- 1Department of Pediatrics, Children’s Cancer Research Center, Kinderklinik München Schwabing, School of Medicine, Technical University of Munich, Munich, Germany
- 2Klinikum der Landeshauptstadt Stuttgart gKAöR, Olgahospital, Stuttgart Cancer Center, Zentrum für Kinder-, Jugend- und Frauenmedizin, Pädiatrie 5 (Pädiatrische Onkologie, Hämatologie, Immunologie), Stuttgart, Germany
- 3Department of Pediatrics, Children’s Cancer Research Institute-S2IRP, St Anna Children’s Hospital, Medical University, Vienna, Austria
- 4Department of Pediatric Hematology/Oncology and BMT, Wroclaw Medical University, Wroclaw, Poland
- 5Department of Pediatric Hematology and Oncology, Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden
- 6Pediatric Hematology and Oncology, University Hospital Zurich, Zurich, Switzerland
- 7Tampere Center for Child, Adolescent and Maternal Health Research, Faculty of Medicine and Health Technology, Tampere University and Tays Cancer Centre, Tampere University Hospital, Tampere, Finland
- 8Department of Pediatrics, Jena University Hospital, Jena, Germany
- 9Department of Pediatric Hematology and Oncology, Universitätsklinikum Frankfurt, Frankfurt, Germany
- 10Institute of Pathology, School of Medicine, Technical University of Munich, Munich, Germany
- 11Department of Pediatric Hematology and Oncology, Universitätsklinikum Tübingen, Tübingen, Germany
- 12Medizinische Fakultät der Universität Tübingen, Tübingen, Germany
Background: Patients with stage IV alveolar rhabdomyosarcoma (RMA) have a 5-year-survival rate not exceeding 30%. Here, we assess the role of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for these patients in comparison to standard-of-care regimens. We also compare the use of HLA-mismatched vs. HLA-matched grafts after reduced vs. myeloablative conditioning regimens, respectively.
Patients and Methods: In this retrospective analysis, we compare event-free survival (EFS), overall survival (OS), and toxicity of HLA-mismatched vs. -matched transplanted patients in uni- and multivariate analyses (total: n = 50, HLA-matched: n = 15, HLA-mismatched: n = 35). Here, the factors age at diagnosis, age at allo-HSCT, sex, Oberlin score, disease status at allo-HSCT, and HLA graft type are assessed. For 29 primarily transplanted patients, three matched non-transplanted patients per one transplanted patient were identified from the CWS registry. Outcomes were respectively compared for OS and EFS. Matching criteria included sex, age at diagnosis, favorable/unfavorable primary tumor site, and metastatic sites.
Results: Median EFS and OS did not differ significantly between HLA-mismatched and -matched patients. In the mismatched group, incidence of acute GvHD was 0.87 (grade III–IV: 0.14) vs. 0.80 in HLA-matched patients (grade III–IV: 0.20). Transplant-related mortality (TRM) of all patients was 0.20 and did not differ significantly between HLA-mismatched and -matched groups. A proportion of 0.58 relapsed or progressed and died of disease (HLA-mismatched: 0.66, HLA-matched: 0.53) whereas 0.18 were alive in complete remission (CR) at data collection. Multivariate and competing risk analyses confirmed CR and very good partial response (VGPR) status prior to allo-HSCT as the only decisive predictor for OS (p < 0.001). Matched-pair survival analyses of primarily transplanted patients vs. matched non-transplanted patients also identified disease status prior to allo-HSCT (CR, VGPR) as the only significant predictor for EFS. Here, OS was not affected, however.
Conclusion: In this retrospective analysis, only a subgroup of patients with good response at allo-HSCT survived. There was no survival benefit of allo-transplanted patients compared to matched controls, suggesting the absence of a clinically relevant graft-versus-RMA effect in the current setting. The results of this analysis do not support further implementation of allo-HSCT in RMA stage IV patients.
Introduction
Alveolar rhabdomyosarcoma (RMA) is a highly malignant pediatric soft tissue sarcoma entity, predominantly characterized by the oncogenic fusion gene forkhead box protein O1 (FOXO1) from chromosome 13 with either paired box protein 3 or 7 (PAX3, PAX7) from chromosome 2 or 1 [t(2;13)(q35;q14) or t(1;13)(p36;q14)] (1–3). Typical clinical manifestation of the primary disease includes extremities, the head and neck region, and other localizations (i.e., perineal and perianal) (4, 5). Unfavorable localization of the primary tumor, such as extremities, implicates a higher risk for relapse and poorer prognosis (6, 7). For non-metastatic RMA, estimated 5-year overall survival (OS) rate is 50%–65% (5, 8). With metastatic disease at diagnosis, 5-year-survival rates do not exceed 30% (6, 9). All rhabdomyosarcoma subtypes present with metastatic bone marrow (BM) involvement in approximately 6% of cases (10). In this disease constellation, 3-year event-free survival (EFS) is about 14% compared to 34% without BM disease (11, 12).
For metastasized disease and/or diagnosis at age > 10 years and/or bone/BM metastases, experimental therapeutic approaches currently under early clinical investigation can be considered CWS-guidance (version 1.6.1). Therefore, allogeneic hematopoietic stem cell transplantation (allo-HSCT) was implemented in some of these patients, hypothesizing the presence of a clinically relevant graft-versus-tumor effect (13, 14). It is unclear whether the use of allo-HSCT yields a survival benefit in the consolidation of prior complete response (CR) vs. non-remission at the time of transplantation as prospective studies are missing (15, 16).
Within the HD CWS-96 study, toxicity rates in patients treated with high-dose (HD) chemotherapy and autologous rescue vs. oral chemotherapy maintenance (OMT) were higher with significantly worse OS outcomes (17). The use of reduced-intensity conditioning regimens (RIC) prior to haploidentical HSCT was growingly implemented to enable a hypothesized graft-versus-RMA effect based on HLA disparity in the last decade.
In this retrospective analysis, we hypothesized that HLA-mismatched compared to HLA-matched allo-HSCT would increase survival rates in RMA patients, indicative of a graft-versus-RMA effect associated with HLA disparity. Furthermore, we hypothesized that a graft-versus-RMA effect would increase EFS and OS of transplanted- vs. matched non-transplanted RMA patients. Primary study objectives in both cases were EFS and OS.
Patients and Methods
Study Design and Data Acquisition
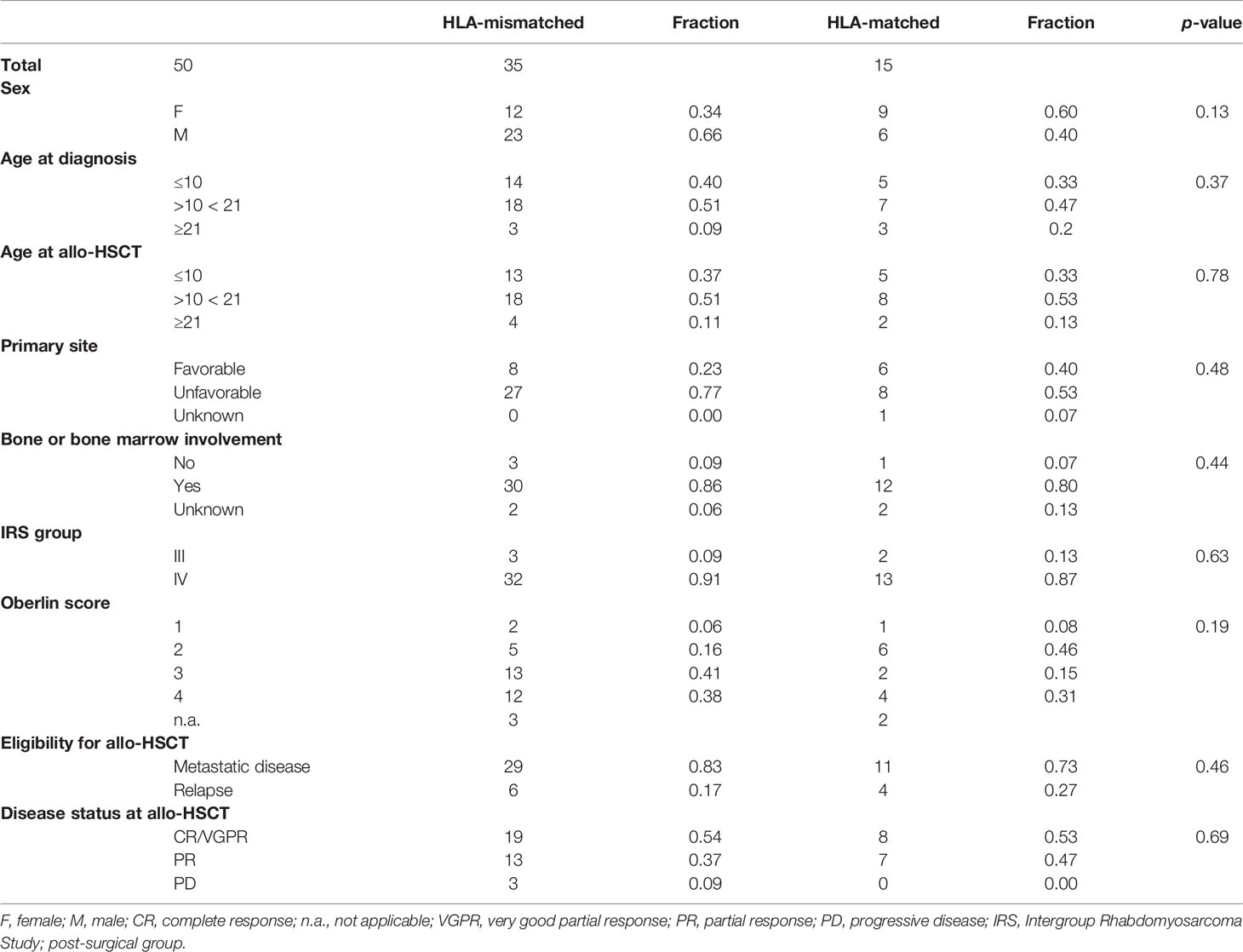
Data were retracted from the Cooperative Weichteilsarkom Studiengruppe (CWS) European soft tissue sarcoma registry (SoTiSaR) after approval by the principal investigators’ institutional ethical commission. For 50 RMA patients (stage III–IV), information concerning patient history and therapeutic interventions were used for further evaluation. All patients were diagnosed with RMA after the year 2000. Database update and last data collection was June 2020. RMA was confirmed by a local and expert pathologist, with genetic confirmation of PAX3/PAX7-FOXO1 fusions in 35 of 38 cases; 3 cases were fusion-negative. Molecular diagnosis was not done in 12 cases.
Definitions
Engraftment was defined as absolute neutrophil count (ANC) ≥0.5 × 109/L. Engraftment failure: ANC <0.5 × 109/L by day +28 after allo-HSCT. When patients succumbed within ≤100 days after allo-HSCT, status of chronic GvHD could not be assessed. Further information on chronic GvHD was not available, due to limited recording in the past. Transplant-related mortality (TRM) was defined as any transplant-related death occurring after allo-HSCT without evidence of underlying disease or disease progression. Death of disease (DOD) was defined as any death related to underlying disease progression or relapse. Response was evaluated as follows: progressive disease (PD) was defined as tumor volume progression ≥50%, partial remission (PR) was defined as tumor volume reduction ≥50%, very good partial response (VGPR) was defined as tumor volume reduction ≥90% or persistence of unclear residuals upon imaging, and complete response (CR) was defined as complete disappearance of all visible disease. Measurement of tumor volume was done according to CWS guidance (1.6.1) using the formula V = π/6 × length × width × thickness. HLA-mismatched was defined as ≤9/10 differing HLA-I and -II alleles. In case of the comparison within HLA-mismatched versus HLA-matched setting, survival data were calculated from the time of 1st allo-HSCT until an event (i.e., relapse—EFS or TRM) or DOD for OS. For matched-pair analysis, survival was calculated from first therapeutic intervention until an event occurred (relapse, TRM, or DOD). Glucksberg criteria were used to grade GvHD.
Patients
The study group consisted of a total of 50 transplanted patients, with 21 female patients (0.42) and 29 male patients (0.58). Median age at diagnosis was 14 years (range 0–24 years) with a median age at allo-HSCT of 14.0 years (range 0–24 years). Comparable to previously published analyses (18), patients were divided into two groups: HLA-mismatched (n = 35) and HLA-matched (n = 15). In the HLA-mismatched group, graft source was sibling in 2 patients, maternal in 17 patients, paternal in 15 patients, and both maternal and paternal in one patient. For HLA-matched patients, graft source was sibling in 14 (sister in 10/14 patients and brother in 4/14 patients). Patients qualified for allo-HSCT due to stage IV metastatic disease and/or age > 10 years and/or bone/bone marrow metastases at diagnosis or relapsed disease. Oberlin scores were used to assess further risk stratification (6). After conditioning therapy, 27/50 (0.54) patients were transplanted in CR or VGPR and 23/50 (0.46) patients were transplanted in PR or PD. Forty out of 50 (0.80) patients received primary allo-HSCT due to their very high-risk disease and/or lack of response to induction therapy. Allo-HSCT as relapse therapy was implemented in 10/50 patients (0.20). As a planned high-dose induction, 5/50 (0.10) received myeloablative chemotherapy and autologous HSCT (HLA-mismatched group = 4, HLA-matched group = 1) prior to allo-HSCT. Sex, age at diagnosis, age at allo-HSCT, primary site (favorable/unfavorable), bone or bone marrow involvement, stage, Oberlin score, eligibility for allo-HSCT, and disease status at allo-HSCT did not differ significantly in between groups A and B (Table 1). Four patients underwent multiple allo-HSCT, either due to relapse after 1st allo-HSCT (n = 2) or engraftment failure of the 1st allograft (n = 2). Mainly, transplants consisted of peripheral blood-derived stem cells (49 of 55 applied allografts, fraction 0.89), and 5/55 allografts (0.09, 1/55 source unknown) were derived from bone marrow. Treatment decisions were in concordance with approvals of institutional review boards and in accordance with the Helsinki Conference Declaration. Informed consent was signed by all patients or their legal guardians before initiation of therapy.
Conditioning Regimens, Graft Manipulation, GvHD Prophylaxis, and Systemic Chemotherapies
Reduced-intensity conditioning (RIC) regimens (n = 37) were mostly based on fludarabine (n = 34) or clofarabine (n = 3), fludarabine monotherapy (n = 1), or in combination with either of the following: busulfan (BU, n = 3), cyclophosphamide (CYC, n = 7), etoposide (ETO, n = 3), melphalan (MEL, n = 31), thiotepa (TT, n = 34), or treosulfan (n = 1). Also, in most cases, either antithymocyte globuline (ATG, n = 11) or muromonab (OKT3, n = 18) was added. Total body irradiation (TBI) was performed in a minority of cases (n = 5). As for myeloablative condition regimens (MAC, n = 11), BU/CYC/ETO (n = 3), BU/TT/CYC (n = 1), BU/MEL/CYC (n = 1), BU/CYC/ATG (n = 1), CYC/TT/ATG (n = 1), CYC/treosulfan/ETO (n = 1), high-dose-ETO/ifosfamide (n = 1), and treosulfan/MEL (n = 1) were applied. TBI was given in 2 cases. Conditioning regimens were unknown in 2 cases.
Graft manipulation for HLA-mismatched grafts mainly consisted of CD3/CD19-depletion (n = 21, with TCRalpha/beta/CD19-depletion instead of CD3/CD19-depletion in 2 cases). CD3/CD19 depletions were combined with CD34 selection in 4 cases. CD34 selection was done alone in 3 cases, or in combination with T-cell depletion (n = 1). T-cell depletion alone was performed in 3 cases. Graft manipulation was unknown in 6 cases. Only one HLA-mismatched graft was not manipulated. HLA-matched grafts were not manipulated (n = 5), selected for CD34+ cells (n = 3), or CD3/CD19-depleted (n = 2). Status was unknown in 5 cases.
GvHD prophylaxis in the HLA-mismatched settings mainly contained mycophenolate mofetil (MMF, n = 27) alone or in combination with either corticosteroid, tacrolimus, post-transplant CYC, or ATG. Also, cyclosporine A (CSA) and methotrexate (MTX) were applied in/as combination. GvHD prophylaxis in the HLA-matched settings mainly composed of CSA (n = 11) alone or in combination with either MMF, tacrolimus, corticosteroid, MTX, sirolimus, or OKT3.
HLA-mismatched patients (n = 35) were treated according to protocols as follows: CWS96 (n = 2), CWS2000P (n = 9), CWS-IV-2002 (n = 6), SoTiSaR (n = 17), and one non-CWS regimen. HLA-matched patients (n = 15) were treated according to the following protocols: CWS96 (n = 6), CWS2000P (n = 5), SoTiSaR (n = 2), MMT-98 (n = 1), and one non-CWS regimen. Transplanted patients utilized for matched-pair analysis (n = 29) received systemic therapies according to CWS96 (n = 2), CWS2000P (n = 9), CWS-IV-2002 (n = 3), and SoTiSaR (n = 15). Non-transplanted patients (n = 87), who served as controls in the matched-pair analysis, were treated according to protocols as follows: CWS96 (n = 11), CWS2000P (n = 29), CWS-IV-2002 (n = 9), and SoTiSaR (n = 38).
Matched-Pair Analysis
Within the CWS database, three matching patients per one transplanted patient were identified for the analysis. For 29 transplanted patients, who received primary allo-HSCT for their high-risk/progressive disease, matching non-transplanted pairs could be identified. Here, 22 transplanted patients received HLA-mismatched grafts and 7 patients received HLA-matched allografts. Matching criteria for further statistical analyses consisted of sex, age at diagnosis, favorable/unfavorable tumor site, and metastatic sites (locations). Primary systemic therapies for transplanted patients were either CEVAIE-based (n = 22) or VAIA-based (n = 7). CEVAIE is an intensive chemotherapy consisting of alternating courses of ifosfamide, vincristine, actinomycin-D (I3VA), carboplatin, epirubicin, vincristine (CVE) and ifosfamide, vincristine, and etoposide (I3VE, CWS guidance v1.6.1). VAIA is another chemotherapy regimen, currently used for the very high-risk group or metastatic stage in past protocols, implementing alternating cycles and combinations of vincristine, adriamycin, ifosfamide, and actinomycin-D. Primary systemic therapies for non-transplanted controls mainly included CEVAIE (n = 55) with O-TI/E (43/55) or were VAIA-based (n = 28) with O-TI/E (21/28), unknown in 4. The specific matching criteria and patients’ characteristics are summarized in Table 4.
Statistical Analyses
End point values were recorded upon the last patient follow-up. Last database update was done in June 2020. Statistical analyses were performed using R studio 1.3.1093 (Public-benefit Corp), R for macOS X Cocoa GUI 4.1.0 (The R foundation for Statistical Computing) and Prism for macOS 9.1.2 (GraphPad Software). Fisher’s exact, Chi-square test, or Chi-square test for trend was applied to compare categorical variables in between study and control groups (for baseline characteristics). In multivariate analysis subdistribution hazard ratios (HR), standard errors, and confidence intervals (CI) are presented when appropriate. Survival probabilities were calculated using Kaplan–Meier curves and compared with a log-rank (Mantel-Cox) test. Cumulative incidence function and multivariate analyses were applied according to Scrucca et al. (19, 20). Statistical significance was attributed to p-values < 0.05.
Results
Engraftment and GvHD
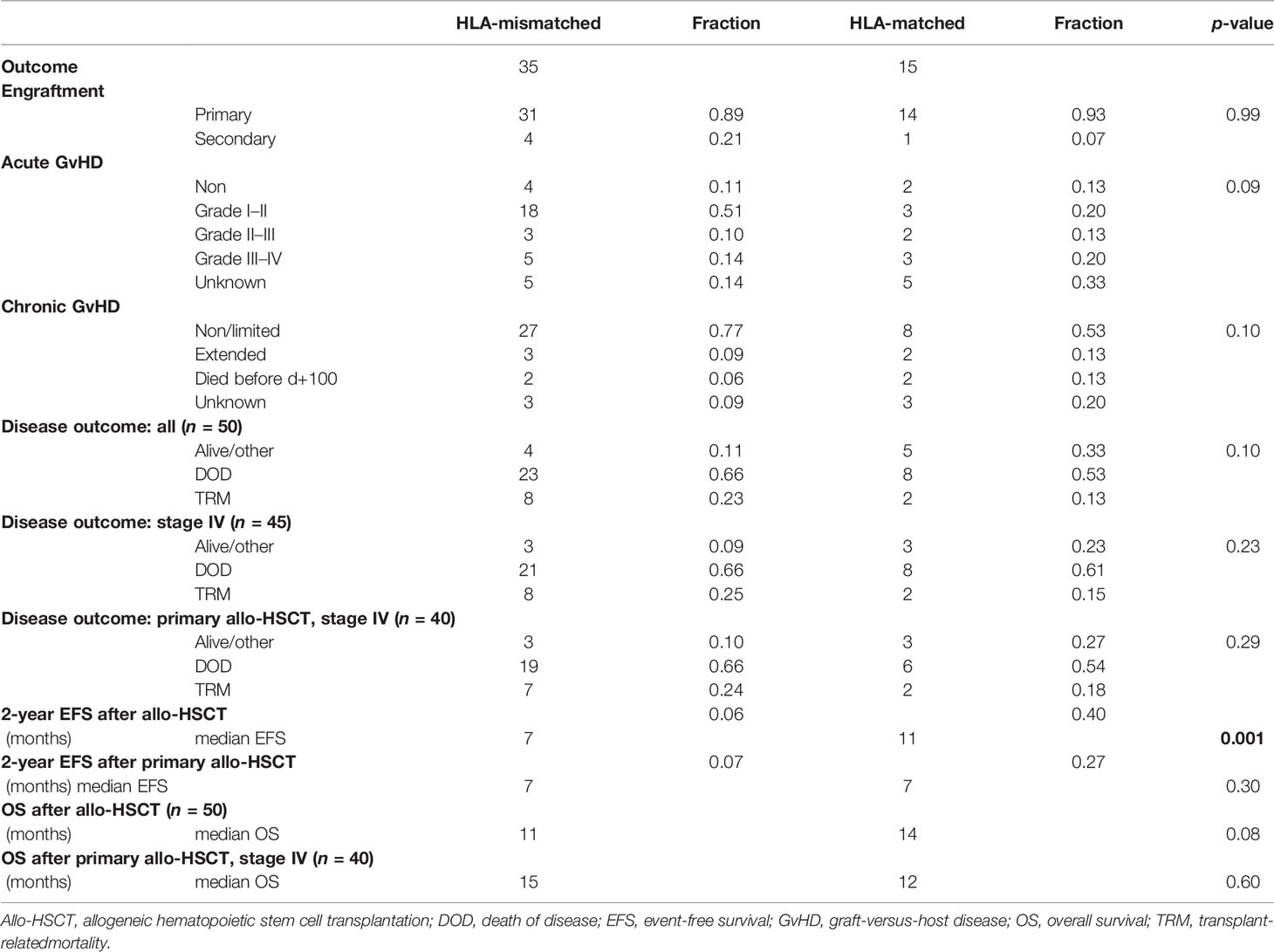
Primary engraftment was stated in 45/50 (0.90) of cases; the rest experienced secondary engraftment. Median time to engraftment was 12 days (HLA-mismatched group, short mismatched: 13, HLA-matched group, short matched: 12). Incidence of acute GvHD in all analyzed patients was 0.85 (mismatched: 0.87 and matched: 0.80). No acute GvHD was reported in 15% of all cases (mismatched: 0.13 and matched: 0.20). Combined incidence with grade I–II acute GvHD was 0.66 (mismatched: 0.73 and matched: 0.50) indicating absent, or little to moderate toxicity in most of the cases. Incidence of extended chronic GvHD mounted up to 0.11 for all patients (mismatched: 0.09 and matched: 0.16). Status was unknown or not assessed in 10/50 cases for acute GvHD and 6/34 cases for chronic GvHD. Incidence of both acute and chronic GvHD did not differ significantly in between groups. More detailed information is provided in Table 2.
Survival and Cause of Death—HLA-Mismatched Versus -Matched Grafts
Next, time intervals from the date of 1st allo-HSCT until occurrence of relapse, DOD, or TRM were compared. At the end of data collection, a total of 9/50 patients (0.18) were alive (or died of reasons unrelated to the primary disease or transplantation, labeled as “other”), 31 patients (0.61) died of primary disease (DOD), and for 10 patients, TRM (0.20) was reported, all without statistically significant differences in between groups. A more detailed overview of disease outcomes is given in Table 2. Mean follow-up time for patients grouped to alive/other was 45.8 months (mismatched: 24.5, matched: 62.8), 15.7 months for DOC (mismatched: 13.4, matched: 22.6), and 11.5 months for TRM (mismatched: 11.1, matched: 13.0), respectively. Although Oberlin risk factors were distributed without statistical significance in between HLA-mismatched and -matched patients, 0.79 of HLA-mismatched patients exhibited an Oberlin score ≥3, whereas only a fraction (0.46) in the HLA-matched group had a score ≥3 (Table 1, Figure 1A). Here, the 3 longest survivors (OS in months per patient: 130, 78, and 77) either had an Oberlin score of 1, or were not metastasized at diagnosis and all three received allo-HSCT as relapse therapy. Additionally, 2/3 had favorable primary tumor localization, 2/3 were younger than 10 years, and 2/3 had VGPR/CR at the time of allo-HSCT. As for the HLA-mismatched group, there was only one patient with a comparable distribution of risk factors and response to induction therapy, who survived for 21 months before DOD. As a consequence, 2-year EFS was significantly higher in the HLA-matched vs. -mismatched group (p-value = 0.001), with a median EFS of 7 months for mismatched and 11 months for matched (p-value = 0.051; median OS—mismatched: 11 months and matched: 14 months, p-value = 0.08) (Table 2). Figure 1B depicts survival curves according to Oberlin score. When analyzing the more homogeneous group of patients with primary allo-HSCT due to stage IV disease at diagnosis (n = 40), 2-year-EFS and median OS did not differ significantly (p-value = 0.46). Here, both groups showed a median EFS of 7 months and median OS for the mismatched group was 15 months versus 12 months for the matched group (p-value = 0.60). As most patients in the matched group received myeloablative conditioning (MAC, 0.86) and most HLA-mismatched cases received reduced-intensity conditioning (RIC, 0.91), we also precluded an effect due to different conditioning regimens (p-value = 0.45; Figure 1C) in a consecutive analysis.
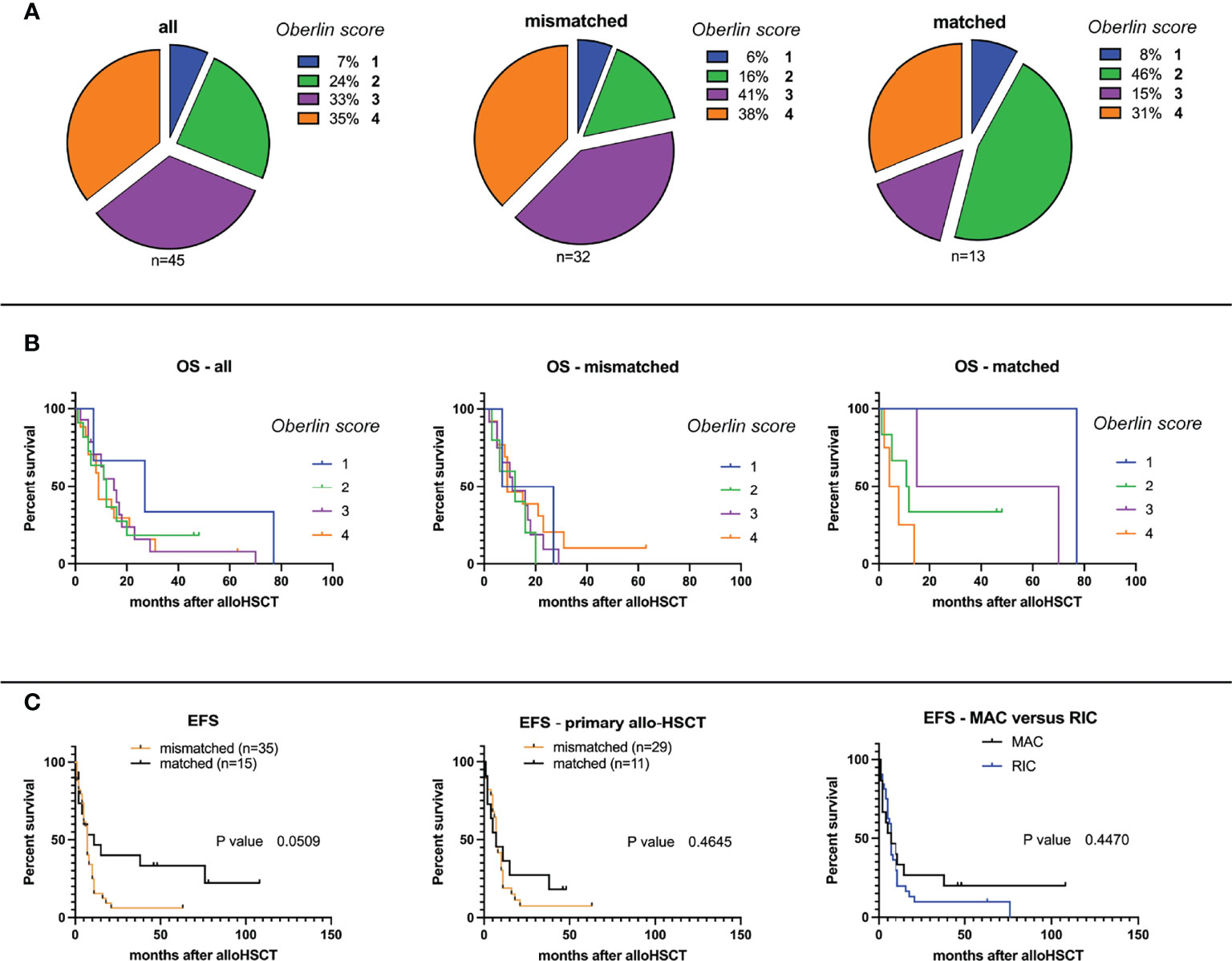
Figure 1 (A) Overall and event-free survival probabilities according to Oberlin score, HLA-mismatch/match, and conditioning regimen. Distribution of Oberlin risk factors (0–4) within study groups for HLA-mismatched and -matched analyses. (B) Overall survival (OS) probabilities after allogeneic hematopoietic stem cell transplantation (allo-HSCT) within study group population: all, HLA-mismatched, and HLA-matched (from left to right). (C) Event-free survival (EFS) probabilities after allo-HSCT for HLA-mismatched versus -matched grafts for all transplanted patients (left), primarily allo-transplanted patients (middle), and separated by myeloablative (MAC) or reduced-intensity conditioning (RIC, right).
As TRM was relatively high, with a fraction of 0.20 in all analyzed cases, we furthermore performed a competing risk analysis by calculating the cumulative incidence function (CIF) for relapse, and separating it from TRM (as it will prevent emergence of later relapse) (19). Equality testing across groups revealed that there was no significant difference for relapse (p-value = 0.27) and TRM (p-value = 0.39). Differences in median OS were not observed even when excluding stage IV patients with TRM from the analysis (Figures 2A, B).
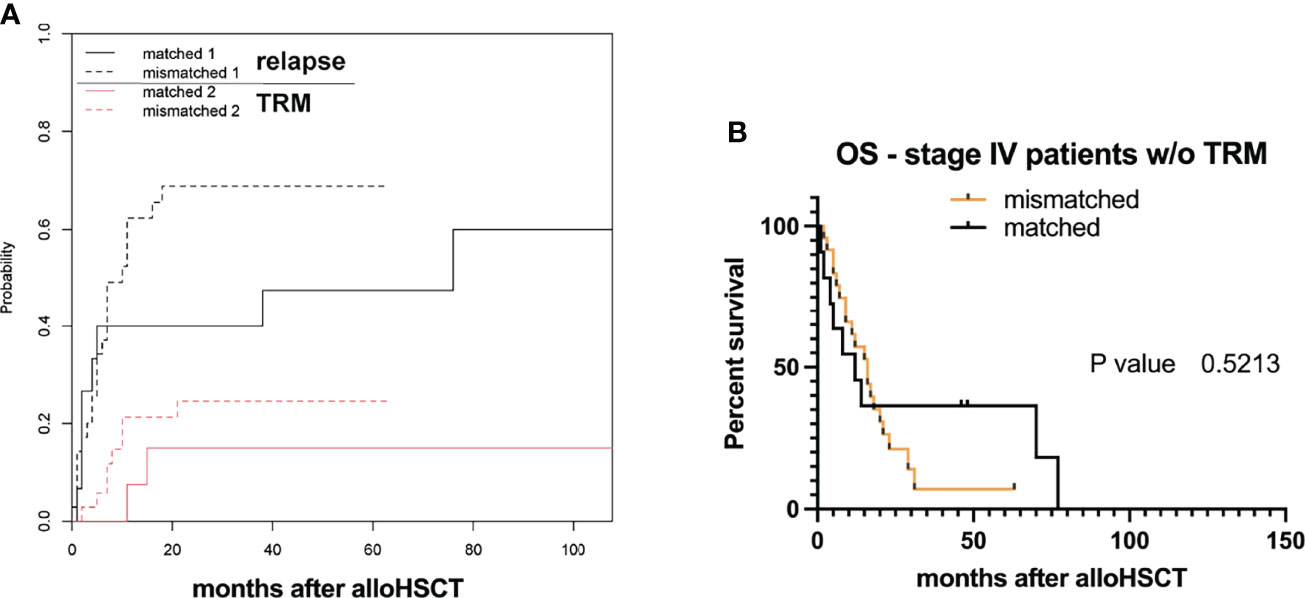
Figure 2 Transplant-related mortality (TRM) and survival. (A) Estimated cumulative incidence curves with relapse and transplant-related mortality (TRM) as competing events after allo-HSCT for HLA-mismatched versus -matched grafts. (B) Overall survival (OS) probabilities after allogeneic hematopoietic stem cell transplantation (allo-HSCT) within study group populations neglecting TRM (w/o without).
Multivariate Regression Modeling
To confirm results of univariate analyses and to further identify additional factors decisive for survival, we performed multivariate regression modeling for competing risks data according to Scrucca et al. (20). Factors with possible influence on EFS and OS were analyzed, namely: age at diagnosis, age at allo-HSCT, Oberlin score (1–4, when applicable), disease status at allo-HSCT (VGPR/CR, PR, and PD), and graft type (HLA mismatch/match).
Multiple models including different combinations of these covariates were fitted and compared (Supplementary Table 1). To select the best fit, the Bayesian information criteria (BIC) were applied. The ultimately selected model included disease status at allo-HSCT and Oberlin score. Only disease status correlated with outcome [p-values with respect to baseline (PD) for PR: p-value < 0.05 for EFS and OS; VGPR/CR: p-value < 0.001 for EFS and OS]. Therefore, CIF was calculated for relapse and death (DOD, TRM) depending on disease status at allo-HSCT (phase: VGPR/CR, PR, or PD, Figure 3). Here, disease status at allo-HSCT significantly correlated with outcomes. A summary of regression modeling for competing risks is provided in Table 3.
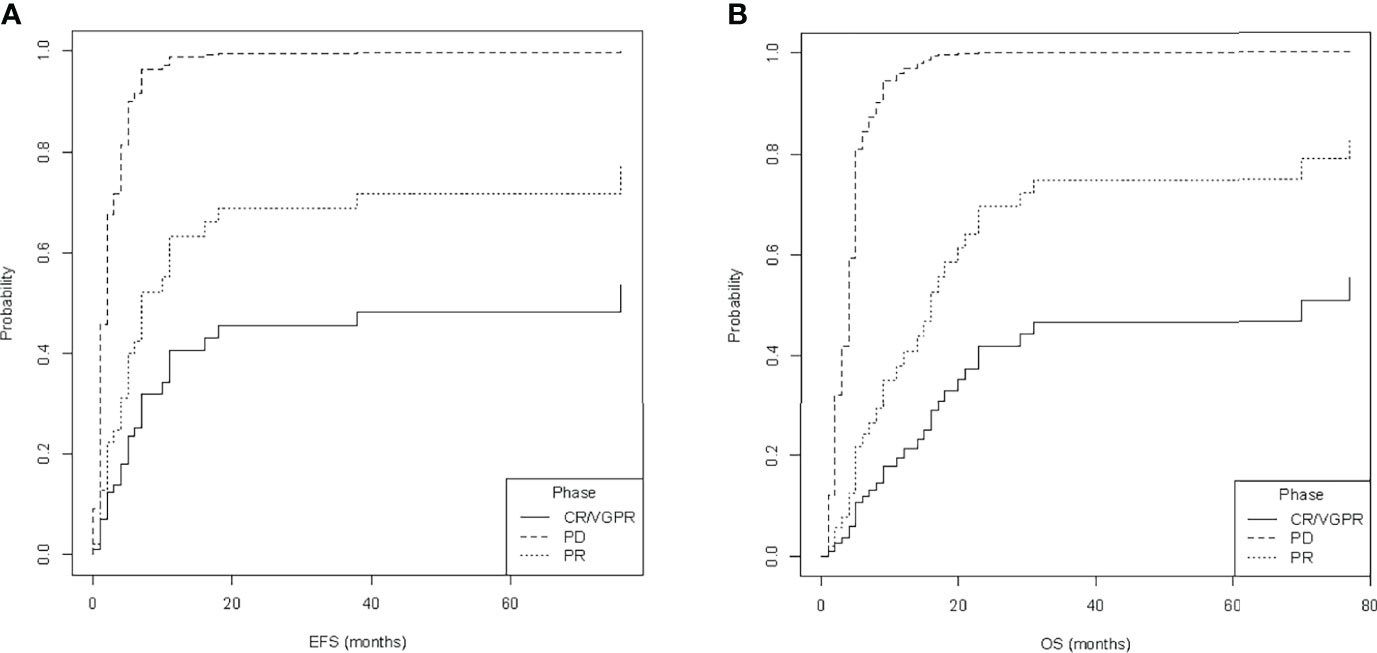
Figure 3 Estimated cumulative incidence of (A) relapse and (B) death, depending on the status at allogeneic hematopoietic stem cell transplantation (phase at allo-HSCT), namely, complete response or very good partial response (CR/VGPR), partial response (PR), and progressive disease (PD). Data of event-free survival (EFS) and overall survival (OS) were used for calculations.
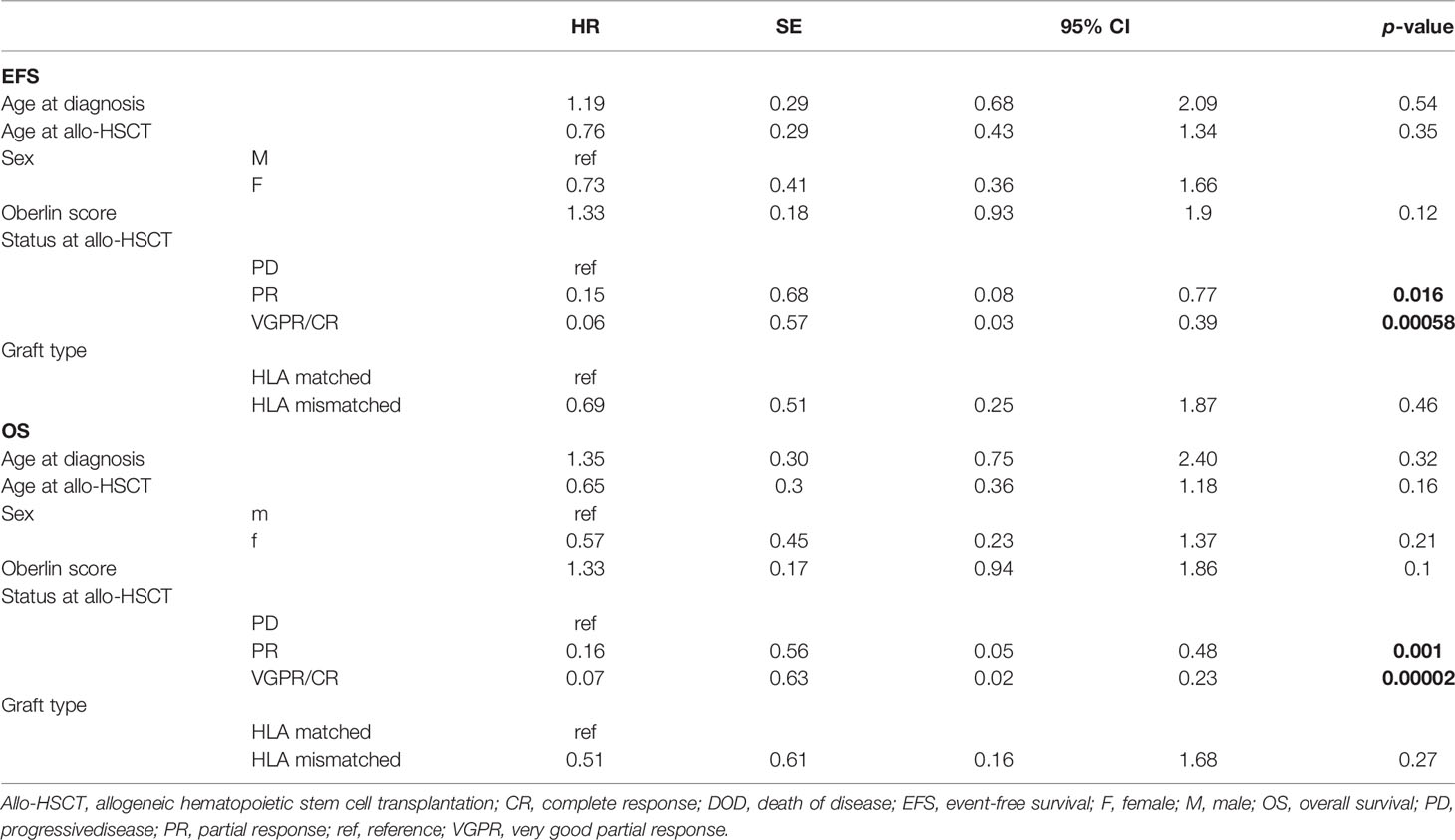
Table 3 Results of univariate and multivariate regression modeling for competing risks: model including all available covariates.
Survival Analysis Compared to Matched Pairs
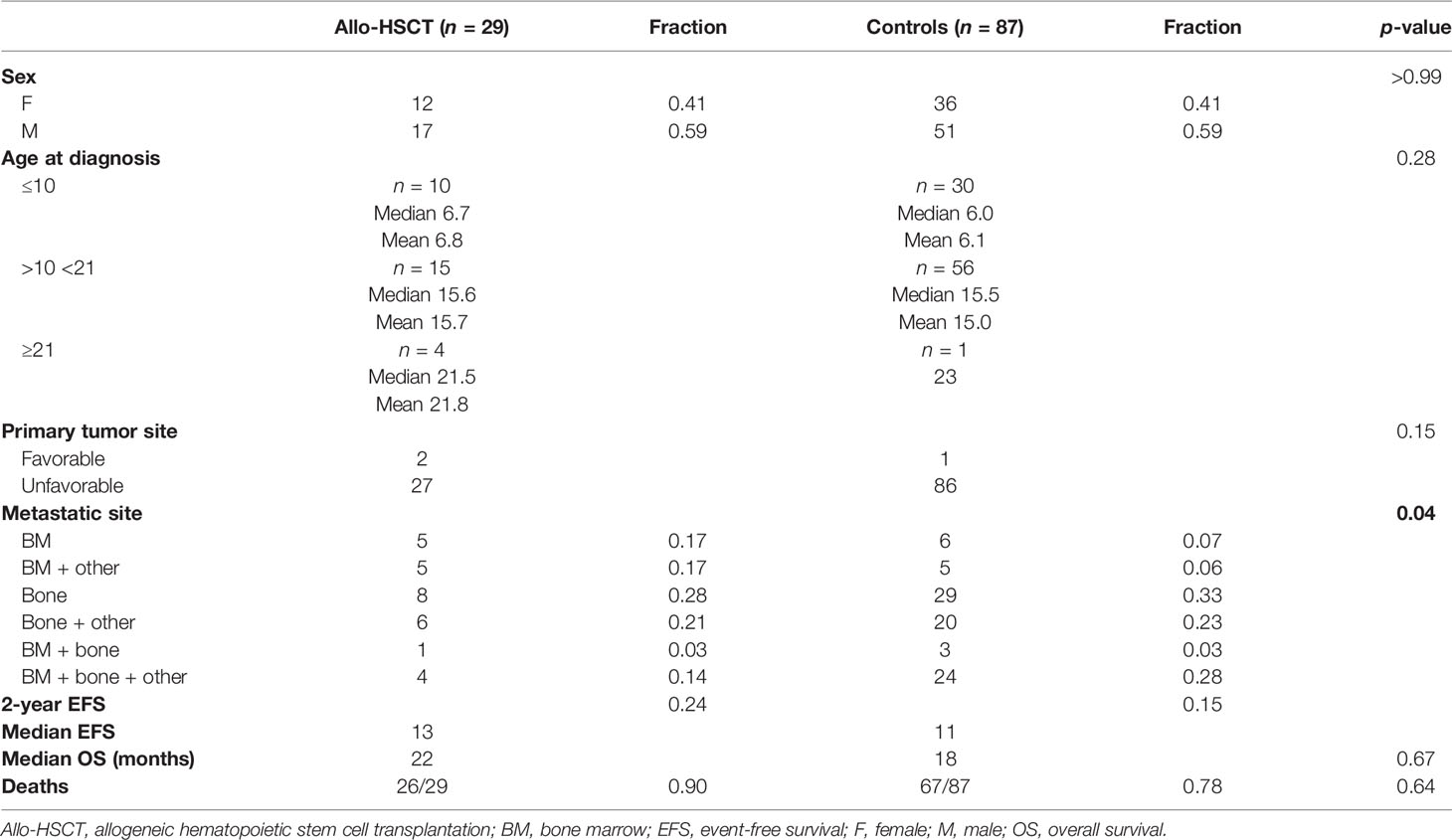
To address the question whether allo-HSCT provides survival benefits compared to standard-of-care therapy, we performed a matched-pair analysis. For 29 primary transplanted patients, matched non-transplanted pairs (3 non-transplanted per 1 transplanted patient) from CWS SoTiSaR were identified and compared for OS and EFS. Matching criteria included sex, age, favorable/unfavorable tumor site, and metastatic site. Table 4 provides relevant patient characteristics according to matching criteria.
For 2 primary transplanted patients, only 2 instead of 3 matched pairs were included for survival analysis. For the 4 transplanted patients aged ≥21 years, 11/12 matched patients were younger than 21 years. Of 2 transplanted patients presented with favorable tumor site at diagnosis, only one favorable matched control was identified.
As distribution in metastatic sites indicated a trend of unequal distribution in between study and control group (tested with Chi-square test for trend, with a tendency towards worse constellation of metastatic sites in the control group), which might influence survival analyses, regression modeling for competing risks was conducted accounting for metastatic site. No significant influence on survival was found (Wald test p-value = 0.08) (20).
EFS probability for study and control groups did not differ significantly (p-value = 0.42) with a median EFS of 13 months for primary transplanted patients versus 11 months for controls (Figure 4A). Similar to EFS, probability to survive (i.e., OS) was not different (p-value = 0.67) with a median OS of 22 months for study group patients versus 18 months for controls (Figure 4B). As status at allo-HSCT was a condition for survival (CR or VGPR) in our previous analyses, we furthermore asked whether patients transplanted in CR/VGPR had a potential benefit from allo-HSCT compared to standard-of-care patients (controls). Indeed, respective patients show an increased survival probability for EFS (median 17 versus 10 months, p-value = 0.04). However, no differences were observed for OS (median 27 versus 17 months, p-value = 0.15, Figures 4C, D), even when neglecting cases of TRM (p-value = 0.12, graph not shown) or only focusing on those who received HLA-mismatched grafts (Kaplan–Meier curve comparison for EFS—p-value = 0.063 and for OS—p-value=0.09, graph not shown).
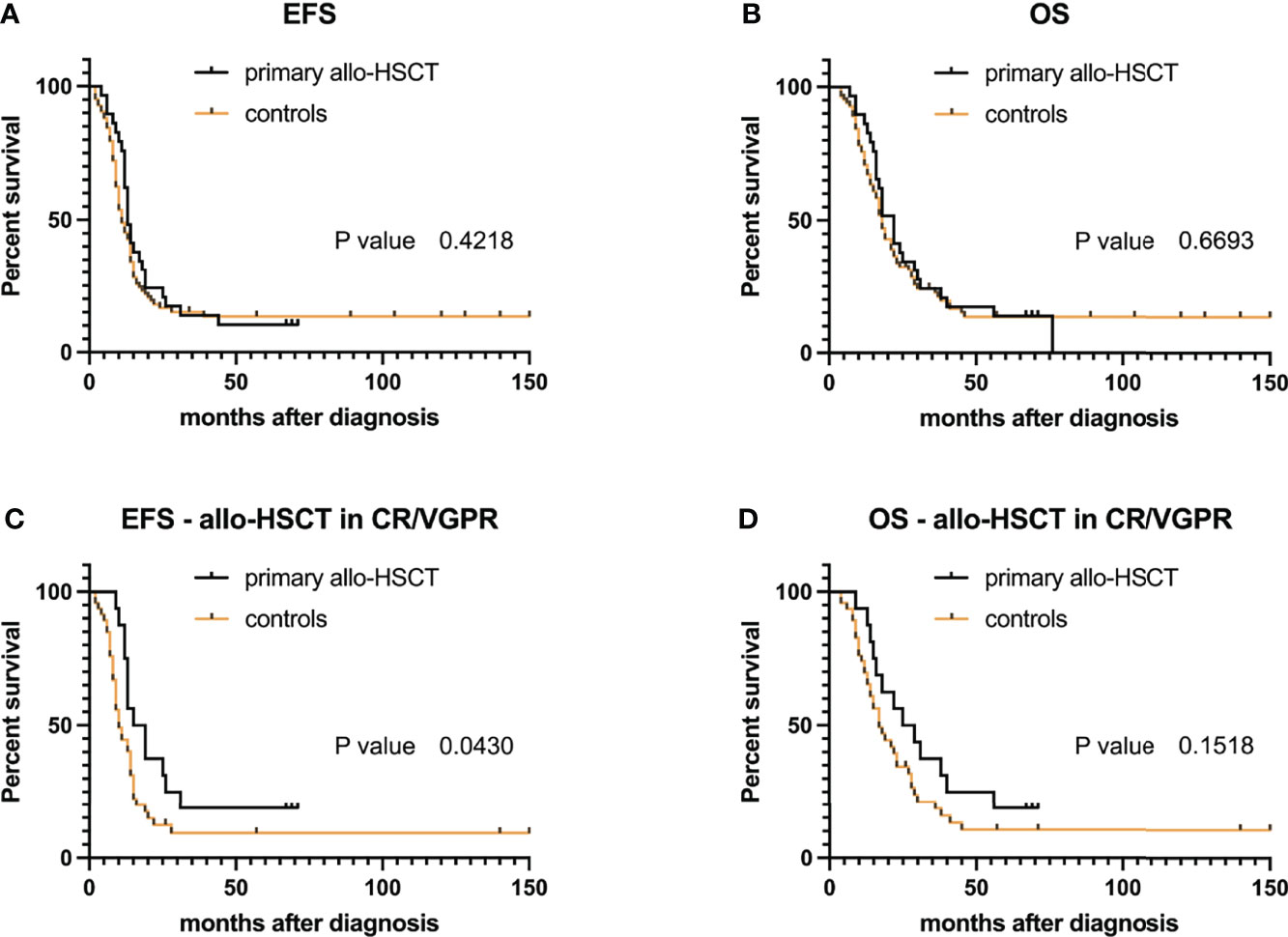
Figure 4 Event-free survival (EFS) and overall survival (OS) probabilities after allogeneic hematopoietic stem cell transplantation (allo-HSCT) compared to matched non-transplanted controls: for all 29 primary transplanted RMA patients (A, B). (C, D) EFS and OS probabilities for patients with CR/VGPR at time of allo-HSCT (n = 16).
Discussion
In this analysis, we hypothesized that (1) HLA-mismatched compared to HLA-matched allo-HSCT leads to survival rates in RMA patients, indicative of a graft-versus-RMA effect. Furthermore, we hypothesized that (2) a clinically relevant graft-versus-RMA effect improves survival in comparison to non-transplanted patients with similar risk factors. Data of patients diagnosed after 2000 were extracted from the CWS registry SoTiSaR. We sought to elaborate whether tumor control by allografts, and in this setting also HLA disparity (mismatched grafts), can be observed. As stage IV disease was the reason for allo-HSCT in most cases, its potential as part of the primary treatment should be evaluated.
In a previous retrospective analysis (15), evaluating the role of allo-HSCT for advanced rhabdomyosarcoma, we did not address the role of HLA-mismatched versus HLA-matched to induce a hypothesized graft-versus-tumor effect due to low frequency of haploidentical transplantations at that time. With better standardization and quality of ex vivo graft manipulation (e.g., CD3/CD19 depletion) (21), there has been a shift towards the use of haploidentical/mismatched grafts. In our analysis, we did not observe significant differences in between both study groups (mismatched vs. matched) in regard to EFS and OS. To minimize selection bias and competing risks, we performed multivariate analyses. Patient populations were small (mismatched n = 35, matched n = 15), imposing certain limitations in the statistical evaluation. Also, the mere fact that most mismatched grafts were T cell-depleted, whereas T-cell depletion was only done in 1/15 HLA-matched grafts, might have masked possible survival benefits in the HLA-mismatched group. An additional limitation constituted the heterogeneous treatment prior to transplantation. However, to our knowledge, this is the first systematic, albeit retrospective, evaluation of RMA patients comparing HLA-mismatched and -matched transplantation settings, as well as assessing a possible survival benefit compared to a non-transplanted control group.
In the present study, incidence of acute and chronic GvHD was comparable to other studies published for RMS (15, 22, 23) or EwS (18). Notably, TRM in our analysis (0.20) is higher than described for RMS by Merker et al. (22) (0.12; 17 of 25 studied patients with RMA) or Thiel et al. (15) (0.13; 23 of 30 studied patients with RMA) but did not differ significantly in between group A and B. The higher incidence of TRM, in comparison to earlier reports, might be due to a different and updated patient population, longer observation periods in our study, and differences among transplantation centers. Nonetheless, this retrospective analysis describes a relatively homogeneous population of 50 RMA patients. Considering TRM despite ex vivo graft manipulation is of utmost importance, although novel techniques for GvHD prophylaxis, such as post-transplant cyclophosphamide, were only implemented in one of the herein reported patients (21, 24). None of the patients with PD at allo-HSCT survived. This is also in line with published reports (15). In our analysis, response to induction therapy is the only reliable predictor for survival. Our data demonstrate that allo-HSCT in RMA patients is not associated with improved survival compared to matched controls (mainly treated with CEVAIE and OMT). Here, all transplanted survivors responded to induction therapy (6/9 patients had CR/VGPR). Additionally, 3/9 survivors were not metastasized at diagnosis, 3/9 had an Oberlin score of 2. Interestingly, one survivor with an Oberlin score of 4 and bone marrow disease was identified in the HLA-mismatched group. This constellation was recently described as fatal (12).
Although our matched-pair analysis revealed a higher probability of EFS for transplanted patients, it did not translate into increased OS. Hence, a clinically relevant graft-versus-RMA effect in our study population was not observed. Similar to high-dose chemotherapy followed by autologous rescue, patients treated with allo-HSCT might fare worse than OMT for IRS group/stage IV patients.
We previously described possible survival benefits from additional post-transplant immune-therapeutic strategies involving donor lymphocyte infusion (DLI) (25). In fact, one of the herein reported patients had BM disease at diagnosis with an Oberlin score of 4, responded very well to induction therapy (CR/VGPR), and survived without relapse at least 63 months after allo-HSCT. In this exceptional case, BM disease was controlled; hence, a graft-versus-tumor effect in this patient to control or maintain CR cannot be excluded.
Genetic engineering of T cells (e.g., with a chimeric antigen receptor, CAR) is a novel and promising approach, which may be implemented in patients with RMS in early clinical trials (NCT00902044). Indeed, in one case report utilizing a HER2 (human epidermal growth factor receptor 2)-specific CAR-T cell product, manufactured from autologous lymphocytes, repetitive application induced CR despite BM involvement. A second relapse after adoptive transfer was again treated with HER2-specific CAR-T cells combined with immune checkpoint blockade. This induced a third CR, which was ongoing for at least 20 months after cessation of T cells (26). Modified DLI with CAR-containing T cells might also be applied as an additive when considering allo-HSCT. This might be helpful to abrogate a T cell-hostile, immunosuppressive tumor microenvironment (27), due to additional cellular effectors in the graft [i.e., NK cells (28)], compared to CAR T-cell monotherapy.
In summary, in this retrospective study, only a subgroup of patients with good response at allo-HSCT survived. For stage IV patients, there was no survival benefit for either HLA-mismatched or HLA-matched transplant settings. Also, allo-transplanted patients with metastasis did not survive longer compared to matched controls, hinting at the absence of a clinically relevant graft-versus-RMA effect in our cohort. The results of this study do not support further implementation of allo-HSCT for stage IV RMA patients, especially with Oberlin scores ≥3, which is in accordance with many experts in the field (22, 29). We would like to emphasize though that this conclusion does not exclude the possible value of DLI in the treatment of RMA patients. The latter aspect may constitute a tool to initiate T-cell-mediated antitumor responses (25). This aspect, as well as the role of allo-HSCT, has not yet been elucidated sufficiently in prospective trials.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: After ethical approval, relevant data were retracted from the Soft Tissue Sarcoma Registry (SoTiSaR) from the European Cooperative Weichteilsarkom Studiengruppe (CWS) containing sensitive information with potentially identifiable human data. Hence, relevant information supporting the conclusions of the manuscript are summarized in tables provided together with the manuscript. Requests to access these datasets should be directed to EH,ZXJpa2EuaGFsbG1lbkBvbGdhaG9zcGl0YWwtc3R1dHRnYXJ0LmRl.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkommission der Technische Universität München. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Conception and design: SS and UT. Provision of study materials or patients: EH, AW, IL, RL, BK, GL, FN, OL, JH, BG, TK, PB, SB, PL, MS-S, and EK. Data analysis and interpretation: SS, EH, FR, HG, CP, EK, and UT. Manuscript writing: SS, HG, and UT. Final manuscript editing: SS, HG, CP, IL, TK, OL, MS-S, EK, and UT. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a grant to SS and UT from the Wilhelm Sander-Stiftung (2021.007.1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors want to express their thanks to all patients and their families, as well as nurses, data managers, and physicians for contributing to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.878367/full#supplementary-material
References
1. Turc-Carel C, Lizard-Nacol S, Justrabo E, Favrot M, Philip T, Tabone E. Consistent Chromosomal Translocation in Alveolar Rhabdomyosarcoma. Cancer Genet Cytogenet (1986) 19(3-4):361–2. doi: 10.1016/0165-4608(86)90069-5
2. Parham DM, Barr FG. Classification of Rhabdomyosarcoma and Its Molecular Basis. Adv Anat Pathol (2013) 20(6):387–97. doi: 10.1097/PAP.0b013e3182a92d0d
3. Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 Paired Box Gene in the Paediatric Solid Tumour Alveolar Rhabdomyosarcoma. Nat Genet (1993) 3(2):113–7. doi: 10.1038/ng0293-113
4. Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol (1995) 13(3):610–30. doi: 10.1200/JCO.1995.13.3.610
5. Maurer HM, Gehan EA, Beltangady M, Crist W, Dickman PS, Donaldson SS, et al. The Intergroup Rhabdomyosarcoma Study-Ii. Cancer (1993) 71(5):1904–22. doi: 10.1002/1097-0142(19930301)71:5<1904::AID-CNCR2820710530>3.0.CO;2-X
6. Oberlin O, Rey A, Lyden E, Bisogno G, Stevens MC, Meyer WH, et al. Prognostic Factors in Metastatic Rhabdomyosarcomas: Results of a Pooled Analysis From United States and European Cooperative Groups. J Clin Oncol (2008) 26(14):2384–9. doi: 10.1200/JCO.2007.14.7207
7. Weigel BJ, Lyden E, Anderson JR, Meyer WH, Parham DM, Rodeberg DA, et al. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol (2016) 34(2):117–22. doi: 10.1200/JCO.2015.63.4048
8. Chen L, Shah HO, Lin JH. Alveolar Rhabdomyosarcoma With Concurrent Metastases to Bone Marrow and Lymph Nodes Simulating Acute Hematologic Malignancy. J Pediatr Hematol Oncol (2004) 26(10):696–7. doi: 10.1097/01.mph.0000140654.50344.92
9. Davicioni E, Anderson MJ, Finckenstein FG, Lynch JC, Qualman SJ, Shimada H, et al. Molecular Classification of Rhabdomyosarcoma–Genotypic and Phenotypic Determinants of Diagnosis: A Report From the Children’s Oncology Group. Am J Pathol (2009) 174(2):550–64. doi: 10.2353/ajpath.2009.080631
10. Weiss AR, Lyden ER, Anderson JR, Hawkins DS, Spunt SL, Walterhouse DO, et al. Histologic and Clinical Characteristics Can Guide Staging Evaluations for Children and Adolescents With Rhabdomyosarcoma: A Report From the Children’s Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol (2013) 31(26):3226–32. doi: 10.1200/JCO.2012.44.6476
11. Lee DH, Park CJ, Jang S, Cho YU, Seo JJ, Im HJ, et al. Clinical and Cytogenetic Profiles of Rhabdomyosarcoma With Bone Marrow Involvement in Korean Children: A 15-Year Single-Institution Experience. Ann Lab Med (2018) 38(2):132–8. doi: 10.3343/alm.2018.38.2.132
12. Bailey KA, Wexler LH. Pediatric Rhabdomyosarcoma With Bone Marrow Metastasis. Pediatr Blood Cancer (2020) 67(5):e28219. doi: 10.1002/pbc.28219
13. Misawa A, Hosoi H, Tsuchiya K, Iehara T, Sawada T, Sugimoto T. Regression of Refractory Rhabdomyosarcoma After Allogeneic Stem-Cell Transplantation. Pediatr Hematol Oncol (2003) 20(2):151–5. doi: 10.1080/0880010390158658
14. Yamazaki F, Osumi T, Shigematsu N, Morioka H, Shimada H. Successful Treatment of Metastatic Rhabdomyosarcoma With Radiochemotherapy and Allogeneic Hematopoietic Stem Cell Transplantation. Jpn J Clin Oncol (2015) 45(2):225–8. doi: 10.1093/jjco/hyu189
15. Thiel U, Koscielniak E, Blaeschke F, Grunewald TG, Badoglio M, Diaz MA, et al. Allogeneic Stem Cell Transplantation for Patients With Advanced Rhabdomyosarcoma: A Retrospective Assessment. Br J Cancer (2013) 109(10):2523–32. doi: 10.1038/bjc.2013.630
16. Doelken R, Weigel S, Schueler F, Doelken G, Beck JF. Poor Outcome of Two Children With Relapsed State Stage IV Alveolar Rhabdomyosarcoma After Allogeneic Stem Cell Transplantation. Pediatr Hematol Oncol (2005) 22(8):699–703. doi: 10.1080/08880010500278806
17. Klingebiel T, Boos J, Beske F, Hallmen E, Int-Veen C, Dantonello T, et al. Treatment of Children With Metastatic Soft Tissue Sarcoma With Oral Maintenance Compared to High Dose Chemotherapy: Report of the HD CWS-96 Trial. Pediatr Blood Cancer (2008) 50(4):739–45. doi: 10.1002/pbc.21494
18. Thiel U, Schober SJ, Ranft A, Gassmann H, Jabar S, Gall K, et al. Correction to: No Difference in Survival After HLA Mismatched Versus HLA Matched Allogeneic Stem Cell Transplantation in Ewing Sarcoma Patients With Advanced Disease. Bone Marrow Transplant (2021) 56(9):2320. doi: 10.1038/s41409-021-01421-8
19. Scrucca L, Santucci A, Aversa F. Competing Risk Analysis Using R: An Easy Guide for Clinicians. Bone Marrow Transplant (2007) 40(4):381–7. doi: 10.1038/sj.bmt.1705727
20. Scrucca L, Santucci A, Aversa F. Regression Modeling of Competing Risk Using R: An in Depth Guide for Clinicians. Bone Marrow Transplant (2010) 45(9):1388–95. doi: 10.1038/bmt.2009.359
21. Schumm M, Lang P, Handgretinger R. Graft Manipulation. In: Carreras E, Dufour C, Mohty M, Kroger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham: Springer (2019). p. 137–42.
22. Merker M, Meister MT, Rettinger E, Jarisch A, Soerensen J, Willasch A, et al. Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation in Patients With High-Risk Soft Tissue Sarcomas: Results of a Single-Center Prospective Trial. Bone Marrow Transplant (2018) 53(7):891–4. doi: 10.1038/s41409-018-0088-6
23. Merker M, Meister MT, Heinze A, Jarisch A, Sorensen J, Huenecke S, et al. Adoptive Cellular Immunotherapy for Refractory Childhood Cancers: A Single Center Experience. Oncotarget (2019) 10(58):6138–51. doi: 10.18632/oncotarget.27242
24. Martinez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol (2017) 35(30):3425–32. doi: 10.1200/JCO.2017.72.6869
25. Schober SJ, von Luettichau I, Wawer A, Steinhauser M, Salat C, Schwinger W, et al. Donor Lymphocyte Infusions in Adolescents and Young Adults for Control of Advanced Pediatric Sarcoma. Oncotarget (2018) 9(32):22741–8. doi: 10.18632/oncotarget.25228
26. Hegde M, Joseph SK, Pashankar F, DeRenzo C, Sanber K, Navai S, et al. Tumor Response and Endogenous Immune Reactivity After Administration of HER2 CAR T Cells in a Child With Metastatic Rhabdomyosarcoma. Nat Commun (2020) 11(1):3549. doi: 10.1038/s41467-020-17175-8
27. Chen L, Oke T, Siegel N, Cojocaru G, Tam AJ, Blosser RL, et al. The Immunosuppressive Niche of Soft-Tissue Sarcomas Is Sustained by Tumor-Associated Macrophages and Characterized by Intratumoral Tertiary Lymphoid Structures. Clin Cancer Res (2020) 26(15):4018–30. doi: 10.1158/1078-0432.CCR-19-3416
28. Schlegel P, Feuchtinger T, Nitschke-Gerard C, Seidel UJ, Lang AM, Kyzirakos C, et al. Favorable NK Cell Activity After Haploidentical Hematopoietic Stem Cell Transplantation in Stage IV Relapsed Ewing’s Sarcoma Patients. Bone Marrow Transplant (2015) 50 Suppl 2:S72–6. doi: 10.1038/bmt.2015.100
Keywords: allogeneic stem cell transplantation, haploidentical, high-risk rhabdomyosarcoma, alveolar rhabdomyosarcoma (RMA) stage IV, matched-pair analysis, graft-versus-host disease, transplant-related mortality
Citation: Schober SJ, Hallmen E, Reßle F, Gassmann H, Prexler C, Wawer A, von Luettichau I, Ladenstein R, Kazanowska B, Ljungman G, Niggli F, Lohi O, Hauer J, Gruhn B, Klingebiel T, Bader P, Burdach S, Lang P, Sparber-Sauer M, Koscielniak E and Thiel U (2022) No Improvement of Survival for Alveolar Rhabdomyosarcoma Patients After HLA-Matched Versus -Mismatched Allogeneic Hematopoietic Stem Cell Transplantation Compared to Standard-of-Care Therapy. Front. Oncol. 12:878367. doi: 10.3389/fonc.2022.878367
Received: 17 February 2022; Accepted: 21 March 2022;
Published: 10 May 2022.
Edited by:
Miguel Angel Diaz, Niño Jesús University Children’s Hospital, SpainReviewed by:
Blanca Molina, Niño Jesús University Children’s Hospital, SpainSebastian Dorin Asaftei, Ospedale Città della Salute e della Scienza, Italy
Richard O’Reilly, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2022 Schober, Hallmen, Reßle, Gassmann, Prexler, Wawer, von Luettichau, Ladenstein, Kazanowska, Ljungman, Niggli, Lohi, Hauer, Gruhn, Klingebiel, Bader, Burdach, Lang, Sparber-Sauer, Koscielniak and Thiel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Johannes Schober, cy5zY2hvYmVyQHR1bS5kZQ==; Uwe Thiel, dXdlLnRoaWVsQHR1bS5kZQ==
 Sebastian Johannes Schober
Sebastian Johannes Schober Erika Hallmen2
Erika Hallmen2 Hendrik Gassmann
Hendrik Gassmann Irene von Luettichau
Irene von Luettichau Bernd Gruhn
Bernd Gruhn Thomas Klingebiel
Thomas Klingebiel Peter Bader
Peter Bader Peter Lang
Peter Lang Uwe Thiel
Uwe Thiel