- 1Department of Biomedicine and Prevention, University of Tor Vergata, Rome, Italy
- 2Santa Lucia Foundation, Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.) Neuro-Oncohematology, Rome, Italy
- 3Department of Biomedicine and Prevention, UniCamillus‐Saint Camillus International University of Health Sciences, Rome, Italy
- 4Department of Experimental Medicine, Tor Vergata University of Rome, Rome, Italy
Acute promyelocytic leukemia (APL) accounts for 10–15% of newly diagnosed acute myeloid leukemias (AML) and is typically caused by the fusion of promyelocytic leukemia with retinoic acid receptor α (RARA) gene. The prognosis is excellent, thanks to the all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) combination therapy. A small percentage of APLs (around 2%) is caused by atypical transcripts, most of which involve RARA or other members of retinoic acid receptors (RARB or RARG). The diagnosis of these forms is difficult, and clinical management is still a challenge for the physician due to variable response rates to ATRA and ATO. Herein we review variant APL cases reported in literature, including genetic landscape, incidence of coagulopathy and differentiation syndrome, frequent causes of morbidity and mortality in these patients, sensitivity to ATRA, ATO, and chemotherapy, and outcome. We also focus on non-RAR rearrangements, complex rearrangements (involving more than two chromosomes), and NPM1-mutated AML, an entity that can, in some cases, morphologically mimic APL.
Introduction
Acute promyelocytic leukemia (APL) was first described in 1957 by Hillestad and accounts for 10–15% of newly diagnosed acute myeloid leukemias (AML), with an incidence of about 1,900 cases per year in Europe (0.12 per 100,000 person-years) (1, 2). APL is characterized by the t(15;17)(q24.1;q.2) balanced translocation, which results in the fusion of promyelocytic leukemia (PML) with retinoic acid receptor α (RARA) gene. This oncogene, found in 98% of cases, causes the transcriptional repression of RARA-targeted genes and the destruction of PML nuclear bodies (PML-NBs), resulting in altered self-renewal, senescence mechanisms, and response to DNA damage (3–7). With the introduction of daunorubicin in 1973, all-trans-retinoic acid (ATRA) in 1988, arsenic trioxide (ATO) in 1997, and, eventually, the chemo-free ATRA+ATO approach in 2006, the disease changed from highly fatal to curable leukemia in most cases (6, 8–14).
Morphologically, APL is characterized by the presence, in the bone marrow (BM) and/or peripheral blood (PB), of immature hypergranular promyelocytes with abundant cytoplasm, irregular nuclei with fine azurophilic granules, and Auer rods in 90% of cases. The immunophenotypic evaluation often shows the expression of myeloid antigen CD13, CD33, CD117, and MPO, while CD34 and HLA-DR, as well as markers of granulocytic differentiation, results are absent or low. A rapid diagnosis of APL may be performed by analyzing the immunocytochemical pattern of the PML protein using the anti-PML monoclonal antibody PG-M3 (15, 16). However, a genetic confirmation of the PML/RARA fusion transcript is mandatory and is carried out by RT-PCR or RT-QLAMP, and it should be performed by turnaround times of 24–48 h in order to warrant a rapid treatment start, which is associated with a reduction in the rate of bleeding complications, the major cause of early death in APL (15, 17–19). Other diagnostic approaches, such as conventional karyotyping and FISH, are useful to identify the t(15;17) translocation (15).
About 2% of APL are characterized by atypical rearrangements, where the RARA is fused to partners other than PML or in which the translocation involves other members of the RAR superfamily (Table 1) (6). These APL-like forms are a tough challenge for the clinician, both because of the difficulty of diagnosis and the generally unfavorable outcome due to diagnostic delays and to the frequent resistance of these forms to treatment commonly used for classical APL (3, 4, 101, 102).
This paper focus on the latter entities in order to summarize knowledge on the molecular landscape and correlations with outcome.
Variant APLs usually have a clinical presentation and morphological and immunophenotypic picture similar to classical APL, including pancytopenia-related symptoms (weakness, fatigue, infection) and bleeding (103). Therefore, if genetic tests result negative or are not readily available, a FISH analysis using RARA, RARB, and RARG probes or conventional karyotype helps to identify possible alternative RAR translocations or other genetic abnormalities. In this context, the morphologic and/or immunophenotypic features of some NPM1-mutated AMLs also resemble APL and will be described in this review.
Pathogenesis, Characteristics, and Management of APL Variants
The pathogenesis of APL has been extensively studied and discussed in previous papers. The chromosomal translocation t(15;17), resulting in the PML-RARA fusion protein, is the main and, probably, the only driver alteration of APL, where additional gene mutations have been reported at a significantly lower rate when compared to other AML subtypes (26, 104, 105). The differentiation block typical of APL results from the destruction of PML nuclear bodies, implicated in DNA replication, transcription, and epigenetic silencing, and from the repression of RARA target differentiation genes by the aberrant recruitment of histone deacetylases (2–4, 105, 106).
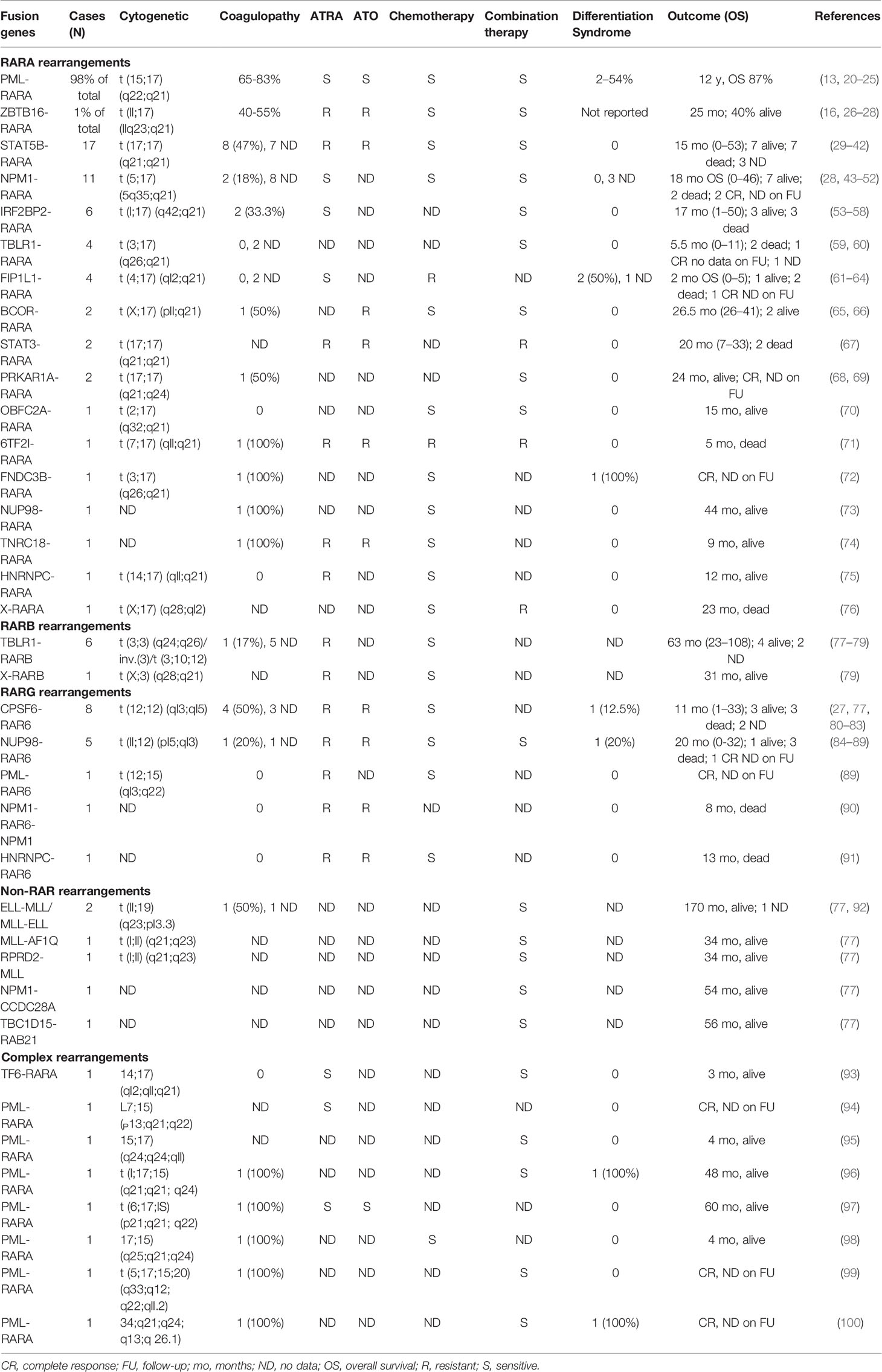
Several rearrangements different from PML-RARA have been described as driver mutations in APL-like AML (Figure 1). In most cases, the RAR gene family is involved (1, 103), including RARB and RARG in addition to RARA, all with a key role in cell development and differentiation (2, 107). Few cases of rearrangements other than RAR, mainly pediatric, have been reported (3, 77).
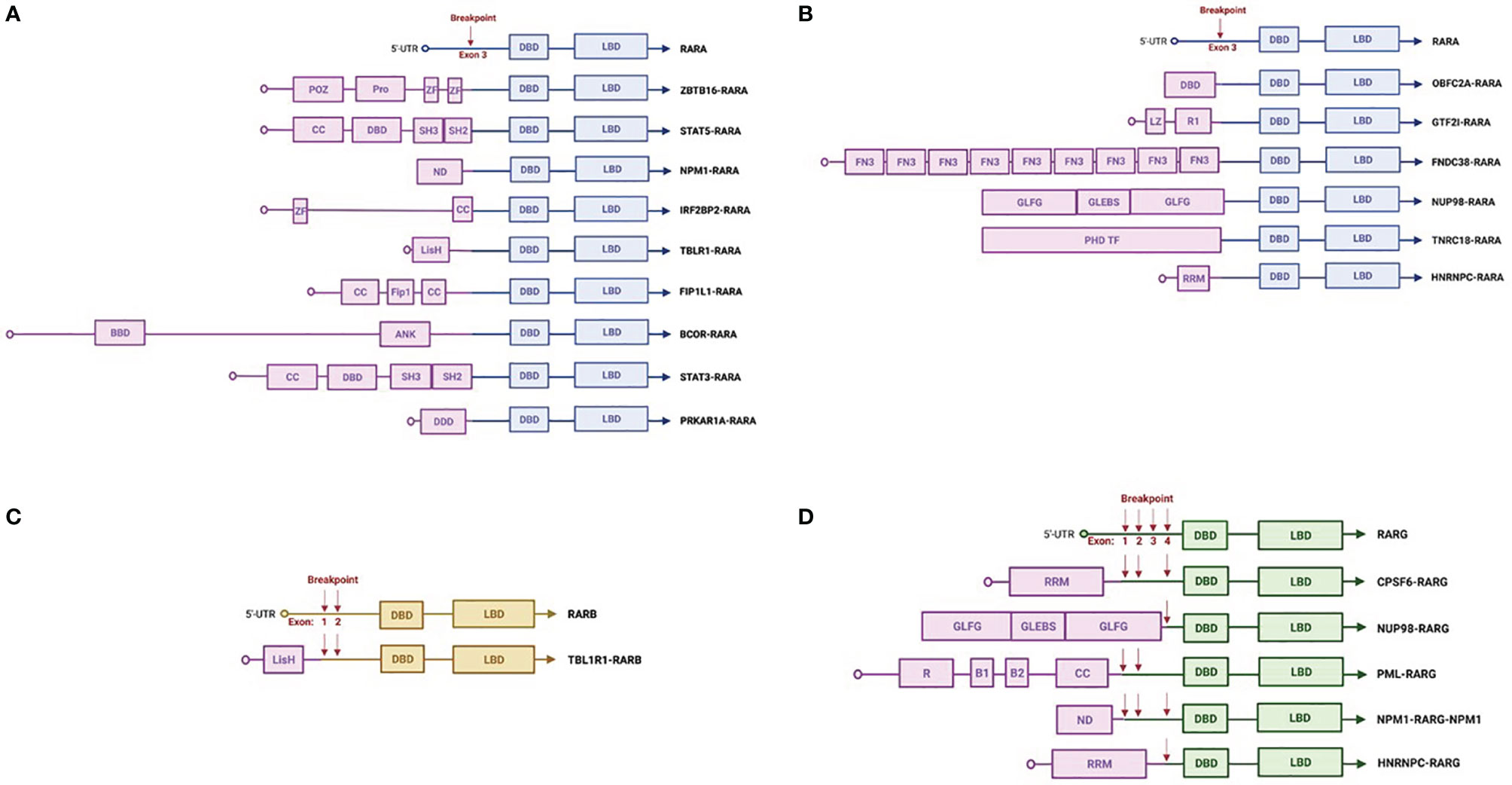
Figure 1 Schematic representations of RARx translocations. Common (A) and rare (B) RARA rearrangements. RARB (C) and RARG (D) rearrangements. The figures were created with Biorender.com. 5′-UTR, 5′-untranslated region; DBD, DNA-binding domain; LBD, ligand-binding domain; CC, coiled coil domain; POZ, BTB/POZ domain; Pro, proline-rich region; Zn, zinc finger domain; SH3, protein–protein interaction domain; SH2, docking domain for phosphorylated tyrosine residues; BBD, BCOR Bcl6-binding domain; ANK, ankyrin repeats; Fip1, FIP1-binding domain for polymerase; LisH, lissencephaly type-1-like homology motif; DDD, dimerization/docking domain of the type I alpha regulatory subunit of cAMP-dependent protein kinase; ND, nucleoplasmin/nucleophosmin domain; LZ, leucine zipper; PHD TF, plant homeodomain finger transcription factor domain; FN3, fibronectin type 3 domain; GLEBS, Gle2/Rae1-binding sequence; GLFG, Gly-Leu-Phe-Gly repeats; R1, I-repeat domains; RRM, RNA recognition motif; R, RING finger domain; B1 and 2, B box.
We reviewed the most frequently reported genetic alterations in APL-like AML since 2010, including cases identified with modern diagnostic standards and treated with current therapeutic strategies (Table 1). Because of clinical relevance, we report data on coagulopathy (main cause of death in APL patients) and differentiation syndrome (DS) (13, 20–22). Coagulopathy is defined as prolonged prothrombin time and/or activated partial thromboplastin time in addition to hypofibrinogenemia and/or increased levels of fibrin degradation products or D-dimer (23–25). We excluded from our analysis cases in which a rearrangement was not identified or cases with a cryptic PML-RARA rearrangement.
RARA Rearrangements
In APL variants, rearrangements involving RARA gene are the most frequently described. The most common translocation, reported in 1% of cases, is the t(11;17)(q23;q21), which fuses RARA with ZBTB16 (formerly known as promyelocytic leukemia zinc finger protein PLZF), a key regulator of physiological and stress-induced myelopoiesis (1–3, 108). Of note is that both the oncogenic fusion transcripts PML-RARA and ZBTB16-RARA are characterized by the identical RARA exon sequence, suggesting a key role of RARA domains in the pathogenesis of this disease. In contrast to classic APL (109, 110), characterized by a low number of additional mutations, this variant presents a complex molecular landscape, similar to other acute myeloid leukemias (AML) (109, 110). Our group showed that ZBTB16-RARA rearranged AML and display several gene mutations commonly identified in AML, including TET2, RUNX1, and CSF3R, and in particular, the most frequent alteration was found in the AT-rich interacting domain containing protein 1A gene (AIRD1A, in 6 out of 7 patients analyzed), a member of the SWI/SNF family of transcriptional regulators, which is a known solid and hematological cancer development driver (26, 111).
Data in literature report a lower incidence of coagulopathy compared to classic APL (40–55%) and a poor outcome, with less than half of the patients surviving at prolonged follow-up (26–28, 102). The therapy with ATRA and ATO is not effective on ZBTB16-RARA AML, and the resistance to ATO is due to the lack of an ATO-binding site, whereas although ATRA induces the degradation of the fusion protein, differentiation and apoptosis do not occur, and there is no clinical response. Indeed DS has been reported in none of the cases (8, 9, 112, 113). The proteolytic activity of ATRA has shown positive results in combination with chemotherapy, which remains the main therapeutic strategy in these patients.
The recent finding of the high prevalence of ARID1A mutations might open new scenarios on the use of targeted drugs since this mutation has been associated with a decrease in intracellular glutathione (GSH). Eprenetapopt (APR-246), a GSH p53-targeting compound inhibitor, has been shown to be effective in both ARID1A-mutated solid tumors and p53-mutated myelodysplastic syndromes and may be active in ZBTB16-RARA AML (10, 11, 114–116).
The signal transducer and activator of transcription 5B (STAT5B) is a member of the STAT family transcription factors and mediates cell differentiation, proliferation, and survival signals induced by various cytokines and hormones (13, 117). STAT5 has been linked to several hematological cancers, in particular lymphoma and acute leukemia (118). Fusion with RARA generates the t(17;17)(q21;q21) and the STAT5B-RARA rearrangement, of which 17 cases have been described (29–41). This variant shares common features with ZBTB16-RARA AML: a moderate incidence of coagulopathy, resistance to ATRA and ATO, and poor outcome (42). Chemotherapy is likewise the best strategy, but hypomethylating agents may also be effective, as shown in a case successfully treated with decitabine and ATRA (29). Given the high relapse rate, patients with STAT5B/RARA-positive leukemia might benefit from hematopoietic stem cell transplantation in the first remission.
The rearrangement of RARA with nucleophosmin 1 [NPM1-RARA, t(5;17)(5q35;q21)], a protein physiologically implicated in genomic stability and DNA repair and one of the most commonly mutated genes in AML, has been frequently reported (101, 102, 119, 120).
These cases have morphologic and phenotypic features similar to PML-RARA-positive APL, but blasts display abundant cytoplasm with small azurophilic granules and regular nuclear outline. A coagulopathy has been rarely reported (2 out of 11 cases), and the prognosis seems better than for the previous variants, probably because of the sensitivity to the drugs used in classic APL. Indeed NPM1-RARA-positive AML are sensitive to ATRA (alone or in combination with chemotherapy, but DS has not been reported (28, 43–52)).
Interferon regulatory factor 2-binding protein 2 (IRF2BP2) plays a role in apoptosis, survival, and cell differentiation (121). In six cases of t(1;17)(q42;q21), IRF2BP2-RARA-rearranged AML has been reported. Coagulopathy occurred in 2 patients; ATRA, alone and in combination with chemotherapy, resulted effective, while no DS has been reported (53–58).
Transducin β-like receptor 1 (TBLR1) plays an important role in stem cell proliferation and differentiation and is involved in the oncogenesis of solid tumors (122). The TBLR1-RARA fusion transcript [t(3;17)(q26;q21)] has been described in 4 cases. ATRA and ATO were successfully used in combination with chemotherapy, and no coagulopathy and DS were observed (59, 60).
Factor interacting with PAPOLA and CPSF1 (FIP1L1), fused with platelet-derived growth factor receptor α (PDGFRα), has been linked to several hematologic malignancies (hypereosinophilic syndrome, chronic eosinophilic leukemia, systemic mastocytosis) (123). The FIP1L1-RARA chimeric protein [t(4;17)(q12;q21)]-positive AML is ATRA-sensitive, and two DS events were reported. Nevertheless, it is an aggressive variant, and half of the reported cases died due to bleeding before the start of therapy or resistance first to chemotherapy and then to ATRA+chemotherapy. No coagulopathy has been reported (61–64).
BCL6 Corepressor (BCOR) is an epigenetic regulator whose alterations recur in solid and hematologic tumors (124). Two BCOR-RARA [t(X;17)(p11;q21)]-positive AML cases have been described. One patient presented coagulopathy that was relieved by ATRA and tamibarotene. Both patients underwent chemotherapy with and without ATRA, and complete response was achieved in both cases. The role of ATRA as monotherapy is therefore uncertain, and ATO resulted ineffective (65, 66).
Signal transducer and activator of transcription 3 (STAT3), a member of the STAT family transcription factors, has functions similar to STAT5 and has been likewise linked to hematological cancers (118). A STAT3-RARA variant [t(17;17)(q21;q21)] has been reported in two cases with unfavorable outcome. Both patients were resistant to ATRA, and one of them was resistant to ATO. One patient, treated with chemotherapy, achieved a temporary complete response (67).
The rearrangement between RARA and protein kinase CAMP-dependent type I regulatory subunit alpha PRKAR1A-RARA, [t(17;17)(q21;q24)], implicated in several cellular functions and in the tumorigenesis of solid cancers, has been described in two cases (125). Both were sensitive to ATRA in combination with chemotherapy (19). One patient presented coagulopathy at onset (68, 69).
Individual cases of rare rearrangements of RARA with the following genes have been described: oligonucleotide/oligosaccharide-binding fold containing 2° [OBFC2A-RARA, t(2;17)(q32;q21)], general transcription factor II-I [GTF2I-RARA, t(7;17)(q11;q21)], fibronectin type III domain-containing protein 3B [FNDC3B-RARA, t(3;17)(q26; q21)], nuclear pore complex protein 98 (NUP98-RARA, cytogenetic analysis not available), trinucleotide repeat containing 18 (TNRC18-RARA, cytogenetic analysis not available), heterogeneous nuclear ribonucleoprotein C (HNRNPC-RARA, t(14;17)(q11;q21)), and chromosome X (X-RARA, t(X;17)(q28;q12)). In most cases, these AMLs resulted resistant to ATRA and ATO. The only exception is a patient with a FNDC3B-RARA-positive AML, who presented DS after 4 days from ATRA initiation and achieved complete response, but there are no data on the long-term response. Chemotherapy was effective, and CR was achieved in all cases, except for one patient with a GTF2I-RARA rearrangement and who experienced early death. Coagulopathy was reported in GTF2I-RARA, FNDC3B-RARA, NUP98-RARA, and TNRC18-RARA-positive AML (70–76).
RARB Rearrangements
TBLR1-RARB rearrangement has been frequently described, with variable cytogenetic features (t(3;3)(q24;q26)/inv.(3)/t(3;10;12)(q26.2;q22;q15)) (77–79). The patients were resistant to ATRA therapy, and no DS occurred, while chemotherapy was effective. One patient presented coagulopathy at the onset of disease. Osumi et al. reported a second type of rearrangement involving RARB, whose partner gene was not detected by whole-genome sequencing [X-RARB: t(X;3)(q28;q21)], which occurred in an AML, which was resistant to ATRA and sensitive to chemotherapy (79).
Thus, in patients with RARB rearrangements, chemotherapy seems a valuable option, as it guarantees good results and favorable outcomes, with all patients alive at a mean follow-up of 56.6 months (range, 23–108 months) (Table 1).
RARG Rearrangements
The most commonly described RARG rearrangement involves the cleavage and polyadenylation specificity factor subunit 6 (CPSF6-RARG, t(12;12)(q13;q15), implicated in solid cancer viability and tumorigenesis and reported in 8 patients (126). Coagulopathy occurred in half of the patients. This variant is not sensitive to treatment with ATRA and ATO, with discrete responses to chemotherapy. Only one case of DS has been described in a patient treated with ATRA+ATO, who died 1 month after diagnosis (27, 77, 80–83).
Five cases of AML with rearrangements between RARG and NUP98 (NUP98-RARG, t(11;12)(p15;q13), have been described. NUP98 is a gene known to fuse with different partner genes in several hematological malignancies (127). Coagulopathy occurred in one patient. ATRA and ATO, alone or in combination, resulted ineffective. One patient presented DS after 14 days from ATRA+ATO initiation, and an in vitro study demonstrated ATRA activity (3). Four patients treated with chemotherapy, one of them combined with ATRA, achieved complete response (84–89).
Three other RARG partner genes have been reported: PML (PML-RARG t(12;15)(q13;q22), NPM1 (NPM1-RARG-NPM1, cytogenetic analysis not available), and heterogeneous nuclear ribonucleoproteins C1/C2 (HNRNPC-RARG, cytogenetic analysis not available). In these cases, no coagulopathy occurred, and the disease was resistant to ATRA and/or ATO (89–91).
Overall, variant APLs with RARG rearrangements are characterized by poor outcome (more than half of the patients died) and resistance to ATRA and ATO, although these drugs have some effect on blasts, as shown by the reported DSs. Therefore, a combined approach with ATRA ± ATO and chemotherapy may be reasonable.
Non-RAR Rearrangements
A few cases of APL variants with RAR-negative rearrangements have been reported, including myeloid/lymphoid or mixed-lineage leukemia and RNA polymerase II elongation factor ELL (ELL-MLL/MLL-ELL, t(11;19)(q23;p13.3), MLL and AF1Q (MLL-AF1Q, (t(1;11)(q21;q23), MLL and regulation of nuclear pre-MRNA domain containing 2 (RPRD2-MLL, t(1;11)(q21;q23), NPM1 and coiled-coil domain containing 28A (NPM1-CCDC28A, cytogenetic analysis not available), TBC1 domain family member 15, and Ras-associated binding 21 (TBC1D15-RAB21, cytogenetic analysis not available) (77, 92).
Most of these genes are known to be involved in leukemogenesis, DNA damage response, and stem cell self-renewal (128–132). All rearrangements have been described in pediatric patients by Zhao et al. ELL-MLL/MLL-ELL has been also described in a young woman, the only one who presented with coagulopathy. The patients were successfully treated with a combination therapy (ATRA, ATO, and chemotherapy) (77, 92).
Of note is that a NPM1-CCDC28A fusion transcript has been recently described in an adult patient whose features, however, resembled more an NPM1-mutated AML than APL (93). The similarities between APL and NPM1-mutated AML will be discussed in the dedicated paragraph.
Complex Rearrangements
Complex rearrangements are translocations involving more than two chromosomes. With the exception of the case reported by Chong et al., all patients presented typical PML-RARA fusion transcripts. Compared with the other atypical transcripts, these patients presented features similar to classic APL: a high percentage of coagulopathy (71%), good response to ATRA, ATO, and/or chemotherapy, discrete incidence of differentiation syndrome (29%), and an excellent prognosis (patients alive at a mean follow-up of 23.8 months; range, 3–60 months). This suggests that karyotypic complexity does not affect the characteristics and outcome of ASPL with typical rearrangements (94–100, 133).
Chong et al. described the only complex rearrangement bearing a fusion transcript different from PML-RARA. It involves RARA and the tropomyosin receptor kinase-fused gene (TGF-RARA, t(3;14;17)(q12;q11;q21), a regulator of intracellular proteins trafficking and fusion partner in solid cancers. This APL variant was also sensitive to ATRA therapy, alone and in combination with chemotherapy, with a favorable outcome. No coagulopathy and DS were observed (94).
Cases of NPM1-Mutated AML With APL-Like Morphology
Recent studies have shown that, in a discrete proportion of cases (up to 30%), NPM1-mutated AML may present clinical and laboratory features similar to APL.
Mason et al. conducted the study with the largest number of cases and included 42 patients. The immunophenotype commonly found in this subset of AMLs was CD117+MPO+CD34-HLA-DR-CD11b-CD13dim/- (134). Furthermore, in this subset of AMLs, the immunocytochemical pattern of the PML protein may resemble APL (135, 136).
In the case series of Rosainz et al., the morphological features of blasts were similar to APL, with Auer Rods found in 2 of the 5 patients and signs of coagulopathy found in all patients. A possible confounding factor may be the frequent leukocytosis in these patients, a feature uncommon in APL and associated, in AML, with an increased risk of DIC. These patients seem to have a better outcome than AML-NPM1 mutated without APL-like features, with significantly longer relapse-free survival (median 64 vs. 9 months) and overall survival (median 81 vs. 20 months) in those who achieved CR (137).
Interestingly, Mason et al., examining next-generation sequencing data, found either TET2 or IDH1/2 mutation in almost all of these patients (98%) (134). El Hajj et al. and Martelli et al. have recently shown that ATRA and ATO can induce differentiation and apoptosis in NPM-1 blasts, suggesting that this combination may also be effective in NPM1-mutated AMLs (135, 138).
Final Considerations
Numerous reports in recent years broadened the knowledge on variant APL prognosis and drug resistance profile. With this literature update, we hope to describe these cases in a complete and detailed manner to support the correct classification of the variants, which, although rare, remain a challenge for the clinician.
A strong suspicion of APL should lead to the prompt initiation of treatment with ATRA and, if the PML-RARA transcript is negative, to further diagnostic investigations. Figure 2 shows a diagnostic algorithm for APL and APL-like AMLs. Frequent monitoring and, possibly, correction of coagulation parameters and blood count are of utmost importance to prevent the onset of coagulopathy. In case of a newly identified transcript, combination therapy may be a valid strategy. Patient management should include careful monitoring of fluid balance, body weight, and any signs of possible SD, which may occur even in the absence of a therapeutic response to ATRA or ATO.
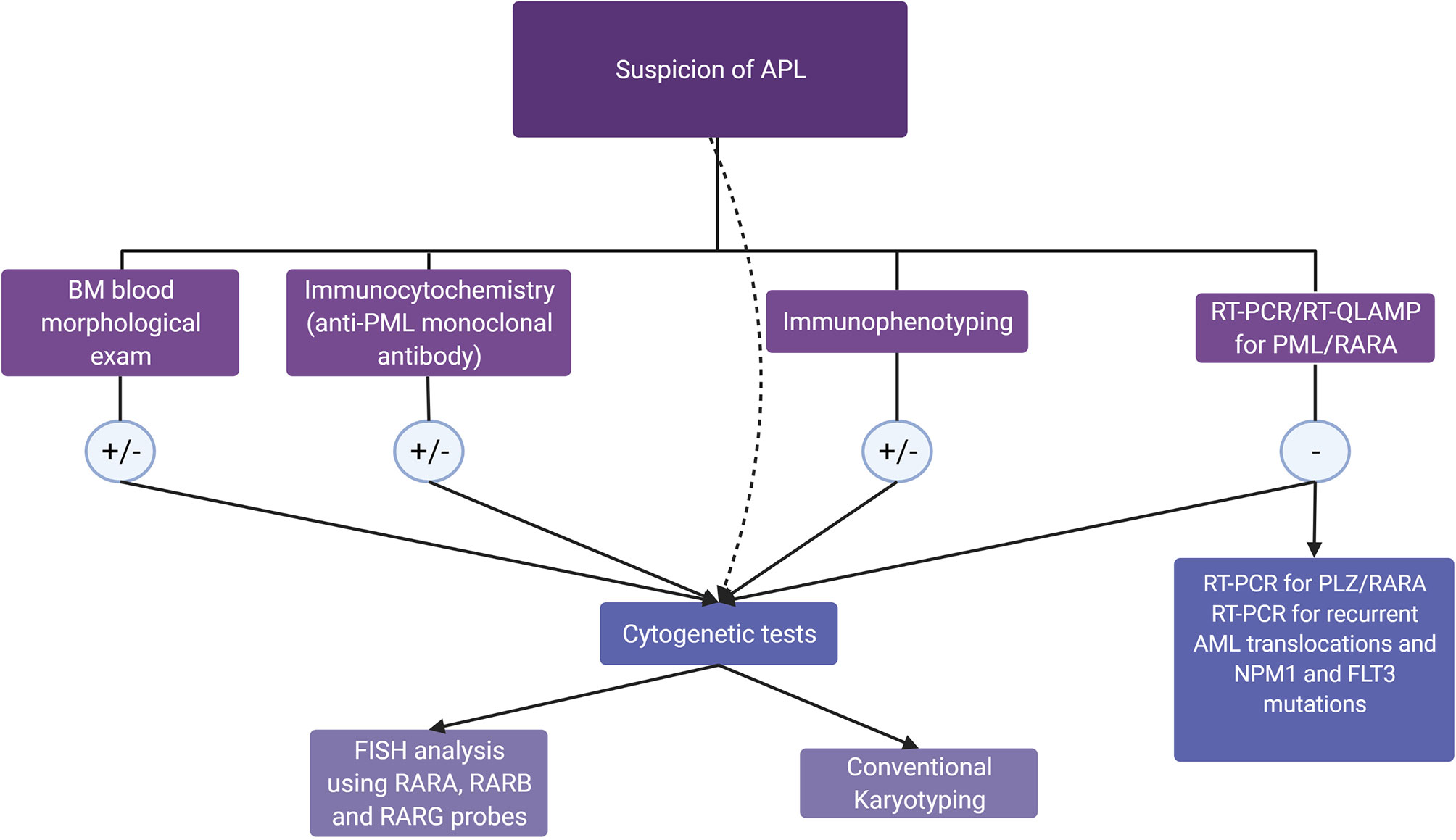
Figure 2 Diagnostic algorithm in the suspicion of acute promyelocytic leukemia (APL) and APL-like acute myeloid leukemias. In patients with morphologic, immunophenotypic, or clinical features raising a suspicion of APL, the guidelines (15) recommend molecular genotyping, which can confirm APL diagnosis in a few hours. If the PML/RARA rearrangement is absent, RT-PCR for recurrent translocations and for PLZF/RARA rearrangement, together with NPM1 and FLT3 mutation testing, should be performed. In all cases, cytogenetic tests, including FISH, allow the diagnostic assessment for RARX rearrangements in 1 to 2 days, while conventional karyotyping will detect karyotype abnormalities in 5–7 days, as recommended by ELN 2017 (139).
Author Contributions
LG, TO, and MTV composed, edited, and finalized the review. ST designed the figure. EF, MD, AR, GF, PP, and MCR reviewed the text. All authors contributed to the article and approved the submitted version.
Funding
Grant from Ministero della Salute, Rome, Italy (Finalizzata 2018, NET-2018-12365935, Personalized medicine program on myeloid neoplasms: characterization of the patient's genome for clinician decision making and systematic collection of real world data to improve quality and health care) to MTV.
Conflict of Interest
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was supported by AIRC 5×1000 call “Metastatic disease: the key unmet need in oncology” to MYNERVA project, #21267 (MYeloid NEoplasms Research Venture AIRC. A detailed description of the MYNERVA project is available at http://www.progettoagimm.it.), PRIN grant N. 2017WXR7ZT to MTV, and the Innovative Medicines Initiative (IMI) 2 project “HARMONY”, no. 116026, to MTV.
References
1. HILLESTAD LK. Acute Promyelocytic Leukemia. Acta Med Scand (1957) 159:189–94. doi: 10.1111/j.0954-6820.1957.tb00124.x
2. Dinmohamed AG, Visser O. Incidence of Acute Promyelocytic Leukemia Across Europe: Results of RARECAREnet-A Population-Based Study. Stem Cell Investig (2019) 6:37. doi: 10.21037/sci.2019.10.03
3. Zhang X, Sun J, Yu W, Jin J. Current Views on the Genetic Landscape and Management of Variant Acute Promyelocytic Leukemia. biomark Res (2021) 9:33. doi: 10.1186/s40364-021-00284-x
4. Geoffroy M-C, de Thé H. Classic and Variants APLs, as Viewed From a Therapy Response. Cancers (Basel) (2020) 12. doi: 10.3390/cancers12040967
5. Dos Santos GA, Kats L, Pandolfi PP. Synergy Against PML-RARa: Targeting Transcription, Proteolysis, Differentiation, and Self-Renewal in Acute Promyelocytic Leukemia. J Exp Med (2013) 210:2793–802. doi: 10.1084/jem.20131121
6. de Thé H, Chen Z. Acute Promyelocytic Leukaemia: Novel Insights Into the Mechanisms of Cure. Nat Rev Cancer (2010) 10:775–83. doi: 10.1038/nrc2943
7. Noguera NI, Catalano G, Banella C, Divona M, Faraoni I, Ottone T, et al. Acute Promyelocytic Leukemia: Update on the Mechanisms of Leukemogenesis, Resistance and on Innovative Treatment Strategies. Cancers (Basel) (2019) 11. doi: 10.3390/cancers11101591
8. Bernard J, Weil M, Boiron M, Jacquillat C, Flandrin G, Gemon M-F. Acute Promyelocytic Leukemia: Results of Treatment by Daunorubicin. Blood. 1973;41(4):489–96. Blood (2016) 128:1779. doi: 10.1182/blood-2016-08-735084
9. Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of All-Trans Retinoic Acid in the Treatment of Acute Promyelocytic Leukemia. Blood (1988) 72:567–72. doi: 10.1182/blood.V72.2.567.567
10. Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of Arsenic Trioxide (As2O3) in the Treatment of Acute Promyelocytic Leukemia (APL): I. As2O3 Exerts Dose-Dependent Dual Effects on APL Cells. Blood (1997) 89:3345–53. doi: 10.1182/blood.V89.9.3345
11. Bernard J, Lasneret J, Chome J, Levy JP, Boiron M. A Cytological And Histological Study Of Acute Premyelocytic Leukaemia. J Clin Pathol (1963) 16:319–24. doi: 10.1136/jcp.16.4.319
12. Jones ME, Saleem A. Acute Promyelocytic Leukemia. A Review of Literature. Am J Med (1978) 65:673–7. doi: 10.1016/0002-9343(78)90856-2
13. Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N Engl J Med (2013) 369:111–21. doi: 10.1056/NEJMoa1300874
14. Gurnari C, De Bellis E, Divona M, Ottone T, Lavorgna S, Voso MT. When Poisons Cure: The Case of Arsenic in Acute Promyelocytic Leukemia. Chemotherapy (2019) 64:238–47. doi: 10.1159/000507805
15. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of Acute Promyelocytic Leukemia: Updated Recommendations From an Expert Panel of the European LeukemiaNet. Blood (2019) 133:1630–43. doi: 10.1182/blood-2019-01-894980
16. Liso V, Bennett J. Morphological and Cytochemical Characteristics of Leukaemic Promyelocytes. Best Pract Res Clin Haematol (2003) 16:349–55. doi: 10.1016/S1521-6926(03)00061-6
17. van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR Analysis of Fusion Gene Transcripts From Chromosome Aberrations in Acute Leukemia for Detection of Minimal Residual Disease. Report of the BIOMED-1 Concerted Action: Investigation of Minimal Residual Disease in Acute Leukemia. Leukemia (1999) 13:1901–28. doi: 10.1038/sj.leu.2401592
18. Spinelli O, Rambaldi A, Rigo F, Zanghì P, D'Agostini E, Amicarelli G, et al. Simple, Rapid and Accurate Molecular Diagnosis of Acute Promyelocytic Leukemia by Loop Mediated Amplification Technology. Oncoscience (2015) 2:50–8. doi: 10.18632/oncoscience.114
19. Gurnari C, Breccia M, Di Giuliano F, Scalzulli E, Divona M, Piciocchi A, et al. Early Intracranial Haemorrhages in Acute Promyelocytic Leukaemia: Analysis of Neuroradiological and Clinico-Biological Parameters. Br J Haematol (2021) 193:129–32. doi: 10.1111/bjh.17018
20. de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, et al. Causes and Prognostic Factors of Remission Induction Failure in Patients With Acute Promyelocytic Leukemia Treated With All-Trans Retinoic Acid and Idarubicin. Blood (2008) 111:3395–402. doi: 10.1182/blood-2007-07-100669
21. Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-Trans-Retinoic Acid, Idarubicin, and IV Arsenic Trioxide as Initial Therapy in Acute Promyelocytic Leukemia (APML4). Blood (2012) 120:1570–80. doi: 10.1182/blood-2012-02-410746
22. Zhu H, Hu J, Chen L, Zhou W, Li X, Wang L, et al. The 12-Year Follow-Up of Survival, Chronic Adverse Effects, and Retention of Arsenic in Patients With Acute Promyelocytic Leukemia. Blood (2016) 128:1525–8. doi: 10.1182/blood-2016-02-699439
23. Stahl M, Tallman MS. Differentiation Syndrome in Acute Promyelocytic Leukaemia. Br J Haematol (2019) 187:157–62. doi: 10.1111/bjh.16151
24. Rodeghiero F, Castaman G, Awisati G, Mandelli F, Barbui T. Treatment of Dic Associated With Apl: Response. Blood (1990) 76:2418. doi: 10.1182/blood.V76.11.2418.2418
25. Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, de la Serna J, et al. Differentiation Syndrome in Patients With Acute Promyelocytic Leukemia Treated With All-Trans Retinoic Acid and Anthracycline Chemotherapy: Characteristics, Outcome, and Prognostic Factors. Blood (2009) 113:775–83. doi: 10.1182/blood-2008-07-168617
26. Fabiani E, Cicconi L, Nardozza AM, Cristiano A, Rossi M, Ottone T, et al. Mutational Profile of ZBTB16-RARA-Positive Acute Myeloid Leukemia. Cancer Med (2021) 10:3839–47. doi: 10.1002/cam4.3904
27. Wen L, Xu Y, Yao L, Wang N, Wang Q, Liu T, et al. Clinical and Molecular Features of Acute Promyelocytic Leukemia With Variant Retinoid Acid Receptor Fusions. Haematoliga (2019) 104:e195–9. doi: 10.3324/haematol.2018.205369
28. Sobas M, Talarn-Forcadell MC, Martínez-Cuadrón D, Escoda L, García-Pérez MJ, Mariz J, et al. PLZF-RAR(α), NPM1-RAR(α), and Other Acute Promyelocytic Leukemia Variants: The PETHEMA Registry Experience and Systematic Literature Review. Cancers (Basel) (2020) 12. doi: 10.3390/cancers12051313
29. Wang L, Yan X, He J. Does Acute Promyelocytic Leukemia Patient With the STAT5B/RARa Fusion Gene Respond Well to Decitabine?: A Case Report and Literature Review. Med (Baltimore) (2020) 99:e22923. doi: 10.1097/MD.0000000000022923
30. Arnould C, Philippe C, Bourdon V, Gr goire MJ, Berger R, Jonveaux P. The Signal Transducer and Activator of Transcription STAT5b Gene is a New Partner of Retinoic Acid Receptor Alpha in Acute Promyelocytic-Like Leukaemia. Hum Mol Genet (1999) 8:1741–9. doi: 10.1093/hmg/8.9.1741
31. Zhang C, Wang Y, Liu B, Gong B, Gong X, Liu Y, et al. Clinical Characteristics of Acute Promyelocytic Leukemia With the STAT5B-RARA Fusion Gene. Blood Cells Mol Dis (2018) 69:71–3. doi: 10.1016/j.bcmd.2017.09.007
32. Wang YY, Hao J, Liu ZY, Weng XQ, Sheng Y, Jiang CL, et al. Novel STAT5B-RARA Fusion Transcript in Acute Promyelocytic Leukemia: Identification and Treatment Response. Leukemia Lymphoma (2015) 56:2731–4. doi: 10.3109/10428194.2015.1007454
33. Chen H, Pan J, Yao L, Wu L, Zhu J, Wang W, et al. Acute Promyelocytic Leukemia With a STAT5b-Rarα Fusion Transcript Defined by Array-CGH, FISH, and RT-PCR. Cancer Genet (2012) 205:327–31. doi: 10.1016/j.cancergen.2012.02.007
34. Kluk MJ, Abo RP, Brown RD, Kuo FC, Dal Cin P, Pozdnyakova O, et al. Myeloid Neoplasm Demonstrating a STAT5B-RARA Rearrangement and Genetic Alterations Associated With All-Trans Retinoic Acid Resistance Identified by a Custom Next-Generation Sequencing Assay. Cold Spring Harb Mol Case Stud (2015) 1:a000307. doi: 10.1101/mcs.a000307
35. Wang A, Cai X, Qiang P, Duan Q. Successful Treatment of a Patient With Acute Promyelocytic Leukemia With a STAT5B/RARA Fusion Gene Using Decitabine. Leuk Lymphoma (2018) 59:763–5. doi: 10.1080/10428194.2017.1357176
36. Jonveaux P, Le Coniat M, Derre J, Flexor MA, Daniel MT, Berger R. Chromosome Microdissection in Leukemia: A Powerful Tool for the Analysis of Complex Chromosomal Rearrangements. Genes Chromosomes Cancer (1996) 15:26–33. doi: 10.1002/(SICI)1098-2264(199601)15:1<26::AID-GCC4>3.0.CO;2-6
37. Kusakabe M, Suzukawa K, Nanmoku T, Obara N, Okoshi Y, Mukai HY, et al. Detection of the STAT5B-RARA Fusion Transcript in Acute Promyelocytic Leukemia With the Normal Chromosome 17 on G-Banding. Eur J Haematol (2008) 80:444–7. doi: 10.1111/j.1600-0609.2008.01042.x
38. Iwanaga E, Nakamura M, Nanri T, Kawakita T, Horikawa K, Mitsuya H, et al. Acute Promyelocytic Leukemia Harboring a STAT5B-RARA Fusion Gene and a G596V Missense Mutation in the STAT5B SH2 Domain of the STAT5B-RARA. Eur J Haematol (2009) 83:499–501. doi: 10.1111/j.1600-0609.2009.01324.x
39. Jovanovic JV, Rennie K, Culligan D, Peniket A, Lennard A, Harrison J, et al. Development of Real-Time Quantitative Polymerase Chain Reaction Assays to Track Treatment Response in Retinoid Resistant Acute Promyelocytic Leukemia. Front Oncol (2011) 1:35. doi: 10.3389/fonc.2011.00035
40. Cahill TJ, Chowdhury O, Myerson SG, Ormerod O, Herring N, Grimwade D, et al. Myocardial Infarction With Intracardiac Thrombosis as the Presentation of Acute Promyelocytic Leukemia: Diagnosis and Follow-Up by Cardiac Magnetic Resonance Imaging. Circulation (2011) 123:e370–2. doi: 10.1161/CIRCULATIONAHA.110.986208
41. Qiao C, Zhang SJ, Chen LJ, Miao KR, Zhang JF, Wu YJ, et al. Identification of the STAT5B-Rarα Fusion Transcript in an Acute Promyelocytic Leukemia Patient Without FLT3, NPM1, C-Kit and C/Ebpα Mutation. Eur J Haematol (2011) 86:442–6. doi: 10.1111/j.1600-0609.2011.01595.x
42. Strehl S, König M, Boztug H, Cooper BW, Suzukawa K, Zhang SJ, et al. All-Trans Retinoic Acid and Arsenic Trioxide Resistance of Acute Promyelocytic Leukemia With the Variant STAT5B-RARA Fusion Gene. Leukemia (2013) 27:1606–10. doi: 10.1038/leu.2012.371
43. Kikuma T, Nakamachi Y, Noguchi Y, Okazaki Y, Shimomura D, Yakushijin K, et al. A New Transcriptional Variant and Small Azurophilic Granules in an Acute Promyelocytic Leukemia Case With NPM1/RARA Fusion Gene. Int J Hematol (2015) 102:713–8. doi: 10.1007/s12185-015-1857-2
44. Corey SJ, Locker J, Oliveri DR, Shekhter-Levin S, Redner RL, Penchansky L, et al. A non-Classical Translocation Involving 17q12 (Retinoic Acid Receptor Alpha) in Acute Promyelocytic Leukemia (APML) With Atypical Features. Leukemia (1994) 8:1350–3.
45. Xu L, Zhao WL, Xiong SM, Su XY, Zhao M, Wang C, et al. Molecular Cytogenetic Characterization and Clinical Relevance of Additional, Complex and/or Variant Chromosome Abnormalities in Acute Promyelocytic Leukemia. Leukemia (2001) 15:1359–68. doi: 10.1038/sj.leu.2402205
46. Grimwade D, Biondi A, Mozziconacci MJ, Hagemeijer A, Berger R, Neat M, et al. Characterization of Acute Promyelocytic Leukemia Cases Lacking the Classic T(15;17): Results of the European Working Party. Groupe Français De Cytogénétique Hématologique, Groupe De Français D’hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOM. Blood (2000) 96:1297–308. doi: 10.1182/blood.V96.4.1297
47. Kanegane H, Nomura K, Abe A, Makino T, Ishizawa S, Shimizu T, et al. Spontaneous Regression of Aleukemic Leukemia Cutis Harboring a NPM/RARA Fusion Gene in an Infant With Cutaneous Mastocytosis. Int J Hematol (2009) 89:86–90. doi: 10.1007/s12185-008-0216-y
48. Otsubo K, Horie S, Nomura K, Miyawaki T, Abe A, Kanegane H. Acute Promyelocytic Leukemia Following Aleukemic Leukemia Cutis Harboring NPM/RARA Fusion Gene. Pediatr Blood Cancer (2012) 59:959–60. doi: 10.1002/pbc.24199
49. Nicci C, Ottaviani E, Luatti S, Grafone T, Tonelli M, Motta MR, et al. Molecular and Cytogenetic Characterization of a New Case of T(5;17)(Q35;Q21) Variant Acute Promyelocytic Leukemia. Leukemia (2005) 19:470–2. doi: 10.1038/sj.leu.2403645
50. Hummel JL, Wells RA, Dubé ID, Licht JD, Kamel-Reid S. Deregulation of NPM and PLZF in a Variant T(5;17) Case of Acute Promyelocytic Leukemia. Oncogene (1999) 18:633–41. doi: 10.1038/sj.onc.1202357
51. Okazuka K, Masuko M, Seki Y, Hama H, Honma N, Furukawa T, et al. Successful All-Trans Retinoic Acid Treatment of Acute Promyelocytic Leukemia in a Patient With NPM/RAR Fusion. Int J Hematol (2007) 86:246–9. doi: 10.1007/BF03006928
52. Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The T(5;17) Variant of Acute Promyelocytic Leukemia Expresses a Nucleophosmin-Retinoic Acid Receptor Fusion. Blood (1996) 87:882–6. doi: 10.1182/blood.V87.3.882.bloodjournal873882
53. Liu Y, Xu F, Hu H, Wen J, Su J, Zhou Q, et al. A Rare Case of Acute Promyelocytic Leukemia With IRF2BP2-RARA Fusion; and Literature Review. Oncol Targ Ther (2019) 12:6157–63. doi: 10.2147/OTT.S217622
54. Alotaibi AS, Abdulrazzaq M, Patel KP, Ravandi F, Konoplev S, Bueso-Ramos C, et al. Acute Promyelocytic Leukemia (APL) With an IRF2BP2-RARA Fusion Transcript: An Aggressive APL Variant. Leukemia Lymphoma (2020) 61:3018–20. doi: 10.1080/10428194.2020.1791853
55. Shimomura Y, Mitsui H, Yamashita Y, Kamae T, Kanai A, Matsui H, et al. New Variant of Acute Promyelocytic Leukemia With IRF2BP2-RARA Fusion. Cancer Sci (2016) 107:1165–8. doi: 10.1111/cas.12970
56. Yin CC, Jain N, Mehrotra M, Zhagn J, Protopopov A, Zuo Z, et al. Identification of a Novel Fusion Gene, IRF2BP2-RARA, in Acute Promyelocytic Leukemia. J Natl Compr Canc Netw (2015) 13:19–22. doi: 10.6004/jnccn.2015.0005
57. Jovanovic JV, Chillón MC, Vincent-Fabert C, Dillon R, Voisset E, Gutiérrez NC, et al. The Cryptic IRF2BP2-RARA Fusion Transforms Hematopoietic Stem/Progenitor Cells and Induces Retinoid-Sensitive Acute Promyelocytic Leukemia. Leukemia (2017) 31:747–51. doi: 10.1038/leu.2016.338
58. Mazharuddin S, Chattopadhyay A, Levy MY, Redner RL. IRF2BP2-RARA T(1;17)(Q42.3;Q21.2) APL Blasts Differentiate in Response to All-Trans Retinoic Acid. Leukemia Lymphoma (2018) 59:2246–9. doi: 10.1080/10428194.2017.1421761
59. Chen Y, Li S, Zhou C, Li C, Ru K, Rao Q, et al. TBLR1 Fuses to Retinoid Acid Receptor α in a Variant T(3;17)(Q26;Q21) Translocation of Acute Promyelocytic Leukemia. Blood (2014) 124:936–45. doi: 10.1182/blood-2013-10-528596
60. Redner RL, Contis LC, Craig F, Evans C, Sherer ME, Shekhter-Levin S. A Novel T(3;17)(P25;Q21) Variant Translocation of Acute Promyelocytic Leukemia With Rearrangement of the RARA Locus. Leukemia (2006) 20:376–9. doi: 10.1038/sj.leu.2404062
61. Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The Seventh Pathogenic Fusion Gene FIP1L1-RARA was Isolated From a T(4;17)-Positive Acute Promyelocytic Leukemia. Haematologica (2008) 93:1414–6. doi: 10.3324/haematol.12854
62. Menezes J, Acquadro F, Perez-Pons de la Villa C, García-Sánchez F, Álvarez S, Cigudosa JC. FIP1L1/RARA With Breakpoint at FIP1L1 Intron 13: A Variant Translocation in Acute Promyelocytic Leukemia. Haematologica1 (2011) 96:1565–6. doi: 10.3324/haematol.2011.047134
63. Wang Y, Rui Y, Shen Y, Li J, Liu P, Lu Q, et al. Myeloid Sarcoma Type of Acute Promyelocytic Leukemia With a Cryptic Insertion of RARA Into FIP1L1: The Clinical Utility of NGS and Bioinformatic Analyses. Front Oncol (2021) 11:688203. doi: 10.3389/fonc.2021.688203
64. Nakanishi T, Nakaya A, Nishio Y, Fujita S, Satake A, Azuma Y, et al. A Variant of Acute Promyelocytic Leukemia With T(4;17)(Q12;Q21) Showed Two Different Clinical Symptoms. Hematol Rep (2019) 11:7971. doi: 10.4081/hr.2019.7971
65. Yamamoto Y, Tsuzuki S, Tsuzuki M, Handa K, Inaguma Y, Emi N. BCOR as a Novel Fusion Partner of Retinoic Acid Receptor Alpha in a T(X;17)(p11;q12) Variant of Acute Promyelocytic Leukemia. Blood (2010) 116:4274–83. doi: 10.1182/blood-2010-01-264432
66. Ichikawa S, Ichikawa S, Ishikawa I, Takahashi T, Fujiwara T, Harigae H. Successful Treatment of Acute Promyelocytic Leukemia With a T(X;17)(p11.4;q21) and BCOR-RARA Fusion Gene. Cancer Genet (2015) 208:162–3. doi: 10.1016/j.cancergen.2015.01.008
67. Yao L, Wen L, Wang N, Liu T, Xu Y, Ruan C, et al. Identification of Novel Recurrent STAT3-RARA Fusions in Acute Promyelocytic Leukemia Lacking T(15;17)(Q22;Q12)/PML-RARA. Blood (2018) 131:935–9. doi: 10.1182/blood-2017-09-807370
68. Catalano A, Dawson MA, Somana K, Opat S, Schwarer A, Campbell LJ, et al. The PRKAR1A Gene is Fused to RARA in a New Variant Acute Promyelocytic Leukemia. Blood (2007) 110:4073–6. doi: 10.1182/blood-2007-06-095554
69. King D, Foucar CE, Ma V, Benitez L, Perissinotti AJ, Marini BL, et al. Identification of Variant APL Translocations PRKAR1A-Rarα and ZBTB16-Rarα (PLZF-Rarα) Through the MI-ONCOSEQ Platform. Cancer Genet (2021) 258–259:57–60. doi: 10.1016/j.cancergen.2021.08.002
70. Won D, Shin SY, Park CJ, Jang S, Chi HS, Lee KH, et al. OBFC2A/RARA: A Novel Fusion Gene in Variant Acute Promyelocytic Leukemia. Blood (2013) 121:1432–5. doi: 10.1182/blood-2012-04-423129
71. Li J, Zhong HY, Zhang Y, Xiao L, Bai LH, Liu SF, et al. GTF2I-RARA is a Novel Fusion Transcript in a T(7;17) Variant of Acute Promyelocytic Leukaemia With Clinical Resistance to Retinoic Acid. Br J Haematol (2015) 168:904–8. doi: 10.1111/bjh.13157
72. Cheng CK, Wang AZ, Wong THY, Wan TSK, Cheung JS, Raghupathy R, et al. FNDC3B is Another Novel Partner Fused to RARA in the T(3;17)(Q26;Q21) Variant of Acute Promyelocytic Leukemia. Blood (2017) 129:2705–9. doi: 10.1182/blood-2017-02-767707
73. Zhu HH, Yang MC, Wang F, Lou YJ, Jin J, Li K, et al. Identification of a Novel NUP98-RARA Fusion Transcript as the 14th Variant of Acute Promyelocytic Leukemia. Am J Hematol (2020) 95:E184–6. doi: 10.1002/ajh.25807
74. Wang Z, Wen L, Zhang L, Xu X, Chen X, Yao L, et al. Identification of a Novel TNRC18-RARA Fusion in Acute Promyelocytic Leukemia Lacking T(15;17)(Q24;Q12)/PML-RARA. Mol Carcinog (2021) 60. doi: 10.1002/mc.23276
75. Liu M, Zhao X, Pan W, Qian Z, Du M, Wang LM, et al. A Novel HNRNPC-RARA Fusion in Acute Promyelocytic Leukaemia Lacking PML-RARA Rearrangement, Sensitive to Venetoclax-Based Therapy. Br J Haematol (2021) 195:e123–8. doi: 10.1111/bjh.17642
76. Wang HP, Xu H, Chen ZM, Tong XM, Qian WB, Jin J. T(X;17) as the Sole Karyotypic Anomaly in a Case of M(3r) Subtype of Acute Promyelocytic Leukemia Without RARalpha Rearrangement. Leukemia Res (2010) 34:e55–7. doi: 10.1016/j.leukres.2009.04.034
77. Zhao J, Liang JW, Xue HL, Shen SH, Chen J, Tang YJ, et al. The Genetics and Clinical Characteristics of Children Morphologically Diagnosed as Acute Promyelocytic Leukemia. Leukemia (2019) 33:1387–99. doi: 10.1038/s41375-018-0338-z
78. Shiba N, Yoshida K, Hara Y, Yamato G, Shiraishi Y, Matsuo H, et al. Transcriptome Analysis Offers a Comprehensive Illustration of the Genetic Background of Pediatric Acute Myeloid Leukemia. Blood Adv (2019) 3:3157–69. doi: 10.1182/bloodadvances.2019000404
79. Osumi T, Tsujimoto SI, Tamura M, Uchiyama M, Nakabayashi K, Okamura K, et al. Recurrent RARB Translocations in Acute Promyelocytic Leukemia Lacking RARA Translocation. Cancer Res (2018) 78:4452–8. doi: 10.1158/0008-5472.CAN-18-0840
80. Zhang Z, Jiang M, Borthakur G, Luan S, Huang X, Tang G, et al. Acute Myeloid Leukemia With a Novel CPSF6-RARG Variant is Sensitive to Homoharringtonine and Cytarabine Chemotherapy. Am J Hematol (2020) 95:E48–51. doi: 10.1002/ajh.25689
81. Liu T, Wen L, Yuan H, Wang Y, Yao L, Xu Y, et al. Identification of Novel Recurrent CPSF6-RARG Fusions in Acute Myeloid Leukemia Resembling Acute Promyelocytic Leukemia. Blood (2018) 131:1870–3. doi: 10.1182/blood-2017-11-818716
82. Qin Y-Z, Huang X-J, Zhu H-H. Identification of a Novel CPSF6-RARG Fusion Transcript in Acute Myeloid Leukemia Resembling Acute Promyelocytic Leukemia. Leukemia (2018) 32:2285–7. doi: 10.1038/s41375-018-0095-z
83. Miller CA, Tricarico C, Skidmore ZL, Uy GL, Lee YS, Hassan A, et al. A Case of Acute Myeloid Leukemia With Promyelocytic Features Characterized by Expression of a Novel RARG-CPSF6 Fusion. Blood Adv (2018) 2:1295–9. doi: 10.1182/bloodadvances.2017014183
84. Such E, Cervera J, Valencia A, Barragán E, Ibañez M, Luna I, et al. A Novel NUP98/RARG Gene Fusion in Acute Myeloid Leukemia Resembling Acute Promyelocytic Leukemia. Blood (2011) 117:242–5. doi: 10.1182/blood-2010-06-291658
85. Such E, Cordón L, Sempere A, Villamón E, Ibañez M, Luna I, et al. In Vitro All-Trans Retinoic Acid Sensitivity of Acute Myeloid Leukemia Blasts With NUP98/RARG Fusion Gene. Ann Hematol (2014) 93:1931–3. doi: 10.1007/s00277-014-2073-5
86. Tao S, Song L, Deng Y, Chen Y, Shi Y, Gan Y, et al. Acute Myeloid Leukemia With NUP98-RARG Gene Fusion Similar to Acute Promyelocytic Leukemia: Case Report and Literature Review. OncoTarg Ther (2020) 13:10559–66. doi: 10.2147/OTT.S273172
87. Zhang X, Li F, Wang J, Suo S, Ling Q, Yu W, et al. Rarγ-Rearrangements Resemble Acute Promyelocytic Leukemia and Benefit From 3 + 7 Regimen. Leukemia Lymphoma (2019) 60:1831–4. doi: 10.1080/10428194.2018.1553302
88. Luo H, Zhang S, Li K, Chen XH, Li YC, Sun Y, et al. A Novel Entity of Acute Myeloid Leukaemia With Recurrent RARG-Rearrangement Resembling Acute Promyelocytic Leukaemia. Leukemia Res (2019) 77:14–6. doi: 10.1016/j.leukres.2018.12.009
89. Ha JS, Do YR, Ki CS, Lee C, Kim DH, Lee W, et al. Identification of a Novel PML-RARG Fusion in Acute Promyelocytic Leukemia. Leukemia (2017) 31:1992–5. doi: 10.1038/leu.2017.167
90. Chen X, Wang F, Zhang Y, Teng W, Cao P, Ma X, et al. A Novel NPM1-RARG-NPM1 Chimeric Fusion in Acute Myeloid Leukaemia Resembling Acute Promyelocytic Leukaemia But Resistant to All-Trans Retinoic Acid and Arsenic Trioxide. Br J Cancer (2019) 120:1023–5. doi: 10.1038/s41416-019-0456-z
91. Su Z, Liu X, Xu Y, Hu W, Zhao C, Zhao H, et al. Novel Reciprocal Fusion Genes Involving HNRNPC and RARG in Acute Promyelocytic Leukemia Lacking RARA Rearrangement. Haematol (2020) 105:e376–8. doi: 10.3324/haematol.2019.244715
92. Zhang X, Huang X, Xu H, Li J, Yu W. MLL-Rearrangement can Resemble Acute Promyelocytic Leukemia. Leukemia Lymphoma (2019) 60:2841–3. doi: 10.1080/10428194.2019.1607328
93. Martelli MP, Rossi R, Venanzi A, Meggendorfer M, Perriello VM, Martino G, et al. Novel NPM1 Exon 5 Mutations and Gene Fusions Leading to Aberrant Cytoplasmic Nucleophosmin in AML. Blood (2021) 138:2696–701. doi: 10.1182/blood.2021012732
94. Chong ML, Cheng H, Xu P, You H, Wang M, Wang L, et al. TFG-RARA: A Novel Fusion Gene in Acute Promyelocytic Leukemia That is Responsive to All-Trans Retinoic Acid. Leukemia Res (2018) 74:51–4. doi: 10.1016/j.leukres.2018.09.012
95. Saiki Y, Saiki H, Uchida A, Uemura Y, Matsunawa M, Isobe Y, et al. [Leukemic Cell Kinetics of APL With a Novel Complex Variant T (12;17;15)(P13;Q21;Q22)]. Rinsho Ketsueki (2020) 61:103–9. doi: 10.11406/rinketsu.61.103
96. Bennour A, Tabka I, Youssef YB, Zaier M, Hizem S, Khelif A, et al. A PML/RARA Chimeric Gene on Chromosome 12 in a Patient With Acute Promyelocytic Leukemia (M4) Associated With a New Variant Translocation: T(12;15;17)(Q24;Q24;Q11). Med Oncol (2013) 30:409. doi: 10.1007/s12032-012-0409-3
97. Lv L, Yang L, Cui H, Ma T. A Complex Translocation (1;17;15) With Spliced Short-Type PML-RARA Fusion Transcripts in Acute Promyelocytic Leukemia: A Case Report. Exp Ther Med (2019) 17:1360–6. doi: 10.3892/etm.2018.7091
98. Zhang YL, Jiang M, Luan SQ, Liu SY, Wan JH, Wan LG, et al. The Novel Three-Way Variant T(6;17;15)(P21;Q21;Q22) in Acute Promyelocytic Leukemia With an FLT3-ITD Mutation: A Case Report. Oncol Lett (2018) 16:6121–5. doi: 10.3892/ol.2018.9413
99. Wang Y, Ma J, Liu X, Liu R, Xu L, Wang L, et al. A Complex Translocation (3;17;15) in Acute Promyelocytic Leukemia Confirmed by Fluorescence in Situ Hybridization. Oncol Lett (2016) 12:4717–9. doi: 10.3892/ol.2016.5280
100. Yamanouchi J, Hato T, Niiya T, Miyoshi K, Azuma T, Sakai I, et al. A New Four-Way Variant T(5;17;15;20)(Q33;Q12;Q22;Q11.2) in Acute Promyelocytic Leukemia. Int J Hematol (2011) 94:395–8. doi: 10.1007/s12185-011-0929-1
101. Iaccarino L, Divona M, Ottone T, Cicconi L, Lavorgna S, Ciardi C, et al. Identification and Monitoring of Atypical PML/RARA Fusion Transcripts in Acute Promyelocytic Leukemia. Genes Chromosomes Cancer (2019) 58:60–5. doi: 10.1002/gcc.22708
102. Cicconi L, Testi AM, Montesinos P, Rego E, Zhu HH, Takahashi H, et al. Characteristics and Outcome of Acute Myeloid Leukemia With Uncommon Retinoic Acid Receptor-Alpha (RARA) Fusion Variants. Blood Cancer J (2021) 11:167. doi: 10.1038/s41408-021-00561-w
103. Adams J, Nassiri M. Acute Promyelocytic Leukemia: A Review and Discussion of Variant Translocations. Arch Pathol Lab Med (2015) 139:1308–13. doi: 10.5858/arpa.2013-0345-RS
104. Vickers M, Jackson G, Taylor P. The Incidence of Acute Promyelocytic Leukemia Appears Constant Over Most of a Human Lifespan, Implying Only One Rate Limiting Mutation. Leukemia (2000) 14:722–6. doi: 10.1038/sj.leu.2401722
105. de Thé H, Pandolfi PP, Chen Z. Acute Promyelocytic Leukemia: A Paradigm for Oncoprotein-Targeted Cure. Cancer Cell (2017) 32:552–60. doi: 10.1016/j.ccell.2017.10.002
106. Lallemand-Breitenbach V, de Thé H. PML Nuclear Bodies. Cold Spring Harb Perspect Biol (2010) 2:a000661. doi: 10.1101/cshperspect.a000661
107. Dollé P. Developmental Expression of Retinoic Acid Receptors (RARs). Nucl Recept Signal (2009) 7:e006. doi: 10.1621/nrs.07006
108. Doulatov S, Notta F, Rice KL, Howell L, Zelent A, Licht JD, et al. PLZF is a Regulator of Homeostatic and Cytokine-Induced Myeloid Development. Genes Dev (2009) 23:2076–87. doi: 10.1101/gad.1788109
109. Madan V, Shyamsunder P, Han L, Mayakonda A, Nagata Y, Sundaresan J, et al. Comprehensive Mutational Analysis of Primary and Relapse Acute Promyelocytic Leukemia. Leukemia (2016) 30:2430. doi: 10.1038/leu.2016.237
110. Iaccarino L, Ottone T, Alfonso V, Cicconi L, Divona M, Lavorgna S, et al. Mutational Landscape of Patients With Acute Promyelocytic Leukemia at Diagnosis and Relapse. Am J Hematol (2019) 94:1091–7. doi: 10.1002/ajh.25573
111. Li J, Wang W, Zhang Y, Cieślik M, Guo J, Tan M, et al. Epigenetic Driver Mutations in ARID1A Shape Cancer Immune Phenotype and Immunotherapy. J Clin Invest (2020) 130:2712–26. doi: 10.1172/JCI134402
112. Rego EM, He LZ, Warrell RPJ, Wang ZG, Pandolfi PP. Retinoic Acid (RA) and As2O3 Treatment in Transgenic Models of Acute Promyelocytic Leukemia (APL) Unravel the Distinct Nature of the Leukemogenic Process Induced by the PML-RARalpha and PLZF-RARalpha Oncoproteins. Proc Natl Acad Sci USA (2000) 97:10173–8. doi: 10.1073/pnas.180290497
113. Koken MH, Daniel MT, Gianni M, Zelent A, Licht J, Buzyn A, et al. Retinoic Acid, But Not Arsenic Trioxide, Degrades the PLZF/RARalpha Fusion Protein, Without Inducing Terminal Differentiation or Apoptosis, in a RA-Therapy Resistant T(11;17)(Q23;Q21) APL Patient. Oncogene (1999) 18:1113–8. doi: 10.1038/sj.onc.1202414
114. Bykov VJ, Zhang Q, Zhang M, Ceder S, Abrahmsen L, Wiman KG. Targeting of Mutant P53 and the Cellular Redox Balance by APR-246 as a Strategy for Efficient Cancer Therapy. Front Oncol (2016) 6:21. doi: 10.3389/fonc.2016.00021
115. Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39:1584–94. doi: 10.1200/JCO.20.02341
116. Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell (2019) 35:177–190.e8. doi: 10.1016/j.ccell.2018.12.009
117. Maurer B, Kollmann S, Pickem J, Hoelbl-Kovacic A, Sexl V. STAT5A and STAT5B-Twins With Different Personalities in Hematopoiesis and Leukemia. Cancers (Basel) (2019) 11. doi: 10.3390/cancers11111726
118. Wingelhofer B, Neubauer HA, Valent P, Han X, Constantinescu SN, Gunning PT, et al. Implications of STAT3 and STAT5 Signaling on Gene Regulation and Chromatin Remodeling in Hematopoietic Cancer. Leukemia (2018) 32:1713–26. doi: 10.1038/s41375-018-0117-x
119. Kunchala P, Kuravi S, Jensen R, McGuirk J, Balusu R. When the Good Go Bad: Mutant NPM1 in Acute Myeloid Leukemia. Blood Rev (2018) 32:167–83. doi: 10.1016/j.blre.2017.11.001
120. Heath EM, Chan SM, Minden MD, Murphy T, Shlush LI, Schimmer AD. Biological and Clinical Consequences of NPM1 Mutations in AML. Leukemia (2017) 31:798–807. doi: 10.1038/leu.2017.30
121. Ramalho-Oliveira R, Oliveira-Vieira B, Viola JPB. IRF2BP2: A New Player in the Regulation of Cell Homeostasis. J Leukoc Biol (2019) 106:717–23. doi: 10.1002/JLB.MR1218-507R
122. Li J, Wang C-Y. TBL1-TBLR1 and Beta-Catenin Recruit Each Other to Wnt Target-Gene Promoter for Transcription Activation and Oncogenesis. Nat Cell Biol (2008) 10:160–9. doi: 10.1038/ncb1684
123. Gilliland G, Cools J, Stover EH, Wlodarska I, Marynen P. FIP1L1-PDGFRalpha in Hypereosinophilic Syndrome and Mastocytosis. Hematol J Off J Eur Haematol Assoc (2004) 5 Suppl 3:S133–7. doi: 10.1038/sj.thj.6200439
124. Astolfi A, Fiore M, Melchionda F, Indio V, Bertuccio SN, Pession A. BCOR Involvement in Cancer. Epigenomics (2019) 11:835–55. doi: 10.2217/epi-2018-0195
125. Bossis I, Stratakis CA. Minireview: PRKAR1A: Normal and Abnormal Functions. Endocrinology (2004) 145:5452–8. doi: 10.1210/en.2004-0900
126. Binothman N, Hachim IY, Lebrun J-J, Ali S. CPSF6 is a Clinically Relevant Breast Cancer Vulnerability Target: Role of CPSF6 in Breast Cancer. EBioMedicine (2017) 21:65–78. doi: 10.1016/j.ebiom.2017.06.023
127. Liu H. NUP98 Rearrangement in B Lymphoblastic Leukemia With Hyperdiploidy. Blood (2020) 136:1011. doi: 10.1182/blood.2020006652
128. Winters AC, Bernt KM. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front Pediatr (2017) 5:4. doi: 10.3389/fped.2017.00004
129. Johnstone RW, Gerber M, Landewe T, Tollefson A, Wold WS, Shilatifard A. Functional Analysis of the Leukemia Protein ELL: Evidence for a Role in the Regulation of Cell Growth and Survival. Mol Cell Biol (2001) 21:1672–81. doi: 10.1128/MCB.21.5.1672-1681.2001
130. Tse W, Meshinchi S, Alonzo TA, Stirewalt DL, Gerbing RB, Woods WG, et al. Elevated Expression of the AF1q Gene, an MLL Fusion Partner, is an Independent Adverse Prognostic Factor in Pediatric Acute Myeloid Leukemia. Blood (2004) 104:3058–63. doi: 10.1182/blood-2003-12-4347
131. Petit A, Ragu C, Soler G, Ottolenghi C, Schluth C, Radford-Weiss I, et al. Functional Analysis of the NUP98-CCDC28A Fusion Protein. Haematologica (2012) 97:379–87. doi: 10.3324/haematol.2011.047969
132. Feldman DE, Chen C, Punj V, Machida K. The TBC1D15 Oncoprotein Controls Stem Cell Self-Renewal Through Destabilization of the Numb-P53 Complex. PloS One (2013) 8:e57312. doi: 10.1371/journal.pone.0057312
133. Powers B, Persons D, Rao D, Woodroof J, Lin TL. High-Risk Microgranular Acute Promyelocytic Leukemia With a Five-Way Complex Translocation Involving PML-RARA. Case Rep Hematol (2015) 2015:343854. doi: 10.1155/2015/343854
134. Mason EF, Kuo FC, Hasserjian RP, Seegmiller AC, Pozdnyakova O. A Distinct Immunophenotype Identifies a Subset of NPM1-Mutated AML With TET2 or IDH1/2 Mutations and Improved Outcome. Am J Hematol (2018) 93:504–10. doi: 10.1002/ajh.25018
135. Martelli MP, Gionfriddo I, Mezzasoma F, Milano F, Pierangeli S, Mulas F, et al. Arsenic Trioxide and All-Trans Retinoic Acid Target NPM1 Mutant Oncoprotein Levels and Induce Apoptosis in NPM1-Mutated AML Cells. Blood (2015) 125:3455–65. doi: 10.1182/blood-2014-11-611459
136. Wu HC, Rérolle D, Berthier C, Hleihel R, Sakamoto T, Quentin S, et al. Actinomycin D Targets NPM1c-Primed Mitochondria to Restore PML-Driven Senescence in AML Therapy. Cancer Discovery (2021) 11(12):3198–213. doi: 10.1158/2159-8290.CD-21-0177
137. Arana Rosainz MJ, Nguyen N, Wahed A, Lelenwa LC, Aakash N, Schaefer K, et al. Acute Myeloid Leukemia With Mutated NPM1 Mimics Acute Promyelocytic Leukemia Presentation. Int J Lab Hematol (2021) 43:218–26. doi: 10.1111/ijlh.13357
138. El Hajj H, Dassouki Z, Berthier C, Raffoux E, Ades L, Legrand O, et al. Retinoic Acid and Arsenic Trioxide Trigger Degradation of Mutated NPM1, Resulting in Apoptosis of AML Cells. Blood (2015) 125:3447–54. doi: 10.1182/blood-2014-11-612416
Keywords: variant acute promyelocytic leukemia, APL-like acute myeloid leukemia, atypical rearrangements, complex rearrangements, genetic landscape
Citation: Guarnera L, Ottone T, Fabiani E, Divona M, Savi A, Travaglini S, Falconi G, Panetta P, Rapanotti M C and Voso MT (2022) Atypical Rearrangements in APL-Like Acute Myeloid Leukemias: Molecular Characterization and Prognosis. Front. Oncol. 12:871590. doi: 10.3389/fonc.2022.871590
Received: 08 February 2022; Accepted: 25 February 2022;
Published: 12 April 2022.
Edited by:
Harinder Gill, University of Hong Kong, ChinaReviewed by:
Maria Paola Martelli, University of Perugia, ItalyCosimo Cumbo, University of Bari Aldo Moro, Italy
Copyright © 2022 Guarnera, Ottone, Fabiani, Divona, Savi, Travaglini, Falconi, Panetta, Rapanotti and Voso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiziana Ottone, dGl6aWFuYS5vdHRvbmVAdW5pcm9tYTIuaXQ=
†These authors have contributed equally to this work
 Luca Guarnera
Luca Guarnera Tiziana Ottone
Tiziana Ottone Emiliano Fabiani
Emiliano Fabiani Mariadomenica Divona1
Mariadomenica Divona1 Giulia Falconi
Giulia Falconi Maria Cristina Rapanotti
Maria Cristina Rapanotti Maria Teresa Voso
Maria Teresa Voso