- Department of Gynecology and Obstetrics, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Purpose: To analyze the relationship between neoadjuvant chemotherapy (NACT) and log odds of positive lymph nodes (LODDS), as well as their prognostic role in advanced ovarian cancer (AOC) patients with optimal cytoreductive surgery.
Patients and Methods: From the SEER database during 2010-2016, we identified 1,012 AOC patients with optimal cytoreductive surgery. Univariable and multivariable logistic regression was performed to identify the relationship between NACT and LODDS. Overall survival (OS) and cancer-specific survival (CSS) were assessed using the Kaplan-Meier method and log-rank test. Univariable and multivariable Cox regression was conducted to determine the independent prognostic factors for OS and CSS.
Results: Factors associated with significantly higher NACT odds included older (≥60 years old), married, tumor size ≥ 15 cm, FIGO IV, and LODDS ≤ 0.1. Multivariable Cox regression model confirmed older (≥60 years old), unmarried, separated, divorced, widowed, mucinous histology type, tumor size ≥ 15 cm, and LODDS > 0.1 were correlated with increased risks of OS and CSS. NACT was not an independent prognostic factor for OS and CSS. In the subgroup analyses, LODDS was an independent prognostic factor for OS and CSS in patients with < 75 years old, married, unmarried, FIGO III, and tumor size < 15 cm.
Conclusion: NACT did not show any survival benefit in AOC patients with optimal cytoreductive surgery, but it may be beneficial in reducing LODDS. Meanwhile, clinicians can use grade of LODDS to predict the prognosis of AOC patients with optimal cytoreductive surgery.
Introduction
Ovarian cancer (OC) is one of the most common malignant tumors and ranks as the fifth cause of cancer death among women in the United States (1). According to the latest cancer statistics, 13,770 patients died of OC, and 21,410 patients were newly diagnosed in the United States in 2021 (2). Given the nonspecific signs and symptoms of OC, such as abdominal bloating, abdominal pain, and unintentional weight loss, most patients are diagnosed at an advanced stage (3, 4).
Standard of care for patients with advanced ovarian cancer (AOC) is primary cytoreductive surgery (PCS) followed by adjuvant chemotherapy (5, 6). The leading prognostic factor in determining survival of patients with AOC is achievement of complete cytoreductive surgery with the aim of macroscopically complete resection of all visible tumor, followed by chemotherapy that includes carboplatin and paclitaxel (7). However, complete cytoreduction surgery may not be achievable in those with heavy peritoneal disease burden. An alternative management option in patients with AOC is initial treatment with neoadjuvant chemotherapy (NACT) to reduce tumor burden followed by interval cytoreductive surgery (ICS) (8). NACT-ICS offers several advantages over PCS, including an increased rate of optimal cytoreduction (no visible residual disease (RD) or RD ≤ 1 cm), lower perioperative morbidity, and higher quality of life in patients with an AOC who would likely not achieve optimal cytoreductive surgery (9, 10). The use of NACT has increased annually by 10.3% between 2011 and 2016 compared with an annual increase of 7.9% between 2006 and 2011 (11). But the clinical use of NACT is also controversial. Compared with PCS, NACT did not show any survival benefit in reported randomized controlled trials (RCT) or Meta analysis (10, 12–15). These results have led to concerns about the adverse effects of NACT.
In clinical practice, lymph node metastasis is one of the major metastases of AOC and an indicator for evaluating recurrence and survival, and a key prognostic factor in determining the development of postoperative treatment plans and follow-up (16). Traditionally, lymph node status is based on positive lymph nodes (PLNs) regardless of the number of resected lymph nodes (RLNs) (17). At present, the lymph node ratio (LNR), defined as the ratio between the number of PLNs and RLNs, is increasingly recognized as a powerful prognostic tool for many cancers (18, 19). The log odds of positive lymph nodes (LODDS) is the logarithm of the ratio of metastatic lymph nodes to negative lymph nodes (log (PLNs+0.5)/(RLNs-PLNs+0.5)) and can identify patients with highly homologous prognoses. It also overcomes the shortcoming of the LNR regardless of the number of lymph nodes examined (20). Recently, LODDS staging was hypothesized to be a better predictor of survival in many cancers compared with LNR and PLN staging (18, 21). Previously, LODDS was used to analyze the prognostic role of mesenteric lymph nodes (MLNs) involvements in AOC (22). However, the prognostic value of LODDS in AOC remains unclear. Therefore, the aim of the current study was to determine the most appropriate nodal staging system for OC patients with optimal cytoreductive surgery. Besides, no retrospective or prospective studies have been conducted to evaluate the relationship between NACT and LODDS. The Surveillance, Epidemiology, and End Results (SEER) cancer database, in the United States, collects information on 34.6% of Americans in 18 registries. Using the SEER database, we extracted details on AOC patients with optimal cytoreductive surgery between 2010 and 2016 to determine the prognostic role of NACT, LODDS, and the relationship between them. These findings will help doctors make better clinical decisions with individualized application of NACT.
Materials and Methods
Data Source and Study Population
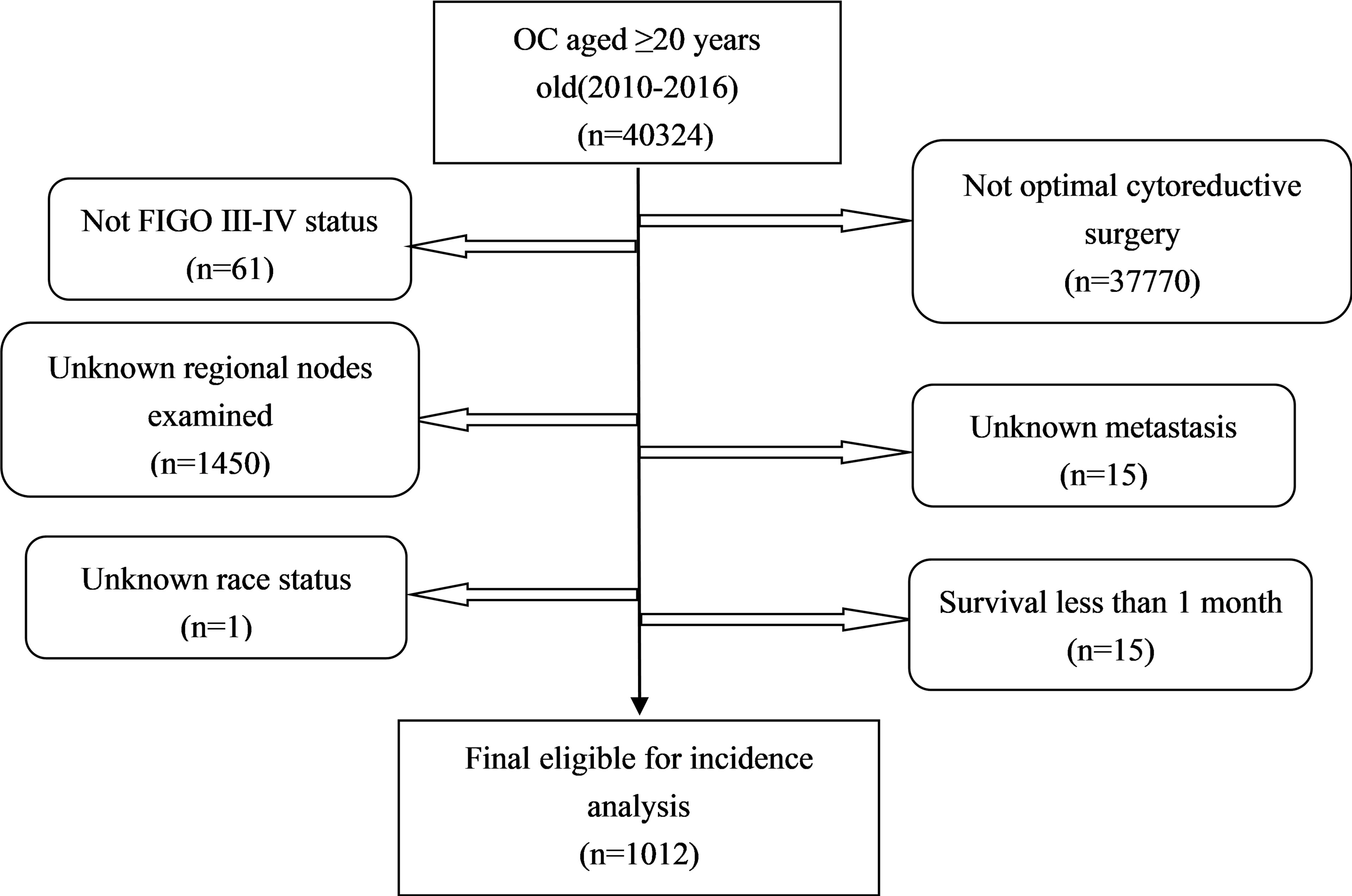
All the primary data were acquired from the SEER database. The SEER∗Stat version 8.3.9 (https://seer.cancer.gov/seerstat/) was used to screen eligible patients who were AOC (ICD-O-3, C56.9) with optimal cytoreductive surgery between 2010 and 2016. We gathered the following information: age at diagnosis, race, year of diagnosis, grade (G1 is equivalent to well differentiated; G2 is equivalent to moderately differentiated; G3 is equivalent to poorly differentiated; G4 is equivalent to undifferentiated), FIGO stage, marital status [other (separated, divorced, widowed)], laterality, Ca125, tumor size, metastasis site, RLNs, PLNs, LODDS, pathological subtype[Third Edition (ICD-O-3) morphology codes(8441/3, 8460/3, 8461/3 for serous; 8380/3 for endometrioid; 8310/3 for clear cell; 8480/3 for mucinous);], vital status, and survival time. The study calculated LODDS by log (PLNs+0.5)/(RLNs-PLNs+0.5). We added 0.05 to both the numerator and denominator to avoid an undefined number. LODDS was classified into LODDS1 (≤ 0.1) and LODDS2 (>0.1). Patients who were diagnosed by autopsy or death certificate with a follow-up shorter than 1 month were excluded. A total of 1,012 patients were eligible for incidence analysis. The primary endpoint was overall survival (OS) and secondary endpoint was cancer-specific survival (CSS). The OS was defined as the survival time in months regardless of the cause of death. The CSS was defined as the time interval from diagnosis of ovarian cancer to ovarian cancer -related death (Figure 1).
Statistical Analysis
PCS and NACT-ICS were compared using the chi-square test (χ2). Univariable and multivariable logistic regression was performed to identify the relationship between NACT and LODDS. Odds ratios (OR) and 95% confidence intervals (CI) were reported from the logistic regression. Univariable and multivariable Cox regression analyses were employed to identify independent predictors associated with survival by reporting the hazard ratios (HR) and 95% CI. Survival comparisons were made using Kaplan–Meier analysis and log-rank tests.
Statistical analyses were all performed using SPSS (version 22.0, IBM Corporation, USA) and R software (version 3.6.3; www.r-project.org/). P < 0.05 (2-sided) was considered significant.
Results
Patient Characteristics
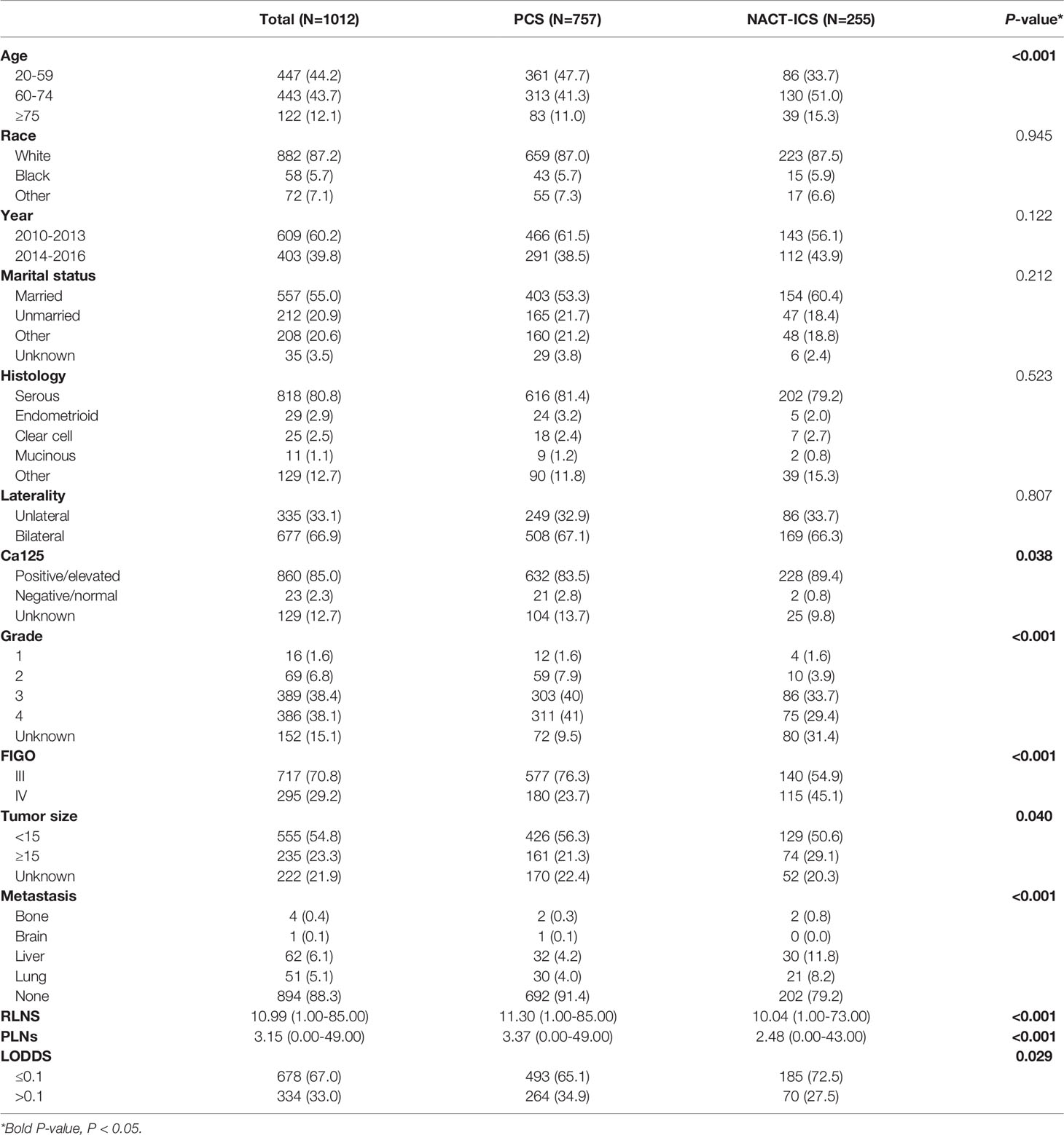
The demographic and clinical characteristics of the 1,012 patients are shown in Table 1. Most of them were white race (87.2%), married (55.0%), serous histologic type (80.8%), bilateral (66.9%), Ca125 positive (85.0%), grade3-4 (76.5%), FIGO III (70.8%), metastatic site of liver (6.1%), tumor size<15 cm (54.8%), LODDS ≤0.1 (67.0%). A total of 255 (25.2%) patients received NACT and 757 (74.8%) did not. There were an equal number of race, year of diagnosis, marital status, histologic type and bilateral in both PCS and NACT-ICS. Patients with older (≥60 years old), lower PLNs, lower LODDS, multiple sites of metastasis, tumor size ≥15 cm and FIGO IV were more likely to receive NACT.
Determinants of NACT
Table 2 shows distributions of patient characteristics according to NACT using univariable and multivariable logistic regression. On the multivariable logistic regression, compared to patients 20-59 years old, those 60-74 years old (OR:1.624, 95%CI: 1.173-2.257), ≥75 years old (OR:1.877, 95%CI: 1.192-3.020), and married patients (vs. unmarried patients, OR: 0.578, 95%CI: 0.381-0.861) had higher odds of NACT. Also, compared to patients with FIGOIII, those with FIGOIV (OR:2.854, 95%CI: 2.091-3.902) were associated with higher odds of NACT. Additionally, compared to tumor size <15 cm, tumor size≥15 cm (OR:1.450, 95%CI: 1.007-2.082) had higher odds of NACT. Further, patients with LODDS >0.1 (OR:0.589, 95%CI: 0.420-0.816) had lower odds of NACT compared to those with LODDS ≤0.1.
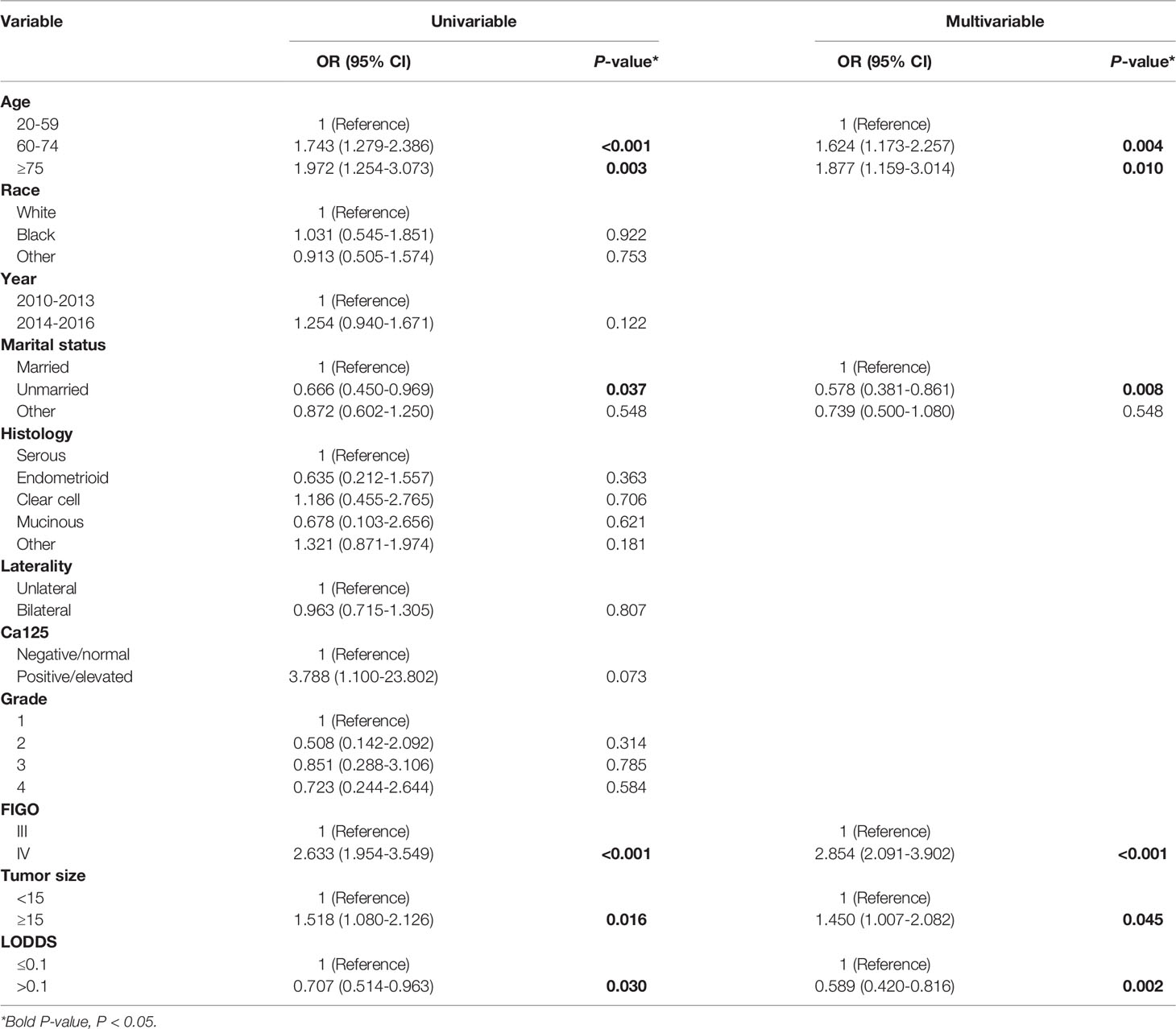
Table 2 Univariable and multivariable logistic regression for associations between patient characteristics and NACT.
The Effects of LODDS and Different Treatment on OS and CSS
Kaplan-Meier curves were performed to analyze the effects of LODDS and different treatment (PCS and NACT-ICS) on the OS and CSS of AOC patients with optimal cytoreductive surgery. Significant differences were seen in OS (5-year OS 42.2% vs. 27.2%), and CSS (5-year CSS 47.3% vs. 29.4%) between LODDS ≤0.1 and LODDS >0.1 groups in general (Figures 2A, B). However, no significant differences were detected in OS (5-year OS 38.2% vs. 31.7%), and CSS (5-year CSS 42.7% vs. 35.1%) between PCS and NACT-ICS groups (Figures 2C, D).

Figure 2 Kaplan–Meier survival curves of LODDS and treatment on OS and CSS. (A) LODDS on OS, (B) LODDS on CSS (1: LODDS ≤ 0.1, 2: LODDS>0.1), (C) treatment on OS, (D) treatment on CSS (0: PCS, 1: NACT-ICS).
Predictors for OS and CSS
In the univariable analysis, age, marital status, histology type, tumor size, and LODDS were all associated with OS and CSS. In the predictors of OS, the multivariable Cox regression model showed that patients 60-74 years old (HR:1.378, 95% CI:1.119-1.696) and ≥75 (HR:1.977, 95% CI: 1.480-2.643) had poorer outcomes compared with those 20-59. Additionally, poor outcomes were seen in unmarried patients (HR:1.512, 95%CI: 1.177-1.942), separated, divorced, or widowed (HR:1.318, 95%CI:1.040-1.671), mucinous histology type (HR:2.754, 95% CI: 1.379-5.501), tumor size ≥15 cm (HR:4.179, 95%CI: 3.285-5.315) or LODDS >0.1 (HR: 1.517, 95%CI: 1.238-1.859) (Table 3). The multivariable Cox regression model also showed the same results in the predictors of CSS (Table 3).
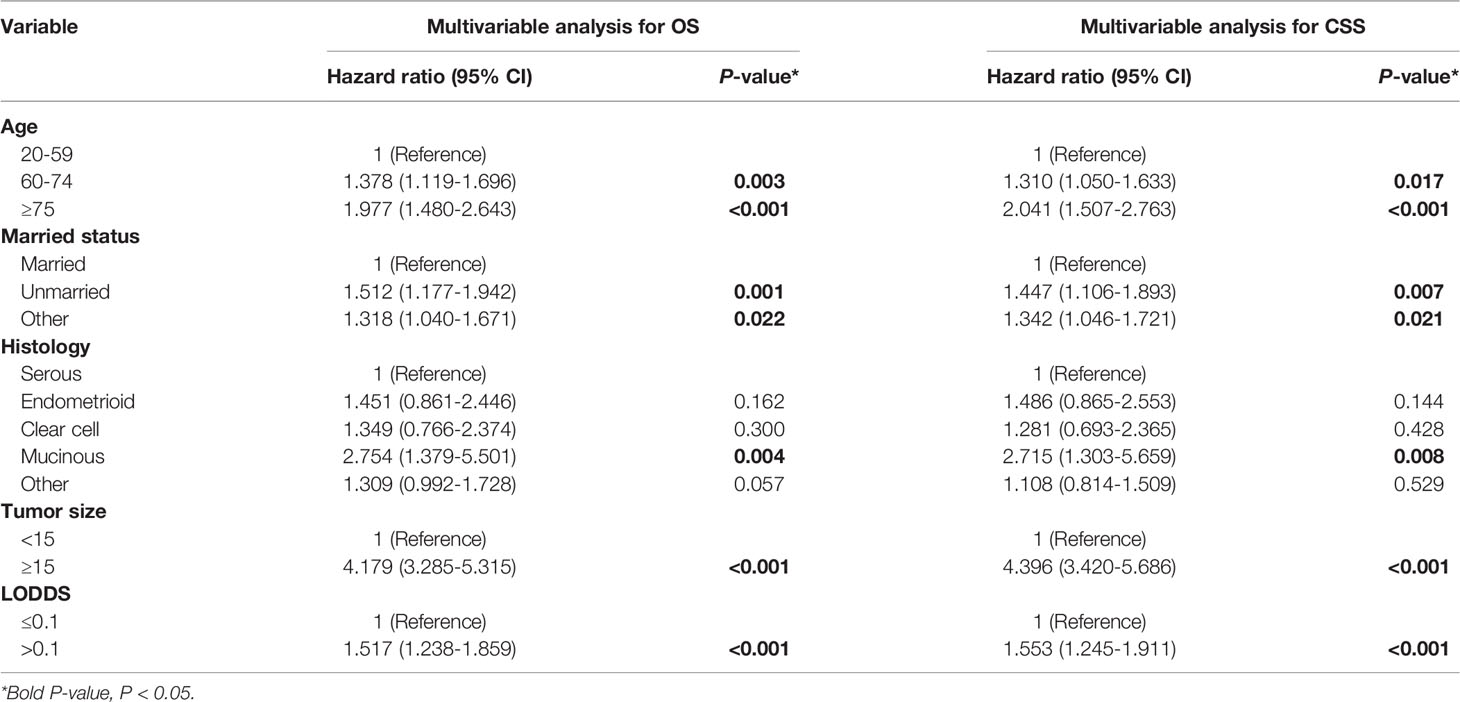
Table 3 Multivariable Cox regression of OS and CSS among AOC patients with optimal cytoreductive surgery.
Subgroup Analysis of the Effects of LODDS on OS and CSS
According to the log-rank tests and Cox regression analysis, we studied the effects of LODDS on the prognosis of AOC patients with optimal cytoreductive surgery. As shown in Table 4, LODDS was found to be an independent prognostic factor of OS in patients 20-59 years old (HR:1.745, 95% CI:1.289-2.364), 60-74 years old (HR:1.363, 95% CI:1.023-1.815), married (HR:1.426, 95% CI:1.095-1.857), unmarried (HR:1.995, 95% CI:1.281-3.107), FIGO III (HR:1.539, 95% CI: 1.212-1.954) or tumor size <15 cm (HR:1.720, 95% CI: 1.311-2.258). The multivariable Cox regression model also showed the same results in the predictors of CSS (Figures 3A–L).

Table 4 Multivariable analysis for LODDS on OS and CSS based on age, marital status, FIGO stage, tumor size and NACT among AOC patients with optimal cytoreductive surgery.
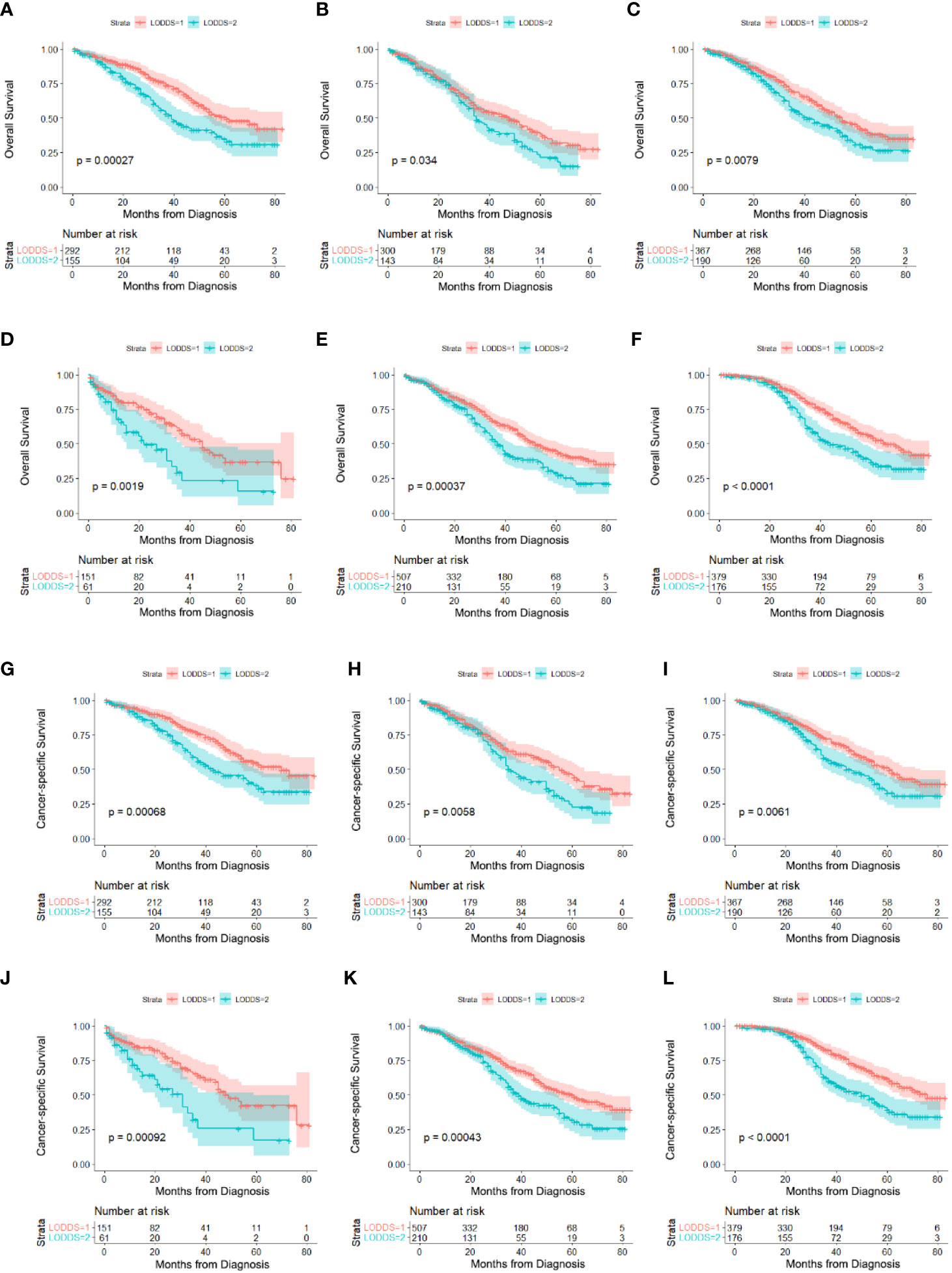
Figure 3 Survival comparison in subgroups of AOC patients with optimal cytoreductive surgery based on LODDS. (A) 20-59 years old on OS, (B) 60-74 years old on OS, (C) married on OS, (D) unmarried on OS, (E) FIGOIII on OS, (F) tumor size<15cm on OS, (G) 20-59 years old on CSS, (H) 60-74 years old on CSS, (I) married on CSS, (J) unmarried on CSS, (K) FIGOIII on CSS, (L) tumor size<15cm on CSS,.
Discussion
Patients with AOC have a poor prognosis and the 5-year survival rate is less than 30% (23). Complete cytoreductive surgery with platinum-based chemotherapy is the standard treatment for patients with AOC (7). The Society of Gynecologic Oncology and American Society of Clinical Oncology issued practice guidelines recommending NACT for patients with high perioperative risk or low likelihood of achieving optimal cytoreductive surgery (5). In our retrospective study, 757 AOC patients with optimal cytoreductive surgery had undergone NACT while 255 did not. Compared with the PCS group, the NACT-ICS group had a higher proportion of older patients, lower PLNs, lower LODDS, multiple sites of metastasis, tumor size ≥15 cm, and FIGO IV. In addition, multivariable logistic regression showed ≥60 years old, married, tumor size ≥15 cm, FIGO IV and LODDS ≤0.1 were associated with higher NACT odds.
Several studies have confirmed that NACT may improve the satisfactory tumor-reducing rate in AOC and suggest that NACT enhances quality of life and lowers the incidence of postoperative adverse events (9, 10). Considering that most patients in the NACT-ICS group were older, later staged, and had poor health, this study believes that NACT is a reasonable treatment option for these AOC patients with difficulty in optimal cytoreductive surgery. However, other studies have found that NACT may increase the risk of platinum resistance and tumor recurrence (24, 25). Our study found NACT was not an independent prognostic factor in AOC patients with optimal cytoreductive surgery and had no significant differences in OS and CSS between PCS and NACT-ICS groups, which agrees with previous studies (10, 12–15).
In general, approximately 60% to 70% of AOC patients with optimal cytoreductive surgery experience peritoneal disease relapse (26). Intraperitoneal chemotherapy when delivered under hyperthermic conditions is termed hyperthermic intraperitoneal chemotherapy (HIPEC), which is a good strategy to address peritoneal disease spread (27). The randomized OVHIPEC study showed that adding HIPEC to ICS improves recurrence-free and OS in patients with stage III OC (28). In addition, the OVHIPEC-2 trial is evaluating HIPEC at PCS and plans to enroll 538 patients with FIGO III epithelial OC (29). Further, HIPEC has been studied in platinum-sensitive recurrent OC patients (30). NCCN guidelines support incorporating this treatment into the counseling of patients considering IDS (1). However, information about whether to use HIPEC was not provided in the SEER database. Combined with the existing data, the research regarding time point, patient selection, drug choice, dose, and duration of HIPEC will be an area of intense research in the future.
The multivariable Cox model showed older (≥60 years old), unmarried, separated, divorced, or widowed, mucinous histology type, tumor size ≥15 cm, or LODDS >0.1 correlated with increased risks of OS and CSS in AOC patients with optimal cytoreductive surgery. Previous studies have shown that marital status is an independent prognostic factor for many cancers (31–33). It is well known that emotional stress down-regulates the cellular immune response and stimulates tumor angiogenesis (34). Separated, divorced, or widowed marital status may limit psychosocial support and personalized care a woman receives. In addition, older patients may have poor health and larger tumors may increase the physical burden, so such patients have lower survival rates. Further, mucinous histology, a rare subtype of epithelial OC, has a poor response to conventional chemotherapy regimens. The early prognosis of this subtype is good, but the prognosis of advanced and recurrent patients is extremely poor (35). Our results showed that LODDS ≤0.1 showed significant survival advantages and higher odds of NACT over patients with LODDS >0.1. We speculate that NACT might be related to a decrease in LODDS. A previous study showed the systematic pelvic and paraaortic lymphadenectomy in AOC patients with normal lymph nodes both before and during surgery was not associated with longer overall or progression-free survival than no lymphadenectomy and was associated with a higher incidence of postoperative complications (36). This suggests that NACT may provide some benefits by reducing the chance of lymphadenectomy. Particularly, stratification analyses were carried out based on several established risk factors for control confounders. LODDS was found to be an independent prognostic factor of OS and CSS in patients <75 years old, married, unmarried, FIGO III and tumor size <15 cm, indicating that LODDS was a meaning prognostic factor. According to these results, clinicians can more reasonably use LODDS grade to predict the prognosis among these patients.
Our research has several limitations. First, information about whether to use HIPEC and regimens and the number of cycles of NACT were insufficient. Second, the SEER database is based on registrations in the United States and whether it applies to other countries or regions is unknown. Despite these inevitable limitations, this study provides an important indication that NACT is not an independent prognostic factor in AOC patients with optimal cytoreductive surgery, but it may contribute to the reduction of LODDS, which is an independent prognostic factor for AOC patients with optimal cytoreductive surgery. And patients with lower LODDS may receive some benefits by reducing the chance of lymphadenectomy.
Data Availability Statement
All the primary data were acquired from the SEER database (https://seer.cancer.gov/seerstat/).
Author Contributions
Study concept and design: R-fA. Data acquisition: Y-mH and J-mY. Data analysis and interpretation: Y-mH and YX. Software: YX. Manuscript preparation: Y-mH and FF. Critical revision: R-fA. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 81972428). The authors also appreciate the SEER database for providing high quality clinical data for our research and AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
References
1. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19(2):191–226. doi: 10.6004/jnccn.2021.0007
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Shampain KL, Wasnik AP. Postneoadjuvant Chemotherapy Response in Advanced Ovarian Cancer: Radiological and Clinical Implications. Acad Radiol (2020) 27(7):958–9. doi: 10.1016/j.acra.2020.02.004
4. Prat J. FIGO Committee on Gynecologic Oncology. FIGO's Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum: Abridged Republication. J Gynecol Oncol (2015) 26(2):87–9. doi: 10.3802/jgo.2015.26.2.87
5. Llueca A, Serra A, Climent MT, Segarra B, Maazouzi Y, Soriano M, et al. Outcome Quality Standards in Advanced Ovarian Cancer Surgery. World J Surg Oncol (2020) 18(1):309. doi: 10.1186/s12957-020-02064-7
6. Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol (2016) 143(1):3–15. doi: 10.1016/j.ygyno.2016.05.022
7. du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. Consensus Statements on the Management of Ovarian Cancer: Final Document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol (2004) 2005) 16 Suppl 8:viii7–viii12. doi: 10.1093/annonc/mdi961
8. Kumar A, Cliby WA. Advanced Ovarian Cancer: Weighing the Risks and Benefits of Surgery. Clin Obstet Gynecol (2020) 63(1):74–9. doi: 10.1097/GRF.0000000000000497
9. Yang L, Zhang B, Xing G, Du J, Yang B, Yuan Q, et al. Neoadjuvant Chemotherapy Versus Primary Debulking Surgery in Advanced Epithelial Ovarian Cancer: A Meta-Analysis of Peri-Operative Outcome. PloS One (2017) 12(10):e0186725. doi: 10.1371/journal.pone.0186725
10. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary Chemotherapy Versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer (CHORUS): An Open-Label, Randomised, Controlled, non-Inferiority Trial. Lancet (2015) 386(9990):249–57. doi: 10.1016/S0140-6736(14)62223-6
11. Knisely AT, St Clair CM, Hou JY, Collado FK, Hershman DL, Wright JD, et al. Trends in Primary Treatment and Median Survival Among Women With Advanced-Stage Epithelial Ovarian Cancer in the US From 2004 to 2016. JAMA Netw Open (2020) 3(9):e2017517. doi: 10.1001/jamanetworkopen.2020.17517
12. Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III Randomised Clinical Trial Comparing Primary Surgery Versus Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer With High Tumour Load (SCORPION Trial): Final Analysis of Peri-Operative Outcome. Eur J Cancer (2016) 59:22–33. doi: 10.1016/j.ejca.2016.01.017
13. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N Engl J Med (2010) 363(10):943–53. doi: 10.1056/NEJMoa0908806
14. Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of Treatment Invasiveness Between Upfront Debulking Surgery Versus Interval Debulking Surgery Following Neoadjuvant Chemotherapy for Stage III/IV Ovarian, Tubal, and Peritoneal Cancers in a Phase III Randomised Trial: Japan Clinical Oncology Group Study Jcog0602. Eur J Cancer (2016) 64:22–31. doi: 10.1016/j.ejca.2016.05.017
15. Bartels HC, Rogers AC, McSharry V, McVey R, Walsh T, O'Brien D, et al. A Meta-Analysis of Morbidity and Mortality in Primary Cytoreductive Surgery Compared to Neoadjuvant Chemotherapy in Advanced Ovarian Malignancy. Gynecol Oncol (2019) 154(3):622–30. doi: 10.1016/j.ygyno.2019.07.011
16. Prat J. FIGO Committee on Gynecologic Oncology. Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum: Abridged Republication of Guidelines From the International Federation of Gynecology and Obstetrics (FIGO). Obstet Gynecol (2015) 126(1):171–4. doi: 10.1097/AOG.0000000000000917
17. Gasimli K, Braicu EI, Nassir M, Richter R, Babayeva A, Chekerov R, et al. Lymph Node Involvement Pattern and Survival Differences of FIGO IIIC and FIGO IIIA1 Ovarian Cancer Patients After Primary Complete Tumor Debulking Surgery: A 10-Year Retrospective Analysis of the Tumor Bank Ovarian Cancer Network. Ann Surg Oncol (2016) 23(4):1279–86. doi: 10.1245/s10434-015-4959-4
18. Gao B, Zhou D, Qian X, Jiang Y, Liu Z, Zhang W, et al. Number of Positive Lymph Nodes Is Superior to LNR and LODDS for Predicting the Prognosis of Pancreatic Neuroendocrine Neoplasms. Front Endocrinol (Lausanne) (2021) 12:613755. doi: 10.3389/fendo.2021.613755
19. Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, et al. The Impact of N-Ratio in Minimizing Stage Migration Phenomenon in Gastric Cancer Patients With Insufficient Number or Level of Lymph Node Retrieved: Results From a Chinese Mono-Institutional Study in 2159 Patients. Ann Oncol (2009) 20(5):897–905. doi: 10.1093/annonc/mdn707
20. Huang X, Niu X, You Z, Long Y, Luo F, Ye H. Comparison of Four Lymph Node Stage Methods for Predicting the Prognosis of Distal Cholangiocarcinoma Patients After Surgery. Front Oncol (2021) 11:779761. doi: 10.3389/fonc.2021.779761
21. Li T, Yang Y, Wu W, Fu Z, Cheng F, Qiu J, et al. Prognostic Implications of ENE and LODDS in Relation to Lymph Node-Positive Colorectal Cancer Location. Transl Oncol (2021) 14(11):101190. doi: 10.1016/j.tranon.2021.101190
22. Berretta R, Capozzi VA, Sozzi G, Volpi L, Ceni V, Melpignano M, et al. Prognostic Role of Mesenteric Lymph Nodes Involvement in Patients Undergoing Posterior Pelvic Exenteration During Radical or Supra-Radical Surgery for Advanced Ovarian Cancer. Arch Gynecol Obstet (2018) 297(4):997–1004. doi: 10.1007/s00404-018-4675-y
23. Seidman JD, Yemelyanova A, Cosin JA, Smith A, Kurman RJ. Survival Rates for International Federation of Gynecology and Obstetrics Stage III Ovarian Carcinoma by Cell Type: A Study of 262 Unselected Patients With Uniform Pathologic Review. Int J Gynecol Cancer (2012) 22(3):367–71. doi: 10.1097/IGC.0b013e31823c6f80
24. Lim MC, Song YJ, Seo SS, Yoo CW, Kang S, Park SY. Residual Cancer Stem Cells After Interval Cytoreductive Surgery Following Neoadjuvant Chemotherapy Could Result in Poor Treatment Outcomes for Ovarian Cancer. Onkologie (2010) 33(6):324–30. doi: 10.1159/000313823
25. Zhang GN, Liu H, Huang JM, Wang L, Zhao JS, Li C, et al. TP53 K351N Mutation-Associated Platinum Resistance After Neoadjuvant Chemotherapy in Patients With Advanced Ovarian Cancer. Gynecol Oncol (2014) 132(3):752–7. doi: 10.1016/j.ygyno.2014.01.028
26. Chang SJ, Bristow RE. Evolution of Surgical Treatment Paradigms for Advanced-Stage Ovarian Cancer: Redefining 'Optimal' Residual Disease. Gynecol Oncol (2012) 125(2):483–92. doi: 10.1016/j.ygyno.2012.02.024
27. Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and Cytoreductive Surgery (CRS) in Ovarian Cancer: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2015) 41(12):1578–89. doi: 10.1016/j.ejso.2015.08.172
28. van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder H, Hermans R, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med (2018) 378(3):230–40. doi: 10.1056/NEJMoa1708618
29. Koole S, van Stein R, Sikorska K, Barton D, Perrin L, Brennan D, et al. Primary Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for FIGO Stage III Epithelial Ovarian Cancer: OVHIPEC-2, a Phase III Randomized Clinical Trial. Int J Gynecol Cancer (2020) 30(6):888–92. doi: 10.1136/ijgc-2020-001231
30. Petrillo M, De Iaco P, Cianci S, Perrone M, Costantini B, Ronsini C, et al. Long-Term Survival for Platinum-Sensitive Recurrent Ovarian Cancer Patients Treated With Secondary Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol (2016) 23(5):1660–5. doi: 10.1245/s10434-015-5050-x
31. Luo P, Zhou JG, Jin SH, Qing MS, Ma H. Influence of Marital Status on Overall Survival in Patients With Ovarian Serous Carcinoma: Finding From the Surveillance Epidemiology and End Results (SEER) Database. J Ovarian Res (2019) 12(1):126. doi: 10.1186/s13048-019-0600-7
32. Randi G, Altieri A, Gallus S, Chatenoud L, Montella M, Franceschi S, et al. Marital Status and Cancer Risk in Italy. Prev Med (2004) 38(5):523–8. doi: 10.1016/j.ypmed.2003.12.004
33. El Ibrahimi S, Pinheiro PS. The Effect of Marriage on Stage at Diagnosis and Survival in Women With Cervical Cancer. Psychooncology (2017) 26(5):704–10. doi: 10.1002/pon.4070
34. Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do Stress Responses Promote Leukemia Progression? An Animal Study Suggesting a Role for Epinephrine and Prostaglandin-E2 Through Reduced NK Activity. PloS One (2011) 6(4):e19246. doi: 10.1371/journal.pone.001
35. Morice P, Gouy S, Leary A. Mucinous Ovarian Carcinoma. N Engl J Med (2019) 380(13):1256–66. doi: 10.1056/NEJMra1813254
Keywords: advanced ovarian cancer, neoadjuvant chemotherapy, optimal cytoreductive surgery, LODDS, prognosis, SEER
Citation: Hou Y-m, Xue Y, Yao J-m, Feng F and An R-f (2022) Relationship Between Neoadjuvant Chemotherapy and Log Odds of Positive Lymph Nodes and Their Prognostic Role in Advanced Ovarian Cancer Patients With Optimal Cytoreductive Surgery. Front. Oncol. 12:878275. doi: 10.3389/fonc.2022.878275
Received: 17 February 2022; Accepted: 30 March 2022;
Published: 16 May 2022.
Edited by:
Michael Kattan, Case Western Reserve University, United StatesReviewed by:
Vito Andrea Capozzi, University Hospital of Parma, ItalyCarlo Ronsini, Università degli Studi della Campania “Luigi Vanvitelli”, Italy
Copyright © 2022 Hou, Xue, Yao, Feng and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-fang An, YW5ydWlmYW5nOTM2QDE2My5jb20=
 Yue-min Hou
Yue-min Hou Rui-fang An
Rui-fang An