
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 14 April 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.868034
This article is part of the Research Topic Enhancing Chemo/Radiosensitivity in Hepato-Pancreatic Biliary Cancers View all 6 articles
Cholangiocarcinoma (CCA) originates from the epithelium of the bile duct and is highly malignant with a poor prognosis. Radical resection is the only treatment option to completely cure primary CCA. Due to the insidious onset of CCA, most patients are already in an advanced stage at the time of the initial diagnosis and may lose the chance of radical surgery. Radiotherapy is an important method of local treatment, which plays a crucial role in preoperative neoadjuvant therapy, postoperative adjuvant therapy, and palliative treatment of locally advanced lesions. However, there is still no unified and clear recommendation on the timing, delineating the range of target area, and the radiotherapy dose for CCA. This article reviews recent clinical studies on CCA, including the timing of radiotherapy, delineation of the target area, and dose of radiotherapy. Further, we summarize large fraction radiotherapy (stereotactic body radiotherapy [SBRT]; proton therapy) in CCA and the development of immunotherapy and the use of targeted drugs combined with radiotherapy.
Cholangiocarcinoma (CCA) is the second-largest hepatic malignancy after hepatocellular carcinoma (HCC), and recently, its incidence is increasing each year. The incidence of CCA varies geographically (1, 2), the incidence in Southeast Asian countries is much higher than that in Western countries. Currently, the reported incidence in China is approximately 6/100,000 people per year (3). According to the anatomical location of tumor occurrence, CCA can be divided into intrahepatic cholangiocarcinoma (ICCA) and extrahepatic cholangiocarcinoma (ECCA). ECCA can be further divided into perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA) (4). CCA has a high degree of malignancy and poor prognosis. The 5-year survival rate of ECCA is 17%, while the 5-year survival rate of ICCA is only 5% (5).
Radical resection is the only cure for primary CCA (4). The method of surgical resection and the area of lymph node dissection depends on the site and the extent of tumor involvement (6). Since the clinical symptoms of CCA lack specificity, most patients would have reached the advanced stage of the disease at the time of diagnosis and lost the chance of radical surgery. Even at an early stage, the postoperative recurrence rates are high (7). Radiotherapy is an important means of local control, known as “the invisible scalpel”. In recent years, with the advancements in imaging and radiotherapy equipment and the progress of radiotherapy technology, the status of radiotherapy in the treatment of CCA has been greatly enhanced, and the importance of radiotherapy has also been clarified in recent studies. This article reviews recent clinical studies on CCA, including the timing of radiotherapy, delineation of the target area, and dose of radiotherapy. Further, we summarize large fraction radiotherapy [stereotactic body radiotherapy (SBRT); proton therapy] in CCA and the development of immunotherapy and the use of targeted drugs combined with radiotherapy.
CCA is highly malignant and progresses rapidly. Although surgery is the only treatment that can achieve a radical cure, radical resection is not advised for all patients, resulting in a 5-year survival rate of only approximately 30% after surgery (3, 4). As early as 2002, Nakeeb et al. conducted a retrospective study using the data of the 10-year postoperative follow-up of patients with ICCA or ECCA and found that the positive postoperative resection margin and lymph node metastasis were vital factors affecting the progression-free survival (PFS) time after surgery for CCA (8). At the same time, postoperative adjuvant chemoradiotherapy resulted in a significant survival benefit compared with surgery alone. Subsequently, Horgan et al. analyzed 20 studies on adjuvant therapy for CCA, and the meta-analysis found that adjuvant chemotherapy or adjuvant chemoradiotherapy had a better survival benefit than surgery alone for patients with positive lymph nodes and R1 resection (OR, 0.49; P ≤ 0.004 and OR, 0.36; P ≤ 0.002) (9). Since then, several retrospective studies or meta-analyses based on ICCA and ECCA have confirmed the crucial role of postoperative adjuvant concurrent chemoradiotherapy in prolonging survival and reducing the risk of death in patients with positive margins or positive lymph nodes (10–13). Therefore, existing guidelines and consensus recommend that postoperative adjuvant radiotherapy or chemoradiotherapy should be considered for ICCA and ECCA patients with positive margins or regional lymph nodes (14).
In addition, there is another landmark study in the history of postoperative adjuvant therapy for CCA, namely, the phase II clinical study, SWOG S0809 (15). To date, this is the only prospective study of adjuvant radiotherapy after CCA. A total of 79 patients with advanced CCA with a postoperative staging of pT2–4, N+ were enrolled in this study, and 25 of them underwent R1 resection. After surgery, all patients received postoperative adjuvant chemotherapy consisting of gemcitabine combined with capecitabine for 2–4 cycles, followed by capecitabine-based concurrent chemoradiotherapy. The dose of radiotherapy was 45 Gy in the lymphatic drainage area and increased to 54 Gy in the tumor bed. For R1 resection, the local dose could be increased to 59.4 Gy. The results showed that the median overall survival (OS) of postoperative radiotherapy for R0 was 34 months, which was higher than that reported in historical studies. Meanwhile, the median OS of R1 patients receiving radiotherapy after surgery was 35 months, and there was no difference in postoperative survival between R1 patients and R0 patients. These results suggest that postoperative radiotherapy and chemotherapy can not only effectively improve the survival of patients undergoing R1 resection but also bring survival benefits to patients in local advanced stages, such as T3 and 4.
Based on the above findings, the 2021 Chinese Society of Clinical Oncology (CSCO) guidelines suggest that postoperative adjuvant radiotherapy or chemoradiotherapy should be considered in ICCA and ECCA patients with positive margins or regional lymph nodes. Meanwhile, it is recommended that postoperative adjuvant radiotherapy should also be considered for patients with postoperative stage PT3–4 for ECCA, but this is not a priority recommendation (14).
Orthotopic liver transplantation (OLT) is an optional treatment method for advanced ICCA, but previous studies have shown that liver transplantation alone could not provide a clear survival benefit. In 1993, the Mayo Clinic introduced neoadjuvant radiotherapy in 19 ICCA patients before OLT, which was followed by external irradiation of 45 Gy followed by intracavitary supplementation of 20–30 Gy, combined with 5-Fu sensitization, followed by 5-Fu chemotherapy until OLT. OLT was successfully implemented in 11 patients; the disease-free survival (DFS) at 3 years was 92% with a median follow-up of 44 months (16). Subsequently, a retrospective analysis of 37 patients with advanced ICCA undergoing OLT from 1985 to 2009 at the Mayo Clinic found that neoadjuvant chemotherapy or chemoradiotherapy resulted in significant survival benefits compared to postoperative adjuvant or surgery alone (5-year DFS: 47% vs. 33% vs. 20%, P=0.03) (17). At the same time, UCLA (the University of California, Los Angeles) Cancer Center conducted a follow-up analysis of the prognosis of ICCA patients undergoing OLT and found that in addition to tumor biological characteristics, lymph node metastasis, and distal metastasis were important factors affecting prognosis after OLT, and neoadjuvant therapy was also an independent factor affecting prognosis. Thus, neoadjuvant chemoradiotherapy is recommended for patients with high recurrence risks. For patients with a tumor length ≤ 6 cm, conventional radiotherapy or short-course large fraction radiotherapy (SBRT 40 Gy/5F) can be chosen based on the experience of the UCLA Cancer Center. While, For tumors larger than 6 cm in length, transarterial chemoembolization is suggested to shrink tumors first (18). These results suggest that neoadjuvant chemoradiotherapy combined with liver transplantation is a new treatment option for advanced ICCA. In addition to OLT, neoadjuvant chemoradiotherapy can also allow patients with initially unresectable locally advanced cholangiocarcinoma to be reclassified as surgical candidates in a substantial proportion. A study conducted by Sumiyoshi et al. found that conventional radiotherapy with a dose of 50 Gy/25F combined with chemotherapy such as S1 or CPT11 for advanced ICCA resulted in a partial response (PR) rate of 57.1%, a radical tumor resection rate of 71%, and a postoperative median DFS of 21.5 months (19). Given this, the CSCO guidelines suggest that neoadjuvant radiotherapy and chemotherapy should be considered for ICCA under the following conditions: intrahepatic lesion ≤ 6 cm, intrahepatic lesion, and lymph node metastasis are within the surgical resection range. In such cases, a conventional radiotherapy dose of 45–50.4 Gy/25–28F or SBRT dose of 40 Gy/5F can be administered; fluorouracil is the main drug in concurrent chemotherapy (14).
The history of neoadjuvant therapy for ECCA dated back to 20 years ago. As early as 1983–1996, the MD Anderson Center conducted a retrospective analysis on the treatment of advanced CCA and found that 9 cases of advanced ECCA were treated with neoadjuvant radiotherapy and chemotherapy, and the radiotherapy dose was 45–50.4 Gy/25–28F combined with 5-FU chemotherapy. The results showed that the R0 resection rate of these 9 patients reached 100%. Pathologic complete response was achieved in 3 cases, suggesting for the first time that neoadjuvant radiotherapy is feasible for advanced ECCA (20). In 2015, Kobayashi et al. conducted a phase I clinical trial of gemcitabine-based neoadjuvant concurrent chemoradiotherapy in patients with advanced ECCA. Twenty-five patients with inoperable advanced ECCA were enrolled in this study. After neoadjuvant chemoradiotherapy, the R0 resection rate was 96%, the 3-year survival rate was 74.6%, and the toxicities associated with treatment were low. This study further confirms the efficacy and safety of neoadjuvant chemoradiotherapy in advanced CCA (21). In a retrospective analysis, Jung et al. and Kobayashi et al. respectively enrolled 57 and 106 ECCA patients with the stage above T3, or with vascular invasion or lymph node metastasis, and administered gemcitabine combined with conventional radiotherapy and compared it with surgery alone. The results showed that neoadjuvant chemoradiotherapy not only effectively achieved preoperative downgrading but also substantially increased DFS and OS (22, 23). Therefore, the CSCO guidelines suggest that preoperative radiotherapy and chemotherapy can be considered for locally advanced ECCA with T3 or higher stages and positive lymph nodes. Based on existing studies, conventional radiotherapy with a dose of 40-45 Gy (1.8–2.0 Gy/F) is recommended as the main treatment, while concurrent chemotherapy drugs recommended are fluorouracil and gemcitabine; further, attention should be paid to relevant toxicity (14).
Although systemic therapy is the preferred treatment for advanced CCA, the prognosis is usually poor, with a median survival of 3 to 9 months (24). Existing studies have shown that symptom-based palliative radiotherapy can alleviate local symptoms and improve local control rates for locally advanced CCA, thereby improving the quality of life of patients with advanced CCA.
Conventional fraction radiotherapy combined with concurrent chemotherapy is a widely accepted palliative therapy. A total of 84 patients with inoperable ICCA were included in the retrospective study conducted by Chen et al. in 2010; 35 of them received external irradiation (dose range of 30–60 Gy; median dose 50 Gy; 1.8–2.0 Gy/F), the median survival (9.5 months vs. 5.1 months, P=0.003) and 1-year survival rate (38.5% vs. 16.4%) were significantly improved in the radiotherapy group compared with the non-radiotherapy group (25). A phase II single-arm study in 2016 included 27 patients with locally advanced ECCA who received gemcitabine combined with cisplatin synchronous radiotherapy (50 Gy). The median follow-up time was 16 months, and the results showed that the 2-year local control rate was 29%, and the 2-year and 3-year OS were 27% and 7%, respectively (26). A prospective exploratory study in South Korea in 2016 enrolled 18 patients with locally advanced CCA who received gemcitabine and cisplatin combined with radiotherapy (45 Gy/25F), and the results showed that the objective response rate of these patients was 27.8%, the median PFS and median OS were 6.8 and 9.6 months, respectively. The result showed that fluorouracil and gemcitabine-based concurrent chemoradiotherapy had a survival benefit in patients with unresectable and metastatic biliary malignancy (27).
Concurrently, existing studies have shown that SBRT has advantages over conventional radiotherapy. In a phase I clinical trial of SBRT conducted by Tse et al. in 2008 for unresectable ICCA patients, the median dose was 32.5 Gy (28.2–48 Gy, 4-9Gy/F), the median survival time was 15.0 months, and the 1-year OS reached 58%, which was significantly improved compared with conventional radiotherapy (28). In a study by Polistina et al. in 2011, the median survival time of hilar CCA patients treated with SBRT (30 Gy/3F) combined with gemcitabine was 35.5 months, and the 2-year OS was 80%, which was much higher than that of patients treated with conventional radiotherapy and chemotherapy alone (29). In 2019, Lee et al. screened 11 studies for the treatment of unresectable advanced CCA, including ECCA and ICCA patients, and the results showed that the overall one-year local control rate was approximately 78.6%, with low radiation-related toxicity (30). Existing studies have shown that the therapeutic efficacy of SBRT is closely related to the biologic equivalent dose (BED) of radiotherapy. A retrospective analysis conducted by Tao et al. in 2016 for unresectable ICCA included 79 patients with a median dose of 58.05 Gy (35–100 Gy/3–30F); the results showed that the high-BED group (> 80.5 Gy) exhibited improved 2-year OS rate (73% vs. 58%) compared to the low-dose group (≤ 80.5 Gy), and confirmed the survival benefit in unresectable CCA patients was associated with dose escalation (31).
Based on the appeal studies, the CSCO guidelines recommend that in patients with unresectable CCA, priority be given to conventional-dose radiotherapy combined with concurrent chemotherapy, especially when extensive lymph node metastasis is present and the radiotherapy target area is large. Patients with localized CCA should be treated with SBRT. The radiation dose in the tumor area and lymphatic drainage area is 45–50.4 Gy, and 1.8–2.0 Gy for a single time. According to patient tolerance, the dose in the tumor area can be increased to 60 Gy or higher, and the dose for organs at risk should be considered in the treatment. For high-dose and low-fraction-radiation therapy such as SBRT, it is recommended to irradiate only the primary tumor and metastatic lymph nodes, and not to include high-risk lymph node drainage areas. At present, there is no unified dose model for SBRT as a standard recommendation, and the dose segmentation can be referred to like 30–50 Gy/3–5F. The determination of single segmentation dose and segmentation time depends on the distance between the target area and organs at risk and the number of organs at risk (14). The current recommendations for radiotherapy for CCA are summarized (Figure 1).
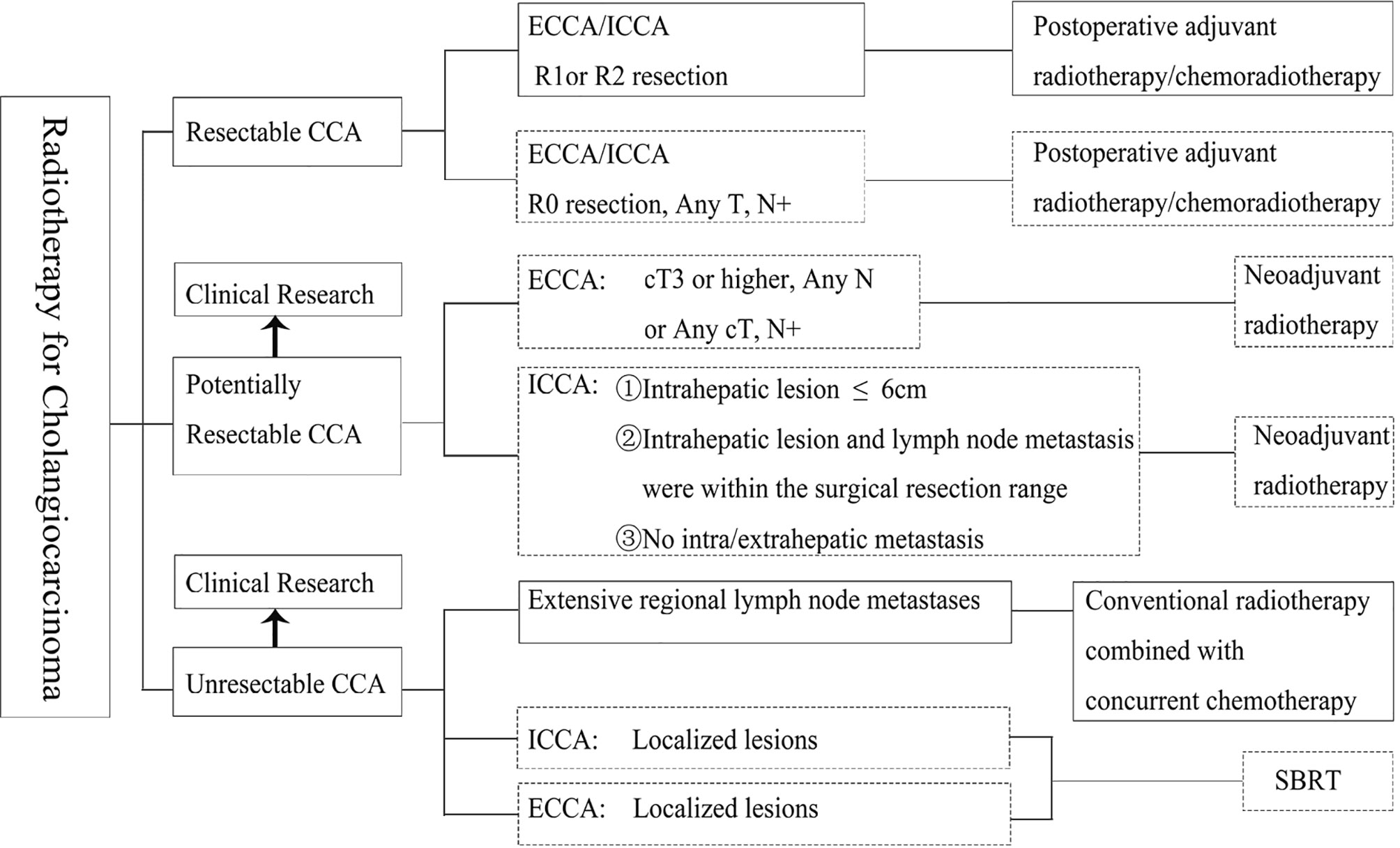
Figure 1 Principles of radiation in different cholangiocarcinoma stages. ECCA, extrahepatic cholangiocarcinoma; ICCA, intrahepatic cholangiocarcinoma; R0, no cancer at resection margins; R1, microscopic residual cancer; R2, macroscopic residual cancer; SBRT, stereotactic body radiation therapy. The boxes with solid lines indicate Category I or II recommendations and the boxes with dotted lines indicate Category III recommendations.
Currently, there is a lack of many prospective studies to guide the decision regarding radiotherapy planning. The timing of radiotherapy depends on the disease status of CCA patients. For patients with resectable CCA, adjuvant radiotherapy should be considered after surgical treatment, and the optimal time to start postoperative adjuvant radiotherapy has not been determined. In SWOG S0809, the phase II clinical trial in 2015, postoperative patients with CCA were treated with gemcitabine combined with capecitabine (biweekly regimen) for 4 cycles, and patients without progression were administered capecitabine combined with radiotherapy (regional lymph node 45 Gy, preoperative tumor bed 54–59.4 Gy), which was widely referenced (15). Among other retrospective studies, in a study on adjuvant radiotherapy for ECCA in 2000, 34 patients were treated with intraoperative radiotherapy, and in 22 of them, external radiotherapy was initiated 4–6 weeks postoperatively (32). In a retrospective study in 2007 that included 34 patients, the median time to start chemoradiotherapy was 53 days (43–62 days) after surgery (33). Therefore, considering the physical recovery of postoperative patients and the results of the current prospective phase II clinical study SWOG S0809 (15) as well as the existing retrospective study results, it can be considered that adjuvant concurrent chemoradiotherapy should be started 8 weeks after surgery. If combined with postoperative adjuvant chemotherapy, 2 to 4 cycles of postoperative adjuvant chemotherapy can be performed first, followed by concurrent chemoradiotherapy (14).
The timing of neoadjuvant radiotherapy for CCA patients with potential resection is inconclusive. It is generally considered to perform the downgrading after neoadjuvant radiotherapy to reevaluate the possibility of surgery, so it needs to be carried out after a multidisciplinary team (MDT) discussion. For patients with unresectable and metastatic CCA, the existing evidence has shown that palliative radiotherapy can improve the quality of life and local control rate in both ICCA and ECCA (31, 34). However, there is no clear definition of the time when palliative radiotherapy should be involved. Based on the existing research, it is recommended that for patients with advanced cholangiocarcinoma, under the condition of acceptable physical condition, palliative radiotherapy is feasible for the lesions with distant organ metastasis, such as liver, lung, bone, and retroperitoneum, when surgery or intervention are impossible, to relieve symptoms and improve local control. The radiotherapy mode (intensity-modulated conformal radiotherapy or SBRT) and the timing of radiotherapy intervention can be implemented with the participation of the MDT (14).
CCA radiotherapy requires accurate definition and delineation of target areas. Accurate delineation of tumor volume can achieve a higher local control rate and reduce radiation-related toxicity. The clinical target volume (CTV) is usually determined based on imaging techniques such as CT or MRI to determine visible tumors. In a 2021 study comparing CT, MR, and PET/MR for CCA target delineation, the gross target volume (GTV) delineated on PET/MR was significantly larger than the target volume delineated on CT and MR, and there was no significant difference between the target delineated on CT and MR. This study showed that compared with CT or MR, targeting CCA based on 18F-FDG PET/MR enables more accurate detection of positive lymph nodes, reducing the risk of missing lymph nodes, and thus accurately defining the GTV (35). However, a unified definition of the target description of CCA is still lacking.
In 2017, Socha et al. further defined the lymph node region of CTV in radiotherapy planning by comparing the postoperative recurrence of the existing research data. In the implementation of past radiotherapy plans, some lymph nodes may be potentially missed, and in ECCA and gallbladder cancer, unnecessary lymph nodes are also included in the target area. therefore, it is necessary to determine the radiotherapy range of high-risk lymph nodes according to the location of the primary tumor (34). In 2017, Marinelli et al. Observed and studied the correlation between the location of primary biliary tract tumor and lymph node involvement rate. They found that for patients with ICCA, the drainage area of high-risk lymph nodes should vary according to the location of primary focus, while for patients with ECCA, the target area should include primary tumor bed and regional lymph nodes, it should be noted that hilar tumors still need to include liver margin and anastomosis (36). Based on the data, the CSCO guidelines recommend that the lymphatic drainage areas be classified according to the tumor site as follows: for ICCA, high-risk lymph node drainage area should include the hilar lymph node, hepatoduodenal lymph node, celiac trunk lymph node, posterior pancreatic head lymph node, mesenteric lymph node, and para-aortic lymph node drainage area. If the primary focal point of ICCA is in the left hepatic lobe, the high-risk lymph node drainage area should include the lesser curvature of the stomach and left gastric lymph node drainage area. For pCCA, it should include the hepatoduodenal lymph nodes, hilar lymph nodes, celiac trunk, epigastric para-aortic lymph nodes and lymph nodes behind the head of the pancreas. While for dCCA, it should include hilar lymph nodes, hepatoduodenal lymph nodes, retro pancreatic lymph nodes, mesenteric lymph nodes, and the abdominal aortic drainage area. For the celiac trunk lymph nodes, their inclusion should be considered based on the imaging evaluation results, considering their low recurrence rate (14, 34, 36). Therefore, the lymph node drainage area at high risk of CCA is shown in Figure 2.
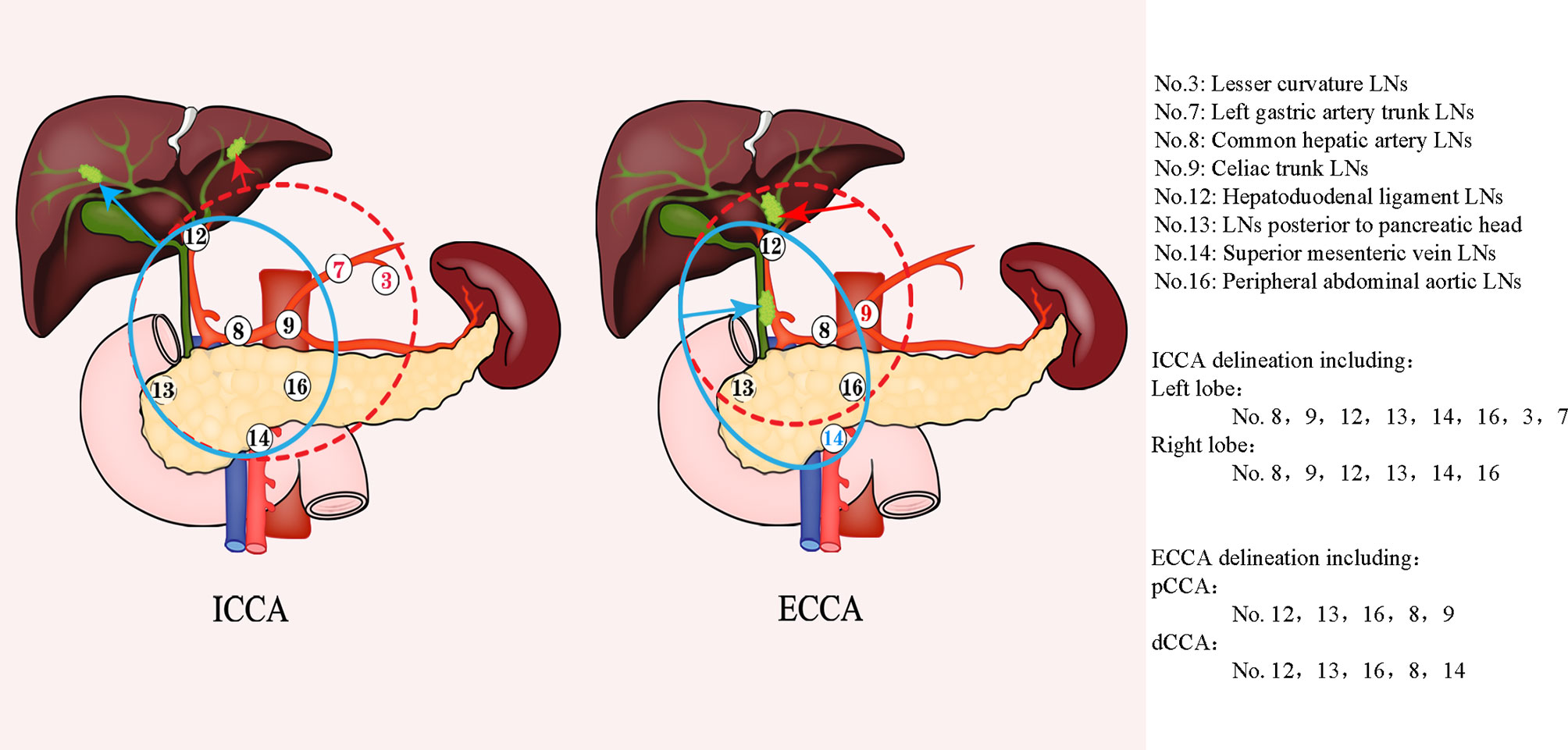
Figure 2 Lymph node delineation in cholangiocarcinoma. The left figure shows the range of LNS with high risk in ICCA, the red dotted line represents the delineation range of LNS for left ICCA, and the blue line represents corresponding LNS for right ICCA; The right figure represents the corresponding the range of LNS with high risk for ECCA, the red dotted line represents the corresponding LNS area for pCCA, and the blue line represents the corresponding lymph node drainage area of dCCA. ECCA, extrahepatic cholangiocarcinoma; ICCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; dCCA, distal cholangiocarcinoma; LNS, lymph node stations.
ICCA usually presents on CT as an unencapsulated homogeneous mass with irregular margins, low density, and irregular peripheral enhancement (37, 38). In 2002, Ebata et al. found that 80 of 253 cases had a microscopically positive margin with a median diffusion distance of 10 mm (39). Similarly, in the 2009 study of Bi et al, analyzed the preoperative imaging and postoperative pathological evaluation and found that the scope of the lesion under the microscope in the pathological evaluation was approximately 0.4–8.0 mm larger than that in imaging (40). Therefore, in the case of conventional radiotherapy, it is suggested that the radiotherapy target area of biliary malignancy should include any tumor area seen in imaging; CTV should be expanded 10 mm based on GTV, the postoperative tumor bed should be included, and anastomosis should be included when the postoperative resection margin is positive. While considering the respiratory mobility and positioning error, PTV should be expanded 5 mm and up and down 7 mm based on CTV. It should be noted that, for high-dose and low-segmentation radiotherapy such as SBRT, it is recommended to irradiate only the primary tumor and metastatic lymph nodes, and not to include the high-risk lymph node drainage areas (14).
Proton radiotherapy can be used as a new technique in the treatment of CCA, thereby protecting normal liver parenchyma and other adjacent organs due to its rapid energy absorption and effective dose reduction (41, 42). Several retrospective and prospective studies have demonstrated superior efficacy of proton therapy in the treatment of cholangiocarcinoma and relevant studies are summarized in Table 1 (43–47). The highest level of evidence to date is a phase II study of high-dose fractionated proton beam therapy for advanced ICCA and HCC in 83 assessable patients, including 37 patients with CCA. The median dose was 58.0 Gray equivalent (GyE). The results showed that the 2-year local control rate of ICCA was 94.1% and the 2-year survival rate was approximately 46.5%, and the incidence of grade 3 or higher radiation-related adverse reactions was only 7.7% in patients with ICCA (45). In 2020, Hung et al. conducted a retrospective analysis of the data of 30 patients with CCA who received proton therapy from 2015 to 2017. The 1-year local control rate of the 30 patients was 88%, and the median PFS was 10.4 months. Among them, 23 patients who received synchronous chemotherapy had a greater survival benefit, and the median PFS was 12.1 months. Meanwhile, the regimen was well tolerated, and the incidence of grade 3 or above adverse reactions was approximately 7% (47). From 2015 to 2017, a prospective study included 30 patients with unresectable CCA, who received large-fraction proton beam therapy with a median dose of 72.6 GyE; the 1-year local control rate was 88%, and 1-year OS was 83% (48). Therefore, compared with conventional radiotherapy, proton therapy has exhibited the advantages of safety and survival benefit.
The development of precision medicine also brings more treatment opportunities for patients with bile duct cancer (49). New drug options are available for advanced CCA, such as the combination of dabrafenib and trametinib has produced promising results for BRAFV600E-mutated CCA (50), and isocitrate dehydrogenase (IDH1) inhibitor has also revealed successful results for biliary tumors (51, 52).
In addition to this, current immunotherapy represented by immune checkpoint inhibitors has shown significant advantages in a variety of malignancies (53). Radiation therapy has a direct cytotoxic effect on tumor cells and can produce certain anti-tumor immune responses by influencing the microenvironment and affecting distant tumor cells by releasing proinflammatory cytokines and chemokines to mobilize systemic immune cells (54). The immunomodulatory effects of radiotherapy have been widely reported in pre-clinical and clinical studies. Radiotherapy releases tumor antigens and facilitates the regulation of immune pathways, increasing tumor antigen presentation, initiating tumor-specific cytotoxic T cells, and enhancing T cell homing. This distant effect can be further enhanced by a combination of radiotherapy and immunization regimens (55). There is preclinical and clinical evidence to indicate that SBRT in combination with immunotherapy is more likely to activate the immune response in the tumor area than conventional radiotherapy (56). In a case report in 2020, a stage IV ICCA patient received radiotherapy combined with 6 cycles of immunotherapy with PD-1 receptor inhibitors to treat lung metastases and intrahepatic lesions. The efficacy of the treatment in the patient was assessed as complete response (CR), and no significant treatment-related adverse reactions were observed. The survival time after combined therapy exceeded 26 months (57). Subsequently, Zhao et al. reported four cases of SBRT combined with immune checkpoint inhibitors in the treatment of ICCA or hilar CCA in 2021, respectively achieving CR, PR, and stable disease, and even one patient who was initially inoperable received surgery (58). Although there is little evidence at present, immunotherapy combined with radiotherapy shows a good prospect, which needs to be further explored. Infiltrating the immune cell distribution and type of bile duct tumor at the same time also has a certain relationship with the prognosis. In EHCC, infiltration of CD4+, CD8+ cytotoxic T lymphocytes, and B lymphocyte/plasma cells could achieve a favorable prognosis, while the increase of macrophages would herald an advanced disease (59). Studies have shown that changes in tumor tissue immune status after radiotherapy are related to therapeutic effect, and patients with persistently high levels of PD-L1 and CD8+ tumor-infiltrating lymphocyte expression in tumor tissues before and after CRT have a poor prognosis (60).
Therefore, the application of immune checkpoint inhibitors or targeted drugs combined with radiotherapy in CCA patients is worthwhile. The relevant clinical studies are summarized in Table 2. Represent studies are the phase II clinical trial of PD-1 inhibitor combined with radiotherapy in the treatment of advanced ICCA in China (NCT03898895), the phase I–II clinical trial of peposertib plus avelumab combined with radiotherapy in the treatment of advanced solid tumors, and hepatobiliary malignancies in the United States (NCT04068194), and the phase I clinical trial (NCT04708067) of bintrafusp alfa combined with high-dose fractionated radiotherapy in the treatment of advanced ICCA. The results of these studies could provide valuable information on such combined therapies.
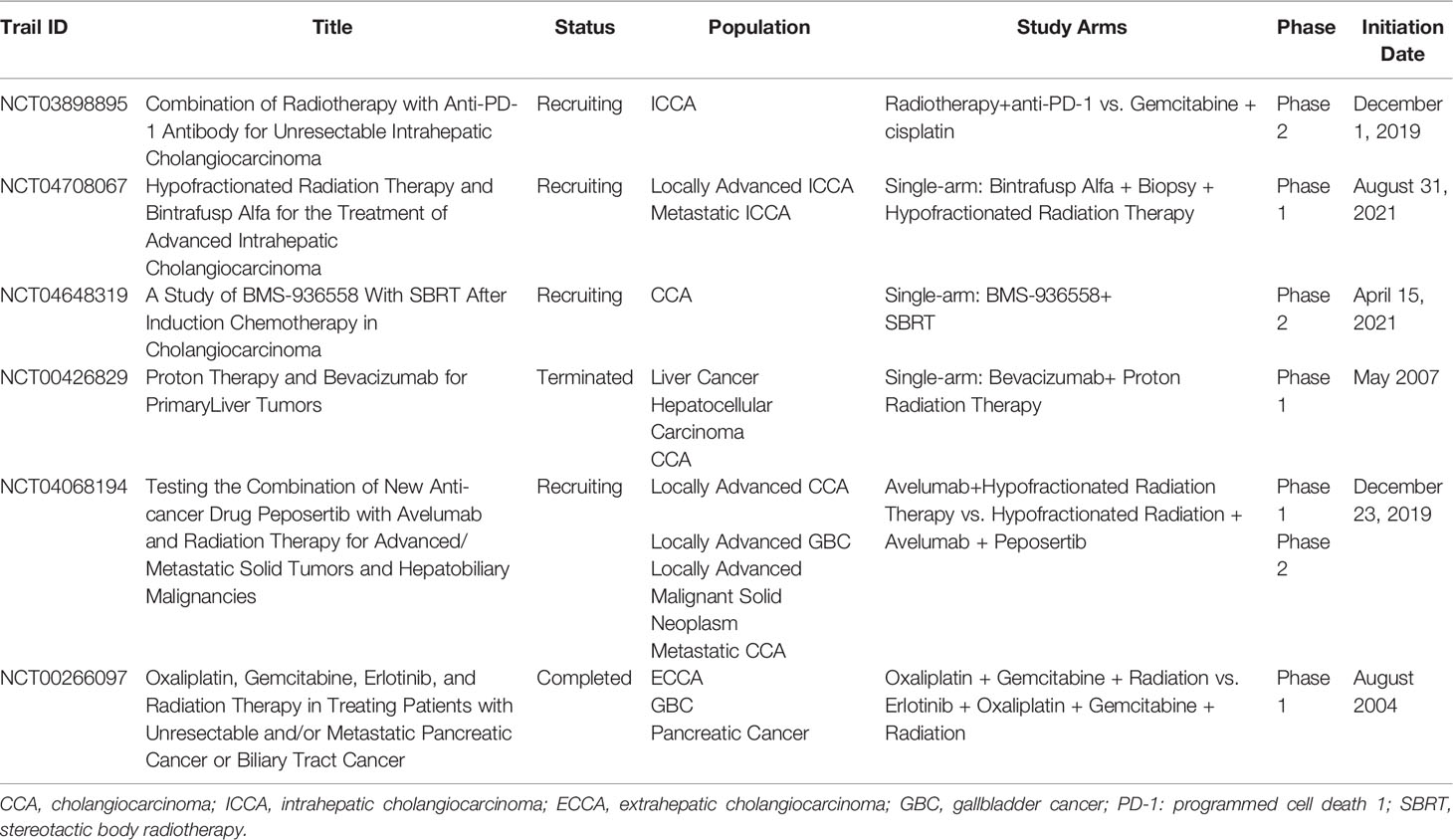
Table 2 Clinical Studies of Immunotherapy or Targeted Therapy Combined with Radiotherapy for cholangiocarcinoma.
The European Society of Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN), and American Society of Clinical Oncology (ASCO) guidelines for hepatobiliary malignancies are still being updated, and the CSCO guidelines for biliary tract cancer have also been launched and updated in recent years. Drug therapy is the mainstay of treatment for advanced cholangiocarcinoma, radiation therapy is still necessary for some patients. The radiotherapy in the existing NCCN, ESMO, and ASCO guidelines is relatively few recommend and lacks a detailed description of radiotherapy mode, dose, and target area. The CSCO guidelines published the first edition of the diagnosis and treatment guidelines for biliary tract cancer in 2020 and elaborated on the relevant content of radiotherapy in detail. In the past two decades, there have been advances in radiotherapy and systemic therapy for CCA, with the survival benefit of postoperative adjuvant radiotherapy for CCA being recognized, and more evidence of SBRT therapy for patients with advanced CCA. The high local recurrence rate in CCA patients with R1 resections provides a basis for postoperative adjuvant radiotherapy. At present, there are many clinical projects under development for CCA radiotherapies, such as a phase III clinical trial in India (NCT02773485) comparing chemotherapy alone with chemotherapy combined with high-dose radiotherapy in the treatment of unresectable CCA, a prospective multicenter study of liver transplantation after neoadjuvant radiotherapy and chemotherapy for unresectable hilar CCA in Spain (NCT04378023), and a phase II trial of SBRT combined with chemotherapy for CCA in Belgium (NCT04648319). It is believed that these clinical projects can provide strong evidence for radiation therapy for CCA.
Nowadays, advances in imaging technology and radiotherapy technology provide prospects for the implementation of precise radiotherapy. In the future, based on tumor biological characteristics, biomarkers, and the direction of tumor microenvironment combined with appropriate imaging and radiotherapy, developing technologies will become the trend in individual precision radiotherapy, further reducing the toxic and side effects of radiotherapy and improving the prognosis of patients with CCA.
HM, YoX and AH contributed to the conception and design of the study. YuX organized the database. NW wrote the first draft of the manuscript. BK, AH, HM, and NW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the Chinese Society of Clinical Oncology (CSCO) (Y-SY201901-0014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the members of the Chinese Society of Clinical Oncology Biliary Oncology Expert Committee under the leadership of Professor Houjie Liang for their efforts to improve the clinical quality in China. We thank all our colleagues from the cancer center, for their kind suggestions and thoughtful discussion. And this manuscript has not been published or presented elsewhere in part or entirety and is not under consideration by another journal.
1. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - Evolving Concepts and Therapeutic Strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
2. Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol (2017) 116:11–31. doi: 10.1016/j.critrevonc.2016.11.012
3. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the Diagnosis and Management of Intrahepatic Cholangiocarcinoma. J Hepatol (2014) 60(6):1268–89. doi: 10.1016/j.jhep.2014.01.021
4. Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver (2017) 11(1):13–26. doi: 10.5009/gnl15568
5. Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, et al. Burden and Centralised Treatment in Europe of Rare Tumours: Results of RARECAREnet—A Population-Based Study. Lancet Oncol (2017) 18(8):1022–39. doi: 10.1016/S1470-2045(17)30445-X
6. Gunasekaran G, Bekki Y, Lourdusamy V, Schwartz M. Surgical Treatments of Hepatobiliary Cancers. Hepatology (2021) 73 Suppl 1:128–36. doi: 10.1002/hep.31325
7. Lamarca A, Edeline J, McNamara MG, Hubner RA, Nagino M, Bridgewater J, et al. Current Standards and Future Perspectives in Adjuvant Treatment for Biliary Tract Cancers. Cancer Treat Rev (2020) 84:101936. doi: 10.1016/j.ctrv.2019.101936
8. Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, et al. Improved Survival in Resected Biliary Malignancies. Surgery (2002) 132(4):555–63; discission 63-4. doi: 10.1067/msy.2002.127555
9. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant Therapy in the Treatment of Biliary Tract Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol (2012) 30(16):1934–40. doi: 10.1200/JCO.2011.40.5381
10. Lee GC, Ferrone CR, Tanabe KK, Lillemoe KD, Blaszkowsky LS, Zhu AX, et al. Predictors of Adjuvant Treatment and Survival in Patients With Intrahepatic Cholangiocarcinoma Who Undergo Resection. Am J Surg (2019) 218(5):959–66. doi: 10.1016/j.amjsurg.2019.02.036
11. Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The Effect of Adjuvant Therapy for Patients With Intrahepatic Cholangiocarcinoma After Surgical Resection: A Systematic Review and Meta-Analysis. PloS One (2020) 15(2):e0229292. doi: 10.1371/journal.pone.0229292
12. Ren B, Guo Q, Yang Y, Liu L, Wei S, Chen W, et al. A Meta-Analysis of the Efficacy of Postoperative Adjuvant Radiotherapy Versus No Radiotherapy for Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. Radiat Oncol (2020) 15(1):15. doi: 10.1186/s13014-020-1459-x
13. Bonet Beltran M, Allal AS, Gich I, Sole JM, Carrio I. Is Adjuvant Radiotherapy Needed After Curative Resection of Extrahepatic Biliary Tract Cancers? A Systematic Review With a Meta-Analysis of Observational Studies. Cancer Treat Rev (2012) 38(2):111–9. doi: 10.1016/j.ctrv.2011.05.003
14. Houjie L, Feng S, Shukui Q, Feng B, Guanghai D, Enxiao L, et al. Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Biliary Tract Cancer 2021. Beijing: People’s Medical Publishing House (2021).
15. Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol (2015) 33(24):2617–22. doi: 10.1200/JCO.2014.60.2219
16. De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, et al. Prolonged Disease-Free Survival After Orthotopic Liver Transplantation Plus Adjuvant Chemoirradiation for Cholangiocarcinoma. Liver Transpl (2000) 6(3):309–16. doi: 10.1053/lv.2000.6143
17. Hong JC, Jones CM, Duffy JP, Petrowsky H, Farmer DG, French S, et al. Comparative Analysis of Resection and Liver Transplantation for Intrahepatic and Hilar Cholangiocarcinoma: A 24-Year Experience in a Single Center. Arch Surg (2011) 146(6):683–9. doi: 10.1001/archsurg.2011.116
18. Sandler KA, Veruttipong D, Agopian VG, Finn RS, Hong JC, Kaldas FM, et al. Stereotactic Body Radiotherapy (SBRT) for Locally Advanced Extrahepatic and Intrahepatic Cholangiocarcinoma. Adv Radiat Oncol (2016) 1(4):237–43. doi: 10.1016/j.adro.2016.10.008
19. Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J Surg (2018) 42(9):2910–8. doi: 10.1007/s00268-018-4558-1
20. McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, et al. Neoadjuvant Chemoradiation for Extrahepatic Cholangiocarcinoma. Am J Surg (1997) 174(6):605–8; discussion 8-9. doi: 10.1016/S0002-9610(97)00203-1
21. Kobayashi S, Tomokuni A, Gotoh K, Takahashi H, Akita H, Marubashi S, et al. Evaluation of the Safety and Pathological Effects of Neoadjuvant Full-Dose Gemcitabine Combination Radiation Therapy in Patients With Biliary Tract Cancer. Cancer Chemother Pharmacol (2015) 76(6):1191–8. doi: 10.1007/s00280-015-2908-3
22. Jung JH, Lee HJ, Lee HS, Jo JH, Cho IR, Chung MJ, et al. Benefit of Neoadjuvant Concurrent Chemoradiotherapy for Locally Advanced Perihilar Cholangiocarcinoma. World J Gastroenterol (2017) 23(18):3301–8. doi: 10.3748/wjg.v23.i18.3301
23. Kobayashi S, Tomokuni A, Gotoh K, Takahashi H, Akita H, Marubashi S, et al. A Retrospective Analysis of the Clinical Effects of Neoadjuvant Combination Therapy With Full-Dose Gemcitabine and Radiation Therapy in Patients With Biliary Tract Cancer. Eur J Surg Oncol (2017) 43(4):763–71. doi: 10.1016/j.ejso.2016.12.008
24. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin Plus Gemcitabine Versus Gemcitabine for Biliary Tract Cancer. N Engl J Med (2010) 362(14):1273–81. doi: 10.1056/NEJMoa0908721
25. Chen YX, Zeng ZC, Tang ZY, Fan J, Zhou J, Jiang W, et al. Determining the Role of External Beam Radiotherapy in Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Analysis of 84 Patients. BMC Cancer (2010) 10:492. doi: 10.1186/1471-2407-10-492
26. Autorino R, Mattiucci GC, Ardito F, Balducci M, Deodato F, Macchia G, et al. Radiochemotherapy With Gemcitabine in Unresectable Extrahepatic Cholangiocarcinoma: Long-Term Results of a Phase II Study. Anticancer Res (2016) 36(2):737–40.
27. Lee KJ, Yi SW, Cha J, Seong J, Bang S, Song SY, et al. A Pilot Study of Concurrent Chemoradiotherapy With Gemcitabine and Cisplatin in Patients With Locally Advanced Biliary Tract Cancer. Cancer Chemother Pharmacol (2016) 78(4):841–6. doi: 10.1007/s00280-016-3143-2
28. Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I Study of Individualized Stereotactic Body Radiotherapy for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol (2008) 26(4):657–64. doi: 10.1200/JCO.2007.14.3529
29. Polistina FA, Guglielmi R, Baiocchi C, Francescon P, Scalchi P, Febbraro A, et al. Chemoradiation Treatment With Gemcitabine Plus Stereotactic Body Radiotherapy for Unresectable, Non-Metastatic, Locally Advanced Hilar Cholangiocarcinoma. Results of a Five Year Experience. Radiother Oncol (2011) 99(2):120–3. doi: 10.1016/j.radonc.2011.05.016
30. Lee J, Yoon WS, Koom WS, Rim CH. Efficacy of Stereotactic Body Radiotherapy for Unresectable or Recurrent Cholangiocarcinoma: A Meta-Analysis and Systematic Review. Strahlenther Onkol (2019) 195(2):93–102. doi: 10.1007/s00066-018-1367-2
31. Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol (2016) 34(3):219–26. doi: 10.1200/JCO.2015.61.3778
32. Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, et al. Benefits of Adjuvant Radiotherapy After Radical Resection of Locally Advanced Main Hepatic Duct Carcinoma. Int J Radiat Oncol Biol Phys (2000) 46(3):581–7. doi: 10.1016/S0360-3016(99)00472-1
33. Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, et al. Adjuvant Concurrent Chemoradiation for Adenocarcinoma of the Distal Common Bile Duct. Int J Radiat Oncol Biol Phys (2007) 68(1):178–82. doi: 10.1016/j.ijrobp.2006.11.048
34. Socha J, Michalak M, Wolakiewicz G, Kepka L. Nodal Areas of Potential Geographic Error in Adjuvant Radiotherapy for Biliary Tract Cancer. Radiother Oncol (2017) 125(2):365–73. doi: 10.1016/j.radonc.2017.09.025
35. Delaby G, Ataeinia B, Wo J, Catalano OA, Heidari P. Impact of 18F-FDG PET/MR Based Tumor Delineation in Radiotherapy Planning for Cholangiocarcinoma. Abdom Radiol (NY) (2021) 46(8):3908–16. doi: 10.1007/s00261-021-03053-4
36. Marinelli I, Guido A, Fuccio L, Farioli A, Panni V, Giaccherini L, et al. Clinical Target Volume in Biliary Carcinoma: A Systematic Review of Pathological Studies. Anticancer Res (2017) 37(3):955–61. doi: 10.21873/anticanres.11404
37. Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, et al. Varying Appearances of Cholangiocarcinoma: Radiologic-Pathologic Correlation. Radiographics (2009) 29(3):683–700. doi: 10.1148/rg.293085729
38. Joo I, Lee JM, Yoon JH. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology (2018) 288(1):7–13. doi: 10.1148/radiol.2018171187
39. Ebata T, Watanabe H, Ajioka Y, Oda K, Nimura Y. Pathological Appraisal of Lines of Resection for Bile Duct Carcinoma. Br J Surg (2002) 89(10):1260–7. doi: 10.1046/j.1365-2168.2002.02211.x
40. Bi AH, Zeng ZC, Ji Y, Zeng HY, Xu C, Tang ZY, et al. Impact Factors for Microinvasion in Intrahepatic Cholangiocarcinoma: A Possible System for Defining Clinical Target Volume. Int J Radiat Oncol Biol Phys (2010) 78(5):1427–36. doi: 10.1016/j.ijrobp.2009.09.069
41. Dawson LA, Ten Haken RK, Lawrence TS. Partial Irradiation of the Liver. Semin Radiat Oncol (2001) 11(3):240–6. doi: 10.1053/srao.2001.23485
42. Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, et al. Proton Beam Therapy for Hepatocellular Carcinoma Adjacent to the Porta Hepatis. Int J Radiat Oncol Biol Phys (2008) 71(2):462–7. doi: 10.1016/j.ijrobp.2007.09.056
43. Makita C, Nakamura T, Takada A, Takayama K, Suzuki M, Ishikawa Y, et al. Clinical Outcomes and Toxicity of Proton Beam Therapy for Advanced Cholangiocarcinoma. Radiat Oncol (2014) 9:26. doi: 10.1186/1748-717X-9-26
44. Ohkawa A, Mizumoto M, Ishikawa H, Abei M, Fukuda K, Hashimoto T, et al. Proton Beam Therapy for Unresectable Intrahepatic Cholangiocarcinoma. J Gastroenterol Hepatol (2015) 30(5):957–63. doi: 10.1111/jgh.12843
45. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol (2016) 34(5):460–8. doi: 10.1200/JCO.2015.64.2710
46. Shimizu S, Okumura T, Oshiro Y, Fukumitsu N, Fukuda K, Ishige K, et al. Clinical Outcomes of Previously Untreated Patients With Unresectable Intrahepatic Cholangiocarcinoma Following Proton Beam Therapy. Radiat Oncol (2019) 14(1):241. doi: 10.1186/s13014-019-1451-5
47. Hung SP, Huang BS, Hsieh CE, Lee CH, Tsang NM, Chang JT, et al. Clinical Outcomes of Patients With Unresectable Cholangiocarcinoma Treated With Proton Beam Therapy. Am J Clin Oncol (2020) 43(3):180–6. doi: 10.1097/COC.0000000000000646
48. Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, et al. Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Semin Liver Dis (2004) 24(2):201–7. doi: 10.1055/s-2004-828896
49. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology (2012) 142(4):1021–31.e15. doi: 10.1053/j.gastro.2011.12.005
50. Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib Plus Trametinib in Patients With BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol (2020) 21(9):1234–43. doi: 10.1016/S1470-2045(20)30321-1
51. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine Alone or in Combination With Cisplatin in Patients With Biliary Tract Cancer: A Comparative Multicentre Study in Japan. Br J Cancer (2010) 103(4):469–74. doi: 10.1038/sj.bjc.6605779
52. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol (2020) 21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1
53. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
54. Demaria S, Formenti SC. Radiation as an Immunological Adjuvant: Current Evidence on Dose and Fractionation. Front Oncol (2012) 2:153. doi: 10.3389/fonc.2012.00153
55. Herrera FG, Bourhis J, Coukos G. Radiotherapy Combination Opportunities Leveraging Immunity for the Next Oncology Practice. CA Cancer J Clin (2017) 67(1):65–85. doi: 10.3322/caac.21358
56. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and Stereotactic Ablative Radiotherapy (ISABR): A Curative Approach? Nat Rev Clin Oncol (2016) 13(8):516–24. doi: 10.1038/nrclinonc.2016.30
57. Liu ZL, Liu X, Peng H, Peng ZW, Long JT, Tang D, et al. Anti-PD-1 Immunotherapy and Radiotherapy for Stage IV Intrahepatic Cholangiocarcinoma: A Case Report. Front Med (Lausanne) (2020) 7:368. doi: 10.3389/fmed.2020.00368
58. Zhao Q, Chen Y, Du S, Yang X, Chen Y, Ji Y, et al. Integration of Radiotherapy With Anti-PD-1 Antibody for the Treatment of Intrahepatic or Hilar Cholangiocarcinoma: Reflection From Four Cases. Cancer Biol Ther (2021) 22(3):175–83. doi: 10.1080/15384047.2020.1834792
59. Marks EI, Yee NS. Immunotherapeutic Approaches in Biliary Tract Carcinoma: Current Status and Emerging Strategies. World J Gastrointest Oncol (2015) 7(11):338–46. doi: 10.4251/wjgo.v7.i11.338
60. Lim YJ, Koh J, Kim S, Jeon SR, Chie EK, Kim K, et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8(+) Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int J Radiat Oncol Biol Phys (2017) 99(5):1216–24. doi: 10.1016/j.ijrobp.2017.07.004
Keywords: cholangiocarcinoma, indications for radiotherapy, target area delineation, radiotherapy dose, radiotherapy mode
Citation: Wang N, Huang A, Kuang B, Xiao Y, Xiao Y and Ma H (2022) Progress in Radiotherapy for Cholangiocarcinoma. Front. Oncol. 12:868034. doi: 10.3389/fonc.2022.868034
Received: 02 February 2022; Accepted: 23 March 2022;
Published: 14 April 2022.
Edited by:
Qian Zhu, Wuhan University, ChinaReviewed by:
Andrea Laurenzi, University Hospital of Bologna Policlinico S. Orsola-Malpighi, ItalyCopyright © 2022 Wang, Huang, Kuang, Xiao, Xiao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ma, MjAwM3hoMTA0M0BodXN0LmVkdS5jbg==; Yong Xiao, MjAwNXhoMDgxNUBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.