- 1Department of First Clinical Medical College, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Gynecology, The First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Background: CA-125 is a clinical biomarker with predictive effect on the prognosis of different cancers. Numerous clinical trials have been conducted to investigate the possibility of using the pretreatment level of CA-125 to predict the prognosis of epithelial ovarian cancer (EOC). However, its value in predicting prognosis remains controversial. The purpose of this meta-analysis was to assess the predictive value of pretreatment CA-125 levels for prognosis in EOC patients.
Methods: We searched the EMBASE, Cochrane library, PubMed and Web of Science databases for studies published up to 3 December 2021, according to specific inclusion and exclusion criteria. The clinical studies that were included investigated the relationship between pretreatment CA-125 levels and ovarian cancer prognosis. Combined hazard ratios (HR) of overall survival (OS) and progression-free survival (PFS) reported in the studies were compared and analyzed using fixed-effects/random-effects models. Sensitivity analysis was used to assess study stability, while Egger’s and Begg’s tests were used to assess publication bias.
Results: This meta-analysis included 23 studies published in 2004 - 2021 with a total of 10,594 EOC patients. Comprehensive analysis demonstrated that the serum level of CA-125 before treatment was significantly correlated with overall survival (OS: HR=1.62, 95%CI=1.270-2.060, p<0.001) and progression-free survival (PFS: HR=1.59, PFS: HR=1.59, 95%CI=1.44~1.76, p<0.001). After comparing data from different FIGO stages and treatments, we discovered that a high pre-treatment serum CA-125 level was associated with a low survival rate.
Conclusion: According to the results of this study, a higher pre-treatment serum CA-125 level is associated with poor survival outcomes, which can be utilized to predict the prognosis of EOC patients. Pre-treatment serum CA-125 level might provide reliable basis for predicting the risk of EOC disease progression. This study is registered with the International Prospective Register of Systematic Reviews (CRD42022300545).
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=300545, identifier [CRD42022300545].
Introduction
Ovarian cancer (OC) is one of the most common malignant tumors in the world, with the highest fatality rate among gynecological malignant tumors with a high degree of malignancy. The 5-year survival rate is less than 45% (1, 2). There are an estimated 290,000 newly diagnosed cases and 180,000 deaths every year around the world (3). Only less than 40% of the women with OC are diagnosed at an early stage due to the lack of typical clinical manifestations and reliable screening approaches. Nowadays, taxane and platinum-based chemotherapy after aggressive cytoreduction therapy is the accepted standard of treatment (4, 5). Despite receiving standard treatment, 75-80% of patients with advanced OC relapsed within 5 years of the initial diagnosis. Various markers have been used in early diagnosis or prognosis prediction, including D-dimer (6), Human epididymis protein 4 (HE4) (7), MMP-2, Survivin, Ki-67, etc. (8, 9). However, the prognosis of OC patients remains poor. Therefore, discovering an accurate and cost-effective predictive approach to evaluate the prognosis of EOC patients before treatment is importance when making the treatment plans.
CA-125 is a transmembrane macromolecule glycoprotein secreted by coelomic epithelial cells. It has become a consensus that CA-125 plays an important role in the prognosis of OC (10–12), and the role of CA-125 in tumorigenesis, metastasis and CA-125-targeted therapy has received great attention. CA-125 has high sensitivity and low specificity because inflammatory environments may promote CA-125 secretion, and non-tumor cells, such as mesothelial cells, and also secrete CA-125 in pro-inflammatory environments (such as ascites) (13). In ovarian cancer, preoperative CA-125 level varies greatly among individuals and may be significantly increased in some patients, which is beneficial for early identification and diagnosis of the tumor. At the same time, CA-125 levels remain in the normal range in more than half of the patients before surgery, making diagnosis and decision-making challenging and confusing for these patients. Numerous studies have been conducted in recent years to investigate the cut-off value of serum CA-125 before treatment and its role as a non-invasive factor in assessing the correlation with EOC prognosis (14–23). Elevated CA-125 level is considered to be a high risk factor for recurrence and death (24–27), however the results were inconsistent. Therefore, we performed this meta-analysis to determine the prognostic significance of the CA-125 level before treatment in EOC patients.
Materials and Methods
Search Strategy
This meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (28). We thoroughly searched the PubMed, Web of Science, Embase and Cochrane Library databases for eligible studies published as of December 3, 2021. The following search phrases were used for literature retrieval: (ovarian cancers OR ovarian neoplasms OR OC OR ovarian tumor OR cancer of ovary OR ovary cancer) AND (CA-125 OR CA 125 OR CA-125 OR Carbohydrate antigen 125 OR Cancer antigen 125) AND (prognosis OR prediction). Detailed search strategies are shown in the online supplementary material. We also searched references of the included articles and grey literature to identify if there were any potential articles to be included. Since the data used in this article were extracted from previous literature, no patient consent or ethical approval was required.
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria: The inclusion criteria were as follows: (1) EOC was diagnosed according to pathological examination or current clinical guidelines; (2) serum CA-125 level was collected before chemotherapy, surgery and neoadjuvant chemotherapy with an explicit cut-off value; (3) articles investigating the relationship between CA-125 and EOC prognosis [including hazard ratio (HR) and corresponding 95% confidence interval (CI) for overall survival (OS) and/or disease-free progression (PFS)], or providing sufficient data to calculate these indicators, including the clinical pathological parameters; (4) published as full-text papers; and (5) the articles were written in English. The exclusion criteria were as follows: (1) reviews, conference abstracts, letters, editorials, expert opinions, case reports, abstracts, and reviews; (2) basic research or animal studies; (3) duplicate publications; and (4) studies on the tumors originating in the gastrointestinal tract and having metastasis to the ovary.
Data Collection, Extraction and Quality Assessment
Two investigators independently collected corresponding data from eligible studies, and any disagreements were resolved through discussions with a third investigator. The following information was extracted: (1) study characteristics including the first author, country of origin, year of publication, number of patients, duration of follow-up, and method of survival analysis; (2) patient characteristics including geographical area, age, metastasis, FIGO stage, treatment and cut-off value; and (3) survival measures including HRs of OS, PFS, DFS and their 95% CIs. The HRs were extracted from multivariate or univariate analyses. If HR values were not provided in the original text, Kaplan-Meier survival curves were used to determine HR values and corresponding CIs (Engauge Digitizer version 10.8). In this meta-analysis, the primary and secondary outcomes were OS and PFS. E-mails were also sent to the corresponding author for the requested data.
Newcastle-Ottawa Scale (NOS) (29) was used to assess the methodological quality of included studies. The studies were graded from 0 to 9 based on cohort selection, comparability, and results. Studies having a score of 6 or above were considered to be of high-quality research. Any disagreements were resolved by a senior researcher.
Statistical Analysis
Pooled HRs and 95% CIs were calculated to assess the relationship between pretreatment CA-125 level and prognosis. The heterogeneity of the included studies was assessed using Cochran’s q-test and Higgins I2 statistic. Data were combined using a random-effects model (DerSimonia-Laird method) if I2>50% or p<0.10 (indicating significant heterogeneity among studies); otherwise a fixed-effects model (Mantel-Haenszel method) was used. To identify sources of heterogeneity between studies, subgroup analyses were conducted based on geographic region, treatment method, and FIGO stage. Furthermore, sensitivity analysis was conducted to assess the impact of individual study data on HRs for OS and PFS. All statistical calculations were performed using STATA MP16 (Stata, College Station, TX, USA). In all the analyses, p<0.05 (two-sided) indicated a statistically significant difference.
Results
Search Results and Characteristics of the Studies
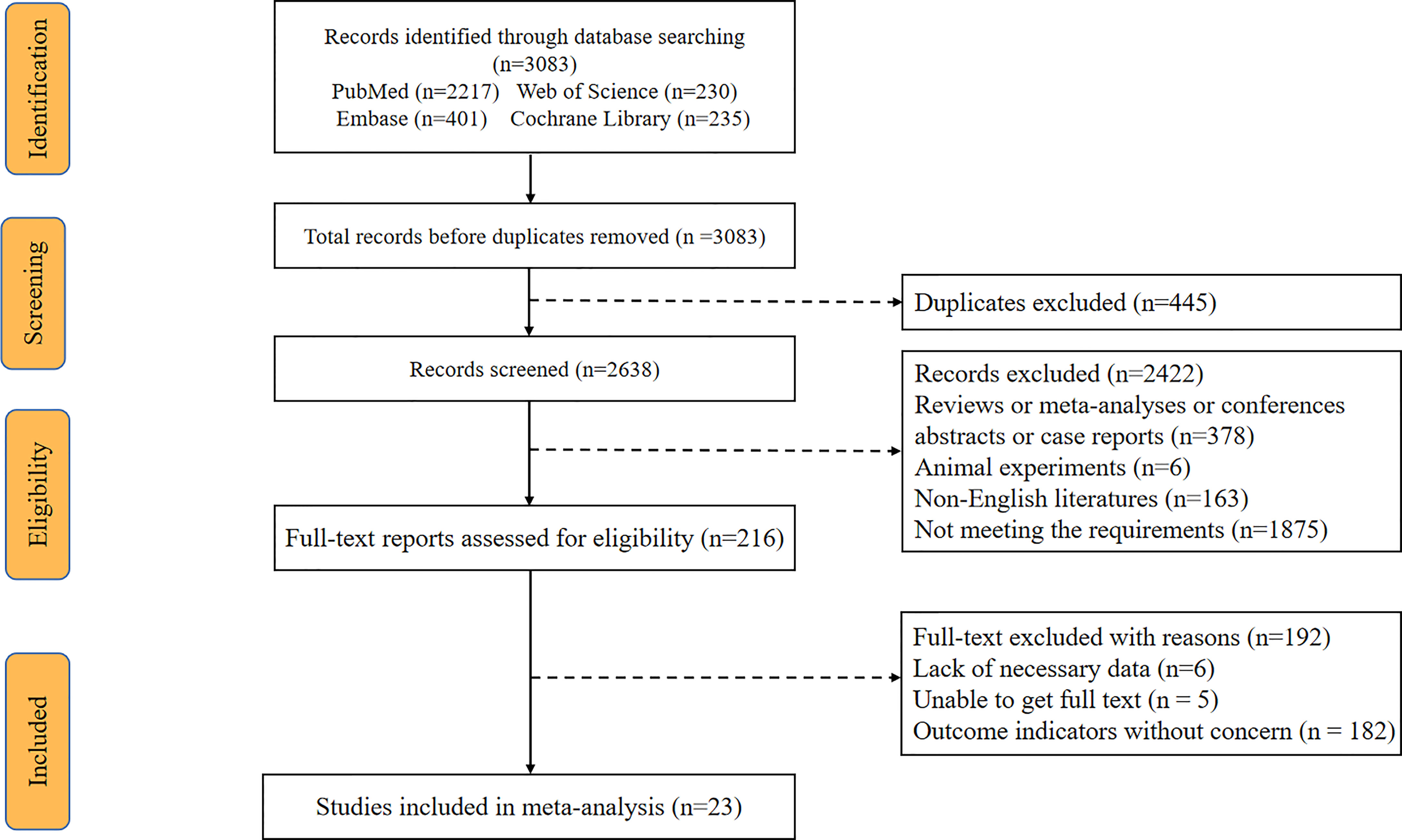
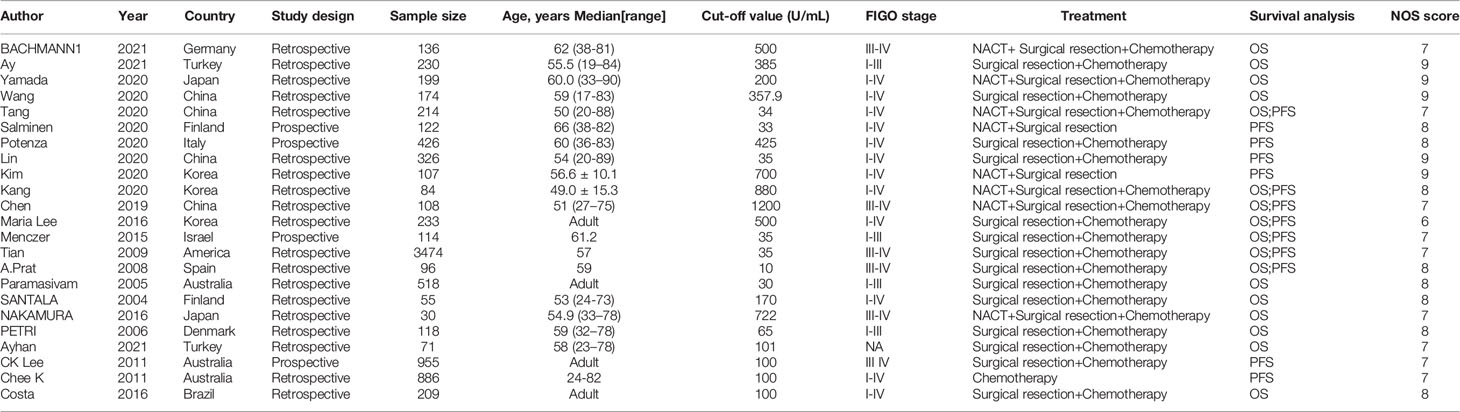
A total of 3083 articles were retrieved from the system’s database, with 445 of them being eliminated owing to dataset duplication. Another 2422 articles were eliminated after analyzing their titles and abstracts. Afterward, 216 articles were reviewed using full-text reading, with 192 of them being eliminated for the following reasons: Six studies lacked required data; five studies were not accessible in full text, and 181 studies did not provide the desired result. There are a total of 24 eligible articles, one which was published in 1995 and was excluded because it was too old (20). Finally, our meta-analysis includes 23 articles published between 2004 and 2021. The flow chart of literature search is shown in Figure 1. The 23 eligible studies included a total of 10,594 patients, with numbers of patients ranging from 30 to 3,474. There were 17 studies exploring the relationship between CA-125 level and OS, and 13 studies providing the relationship between CA-125 and PFS. The cutoff value was between 33 and 1200. There were 20 retrospective cohort studies and 3 prospective cohort studies out of the 23 eligible studies. Four studies were from Europe, 10 from Asia, 2 from the Americas and 1 from Oceania. In 13 studies, HR was recorded in univariate analysis. In 10 trials, HR was measured using multivariate analysis, while in 5 studies, HR was obtained using survival curves. The main characteristics of the 23 included studies are shown in Table 1. The NOS scores of all the studies were between 6 and 9. The included studies all had a high NOS score ≥ 6.
The Effect of CA-125 Level on the Prognosis of OS in EOC Patients
We analyzed the data from 6063 EOC cancer patients in 17 studies (6, 14, 15, 17–19, 23, 24, 26, 27, 30–36) to determine the relationship between pretreatment serum CA-125 level and OS. A random-effects model was employed based on the results of the heterogeneity analysis (I2 = 86.40%, p <0.001). The results presented that increased serum CA-125 level before treatment was associated with poorer OS (HR=1.62, 95%CI=1.270-2.060, p<0.001, Figure 2 and Table S2), with a statistically significant difference. The heterogeneity of HR was significant in the OS group, thus subgroup analyses were performed based on FIGO stage, and treatment strategy to identify the sources of heterogeneity. Since one of the studies did not explicitly state the FIGO staging (33), no subgroup analysis of FIGO staging was performed. The results suggested that higher heterogeneity might be attributed to differences in geographic regions, tumor stages, and treatment strategies. However, the final results showed that higher pre-treatment CA-125 level was consistently correlated with poorer OS regardless of the grouping (Figure 3 and Table S2).

Figure 2 Forest plot of the association of CA-125 with OS in EOC cancer patients. CI, confidence interval; HR, hazard ratio.
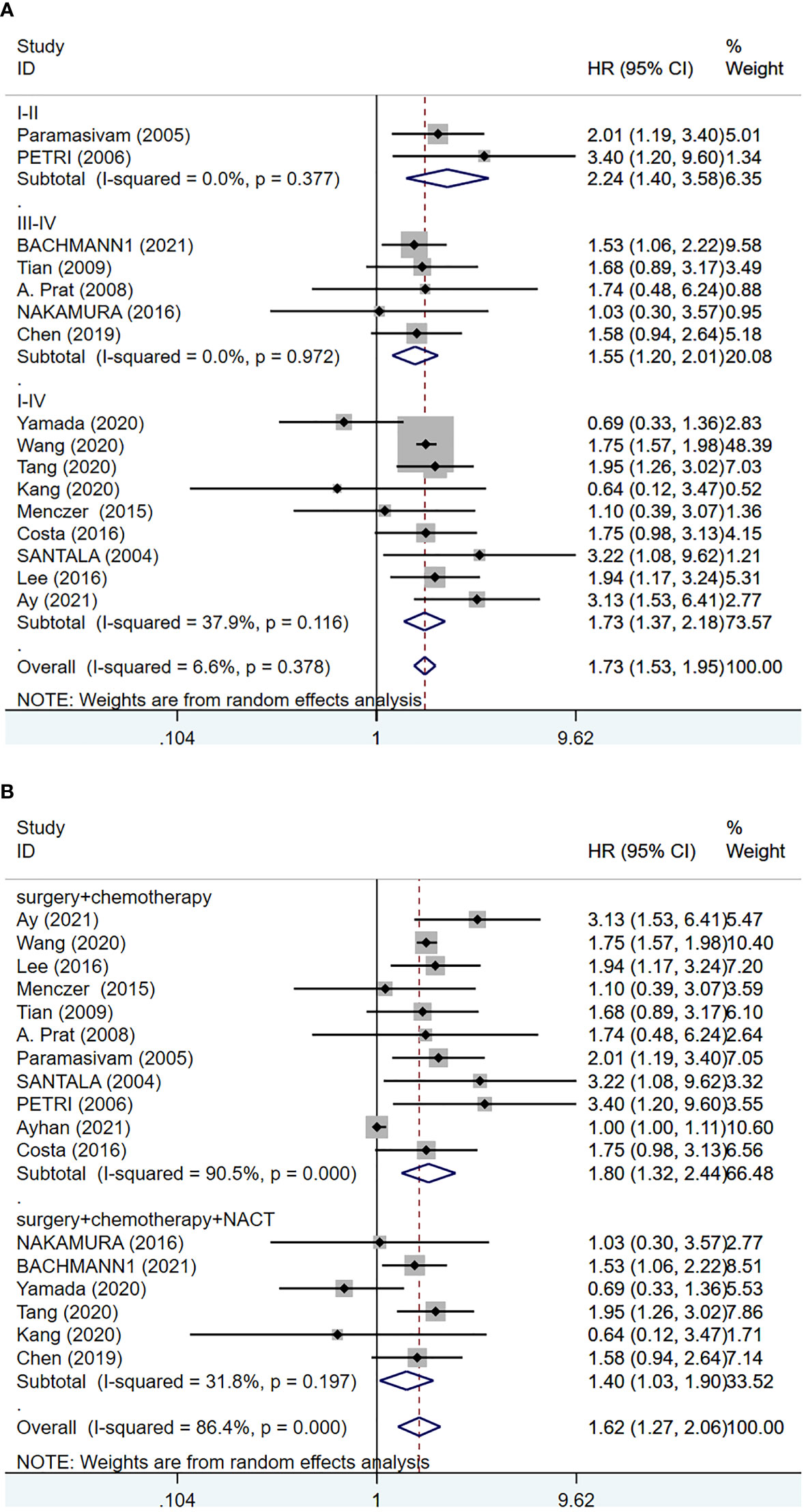
Figure 3 Forest plot of the relationship between CA-125 level and clinical factors in EOC patients. (A) FIGO stage (I-II versus III-IV versus I-IV); (B) treatment (surgery + chemotherapy versus surgery + chemotherapy + NACT).
The Predictive Effect of CA-125 Level on the Prognosis of PFS in EOC Patients
Data from 13 studies with a total of 7145 patients were retrieved and utilized in the PFS analysis (16–18, 21, 22, 25, 26, 30–32, 36–38). The integrated data showed that increased serum CA-125 level before treatment was associated with poor PFS (HR=1.59, 95%CI=1.44-1.76, p<0.001, Figure 4). There was no heterogeneity between studies (I2 = 0.0%, p=0.612). Therefore, a fixed-effects model was used. No subgroup analysis was done due to the small number of studies reporting PFS.
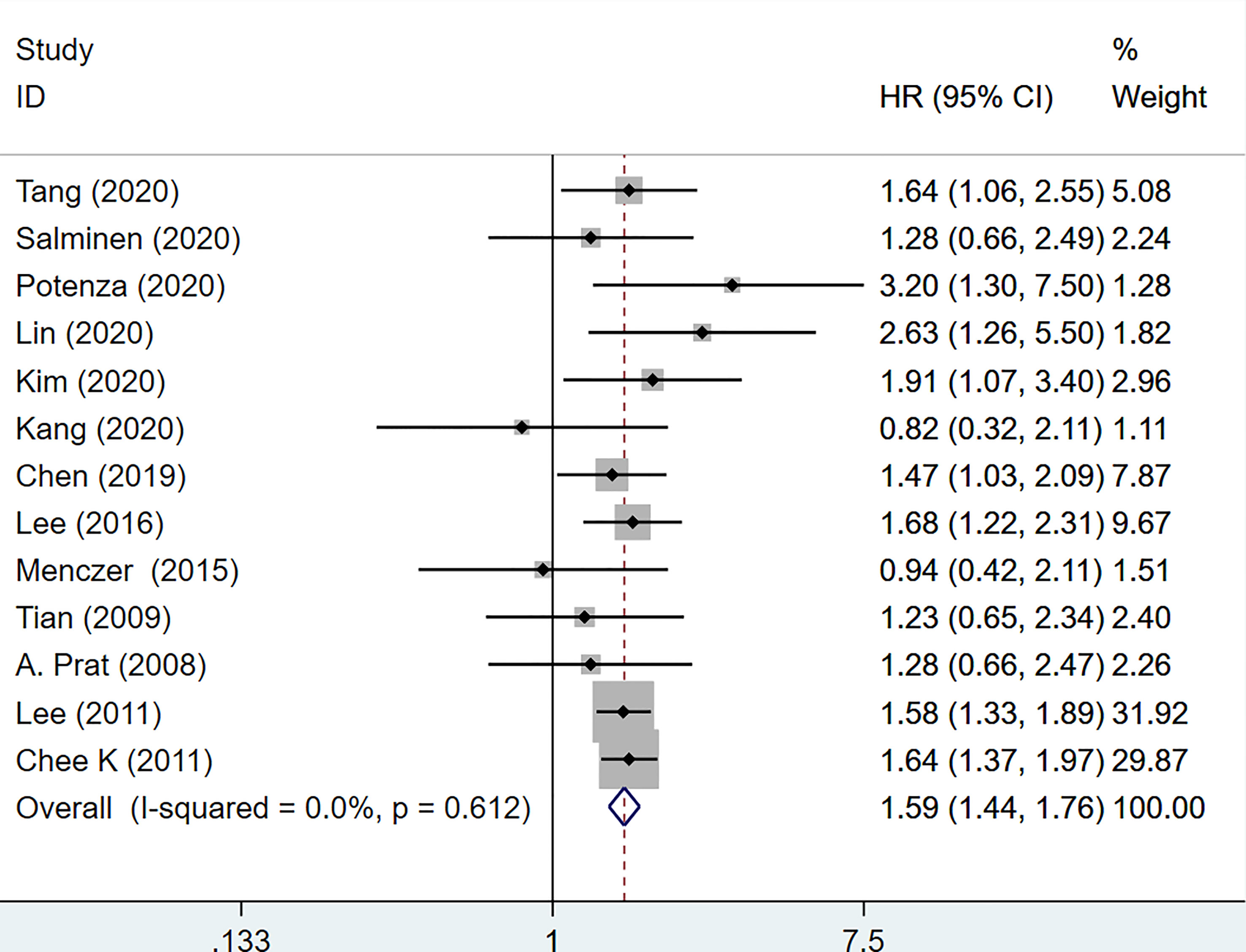
Figure 4 Forest plot of the association of CA-125 level with PFS in EOC patients. CI, confidence interval; HR, hazard ratio.
Sensitivity Analysis
Sensitivity analysis was carried out after merging the included literatures. After removing the included literatures one by one, the effect size in the remaining literatures were combined again. The results showed that there was no significant change in the combined HR and 95% CI, indicating that the results of this meta-analysis are relatively stable and reliable (Figure S1).
Publication Bias
Begg’s and Egger’s tests were used to test whether there was publication bias in the included literature. Figure S2 illustrates that when OS was evaluated for publication bias, Egger’s test revealed a statistically significant difference (p<0.05), indicating that there was publication bias in the results of this study. The reason might be that the authors chose to withdraw the manuscript, delay or omit publication because the outcomes was regarded as negative and meaningless, resulting in fewer reports of relevant results being published. In order to further evaluate the impact of publication bias on the results, trim-and-fill method was used. After one missing study was added, it was found that there was no significant change in the combined effect size, the cut-and-fill method yielded an HR of 1.573 (95%CI: 1.243-1.990). This result indicated that the impact of publication bias was small and the results were relatively stable. The results of other studies showed no statistical significance, suggesting that there was no publication bias. The funnel plot for the trim-and-fill method is shown in Figure S3.
Discussion
Previous studies have proven the relevance of pre-treatment CA-125 levels in predicting EOC prognosis, and this has been consistently verified in guiding clinical practice and prolonging patient survival. However, since there is no consensus on the fixed cut-off value for pre-treatment serum CA-125 concentration, resulting in inconsistent and inconclusive findings, we reviewed published articles to assess the potential for clinical application of pre-treatment CA-125 in EOC. To the best of our knowledge, this is the first systematic review to investigate the prognostic value of pre-treatment CA-125 in EOC patients. In this study, we sought to explore whether the pre-treatment serum CA-125 level could be used as a prognostic indicator of EOC to help predict the best cytoreductive surgery, reduce surgical complications and economic burden, and better evaluate the prognosis of EOC patients. We collected data from 23 studies involving 10,594 patients, and pooled analysis showed that elevated pre-treatment serum CA-125 level was significantly associated with poor prognosis in patients with EOC. In addition, subgroup analysis was performed according to FIGOstage and treatment. The results of all subgroup analyses showed that there was no significant difference in prognosis across different subgroups (p>0.05). The pre-treatment serum CA-125 level had predictive significance for the prognosis regardless of whether the tumor was at an early or advanced stage or if NACT was employed. Elevated CA-125 may be a strong prognostic marker for disease progression, tumor recurrence, and long-term survival outcomes including OS and PFS.
In recent years, the tumor microenvironment has received increasing attention in the field of tumor immunology. The mechanism of CA-125 affecting prognosis may be related to the promotion of metastasis of cancer cells to other sites. CA-125 is a repeated peptide epitope of the mucin MUC16 (39), which binds with high affinity to Mesothelin, a protein lining the peritoneal mesothelium (40), and promotes the attachment of cancer cells to the mesothelial lining, leading to peritoneal metastasis of ovarian cancer cells (41). This mechanism is thought to be related to the interaction of phosphorylation of MMP-7 and p38 mitogen-activated protein kinase (MAPK), increased expression of β-catenin, and translocation of p120ctn (42). In addition, MUC16 has been shown to inhibit the innate immune response to OC cells by directly inhibiting natural killer (NK) cells, helping cancer cells evade the host immune response. The mechanism of action is as follows: MUC16 down-regulates CD16 on NK cells and interacts with Siglec-9 on its surface to inhibit the formation of synapses between NK cells and ovarian cancer cells (41, 43–45), thus inhibiting the anticancer immune response. Increased and decreased serum CA-125 levels are associated with progression and regression of EOC (13). CA-125 at two different concentrations had a significant effect on OC cell migration, and the stimulating effect of CA-125 on cell migration appears to be mediated through the Wnt signaling pathway. Dickkopf-related protein-1 (DKK-1), a Wnt antagonist, has been shown to inhibit cancer cell migration, and its expression in OC tissues is lower than in normal tissues. The majority of OC patients have high serum CA-125 levels, and CA-125 enhances ovarian cancer cell migration by decreasing DKK-1 expression and activating the serum- and glucocorticoid-regulated kinase 3 (SGK3)/forkhead box O3 pathway (FOXO3). CA-125 induces metastasis of OC cells to axillary and inguinal lymphoid tissues (12, 46), resulting in a poor prognosis. CA-125 may play a wide range of rolesin the humoral immunosuppression of cancer by directly binding to a subset of tumor-targeting antibodies and blocking their immune effector functions (47). Hence, elevated CA-125 implies a predominance of tumor-promoting activity in the tumor microenvironment, which ultimately leads to a poor prognosis.
Several previous meta-analyses and retrospective analyses have investigated at the prognostic role of CA-125 in solid tumors. Overexpression of MUC16 is associated with poor prognosis in various malignancies (48–51). CA-125 was found to be an excellent prognostic indicator in a recent investigation on non-gynecologic cancer metastasis to the ovary (52), and another study revealed that CA-125 was a prognostic factor for survival in patients with gynecologic tumor brain metastases (53). Several other studies have also shown that pre-treatment serum CA-125 is an independent prognostic factor in patients with bladder urothelial carcinoma (UCB) (54), pancreatic ductal adenocarcinoma (55), and renal cell carcinoma (56). Here, we demonstrate the prognostic efficacy of CA-125 relative to OS and PFS in EOC, which is consistent with findings in other cancer types.
Due to the biological heterogeneity of EOC, which is not a single disease entity but a group of heterogeneous tumors with different clinicopathological features, different pre-treatment CA-125 levels have been found in patients with different EOC subtypes, such as low levels in mucinous and clear cell carcinomas. Although the included studies did not use a consistent cut-off value for pre-treatment serum CA-125 concentration, most studies found that higher levels of CA-125 in the normal range were associated with a higher risk ratio for recurrence or death (57).
The society of gynecologic oncology and the American society of clinical oncology consider that women with high perioperative risk or a low probability of tumor cytoreduction to a residual lesion <1cm (ideally no lesion visible) should receive NACH. Women who are eligible for PDS and have potentially resectable disease may receive either NACH or PDS. However, PDS is preferred if the likelihood of achieving cytoreduction to <1cm (ideally no visible disease) at an acceptable incidence is high (58). Many studies have been conducted to determine whether pre-treatment serum CA-125 predicts optimal PDS or IDS in patients with EOC, but the results have been inconsistent.
The study by Merlo et al. indicated that higher pre-treatment CA-125 levels were less likely to achieve optimal cytoreduction. At the cut-off value of 500IU/ml, 58% of patients with advanced EOC achieved complete or at least optimal cytoreductive surgery, and at the cut-off value of 250IU/ml, the probability increased to 74%. Merlo concluded that a cut-off value of 500IU/ml was an ideal threshold for predicting PDS success (59), which was consistent with the result of previous research. The study by Rodriguez et al. showed that patients with a pre-treatment CA-125 level of ≤100U/mL were more likely to undergo cytoreductive surgery without residual lesions (60). Lin et al, on the other hand, proposed that a pretreatment serum CA-125 level of ≥35 U/mL is an independent prognostic factor in epithelial ovarian cancer and that elevated levels should be regarded as a high risk of recurrence and mortality (25).
Based on our findings and those of other relevant studies, pre-treatment CA-125 may contribute to the prognosis of cancer and the development of clinical treatment strategies. For example, EOC patients with low pre-treatment CA-125 may benefit more from PDS, NACT, or other cancer-related treatments than EOC patients with high CA-125. The prognostic relevance of pre-treatment CA-125 has attracted clinical attention, and in order to benefit EOC patients, ongoing and systematic studies are still necessary to fully understand and utilize CA-125.
There are several limitations to this study. First, the number of studies we included was relatively small. Second, we were unable to perform further subgroup analyses due to the diversity of cut-off values defined in each study. Third, most of the enrolled studies were retrospective and extracted several HRs from univariate analyses, which may lead to overestimated effect sizes. Therefore, this study may have information bias, selection bias, and misclassification bias, and the optimal cut-off value cannot be determined. Larger-scale prospective studies are needed to confirm the significance of pretreatment CA-125 in predicting the prognosis of EOC and to provide evidence for its clinical application. In summary, according to the current study, the pre-treatment CA-125 level has some certain significance in predicting the prognosis of EOC. The results of this study may help to determine whether patients may benefit from surgery, chemotherapy, and/or neoadjuvant chemotherapy.
Conclusion
This meta-analysis has shown that higher pre-treatment CA-125 levels are significantly associated with poorer OS and PFS in patients with EOC. We suggest that CA-125 should be monitored to guide prognosis and provide reliable information on the risk of disease progression in EOC. Nevertheless, due to some limitations of this study, large multi-center prospective trials are needed to examine the prognostic role of pretreatment CA-125 in EOC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
QW designed this study. QW and SZ contributed to literature retrieval, review and data extraction. SZ and XL conducted the statistical analyses. QW, and XL contributed to the manuscript drafting. QW, and XL contributed to the manuscript revision. XF provided fund help. All authors contributed to the article and approved the submitted version.
Funding
Project supported by the National Natural Science Foundation of China (Grant NO.81973894).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to the classmates who completed the article with me, and my graduate advisor.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.868061/full#supplementary-material
References
1. Webb PM, Jordan SJ. Epidemiology of Epithelial Ovarian Cancer. Best Pract Res Clin Obstet Gynaecol (2017) 41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006
2. Eisenhauer EA. Real-World Evidence in the Treatment of Ovarian Cancer. Ann Oncol (2017) 28(suppl_8):viii61–5. doi: 10.1093/annonc/mdx443
3. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
4. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta-Analysis. J Clin Oncol (2002) 20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248
5. Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved Optimal Cytoreduction Rates for Stages IIIC and IV Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer: A Change in Surgical Approach. Gynecol Oncol (2004) 94(3):650–4. doi: 10.1016/j.ygyno.2004.01.029
6. Yamada Y, Kawaguchi R, Iwai K, Niiro E, Morioka S, Tanase Y, et al. Preoperative Plasma D-Dimer Level Is a Useful Prognostic Marker in Ovarian Cancer. J Obstet Gynaecol (2020) 40(1):102–6. doi: 10.1080/01443615.2019.1606176
7. Rong Y, Li L. Early Clearance of Serum HE4 and CA-125 in Predicting Platinum Sensitivity and Prognosis in Epithelial Ovarian Cancer. J Ovarian Res (2021) 14(1):2. doi: 10.1186/s13048-020-00759-9
8. Jia H, Zhang Q, Liu F, Zhou D. Prognostic Value of MMP-2 for Patients With Ovarian Epithelial Carcinoma: A Systematic Review and Meta-Analysis. Arch Gynecol Obstet (2017) 295(3):689–96. doi: 10.1007/s00404-016-4257-9
9. Kucukgoz Gulec U, Gumurdulu D, Guzel AB, Paydas S, Seydaoglu G, Acikalin A, et al. Prognostic Importance of Survivin, Ki-67, and Topoisomerase Iiα in Ovarian Carcinoma. Arch Gynecol Obstet (2014) 289(2):393–8. doi: 10.1007/s00404-013-3000-z
10. Shen Y, Li L. Serum HE4 Superior to CA-125 in Predicting Poorer Surgical Outcome of Epithelial Ovarian Cancer. Tumour Biol (2016) 37(11):14765–72. doi: 10.1007/s13277-016-5335-0
11. Zwakman N, van de Laar R, Van Gorp T, Zusterzeel PL, Snijders MP, Ferreira I, et al. Perioperative Changes in Serum CA-125 Levels: A Prognostic Factor for Disease-Specific Survival in Patients With Ovarian Cancer. J Gynecol Oncol (2017) 28(1):e7. doi: 10.3802/jgo.2017.28.e7
12. Yuan Q, Song J, Yang W, Wang H, Huo Q, Yang J, et al. The Effect of CA-125 on Metastasis of Ovarian Cancer: Old Marker New Function. Oncotarget (2017) 8(30):50015–22. doi: 10.18632/oncotarget.18388
13. Matte I, Garde-Granger P, Bessette P, Piché A. Ascites From Ovarian Cancer Patients Stimulates MUC16 Mucin Expression and Secretion in Human Peritoneal Mesothelial Cells Through an Akt-Dependent Pathway. BMC Cancer (2019) 19(1):406. doi: 10.1186/s12885-019-5611-7
14. Bachmann R, Brucker S, Stäbler A, Kramer B, Ladurner R, Konigsrainer A, et al. Prognostic Relevance of High Pretreatment CA-125 Levels in Primary Serous Ovarian Cancer. Mol Clin Oncol (2021) 14(1):8. doi: 10.3892/mco.2020.2170
15. Ay S, Ozyukseler DT, Dulgar O, Basak M, Yildirim ME, Gumus M. Initial Ca 125 Value as a Predictive Marker for High-Grade Serous Ovarian Cancer. J Coll Physicians Surg Pak (2021) 30(6):651–6. doi: 10.29271/jcpsp.2021.06.651
16. Salminen L, Nadeem N, Jain S, Grènman S, Carpén O, Hietanen S, et al. A Longitudinal Analysis of CA-125 Glycoforms in the Monitoring and Follow Up of High Grade Serous Ovarian Cancer. Gynecol Oncol (2020) 156(3):689–94. doi: 10.1016/j.ygyno.2019.12.025
17. Lee M, Chang MY, Yoo H, et al. Clinical Significance of CA-125 Level After the First Cycle of Chemotherapy on Survival of Patients With Advanced Ovarian Cancer. Yonsei Med J (2016) 57(3):580–7. doi: 10.3349/ymj.2016.57.3.580
18. Menczer J, Ben-Shem E, Golan A, Levy T. The Significance of Normal Pretreatment Levels of CA-125 (<35 U/mL) in Epithelial Ovarian Carcinoma. Rambam Maimonides Med J (2015) 6(1):e0005. doi: 10.5041/RMMJ.10180
19. Paramasivam S, Tripcony L, Crandon A, Quinn M, Hammond I, Marsden D, et al. Prognostic Importance of Preoperative CA-125 in International Federation of Gynecology and Obstetrics Stage I Epithelial Ovarian Cancer: An Australian Multicenter Study. J Clin Oncol (2005) 23(25):5938–42. doi: 10.1200/JCO.2005.08.151
20. Nagele F, Petru E, Medl M, Kainz C, Graf AH, Sevelda P. Preoperative CA 125: An Independent Prognostic Factor in Patients With Stage I Epithelial Ovarian Cancer. Obstet Gynecol (1995) 86(2):259–64. doi: 10.1016/0029-7844(95)00126-c
21. Lee CK, Simes RJ, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic Nomogram to Predict Progression-Free Survival in Patients With Platinum-Sensitive Recurrent Ovarian Cancer. Br J Cancer (2011) 105(8):1144–50. doi: 10.1038/bjc.2011.364
22. Lee CK, Friedlander M, Brown C, Gebski VJ, Georgoulopoulos A, Vergote I, et al. Early Decline in Cancer Antigen 125 as a Surrogate for Progression-Free Survival in Recurrent Ovarian Cancer. J Natl Cancer Inst (2011) 103(17):1338–42. doi: 10.1093/jnci/djr282
23. da Costa AA, Valadares CV, Mantoan H, Saito A, Salvadori MM, Guimarães AP, et al. The Value of Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer and Application of a Prognostic Score. Int J Gynecol Cancer (2016) 26(3):449–55. doi: 10.1097/IGC.0000000000000649
24. Wang H, Shen Y, Ding X, Ding Y, Cheng L, Lai J. Prognostic Significance of Preoperative Circulating FAR and FCI Scores in Patients With Ovarian Cancer. Clin Chim Acta (2020) 509:252–7. doi: 10.1016/j.cca.2020.06.034
25. Lin YH, Wu CH, Fu HC, Chen YJ, Chen YY, Ou YC, et al. Prognostic Significance of Elevated Pretreatment Serum Levels of CEA and CA-125 in Epithelial Ovarian Cancer. Cancer Biomark (2020) 28(3):285–92. doi: 10.3233/CBM-201455
26. Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, et al. CA-125 Change After Chemotherapy in Prediction of Treatment Outcome Among Advanced Mucinous and Clear Cell Epithelial Ovarian Cancers: A Gynecologic Oncology Group Study. Cancer (2009) 115(7):1395–403. doi: 10.1002/cncr.24152
27. Petri AL, Høgdall E, Christensen IJ, Kjaer SK, Blaakaer J, Høgdall CK. Preoperative CA-125 as a Prognostic Factor in Stage I Epithelial Ovarian Cancer. APMIS (2006) 114(5):359–63. doi: 10.1111/j.1600-0463.2006.apm_397.x
28. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
29. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
30. Prat A, Parera M, Peralta S, Perez-Benavente MA, Garcia A, Gil-Moreno A, et al. Nadir CA-125 Concentration in the Normal Range as an Independent Prognostic Factor for Optimally Treated Advanced Epithelial Ovarian Cancer. Ann Oncol (2008) 19(2):327–31. doi: 10.1093/annonc/mdm495
31. Tang Y, Hu HQ, Tang FX, Lin D, Shen R, Deng L, et al. Combined Preoperative LMR and CA125 for Prognostic Assessment of Ovarian Cancer. J Cancer (2020) 11(11):3165–71. doi: 10.7150/jca.42477
32. Chen JP, Huang QD, Wan T, Tu H, Gu HF, Cao JY, et al. Combined Score of Pretreatment Platelet Count and CA125 Level (PLT-CA125) Stratified Prognosis in Patients With FIGO Stage IV Epithelial Ovarian Cancer. J Ovarian Res (2019) 12(1):72. doi: 10.1186/s13048-019-0544-y
33. Ayhan A, Akilli H. Prognostic Factors Associated With Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy in Recurrent Ovarian Cancer. Int J Gynaecol Obstet (2021) 152(2):202–7. doi: 10.1002/ijgo.13410
34. Santala M, Risteli J, Kauppila A. Comparison of Carboxyterminal Telopeptide of Type I Collagen (ICTP) and CA 125 as Predictors of Prognosis in Ovarian Cancer. Anticancer Res (2004) 24(2C):1057–62.
35. Nakamura K, Nagasaka T, Nishida T, Haruma T, Ogawa C, Kusumoto T, et al. Neutrophil to Lymphocyte Ratio in the Pre-Treatment Phase of Final-Line Chemotherapy Predicts the Outcome of Patients With Recurrent Ovarian Cancer. Oncol Lett (2016) 11(6):3975–81. doi: 10.3892/ol.2016.4513
36. Kang JH, Lai YL, Cheng WF, Kim HS, Kuo KT, Chen YL, et al. Clinical Factors Associated With Prognosis in Low-Grade Serous Ovarian Carcinoma: Experiences at Two Large Academic Institutions in Korea and Taiwan. Sci Rep (2020) 10(1):20012. doi: 10.1038/s41598-020-77075-1
37. Potenza E, Parpinel G, Laudani ME, Macchi C, Fuso L, Zola P. Prognostic and Predictive Value of Combined HE-4 and CA-125 Biomarkers During Chemotherapy in Patients With Epithelial Ovarian Cancer. Int J Biol Markers (2020) 35(4):20–7. doi: 10.1177/1724600820955195
38. Kim SI, Jung M, Dan K, Lee S, Lee C, Kim HS, et al. Proteomic Discovery of Biomarkers to Predict Prognosis of High-Grade Serous Ovarian Carcinoma. Cancers (Basel) (2020) 12(4):790. doi: 10.3390/cancers12040790
39. Yin BW, Lloyd KO. Molecular Cloning of the CA-125 Ovarian Cancer Antigen: Identification as a New Mucin, MUC16. J Biol Chem vol (2001) 276:29. doi: 10.1074/jbc.M103554200
40. Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of Ovarian Cancer Antigen CA-125/MUC16 to Mesothelin Mediates Cell Adhesion. J Biol Chem (2004) 279(10):9190–8. doi: 10.1074/jbc.M312372200
41. Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al. Mesothelin-MUC16 Binding Is a High Affinity, N-Glycan Dependent Interaction That Facilitates Peritoneal Metastasis of Ovarian Tumors. Mol Cancer (2006) 5(1):50. doi: 10.1186/1476-4598-5-50
42. Boivin M, Lane D, Piché A, Rancourt C. CA-125 (MUC16) Tumor Antigen Selectively Modulates the Sensitivity of Ovarian Cancer Cells to Genotoxic Drug-Induced Apoptosis. Gynecol Oncol (2009) 115(3):407–13. doi: 10.1016/j.ygyno.2009.08.007
43. Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, André S, et al. Identification of Siglec-9 as the Receptor for MUC16 on Human NK Cells, B Cells, and Monocytes. Mol Cancer (2010) 9:118. doi: 10.1186/1476-4598-9-118
44. Belisle JA, Gubbels JA, Raphael CA, Migneault M, Rancourt C, Connor JP, et al. Peritoneal Natural Killer Cells From Epithelial Ovarian Cancer Patients Show an Altered Phenotype and Bind to the Tumour Marker MUC16 (Ca125). Immunology (2007) 122(3):418–29. doi: 10.1111/j.1365-2567.2007.02660.x
45. Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, et al. Potent Suppression of Natural Killer Cell Response Mediated by the Ovarian Tumor Marker CA125. Gynecol Oncol (2005) 99(3):704–13. doi: 10.1016/j.ygyno.2005.07.030
46. Huo Q, Xu C, Shao Y, Yu Q, Huang L, Liu Y, et al. Free CA-125 Promotes Ovarian Cancer Cell Migration and Tumor Metastasis by Binding Mesothelin to Reduce DKK1 Expression and Activate the SGK3/FOXO3 Pathway. Int J Biol Sci (2021) 17(2):574–88. doi: 10.7150/ijbs.52097
47. Nicolaides NC, Schweizer C, Somers EB, Wang W, Fernando S, Ross EN, et al. CA-125 Suppresses Amatuximab 32. Immune-Effector Function and Elevated Serum Levels Are Associated With Reduced Clinical Response in First Line Mesothelioma Patients. Cancer Biol Ther (2018) 19(7):622–30. doi: 10.1080/15384047.2018.1449614
48. Shimizu A, Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, et al. Coexpression of MUC16 and Mesothelin Is Related to the Invasion Process in Pancreatic Ductal Adenocarcinoma. Cancer Sci (2012) 103(4):739–46. doi: 10.1111/j.1349-7006.2012.02214.x
49. Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci Rep (2013) 3:1870. doi: 10.1038/srep01870
50. Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, Koriyama C, et al. Pathobiological Implications of MUC16/CA125 Expression in Intrahepatic Cholangiocarcinoma-Mass Forming Type. Pathobiology (2012) 79(2):101–6. doi: 10.1159/000335164
51. Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, et al. Oncogenic KRAS Targets MUC16/CA125 in Pancreatic Ductal Adenocarcinoma. Mol Cancer Res (2017) 15(2):201–12. doi: 10.1158/1541-7786.MCR-16-0296
52. Kikkawa F, Shibata K, Ino K, Nomura S, Kajiyama H, Suzuki T, et al. Preoperative Findings in Non-Gynecologic Carcinomas Metastasizing to the Ovaries. Gynecol Obstet Invest (2002) 54(4):221–7. doi: 10.1159/000068388
53. Nagtegaal SHJ, Hulsbergen AFC, van Dorst EBL, Kavouridis VK, Jessurun CAC, Broekman MLD, et al. Age, Pathology and CA-125 Are Prognostic Factors for Survival in Patients With Brain Metastases From Gynaecological Tumours. Clin Transl Radiat Oncol (2020) 24:11–5. doi: 10.1016/j.ctro.2020.05.001
54. Laukhtina E, Pradere B, Mori K, Schuettfort VM, Quhal F, Mostafaei H, et al. Prognostic Blood-Based Biomarkers in Patients Treated With Neoadjuvant Chemotherapy for Urothelial Carcinoma of the Bladder: A Systematic Review. Urol Oncol (2021) 39(8):471–9. doi: 10.1016/j.urolonc.2021.03.005
55. Mehta S, Bhimani N, Gill AJ, Samra JS, Sahni S, Mittal A, et al. Serum Biomarker Panel for Diagnosis and Prognosis of Pancreatic Ductal Adenocarcinomas. Front Oncol (2021) 11:708963. doi: 10.3389/fonc.2021.708963
56. Grankvist K, Ljungberg B, Rasmuson T. Evaluation of Five Glycoprotein Tumour Markers (CEA, CA-50, CA-19-9, CA-125, CA-15-3) for the Prognosis of Renal-Cell Carcinoma. Int J Cancer (1997) 74(2):233–6. doi: 10.1002/(sici)1097-0215(19970422)74:2<233::aid-ijc17>3.0.co;2-e
57. Chen X, Zhang J, Cheng W, Chang DY, Huang J, Wang X, et al. CA-125 Level as a Prognostic Indicator in Type I and Type II Epithelial Ovarian Cancer. Int J Gynecol Cancer (2013) 23(5):815–22. doi: 10.1097/IGC.0b013e31828f7a24
58. Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2016) 34(28):3460–73. doi: 10.1200/JCO.2016.68.6907
59. Merlo S, Besic N, Drmota E, Kovacevic N. Preoperative Serum CA-125 Level as a Predictor for the Extent of Cytoreduction in Patients With Advanced Stage Epithelial Ovarian Cancer. Radiol Oncol (2021) 55(3):341–6. doi: 10.2478/raon-2021-0013
Keywords: CA-125, ovarian cancer, prognosis, meta-analysis, systematic review, neoadjuvant chemotherapy, overall survival, progress-free survival
Citation: Wang Q, Feng X, Liu X and Zhu S (2022) Prognostic Value of Elevated Pre-treatment Serum CA-125 in Epithelial Ovarian Cancer: A Meta-Analysis. Front. Oncol. 12:868061. doi: 10.3389/fonc.2022.868061
Received: 02 February 2022; Accepted: 11 March 2022;
Published: 07 April 2022.
Edited by:
Andrea Tinelli, Moscow Institute of Physics and Technology, RussiaReviewed by:
Fuat Demirkiran, Istanbul University-Cerrahpasa, TurkeyCarlo Ronsini, Università degli Studi della Campania “Luigi Vanvitelli”, Italy
Copyright © 2022 Wang, Feng, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Feng, RG9jdG9yZnhsQDE2My5jb20=
 Qingyi Wang
Qingyi Wang Xiaoling Feng
Xiaoling Feng Xiaofang Liu1
Xiaofang Liu1