- 1College of Computer Science and Technology, Harbin Engineering University, Harbin, China
- 2Key Laboratory of Symbolic Computation and Knowledge Engineering of Ministry of Education, Jilin University, Changchun, China
- 3School of Mathematics, Harbin Institute of Technology, Harbin, China
Introduction: Immune checkpoint inhibitors (ICIs) have been approved to prolong overall survival (OS), compared to other treatments. However, the recent studies reported consistent and inconsistent results. Hence, we conducted this meta-analysis to evaluate the efficacy of ICIs.
Materials and Methods: The articles were identified by searching PubMed, Embase, and Google Scholar published up to December 2021. A total of 12,126 participants (6,450 cases and 5,676 controls) were involved in the meta-analysis. Median OS and median progression-free survival (PFS) were selected to evaluate the efficacy of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed death ligand 1 (PD-L1) inhibitors (ipilimumab, nivolumab or pembrolizumab, and atezolizumab, respectively). Utilizing the random-effect model, hazard ratios (HRs) with 95 confidence intervals (CIs) were calculated by R software.
Results: We observed a significant association between cancer patients and ICIs in OS (HR = 0.79, CI = 0.74–0.84) and PFS (HR = 0.80, CI = 0.75–0.86).
Conclusions: The meta-analysis suggested that ICIs were associated with obvious improvements in PFS and OS compared with non-ICI therapies.
Introduction
Cancer, an enormous burden on society, is one of the main reasons of death in both developed and developing countries. According to the global cancer statistics, there were about 19.3 million new cancer cases and nearly 10.0 million cancer deaths in 2020 worldwide (1). The immune system can recognize and prepare to eliminate cancer but is controlled by inhibitory receptors and ligands (2). Immune checkpoints are regulatory pathways in the immunome that inhibit a part of an active immune response against a specific target or a group of targets (3). These immune checkpoint pathways are often able to keep self-tolerance and limit incidental tissue damage during the antimicrobial immune response; thus, immune destruction can be averted by cancers. There is no doubt that tumors co-opt certain immune checkpoint pathways as a main mechanism of immune resistance, especially against T cells that are specific for tumor antigens (4).
Immune checkpoint inhibitors (ICIs), which regain the efficacy of tumor-specific T cells in the tumor microenvironment, enhancing the immune system’s ability to recognize and eradicate tumors, are breakthroughs in the treatment of cancer and have made significant advances in both hematological and solid tumor oncology (5). They have been approved for use in melanoma, bladder cancer, non-small cell lung cancer (NSCLC), stomach cancer, renal cell carcinoma (RCC) and head and neck squamous cell carcinoma and will be approved for other types in the foreseeable future tumors (6, 7).
The US Food and Drug Administration (FDA) approved ipilimumab as the first CTLA-4 inhibitor of advanced melanoma. Nivolumab and pembrolizumab were the first of two PD-1 inhibitors approved for advanced melanoma, and atezolizumab was the first programmed death ligand 1 (PD-L1) inhibitor approved by the FDA (8–11).
Recent studies showed that ICIs could prolong the overall survival (OS) of cancer patients, compared with placebo, dacarbazine, everolimus, paclitaxel, chemotherapy, and other therapy methods or drugs (12–24). However, the studies reported inconsistent results. In 2013, Reck et al. randomly assigned 130 SCLC patients to receive paclitaxel with placebo (control) or ipilimumab 10 mg/kg in two alternative regimens, concurrent ipilimumab or phased ipilimumab, and declared that ipilimumab did not prolong the overall survival (OS) of SCLC patients (25). In 2014, Kwon et al. did a double-blind, multicenter, randomized, phase 3 trial with 799 metastatic castration-resistant prostate cancer (399 to ipilimumab and 400 to placebo) patients and reported that no obvious difference in overall survival was found between the ipilimumab group and the placebo group (26). In 2016, Beer et al. randomly assigned 400 and 202 metastatic castration-resistant prostate cancer patients to ipilimumab and to placebo, respectively, and discovered that ipilimumab did not increase the overall survival (OS) of patients with metastatic castration-resistant prostate cancer (27). Reck et al. randomly assigned 478 small-cell lung cancer (SCLC) patients to the chemotherapy plus ipilimumab group and 476 SCLC patients to the chemotherapy plus placebo group and got a conclusion that ipilimumab plus chemotherapy did not prolong OS compared with chemotherapy alone in SCLC patients (28). Larkin et al. randomly assigned 272 melanoma patients to the nivolumab group and 133 melanoma patients to chemotherapy and found that nivolumab did not prolong OS compared with chemotherapy alone in SCLC (29). Owonikoko et al. randomly assigned 278 SCLC patients to the ipilimumab group and 278 SCLC patients to the chemotherapy group and found that ipilimumab did not prolong OS compared with chemotherapy alone in SCLC patients (30). Spigel et al. randomly assigned 284 SCLC patients to the nivolumab group and 285 SCLC patients to the chemotherapy group and got a conclusion that nivolumab did not prolong OS compared with chemotherapy alone in SCLC patients (31). Hence, to get a more convincing result, we performed a meta-analysis to study the efficacy of ipilimumab, nivolumab, pembrolizumab, and atezolizumab, compared to other therapies or other drugs.
Materials and Methods
Search Strategy
We identified all randomized clinical trials that compared ipilimumab, nivolumab, pembrolizumab, or atezolizumab with the non-immunotherapy control arms from January 1, 2007, to December 31, 2021. The articles we collected were searched by using the keywords “overall survival” or “OS,” “progression-free survival” or “PFS,” “immune checkpoint inhibitors” or “immune checkpoint blockage” or “ICIs” or “ipilimumab” or “nivolumab” or “pembrolizumab” or “atezolizumab” in the PubMed, Google Scholar and Embase databases. The articles we selected were written in English.
Study Selection Criteria
Trials were eligible for inclusion if they met the following criteria: (1) trials that involved patients must receive cancer treatment; (2) trials that had adequate data available including OS and PFS; (3) trials were phase 2 or phase 3 randomized clinical trials (RCTs); and (4) the articles published must be written in English.
Data Extraction
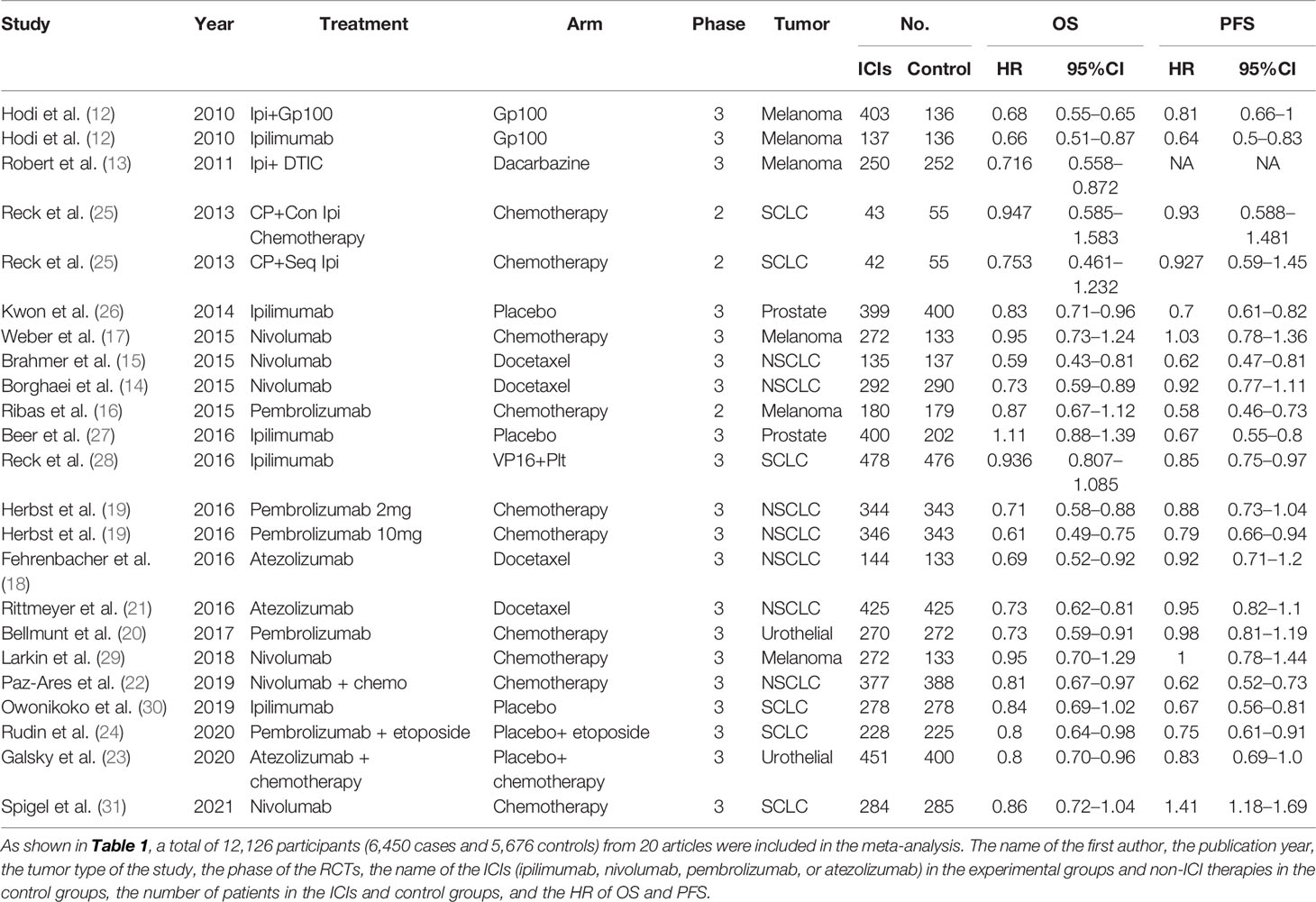
We extracted the following information from each study and selected the items including first author’s last name, year of publication, phase of RCTs, the name of the ICIs (ipilimumab, nivolumab, pembrolizumab or atezolizumab) and control arms, number of patients ICIs and control groups, and the hazard ratios (HRs) of OS and PFS. All the duplicated studies were excluded.
Statistics Analysis
To calculate the overall incidence and HR of OS and PFS, we combined estimates by exploiting the fixed-effect model with the Mantel and Haenszel method and by employing the random-effect models with the DerSimonian and Laird method. The statistical analysis was performed with the R software package named Meta. The HR with 95% confidence interval (CI) was calculated to access the association between overall survival and ICIs.
Two quantities, Cochran’s Q and I2, were used to access the heterogeneity in different types of ICIs groups and subgroups. Statistical heterogeneity was assessed using Cochrane’s Q statistic, and the p value ranging from 0% to 100%, to measure the significance level of inconsistency. If the value of value I2 is less than 50%, or the p value of heterogeneity is greater than 0.10, the fixed-effect model is applied, otherwise the random effect model is employed. After the heterogeneity test, we exploited the R meta package to conduct the meta-analysis with the random-effect model.
Egger’s test (32) and Begg’s test (33) were selected to access the publication bias for OS and PFS. When a two-tailed p value was less than 0.05, the publication bias was extremely significant. Moreover, the potential publication bias was assessed by Begg’s funnel plots to check the relative symmetry of the overall estimated individual study estimates.
Results
Literature Search
A flowchart for the article selection is shown in Figure 1. Through the search strategy, a total of 953 articles were identified. According to the title and abstract, 675 articles were primarily deleted, 193 articles were removed because they were letters, reviews, and other articles, 41 articles were excluded as they were not randomized clinical trials (RCTs), 12 articles were removed because they did not meet our case–control method, and 4 articles were deleted because studies were not phase 2 or phase 3 RCTs. Then 28 were remained, and 8 articles were removed because of the publication bias test. Finally, 20 articles were left, including 7 ipilimumab articles, 6 nivolumab articles, 4 pembrolizumab articles, and 3 atezolizumab articles, respectively (shown in Table 1). Moreover, the data we extracted from the articles were accessed in clinical trial databases with specific identifiers.
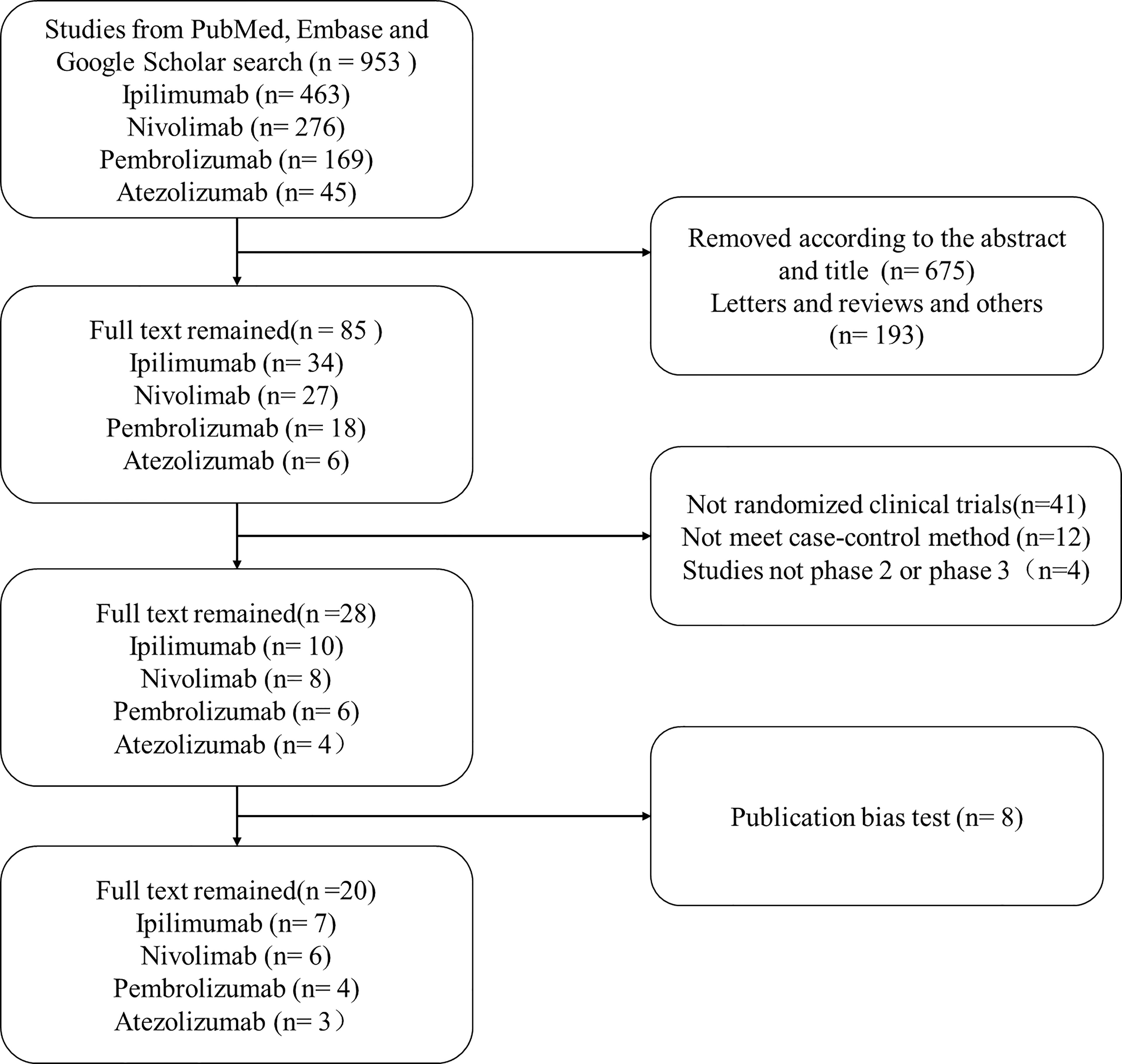
Figure 1 The flowchart of collecting articles. Through the search strategy, a total of 953 articles were identified. According to the title and abstract, 675 articles were primarily deleted, 193 articles were removed because they were letters, reviews, and other articles, 41 articles were excluded as they were non-randomized clinical trials, 12 articles were removed because they did not meet our case–control method, and 4 articles were deleted because studies were not phase 2 or phase 3 RCTs. Then 28 were remained. Moreover, 8 articles were removed after the publication bias test.
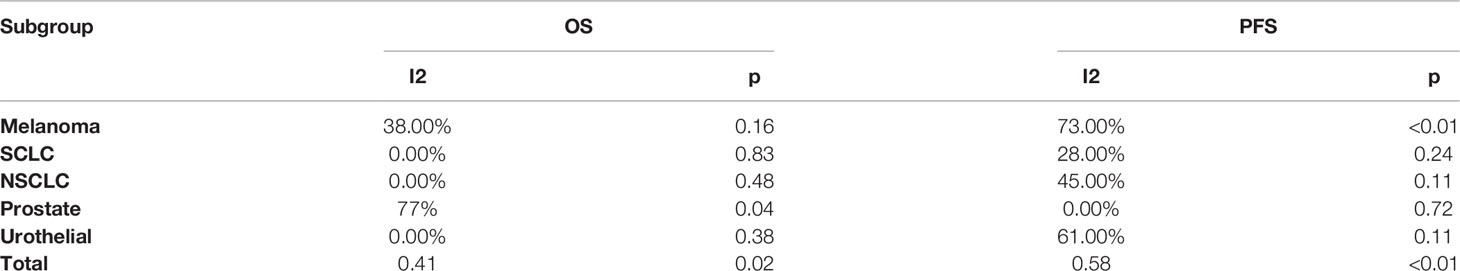
Heterogeneity Test
The summary result of heterogeneity is directly shown in Table 2. In the OS group, we found little heterogeneity in total with I2 = 41%, p = 0.02, and we chose to select the random-effect model according to the method we used. In the tumor subgroup heterogeneity test, we did not find obvious significant heterogeneity in the melanoma (I2 = 38%, p = 0.16), SCLC (I2 = 0%, p=0.83), NSCLC (I2 = 0%, p=0.48), and urothelial cancer (I2 = 0%, p = 0.73) subgroups, but a significant heterogeneity was found in the prostate cancer (I2 = 77%, p = 0.04) subgroup. In the PFS group, we also found heterogeneity in total with I2 = 58%, p < 0.01, and we chose to select the random-effect model according to the method we used. In the tumor subgroup heterogeneity test, we did not find obvious significant heterogeneity in the SCLC (I2 = 28%, p = 0.24), NSCLC (I2 = 45%, p = 0.11) and prostate cancer (I2 = 0%, p = 0.72) subgroups, but a significant heterogeneity was found in the melanoma (I2 = 73%, p < 0.01) and urothelial cancer (I2 = 61%, p = 0.11) subgroups.
Publication Bias Analysis and Sensitivity Analysis
The p-values of Begg’s test and Egger’s test were applied for OS and PFS. We did not find publication bias in OS by Begg’s test (p = 0.5436) and Egger’s test (p = 0.6849), and in PFS by Begg’s test (p = 0.9483) and Egger’s test (p = 0.9774). The result of the OS and PFS publication bias analysis is directly reflected in Figure 2 by using Begg’s funnel plot.
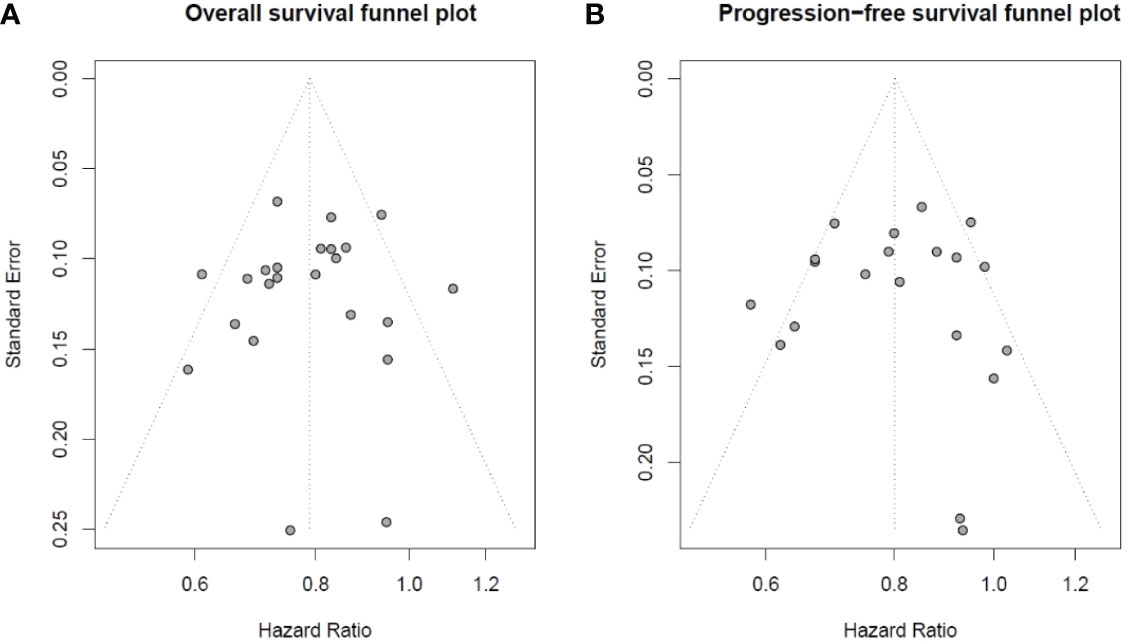
Figure 2 Begg’s funnel plot of overall survival and progression-free survival studies: (A) Begg’s funnel plot of overall survival studies to evaluate publication bias. (B) Begg’s funnel plot of progression-free survival studies to evaluate publication bias.
Association of ICIs With Overall Survival
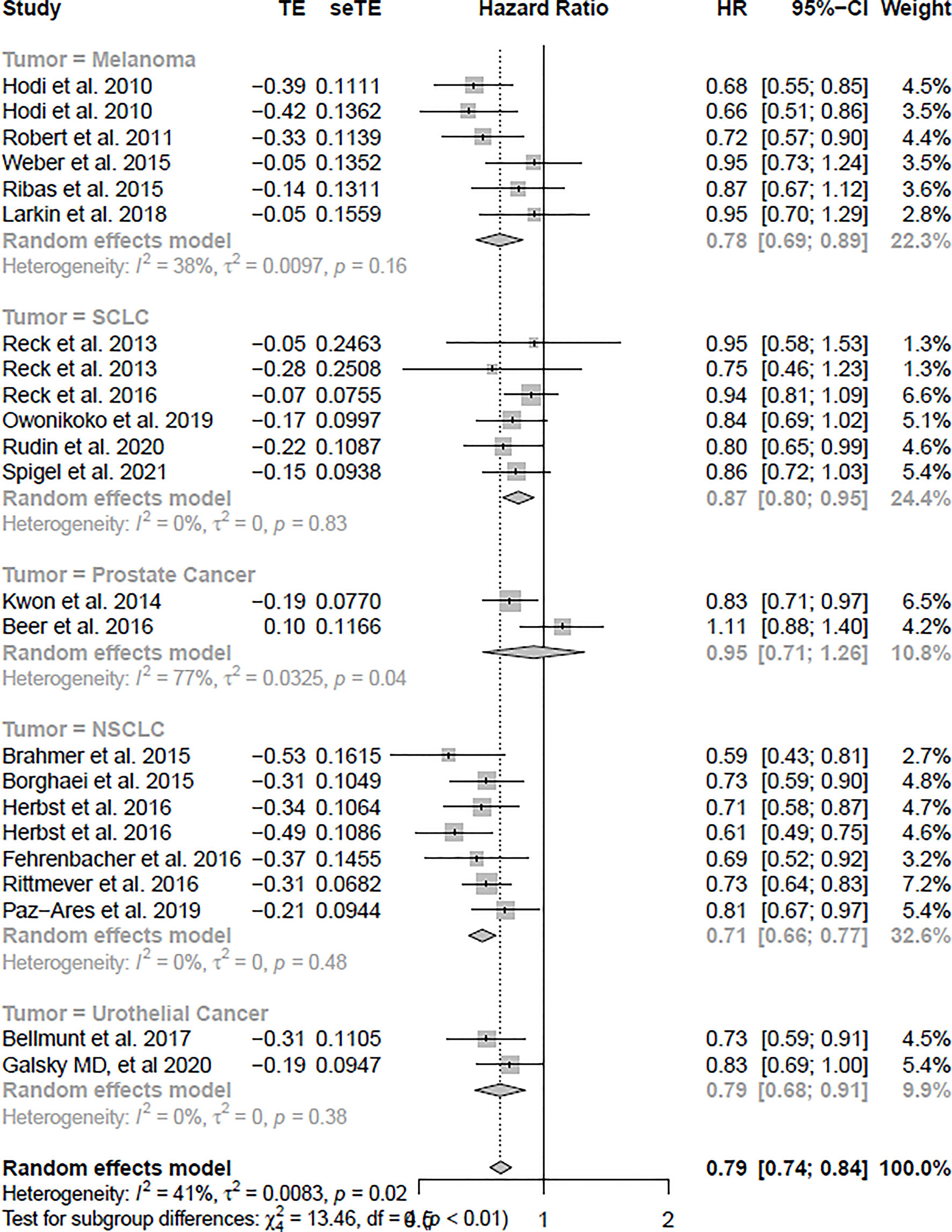
The OS analysis was included in 23 studies, and the PFS analysis was included in 20 studies (shown in Table 1). Figure 3 shows the results of OS, and Figure 4 shows the results of PFS. Table 3 shows the summary of the melanoma, SCLC, NSCLC, prostate cancer, and urothelial cancer (I2 = 0%, p = 0.73) subgroups, but a significant heterogeneity was found in the prostate cancer subgroup meta-analysis and overall meta-analysis.
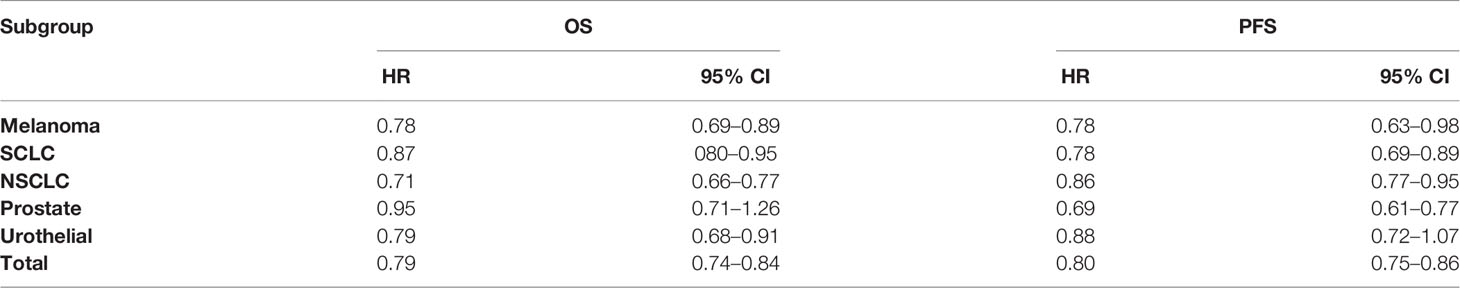
In the OS analysis, the ICIs were associated with substantially ameliorated OS (HR = 0.79, CI = 0.74–0.84), compared with non-ICI therapies. In the subgroup analyses, melanoma, SCLC, NSCLC, and urothelial cancer patients treated with ICIs were associated more with OS compared with non-ICI therapies (HR = 0.78, CI = 0.69–0.89; HR = 0.87, CI = 0.80–0.95; HR = 0.71, CI = 0.66–0.77; HR = 0.79, CI = 0.68–0.91), respectively. However, prostate cancer was not significantly associated with improved OS (HR = 0.95, CI = 0.71–1.26).
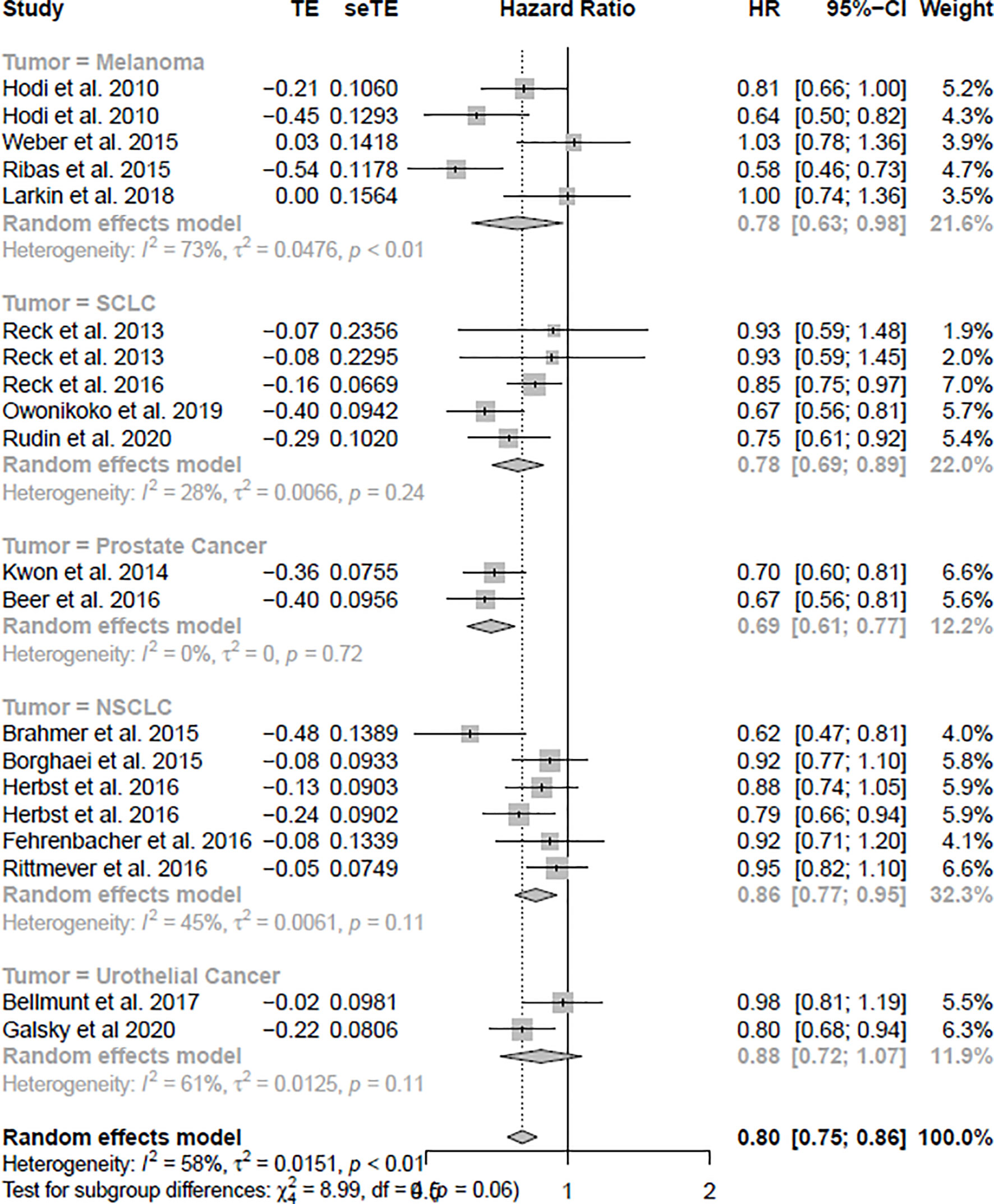
Association of ICIs With Progression-Free Survival
In the PFS analysis, the ICIs were associated with significantly improved PFS (HR = 0.80, CI = 0.75–0.86), compared with non-ICI therapies. In subgroup analyses, melanoma, SCLC, NSCLC, and prostate cancer patients treated with ICIs were associated more with PFS compared with non-ICI therapies (HR = 0.78, CI = 0.63–0.98; HR = 0.78, CI = 0.69–0.89; HR = 0.86, CI = 0.77–0.95; HR = 0.69, CI = 0.61–0.77), respectively. However, urothelial cancer was not significantly associated with improved PFS (HR = 0.88, CI = 0.72–1.05).
Discussion
In our meta-analysis, a total of 12,126 participants (6,450 cases and 5,676 controls), treated with ICIs and non-ICI arms, from 20 articles were included.
In total, among 12,126 patients in our meta-analysis, 2,423 patients (1,514 cases and 969 controls) were included into the melanoma subgroup, 2,727 patients (1,353 cases and 1,374 controls) were included into the SCLC subgroup, 4,122 patients (2,063 cases and 2,059 controls) were included into the NSCLC subgroup, 1,401 patients (799 cases and 602 controls) were included into the prostate subgroup, and 1,393 patients (721 cases and 672 controls) were included into the urothelial cancer subgroup.
To our knowledge, this is the comprehensive meta-analysis to assess the efficacy of ICIs (ipilimumab, pembrolizumab, nivolumab, and atezolizumab) in different types of tumors, including melanoma, SCLC, NSCLC, prostate cancer, and urothelial cancer. Results of trials on ICIs have been published, while the clinical value of ICIs is still controversial. To further investigate the efficacy of ICIs, we made five subgroups of melanoma, SCLC, NSCLC, prostate cancer, and urothelial cancer with OS and PFS.
The pooled analyses indicated that ICIs were associated with obviously ameliorated PFS and OS compared with non-ICI arms. In OS subgroup analyses, melanoma, SCLC, NSCLC, and urothelial cancer patients treated with ICIs were associated more with OS compared with non-ICI therapies. However, prostate cancer was not significantly associated with improved OS. In PFS subgroup analyses, melanoma, SCLC, NSCLC, and prostate cancer patients treated with ICIs were associated more with PFS compared with non-ICI therapies. However, urothelial cancer was not significantly associated with improved PFS.
However, the meta-analysis had some limitations. To begin with, the number of participants in our meta-analysis was 12,126, and more studies should be added to this meta-analysis. Second, some heterogeneity existed in this meta-analysis, especially in the PFS group. It should be solved in the further study. Besides, more studies should be added into the prostate cancer patients and urothelial cancer subgroups.
Conclusions
This meta-analysis got a conclusion that immune checkpoint inhibitors were associated with obviously ameliorated PFS and OS compared with non-ICI therapies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WD and LX wrote the manuscript. WD and XY collected the data and conducted the experiment. LX performed the project. WD interpreted the results. WD and LX developed the analytical tools. All authors contributed to the article and approved the submitted version.
Funding
This research was funded in part by the National Natural Science Foundation of China, grant number 62172122, and the Fundamental Research Funds for the Central Universities, Jilin University, grant number 93K172021K04.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XL declared a shared affiliation with the author XL to the handling editor at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.876098/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Paucek RD, Baltimore D, Li G. The Cellular Immunotherapy Revolution: Arming the Immune System for Precision Therapy. Trends Immunol (2019) 40(4):292–309. doi: 10.1016/j.it.2019.02.002
3. Li L, Jiang M, Qi L, Wu Y, Song D, Gan J, et al. Pyroptosis, a New Bridge to Tumor Immunity. Cancer Sci (2021) 112(10):3979–94. doi: 10.1111/cas.15059
4. Schlotter CM, Tietze L, Vogt U, Heinsen CV, Hahn A. Ki67 and Lymphocytes in the Pretherapeutic Core Biopsy of Primary Invasive Breast Cancer: Positive Markers of Therapy Response Prediction and Superior Survival. Hormone Mol Biol Clin Invest (2017) 32(2):20170022. doi: 10.1515/hmbci-2017-0022
5. Zhou Z, Li M. Evaluation of BRCA1 and BRCA2 as Indicators of Response to Immune Checkpoint Inhibitors. JAMA Netw Open (2021) 4(5):e217728. doi: 10.1001/jamanetworkopen.2021.7728
6. Marrone KA, Ying W, Naidoo J. Immune-Related Adverse Events From Immune Checkpoint Inhibitors. Clin Pharmacol Ther (2016) 100(3):242–51. doi: 10.1002/cpt.394
7. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive Meta-Analysis of Key Immune-Related Adverse Events From CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res (2017) 5(4):312–8. doi: 10.1158/2326-6066.CIR-16-0237
9. Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, et al. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Nonsmall Cell Lung Cancer With Progression on or After Platinum-Based Chemotherapy. Oncologist (2016) 21(5):634–42. doi: 10.1634/theoncologist.2015-0507
10. Ma W, Gilligan BM, Yuan J, Li T. Current Status and Perspectives in Translational Biomarker Research for PD-1/PD-L1 Immune Checkpoint Blockade Therapy. J Hematol Oncol (2016) 9:47. doi: 10.1186/s13045-016-0277-y
11. Hazarika M, Chuk MK, Theoret MR, Mushti S, He K, Weis SL, et al. US FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab. Clin Cancer Res (2017) 23(14):3484–8. doi: 10.1158/1078-0432.CCR-16-0712
12. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
13. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364(26):2517–26. doi: 10.1056/NEJMoa1104621
14. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
15. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-SmallCell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
16. Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab Versus Minvestigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol (2015) 16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2
17. Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Melanoma Who Progressed After Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2015) 16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8
18. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
19. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
20. Bellmunt J, De Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
21. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Nonsmall-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
22. Paz-Ares L, Ciuleanu TE, Yu X, Salman PA. LBA3 Nivolumab (NIVO)+ Platinum-Doublet Chemotherapy (Chemo) vs Chemo as First-Line (1l) Treatment (Tx) for Advanced Non-Small Cell Lung Cancer (aNSCLC): CheckMate 227-Part 2 Final Analysis. Ann Oncol (2019) 30:xi67–8. doi: 10.1093/annonc/mdz453.004
23. Galsky MD, Arija JÁV, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab With or Without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (2020) 10236:1547–57. doi: 10.1016/S0140-6736(20)30230-0
24. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38(21):2369. doi: 10.1200/JCO.20.00793
25. Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab Incombination With Paclitaxel and Carboplatin as First-Line Therapy in Extensive-Disease-Small-Cell Lung Cancer: Results From a Randomized, Double-Blind, Multicenter Phase 2 Trial. Ann Oncol (2013) 24(1):75–83. doi: 10.1093/annonc/mds213
26. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, Van den Eertwegh AJM, et al. Ipilimumab Versus Placebo After Radiotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer That had Progressed After Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, DoubleBlind, Phase 3 Trial. Lancet Oncol (2014) 15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5
27. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol (2016) 35(1):40–7. doi: 10.1200/JCO.2016.69.1584
28. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol (2016) 34(31):3740–8. doi: 10.1200/JCO.2016.67.6601
29. Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol (2018) 36(4):383. doi: 10.1200/JCO.2016.71.8023
30. Owonikoko TK, Kim HR, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab (Nivo) PlusIpilimumab (Ipi), Nivo, or Placebo (Pbo) as Maintenance Therapy in Patients (Pts) With Extensive Disease Small Cell Lung Cancer (ED-SCLC) After First-Line (1l) Platinum-Based Chemotherapy (Chemo): Results From the Double-Blind, Randomized Phase III CheckMate 451 Study. Ann Oncol (2019) 30:ii77. doi: 10.1093/annonc/mdz094
31. Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-Line Nivolumab in Relapsed Small-Cell Lung Cancer: CheckMate 331. Ann Oncol (2021) 32.5:631–41. doi: 10.1016/j.annonc.2021.01.071
32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
Keywords: immune checkpoint inhibitors, meta-analysis, cytotoxic T-lymphocyte-associated protein 4, overall survival, programmed cell death 1, progression-free survival, programmed death ligand 1
Citation: Xu L, Yan X and Ding W (2022) Meta-Analysis of Efficacy From CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Front. Oncol. 12:876098. doi: 10.3389/fonc.2022.876098
Received: 15 February 2022; Accepted: 17 March 2022;
Published: 28 April 2022.
Edited by:
Liang Cheng, Harbin Medical University, ChinaCopyright © 2022 Xu, Yan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyue Ding, d3lkaW5nMDUwMUBob3RtYWlsLmNvbQ==
 Li Xu
Li Xu Xin Yan
Xin Yan Weiyue Ding
Weiyue Ding