
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 04 April 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.868654
This article is part of the Research TopicCase Reports in Molecular and Cellular Oncology : 2022View all 10 articles
Gastric cancer is one of the most common malignant tumors and patients show a short survival, those combined with bone marrow invasion have a median survival of only 37 days. Here we reported the treatment of a 47-year-old male with advanced gastric cancer and complicated with bone marrow invasion and extensive metastases, who did not tolerate chemotherapy, under monotherapy with savolitinib, a MET receptor tyrosine kinase inhibitor. Before treatment, the patient was in severe pain and presented with thrombocytopenia and hemorrhagic anemia. Savolitinib was given based on amplification and rearrangement of the MET gene in his tumor. After savolitinib treatment, the patient’s condition promptly improved, efficacy evaluation indicated partial remission, and the patient was alive and remained progression-free at 15 weeks at the time of reporting. No obvious adverse reactions occurred. Besides, another case of a female gastric cancer patient with MET amplification who received savolitinib monotherapy as a third-line treatment that remained progression-free at 12 weeks was also reported. This report provides a new reference for understanding MET abnormalities in gastric cancer and offers a possibility for future application of MET tyrosine kinase inhibitors in the therapy of gastric cancer with MET abnormalities. Also, it suggests that sequencing of MET can be considered a routine target in advanced gastric cancer patients.
Gastric cancer (GC) is the fifth most common cancer and ranks third in cancer-related deaths (1). The median overall survival (OS) of patients with advanced GC is less than one year with combination chemotherapy (2) but if bone marrow invasion is present, the median OS is shortened to 37 days (3).
The MET gene-encoded protein c-mesenchymal-epithelial transition factor (MET, also known as hepatocyte growth factor (HGF) receptor) is a tyrosine kinase receptor that modulates cell proliferation, growth, survival, apoptosis, and epithelial-mesenchymal transition. Abnormal activation of MET, which promotes tumor cell proliferation and metastasis, involves MET exon 14 skipping mutation, MET amplification, and MET protein overexpression (4). Multiple studies have shown a positive relation between MET amplification and overexpression, and the median OS is shortened in GC patients with these abnormalities, which are considered poor prognostic factors (5–8). Savolitinib is a selective MET tyrosine kinase inhibitor (TKI) being developed for the treatment of multiple cancers. In June 2021, savolitinib was approved in China to treat progressive or intolerant local advanced or metastatic non-small cell lung cancer (NSCLC) with MET exon 14 skipping mutation after platinum-containing chemotherapy (9).
In this study, we reported an advanced GC patient with bone marrow invasion and extensive metastasis. Next-generation sequencing revealed that his tumor had MET amplification and rearrangement. After savolitinib treatment, his clinical symptoms promptly improved, no obvious adverse reactions occurred, and he was alive and remained progression-free at 15 weeks at the time of reporting. Meanwhile, another case of a female GC patient with MET amplification who received savolitinib monotherapy as a third-line treatment that remained progression-free at 12 weeks was also reported here. Our report demonstrated the effectiveness of savolitinib in the therapy of advanced GC with MET abnormalities.
A 47-year-old male was admitted for “low back pain and weight loss for 20 days”. He had a six-year history of type 2 diabetes mellitus and no other significant past or family medical histories. He was unable to walk at admission, with a Karnofsky performance status (KPS) score of 60 points and a pain number rating scale (NRS) score (0 to 10 points) of 8 points. Physical examination showed systemic mucosal pallor, gingival bleeding, a few ecchymoses on the right lower abdomen skin where insulin was injected, and lumbosacral vertebral tenderness. Blood test indicated severe low platelets (14 × 109/L), hemoglobin (66 g/L), and normal leukocytes (6.14 × 109/L). Tumor markers evaluation showed elevated CEA (103.30 µg/L), CA125 (36.00 U/mL), and CA19-9 (1321.73 U/mL). Enhanced computed tomography (CT) evidenced that the gastric fundus and body were thickened (Figure 1A), and multiple lymph node metastases were found in perigastric, liver hilar, retroperitoneal, mediastinal, and bilateral lung hilar regions (Figure 1B). Extensive mixed bone metastases throughout the body (supraorbital margin of right frontal bone, bilateral clavicles, bilateral scapulae, multiple ribs, thoracic, lumbar and sacral vertebrae, pelvic bones, right humerus, and bilateral upper femurs) were observed (Figure 1B). Pathological biopsy with gastroscopy (Figure 1C) indicated poorly differentiated adenocarcinoma (Figure 1D), and immunohistochemistry (IHC) of cancer cells showed HER2 negative, PD-L1 combined positive score (CPS) = 3 (Dako 22C3 antibody). Fluorescence in-situ hybridization showed negative Epstein Barr Virus ambiguous. A bone marrow smear (of the left posterior superior iliac spine) showed a large number of metastatic cancer cells distributed in pile, absence of megakaryocytes, and few platelets (Figure 1E), and a bone marrow biopsy showed cancer cells striped or scattered between bone trabeculae (Figure 1F). A tumor next-generation sequencing (by Geneseeq Technology Inc.) showed MET amplification (copies in biopsy and plasma: 19.7 and 18.1 respectively), and MET-suppression of tumorigenicity 7 (ST7) rearrangement (MET: exon 14 - ST7: exon 2, in 0.8% biopsy and 5.1% plasma respectively; Figures 2A, B and Supplementary Figure 1), microsatellite stable, tumor mutation burden 6.3 mutations/Mb. Clinical diagnosis of this patient indicated stage IVB GC (complicated with bone marrow invasion, extensive bone and lymph node metastases, with MET amplification and rearrangement at cT4aN3M1).
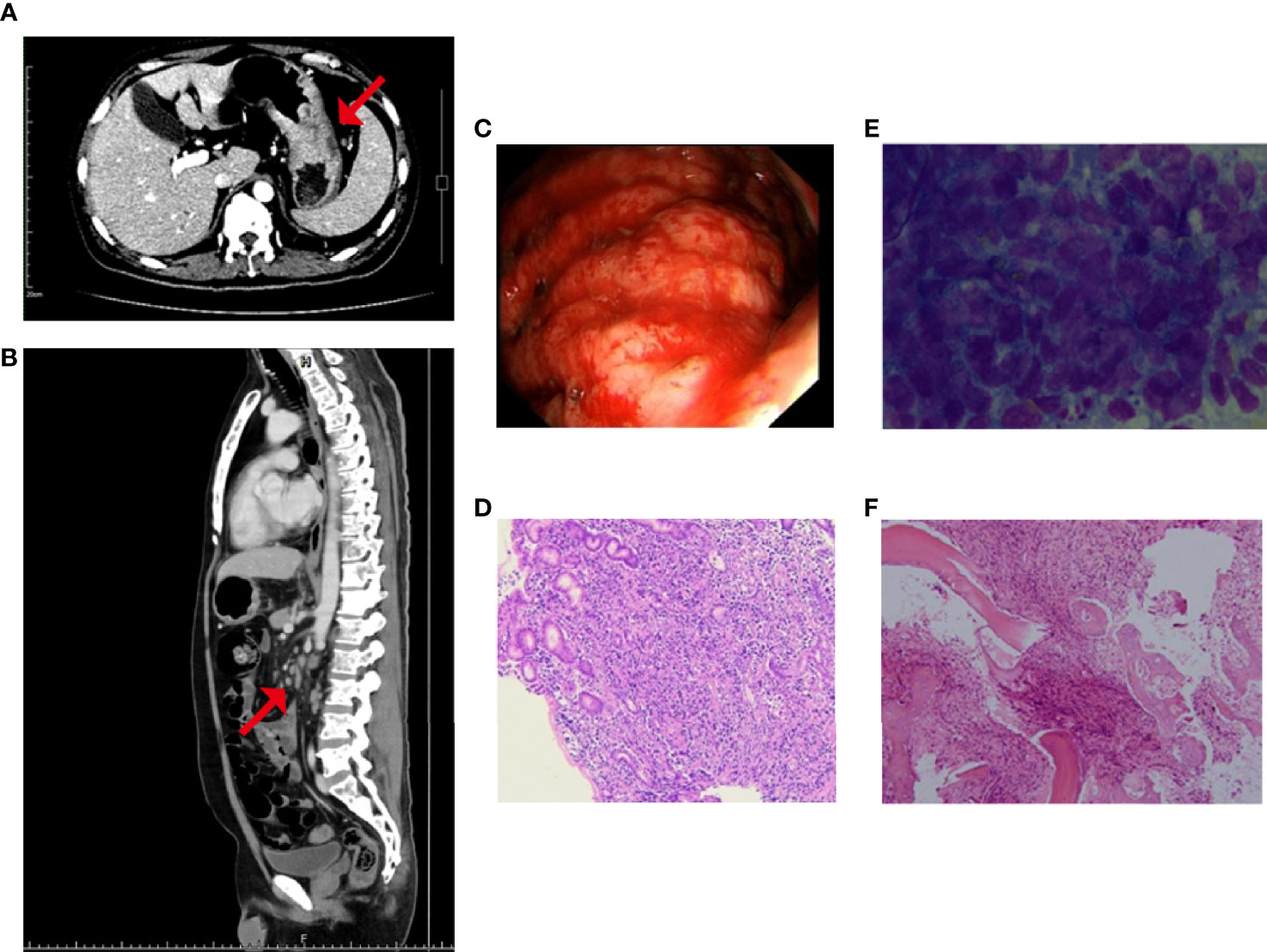
Figure 1 Before treatment, enhanced CT showed (A) thickening of gastric wall and (B) multiple enlarged abdominal lymph nodes, as well as mixed bone metastasis of the vertebras; the red arrow represents the location of the lesion; (C) Gastroscopy showed the lesion was found in the greater curvature of upper gastric body, about 5 cm × 8 cm in size, with uneven surface and bloody substances; and Hematoxylin-eosin staining of (D) the gastric biopsy, (E) the bone marrow smear, and (F) the bone marrow biopsy.
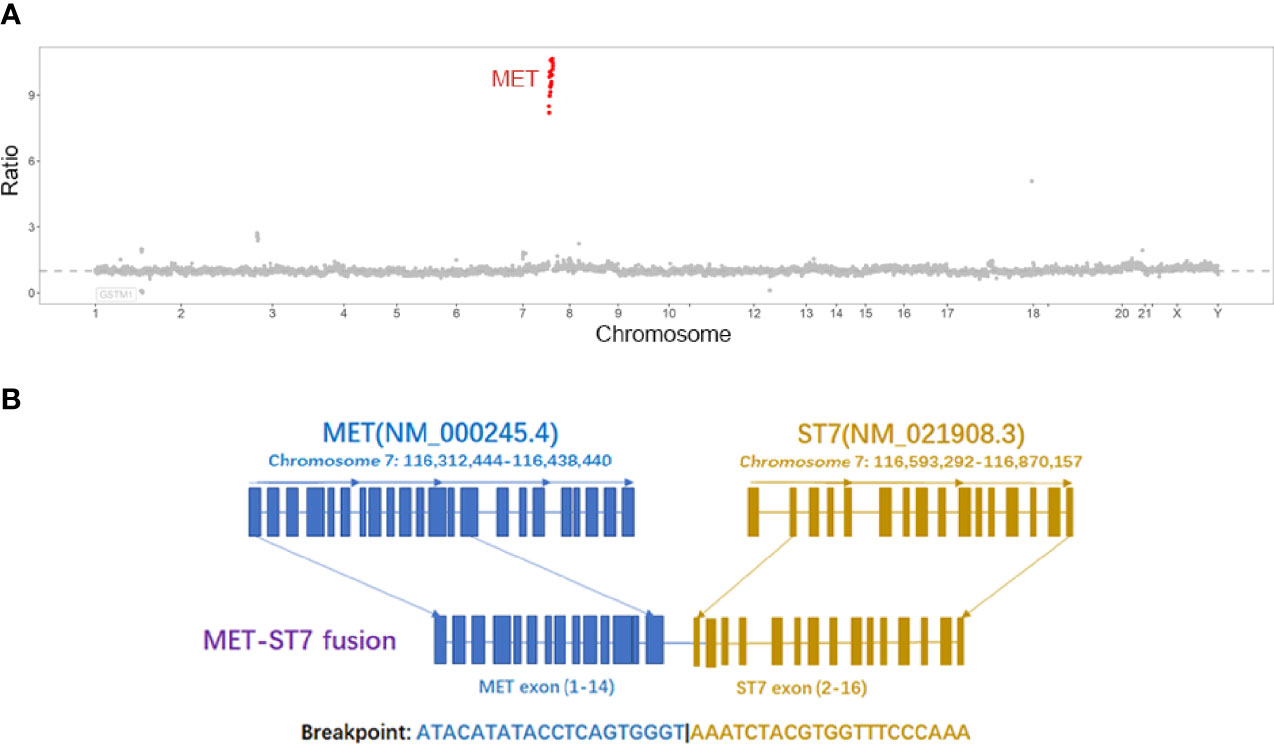
Figure 2 (A) The copy number ratio of MET to centromere of chromosome 7 in tissues, each red point represents an exon of MET; (B) MET-ST7 fusion diagram.
After admission, our patient received repeated transfusions of erythrocyte, platelets, and drugs for promoting platelet production (recombinant human thrombopoietin, interleukin-11, and avatrombopag) plus supportive treatments. His hemoglobin and platelets slightly and transiently increased after blood transfusion but rapidly decreased. Eleven days after admission, the patient received one dose of 240 mg nivolumab. However, the transfusion effect declined after repeated platelet transfusion probably due to the positive anti-platelet antibodies. To block the effect of platelet antibodies, intravenous immunoglobulin (IVIG, 0.5 g/kg) was infused several times before subsequent platelet transfusion. Sixteen days after admission, induction chemotherapy with low-dose tegafur-gimeracil-oteracil potassium (i.e., S-1, 40 mg, twice daily) was started when no bleeding symptoms were shown. However, three days later (nineteen days after admission), dark stools and nasal mucosal bleeding appeared, and platelet count dropped to 12 × 109/L, thus S-1 was stopped. Twenty-one days after admission, the patient further developed fever, pulmonary infection, and acute left heart failure, which represented an extremely critical condition. Considering his intolerance of traditional chemotherapy as well as the significant abnormalities of the amplification and rearrangement of MET in his tumor, savolitinib was given to the patient (400 mg/d × 4 d, followed by 600 mg/d orally to date) along with antibiotics and supportive treatments. After savolitinib treatment for four days, platelets recovered to 27 × 109/L. Bone pain symptoms were significantly relieved and the dosage of analgesics was reduced. Eighteen days after savolitinib treatment, platelets recovered to 81 × 109/L, the thrombopoietic agents were stopped, then the patient was discharged. Fifty-five days after savolitinib treatment, the patient returned for a follow-up session. He was able to walk, had a KPS score of 80 points, and a pain NRS score of one point. Physical examination showed no signs of skin petechiae, ecchymoses, and lumbosacral vertebral tenderness. Blood test indicated normal platelets (188 × 109/L), hemoglobin (111 g/L), and leukocytes (Figures 3A, B). Tumor markers significantly decreased (CEA 2.71 µg/L, CA125 17.2 U/mL, and CA19-9 35.04 U/mL). CT re-examination showed that gastric fundus and body wall were thinner (Figure 3C), and partial lymph node metastases were shrunk (Figure 3D). Bone marrow smear re-examination showed the absence of metastatic cancer cells (Figure 3E), and bone marrow biopsies showed interstitial fibrous hyperplasia with foam cell infiltration, suggesting post-treatment changes (Figure 3F). No obvious adverse reaction was shown. The patient achieved partial remission (PR) based on Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1. At the time of reporting, the patient was alive and remained progression-free at 15 weeks.
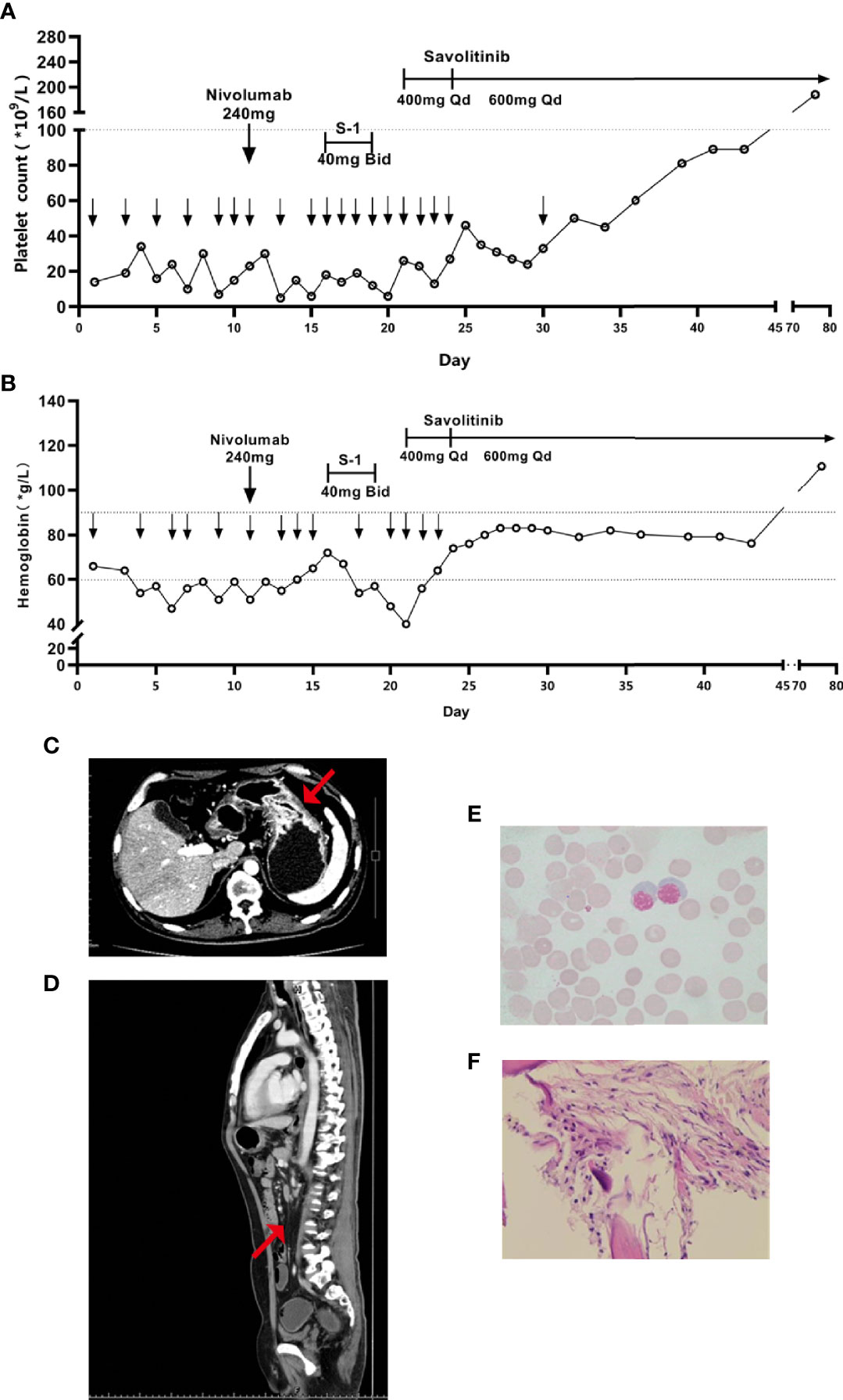
Figure 3 Variation trends of (A) platelet and (B) hemoglobin since admission, the lower downward arrow represents the date of platelet and hemoglobin transfusion, respectively. After savolitinib treatment, enhanced CT showed that (C) the thickness of gastric wall was thinner and (D) the abdominal lymph nodes were shrunk and fewer, the red arrow represents the location of the lesion; and post-treatment Hematoxylin-eosin staining of (E) the bone marrow smear and (F) the bone marrow biopsy.
During the preparation of this manuscript, in our department, there was another 39-year-old female patient with advanced GC was given savolitinib. At initial admission, her pathological biopsy with gastroscopy indicated poorly differentiated adenocarcinoma, and IHC of cancer cells showed HER2 and PD-L1 CPS negative. A tumor next-generation sequencing showed MET amplification (9.1 copies in biopsy), microsatellite stable, and tumor mutation burden 3.95 mutations/Mb. She was diagnosed as cT4aN3M1 stage IVB GC (complicated with bilateral ovarian metastases (Krukenburg tumors), extensive lymph node metastases in perigastric, retroperitoneal, and mesenteric regions, massive abdominal-pelvic ascites, with MET amplification). After an 8-month first-line therapy of nivolumab plus S-1 and oxaliplatin (5-month for oxaliplatin), and a 4-month second-line chemotherapy of paclitaxel-albumin, she had a KPS score of 70 points and a pain NRS score of 4 points (abdominal pain), her primary tumor lesion and metastases markedly progressed both in size and quantity, with multiple new metastases in bones. She could not tolerate continued chemotherapy. Considering that MET amplification was detected in her tumor (9.1 copies), she received savolitinib monotherapy as a third-line treatment. By the eighth week, she had a KPS score of 90 points, abdominal pain relieved (NRS score of 0 points), and gained 10 kg in body weight. CA19-9 dropped from > 12000 U/ml pretreatment to 2704.83 U/ml, CEA from 26.27 ug/L to 6.73 ug/L. CT re-examination showed PR (Supplementary Figure 2), no obvious adverse reaction was shown. At the time of reporting, she was alive and remained progression-free at 12 weeks.
At present, systemic chemotherapy (including platinum and/or fluorouracil, paclitaxel, irinotecan, etc), immunotherapy, and targeted therapy are mainly used to treat advanced GC (10). This provided theoretical evidence for nivolumab and S-1 induction chemotherapy, which were initially selected for the male patient reported in this study. However, nivolumab efficacy was probably not achieved because of repeated infusions of IVIG.
IVIG, which contains large amounts of gamma immunoglobulin (IgG) antibodies, is often needed for the therapy of autoimmune diseases based on its contribution to anti-inflammatory and immunomodulatory activities (11). In clinical practices regarding immunotherapy-related adverse events, some patients require immunosuppressive agents including IVIG besides steroids to reduce inflammatory reactions (12–16). One of its regulation mechanisms is that IVIG can compete with pathological autoantibodies for binding the neonatal Fc receptor (FcRn). The FcRn binds serum IgG that has been endocytosed by myeloid cells or endothelial cells, and recycles the IgG to the cell surface, avoiding IgG catabolism by the lysosome (11, 17, 18). In the absence of FcRn, the half-life of IgG is reduced (19, 20). In this reported case, the transfusion effect declined after repeated platelet transfusion, which was an unfavorable condition for the patient, and we detected positive anti-platelet antibodies in the serum. We used repeated IVIG to accelerate clearance of anti-platelet antibodies. However, IVIG also could compete with nivolumab, a kind of monoclonal IgG antibody that blocks PD-1 receptors of T cells to unleash anti-tumor effect, for binding FcRn, probably resulting in the accelerated clearance of nivolumab with a shortened half-life. It is unclear at present to what extent the use of IVIG contributes to the reduction of anti-tumor efficacy of PD-1 antibodies.
Pharmacokinetics of a single dose of S-1 showed that the mean half-life of its active components are between 1.9-13.1 hours (21). Our patient received 40 mg of S-1 twice daily, which was lower than the regular dose of 60 mg according to his body surface area. Moreover, the patient suffered from thrombocytopenia, acute gastrointestinal bleeding, pulmonary infection, and heart failure. He could not tolerate continued chemotherapy after S-1 treatment for only three days.
When no standard treatments were available, the significant abnormal MET in the tumor of this patient attracted our attention. MET is located on 7q21-31. Since the 1980s, MET exon 14 skipping mutation, MET amplification, and MET protein overexpression have been associated with tumor cell proliferation and metastasis (4). In recent years, great progress has been made in research on small molecule selective MET-tyrosine kinase inhibitors (TKI), represented by savolitinib, capmatinib, and tepotinib (9, 22, 23).
Savolitinib, which showed good antitumor activities in the human glioma xenograft model, was firstly reported in 2014 as one of the inventions of a series of novel MET inhibitors (24). Being a type I small-molecule MET-TKI, savolitinib efficiently binds to the active protein kinase conformation (Asp-Phe-Gly (DFG)-in) of MET, leading to the inhibition of MET phosphorylation and downstream signaling (25). Gavine et al. reported that in a panel of GC patient-derived tumor xenograft models, savolitinib demonstrated significant anti-tumor efficacy only in MET-amplified models through the inhibition of phospho-MET and downstream signaling via ERK and AKT pathways (26).
To date, therapy targeting MET is mostly reported in the treatment of lung cancer, whose overall incidence of MET exon 14 skipping mutation as a primary driver mutation is 3% to 4%, whereas acquired MET amplification is detected after 5% to 20% epidermal growth factor receptor (EGFR)-TKI resistance (27). In a study conducted in 70 cases of advanced NSCLC patients with MET exon 14 skipping mutation, savolitinib as second-line treatment achieved an overall response rate (ORR) of 42.9%, a median progression-free survival (PFS) of 6.8 months, and a median OS of 12.5 months (28). The phase 1b TATTON study showed that combined therapy of osimertinib (an EGFR-TKI) and savolitinib, in patients with MET amplification secondary to EGFR mutation following EGFR-TKI treatment, achieved an ORR of 48% to 64% (29).
In addition to lung cancer, savolitinib is also applied for the treatment of papillary renal cell carcinoma (PRCC) and GC which is related to MET abnormalities. In a single-arm phase II study (NCT02127710) of PRCC patients treated with savolitinib, patients in the MET-driven subgroup (defined as chromosome 7 copy gain, focal MET or HGF gene amplification ≥ 6 copies, or MET kinase domain mutations > 5% allele frequency) had significant longer median PFS and higher ORR than the MET-independent subgroup (6.2 vs 1.4 months and 18% vs 0%, respectively) (30). A phase III randomized study (SAVOIR) designed solely for metastatic MET-driven PRCC patients favored savolitinib over sunitinib with a numerically higher response rate (27% vs 7%) (31). However, in the phase II SWOG1500 study of PRCC patients unselected for MET status, patients treated with savolitinib showed an increased risk of progression compared to those with sunitinib (32). These indicated that savolitinib exerts its highly selective antitumor activity through a MET-dependent manner.
MET amplification is present 1% to 10% GC (27). Previous studies revealed that MET amplification is involved in invasion, metastasis, advanced stage, and poor prognosis of GC. For instance, An et al. reported that the median PFS and OS of patients carrying MET amplification were 3.6 and 5.7 months, respectively, whereas those of patients without MET amplification were 6.9 and 15.5 months (33). Lee et al. found that the survival rate of GC patients with MET copy number greater than 4 (incidence 21.1%) was distinctly lower than that of patients with a lower copy number (8).
The VIKTORY phase II clinical trial of advanced GC confirmed that the ORR of patients with MET copy number greater than 10 (incidence 3.5%) treated by savolitinib monotherapy was 50%, and their PFS were 4-6 months (34). In a phase I study of capmatinib monotherapy for multiple tumors with MET overexpression or gene amplification, 2 of 9 patients with GC achieved stable disease (SD) (35). In a phase I clinical trial of tepotinib monotherapy in the treatment of multiple tumors (no limitation to MET status), 1 of 2 patients with GC achieved SD (36). At present, a single-arm phase II clinical trial (NCT04923932) of savolitinib for treating locally advanced or metastatic gastric cancer and esophagogastric junction adenocarcinoma with MET gene amplifications is ongoing.
Our male patient also had a MET-ST7 rearrangement. ST7 locates at 7q31, which is close to MET. ST7 is often present with loss of heterozygosity (LOH), rather than point mutation in multiple tumors, and is believed to be a tumor suppressor gene (37–39). In a study carried out in ALK-rearranged positive NSCLC patients receiving ALK-TKI treatment, it was found that 3% exhibited secondary MET-ST7 rearrangement (MET exons 2-21-ST7 exon 1; the fusion site was different from that of our patient). Introducing the rearranged gene to ALK-TKI-sensitive cell line induced resistance to ALK inhibitors, and this resistance was reversed by combining treatment with MET-TKIs (crizotinib, capmatinib, or savolitinib) (40). These findings indicate that MET-ST7 rearrangement may be pivotal in the development of cancer.
The male case reported in this article is an advanced GC patient with bone marrow invasion and extensive metastases, whose MET was highly amplified and co-existed with MET-ST7 rearrangement. In the situation that routine chemotherapy was not well tolerated and the patient’s condition was extremely critical, considering the significant abnormalities of his MET gene as well as the published clinical data and drug accessibility, savolitinib monotherapy was selected. After savolitinib treatment, the patient’s condition promptly improved, his platelets returned to normal, anemia and bone marrow invasion disappeared. Tumor efficacy evaluation indicated PR and maintained over 15 weeks, no obvious adverse reactions occurred. We cannot completely rule out the possibility that the treatment effect is the result of a combination of all three treatments (nivolumab, S-1, and savolitinib), this is the limitation of our report. But taking into account that the patient received only one dose of nivolumab and 3-day low-dose S-1 before savolitinib treatment, accompanied by repeated IVIG which could accelerate nivolumab’s clearance, his condition was still rapidly deteriorating, we believe that the anti-tumor activity of savolitinib played a major role in his treatment strategy.
In general, this report of savolitinib monotherapy validated the prominent efficacy of savolitinib in the treatment of advanced GC with MET amplification, providing a potential and effective treatment option for such patients.
Here we report the treatment of aggressive advanced GC patients with MET abnormalities, who responded to savolitinib monotherapy promptly and positively. It provides a new reference for understanding MET abnormalities in GC, and offers a possibility for future application of MET-TKIs in GC patients. Also, it suggests that sequencing of MET can be considered a routine target in advanced GC patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
WY, LS, ZZ, and MD contributed to the implement of the treatment. WY and LH contributed to the collection, analysis and interpretation of data, drafting and revision of the manuscript. SY contributed to the conception of the treatment, revision and approval of the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No.81702312) in the medical writing assistance and publication fees of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.868654/full#supplementary-material
Supplementary Figure 1 | Base map of MET-ST7 fusion site.
Supplementary Figure 2 | (A) Gastroscopy showed an ulcer lesion (black arrow) with uneven surface was found in the lesser curvature of gastric fundus, about 2.5 cm × 2 cm in size; a submucosal bulge (white arrow, proved to be poorly differentiated adenocarcinoma as well) was found at the junction of gastric fundus and body, about 1.5 cm in diameter; (B) Hematoxylin-eosin staining of the gastric biopsy from the ulcer lesion; Enhanced CT images of the gastric wall (C) at diagnosis, (D) progression after two lines of chemotherapy, and (E) after savolitinib treatment for 8 weeks. The red arrow represents the location of the lesion.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric Cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/s0140-6736(20)31288-5
3. Kwon JY, Yun J, Kim HJ, Kim KH, Kim SH, Lee SC, et al. Clinical Outcome of Gastric Cancer Patients With Bone Marrow Metastases. Cancer Res Treat (2011) 43(4):244–9. doi: 10.4143/crt.2011.43.4.244
4. Malik R, Mambetsariev I, Fricke J, Chawla N, Nam A, Pharaon R, et al. MET Receptor in Oncology: From Biomarker to Therapeutic Target. Adv Cancer Res (2020) 147:259–301. doi: 10.1016/bs.acr.2020.04.006
5. Zhang J, Guo L, Liu X, Li W, Ying J. MET Overexpression, Gene Amplification and Relevant Clinicopathological Features in Gastric Adenocarcinoma. Oncotarget (2017) 8(6):10264–73. doi: 10.18632/oncotarget.14382
6. Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, et al. MET in Gastric Carcinomas: Comparison Between Protein Expression and Gene Copy Number and Impact on Clinical Outcome. Br J Cancer (2012) 107(2):325–33. doi: 10.1038/bjc.2012.237
7. Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D’Emidio S, et al. Genetic Activation of the MET Pathway and Prognosis of Patients With High-Risk, Radically Resected Gastric Cancer. J Clin Oncol (2011) 29(36):4789–95. doi: 10.1200/jco.2011.36.7706
8. Lee J, Seo JW, Jun HJ, Ki CS, Park SH, Park YS, et al. Impact of MET Amplification on Gastric Cancer: Possible Roles as a Novel Prognostic Marker and a Potential Therapeutic Target. Oncol Rep (2011) 25(6):1517–24. doi: 10.3892/or.2011.1219
9. Markham A. Savolitinib: First Approval. Drugs (2021) 81(14):1665–70. doi: 10.1007/s40265-021-01584-0
10. Joshi SS, Badgwell BD. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
11. Schwab I, Nimmerjahn F. Intravenous Immunoglobulin Therapy: How Does Igg Modulate the Immune System? Nat Rev Immunol (2013) 13(3):176–89. doi: 10.1038/nri3401
12. Ahmad S, Lewis M, Corrie P, Iddawela M. Ipilimumab-Induced Thrombocytopenia in a Patient With Metastatic Melanoma. J Oncol Pharm Pract (2012) 18(2):287–92. doi: 10.1177/1078155211411001
13. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical Neurological Complications of Ipilimumab Therapy in Patients With Metastatic Melanoma. Neuro Oncol (2014) 16(4):589–93. doi: 10.1093/neuonc/nou001
14. Spain L, Diem S, Larkin J. Management of Toxicities of Immune Checkpoint Inhibitors. Cancer Treat Rev (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
15. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol (2016) 2(10):1346–53. doi: 10.1001/jamaoncol.2016.1051
16. Akhtari M, Waller EK, Jaye DL, Lawson DH, Ibrahim R, Papadopoulos NE, et al. Neutropenia in a Patient Treated With Ipilimumab (Anti-CTLA-4 Antibody). J Immunother (2009) 32(3):322–4. doi: 10.1097/CJI.0b013e31819aa40b
17. Junghans RP, Anderson CL. The Protection Receptor for Igg Catabolism is the Beta2-Microglobulin-Containing Neonatal Intestinal Transport Receptor. Proc Natl Acad Sci USA (1996) 93(11):5512–6. doi: 10.1073/pnas.93.11.5512
18. Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete Fcrn Dependence for Intravenous Ig Therapy in Autoimmune Skin Blistering Diseases. J Clin Invest (2005) 115(12):3440–50. doi: 10.1172/jci24394
19. Hansen RJ, Balthasar JP. Intravenous Immunoglobulin Mediates an Increase in Anti-Platelet Antibody Clearance via the Fcrn Receptor. Thromb Haemost (2002) 88(6):898–9. doi: 10.1055/s-0037-1613331
20. Hansen RJ, Balthasar JP. Effects of Intravenous Immunoglobulin on Platelet Count and Antiplatelet Antibody Disposition in a Rat Model of Immune Thrombocytopenia. Blood (2002) 100(6):2087–93. doi: 10.1182/blood.V100.6.2087
21. Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, et al. Pharmacokinetic Study of s-1, a Novel Oral Fluorouracil Antitumor Drug. Clin Cancer Res (1999) 5(8):2000–5.
22. Markham A. Tepotinib: First Approval. Drugs (2020) 80(8):829–33. doi: 10.1007/s40265-020-01317-9
23. Dhillon S. Capmatinib: First Approval. Drugs (2020) 80(11):1125–31. doi: 10.1007/s40265-020-01347-3
24. Jia H, Dai G, Weng J, Zhang Z, Wang Q, Zhou F, et al. Discovery of (s)-1-(1-(Imidazo[1,2-a]Pyridin-6-Yl)Ethyl)-6-(1-Methyl-1H-Pyrazol-4-Yl)-1H-[1,2, 3]Triazolo[4,5-B]Pyrazine (Volitinib) as a Highly Potent and Selective Mesenchymal-Epithelial Transition Factor (C-Met) Inhibitor in Clinical Development for Treatment of Cancer. J Med Chem (2014) 57(18):7577–89. doi: 10.1021/jm500510f
25. Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET Pathway Aberrations as Diagnostic, Prognostic, and Predictive Biomarkers in Human Cancers. Crit Rev Clin Lab Sci (2019) 56(8):533–66. doi: 10.1080/10408363.2019.1653821
26. Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, et al. Volitinib, a Potent and Highly Selective C-Met Inhibitor, Effectively Blocks C-Met Signaling and Growth in C-MET Amplified Gastric Cancer Patient-Derived Tumor Xenograft Models. Mol Oncol (2015) 9(1):323–33. doi: 10.1016/j.molonc.2014.08.015
27. Guo R, Luo J, Chang J, Rekhtman N, Arcila M, Drilon A. MET-Dependent Solid Tumours - Molecular Diagnosis and Targeted Therapy. Nat Rev Clin Oncol (2020) 17(9):569–87. doi: 10.1038/s41571-020-0377-z
28. Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-Daily Savolitinib in Chinese Patients With Pulmonary Sarcomatoid Carcinomas and Other Non-Small-Cell Lung Cancers Harbouring MET Exon 14 Skipping Alterations: A Multicentre, Single-Arm, Open-Label, Phase 2 Study. Lancet Respir Med (2021) 9(10):1154–64. doi: 10.1016/s2213-2600(21)00084-9
29. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib Plus Savolitinib in Patients With EGFR Mutation-Positive, MET-Amplified, Non-Small-Cell Lung Cancer After Progression on EGFR Tyrosine Kinase Inhibitors: Interim Results From a Multicentre, Open-Label, Phase 1b Study. Lancet Oncol (2020) 21(3):373–86. doi: 10.1016/s1470-2045(19)30785-5
30. Choueiri TK, Plimack E, Arkenau HT, Jonasch E, Heng DYC, Powles T, et al. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol (2017) 35(26):2993–3001. doi: 10.1200/JCO.2017.72.2967
31. Choueiri TK, Heng DYC, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(8):1247–55. doi: 10.1001/jamaoncol.2020.2218
32. Pal SK, Tangen C, Thompson IM, Balzer-Haas N, George DJ, Heng DYC, et al. A Comparison of Sunitinib With Cabozantinib, Crizotinib, and Savolitinib for Treatment of Advanced Papillary Renal Cell Carcinoma: A Randomised, Open-Label, Phase 2 Trial. Lancet (2021) 397(10275):695–703. doi: 10.1016/s0140-6736(21)00152-5
33. An X, Wang F, Shao Q, Wang FH, Wang ZQ, Wang ZQ, et al. MET Amplification Is Not Rare and Predicts Unfavorable Clinical Outcomes in Patients With Recurrent/Metastatic Gastric Cancer After Chemotherapy. Cancer (2014) 120(5):675–82. doi: 10.1002/cncr.28454
34. Lee J, Kim ST, Kim K, Lee H, Kozarewa I, Mortimer PGS, et al. Tumor Genomic Profiling Guides Patients With Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov (2019) 9(10):1388–405. doi: 10.1158/2159-8290.CD-19-0442
35. Bang YJ, Su WC, Schuler M, Nam DH, Lim WT, Bauer TM, et al. Phase 1 Study of Capmatinib in MET-Positive Solid Tumor Patients: Dose Escalation and Expansion of Selected Cohorts. Cancer Sci (2020) 111(2):536–47. doi: 10.1111/cas.14254
36. Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, et al. Phase I Trial of the MET Inhibitor Tepotinib in Japanese Patients With Solid Tumors. Jpn J Clin Oncol (2020) 50(8):859–66. doi: 10.1093/jjco/hyaa042
37. Yoshimura S, Yamada T, Ohwada S, Koyama T, Hamada K, Tago K, et al. Mutations in the ST7/RAY1/HELG Locus Rarely Occur in Primary Colorectal, Gastric, and Hepatocellular Carcinomas. Br J Cancer (2003) 88(12):1909–13. doi: 10.1038/sj.bjc.6600942
38. Dong SM, Sidransky D. Absence of ST7 Gene Alterations in Human Cancer. Clin Cancer Res (2002) 8(9):2939–41.
39. Zenklusen JC, Conti CJ, Green ED. Mutational and Functional Analyses Reveal That ST7 Is a Highly Conserved Tumor-Suppressor Gene on Human Chromosome 7q31. Nat Genet (2001) 27(4):392–8. doi: 10.1038/86891
Keywords: savolitinib, MET gene, advanced gastric cancer, bone marrow invasion, case report
Citation: Ye W, He L, Su L, Zheng Z, Ding M and Ye S (2022) Case Report: Prompt Response to Savolitinib in a Case of Advanced Gastric Cancer With Bone Marrow Invasion and MET Abnormalities. Front. Oncol. 12:868654. doi: 10.3389/fonc.2022.868654
Received: 03 February 2022; Accepted: 14 March 2022;
Published: 04 April 2022.
Edited by:
Aamir Ahmad, University of Alabama at Birmingham, United StatesReviewed by:
Tabish Hussain, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2022 Ye, He, Su, Zheng, Ding and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Ye, eWVzaGVuZzJAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.