- 1Cancer Center, Department of Pulmonary and Critical Care Medicine, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou, China
- 2Department of Medical Oncology, Shouguang Hospital of Traditional Chinese Medicine, Shouguang, China
- 3Division of Cancer, Department of Surgery and Cancer, Imperial College London, London, United Kingdom
- 4Xcovery Holdings, Palm Beach Gardens, FL, United States
- 5Department of Medical Oncology, Lung Cancer and Gastrointestinal Unit, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Changsha, China
- 6Department of Thoracic Surgery, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Anaplastic lymphoma kinase (ALK) alterations in non-small cell lung cancer (NSCLC) can be effectively treated with a variety of ALK-targeted drugs. After the approval of the first-generation ALK inhibitor crizotinib which achieved better results in prolonging the progression-free survival (PFS) compared with chemotherapy, a number of next-generation ALK inhibitors have been developed including ceritinib, alectinib, brigatinib, and ensartinib. Recently, a potent, third-generation ALK inhibitor, lorlatinib, has been approved by the Food and Drug Administration (FDA) for the first-line treatment of ALK-positive (ALK+) NSCLC. These drugs have manageable toxicity profiles. Responses to ALK inhibitors are however often not durable, and acquired resistance can occur as on-target or off-target alterations. Studies are underway to explore the mechanisms of resistance and optimal treatment options beyond progression. Efforts have also been undertaken to develop further generations of ALK inhibitors. This review will summarize the current situation of targeting the ALK signaling pathway.
1 Background
1.1 ALK Signaling Pathway
NSCLC accounts for around 80% of lung cancers, with ALK+ NSCLC accounting for 3%–7% of these (1). ALK is a proto-oncogene which encodes anaplastic lymphoma kinase that is primarily expressed in the nervous system. ALK signaling is activated in cancer cells primarily through three mechanisms: gene fusions, gene amplification, and activating point mutations (2). ALK rearrangements were first identified in 2007 in NSCLC, where the 3′ region of the ALK gene was fused with the 5′ sequence of the echinoderm microtubule-associated protein-like 4 (EML4) gene. The rearrangement results in the expression of the EML4-ALK fusion protein (3). Many kinds of ALK fusion genes have been found in multiple cancer types (4). In ALK fusions, the partner drives ALK activity at the level of gene expression and through multimerization of the ALK kinase domain, which is presumed to promote several biological functions including cell differentiation, proliferation, and anti-apoptosis (5). ALK can activate signaling cascades, such as the mitogen-activated protein kinase (MAPK), (phosphatidylinositol 3−kinase) PI3K/(protein kinase B) AKT, MEK/ERK kinase 2/3 (MEKK2/3), Crk-like/CRK SH3 domain-binding guanine nucleotide-releasing factor (CRKL/C3G), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and mitogen-activated protein kinase kinase 5-extracellular signal-regulated kinase 5 (MEK5-ERK5) pathways (6).
1.2 Diagnosis of ALK Rearrangement
The ALK locus is prone to translocation, and more than 20 different ALK fusion protein partners have been discovered (5). The detection of ALK rearrangements is widely recognized in NSCLC. Different methods are now available, with immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) representing validated diagnostic techniques for the assessment of ALK status (7, 8). As chromogenic in situ hybridization (CISH) allows concurrent analysis of histological features and gene rearrangement of the tumors, it is also a useful method in assessing ALK status (9). Next-generation sequencing (NGS) can detect a fusion between any partners, which makes it advantageous. Multiplexed PCR amplicon-based targeted NGS interrogates fusion transcripts involving many known driver genes and partners (10). Furthermore, NGS is able to assess multiple other genes simultaneously with great sensitivity.
Other than identification of ALK rearrangements from tissue biopsy, non-invasive genotyping of circulating tumor nucleic acids has gained attention as an alternative strategy. Compared to mutations and insertions/deletions, ALK rearrangements are more complex as they incorporate diverse breakpoints and multiple fusion partners (11). As DNA shedding in plasma of patients with advanced disease increases, the sensitivity of ALK fusion detection in ctDNA improves at disease progression (12, 13). The longitudinal ctDNA assays for early detection of disease progression in ALK+ patients receiving treatment is under intense investigation.
1.3 Characteristics of ALK+ NSCLC Patients
ALK+ NSCLC patients tend to be younger, with no smoking history, and have adenocarcinoma as the most common histological subtype (14). A recent meta-analysis confirmed that there is an increased incidence of thromboembolism in ALK+ NSCLC patients as compared to non-ALK+ patients (15). Real-world data also suggested an increased risk of venous thromboembolism in ALK-rearranged NSCLC patients (16, 17).
Advanced ALK+ NSCLC has different imaging features of primary tumor and metastatic patterns from those of EGFR+ or wild-type NSCLC (18). ALK+ NSCLC often presents with central tumor location, large pleural effusion, and absence of a pleural tail (19). ALK+ tumors are also prone to nodal metastasis and lymphangitic carcinomatosis. The radiological features can clinically help discriminate ALK+ from ALK- tumors, but genetic evidence is always required.
1.4 ALK Variants and Fusion Partners
ALK variants have been reported to influence the efficacy of ALK TKIs, but results were inconsistent. A prospective study from Camidge et al. did not find that different ALK variants would impact PFS for first-line alectinib or crizotinib (20). In two other studies, ALK V3a/b had a worse OS (21, 22). A recent study also suggested a prognostic role of ALK variants on treatment outcome (23). In that study, 64 ALK variants were identified in 59 patients, with V1 (32.8%) and V3a/b (28.1%) being the most common. Patients with non-V3a/b showed a trend toward longer OS. Meanwhile, although ALK+ NSCLC patients have a high PD-L1 expression rate, there is no significant association with ALK variant subtypes (23). A meta-analysis suggested that there was no significant difference of patients with the V1 variant from non-V1 in terms of PFS and OS, while V3 was associated with shorter OS (24). However, a propensity score analysis did not find a difference of ALK variants regarding clinical features and outcomes (25), which was consistent with sensitivity of ALK variants to alectinib in ALK-transformed cells (26). The molecular link between ALK variants, the differential response to TKIs, and resistance mutations support NGS-based detection of ALK status to guide treatment strategies (27).
Other than ALK variants, other ALK fusion partners include ATIC-ALK, RANBP2-ALK, NPM1-ALK, TFG-ALK, KIF5B-ALK, SQSTM1-ALK, TPM4-ALK, and CLTC-ALK (28). Their responses to ALK TKIs have been reported in several case reports, some of which were associated with better prognosis (29).
The impact of 5′-ALK on the efficacy of crizotinib was reported (30). Compared with 3′-ALK fusion alone, patients with non-reciprocal/reciprocal ALK translocation had a higher incidence of central nervous system (CNS) metastasis at baseline. Harboring non-reciprocal/reciprocal ALK translocation was an independent predictor of worse PFS for crizotinib-treated ALK\+ NSCLC.
1.5 Treatment Modality
As ALK+ NSCLC is a gene fusion-driven cancer, tyrosine kinase inhibitors (TKIs) have been developed to treat this unique disease. Currently, six ALK-target agents have been approved to treat advanced ALK+ NSCLC, including crizotinib, alectinib, ceritinib, ensartinib, brigatinib, and lorlatinib. These targeted agents induce durable responses and improve survival outcomes. Treatment with ALK inhibitors is recognized as the standard of care for advanced ALK+ NSCLC.
2 ALK Targeted Therapies in NSCLC
2.1 First-Generation ALK TKI
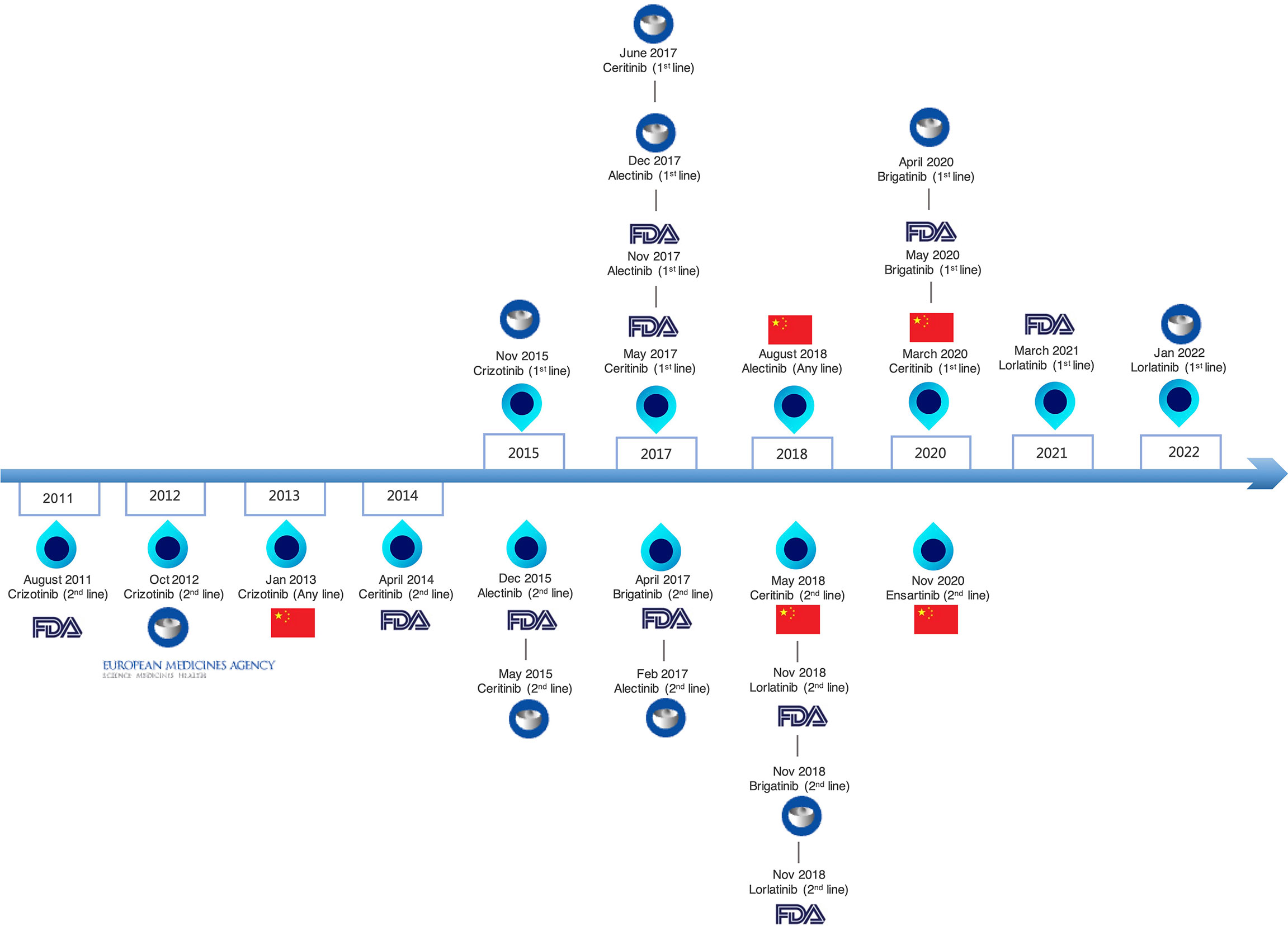
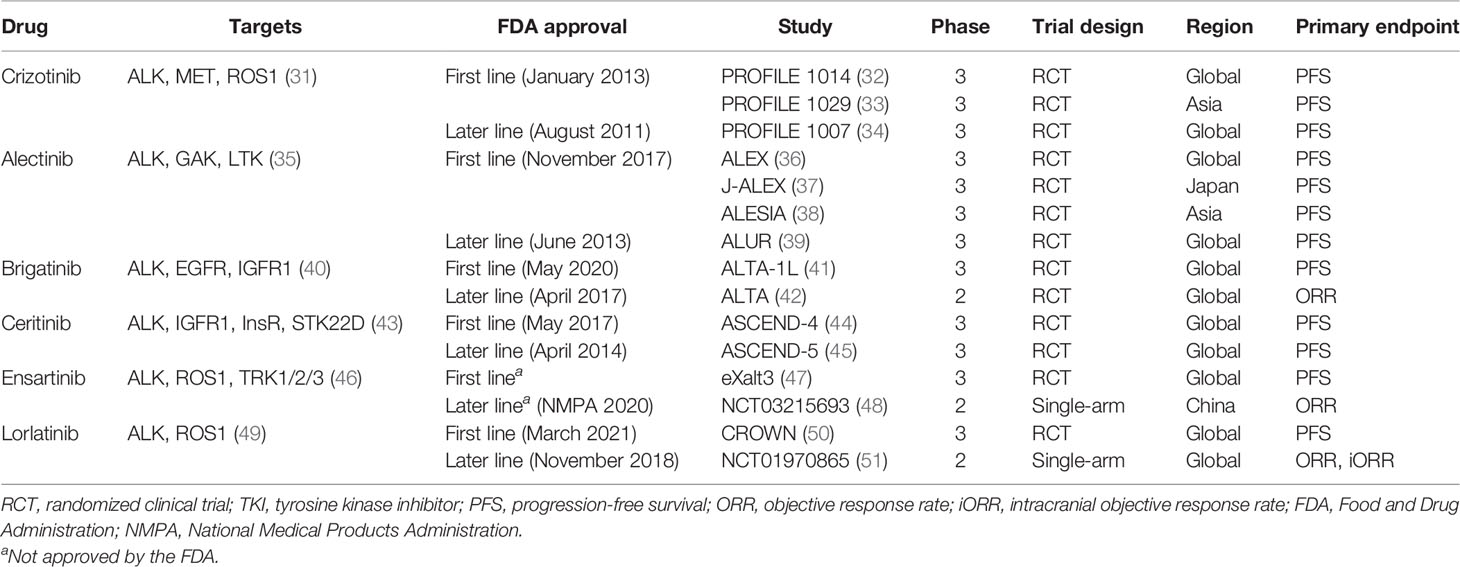
The six currently approved ALK TKIs for advanced ALK+ NSCLC were classified into three generations (Figure 1). The drug targets, approved indication by FDA, trial design, and primary endpoint of clinical trials are summarized in Table 1, which can help illustrate the currently available ALK-TKIs. The development of crizotinib, a first-in-class and first-generation ALK TKI, revolutionized the treatment of ALK+ NSCLC (52). Crizotinib is a small-molecule inhibitor of the receptor tyrosine kinases ALK, ROS1, and c-MET. In phase I and II studies, crizotinib demonstrated durable responses in advanced ALK-positive NSCLC patients (53, 54), leading to the accelerated FDA approval in 2016. In a phase III study PROFILE 1007, crizotinib showed improved PFS compared with chemotherapy in first-line and previously treated patients (34). However, the pharmacokinetic failure to crizotinib is mainly due to its poor blood–brain barrier penetration, and CNS is a common site of progression with crizotinib (55). Crizotinib-treated patients will virtually develop acquired resistance. L1196M, and G1269A, and C1156Y mutations alter the structure of the ATP-binding pocket and thus prevent crizotinib from binding to ALK (56).
2.2 Second-Generation ALK TKIs
The second-generation ALK-TKIs alectinib, ceritinib, ensartinib, and brigatinib were developed to overcome crizotinib resistance, and they exhibited potent activity to crizotinib-resistant ALK+ NSCLC patients.
2.2.1 Alectinib
Alectinib is a next-generation inhibitor that is highly selective for ALK (57). Alectinib, which is not a P-glycoprotein substrate, has a better penetration to the blood–brain barrier compared with crizotinib (58). Alectinib was approved by the FDA for second-line treatment in 2015 based on two single-arm trials (NP28761 and NP28673) including 225 patients treated with alectinib 600 mg orally twice daily (59). The J-ALEX trial was the first study to show that the second-generation ALK inhibitor alectinib provides a PFS advantage and is more tolerable than crizotinib with the dose of 300 mg twice daily (37). Alectinib was approved by the FDA for the first-line treatment of ALK+ NSCLC in 2017 based on the phase III ALEX trial with alectinib 600 mg twice daily (36). In a final analysis of the J-ALEX study, compared to crizotinib, alectinib did not achieve overall survival (OS) benefit (60), reflecting that the crossover to the post first-line treatment might greatly influence OS, especially in ALK+ NSCLC who could get significant benefit from all ALK TKIs. A prospective real-world study investigated the strategy of switching to alectinib in ALK+ NSCLC patients that did not experience disease progression with initial crizotinib (61). The results indicated that an early switch from crizotinib to alectinib might be a viable option and may promote better treatment compliance.
Data from J-ALEX suggested that compared with ALEX wherein 600 mg twice daily was used, alectinib 300 mg twice daily did not produce a markedly different primary outcome of PFS in a Japanese population. Since alectinib 300 mg twice daily will produce fewer adverse events (AEs) and fewer treatment interruptions, the lower dose is therefore an attractive approach in the study population (62). The on-target resistance of the mechanism of alectinib is related with emergence of G1202R and I1171N/S/T mutations (63).
2.2.2 Brigatinib
In preclinical models, brigatinib (AP26113) has been shown to overcome resistance to first- and second-generation ALK TKIs (40). In crizotinib-treated (ALTA trial) and crizotinib-naïve (ALTA-1L trial) patients with ALK+ NSCLC, brigatinib has shown promising antitumor activity, including substantial activity against central nervous system (CNS) metastases (41, 64). In the final analysis of ALTA-1L, brigatinib demonstrated superior efficacy over crizotinib regardless of ALK fusion variant or TP53 mutation status, especially in patients with baseline brain metastases (65). In a network meta-analysis, brigatinib ranked the highest by efficacy in the CNS metastasis subgroup compared with alectinib, while alectinib ranked the highest by efficacy in the overall population (66). In general, brigatinib is well tolerated; however, the early-onset pulmonary toxicity has raised some concerns. The ATOMIC ARI-AT-002 trial (NCT02706626) is ongoing to evaluate the efficacy of brigatinib against ALK-resistant mutations after second-generation ALK inhibitor treatment other than brigatinib in patients with ALK+ NSCLC (67). A phase III ALTA-3 trial (NCT03596866) comparing brigatinib versus alectinib in the first-line ALK+ NSCLC is also ongoing (68).
2.2.3 Ceritinib
Ceritinib obtained FDA approval for the treatment of ALK-positive patients who progressed or were intolerant to crizotinib in 2014, and as a first-line therapy in 2017. Approval was based on the ASCEND-1 (69) and ASCEND-2 studies (70). In the phase II ASCEND-2 study, crizotinib-pretreated ALK+ NSCLC received ceritinib at a standard dose of 750 mg daily and achieved an objective response rate (ORR) of 38.6% (70). A phase I, three-arm ASCEND-8 study demonstrated that ceritinib 450 mg with food showed similar efficacy and less gastrointestinal toxicity compared to 750-mg fasted (71). Two randomized Phase III trials compared ceritinib vs. standard chemotherapy in the first-line (ASCEND-4) (44) or second-line (ASCEND-5) setting (45). However, the toxicity profile of ceritinib from ASCEND-4 and ASCEND-5 indicated a higher frequency of dose interruptions and modifications due to adverse events (AEs) compared to chemotherapy. Real-world data comparing ceritinib versus alectinib in ALK+NSCLC found that alectinib exposure was associated with longer OS compared with ceritinib in ALK+ NSCLC (72). The pharmacokinetic (PK) data from the ASCEND-8 study (71) led to the FDA approval of ceritinib 450 mg QD, administered with food.
2.2.4 Ensartinib
Ensartinib (X-396) is an aminopyridazine-based small molecule that inhibits ALK. Furthermore, ensartinib has reported some activity against ROS1, AXL, and cMET (73). In a phase 1/2 trial, ensartinib has shown promising clinical activity in ALK+ NSCLC (46). A single-arm phase 2 trial investigating ensartinib in second-line ALK+ NSCLC demonstrated an ORR of 52% (48), which led to its approval by the National Medical Products Administration (NMPA) of China. The phase III eXalt3 study comparing ensartinib versus crizotinib for the first-line treatment of ALK+ NSCLC demonstrated that ensartinib is superior to crizotinib in both systemic and intracranial diseases (47). Of note, crossover was not allowed in this trial. A dynamic sequencing of circulating tumor DNA (ctDNA) in ensartinib-resistant ALK+ NSCLC patients revealed that ALK-dependent resistance mechanisms of ensartinib were mainly due to G1269A, G1202R, and E1210K mutations (74).
2.3 Third-Generation ALK TKI
Approximately half of resistance to second-generation ALK-TKIs is associated with secondary mutations in the ALK kinase domain (75). Lorlatinib is a 3rdz-generation ALK TKI and is a small and compact macrocyclic inhibitor. The macrocyclic formation had an improved metabolic stability and a low frequency of P-glycoprotein-mediated efflux in vitro. Diverse compound ALK mutations were identified in lorlatinib-resistant cells or patient samples after sequential ALK-TKI treatments (76, 77). Lorlatinib can inhibit G1202R mutation, but not compound mutations (78). Lorlatinib was approved by the FDA in 2018 for the second- or third-line treatment of ALK+ NSCLC (51). The phase III CROWN study comparing lorlatinib versus crizotinib achieved the best-in-class differential PFS benefit of HR 0.28 (50), which led to its first-line approval of the FDA in March 2021. Crossover was not allowed in the CROWN study. This result may redefine the new potential standard of care in the first-line setting. As there are no head-to-head comparisons of lorlatinib to second-generation ALK TKIs, debates were raised regarding whether lorlatinib is the best first-line treatment for ALK+ NSCLC (79, 80). Compared with alectinib, lorlatinib was associated with a higher incidence of grade 3 or higher AEs (81) mostly related to its higher penetration in the CNS.
2.4 Fourth-Generation ALK TKIs Under Investigation
The sequential use of ALK TKIs which is active to ALK “single mutant” will lead to double ALK resistance mutations. Fourth-generation ALK TKIs such as TPX-0131 and NVL-655 have been developed, which are “double mutant active.” TPX-0131 is a compact macrocyclic inhibitor, which was designed to fit completely in the ATP-binding pocket. It may reduce the susceptibility to a variety of ALK TKI-resistant mutations, including solvent front, hinge region, gatekeeper, and compound mutations (82). Other than being sensitive to most single resistant mutations, TPX-0131 is effective for compound mutations such as G1202R+L1198F, G1202R+L1196M, L1196M+ L1198F, and G1202R+C1156F. Another 4th-generation ALK TKI, NVL-655, is a brain-penetrant small-molecule inhibitor with activity against solvent front drug-resistance mutations, such as G1202R, G1202R+L1196M, and G1202R+G1269A (83). Furthermore, NVL-655 displayed brain penetrance to open up the potential to treat brain metastases while avoiding off-target CNS adverse events.
2.5 Other ALK TKIs
Entrectinib is a selective inhibitor of TRKA/B/C, ALK, and ROS1 (84). Combined results from two phase I/II basket trials (ALKA-372-001 and the STARTRK-1 trial) suggested that entrectinib was well tolerated and active against ALK+ NSCLC (85). A phase II basket trial STARTRK-2 (NCT02568267) is currently ongoing to evaluate entrectinib for the treatment of patients with NTRK, ROS1, and ALK gene rearrangements. Repotrectinib (TPX-0005) is a rationally designed macrocyclic TKI developed to inhibit ALK, ROS-1, and TRKA-C (86). It is smaller than lorlatinib and has a high activity in CNS. The TREDENT-1 study (NCT03093116) for repotrectinib showed encouraging data in ALK+ NSCLC patients (87).
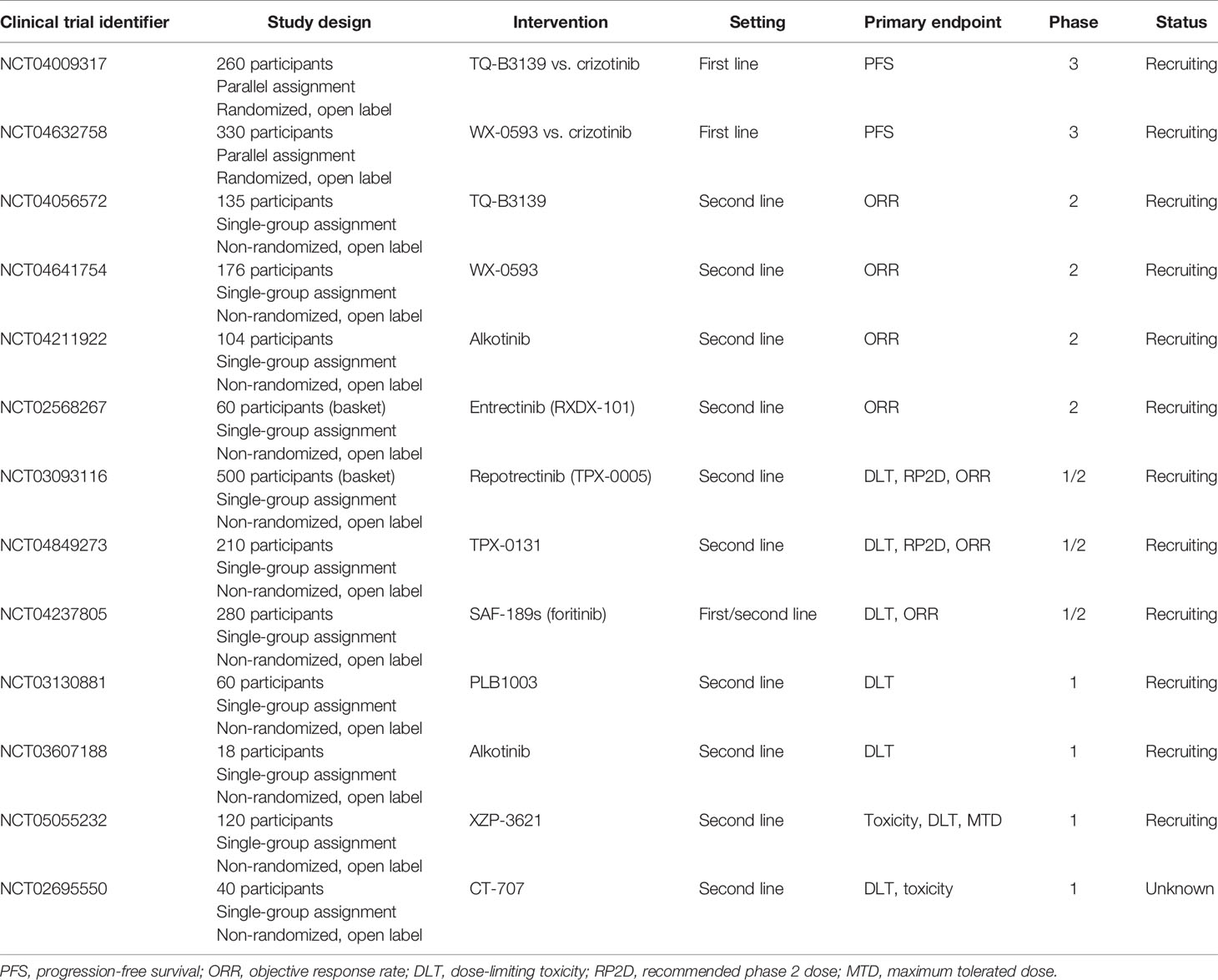
Other novel ALK TKIs include TQ-B3139 (88), WX-0593 (89), PLB-1003 (90), SAF-189s (91), and CT-707 (92). Several other ALK TKIs are under preclinical investigation, such as gilteritinib (93) and XMU-MP-5 (94). An ALK proteolysis-targeting chimeric (PROTAC) degrader is also under development. The six different ALK PROTACs are all based on the second-generation ALK-TKIs, including ceritinib-based (95–98), TAE684-based (96), and brigatinib-based ALK PROTACs (99). During this process, kinase mutations and off-target effects may occur, which is a major clinical challenge (100). The ongoing clinical trials investigating novel-generation ALK TKIs in ALK+ NSCLC are summarized in Table 2 (up to December 18, 2021).
2.6 Treatment Options Other Than ALK TKIs
2.6.1 Chemotherapy
As chemotherapy has limited efficacy in ALK+ NSCLC after failure of a second-generation ALK TKI, combination therapy with ALK TKI and chemotherapy has been proposed in ALK+ NSCLC refractory to at least one second-generation ALK TKI. This strategy has been proved to be a possible choice by several studies. Crizotinib plus pemetrexed in ALK+ NSCLC patients with multiple CNS metastases demonstrated better efficacy than monotherapy (101). Chemotherapy in combination with ALK TKI proved to be of higher efficacy, suggesting a potential role for ongoing ALK inhibition (102).
2.6.2 Anti-Angiogenic Drugs
Anti-angiogenic drugs have also been investigated in ALK+ NSCLC. Vascular endothelial growth factor (VEGFR) expression has been reported to be upregulated in ALK+ NSCLC, which induces resistance to ALK TKIs (103). A single-arm study of involving 12 patients of ALK+ NSCLC demonstrated that crizotinib plus bevacizumab showed benefit in first-line ALK+ NSCLC, with an acceptable safety profile (104). In another phase 1/2 single-arm trial, alectinib plus bevacizumab was also well tolerated (105).
2.6.3 Immune Checkpoint Inhibitors
The PD-L1-positive and strongly positive rates among ALK+ NSCLC patients were 46.7%–50% and 13.3%–16%, respectively (23, 106). Studies have shown that the ALK oncoprotein is able to upregulate PD-L1 expression in lung cancer cells. Upregulation of PD-L1 by EML4-ALK was mediated by activating MEK-ERK and PI3K-AKT signaling pathways in NSCLC, which suggests a link between oncogene and PD-L1 expression (107). The expression of PD-L1 in ALK+ NSCLC has brought immunotherapy drugs such as immune checkpoint inhibitors (ICIs) into consideration for ALK+ NSCLC. A real-world analysis of ICIs in ALK+ NSCLC patients from a Flatiron Health electronic health record demonstrated limited efficacy of ICIs provided either before or after TKIs (108). Recent evidence indicated new roles of ALK and its genetic aberrations in immune evasion and in innate and cell-mediated immunity (109). The tumor microenvironment of ALK+ NSCLC suggested a poorly immunogenic “immune desert” of ALK+ NSCLC that also prevents the successful use of immune checkpoint inhibitors (ICI) (110). Furthermore, the toxicity of ICI for ALK+ NSCLC patients was too high. The sequential use of ICIs and crizotinib has also been reported with an increased risk of hepatotoxicity in retrospective studies (111). The challenge to researchers is not only to improve the efficacy of ICI in ALK+ NSCLC but also to find immunotherapeutic drugs that have acceptable toxicity in combination regimens.
2.6.4 Radiotherapy
There are no firm data for concurrent usage of ALK TKIs and radiotherapy. However, radiotherapy acts as a salvage treatment for patients who have oligoprogressive metastatic disease while under targeted therapy (112). In oligoprogressive diseases of ALK+ lung cancer, continuation of ALK TKIs with local ablative therapy should be considered for sustained control, which can potentially eradicate resistant cancer cell clones and confer survival benefit (113). Ablative and hypofractionated radiotherapy is one strategy for ALK+ lung cancer, since many ALK+ NSCLC patients treated with ALK TKIs experienced local disease progression (114). Timing of radiotherapy remains unclear, especially under different clinical settings. Furthermore, the safety of the combination of ALK TKIs and radiotherapy is unclear (115). Case reports using radiotherapy combined with alectinib and lorlatinib presented radiation-induced CNS necrosis, and this toxicity remains long after radiation (116, 117).
3 Discussion
3.1 How to Choose the Optimal First-Line Treatment?
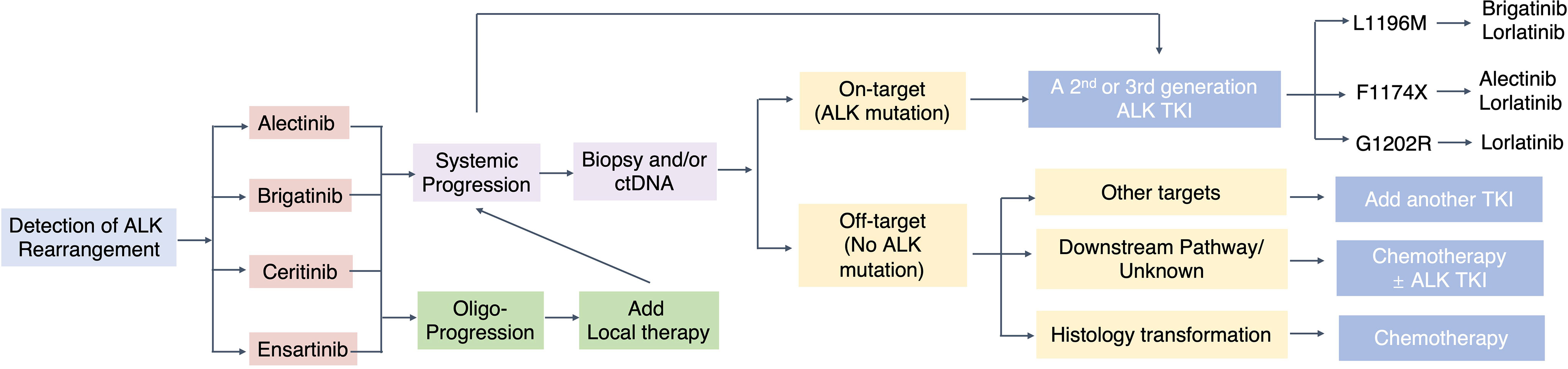
There is a continuous debate regarding the choice of the optimal upfront ALK TKI for the first-line treatment of ALK+ NSCLC, the subsequent sequencing strategies, and whether these considerations should be based on specific on-target ALK resistance mutations or not. Our recently published Bayesian network meta-analysis has compared the efficacy and safety of 6 ALK TKIs and chemotherapy in the first-line setting (118). Regarding PFS benefit for the first-line setting, lorlatinib ranks first, while the toxicity of lorlatinib needs to be paid attention to. However, the goal of treating advanced ALK+ NSCLC should not just be limited to improve median PFS in the first-line setting. There is no consensus on how to best sequence the ALK TKIs which are “single mutant active.” Some advocate using second-generation ALK TKIs due to their favorable toxicity profile, while leaving lorlatinib, the only third-generation ALK TKI, for salvage treatment (Figure 2).
ALK+ NSCLC has a high tendency for brain metastases compared to non-oncogene-driven NSCLC subtypes (119). Compared with first-generation ALK TKI, second- and third-generation ALK TKIs have a better efficacy of brain metastases. Ceritinib demonstrated an intracranial ORR of 35%–73% and an intracranial disease control rate (DCR) of 61%–86% in ALK-TKI naïve and -pretreated patients (44, 45, 69, 70). The intracranial ORR and intracranial DCR of alectinib in clinical trials were 54%–81% and 78%–90%, respectively (36, 39, 120, 121). Brigatinib showed an encouraging activity in the CNS, with an intracranial ORR of 42%–73% and an intracranial DCR of 83%–93% (42). A meta-analysis investigated the role of ALK TKIs in the treatment of ALK+ NSCLC patients with brain metastases, who had been pretreated with radiotherapy or not and/or chemotherapy (122). The results also confirmed better intracranial control with second-generation ALK TKIs (alectinib, brigatinib, and ceritinib) compared with crizotinib. Ensartinib demonstrated an intracranial ORR of 63.6%–70% and an intracranial DCR of 98%–100% (47, 48). Lorlatinib had an intracranial ORR of 61%–66% in the first-line setting (50). Lorlatinib also showed substantial intracranial activity in second-generation ALK TKI-pretreated patients, with or without baseline CNS metastases (123, 124). This evidence suggested that withholding brain radiotherapy in patients with asymptomatic brain metastases and use of radiotherapy during progression could be an option. Prospective trials are warranted to confirm the validity of this strategy.
3.2 Resistance Mechanism of ALK TKIs
There are two main categories of resistance mechanisms to ALK TKIs, namely, on-target alterations such as ALK mutation/gene amplification and off-target changes such as bypass signaling pathways (75). Substitution with ALK-destabilizing mutations could activate the ALK signaling pathway, which confers drug resistance to inhibitors (125). Inherent ALK resistance mutations are only found in a proportion of patients with acquired resistance to ALK-TKI, for first- and second-generation ALK-TKIs. ALK mutations such as somatic kinase domain mutations are the primary resistant mechanism. Two major ALK mutations after first-generation ALK TKI crizotinib were L1196M (7%) and G1269A (4%) (75), which alter 3D conformation and hinder TKI binding (126). Resistance to second-generation ALK TKIs is associated with specific mutations, such as G1202R, I1171N, S1206Y, and E1201K, for which not all TKIs are equally effective. In patient samples post-ceritinib, secondary mutations were detected in 56% of the cases, with 17% of double mutations: G1202R (21%), F1174 C/L (17%), and C1156Y (8%) (75). Acquired mutations of alectinib have been identified in 53% of the patients: G1202R (29%), I1171T/S (12%), V11180L (6%), and L1196M (6%) (75). Although brigatinib showed activity against G1202R, which is a frequent mutation associated with alectinib-resistant cancer (127), it is worth noting that G1202R has also been detected in brigatinib-resistant samples, raising the question of how clinically useful brigatinib is against this solvent front mutation (128). Of note, G1202R was not the most common ALK mutation in ensartinib-resistant patients, in which G1269A (6.6%) was the more identified than G1202R (2.8%) among 14.2% of the patients with secondary ALK mutations post second-line ensartinib (74). On-target resistance to the third-generation ALK inhibitor lorlatinib is primarily mediated by compound ALK mutations (129). Interestingly, some compound mutations that lead to lorlatinib resistance result in re-sensitization to first- or second-generation ALK TKIs, such as I1171N + L1256F, and C1156Y + L1198F which lead to re-sensitization to alectinib and crizotinib, respectively (76, 130). Patients with secondary ALK mutations refractory to the previous ALK TKI can be treated with other ALK TKIs. This re-sensitization phenomenon supported the sequential and possibly alternating use of different ALK TKIs.
ALK-independent mechanisms are only partially understood and particularly challenging, as they may result in refractoriness to further ALK inhibition. ALK-independent resistance mechanisms involve bypass pathways, such as EGFR, cMET, and AXL, or histological transformation into small cell lung cancer (SCLC) (131–133). Mechanisms of resistance to novel generation ALK TKIs are complex and diverse, reflecting the selective genetic pressure of drugs (134). In a prospective MATCH-R study, adaptive mechanisms driving resistance to lorlatinib were explored by a longitudinal assessment of tumor biopsies and ctDNA and the development of patient-derived xenograft (PDX) and cell lines (135). Epithelial–mesenchymal transition (EMT) mediated resistance in two patient-derived cell lines, and a novel bypass mechanism of resistance caused by NF2 loss-of-function mutations was described.
3.3 Toxicity Considerations
Clinical trials have established that ALK TKIs are generally safe and well tolerated. First-generation crizotinib has demonstrated a spectrum of toxicities, such as visual disorders (diplopia, photopsia, blurred vision), as well as QTc prolongation and bradycardia, while most of the AEs are grades 1–2 (136). Gastrointestinal toxicities were associated with different ALK TKIs, such as vomiting, nausea, and diarrhea. Brigatinib was characterized by a peculiar and early-onset interstitial lung toxicity (137). The most common AEs of lorlatinib were notably hypercholesterolemia (81%) and hypertriglyceridemia (60%), with cases of grade 3–4 toxicities occurring in 16% of patients. Special AEs of lorlatinib include CNS effects such as changes in mood, mental status, and peripheral neuropathy (138). Although different ALK TKIs share some common AEs, they have some unique toxicities, which should be taken into account to identify the right drug for the right patient. Finding ways to tackle these toxicities will play an essential role in drug strategies for ALK+ NSCLC patients.
A list of different parameters could potentially affect the interpretation of toxicity (139). Among them, the drug dose is one of the reasons which influence the tolerability and toxicity of ALK TKIs. As toxicity is related to drug dose, fewer toxicities were noted with the 300-mg dose than with the 600-mg dose of alectinib (62). Exposure-response analyses indicated that a lower dose of alectinib and crizotinib could result in diminishing treatment efficacy (140). Therefore, monitoring drug dose and toxicity might influence the treatment outcome of patients receiving ALK TKIs.
3.4 Beyond Advanced NSCLC
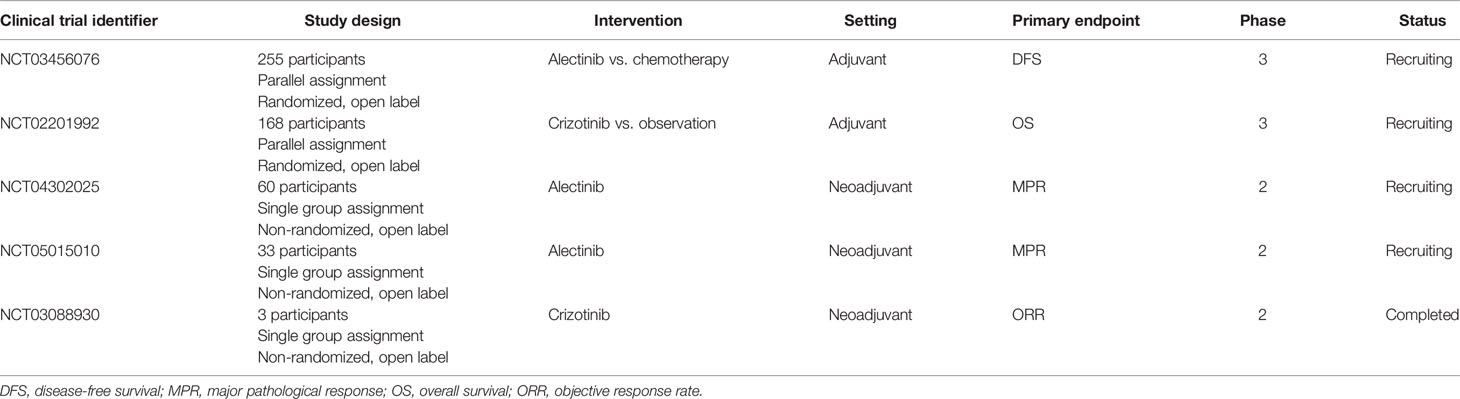
The treatment strategy of advanced ALK+ NSCLC has brought ALK-targeted therapy into early and locoregional (N2) stages. As acquired resistance of targeting ALK in the advanced stage setting emerges inevitably, TKIs are able to inhibit cancer cell proliferation, hinder tumor growth, and control cancer metastasis, but not to eradicate or cure the disease. There are no clear data regarding the frequency in early-stage or locoregional disease (141). Inhibiting the ALK signaling pathway at earlier stages still faces many challenges. Neoadjuvant and adjuvant ALK TKIs in ALK+ NSCLC have yielded mixed results (142). Table 3 shows the clinical trials of ALK TKIs in neoadjuvant and adjuvant settings (up to December 18, 2021).
4 Conclusions
In this “precision medicine” era, although the detection of oncogenes is common practice and the administration of targeted agents is a recognized option, molecular results should be interpreted with caution. The integration of the roles including pathologists, molecular biologists, and clinicians is needed. The treatment algorithm of ALK+ NSCLC is becoming more complex. New-generation TKIs have better CNS penetration across the blood–brain barrier, resulting in superior intracranial response rates and preventing brain metastases. A head-to-head comparison between all ALK TKIs is still lacking, but novel ALK TKIs are being developed to overcome resistance to currently available ALK TKIs, hypothesizing a defined sequential ALK TKI strategy in this disease. After failure of targeted therapies, chemotherapy might still be a valid option, while the role of immunotherapy is yet to be clarified. Overcoming the challenges for the development of more potent drugs will be essential to improving the survival rate of ALK+ NSCLC in the future.
Author Contributions
Conceptualization, LP and YZ. Writing—original draft preparation, LP and ZY. Writing—review and editing, LP, JS, GS, and YS. Supervision, JS and ZY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Administration of Traditional Chinese Medicine of Zhejiang Province (grant number: 2022ZA021) and a grant from the Medical Science Research Foundation of Health Bureau of Zhejiang Province (grant number: 2022KY545).
Conflict of Interest
GS is employed by, and holds stock in, Xcovery Holdings, Inc. JS is Editor-in-Chief of Oncogene and has sat on SABs for Vaccitech, Heat Biologics, Eli Lilly, Alveo Technologies, Pear Bio, Agenus, Equilibre Biopharmaceuticals, Graviton Bioscience Corporation, Celltrion, Volvox, Certis, Greenmantle, vTv Therapeutics, APIM Therapeutics, Bryologyx and Benevolent AI. He has consulted with Lansdowne partners and Vitruvian. He chairs the Board of Directors for Xerion and previously BB Biotech Healthcare Trust PLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Pharmacube for helping collecting clinical trial data.
References
1. Addeo A, Tabbo F, Robinson T, Buffoni L, Novello S. Precision Medicine in ALK Rearranged NSCLC: A Rapidly Evolving Scenario. Crit Rev Oncol Hematol (2018) 122:150–6. doi: 10.1016/j.critrevonc.2017.12.015
2. Bayliss R, Choi J, Fennell DA, Fry AM, Richards MW. Molecular Mechanisms That Underpin EML4-ALK Driven Cancers and Their Response to Targeted Drugs. Cell Mol Life Sci (2016) 73(6):1209–24. doi: 10.1007/s00018-015-2117-6
3. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature (2007) 448(7153):561–6. doi: 10.1038/nature05945
4. Hallberg B, Palmer RH. Mechanistic Insight Into ALK Receptor Tyrosine Kinase in Human Cancer Biology. Nat Rev Cancer (2013) 13(10):685–700. doi: 10.1038/nrc3580
5. Hallberg B, Palmer RH. The Role of the ALK Receptor in Cancer Biology. Ann Oncol (2016) 27 Suppl 3:iii4–15. doi: 10.1093/annonc/mdw301
6. Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a Kinase Gene, ALK, to a Nucleolar Protein Gene, NPM, in Non-Hodgkin's Lymphoma. Science (1994) 263(5151):1281–4. doi: 10.1126/science.8122112
7. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian Adapted Clinical Practice Guidelines for the Management of Patients With Metastatic Non-Small-Cell Lung Cancer: A CSCO-ESMO Initiative Endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(2):171–210. doi: 10.1093/annonc/mdy554
8. Gao X, Sholl LM, Nishino M, Heng JC, Janne PA, Oxnard GR. Clinical Implications of Variant ALK FISH Rearrangement Patterns. J Thorac Oncol (2015) 10(11):1648–52. doi: 10.1097/JTO.0000000000000665
9. Kim H, Yoo SB, Choe JY, Paik JH, Xu X, Nitta H, et al. Detection of ALK Gene Rearrangement in Non-Small Cell Lung Cancer: A Comparison of Fluorescence in Situ Hybridization and Chromogenic in Situ Hybridization With Correlation of ALK Protein Expression. J Thorac Oncol (2011) 6(8):1359–66. doi: 10.1097/JTO.0b013e31821cfc73
10. Beadling C, Wald AI, Warrick A, Neff TL, Zhong S, Nikiforov YE, et al. A Multiplexed Amplicon Approach for Detecting Gene Fusions by Next-Generation Sequencing. J Mol Diagn (2016) 18(2):165–75. doi: 10.1016/j.jmoldx.2015.10.002
11. Dagogo-Jack I, Ritterhouse LL. The Role of Plasma Genotyping in ALK- and ROS1-Rearranged Lung Cancer. Transl Lung Cancer Res (2020) 9(6):2557–70. doi: 10.21037/tlcr-2019-cnsclc-09
12. Dagogo-Jack I, Brannon AR, Ferris LA, Campbell CD, Lin JJ, Schultz KR, et al. Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors Through Longitudinal Analysis of Circulating Tumor DNA. JCO Precis Oncol (2018) 2018:PO.17.00160. doi: 10.1200/PO.17.00160
13. Mezquita L, Swalduz A, Jovelet C, Ortiz-Cuaran S, Howarth K, Planchard D, et al. Clinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients With Non-Small-Cell Lung Cancer. JCO Precis Oncol (2020) 4:PO.19.00281. doi: 10.1200/PO.19.00281
14. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol (2009) 27(26):4247–53. doi: 10.1200/JCO.2009.22.6993
15. Zhu VW, Zhao JJ, Gao Y, Syn NL, Zhang SS, Ou SI, et al. Thromboembolism in ALK+ and ROS1+ NSCLC Patients: A Systematic Review and Meta-Analysis. Lung Cancer (2021) 157:147–55. doi: 10.1016/j.lungcan.2021.05.019
16. Roopkumar J, Poudel SK, Gervaso L, Reddy CA, Velcheti V, Pennell NA, et al. Risk of Thromboembolism in Patients With ALK and EGFR-Mutant Lung Cancer: A Cohort Study. J Thromb Haemost (2020) 19(3):822–29. doi: 10.1111/jth.15215
17. Al-Samkari H, Leiva O, Dagogo-Jack I, Shaw A, Lennerz J, Iafrate AJ, et al. Impact of ALK Rearrangement on Venous and Arterial Thrombotic Risk in NSCLC. J Thorac Oncol (2020) 15(9):1497–506. doi: 10.1016/j.jtho.2020.04.033
18. Mendoza DP, Lin JJ, Rooney MM, Chen T, Sequist LV, Shaw AT, et al. Imaging Features and Metastatic Patterns of Advanced ALK-Rearranged Non-Small Cell Lung Cancer. AJR Am J Roentgenol (2020) 214(4):766–74. doi: 10.2214/AJR.19.21982
19. Yamamoto S, Korn RL, Oklu R, Migdal C, Gotway MB, Weiss GJ, et al. ALK Molecular Phenotype in Non-Small Cell Lung Cancer: CT Radiogenomic Characterization. Radiology (2014) 272(2):568–76. doi: 10.1148/radiol.14140789
20. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol (2019) 14(7):1233–43. doi: 10.1016/j.jtho.2019.03.007
21. Christopoulos P, Endris V, Bozorgmehr F, Elsayed M, Kirchner M, Ristau J, et al. EML4-ALK Fusion Variant V3 Is a High-Risk Feature Conferring Accelerated Metastatic Spread, Early Treatment Failure and Worse Overall Survival in ALK(+) Non-Small Cell Lung Cancer. Int J Cancer (2018) 142(12):2589–98. doi: 10.1002/ijc.31275
22. Su Y, Long X, Song Y, Chen P, Li S, Yang H, et al. Distribution of ALK Fusion Variants and Correlation With Clinical Outcomes in Chinese Patients With Non-Small Cell Lung Cancer Treated With Crizotinib. Target Oncol (2019) 14(2):159–68. doi: 10.1007/s11523-019-00631-x
23. Chang GC, Yang TY, Chen KC, Hsu KH, Huang YH, Su KY, et al. ALK Variants, PD-L1 Expression, and Their Association With Outcomes in ALK-Positive NSCLC Patients. Sci Rep (2020) 10(1):21063. doi: 10.1038/s41598-020-78152-1
24. Wang S, Luo R, Shi Y, Han X. The Impact of the ALK Fusion Variant on Clinical Outcomes in EML4-ALK Patients With NSCLC: A Systematic Review and Meta-Analysis. Future Oncol (2022) 18(3):385–402. doi: 10.2217/fon-2021-0945
25. Batra U, Sharma M, Nathany S, Jain P, Soni S, Mehta A. Are All ALK Variants Created Equal? Clinicopathologic Features and Outcomes: A Propensity-Matched Study. Int J Clin Oncol (2021) 26(7):1221–8. doi: 10.1007/s10147-021-01916-w
26. Furugaki K, Harada N, Yoshimura Y. Sensitivity of Eight Types of ALK Fusion Variant to Alectinib in ALK-Transformed Cells. Anticancer Drugs (2021) 33(2):124–31. doi: 10.1097/CAD.0000000000001249
27. Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol (2018) 36(12):1199–206. doi: 10.1200/JCO.2017.76.2294
28. Wu W, Haderk F, Bivona TG. Non-Canonical Thinking for Targeting ALK-Fusion Onco-Proteins in Lung Cancer. Cancers (Basel) (2017) 9(12):164. doi: 10.3390/cancers9120164
29. Kang J, Zhang XC, Chen HJ, Zhong WZ, Xu Y, Su J, et al. Complex ALK Fusions Are Associated With Better Prognosis in Advanced Non-Small Cell Lung Cancer. Front Oncol (2020) 10:596937. doi: 10.3389/fonc.2020.596937
30. Zhang Y, Zeng L, Zhou C, Li Y, Wu L, Xia C, et al. Detection of Nonreciprocal/Reciprocal ALK Translocation as Poor Predictive Marker in Patients With First-Line Crizotinib-Treated ALK-Rearranged NSCLC. J Thorac Oncol (2020) 15(6):1027–36. doi: 10.1016/j.jtho.2020.02.007
31. Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB. Preclinical Rationale for Use of the Clinically Available Multitargeted Tyrosine Kinase Inhibitor Crizotinib in ROS1-Translocated Lung Cancer. J Thorac Oncol (2012) 7(7):1086–90. doi: 10.1097/JTO.0b013e3182570919
32. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med (2014) 371(23):2167–77. doi: 10.1056/NEJMoa1408440
33. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib Versus Chemotherapy in East Asian Patients With ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol (2018) 13(10):1539–48. doi: 10.1016/j.jtho.2018.06.012
34. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib Versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N Engl J Med (2013) 368(25):2385–94. doi: 10.1056/NEJMoa1214886
35. Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell (2011) 19(5):679–90. doi: 10.1016/j.ccr.2011.04.004
36. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(9):829–38. doi: 10.1056/NEJMoa1704795
37. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib Versus Crizotinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet (2017) 390(10089):29–39. doi: 10.1016/S0140-6736(17)30565-2
38. Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib Versus Crizotinib in Untreated Asian Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer (ALESIA): A Randomised Phase 3 Study. Lancet Respir Med (2019) 7(5):437–46. doi: 10.1016/S2213-2600(19)30053-0
39. Novello S, Mazieres J, Oh IJ, de Castro J, Migliorino MR, Helland A, et al. Alectinib Versus Chemotherapy in Crizotinib-Pretreated Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small-Cell Lung Cancer: Results From the Phase III ALUR Study. Ann Oncol (2018) 29(6):1409–16. doi: 10.1093/annonc/mdy121
40. Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res (2016) 22(22):5527–38. doi: 10.1158/1078-0432.CCR-16-0569
41. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib Versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2027–39. doi: 10.1056/NEJMoa1810171
42. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol (2017) 35(22):2490–8. doi: 10.1200/JCO.2016.71.5904
43. Nishio M, Murakami H, Horiike A, Takahashi T, Hirai F, Suenaga N, et al. Phase I Study of Ceritinib (LDK378) in Japanese Patients With Advanced, Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer or Other Tumors. J Thorac Oncol (2015) 10(7):1058–66. doi: 10.1097/JTO.0000000000000566
44. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-Line Ceritinib Versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet (2017) 389(10072):917–29. doi: 10.1016/S0140-6736(17)30123-X
45. Shaw AT, Kim TM, Crino L, Gridelli C, Kiura K, Liu G, et al. Ceritinib Versus Chemotherapy in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(7):874–86. doi: 10.1016/S1470-2045(17)30339-X
46. Horn L, Infante JR, Reckamp KL, Blumenschein GR, Leal TA, Waqar SN, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results From a First-In-Human Phase I/II, Multicenter Study. Clin Cancer Res (2018) 24(12):2771–9. doi: 10.1158/1078-0432.CCR-17-2398
47. Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol (2021) 7(11):1617–25. doi: 10.1001/jamaoncol.2021.3523
48. Yang Y, Zhou J, Zhou J, Feng J, Zhuang W, Chen J, et al. Efficacy, Safety, and Biomarker Analysis of Ensartinib in Crizotinib-Resistant, ALK-Positive Non-Small-Cell Lung Cancer: A Multicentre, Phase 2 Trial. Lancet Respir Med (2020) 8(1):45–53. doi: 10.1016/S2213-2600(19)30252-8
49. Zou HY, Friboulet L, Kodack DP, Engstrom LD, Li Q, West M, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell (2015) 28(1):70–81. doi: 10.1016/j.ccell.2015.05.010
50. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med (2020) 383(21):2018–29. doi: 10.1056/NEJMoa2027187
51. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer: Results From a Global Phase 2 Study. Lancet Oncol (2018) 19(12):1654–67. doi: 10.1016/S1470-2045(18)30649-1
52. Blackhall F, Cappuzzo F. Crizotinib: From Discovery to Accelerated Development to Front-Line Treatment. Ann Oncol (2018) 29(4):1073. doi: 10.1093/annonc/mdx121
53. Blackhall F, Ross Camidge D, Shaw AT, Soria JC, Solomon BJ, Mok T, et al. Final Results of the Large-Scale Multinational Trial PROFILE 1005: Efficacy and Safety of Crizotinib in Previously Treated Patients With Advanced/Metastatic ALK-Positive Non-Small-Cell Lung Cancer. ESMO Open (2017) 2(3):e000219. doi: 10.1136/esmoopen-2017-000219
54. Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and Safety of Crizotinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer: Updated Results From a Phase 1 Study. Lancet Oncol (2012) 13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3
55. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF Concentration of the Anaplastic Lymphoma Kinase Inhibitor Crizotinib. J Clin Oncol (2011) 29(15):e443–5. doi: 10.1200/JCO.2010.34.1313
56. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK Mutations in Lung Cancer That Confer Resistance to ALK Inhibitors. N Engl J Med (2010) 363(18):1734–9. doi: 10.1056/NEJMoa1007478
57. Santarpia M, Altavilla G, Rosell R. Alectinib: A Selective, Next-Generation ALK Inhibitor for Treatment of ALK-Rearranged Non-Small-Cell Lung Cancer. Expert Rev Respir Med (2015) 9(3):255–68. doi: 10.1586/17476348.2015.1009040
58. Lin JJ, Jiang GY, Joshipura N, Ackil J, Digumarthy SR, Rincon SP, et al. Efficacy of Alectinib in Patients With ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J Thorac Oncol (2019) 14(4):683–90. doi: 10.1016/j.jtho.2018.12.002
59. Larkins E, Blumenthal GM, Chen H, He K, Agarwal R, Gieser G, et al. FDA Approval: Alectinib for the Treatment of Metastatic, ALK-Positive Non-Small Cell Lung Cancer Following Crizotinib. Clin Cancer Res (2016) 22(21):5171–6. doi: 10.1158/1078-0432.CCR-16-1293
60. Yoshioka H, Hida T, Nokihara H, Morise M, Kim YH, Azuma K, et al. Final OS Analysis From the Phase III J-Alex Study of Alectinib (ALC) Versus Crizotinib (CRZ) in Japanese ALK-Inhibitor Naïve ALK-Positive Non-Small Cell Lung Cancer (ALK+ NSCLC). J Clin Oncol (2021) 39(15_suppl):9022–2. doi: 10.1200/JCO.2021.39.15_suppl.9022
61. Pan Y, Xiao W, Ye F, Wang H, Shen Y, Yu X, et al. Outcomes of Switching From Crizotinib to Alectinib in Patients With Advanced Non-Small Cell Lung Cancer With Anaplastic Lymphoma Kinase Fusion. Ann Transl Med (2021) 9(12):1014. doi: 10.21037/atm-21-2769
62. Sacdalan DB, Lucero JA. Revisiting a Lower Starting Dose of Alectinib in ALK-Positive Non-Small Cell Lung Cancer. Cancer Treat Res Commun (2021) 27:100319. doi: 10.1016/j.ctarc.2021.100319
63. Yanagitani N, Uchibori K, Koike S, Tsukahara M, Kitazono S, Yoshizawa T, et al. Drug Resistance Mechanisms in Japanese Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer and the Clinical Responses Based on the Resistant Mechanisms. Cancer Sci (2020) 111(3):932–9. doi: 10.1111/cas.14314
64. Huber RM, Hansen KH, Paz-Ares Rodriguez L, West HL, Reckamp KL, Leighl NB, et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-Up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J Thorac Oncol (2020) 15(3):404–15. doi: 10.1016/j.jtho.2019.11.004
65. Camidge D, Kim HR, Ahn M-J, Yang J, Han J-Y, Hochmair M, et al. Brigatinib Versus Crizotinib in Anaplastic Lymphoma Kinase (ALK) Inhibitor–Naive Advanced ALK-Positive Non–Small Cell Lung Cancer: Final Results of the Phase 3 ALTA-1l Trial. J Thorac Oncol (2021) 16:2091–108. doi: 10.1016/j.jtho.2021.07.035
66. Ando K, Akimoto K, Sato H, Manabe R, Kishino Y, Homma T, et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer With or Without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers (Basel) (2020) 12(4):942. doi: 10.3390/cancers12040942
67. Stinchcombe TE, Doebele RC, Wang X, Gerber DE, Horn L, Camidge DR. Preliminary Clinical and Molecular Analysis Results From a Single-Arm Phase 2 Trial of Brigatinib in Patients With Disease Progression After Next-Generation ALK Tyrosine Kinase Inhibitors in Advanced ALK+ NSCLC. J Thorac Oncol (2020) 16(1):156–61. doi: 10.1016/j.jtho.2020.09.018
68. Popat S, Liu G, Lu S, Song G, Ma X, Yang JC. Brigatinib vs Alectinib in Crizotinib-Resistant Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer (ALTA-3). Future Oncol (2021) 17(32):4237–47. doi: 10.2217/fon-2021-0608
69. Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and Safety of Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-1): Updated Results From the Multicentre, Open-Label, Phase 1 Trial. Lancet Oncol (2016) 17(4):452–63. doi: 10.1016/S1470-2045(15)00614-2
70. Crino L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol (2016) 34(24):2866–73. doi: 10.1200/JCO.2015.65.5936
71. Cho BC, Obermannova R, Bearz A, McKeage M, Kim DW, Batra U, et al. Efficacy and Safety of Ceritinib (450 Mg/D or 600 Mg/D) With Food Versus 750-Mg/D Fasted in Patients With ALK Receptor Tyrosine Kinase (ALK)-Positive NSCLC: Primary Efficacy Results From the ASCEND-8 Study. J Thorac Oncol (2019) 14(7):1255–65. doi: 10.1016/j.jtho.2019.03.002
72. Wilkinson S, Gupta A, Scheuer N, Mackay E, Arora P, Thorlund K, et al. Assessment of Alectinib vs Ceritinib in ALK-Positive Non-Small Cell Lung Cancer in Phase 2 Trials and in Real-World Data. JAMA Netw Open (2021) 4(10):e2126306. doi: 10.1001/jamanetworkopen.2021.26306
73. Menichincheri M, Ardini E, Magnaghi P, Avanzi N, Banfi P, Bossi R, et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), C-Ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) Inhibitor. J Med Chem (2016) 59(7):3392–408. doi: 10.1021/acs.jmedchem.6b00064
74. Yang Y, Huang J, Wang T, Zhou J, Zheng J, Feng J, et al. Decoding the Evolutionary Response to Ensartinib in Patients With ALK-Positive NSCLC by Dynamic Circulating Tumor DNA Sequencing. J Thorac Oncol (2021) 16(5):827–39. doi: 10.1016/j.jtho.2021.01.1615
75. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
76. Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med (2016) 374(1):54–61. doi: 10.1056/NEJMoa1508887
77. Yoda S, Lin JJ, Lawrence MS, Burke BJ, Friboulet L, Langenbucher A, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov (2018) 8(6):714–29. doi: 10.1158/2159-8290.CD-17-1256
78. Basit S, Ashraf Z, Lee K, Latif M. First Macrocyclic 3(Rd)-Generation ALK Inhibitor for Treatment of ALK/ROS1 Cancer: Clinical and Designing Strategy Update of Lorlatinib. Eur J Med Chem (2017) 134:348–56. doi: 10.1016/j.ejmech.2017.04.032
79. Camidge DR. Lorlatinib Should Not be Considered as the Preferred First-Line Option in Patients With Advanced ALK Rearranged NSCLC. J Thorac Oncol (2021) 16(4):528–31. doi: 10.1016/j.jtho.2020.12.022
80. Nagasaka M, Ou SI. Lorlatinib Should Be Considered as the Preferred First-Line Option in Patients With Advanced ALK-Rearranged NSCLC. J Thorac Oncol (2021) 16(4):532–6. doi: 10.1016/j.jtho.2020.12.021
81. Ando K, Manabe R, Kishino Y, Kusumoto S, Yamaoka T, Tanaka A, et al. Comparative Efficacy and Safety of Lorlatinib and Alectinib for ALK-Rearrangement Positive Advanced Non-Small Cell Lung Cancer in Asian and Non-Asian Patients: A Systematic Review and Network Meta-Analysis. Cancers (Basel) (2021) 13(15):3704. doi: 10.3390/cancers13153704
82. Murray BW, Zhai D, Deng W, Zhang X, Ung J, Nguyen V, et al. TPX-0131, a Potent CNS-Penetrant, Next-Generation Inhibitor of Wild-Type ALK and ALK-Resistant Mutations. Mol Cancer Ther (2021) 20(9):1499–507. doi: 10.1158/1535-7163.MCT-21-0221
83. Pelish H, Tangpeerachaikul A, Kohl N, Porter J, Shair M, Horan J. NUV-655 (NVL-655) Is a Selective, Brain-Penetrant ALK Inhibitor With Antitumor Activity Against the Lorlatinib-Resistant G1202R/L1196M Compound Mutation. Cancer Res (2021) 81(13_Supplement):1468. doi: 10.1158/1538-7445.AM2021-1468
84. Ardini E, Menichincheri M, Banfi P, Bosotti R, De Ponti C, Pulci R, et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor With Activity in Multiple Molecularly Defined Cancer Indications. Mol Cancer Ther (2016) 15(4):628–39. doi: 10.1158/1535-7163.MCT-15-0758
85. Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results From Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov (2017) 7(4):400–9. doi: 10.1158/2159-8290.CD-16-1237
86. Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov (2018) 8(10):1227–36. doi: 10.1158/2159-8290.CD-18-0484
87. Cho B, Drilon A, Doebele R, Kim D-S, Lin J, Lee J, et al. Safety and Preliminary Clinical Activity of Repotrectinib in Patients With Advanced ROS1 Fusion-Positive Non-Small Cell Lung Cancer (TRIDENT-1 Study). J Clin Oncol (2019) 37:9011–1. doi: 10.1200/JCO.2019.37.15_suppl.9011
88. Ma Y, Yang N, Li S, Zhao H, Li L, Yang H, et al. A Phase I, Dose-Escalation and Expansion Study of TQ-B3139, a Novel ALK TKI, in Chinese ALK or ROS1 Positive Advanced Non-Small Cell Lung Cancer (NSCLC). J Clin Oncol (2020) 38:9585–5. doi: 10.1200/JCO.2020.38.15_suppl.9585
89. Shi Y, Fang J, Hao X, Zhang S, Liu Y, Wang L, et al. Safety and activity of WX-0593 (Iruplinalkib) in Patients With ALK- or ROS1-Rearranged Advanced Non-Small Cell Lung Cancer: A Phase 1 Dose-Escalation and Dose-Expansion Trial. Signal Transduct Target Ther (2022) 7(1):25. doi: 10.1038/s41392-021-00841-8
90. Han B, Chu T, Qian J, Yan B, Zhang Y, Chang Q. Preliminary Results of Second Generation ALK Inhibitor PLB1003: A Phase La Study. J Thorac Oncol (2019) 14:S651. doi: 10.1016/j.jtho.2019.08.1375
91. Xia ZJ, Ji YC, Sun DQ, Peng X, Gao YL, Fang YF, et al. SAF-189s, A Potent New-Generation ROS1 Inhibitor, Is Active Against Crizotinib-Resistant ROS1 Mutant-Driven Tumors. Acta Pharmacol Sin (2020) 42(6):998–1004. doi: 10.1038/s41401-020-00513-3
92. Song P, Zhang X, Yang D, Wang H, Si X, Zhang L. Single-Center Study to Determine the Safety and Efficacy of CT-707 in Chinese Patients With Advanced Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer. Thorac Cancer (2020) 11(5):1216–23. doi: 10.1111/1759-7714.13376
93. Mizuta H, Okada K, Araki M, Adachi J, Takemoto A, Kutkowska J, et al. Gilteritinib Overcomes Lorlatinib Resistance in ALK-Rearranged Cancer. Nat Commun (2021) 12(1):1261. doi: 10.1038/s41467-021-21396-w
94. Lu Y, Fan Z, Zhu SJ, Huang X, Zhuang Z, Li Y, et al. A New ALK Inhibitor Overcomes Resistance to First- and Second-Generation Inhibitors in NSCLC. EMBO Mol Med (2021) 14(1):e14296. doi: 10.15252/emmm.202114296
95. Kang CH, Lee DH, Lee CO, Du Ha J, Park CH, Hwang JY. Induced Protein Degradation of Anaplastic Lymphoma Kinase (ALK) by Proteolysis Targeting Chimera (PROTAC). Biochem Biophys Res Commun (2018) 505(2):542–7. doi: 10.1016/j.bbrc.2018.09.169
96. Powell CE, Gao Y, Tan L, Donovan KA, Nowak RP, Loehr A, et al. Chemically Induced Degradation of Anaplastic Lymphoma Kinase (ALK). J Med Chem (2018) 61(9):4249–55. doi: 10.1021/acs.jmedchem.7b01655
97. Zhang C, Han XR, Yang X, Jiang B, Liu J, Xiong Y, et al. Proteolysis Targeting Chimeras (PROTACs) of Anaplastic Lymphoma Kinase (ALK). Eur J Med Chem (2018) 151:304–14. doi: 10.1016/j.ejmech.2018.03.071
98. Wang Y, Han L, Liu F, Yang F, Jiang X, Sun H, et al. Targeted Degradation of Anaplastic Lymphoma Kinase by Gold Nanoparticle-Based Multi-Headed Proteolysis Targeting Chimeras. Colloids Surf B Biointerfaces (2020) 188:110795. doi: 10.1016/j.colsurfb.2020.110795
99. Sun N, Ren C, Kong Y, Zhong H, Chen J, Li Y, et al. Development of a Brigatinib Degrader (SIAIS117) as a Potential Treatment for ALK Positive Cancer Resistance. Eur J Med Chem (2020) 193:112190. doi: 10.1016/j.ejmech.2020.112190
100. Song X, Zhong H, Qu X, Yang L, Jiang B. Two Novel Strategies to Overcome the Resistance to ALK Tyrosine Kinase Inhibitor Drugs: Macrocyclic Inhibitors and Proteolysis-Targeting Chimeras. MedComm (2020) (2021) 2(3):341–50. doi: 10.1002/mco2.42
101. Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-Dose Pemetrexed in Combination With High-Dose Crizotinib for the Treatment of Refractory CNS Metastases in ALK-Rearranged Non-Small-Cell Lung Cancer. J Thorac Oncol (2013) 8(1):e3–5. doi: 10.1097/JTO.0b013e3182762d20
102. Lin JJ, Schoenfeld AJ, Zhu VW, Yeap BY, Chin E, Rooney M, et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol (2020) 15(2):258–65. doi: 10.1016/j.jtho.2019.10.014
103. Chen Y, Ma G, Su C, Wu P, Wang H, Song X, et al. Apatinib Reverses Alectinib Resistance by Targeting Vascular Endothelial Growth Factor Receptor 2 and Attenuating the Oncogenic Signaling Pathway in Echinoderm Microtubule-Associated Protein-Like 4-Anaplastic Lymphoma Kinase Fusion Gene-Positive Lung Cancer Cell Lines. Anticancer Drugs (2018) 29(10):935–43. doi: 10.1097/CAD.0000000000000667
104. Huang Z, Xiong Q, Cui Z, Tao H, Zhang S, Wang L, et al. Efficacy and Safety of Crizotinib Plus Bevacizumab in ALK/ROS-1/C-MET Positive Non-Small Cell Lung Cancer: An Open-Label, Single-Arm, Prospective Observational Study. Am J Transl Res (2021) 13(3):1526–34.
105. Lin JJ, Muzikansky A, Kennedy E, Kuberski H, Stober LL, Wanat AC, et al. Safety and Activity of Alectinib Plus Bevacizumab in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase I/II Study. ESMO Open (2021) 7(1):100342. doi: 10.1016/j.esmoop.2021.100342
106. Tseng JS, Yang TY, Wu CY, Ku WH, Chen KC, Hsu KH, et al. Characteristics and Predictive Value of PD-L1 Status in Real-World Non-Small Cell Lung Cancer Patients. J Immunother (2018) 41(6):292–9. doi: 10.1097/CJI.0000000000000226
107. Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res (2015) 21(17):4014–21. doi: 10.1158/1078-0432.CCR-15-0016
108. Jahanzeb M, Lin HM, Pan X, Yin Y, Baumann P, Langer CJ. Immunotherapy Treatment Patterns and Outcomes Among ALK-Positive Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer (2020) 22(1):49–57. doi: 10.1016/j.cllc.2020.08.003
109. Wang L, Lui VWY. Emerging Roles of ALK in Immunity and Insights for Immunotherapy. Cancers (Basel) (2020) 12(2):426. doi: 10.3390/cancers12020426
110. Budczies J, Kirchner M, Kluck K, Kazdal D, Glade J, Allgauer M, et al. Deciphering the Immunosuppressive Tumor Microenvironment in ALK- and EGFR-Positive Lung Adenocarcinoma. Cancer Immunol Immunother (2021) 71(2):251–65. doi: 10.1007/s00262-021-02981-w
111. Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT, et al. Increased Hepatotoxicity Associated With Sequential Immune Checkpoint Inhibitor and Crizotinib Therapy in Patients With Non-Small Cell Lung Cancer. J Thorac Oncol (2019) 14(1):135–40. doi: 10.1016/j.jtho.2018.09.001
112. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and Classification of Oligometastatic Disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus Recommendation. Lancet Oncol (2020) 21(1):e18–28. doi: 10.1016/S1470-2045(19)30718-1
113. Wrona A, Dziadziuszko R, Jassem J. Combining Radiotherapy With Targeted Therapies in Non-Small Cell Lung Cancer: Focus on Anti-EGFR, Anti-ALK and Anti-Angiogenic Agents. Transl Lung Cancer Res (2021) 10(4):2032–47. doi: 10.21037/tlcr-20-552
114. Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, et al. Stereotactic Radiation Therapy can Safely and Durably Control Sites of Extra-Central Nervous System Oligoprogressive Disease in Anaplastic Lymphoma Kinase-Positive Lung Cancer Patients Receiving Crizotinib. Int J Radiat Oncol Biol Phys (2014) 88(4):892–8. doi: 10.1016/j.ijrobp.2013.11.010
115. Antoni D, Burckel H, Noel G. Combining Radiation Therapy With ALK Inhibitors in Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer (NSCLC): A Clinical and Preclinical Overview. Cancers (Basel) (2021) 13(10):2394. doi: 10.3390/cancers13102394
116. Ou SH, Weitz M, Jalas JR, Kelly DF, Wong V, Azada MC, et al. Alectinib Induced CNS Radiation Necrosis in an ALK+NSCLC Patient With a Remote (7 Years) History of Brain Radiation. Lung Cancer (2016) 96:15–8. doi: 10.1016/j.lungcan.2016.03.008
117. Zhu VW, Nagasaka M, Kubota T, Raval K, Robinette N, Armas O, et al. Symptomatic CNS Radiation Necrosis Requiring Neurosurgical Resection During Treatment With Lorlatinib in ALK-Rearranged NSCLC: A Report of Two Cases. Lung Cancer (Auckl) (2020) 11:13–8. doi: 10.2147/LCTT.S224991
118. Peng L, Lu D, Xia Y, Hong S, Selvaggi G, Stebbing J, et al. Efficacy and Safety of First-Line Treatment Strategies for Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Bayesian Network Meta-Analysis. Front Oncol (2021) 11:754768. doi: 10.3389/fonc.2021.754768
119. Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol (2016) 34(2):123–9. doi: 10.1200/JCO.2015.62.0138
120. Gadgeel SM, Shaw AT, Govindan R, Gandhi L, Socinski MA, Camidge DR, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2016) 34(34):4079–85. doi: 10.1200/JCO.2016.68.4639
121. Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and Activity of Alectinib Against Systemic Disease and Brain Metastases in Patients With Crizotinib-Resistant ALK-Rearranged Non-Small-Cell Lung Cancer (AF-002JG): Results From the Dose-Finding Portion of a Phase 1/2 Study. Lancet Oncol (2014) 15(10):1119–28. doi: 10.1016/S1470-2045(14)70362-6
122. Petrelli F, Lazzari C, Ardito R, Borgonovo K, Bulotta A, Conti B, et al. Efficacy of ALK Inhibitors on NSCLC Brain Metastases: A Systematic Review and Pooled Analysis of 21 Studies. PloS One (2018) 13(7):e0201425. doi: 10.1371/journal.pone.0201425
123. Bauer TM, Shaw AT, Johnson ML, Navarro A, Gainor JF, Thurm H, et al. Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression With Lorlatinib in Patients With Previously Treated ALK-Positive Non-Small-Cell Lung Cancer. Target Oncol (2020) 15(1):55–65. doi: 10.1007/s11523-020-00702-4
124. Felip E, Shaw AT, Bearz A, Camidge DR, Solomon BJ, Bauman JR, et al. Intracranial and Extracranial Efficacy of Lorlatinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer Previously Treated With Second-Generation ALK TKIs. Ann Oncol (2021) 32(5):620–30. doi: 10.1016/j.annonc.2021.02.012
125. Li J, Huang Y, Wu M, Wu C, Li X, Bao J. Structure and Energy Based Quantitative Missense Variant Effect Analysis Provides Insights Into Drug Resistance Mechanisms of Anaplastic Lymphoma Kinase Mutations. Sci Rep (2018) 8(1):10664. doi: 10.1038/s41598-018-28752-9
126. Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of Resistance to Crizotinib in Patients With ALK Gene Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res (2012) 18(5):1472–82. doi: 10.1158/1078-0432.CCR-11-2906
127. Lin JJ, Zhu VW, Schoenfeld AJ, Yeap BY, Saxena A, Ferris LA, et al. Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC. J Thorac Oncol (2018) 13(10):1530–8. doi: 10.1016/j.jtho.2018.06.005
128. Bordi P, Tiseo M, Rofi E, Petrini I, Restante G, Danesi R, et al. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin Lung Cancer (2017) 18(6):692–7. doi: 10.1016/j.cllc.2017.04.013
129. Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2019) 37(16):1370–9. doi: 10.1200/JCO.18.02236
130. Okada K, Araki M, Sakashita T, Ma B, Kanada R, Yanagitani N, et al. Prediction of ALK Mutations Mediating ALK-TKIs Resistance and Drug Re-Purposing to Overcome the Resistance. EBioMedicine (2019) 41:105–19. doi: 10.1016/j.ebiom.2019.01.019
131. Fujita S, Masago K, Katakami N, Yatabe Y. Transformation to SCLC After Treatment With the ALK Inhibitor Alectinib. J Thorac Oncol (2016) 11(6):e67–72. doi: 10.1016/j.jtho.2015.12.105
132. Yamada T, Takeuchi S, Nakade J, Kita K, Nakagawa T, Nanjo S, et al. Paracrine Receptor Activation by Microenvironment Triggers Bypass Survival Signals and ALK Inhibitor Resistance in EML4-ALK Lung Cancer Cells. Clin Cancer Res (2012) 18(13):3592–602. doi: 10.1158/1078-0432.CCR-11-2972
133. Yun MR, Lim SM, Kim SK, Choi HM, Pyo KH, Kim SK, et al. Enhancer Remodeling and MicroRNA Alterations Are Associated With Acquired Resistance to ALK Inhibitors. Cancer Res (2018) 78(12):3350–62. doi: 10.1158/0008-5472.CAN-17-3146
134. Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment With Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res (2019) 25(22):6662–70. doi: 10.1158/1078-0432.CCR-19-1436
135. Recondo G, Mezquita L, Facchinetti F, Planchard D, Gazzah A, Bigot L, et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res (2020) 26(1):242–55. doi: 10.1158/1078-0432.CCR-19-1104
136. Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36(22):2251–8. doi: 10.1200/JCO.2017.77.4794
137. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and Safety of Brigatinib in ALK-Rearranged Non-Small-Cell Lung Cancer and Other Malignancies: A Single-Arm, Open-Label, Phase 1/2 Trial. Lancet Oncol (2016) 17(12):1683–96. doi: 10.1016/S1470-2045(16)30392-8
138. Nagasaka M, Ge Y, Sukari A, Kukreja G, Ou SI. A User's Guide to Lorlatinib. Crit Rev Oncol Hematol (2020) 151:102969. doi: 10.1016/j.critrevonc.2020.102969
139. Simons EA, Smith DE, Gao D, Camidge DR. Variation in Toxicity Reporting Methods for Early Phase Lung Cancer Treatment Trials at Oncology Conferences. J Thorac Oncol (2020) 15(9):1425–33. doi: 10.1016/j.jtho.2020.04.020
140. Morcos PN, Nueesch E, Jaminion F, Guerini E, Hsu JC, Bordogna W, et al. Exposure-Response Analysis of Alectinib in Crizotinib-Resistant ALK-Positive Non-Small Cell Lung Cancer. Cancer Chemother Pharmacol (2018) 82(1):129–38. doi: 10.1007/s00280-018-3597-5
141. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28(suppl_4):iv1–21. doi: 10.1093/annonc/mdx222
Keywords: lung cancer, ALK, rearrangement, tyrosine kinase inhibitor, resistance
Citation: Peng L, Zhu L, Sun Y, Stebbing J, Selvaggi G, Zhang Y and Yu Z (2022) Targeting ALK Rearrangements in NSCLC: Current State of the Art. Front. Oncol. 12:863461. doi: 10.3389/fonc.2022.863461
Received: 27 January 2022; Accepted: 08 March 2022;
Published: 06 April 2022.
Edited by:
Chunxia Su, Shanghai Pulmonary Hospital, ChinaReviewed by:
Wenhua Liang, First Affiliated Hospital of Guangzhou Medical University, ChinaZhengfei Zhu, Fudan University, China
Copyright © 2022 Peng, Zhu, Sun, Stebbing, Selvaggi, Zhang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhentao Yu, eXV6aHRhb0Bob3RtYWlsLmNvbQ==; Yongchang Zhang, emhhbmd5b25nY2hhbmdAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Ling Peng
Ling Peng Liping Zhu
Liping Zhu Yilan Sun1
Yilan Sun1 Yongchang Zhang
Yongchang Zhang