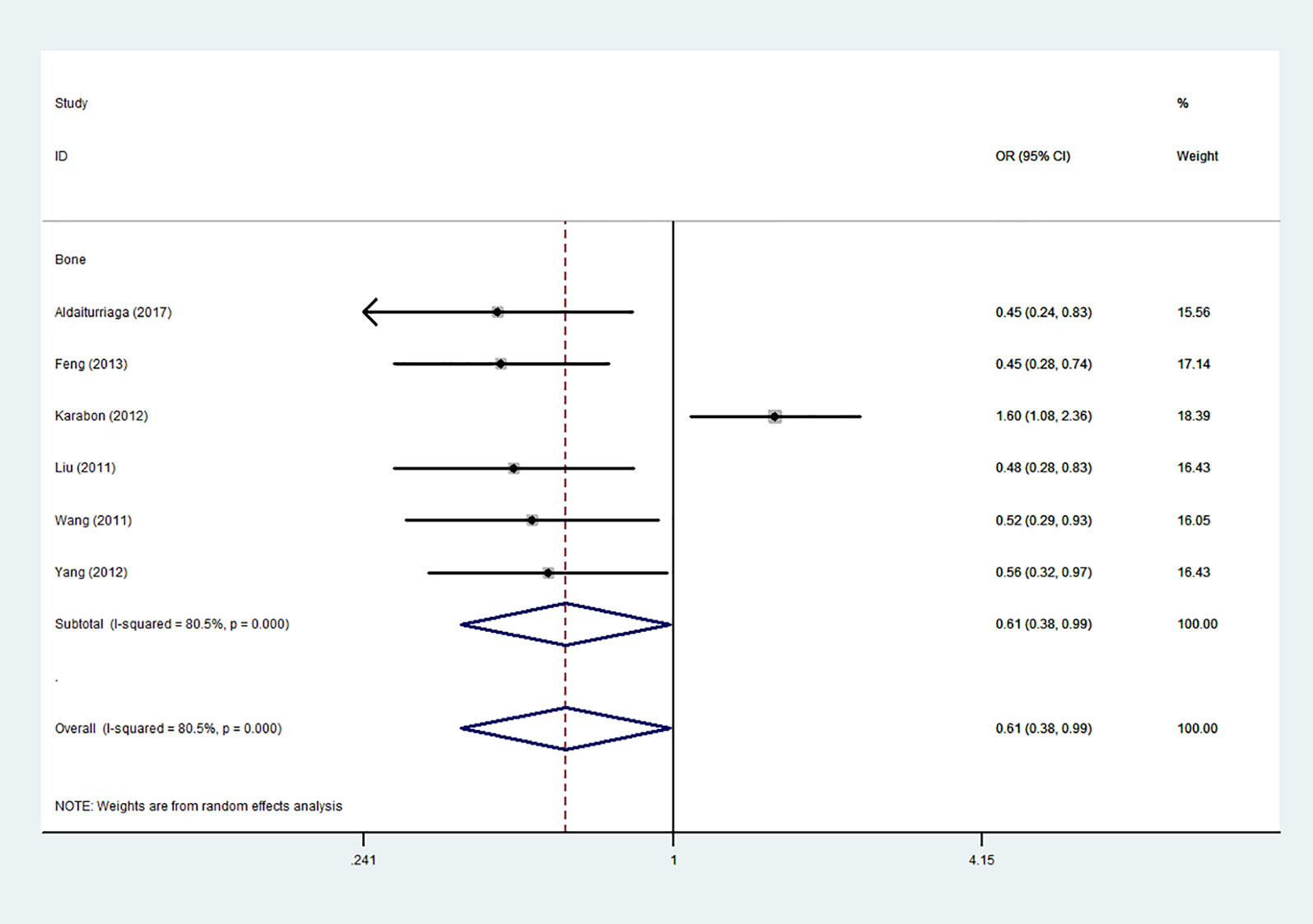
Figure 7 Forest plot of the association between the CTLA-4 gene rs231775 polymorphism and bone cancer risk (G-allele vs. A-allele).
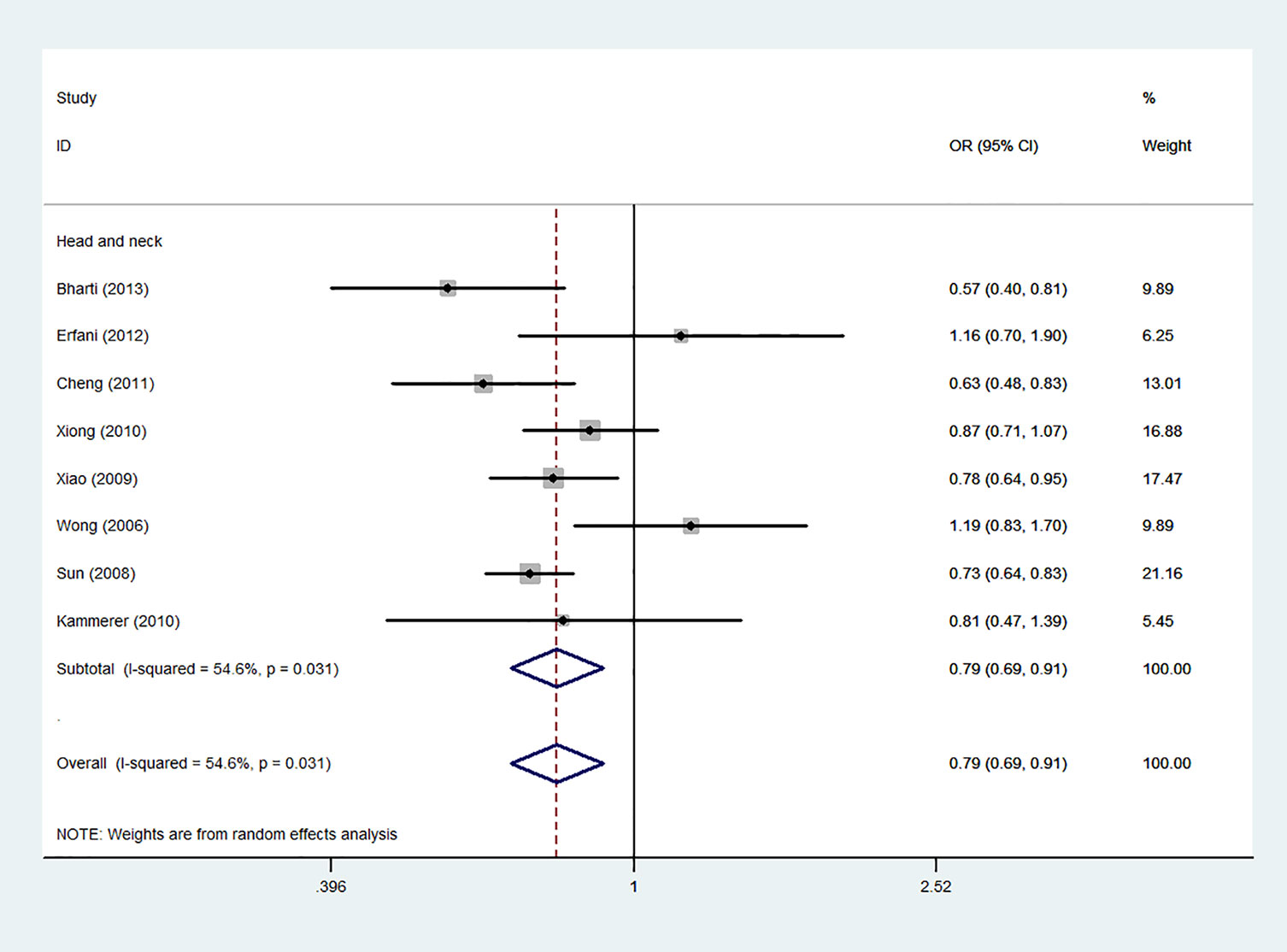
Figure 8 Forest plot of the association between the CTLA-4 gene rs231775 polymorphism and head and neck cancer risk (G-allele vs. A-allele).
We also classified tumors into five systems and observed a significant association between the polymorphism and digestive tract cancer (GG+GA vs. AA, OR = 1.32, 95%CI = 1.04-1.67, Pheterogeneity< 0.001, P = 0.022), however, decreased associations were observed in three kinds of systems (orthopedic tumor: GG vs. AA: OR = 0.68, 95%CI = 0.46-0.99, Pheterogeneity= 0.001, P = 0.048; urinary tract tumor: GG+GA vs. AA, OR = 0.55, 95%CI = 0.42-0.71, Pheterogeneity< 0.001, P < 0.001; gynecological tumor: G-allele vs. A-allele, OR = 0.87, 95%CI = 0.78-0.99, Pheterogeneity= 0.023, P = 0.028). In spite of variations in the frequency of occurrence of this sequence variant among ethnic groups, decreased cancer risk in both Asian (GG vs. GA+ AA, OR = 0.87, 95%CI = 0.81-0.95, Pheterogeneity< 0.001, P=0.001, Figure 9) and Caucasian (GG vs. GA+ AA, OR = 0.89, 95%CI = 0.81-0.97, Pheterogeneity= 0.051, P=0.010, Figure 10) populations was observed. On the basis of stratification by source of control, we evaluated an OR for the rs231775 polymorphism of CTLA-4, and found a decreased association in a recessive genetic model (HB: OR = 0.88, 95%CI = 0.77-1.00, Pheterogeneity< 0.001, P = 0.046; PB: OR = 0.86, 95%CI = 0.81-0.93, Pheterogeneity< 0.001, P <0.001) (Table 2).
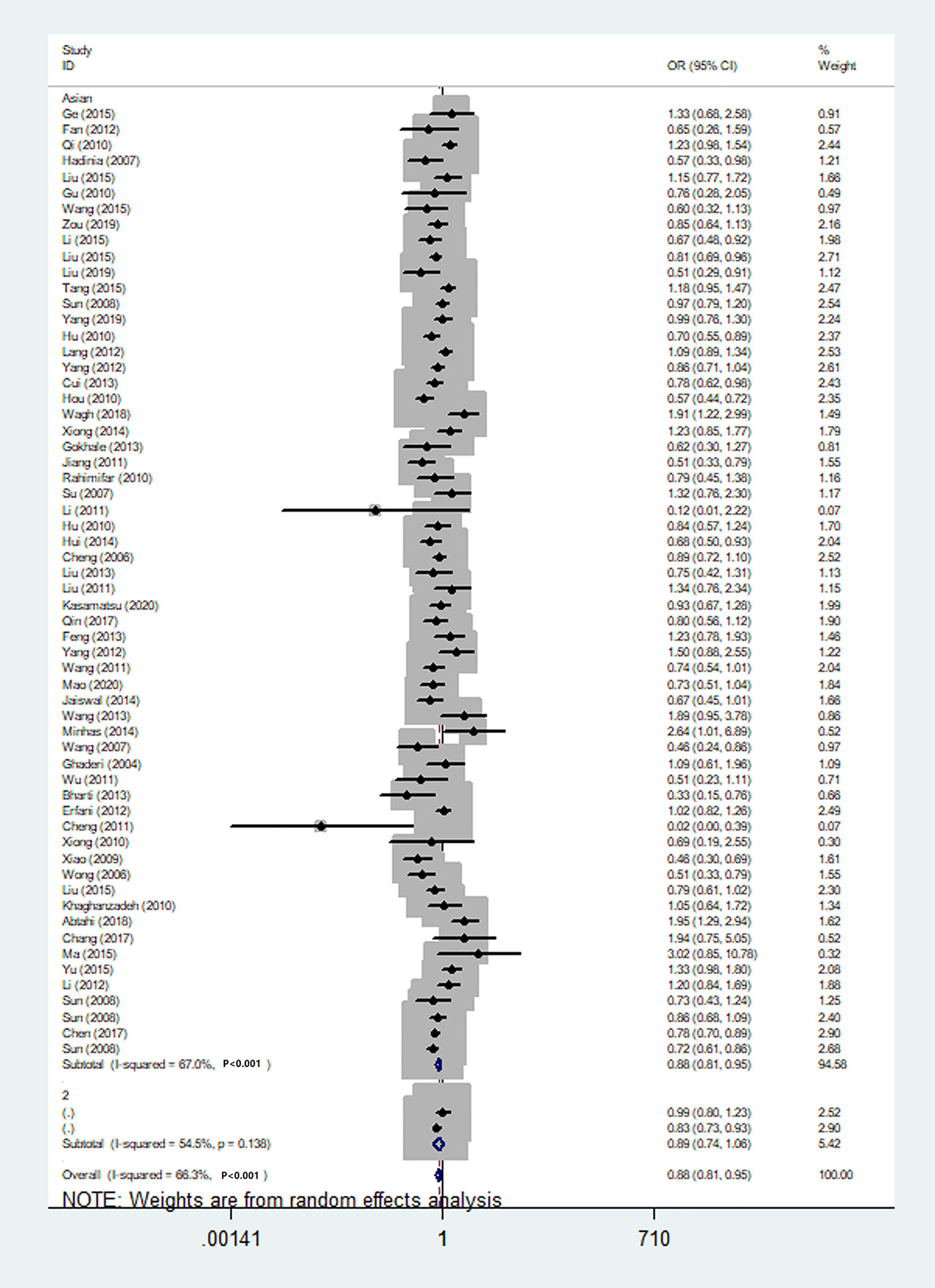
Figure 9 Forest plot of cancer risk associated with the CTLA-4 gene rs231775 polymorphism in Asians (G-allele vs. A-allele model).
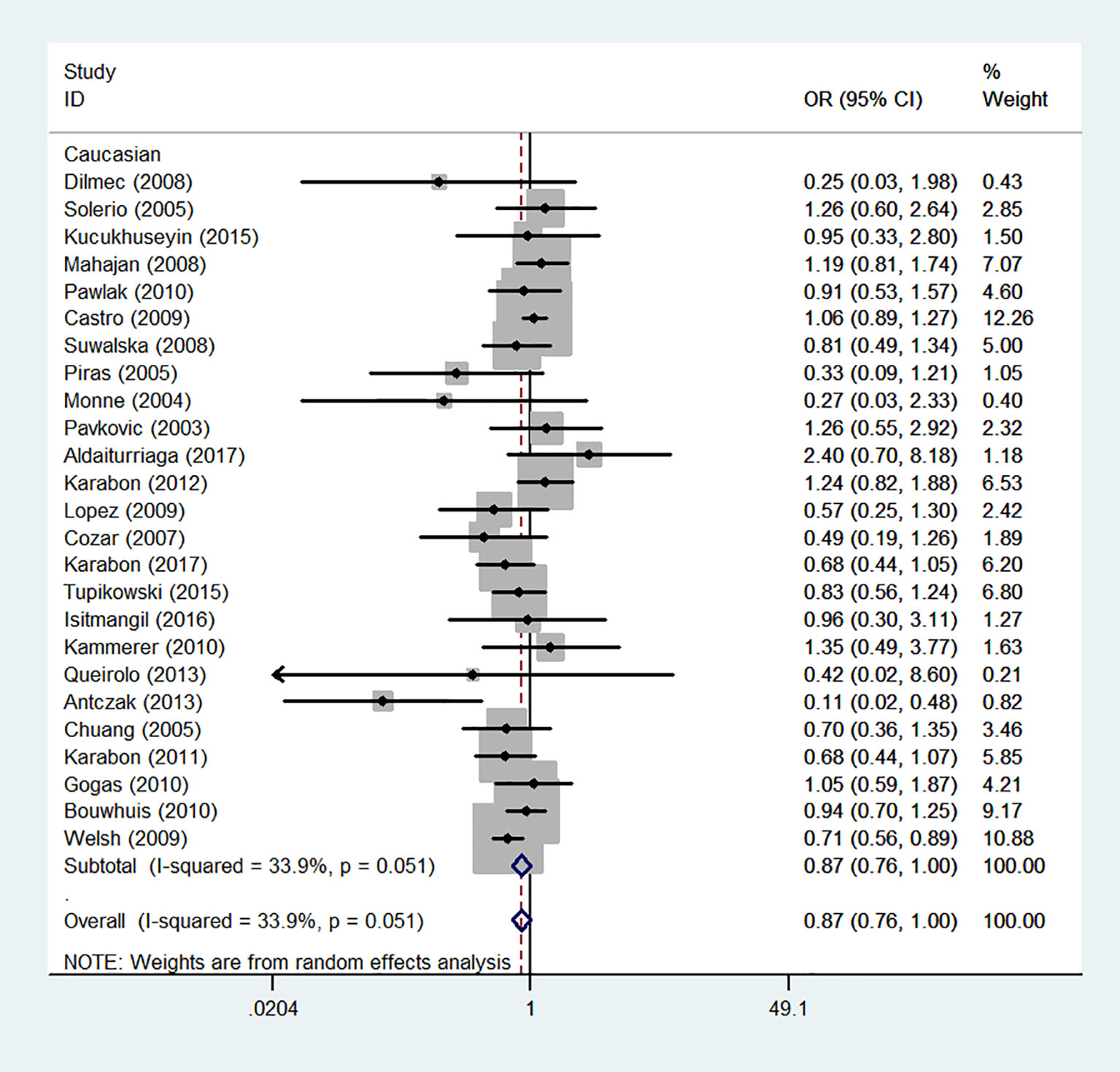
Figure 10 Forest plot of cancer risk associated with the CTLA-4 gene rs231775 polymorphism in Caucasians (G-allele vs. A-allele model).
Meta-Regression
Based on the year of publication, ethnicity, genotype methods, and source of control, a meta-regression analysis indicated that there was a significant association for the allele model (A-allele vs. G-allele) with a regression coefficient of 0.131, 0.464, 0.635, and 0.420, respectively, this suggests that the heterogeneity from the rs231775 polymorphism in cancer could not result from the year of publication, ethnicity, source of control, or genotype methods subgroups (Figures 11A–D) if the heterogeneity was found in the current study.
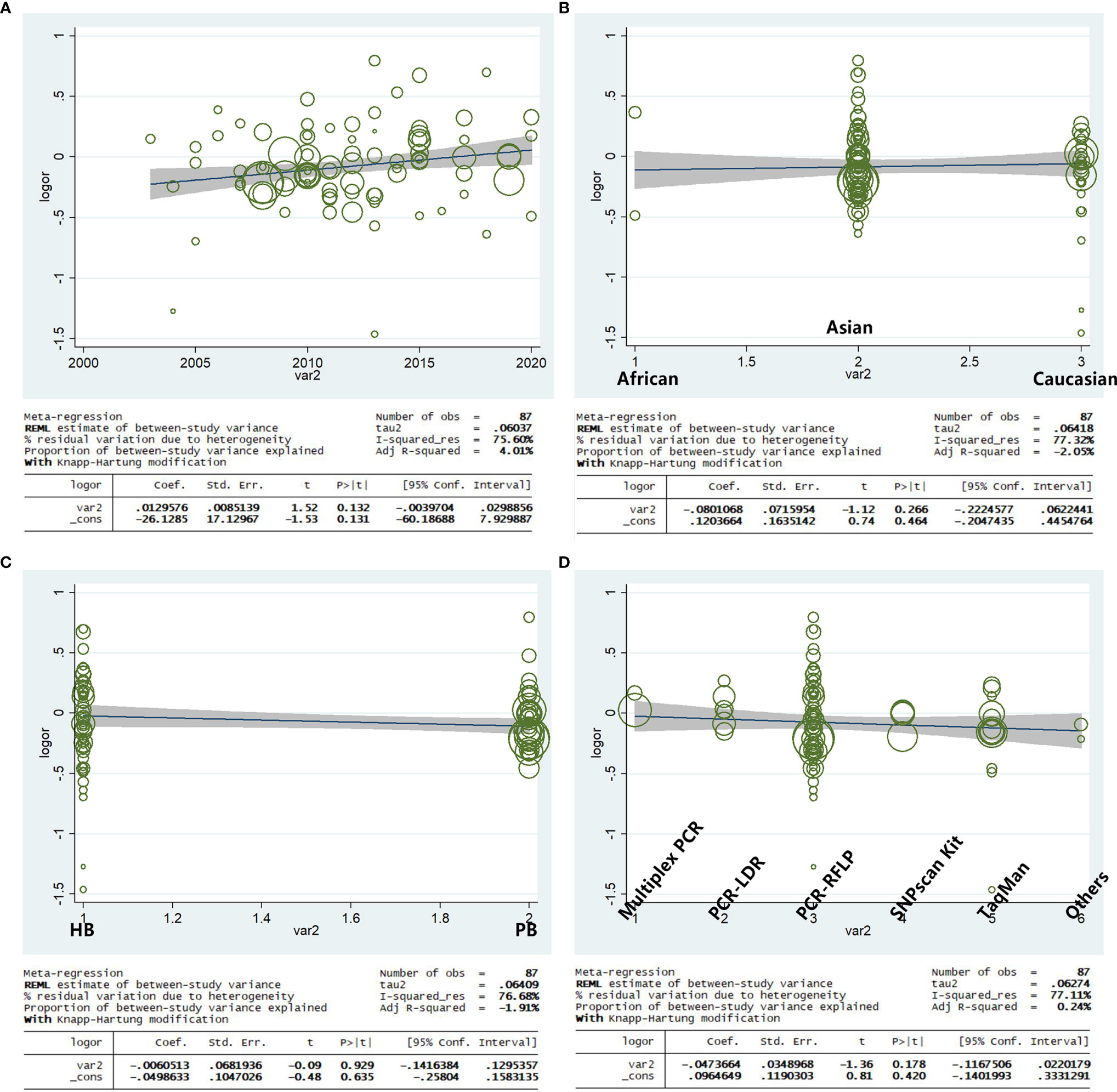
Figure 11 Random-effect meta-regression of log odds ratio vs. publication year (A), regular ethnicity (B), source of control (C), and genotype methods (D), respectively.
Discussion
Nearly 9 million people die of cancer each year worldwide (103). In the challenge of cancer treatment, immunotherapy has attracted remarkable interest among scientists because of its ability to kill tumor cells directly (14, 104). The Treg cell population expresses a number of immune-modulatory receptors, including CTLA-4, programmed cell death protein 1, and the vascular endothelial growth factor receptor (105). Activated T and Treg cells (106) express CTLA-4. Atkins et al. demonstrated improvement in the rate of survival of non-small cell lung cancer, renal cell carcinoma, melanoma, and head and neck squamous cell cancer by blocking the CTLA-4 immune checkpoint, which showed that the CTLA-4 gene is a promising target gene in the future treatment for cancer (107).
Previously, several meta-analyses were focused on the CTLA-4 polymorphisms, which showed the vital role of CTLA-4 in the susceptibility to many diseases, such as cancer. It was documented that the immune related gene CTLA-4 rs5742909 polymorphism had a significantly increased association with cervical carcinogenesis. Dai et al. found the CTLA-4 rs3087243 polymorphism may reduce breast cancer risk, however, rs4553808 may increase breast cancer risk in different ethnicity or genetic models (108, 109). Another polymorphism rs231775 is the most common SNP that has been reported in many tumors, however, a clear conclusion has not been gained yet despite few meta-analyses (110, 111).
Based on 87 case-control studies, we carried out a meta-analysis, which showed CTLA-4 rs231775 polymorphism plays an important role in cancer risks. According to the results, CTLA-4 rs231775 is strongly associated with the maximum cancer risk. Second, both Asian and Caucasian populations were significantly less likely to develop cancer when individuals carry the rs231775 G-allele. Last, individuals with the rs231775G allele may be at a lower risk for cancer in both HB and PB studies. The results of these studies recommend that the rs231775 polymorphism may contribute to cancer development. Next, based on the stratified cancer type analysis, CTLA-4 rs231775 polymorphism was found to be a risk factor for thyroid cancer and colorectal cancer; that is, in individuals carrying the G-allele, the risk of being diagnosed with cancer is increased; on the other hand, it proved to be a protective factor for liver cancer, breast cancer, cervical cancer, head and neck cancer, bone cancer, and pancreatic cancer, in other words, individuals carrying G-allele may have a lower risk of being diagnosed with cancer. However, no association was detected between this SNP and myeloma, bladder cancer, gastric cancer, lung cancer, renal cancer, leukemia, lymphoma, or melanoma. Some of the reasons why the same gene polymorphism plays different roles in different cancer types may be the difference in the pathogenesis of each kind of cancer, and the same gene and its polymorphism may have different functions and susceptibility.
Gene polymorphisms have the important property of their incidence varying widely across different ethnic populations or races. Based on the subgroup analysis by ethnicity, CTLA-4 rs231775 polymorphism was observed to be significantly associated with lower cancer risks in Asians and Caucasians, but not Africans, suggesting genetic diversity across ethnic groups. This difference can be explained by two factors: genetic and environmental differences among different ethnic groups, and linkage disequilibrium patterns between different populations. Polymorphisms may be related to the presence of closer causal variants in varying populations.
The meta-analysis we performed has certain limitations. To begin with, interactions between gene-environment, gene-gene, or different polymorphic loci of the same gene can modulate the risk for cancer, so researchers should investigate these factors in the future. Moreover, other covariates such as age, sex, family history, environmental factors, cancer stage, and lifestyle should be considered. Furthermore, the control group did not comprise strictly healthy controls. Even so, the meta-analysis we conducted has two advantages. First, data from numerous studies were pooled, significantly increasing the power of the analysis. Second, our selection criteria led to a satisfactory quality of case-control studies that are included in the current meta-analysis. Finally, the strength of the current study as per the software is ‘1’, which indicates the conclusions from our study are convincing and clear.
Conclusion
The meta-analysis in the current study suggests a significant association between CTLA-4 rs231775 polymorphism and some types of cancer and overall risk for cancer. Consequently, more large-scale studies, which are well-designed, are needed, with a focus on gene-environment and gene-gene interactions. Future research should provide a more comprehensive clarity of the association between the CTLA-4 rs231775 polymorphism and the risk of developing cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical review and approval was not required for this animal study, in accordance with the local legislation and institutional requirements.
Author Contributions
HW, YF, and HZ were major contributors in writing the manuscript. HW and YF created all the figures. HZ performed the literature search. LZ, YC and YM made substantial contributions to the design of the manuscript and revised it critically for important intellectual content. All authors have read and approved the final version of this manuscript.
Funding
This work was supported by National Natural Science Foundation (No. 81802576), Wuxi Commission of Health and Family Planning (No. T202024, J202012, Z202011), the Science and Technology Development Fund of Wuxi (No. WX18IIAN024, N20202021), and Jiangnan University Wuxi School of Medicine (No. 1286010242190070) and Wuxi “Taihu Talent Program”-High-end Talent in Medical and Healthentalent plan of Taihu Lake in Wuxi (Double Hundred Medical Youth Professionals Program) from Health Committee of Wuxi (No. BJ2020061).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful for the guidance of Professor YC.
References
1. Bray F, Laversame M, Weiderpass E. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer (2021) 127(16):3029–30. doi: 10.1002/cncr.33587
2. (WHO)., W.H.O. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region 2000-2019 (2020). WHO (Accessed December 11, 2020).
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Sud A, Kinnersley B, Houlston RS. Genome-Wide Association Studies of Cancer: Current Insights and Future Perspectives. Nat Rev Cancer (2017) 17(11):692–704. doi: 10.1038/nrc.2017.82
5. Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet (2017) 101(1):5–22. doi: 10.1016/j.ajhg.2017.06.005
6. Gersten O, Wilmoth JR. The Cancer Transition in Japan Since 1951. Demogr Res (2002) 7:271–306. doi: 10.4054/DemRes.2002.7.5
7. Omran AR. The Epidemiologic Transition. A Theory of the Epidemiology of Population Change. 1971. Bull World Health Organ (2001) 79(2):161–70.
8. Shin JH, Jeong J, Maher SE, Lee HW, Lim J, Bothwell ALM. Colon Cancer Cells Acquire Immune Regulatory Molecules From Tumor-Infiltrating Lymphocytes by Trogocytosis. Proc Natl Acad Sci USA (2021) 118(48):e2110241118. doi: 10.1073/pnas.2110241118
9. Li J, Wang W, Sun Y, Zhu Y. CTLA-4 Polymorphisms and Predisposition to Digestive System Malignancies: A Meta-Analysis of 31 Published Studies. World J Surg Oncol (2020) 18(1):55. doi: 10.1186/s12957-020-1806-2
10. Zhuo C, Yi T, Wei C, Wu X, Cen X, Feng S, et al. Association of Cytotoxic T Lymphocyte-Associated Protein 4 Gene -1772T/C Polymorphism With Gastric Cancer Risk: A Prisma-Compliant Meta-Analysis. Medicine (Baltimore) (2020) 99(50):e23542. doi: 10.1097/md.0000000000023542
11. Gouda NS, Fawzy MS, Toraih EA. Impact of Cytotoxic T-Lymphocyte-Associated Protein 4 Codon 17 Variant and Expression on Vitiligo Risk. J Clin Lab Anal (2021) 35(6):e23777. doi: 10.1002/jcla.23777
12. Qi YY, Zhao XY, Liu XR, Wang YN, Zhai YL, Zhang XX, et al. Lupus Susceptibility Region Containing CTLA4 Rs17268364 Functionally Reduces CTLA4 Expression by Binding EWSR1 and Correlates IFN-α Signature. Arthritis Res Ther (2021) 23(1):279. doi: 10.1186/s13075-021-02664-y
13. Zhou B, Chen M, Shang S, Zhao J. Association of CTLA-4 Gene Polymorphisms and Alopecia Areata: A Systematic Review and Meta-Analysis. Biomarkers (2022) 1–11. doi: 10.1080/1354750x.2022.2046855
14. Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers (Basel) (2021) 13(6):1440. doi: 10.3390/cancers13061440
15. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
16. Mantel N, Haenszel W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. J Natl Cancer Inst (1959) 22(4):719–48.
17. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
18. Hayashino Y, Noguchi Y, Fukui T. Systematic Evaluation and Comparison of Statistical Tests for Publication Bias. J Epidemiol (2005) 15(6):235–43. doi: 10.2188/jea.15.235
19. Shieh G. Power and Sample Size Calculations for Comparison of Two Regression Lines With Heterogeneous Variances. PloS One (2018) 13(12):e0207745. doi: 10.1371/journal.pone.0207745
20. Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the Risk of Ketoacidosis Due to Sodium-Glucose Cotransporter (SGLT)-2 Inhibitors in Patients With Type 1 Diabetes: A Meta-Analysis and Meta-Regression. PloS Med (2020) 17(12):e1003461. doi: 10.1371/journal.pmed.1003461
21. Ge J, Zhu L, Zhou J, Li G, Li Y, Li S, et al. Association Between Co-Inhibitory Molecule Gene Tagging Single Nucleotide Polymorphisms and the Risk of Colorectal Cancer in Chinese. J Cancer Res Clin Oncol (2015) 141(9):1533–44. doi: 10.1007/s00432-015-1915-4
22. Fan Z, Fu J, Chen T, Wang G. A Study of Relationship Between Cytotoxic T−lymphocyte Antigen−4+49A>G Gene Polymorphism and Colorectal Cancer. Heilongjiang Med J (2012) 36:810–1. doi: 10.3969/j.issn.1004-5775.2012.11.003
23. Qi P, Ruan CP, Wang H, Zhou FG, Xu XY, Gu X, et al. CTLA-4 +49a>G Polymorphism is Associated With the Risk But Not With the Progression of Colorectal Cancer in Chinese. Int J Colorectal Dis (2010) 25(1):39–45. doi: 10.1007/s00384-009-0806-z
24. Hadinia A, Hossieni SV, Erfani N, Saberi-Firozi M, Fattahi MJ, Ghaderi A. CTLA-4 Gene Promoter and Exon 1 Polymorphisms in Iranian Patients With Gastric and Colorectal Cancers. J Gastroenterol Hepatol (2007) 22(12):2283–7. doi: 10.1111/j.1440-1746.2007.04862.x
25. Liu Z, Song Z, Sun J, Sun F, Li C, Sun J, et al. Association Between CTLA-4 Rs231775 Polymorphism and Hepatocellular Carcinoma Susceptibility. Int J Clin Exp Pathol (2015) 8(11):15118–22.
26. Gu X, Qi P, Zhou F, Ji Q, Wang H, Dou T, et al. +49g > A Polymorphism in the Cytotoxic T-Lymphocyte Antigen-4 Gene Increases Susceptibility to Hepatitis B-Related Hepatocellular Carcinoma in a Male Chinese Population. Hum Immunol (2010) 71(1):83–7. doi: 10.1016/j.humimm.2009.09.353
27. Wang L, Jing F, Su D, Zhang T, Yang B, Jiao S, et al. Association Between CTLA-4 Rs231775 Polymorphism and Risk of Colorectal Cancer: A Meta Analysis. Int J Clin Exp Med (2015) 8(1):650–7.
28. Dilmec F, Ozgonul A, Uzunkoy A, Akkafa F. Investigation of CTLA-4 and CD28 Gene Polymorphisms in a Group of Turkish Patients With Colorectal Cancer. Int J Immunogenet (2008) 35(4-5):317–21. doi: 10.1111/j.1744-313X.2008.00782.x
29. Solerio E, Tappero G, Iannace L, Matullo G, Ayoubi M, Parziale A, et al. CTLA4 Gene Polymorphism in Italian Patients With Colorectal Adenoma and Cancer. Dig Liver Dis (2005) 37(3):170–5. doi: 10.1016/j.dld.2004.10.009
30. Zou C, Qiu H, Tang W, Wang Y, Lan B, Chen Y. CTLA4 Tagging Polymorphisms and Risk of Colorectal Cancer: A Case-Control Study Involving 2,306 Subjects. Onco Targets Ther (2018) 11:4609–19. doi: 10.2147/OTT.S173421
31. Li X, Zhao L, Zhong H, Fang T, Liu W. Correlative Study on CTLA−4+49a>G Polymorphism Gene Polymorphism and Colorectal Cancer. Prog Mod BioMed (2015) 15:2−824. doi: 10.13241/j.cnki.pmb.2015.05.007
32. Liu C, Wang Y, Jiang H, Tang W, Chen S, Kang M, et al. Association Between Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) +49 G>A (Rs231775) Polymorphism and Esophageal Cancer: From a Case-Control Study to a Meta-Analysis. Int J Clin Exp Med (2015) 8(10):17664–73.
33. Liu J, Tang W, Lin W, Wang Y, Chen Y, Wang J, et al. Lack of Association Between CTLA-4 Genetic Polymorphisms and Noncardiac Gastric Cancer in a Chinese Population. DNA Cell Biol (2019) 38(5):443–8. doi: 10.1089/dna.2018.4555
34. Tang W, Wang Y, Chen S, Lin J, Chen B, Yu S, et al. Investigation of Cytotoxic T-Lymphocyte Antigen 4 Polymorphisms in Gastric Cardia Adenocarcinoma. Scand J Immunol (2016) 83:212–8. doi: 10.1111/sji.12409
35. Sun T, Zhou Y, Yang M, Hu Z, Tan W, Han X, et al. Functional Genetic Variations in Cytotoxic T-Lymphocyte Antigen 4 and Susceptibility to Multiple Types of Cancer. Cancer Res (2008) 68(17):7025–34. doi: 10.1158/0008-5472.CAN-08-0806
36. Yang J, Liu J, Chen Y, Tang W, Liu C, Sun Y, et al. Association of CTLA-4 Tagging Polymorphisms and Haplotypes With Hepatocellular Carcinoma Risk: A Case-Control Study. Medicine (Baltimore) (2019) 98(29):e16266. doi: 10.1097/MD.0000000000016266
37. Hu L, Liu J, Chen X, Zhang Y, Liu L, Zhu J, et al. CTLA-4 Gene Polymorphism +49 a/G Contributes to Genetic Susceptibility to Two Infection-Related Cancers-Hepatocellular Carcinoma and Cervical Cancer. Hum Immunol (2010) 71(9):888–91. doi: 10.1016/j.humimm.2010.05.023
38. Lang C, Chen L, Li S. Cytotoxic T-Lymphocyte Antigen-4 +49G/A Polymorphism and Susceptibility to Pancreatic Cancer. DNA Cell Biol (2012) 31(5):683–7. doi: 10.1089/dna.2011.1417
39. Yang M, Sun T, Zhou Y, Wang L, Liu L, Zhang X, et al. The Functional Cytotoxic T Lymphocyte-Associated Protein 4 49G-to-A Genetic Variant and Risk of Pancreatic Cancer. Cancer (2012) 118(19):4681–6. doi: 10.1002/cncr.27455
40. Cui J, Ma H, Cui W, Ma W, Qi Y. Association of Cytotoxic T Lymphocyte Antigen-4 Gene Polymorphism With Susceptibility on Colorectal Cancer. Chin J Curr Adv Gen Surg (2013) 16(2):127–30. doi: 10.3969/j.issn.1009-9905.2013.02.013
41. Hou R, Cao B, Chen Z, Li Y, Ning T, Li C, et al. Association of Cytotoxic T Lymphocyte-Associated Antigen-4 Gene Haplotype With the Susceptibility to Gastric Cancer. Mol Biol Rep (2010) 37(1):515–20. doi: 10.1007/s11033-009-9705-1
42. Kucukhuseyin O, Turan S, Yanar K, Arikan S, Duzkoylu Y, Aydin S, et al. Individual and Combined Effects of CTLA4-CD28 Variants and Oxidant-Antioxidant Status on the Development of Colorectal Cancer. Anticancer Res (2015) 35(10):5391–400.
43. Mahajan R, El-Omar EM, Lissowska J, Grillo P, Rabkin CS, Baccarelli A, et al. Genetic Variants in T Helper Cell Type 1, 2 and 3 Pathways and Gastric Cancer Risk in a Polish Population. Jpn J Clin Oncol (2008) 38(9):626–33. doi: 10.1093/jjco/hyn075
44. Wagh P, Kulkarni P, Kerkar S, Warke H, Chaudhari H, Deodhar K, et al. Polymorphisms in Cytotoxic T-Lymphocyte Associated Antigen 4 Gene Does Not Affect Scytotoxic T-Lymphocyte Associated Antigen 4 Levels in Human Papillomavirus-Infected Women With or Without Cervical Cancer. Indian J Med Microbiol (2018) 36(2):207–10. doi: 10.4103/ijmm.IJMM_17_220
45. Xiong YH, He L, Fei J. Genetic Variations in Cytotoxic T-Lymphocyte Antigen-4 and Susceptibility to Cervical Cancer. Int Immunopharmacol (2014) 18(1):71–6. doi: 10.1016/j.intimp.2013.10.018
46. Gokhale P, Kerkar S, Tongaonkar H, Salvi V, Mania-Pramanik J. CTLA-4 Gene Polymorphism at Position +49 a>G in Exon 1: A Risk Factor for Cervical Cancer in Indian Women. Cancer Genet (2013) 206(5):154–61. doi: 10.1016/j.cancergen.2013.04.003
47. Jiang L, Luo RY, Zhang W, Wang LR, Wang F, Cheng YX. [Single Nucleotide Polymorphisms of CTLA4 Gene and Their Association With Human Cervical Cancer]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi (2011) 28(3):313–7. doi: 10.3760/cma.j.issn.1003-9406.2011.03.017
48. Rahimifar S, Erfani N, Sarraf Z, Ghaderi A. Ctla-4 Gene Variations may Influence Cervical Cancer Susceptibility. Gynecol Oncol (2010) 119(1):136–9. doi: 10.1016/j.ygyno.2010.06.006
49. Su TH, Chang TY, Lee YJ, Chen CK, Liu HF, Chu CC, et al. CTLA-4 Gene and Susceptibility to Human Papillomavirus-16-Associated Cervical Squamous Cell Carcinoma in Taiwanese Women. Carcinogenesis (2007) 28(6):1237–40. doi: 10.1093/carcin/bgm043
50. Pawlak E, Karabon L, Wlodarska-Polinska I, Jedynak A, Jonkisz A, Tomkiewicz A, et al. Influence of CTLA-4/CD28/ICOS Gene Polymorphisms on the Susceptibility to Cervical Squamous Cell Carcinoma and Stage of Differentiation in the Polish Population. Hum Immunol (2010) 71(2):195–200. doi: 10.1016/j.humimm.2009.11.006
51. Li H, Zhou YF, Guo HY, Sun T, Zhang WH, Lin DX. Association Between CTLA-4 Gene Polymorphism and Susceptibility to Cervical Cancer. Zhonghua Zhong Liu Za Zhi (2011) 33(9):681–4. doi: 10.3760/cma.j.issn.0253-3766.2011.09.009
52. Castro FA, Haimila K, Sareneva I, Schmitt M, Lorenzo J, Kunkel N, et al. Association of HLA-DRB1, Interleukin-6 and Cyclin D1 Polymorphisms With Cervical Cancer in the Swedish Population–a Candidate Gene Approach. Int J Cancer (2009) 125(8):1851–8. doi: 10.1002/ijc.24529
53. Khorshied MM, Gouda HM, Khorshid OM. Association of Cytotoxic T-Lymphocyte Antigen 4 Genetic Polymorphism, Hepatitis C Viral Infection and B-Cell non-Hodgkin Lymphoma: An Egyptian Study. Leuk Lymphoma (2014) 55(5):1061–6. doi: 10.3109/10428194.2013.820294
54. Hui L, Lei Z, Peng Z, Ruobing S, Fenghua Z. Polymorphism Analysis of CTLA-4 in Childhood Acute Lymphoblastic Leukemia. Pak J Pharm Sci (2014) 27(4 Suppl):1005–13. doi: 10.4314/tjpr.v13i7.24
55. Cheng TY, Lin JT, Chen LT, Shun CT, Wang HP, Lin MT, et al. Association of T-Cell Regulatory Gene Polymorphisms With Susceptibility to Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. J Clin Oncol (2006) 24(21):3483–9. doi: 10.1200/JCO.2005.05.5434
56. Suwalska K, Pawlak E, Karabon L, Tomkiewicz A, Dobosz T, Urbaniak-Kujda D, et al. Association Studies of CTLA-4, CD28, and ICOS Gene Polymorphisms With B-Cell Chronic Lymphocytic Leukemia in the Polish Population. Hum Immunol (2008) 69(3):193–201. doi: 10.1016/j.humimm.2008.01.014
57. Piras G, Monne M, Uras A, Palmas A, Murineddu M, Arru L, et al. Genetic Analysis of the 2q33 Region Containing CD28-CTLA4-ICOS Genes: Association With non-Hodgkin's Lymphoma. Br J Haematol (2005) 129(6):784–90. doi: 10.1111/j.1365-2141.2005.05525.x
58. Monne M, Piras G, Palmas A, Arru L, Murineddu M, Latte G, et al. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Gene Polymorphism and Susceptibility to non-Hodgkin's Lymphoma. Am J Hematol (2004) 76(1):14–8. doi: 10.1002/ajh.20045
59. Pavkovic M, Georgievski B, Cevreska L, Spiroski M, Efremov DG. CTLA-4 Exon 1 Polymorphism in Patients With Autoimmune Blood Disorders. Am J Hematol (2003) 72(2):147–9. doi: 10.1002/ajh.10278
60. Liu J, Liu J, Song B, Wang T, Liu Y, Hao J, et al. Genetic Variations in CTLA-4, TNF-Alpha, and LTA and Susceptibility to T-Cell Lymphoma in a Chinese Population. Cancer Epidemiol (2013) 37(6):930–4. doi: 10.1016/j.canep.2013.08.011
61. Liu Y, He Z, Feng D, Shi G, Gao R, Wu X, et al. Cytotoxic T-Lymphocyte Antigen-4 Polymorphisms and Susceptibility to Osteosarcoma. DNA Cell Biol (2011) 30(12):1051–5. doi: 10.1089/dna.2011.1269
62. Kasamatsu T, Awata M, Ishihara R, Murakami Y, Gotoh N, Matsumoto M, et al. PDCD1 and PDCD1LG1 Polymorphisms Affect the Susceptibility to Multiple Myeloma. Clin Exp Med (2020) 20(1):51–62. doi: 10.1007/s10238-019-00585-4
63. Qin XY, Lu J, Li GX, Wen L, Liu Y, Xu LP, et al. CTLA-4 Polymorphisms Are Associated With Treatment Outcomes of Patients With Multiple Myeloma Receiving Bortezomib-Based Regimens. Ann Hematol (2018) 97(3):485–95. doi: 10.1007/s00277-017-3203-7
64. Bilbao-Aldaiturriaga N, Patino-Garcia A, Martin-Guerrero I, Garcia-Orad A. Cytotoxic T Lymphocyte-Associated Antigen 4 Rs231775 Polymorphism and Osteosarcoma. Neoplasma (2017) 64(2):299–304. doi: 10.4149/neo_2017_218
65. Feng D, Yang X, Li S, Liu T, Wu Z, Song Y, et al. Cytotoxic T-Lymphocyte Antigen-4 Genetic Variants and Risk of Ewing's Sarcoma. Genet Test Mol Biomarkers (2013) 17(6):458–63. doi: 10.1089/gtmb.2012.0488
66. Yang S, Wang C, Zhou Y, Sun G, Zhu D, Gao S. Cytotoxic T-Lymphocyte Antigen-4 Polymorphisms and Susceptibility to Ewing's Sarcoma. Genet Test Mol Biomarkers (2012) 16(10):1236–40. doi: 10.1089/gtmb.2012.0129
67. Wang W, Wang J, Song H, Liu J, Song B, Cao X. Cytotoxic T-Lymphocyte Antigen-4 +49G/A Polymorphism is Associated With Increased Risk of Osteosarcoma. Genet Test Mol Biomarkers (2011) 15(7-8):503–6. doi: 10.1089/gtmb.2010.0264
68. Karabon L, Pawlak-Adamska E, Tomkiewicz A, Jedynak A, Kielbinski M, Woszczyk D, et al. Variations in Suppressor Molecule Ctla-4 Gene are Related to Susceptibility to Multiple Myeloma in a Polish Population. Pathol Oncol Res (2012) 18(2):219–26. doi: 10.1007/s12253-011-9431-6
69. Mao F, Niu XB, Gu S, Ji L, Wei BJ, Wang HB. CTLA-4 +49a/G Polymorphism Increases the Susceptibility to Bladder Cancer in Chinese Han Participants: A Case-Control Study. Dis Markers (2020) 2020:8143158. doi: 10.1155/2020/8143158
70. Jaiswal PK, Singh V, Mittal RD. Cytotoxic T Lymphocyte Antigen 4 (CTLA4) Gene Polymorphism With Bladder Cancer Risk in North Indian Population. Mol Biol Rep (2014) 41(2):799–807. doi: 10.1007/s11033-013-2919-2
71. Wang L, Su G, Zhao X, Cai Y, Cai X, Zhang J, et al. Association Between the Cytotoxic T-Lymphocyte Antigen 4 +49A/G Polymorphism and Bladder Cancer Risk. Tumour Biol (2014) 35(2):1139–42. doi: 10.1007/s13277-013-1152-x
72. Saenz Lopez P, Vazquez Alonso F, Romero JM, Carretero R, Tallada Bunuel M, Ruiz Cabello F, et al. [Polymorphisms in Inflammatory Response Genes in Metastatic Renal Cancer]. Actas Urol Esp (2009) 33(5):474–81. doi: 10.1016/s0210-4806(09)74180-4
73. Cozar JM, Romero JM, Aptsiauri N, Vazquez F, Vilchez JR, Tallada M, et al. High Incidence of CTLA-4 AA (CT60) Polymorphism in Renal Cell Cancer. Hum Immunol (2007) 68(8):698–704. doi: 10.1016/j.humimm.2007.05.002
74. Karabon L, Tupikowski K, Tomkiewicz A, Partyka A, Pawlak-Adamska E, Wojciechowski A, et al. Is the Genetic Background of Co-Stimulatory CD28/CTLA-4 Pathway the Risk Factor for Prostate Cancer? Pathol Oncol Res (2017) 23(4):837–43. doi: 10.1007/s12253-016-0180-4
75. Tupikowski K, Partyka A, Kolodziej A, Dembowski J, Debinski P, Halon A, et al. CTLA-4 and CD28 Genes' Polymorphisms and Renal Cell Carcinoma Susceptibility in the Polish Population–a Prospective Study. Tissue Antigens (2015) 86(5):353–61. doi: 10.1111/tan.12671
76. Babteen NA, Fawzy MS, Alelwani W, Alharbi RA, Alruwetei AM, Toraih EA, et al. Signal Peptide Missense Variant in Cancer-Brake Gene CTLA4 and Breast Cancer Outcomes. Gene (2020) 737:144435. doi: 10.1016/j.gene.2020.144435
77. Minhas S, Bhalla S, Shokeen Y, Jauhri M, Saxena R, Verma IC, et al. Lack of Any Association of the CTLA-4 +49 G/A Polymorphism With Breast Cancer Risk in a North Indian Population. Asian Pac J Cancer Prev (2014) 15(5):2035–8. doi: 10.7314/apjcp.2014.15.5.2035
78. Wang L, Li D, Fu Z, Li H, Jiang W, Li D. Association of CTLA-4 Gene Polymorphisms With Sporadic Breast Cancer in Chinese Han Population. BMC Cancer (2007) 7:173. doi: 10.1186/1471-2407-7-173
79. Ghaderi A, Yeganeh F, Kalantari T, Talei AR, Pezeshki AM, Doroudchi M, et al. Cytotoxic T Lymphocyte Antigen-4 Gene in Breast Cancer. Breast Cancer Res Treat (2004) 86(1):1–7. doi: 10.1023/B:BREA.0000032918.89120.8e
80. Wu Q, Zhan X, Dou T, Chen H, Fan W, Zhou K, et al. CTLA4 A49G Polymorphism Shows Significant Association With Glioma Risk in a Chinese Population. Biochem Genet (2011) 49(3-4):190–201. doi: 10.1007/s10528-010-9398-0
81. Bharti V, Mohanti BK, Das SN. Functional Genetic Variants of CTLA-4 and Risk of Tobacco-Related Oral Carcinoma in High-Risk North Indian Population. Hum Immunol (2013) 74(3):348–52. doi: 10.1016/j.humimm.2012.12.008
82. Erfani N, Haghshenas MR, Hoseini MA, Hashemi SB, Khademi B, Ghaderi A. Strong Association of CTLA-4 Variation (CT60A/G) and CTLA-4 Haplotypes With Predisposition of Iranians to Head and Neck Cancer. Iran J Immunol (2012) 9(3):188–98
83. Cheng XL, Ning T, Xu CQ, Li Y, Zhu BS, Hou FT, et al. Haplotype Analysis of CTLA4 Gene and Risk of Esophageal Squamous Cell Carcinoma in Anyang Area of China. Hepatogastroenterology (2011) 58(106):432–7. doi: 10.1016/j.dld.2010.10.013
84. Xiao M, Qi F, Chen X, Luo Z, Zhang L, Zheng C, et al. Functional Polymorphism of Cytotoxic T-Lymphocyte Antigen 4 and Nasopharyngeal Carcinoma Susceptibility in a Chinese Population. Int J Immunogenet (2010) 37(1):27–32. doi: 10.1111/j.1744-313X.2009.00888.x
85. Wong YK, Chang KW, Cheng CY, Liu CJ. Association of CTLA-4 Gene Polymorphism With Oral Squamous Cell Carcinoma. J Oral Pathol Med (2006) 35(1):51–4. doi: 10.1111/j.1600-0714.2005.00377.x
86. Liu HN, Su JL, Zhou SH, Liu LJ, Qie P. Cytotoxic T Lymphocyte-Associated Antigen-4 +49A>G Polymorphism and the Risk of non-Small Cell Lung Cancer in a Chinese Population. Int J Clin Exp Med (2015) 8(7):11519–23.
87. Khaghanzadeh N, Erfani N, Ghayumi MA, Ghaderi A. CTLA4 Gene Variations and Haplotypes in Patients With Lung Cancer. Cancer Genet Cytogenet (2010) 196(2):171–4. doi: 10.1016/j.cancergencyto.2009.09.001
88. Abtahi S, Izadi Jahromi F, Dabbaghmanesh MH, Malekzadeh M, Ghaderi A. Association Between CTLA-4 + 49a > G and - 318C > T Single-Nucleotide Polymorphisms and Susceptibility to Thyroid Neoplasm. Endocrine (2018) 62(1):159–65. doi: 10.1007/s12020-018-1663-8
89. Chang DF, Chen XH, Huang J, Sun YM, Zhu DY, Xu ZQ. CTLA-4 Gene Polymorphisms Associate With Efficacy of Postoperative Radioiodine-131 for Differentiated Thyroid Carcinoma. Future Oncol (2017) 13(12):1057–68. doi: 10.2217/fon-2016-0399
90. Ma Y, Liu X, Zhu J, Li W, Guo L, Han X, et al. Polymorphisms of Co-Inhibitory Molecules (CTLA-4/PD-1/PD-L1) and the Risk of non-Small Cell Lung Cancer in a Chinese Population. Int J Clin Exp Med (2015) 8(9):16585–91.
91. Isitmangil G, Gurleyik G, Aker FV, Coskun C, Kucukhuseyin O, Arikan S, et al. Association of CTLA4 and CD28 Gene Variants and Circulating Levels of Their Proteins in Patients With Breast Cancer. In Vivo (2016) 30(4):485–93.
92. Kammerer PW, Toyoshima T, Schoder F, Kammerer P, Kuhr K, Brieger J, et al. Association of T-Cell Regulatory Gene Polymorphisms With Oral Squamous Cell Carcinoma. Oral Oncol (2010) 46(7):543–8. doi: 10.1016/j.oraloncology.2010.03.025
93. Queirolo P, Morabito A, Laurent S, Lastraioli S, Piccioli P, Ascierto PA, et al. Association of CTLA-4 Polymorphisms With Improved Overall Survival in Melanoma Patients Treated With CTLA-4 Blockade: A Pilot Study. Cancer Invest (2013) 31(5):336–45. doi: 10.3109/07357907.2013.793699
94. Antczak A, Pastuszak-Lewandoska D, Gorski P, Domanska D, Migdalska-Sek M, Czarnecka K, et al. Ctla-4 Expression and Polymorphisms in Lung Tissue of Patients With Diagnosed non-Small-Cell Lung Cancer. BioMed Res Int (2013) 2013:576486. doi: 10.1155/2013/576486
95. Chuang WY, Strobel P, Gold R, Nix W, Schalke B, Kiefer R, et al. A CTLA4high Genotype is Associated With Myasthenia Gravis in Thymoma Patients. Ann Neurol (2005) 58(4):644–8. doi: 10.1002/ana.20577
96. Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J. Cytotoxic T Lymphocyte Antigen 4 Expression in Human Breast Cancer: Implications for Prognosis. Cancer Immunol Immunother (2015) 64(7):853–60. doi: 10.1007/s00262-015-1696-2
97. Li D, Zhang Q, Xu F, Fu Z, Yuan W, Li D, et al. Association of CTLA-4 Gene Polymorphisms With Sporadic Breast Cancer Risk and Clinical Features in Han Women of Northeast China. Mol Cell Biochem (2012) 364(1-2):283–90. doi: 10.1007/s11010-012-1228-8
98. Chen S, Wang Y, Chen Y, Lin J, Liu C, Kang M, et al. Investigation of Cytotoxic T-Lymphocyte Antigen-4 Polymorphisms in non-Small Cell Lung Cancer: A Case-Control Study. Oncotarget (2017) 8(44):76634–43. doi: 10.18632/oncotarget.20638
99. Karabon L, Pawlak E, Tomkiewicz A, Jedynak A, Passowicz-Muszynska E, Zajda K, et al. CTLA-4, CD28, and ICOS Gene Polymorphism Associations With non-Small-Cell Lung Cancer. Hum Immunol (2011) 72(10):947–54. doi: 10.1016/j.humimm.2011.05.010
100. Gogas H, Dafni U, Koon H, Spyropoulou-Vlachou M, Metaxas Y, Buchbinder E, et al. Evaluation of Six CTLA-4 Polymorphisms in High-Risk Melanoma Patients Receiving Adjuvant Interferon Therapy in the He13A/98 Multicenter Trial. J Transl Med (2010) 8:108. doi: 10.1186/1479-5876-8-108
101. Bouwhuis MG, Gast A, Figl A, Eggermont AM, Hemminki K, Schadendorf D, et al. Polymorphisms in the CD28/CTLA4/ICOS Genes: Role in Malignant Melanoma Susceptibility and Prognosis? Cancer Immunol Immunother (2010) 59(2):303–12. doi: 10.1007/s00262-009-0751-2
102. Welsh MM, Applebaum KM, Spencer SK, Perry AE, Karagas MR, Nelson HH. CTLA4 Variants, UV-Induced Tolerance, and Risk of non-Melanoma Skin Cancer. Cancer Res (2009) 69(15):6158–63. doi: 10.1158/0008-5472.CAN-09-0415
103. Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer Statistics for Adolescents and Young Adults 2020. CA Cancer J Clin (2020) 70(6):443–59. doi: 10.3322/caac.21637
104. Speiser DE, Ho PC, Verdeil G. Regulatory Circuits of T Cell Function in Cancer. Nat Rev Immunol (2016) 16(10):599–611. doi: 10.1038/nri.2016.80
105. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. Pillars Article: CTLA-4 can Function as a Negative Regulator of T Cell Activation. Immunity (1994) 1:405–13. doi: 10.1016/1074-7613(94)90071-X
106. Buchbinder E, Hodi FS. Cytotoxic T Lymphocyte Antigen-4 and Immune Checkpoint Blockade. J Clin Invest (2015) 125(9):3377–83. doi: 10.1172/jci80012
107. Matheu MP, Othy S, Greenberg ML, Dong TX, Schuijs M, Deswarte K, et al. Imaging Regulatory T Cell Dynamics and CTLA4-Mediated Suppression of T Cell Priming. Nat Commun (2015) 6:6219. doi: 10.1038/ncomms7219
108. Dai Z, Tian T, Wang M, Liu X, Lin S, Yang P, et al. CTLA-4 Polymorphisms Associate With Breast Cancer Susceptibility in Asians: A Meta-Analysis. PeerJ (2017) 5:e2815. doi: 10.7717/peerj.2815
109. Das AP, Saini S, Agarwal SM. A Comprehensive Meta-Analysis of non-Coding Polymorphisms Associated With Precancerous Lesions and Cervical Cancer. Genomics (2022) 114(3):110323. doi: 10.1016/j.ygeno.2022.110323
110. Hu P, Liu Q, Deng G, Zhang J, Liang N, Xie J, et al. The Prognostic Value of Cytotoxic T-Lymphocyte Antigen 4 in Cancers: A Systematic Review and Meta-Analysis. Sci Rep (2017) 7:42913. doi: 10.1038/srep42913
Keywords: cancer, cytotoxic T-lymphocyte antigen 4, polymorphism, tumor marker, meta-analysis
Citation: Wan H, Zhou H, Feng Y, Chen Y, Zhu L and Mi Y (2022) Comprehensive Analysis of 29,464 Cancer Cases and 35,858 Controls to Investigate the Effect of the Cytotoxic T-Lymphocyte Antigen 4 Gene rs231775 A/G Polymorphism on Cancer Risk. Front. Oncol. 12:878507. doi: 10.3389/fonc.2022.878507
Received: 18 February 2022; Accepted: 28 March 2022;
Published: 04 May 2022.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesReviewed by:
Azza Mahmoud Kamel, Cairo University, EgyptMujeeb Zafar Banday, Government Medical College (GMC), India
Copyright © 2022 Wan, Zhou, Feng, Chen, Zhu and Mi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijie Zhu, am5keGZ5emxqQDE2My5jb20=; Yuanyuan Mi, bWluaWFvMTk4NEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Hongyuan Wan
Hongyuan Wan Hangsheng Zhou
Hangsheng Zhou Yanyan Feng
Yanyan Feng Yongquan Chen
Yongquan Chen Lijie Zhu
Lijie Zhu Yuanyuan Mi
Yuanyuan Mi