- 1Holden Comprehensive Cancer Center, University of Iowa Hospitals and Clinics, Iowa City, IA, United States
- 2Pfizer Inc., Hellas, Greece
- 3Pfizer Inc., New York, NY, United States
Background: Combination axitinib plus pembrolizumab is a standard of care in the first-line treatment of patients with advanced clear cell renal cell carcinoma (RCC). This analysis describes the clinical characteristics, treatment management and outcomes of patients receiving first-line (1L) axitinib plus pembrolizumab in a real-world US setting.
Methods: Electronic health record (EHR)-derived data from the Flatiron Health Database, which includes ~280 cancer clinics across 800 sites in the US, were used. Patients had confirmed Stage IV or metastatic RCC and initiated 1L axitinib plus pembrolizumab on or after 1/1/2018 to 3/31/2021. Outcomes were best overall response rate; real-world progression-free survival (rwPFS) and overall survival (OS) at landmark time periods (3, 6, 9, and 12 months). Therapy management (TM) included dose hold, dose change and discontinuation. Data are reported as medians (IQR) unless otherwise noted.
Results: 355 patients received 1L axitinib plus pembrolizumab, with median follow-up of 9.7 (0.1–24.3) months. IMDC Risk Score was favorable, intermediate, and poor in 27 (7.6%), 126 (35.5%), and 76 (21.4%) patients, respectively (23.4% intermediate/poor, 12.1% unknown). 270 patients (76.1%) received only 1L axitinib plus pembrolizumab and 85 patients (24.3%) received ≥1 subsequent line of treatment; cabozantinib was the most frequent subsequent line of treatment (47.9%). rwPFS at 3 months and 1 year was 77.2% and 39.3%, respectively. OS ranged from 90.8% at 3 months to 73.5% at 1 year. Best overall response rate was 47.9%. Toxicity was the most common reason for first TM events of dose hold, change and discontinuation at, 58.6%, 58.5%, and 45.8%, respectively. Over 80% of patients with TM were able to continue with 1L axitinib plus pembrolizumab.
Conclusions: In a real-world setting, axitinib plus pembrolizumab was effective as a 1L treatment for patients with advanced RCC. Dose holds, changes and discontinuation were driven by treatment-related toxicity. Dose holds may represent an effective TM strategy to toxicity.
Introduction
In 2020, there were over 430,000 new cases of kidney cancer and approximately 180,000 associated deaths (1). Renal cell carcinoma (RCC) represents nearly 90% of kidney cancers and an estimated 75% of RCC cases of the clear cell histological subtype (2, 3). The 5-year relative survival rate for patients with localized kidney cancer is almost 93%; however for patients with advanced or metastatic disease, there is a dramatic fall to 14% (4).
The treatment of advanced RCC has recently taken substantial steps forward and continues to rapidly evolve. Existing and emerging first-line regimens include anti-angiogenic and/or immunosuppressive agents. These include vascular endothelial growth factor-receptor (VEGF-R) tyrosine kinase inhibitors (TKI; axitinib, cabozantinib, lenvatinib), and immune checkpoint inhibitors (avelumab, ipilimumab, nivolumab, and pembrolizumab). Treatment selection is partly based on the presence of risk factors as defined by prognostic models, predominantly the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model and the Memorial Sloan Kettering Cancer Center model (5, 6). These models classify patients as favorable, intermediate or poor risk according to the number of risk factors. Combination treatment standards of care (preferred regimens) for patients of any IMDC risk include the combinations axitinib plus pembrolizumab (KEYNOTE-426) (7, 8), cabozantinib plus nivolumab (CheckMate 9ER) (9), and lenvatinib plus pembrolizumab (CLEAR trial) (10). In addition to the aforementioned combinations, nivolumab plus ipilimumab (CheckMate 214) (11, 12) is a preferred first-line treatment option for patients of IMDC intermediate or poor risk (13–15).
The axitinib plus pembrolizumab combination was approved for the first-line treatment of advanced RCC by the FDA in April, 2019, based on the results of the KEYNOTE-426 phase 3 trial (7, 8). In an extended follow-up of the phase 3 trial, axitinib plus pembrolizumab showed sustained clinical benefit compared with single agent sunitinib in both overall survival (OS; median not reached with pembrolizumab and axitinib vs. 35·7 months [95% CI 33·3–not reached] with sunitinib) and median progression-free survival (PFS; 15·4 months [12·7–18·9] vs. 11·1 months [9·1–12·5]; p<0·0001) (7).
In the KEYNOTE-426 phase 3 extended follow-up, treatment-related adverse events led to approximately one fifth of patients discontinuing axitinib treatment and nearly two thirds requiring treatment interruption (7). Understanding the clinical characteristics of patients with advanced RCC treated with axitinib plus pembrolizumab may help identify populations and strategies to optimize treatment duration and potentially improve clinical outcomes. This analysis aimed to describe the demographic and clinical characteristics, treatment patterns, and therapy management of patients with advanced RCC treated with first-line combination axitinib plus pembrolizumab in a US real-world setting.
Methods
Data Source and Patients
EHR-derived data from the nationwide Flatiron Health de-identified database, which includes approximately 280 cancer clinics across ~800 sites of care in the US. Eligible patients had an RCC diagnosis (ICD9 189.x or ICD10 C64x or C654x), with evidence of stage IV or recurrent metastatic RCC with a metastatic diagnosis date on or after January 1, 2011. Patients were included if they were 18 years or older in the year of the index first line therapy prescription, had evidence of pathology consistent with RCC, and had at ≥2 clinic encounters on different days to be included in the study. Among these, 355 patients initiating first-line axitinib + pembrolizumab on or after 1/1/2018 to 3/31/2021, with no prior aRCC treatment were identified for the study. First line therapy cohorts were explored regardless of follow-up time available, except for real-world PFS (rwPFS) and real-world OS (OS) where patients were censored (see below). Baseline characteristics are considered as of the index date, which was defined as the date of first prescription of first-line therapy. This study used only de-identified EHR-derived data. The protocol from Flatiron Health governing data collection had IRB approval with a waiver of informed consent, in accordance with the Health Insurance Portability and Accountability Act.
Derived Variables and Outcomes
IMDC Risk Score was derived by Flatiron Health from individual data components as available in the EHR. When missing data did not allow patients to confidently be grouped into ‘Favorable’, ‘Intermediate’, or ‘Poor’ risk status according to the validated algorithm, Flatiron Health used the additional classifications of ‘Poor/Intermediate’ and ‘Unknown’ Risk.
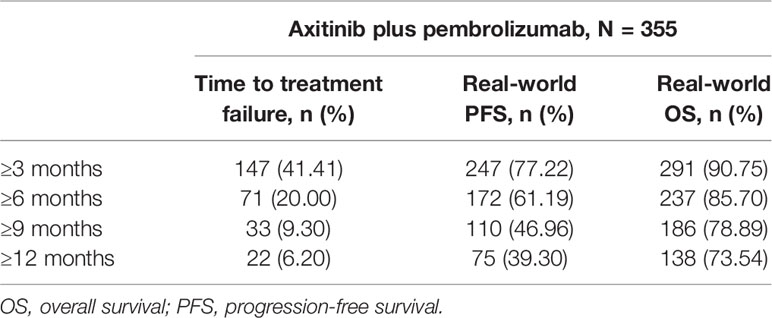
Patient follow-up was defined as time from index date to last recorded activity. Treatment duration was calculated as the time from first treatment date to last treatment date, regardless of any gaps in treatment. Time to treatment failure (TTF) was defined as the time from treatment initiation until treatment discontinuation or therapy change (next line of therapy – switch or augmentation), end of enrolment, or death. Patients were censored in the analysis of rwPFS and OS if they did not experience a respective clinical event (rwPFS: progression/death; OS: death) as of the last confirmed structured or unstructured activity date, and were still alive at the study cut-off date.
Real-world response was defined using response assessment categories that are abstracted based on the healthcare provider’s qualitative description of response to therapy. Best overall response was defined as the maximum response to therapy to first-line therapy (complete response plus partial response). Therapy management events, as documented in the EHR, were defined as dose hold, dose change and discontinuation. For patients with more than one reason for therapy management, a hierarchy was used to select the reason: toxic effect of treatment > progression > cancer-related symptoms not due to treatment > non-cancer related medical issue > financial > patient request > no evidence of disease > insufficient response > other > unknown. Subsequent clinical events such as treatment switches after toxicity-related therapy management were also captured.
Statistical Analysis
Descriptive statistics were used to summarize patient demographic and clinical characteristics, treatment patterns and therapy management, and to tabulate landmark rwPFS TTF and OS. The Kaplan-Meier method was used to estimate rwPFS, rwTFF, and OS curves.
Results
Patients
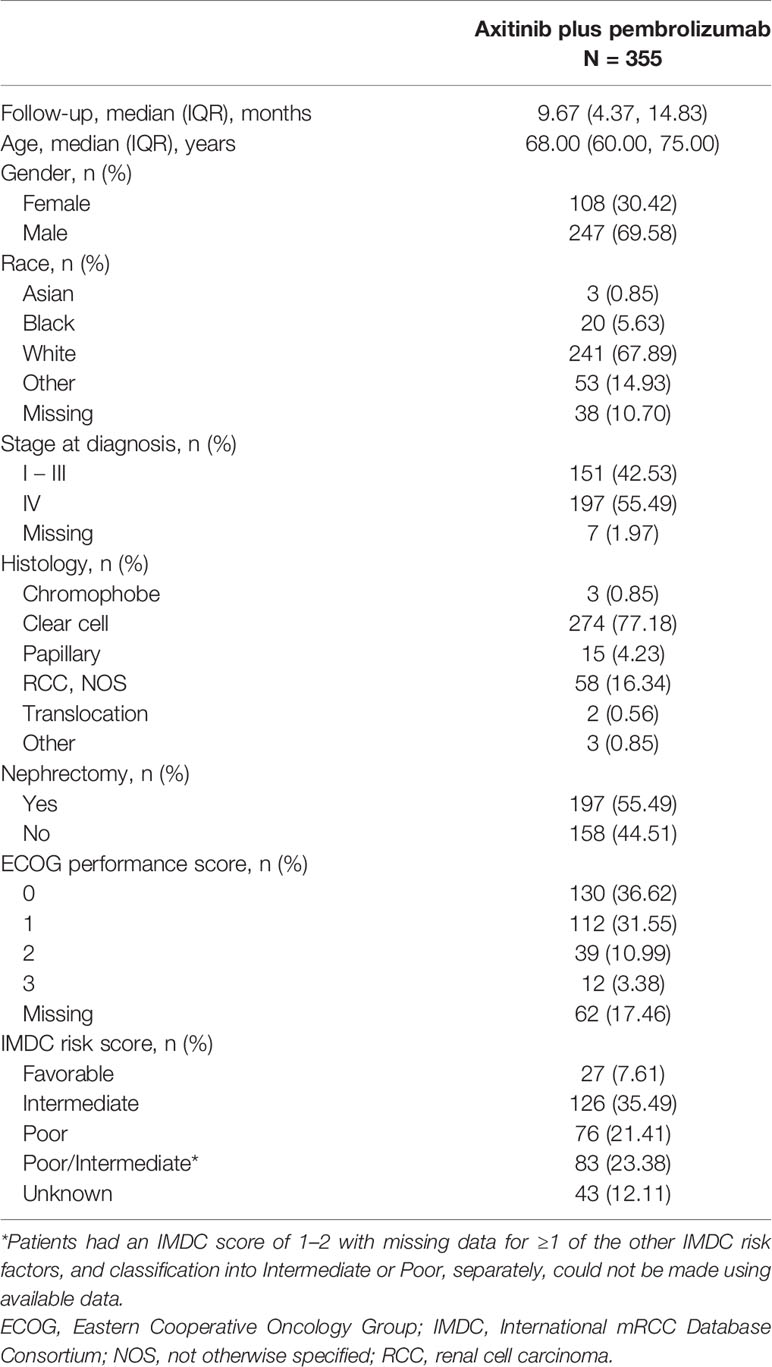
Three hundred and fifty-five patients received first-line axitinib plus pembrolizumab, with a median (IQR) follow-up of 9.67 (4.37-14.83) months. Patient demographics and clinical characteristics are summarized in Table 1. Overall, the majority of patients were of white ethnicity (67.89%), male (69.58%), and median age was 68.00 (60.00-75.00) years. Patients were predominantly treated at community-based practices (n=330, 92.96%), with 25 patients (7.04%) treated at academic-based practices.
At diagnosis, 55.49% of patients had Stage IV, 42.54% had Stage I - III RCC (1.97% Stage at diagnosis unknown), and 77.18% of patients had clear cell histology. More than half of patients had undergone a nephrectomy, almost 70% of patients had a ECOG performance score of 0 or 1, and the largest prognostic group was intermediate IMDC risk (35.5%; Table 1).
Treatment Characteristics
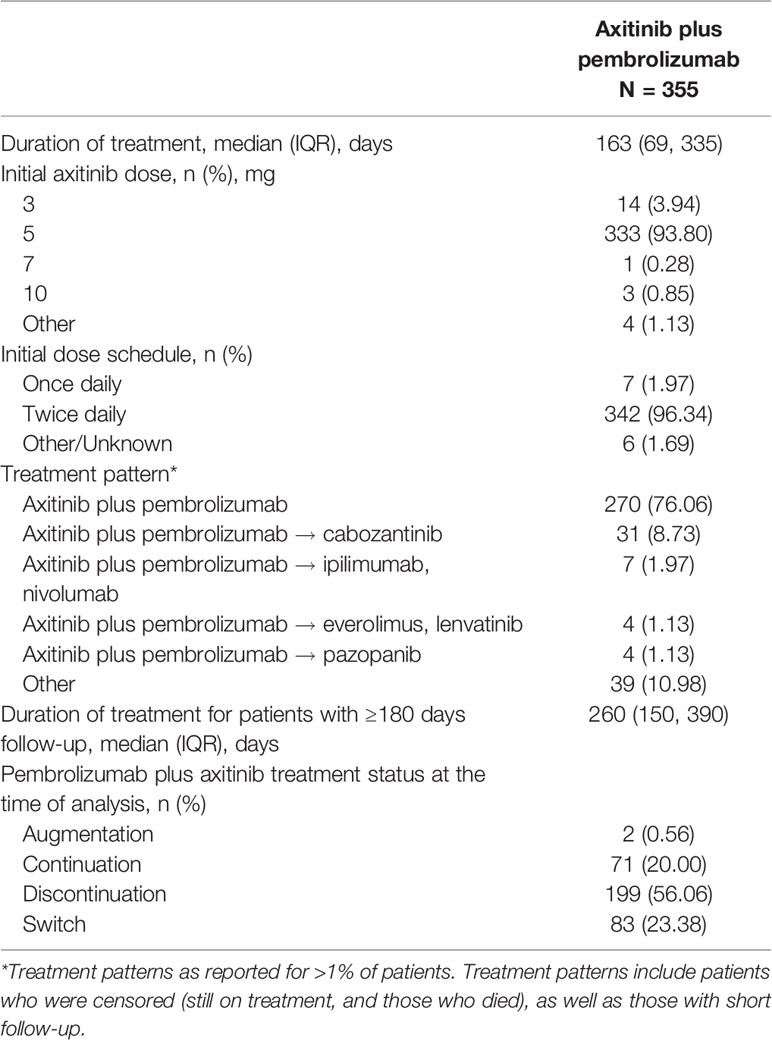
At the time of analysis, 270 patients (76.06%) had initiated first-line therapy with axitinib plus pembrolizumab (Table 2) and had not received subsequent therapy after first-line treatment. Over 50% of all patients had discontinued axitinib plus pembrolizumab treatment at the time of analysis, whereas 20% continued with axitinib plus pembrolizumab treatment (Table 2). The majority of patients received axitinib plus pembrolizumab according to the recommended dose (axitinib 5mg; 93.8%) and dose schedule (twice daily; 96.3%) (Table 2). Median treatment duration was 163 (IQR 69–335) days (censored and uncensored). After controlling for consistent follow-up of 180 days, median treatment duration for 239 patients was 269 (IQR 150-390) (Table 2).
Eighty-five patients (23.9%) received more than one line of therapy, with VEGF-R inhibitors as the most common second-line treatment (51/85 patients, 60.0% in Table 2). Cabozantinib was the most frequently used second-line agent (43/85 patients, 50.1%), followed by nivolumab plus ipilimumab (9/85 patients, 10.6% in Table 2).
Clinical Events and Treatment Effectiveness
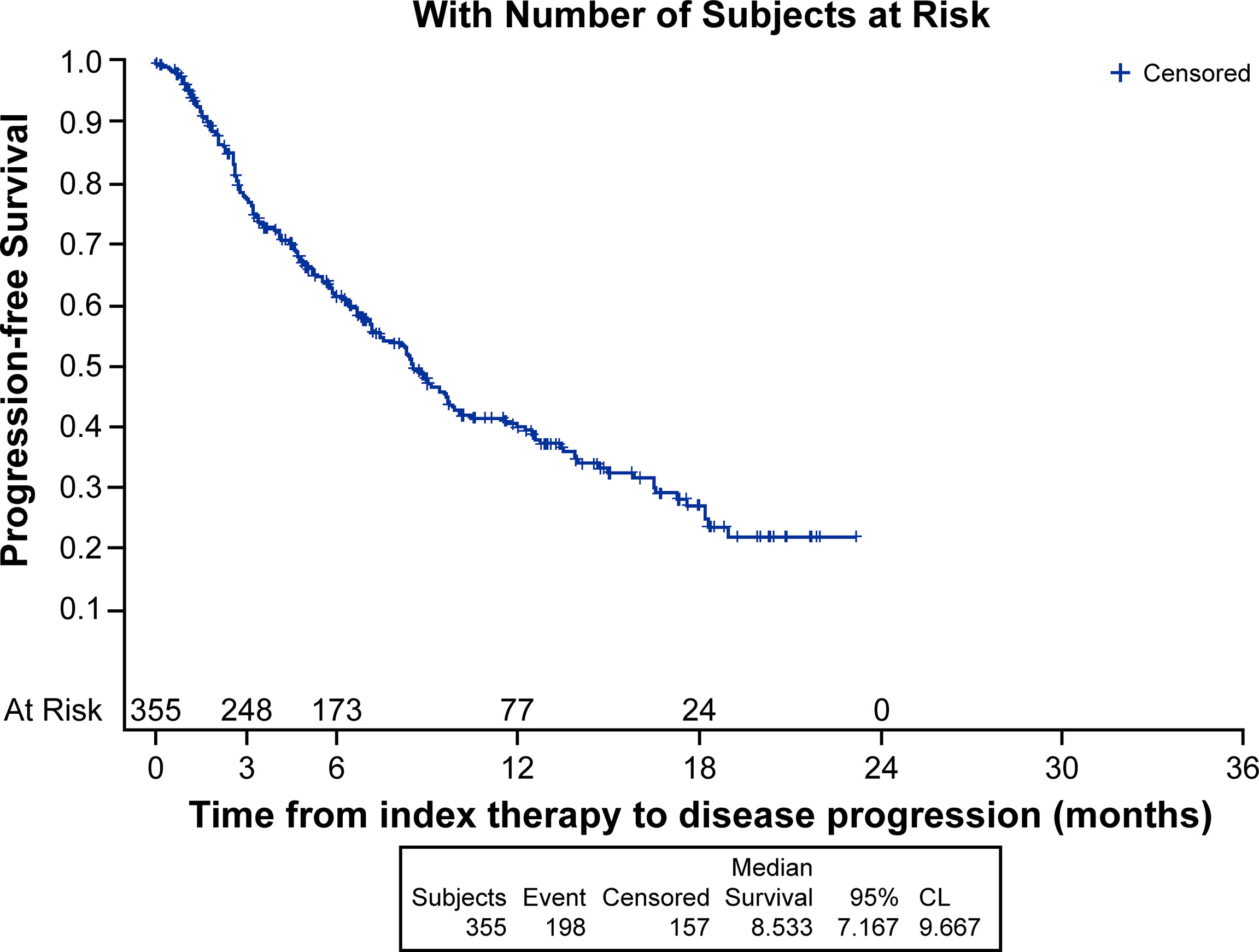
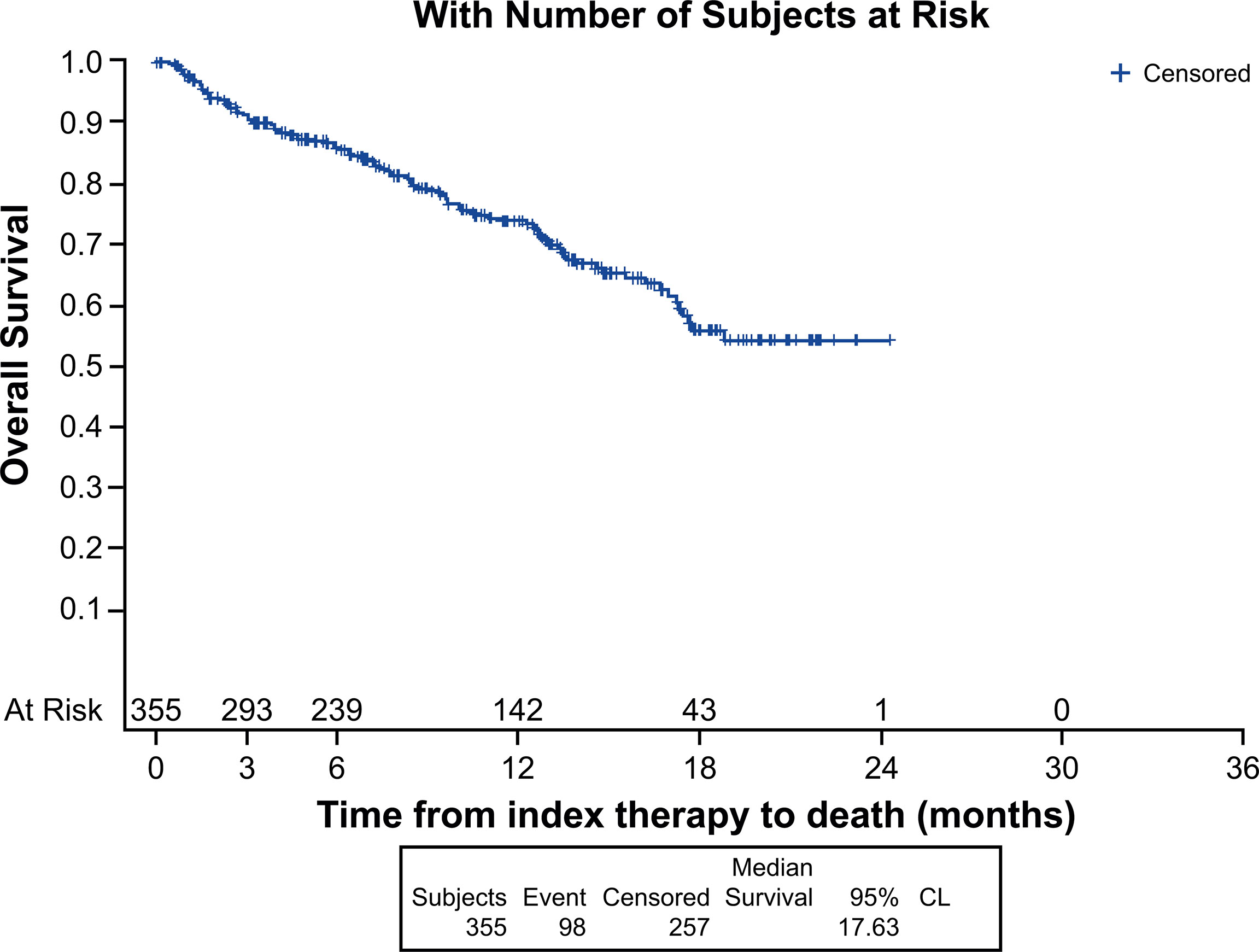
Median (95% CI) Kaplan-Meier estimates of TTF (17.75% censored) [figure not shown] and rwPFS (44% censored) were 2.70 (2.20, 3.00) and 8.53 (7.17, 9.67) (Figure 1) months, respectively. Median OS (257 censored) was not reached (Figure 2). Table 3 describes TTF, rwPFS, and OS during follow-up at specified landmark timepoints (3, 6, 9, and 12 months). Patient response to treatment is summarized in Figure 3. The best overall response rate to axitinib plus pembrolizumab was 47.9% (170/355 patients); complete response was observed in 4.23% of patients and partial response observed in 46.20%.
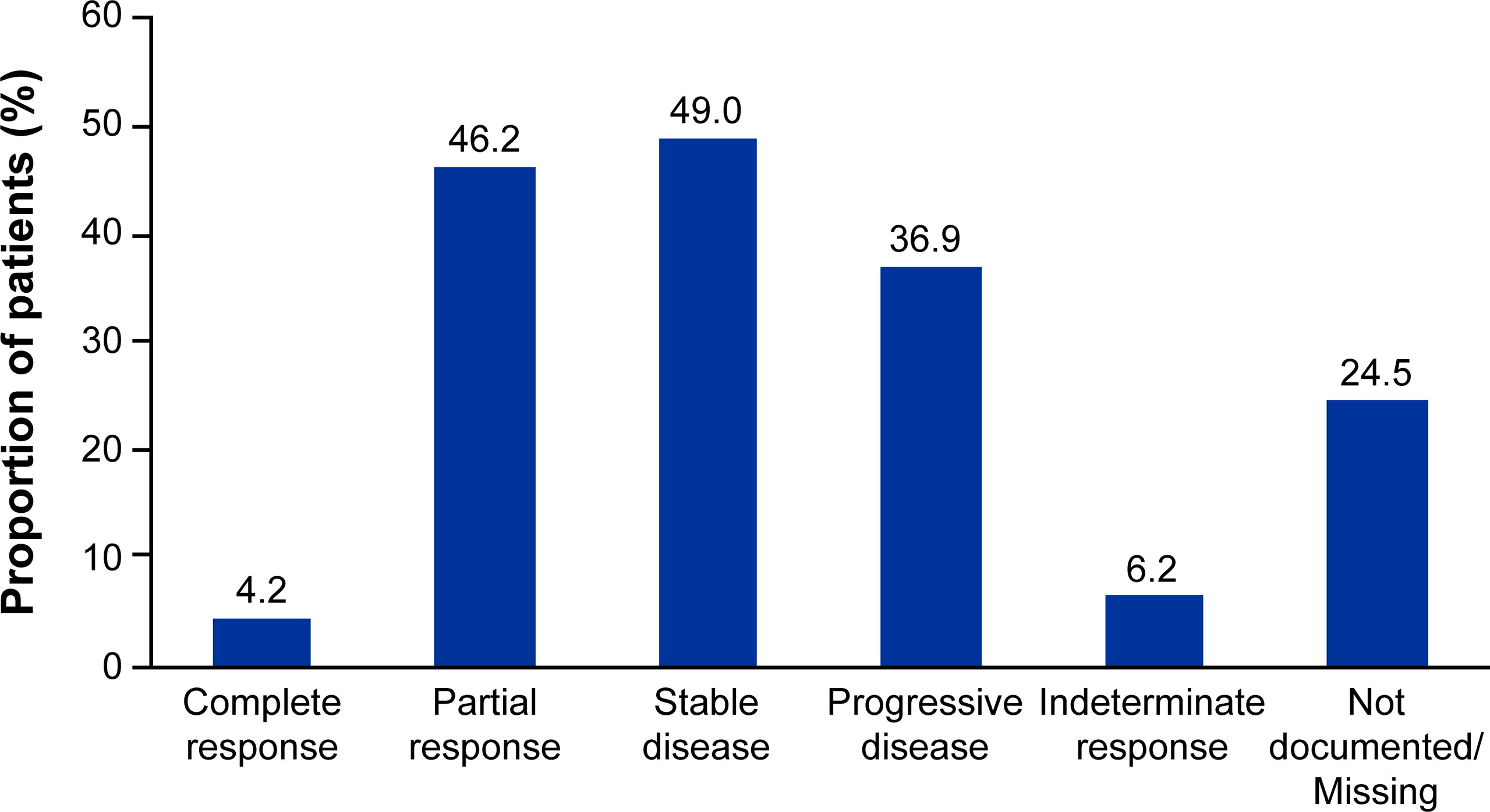
Figure 3 Patient response to first-line axitinib plus pembrolizumab treatment. Responses are not mutually exclusive and represent patient responses throughout the entire follow-up period.
Therapy Management and Subsequent Events
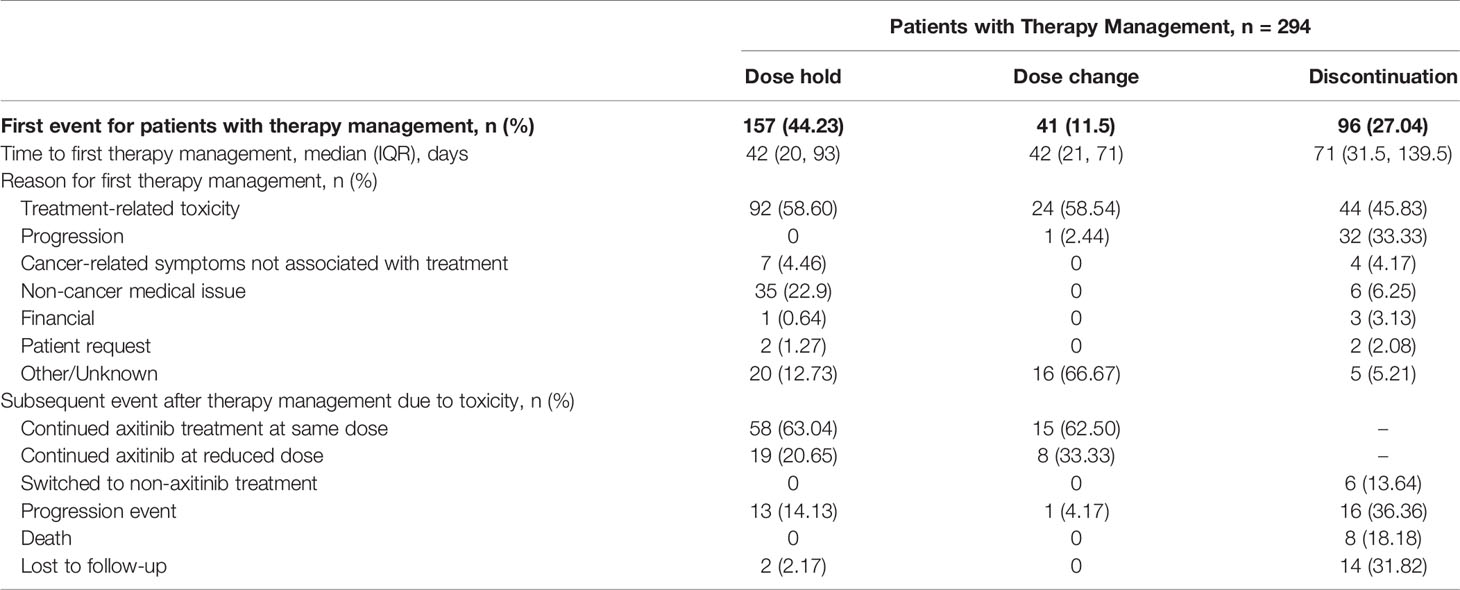
Median duration of treatment for patients without treatment modifications was nominally shorter compared to patients who received treatment modifications at 122 and 176 days, respectively. The most frequent first therapy management was dose hold (157 patients, 44.23%), followed by discontinuation (96 patients, 27.04%) and then dose change (41 patients, 11.55%). For each type of therapy management, toxicity related to treatment was the most common reason (Table 4). Time to first therapy management was similar for dose hold (42 days) and dose change (42 days), but nominally longer for discontinuation (71 days). Subsequent events following therapy management due to toxicity of therapy are summarized in Figure 4. The majority of patients who had a dose hold or change as their first therapy management (due to toxicity of therapy) continued treatment with axitinib plus pembrolizumab at their current dose, or at a reduced dose after therapy management (Table 4). For patients whose first therapy management was discontinuation due to toxicity of therapy, 13.6% (6/44) switched to another treatment (Table 4).
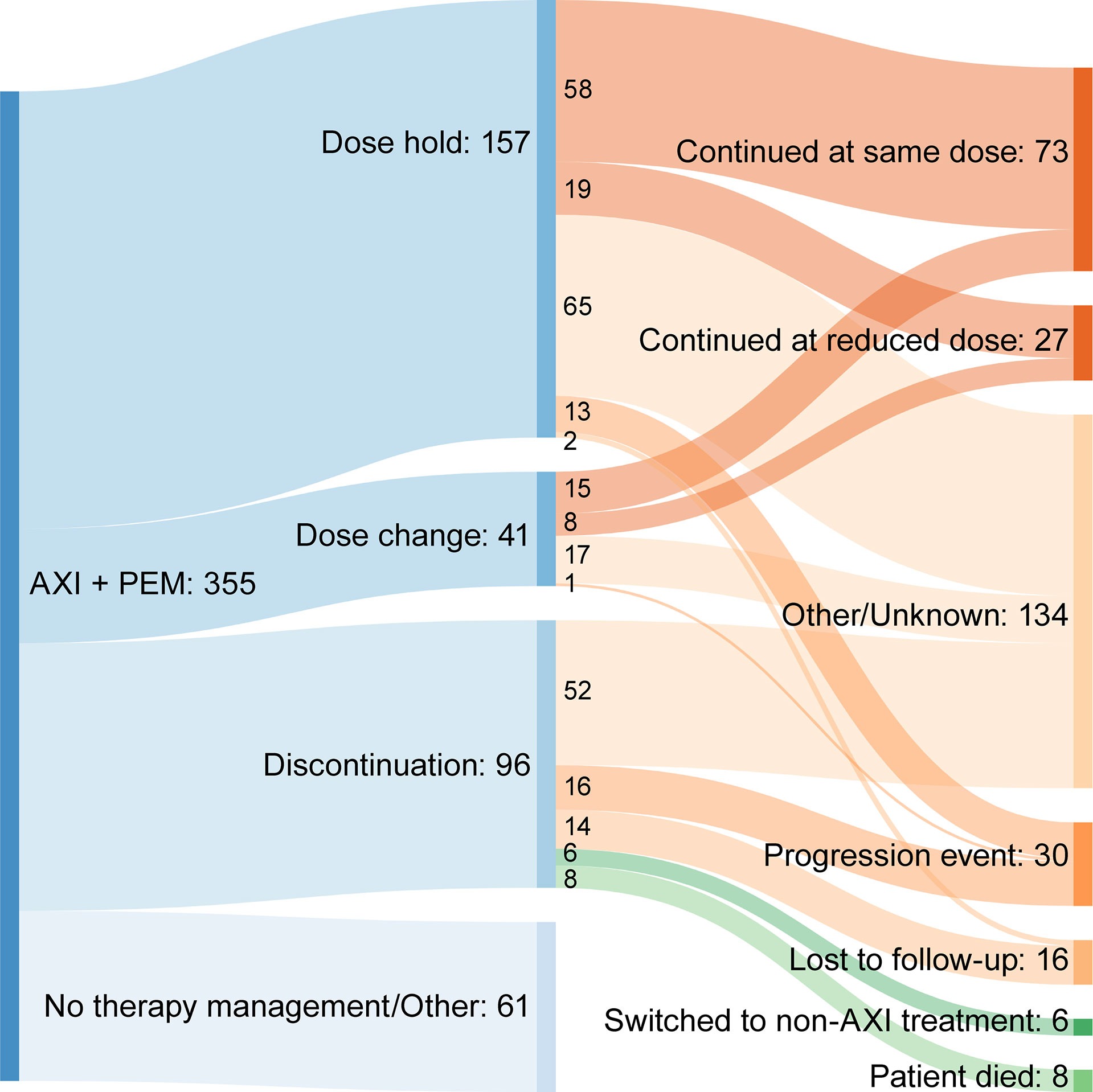
Figure 4 Sankey diagram summarizing subsequent events following therapy management due to toxicity. AXI + PEM, axitinib plus pembrolizumab.
Discussion
The introduction of combination anti-angiogenic agents with immunotherapy agents to treat patients with advanced RCC has provided substantial clinical benefit; however, this has also added to the complexity of managing treatment-related toxicity. For TKIs, such as axitinib, treatment-related toxicity may be managed by dose holds or reduction, whereas, for immunotherapy agents such as pembrolizumab, toxicity management may include the administration of immunosuppressants, such as corticosteroids (16). Some of the treatment-related adverse events associated with anti-angiogenic TKIs are similar to those seen with immunotherapy agents and may be additive as well as overlapping, which adds to the complexity of identifying the etiology and managing accordingly (16). In our analysis, the most common first therapy management for patients receiving axitinib plus pembrolizumab was dose hold (44.2%), followed by discontinuation (27.0%) and then dose change (11.5%). In each instance, the most frequent reason for therapy management was treatment-related toxicity. Although the etiology of the treatment-related toxicity was not recorded in our analysis, our data support the use of dose hold as an effective means of treatment management. Following dose hold, nearly 85% of patients with a dose hold due to toxicity of therapy continued their current axitinib dose, or continued axitinib combination therapy at a modified dose or schedule. Similarly, for patients whose first therapy management was dose modification (reduction), over 90% with a dose modification due to toxicity of therapy were able to continue receiving axitinib plus pembrolizumab. These results are of supportive of the implications of previous research suggesting that dose titration may be a potential means for improving outcomes (17).
Patients who discontinued treatment as a first therapy management had a median time to discontinuation of 71 days, representing approximately two cycles of axitinib treatment. Almost half of these discontinuations were due to treatment-related toxicity. This represents a sizable proportion of patients who discontinue before any other therapy management strategies are employed. Following axitinib plus pembrolizumab discontinuation due to toxicity, almost 14% switched to another treatment after discontinuing. These patients may represent a group who may benefit from optimized therapy management. As several different combinations of immunotherapy and TKIs are approved in metastatic RCC and other malignancies, it is critical for the prescribing providers to be comfortable managing any adverse events and be familiar with the strategies of holding and modifying the responsible agents according to the grade of toxicities and prior to premature discontinuation, which may negatively impact the clinical outcome.
To our knowledge, this analysis is the first-real-world data reporting patient characteristics, treatment management and outcomes in patients with advanced RCC who received first-line axitinib plus pembrolizumab. Randomized controlled trials (RCTs) generally utilize stringent eligibility criteria to ensure high internal validity to help answer a specific clinical question. As a result, the RCT population of patients do not always share the same diversity as those seen in a real-world clinical setting (18). In real-world practice, patients with advanced RCC treated with anti-angiogenic TKIs have been shown to be of less favorable prognosis, have more co-morbidities and worse outcomes compared with patients enrolled on RCTs (19, 20). Consistently, there were fewer patients from our analysis of predominantly community-based US clinical practices, who were of IMDC favorable risk (7.6%), compared with those who enrolled on the pivotal phase 3 trial (32%) (7, 8). Notably, 12.1% of patients in our analysis were of unknown IMDC risk score and 23.4% could not confidently be classed as only intermediate or poor risk due to missing data. This means that it is possible >50% of the analyzed population may have been of poor IMDC risk. Despite this, response to axitinib plus pembrolizumab in our analysis was of a similar magnitude compared with that reported in KEYNOTE-426 (ORR, 47.9% vs. 60%, respectively) (7). KEYNOTE-426 also noted the most common reason for discontinuation of therapy among all patients in the analysis was disease progression (7). This analysis, however, did not present the most common reason for discontinuation among all patients who discontinued; rather, but reported the most common reasons for discontinuation only when discontinuation was the first therapy management ‘event’ a patient experienced after initiation of first-line axitinib plus pembrolizumab. In a separate analysis that utilized the Flatiron database, patients with advanced RCC treated with first-line axitinib plus pembrolizumab had 12-month rwPFS and OS of 41.4% and 68.5%, respectively (21). These estimates are similar to the wPFS (39.3%) and OS (73.5%) at 12 months reported in our analysis.
The main limitation of this analysis was the short follow-up period of approximately 10 months. This resulted in a relatively short duration of treatment and the number of patients with ≥180 days follow-up was insufficient to allow for a meaningful analysis of therapy management events. This impacted the analysis of outcomes and treatment patterns. The inclusion of patients with short follow-up may have artificially decreased the proportion of first-line axitinib plus pembrolizumab patients who advanced to subsequent treatment. It should also be noted that for therapy management (dose hold, change, discontinuation), the denominator was patients who had a therapy management event attributable to toxicity of treatment, not patients who experienced toxicity of treatment. Specific adverse events, including those indicating toxicity of treatment, were not explicitly captured. Therefore, the category of patients who received therapy management may not represent all patients who experienced toxicity due to therapy. As with many real-world studies, missing data, or data elements not captured, may suggest that some conclusions drawn from this analysis need further investigation. Such examples relevant to this analysis are the lack of information from this RWD source about the absence or presence of sarcomatoid tumor features, or type and grade of toxicity leading to treatment discontinuation. Nevertheless, this analysis provides an important insight into the clinical characteristics and therapy management of patients with advanced RCC receiving axitinib plus pembrolizumab in a real-world setting. Furthermore, it identifies dose hold as a potentially effective treatment management strategy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Flatiron Health governing data collection IRB with a waiver of informed consent, in accordance with the Health Insurance Portability and Accountability Act. This study used only de-identified EHR-derived data. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and design: All authors. Performed research: All authors. Contributed new reagents or analytic tools: N/A. Analysis and interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that this study received funding from Pfizer Inc. RL, BL, GS, GZ, DT, AV, and DR were employees of Pfizer Inc. The funder had the following involvement with the study: Pfizer-affiliated authors were involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Medical writing support was provided by Leon Adams, PhD, of Engage Scientific Solutions, and was funded was by Pfizer.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The Epidemiology of Renal Cell Carcinoma. Eur Urol (2011) 60(4):615–21. doi: 10.1016/j.eururo.2011.06.049
3. Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, et al. Understanding Pathologic Variants of Renal Cell Carcinoma: Distilling Therapeutic Opportunities From Biologic Complexity. Eur Urol (2015) 67(1):85–97. doi: 10.1016/j.eururo.2014.04.029
4. Institute NCCancer Stat Facts: Kidney and Renal Pelvis Cancer (2021). Available at: https://seer.cancer.gov/statfacts/html/kidrp.html (Accessed October, 2021).
5. Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External Validation and Comparison With Other Models of the International Metastatic Renal-Cell Carcinoma Database Consortium Prognostic Model: A Population-Based Study. Lancet Oncol (2013) 14(2):141–8. doi: 10.1016/S1470-2045(12)70559-4
6. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. J Clin Oncol (2002) 20(1):289–96. doi: 10.1200/JCO.2002.20.1.289
7. Powles T, Plimack ER, Soulieres D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab Plus Axitinib Versus Sunitinib Monotherapy as First-Line Treatment of Advanced Renal Cell Carcinoma (KEYNOTE-426): Extended Follow-Up From a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2020) 21(12):1563–73. doi: 10.1016/S1470-2045(20)30436-8
8. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
9. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab Plus Cabozantinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
10. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib Plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med (2021) 384(14):1289–300. doi: 10.1056/NEJMoa2035716
11. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab Plus Ipilimumab Versus Sunitinib for First-Line Treatment of Advanced Renal Cell Carcinoma: Extended 4-Year Follow-Up of the Phase III CheckMate 214 Trial. ESMO Open (2020) 5(6):e001079. doi: 10.1136/esmoopen-2020-001079
12. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
13. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann Oncol (2019) 30(5):706–20. doi: 10.1093/annonc/mdz056
14. ESMO. Eupdate – Renal Cell Carcinoma Treatment Recommendations (2021). Available at: https://www.esmo.org/guidelines/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations-4 (Accessed October, 2021).
15. NCCN. Clinical Practice Guidelines Oncology: Kidney Cancer Version 2.2022 (2021). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1440 (Accessed October, 2021).
16. Grunwald V, Voss MH, Rini BI, Powles T, Albiges L, Giles RH, et al. Axitinib Plus Immune Checkpoint Inhibitor: Evidence- and Expert-Based Consensus Recommendation for Treatment Optimisation and Management of Related Adverse Events. Br J Cancer (2020) 123(6):898–904. doi: 10.1038/s41416-020-0949-9
17. Rini BI, Melichar B, Ueda T, Grunwald V, Fishman MN, Arranz JA, et al. Axitinib With or Without Dose Titration for First-Line Metastatic Renal-Cell Carcinoma: A Randomised Double-Blind Phase 2 Trial. Lancet Oncol (2013) 14(12):1233–42. doi: 10.1016/S1470-2045(13)70464-9
18. Rothwell PM. Commentary: External Validity of Results of Randomized Trials: Disentangling a Complex Concept. Int J Epidemiol (2010) 39(1):94–6. doi: 10.1093/ije/dyp305
19. Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of Patients With Metastatic Renal Cell Carcinoma That do Not Meet Eligibility Criteria for Clinical Trials. Ann Oncol (2014) 25(1):149–54. doi: 10.1093/annonc/mdt492
20. Mitchell AP, Harrison MR, Walker MS, George DJ, Abernethy AP, Hirsch BR. Clinical Trial Participants With Metastatic Renal Cell Carcinoma Differ From Patients Treated in Real-World Practice. J Oncol Pract (2015) 11(6):491–7. doi: 10.1200/JOP.2015.004929
21. Zarrabi K, Handorf EA, Miron B, Zibelman MR, Anari F, Ghatalia P, et al. Real-World Outcomes in Patients With Metastatic Clear Cell Renal Cell Carcinoma Receiving Front-Line Axitinib Plus Pembrolizumab Versus Ipilimumab Plus Nivolumab. J Clin Oncol (2021) 39(15_suppl):4551. doi: 10.1200/JCO.2021.39.15_suppl.4551
Keywords: axitinib, combination, renal cell carcinoma, real-world, therapy management
Citation: Zakharia Y, Thomaidou D, Li B, Siu G, Levin R, Vlahiotis A, Rao D and Zanotti G (2022) Real-World Therapy Management and Outcomes of First-Line Axitinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma in the United States. Front. Oncol. 12:861189. doi: 10.3389/fonc.2022.861189
Received: 24 January 2022; Accepted: 21 March 2022;
Published: 19 May 2022.
Edited by:
Marijo Bilusic, University of Miami Health System, United StatesReviewed by:
Rosa M. Nadal, National Heart, Lung, and Blood Institute (NIH), United StatesRaffaele Ratta, Hôpital Foch, France
Javier Molina-Cerrillo, Ramón y Cajal University Hospital, Spain
Copyright © 2022 Zakharia, Thomaidou, Li, Siu, Levin, Vlahiotis, Rao and Zanotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yousef Zakharia, eW91c2VmLXpha2hhcmlhQHVpb3dhLmVkdQ==
 Yousef Zakharia
Yousef Zakharia Despina Thomaidou2
Despina Thomaidou2 Rebecca Levin
Rebecca Levin