- 1Department of Neurosurgery, Tangdu Hospital, Airforce Medical University, Xi’an, China
- 2Department of Health Statistics, Airforce Medical University, Xi’an, China
- 3National Time Service Center, Chinese Academy of Sciences, Xi’an, China
- 4School of Optoelectronics, University of Chinese Academy of Sciences, Beijing, China
Objective: To design a multidisciplinary enhanced recovery after surgery (ERAS) protocol for glioma patients undergoing elective craniotomy and evaluate its clinical efficacy and safety after implementation in a tertiary neurosurgical center in China.
Methods: ERAS protocol for glioma patients was developed and modified based on the best available evidence. Patients undergoing elective craniotomy for treatment of glioma between September 2019 to May 2021 were enrolled in a randomized clinical trial comparing a conventional neurosurgical perioperative care (control group) to an ERAS protocol (ERAS group). The primary outcome was postoperative hospital length of stay (LOS). Secondary outcomes were 30-day readmission rate, postoperative complications, duration of the drainage tube, time to first oral fluid intake, time to ambulation and functional recovery status.
Results: A total of 151 patients were enrolled (ERAS group: n = 80; control group: n = 71). Compared with the control group, postoperative LOS was significantly shorter in the ERAS group (median: 5 days vs. 7 days, p<0.0001). No 30-day readmission or reoperation occurred in either group. The time of first oral intake, urinary catheter removal within 24 h and early ambulation on postoperative day (POD) 1 were earlier and shorter in the ERAS group compared with the control group (p<0.001). No statistical difference was observed between the two groups in terms of surgical- and nonsurgical-related complications. Functional recovery in terms of Karnofsky Performance Status (KPS) scores both at discharge and 30-day follow-up was similar in the two groups. Moreover, no significant difference was found between the two groups in the Hospital Anxiety and Depression Scale (HADS) scores.
Conclusion: The implementation of the ERAS protocol for glioma patients offers significant benefits over conventional neurosurgical perioperative management, as it is associated with enhancing postoperative recovery, without additional perioperative complications and risks.
Clinical Trial Registration: Chinese Clinical Trial Registry (http://www.chictr.org.cn/showproj.aspx?proj=42016), identifier ChiCTR1900025108
Introduction
Glioma is the most frequent primary malignant brain tumor in adults. Despite significant advances in treatment, the prognosis remains poor. Glioblastoma (GBM) is the most common and lethal subtype, accounting for 49.1% of primary malignant brain tumors. Its median survival time is about 14.4 months (1). Advancements in standard treatment regimens, including maximal safe resection, radiotherapy with concomitant temozolomide (TMZ), maintenance chemotherapy, tumor treatment field (TTF), immunotherapy and targeted therapy, have improved survival rates in patients with low- and high-grade gliomas in the past few decades. Therefore, assessment and improvement of the patient’s functional status in each treatment section have been emphasized.
Enhanced recovery after surgery (ERAS) protocols have been widely adopted in diverse surgical specialties. As a patient-centered perioperative model, ERAS offers several clinical advantages, including reduced surgical stress response, accelerated postoperative recovery, shortened hospital length of stay (LOS) and improved overall satisfaction (2, 3). In addition, our previous study confirmed the feasibility and safety of the ERAS protocol in neurosurgery (4). However, despite the diversity of neurosurgical diseases, there is a paucity of literature on ERAS protocols for various neurological diseases, especially gliomas.
Herein, we designed and implemented a multidisciplinary ERAS protocol for glioma patients undergoing elective craniotomy and evaluated its clinical efficacy and safety in a large tertiary hospital in China. The effect of ERAS protocol on perioperative rehabilitation was explored to provide evidence for the establishment of disease-specific neurosurgical ERAS protocol for patients with gliomas.
Materials and methods
Patient Enrollment
This registry study was conducted in the Department of Neurosurgery, Tangdu Hospital, Air Force Military Medical University (Xi’an, China). The study protocol was approved by the institutional ethics committee of Tangdu Hospital (No. 201907-10) and registered at the Chinese Clinical Trial Registry (ChiCTR1900025108, http://www.chictr.org.cn/showproj.aspx?proj=42016) before implementation. Written informed consent for participation was obtained from all participants. The protocol adheres to the principles outlined in the US Code of Federal Regulations, Title 45, Part 46, Protection of Human Subjects, revised June 23, 2005, and the World Medical Association Declaration of Helsinki.
From September 2019 to May 2021, consecutive patients who underwent elective craniotomy for treatment of glioma at Tangdu Hospital were recruited. The inclusion criteria were as follows: aged 18-65 years old, Karnofsky Performance Score (KPS) ≥70, initially diagnosed supratentorial glioma and lesion involving less than 3 lobes. Patients who needed emergency surgery and with radio- or chemotherapy history were excluded.
From September 2019 to August 2020, patients received a standard neurosurgical care protocol (control group). In June 2020, a neurosurgical ERAS protocol was implemented for glioma patients who received elective craniotomy (ERAS group). Primary outcomes were postoperative hospital length of stay (LOS) and total hospitalization costs. Secondary outcomes were 30-day readmission rate, postoperative complications, duration of the drainage tube, time to first oral fluid intake, time to ambulation and functional recovery status. All patients were followed for 30 days after hospital discharge.
ERAS Protocol for Gliomas
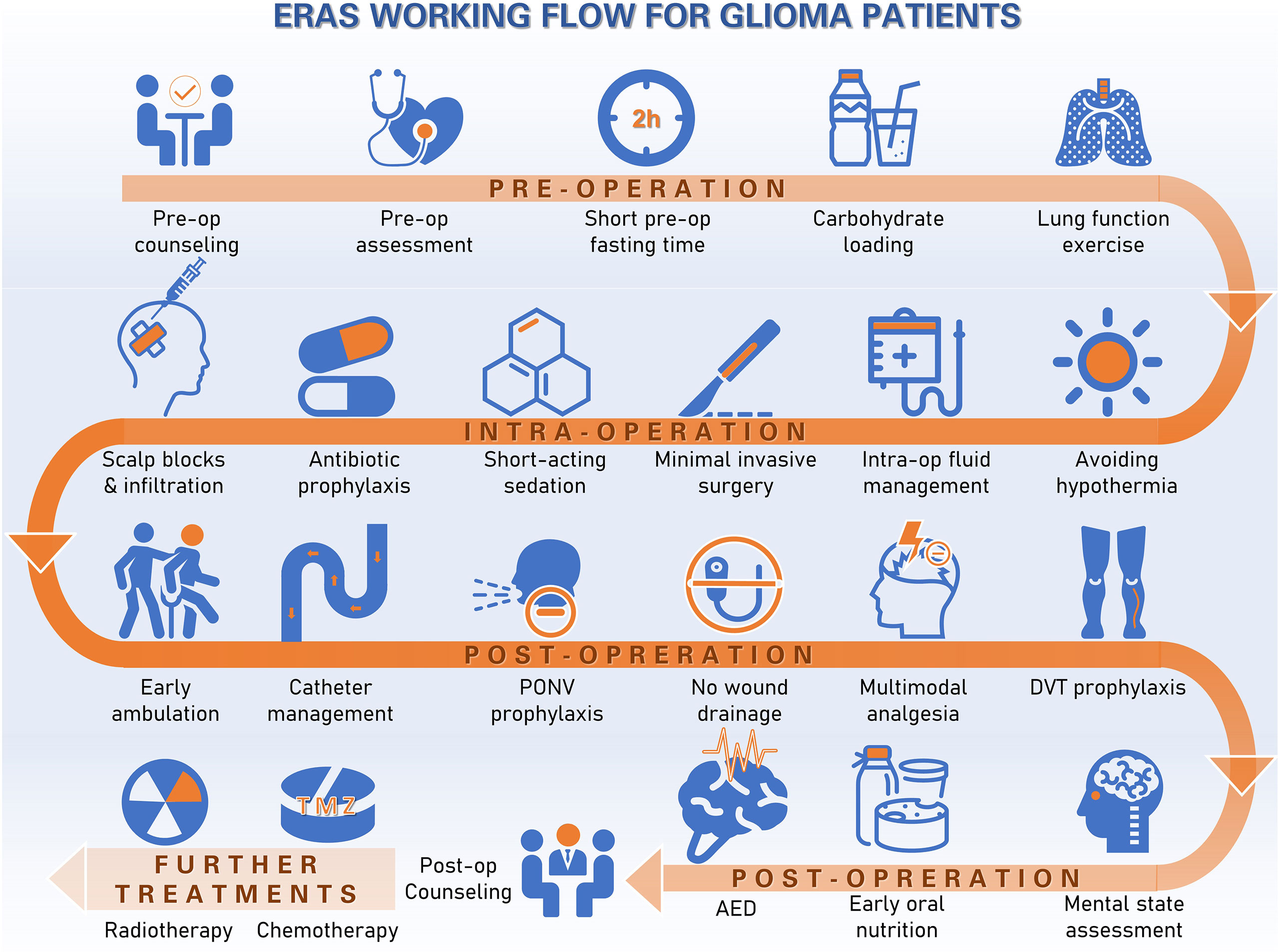
Details of our institutional ERAS protocol have been previously described (4). The protocol complies with the ERAS society research reporting guidelines (5). Briefly, our ERAS protocol for glioma patients consists of three sections: preoperative, intraoperative and postoperative intervention. Key items include patient and family education, baseline functional and nutritional assessment, smoking and alcohol abstinence, limited fasting, carbohydrate loading, no long-acting sedation, antibiotics prophylaxis, scalp block and local incision analgesia, hypothermia avoidance, goal-directed fluid balance, minimally invasive approaches and techniques for the craniotomy, early termination of the drainage tube, postoperative nausea and vomiting (PONV) assessment, preventive opioid-sparing multimodal analgesia, early mobilization and ambulation, deep vein thrombosis (DVT) prophylaxis and early oral feeding within 24 h. The glioma ERAS protocol was further modified to include extra measures focusing on stressor evaluation, such as mental status. The working flow of ERAS protocol for gliomas was summarized in Figure 1.
The hospital anxiety and depression scale (HADS) was applied twice to grade the patient’s psychological status at admission and discharge. HADS has comprised two subcomponents: depression (HADS-D) and anxiety (HADS-A) (6). Changes in HADS scores from admission to discharge were calculated. Minimum clinically important difference (MCID) was also used to detect the changes in HADS scores to assess the mental health of glioma patients. A 1.7-point reduction was set as the indicator value (7).
Perioperative management of patients in the control group was conducted based on the current standard neurosurgical care at our institution. Some elements routinely implemented in clinical practice (e.g., antibiotic prophylaxis, smoking cessation education, etc.) were also applied in the control group.
Patients were discharged according to well-defined discharge criteria, including full consciousness, adequate pain control, body temperature within normal range, ability to take adequate food without the need for intravenous nutrition, effective wound healing and major laboratory tests within normal limits. A discharge readiness assessment was conducted by a senior attending neurosurgeon who was not involved in the study. Clinical or telephone follow-ups were conducted one month after discharge. Adverse events data and KPS scores were collected during follow-up to evaluate the functional status of patients.
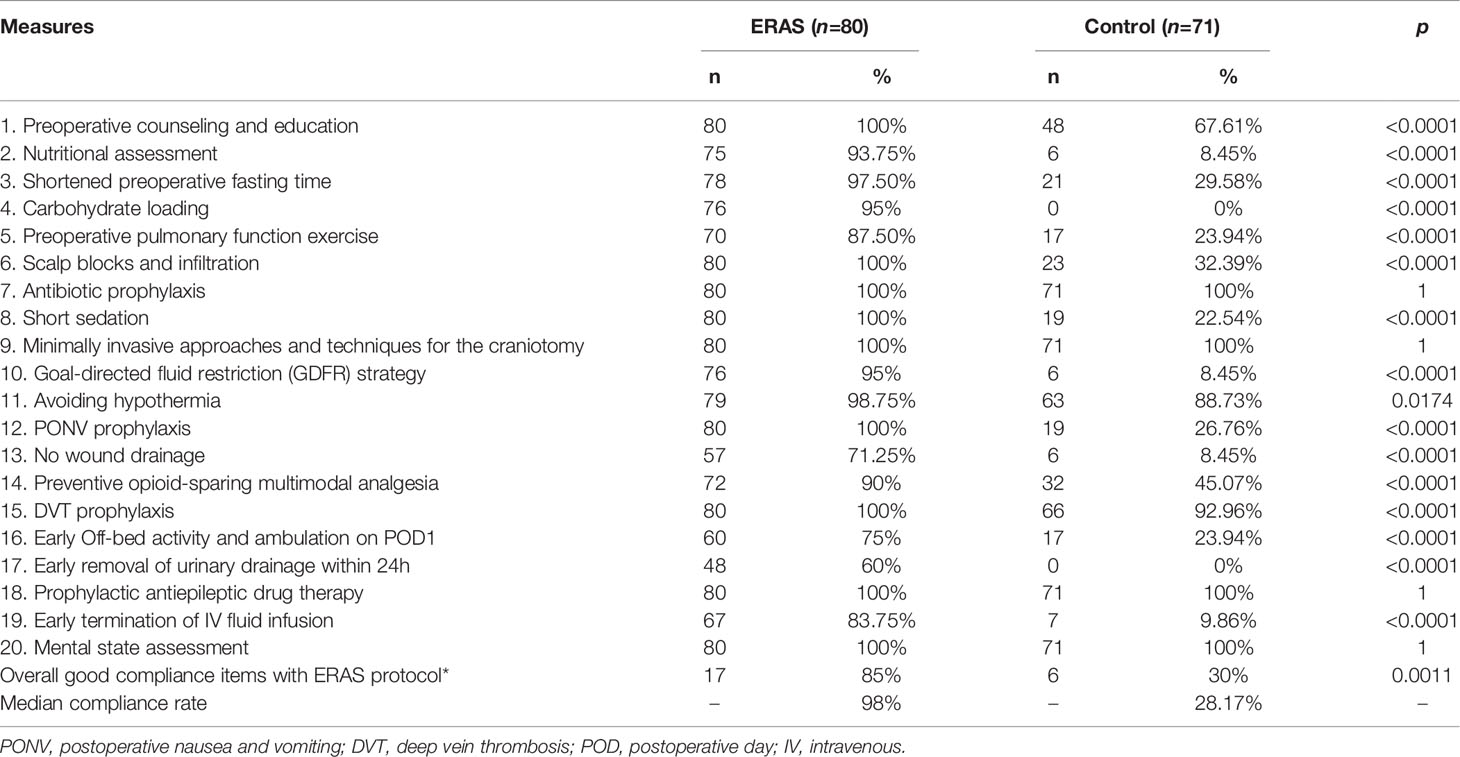
The medical record system and the ERAS Record Sheet were used to collect perioperative data. Overall compliance to 20 key ERAS measures was assessed and expressed as a percentage. Good compliance was defined as >80% score per item and/or per patient.
In order to analyze the effect of surgical start time, two surgical cohorts were created based on patients receiving surgery before or after 2 PM. Early cohort (before 2 PM) and later cohort (after 2 PM) were stratified in both groups.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median [interquartile range (IQR)], and categorical data were presented as numbers (percentages). The student’s t-test was used to compare two groups of normally distributed continuous data. The Mann-Whitney U test was used to compare differences between non-normally distributed variables. The chi-square tests or Fisher exact tests were used to compare categorical variables. Statistical significance was defined as p<0.05. All statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA).
Results
Patient Sociodemographic and Clinical Characteristics
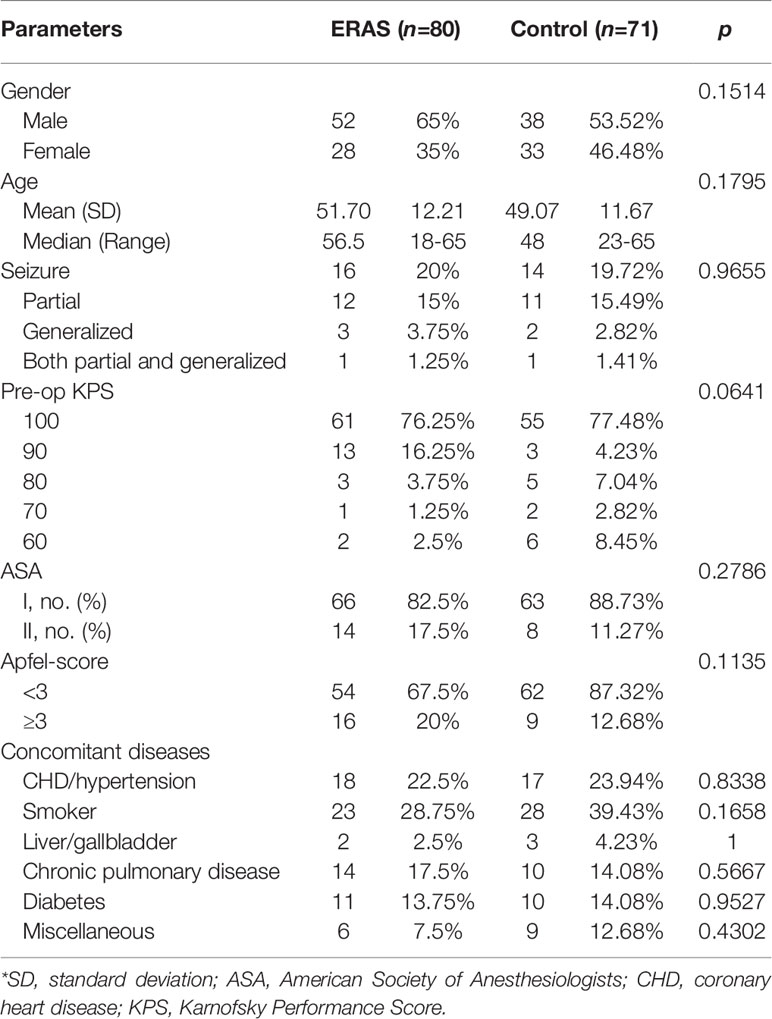
A total of 166 patients diagnosed with glioma and received neurosurgical treatment were recruited. Nine patients declined informed consent. Five patients were lost clinical follow-up due to long distance or COVID-19 travel restrictions. One patient was lost follow-up due to refuse phone or other connection. A total of 151 patients were included in the final analysis (80 in the ERAS group and 71 in the control group, Diagram 1). No significant difference was found in baseline clinical characteristics between the two groups (Table 1).
The average age was 51.7 ± 12.21 in the ERAS group and 49.07 ± 11.67 in the control group (p = 0.1795). There were more males than females in both groups, but without notable differences [ERAS group: 52 males (65%) and 28 females (35%); control group: 38 males (53.52%) and 33 females (46.48%)]. No significant difference was found for concomitant diseases (Table 1).
For glioma pathology diagnosis, WHO grade IV GBM was the major type in both groups, including 43 cases (53.75%) in the ERAS group and 44 cases (61.97%) in the control group. The tumor location was summarized in Table 2. Gliomas mainly invaded the frontal lobe [ERAS group: 19 cases (23.75%); control group: 23 cases (32.39%)], temporal lobe [ERAS group: 12 cases (15%); control group: 7 cases (9.86%)] and parietal lobe [ERAS group: 8 cases (10%); control group: 5 cases (7.04%)]. No significant difference was found between the two groups regarding pathological diagnosis (p = 0.8055).
Compliance With the ERAS Protocol
The target compliance rate for the ERAS protocol in our center was above 80%. Key measures of the ERAS protocol for glioma patients are summarized in Table 3. The overall median overall compliance rate was 98% in the ERAS group and 28.17% in the control group (p<0.001). For the purpose of analysis, we also divided the ERAS protocol into pre-operative (5 items), intra-operative (6 items) and post-operative (9 items) measures (Figure 2).
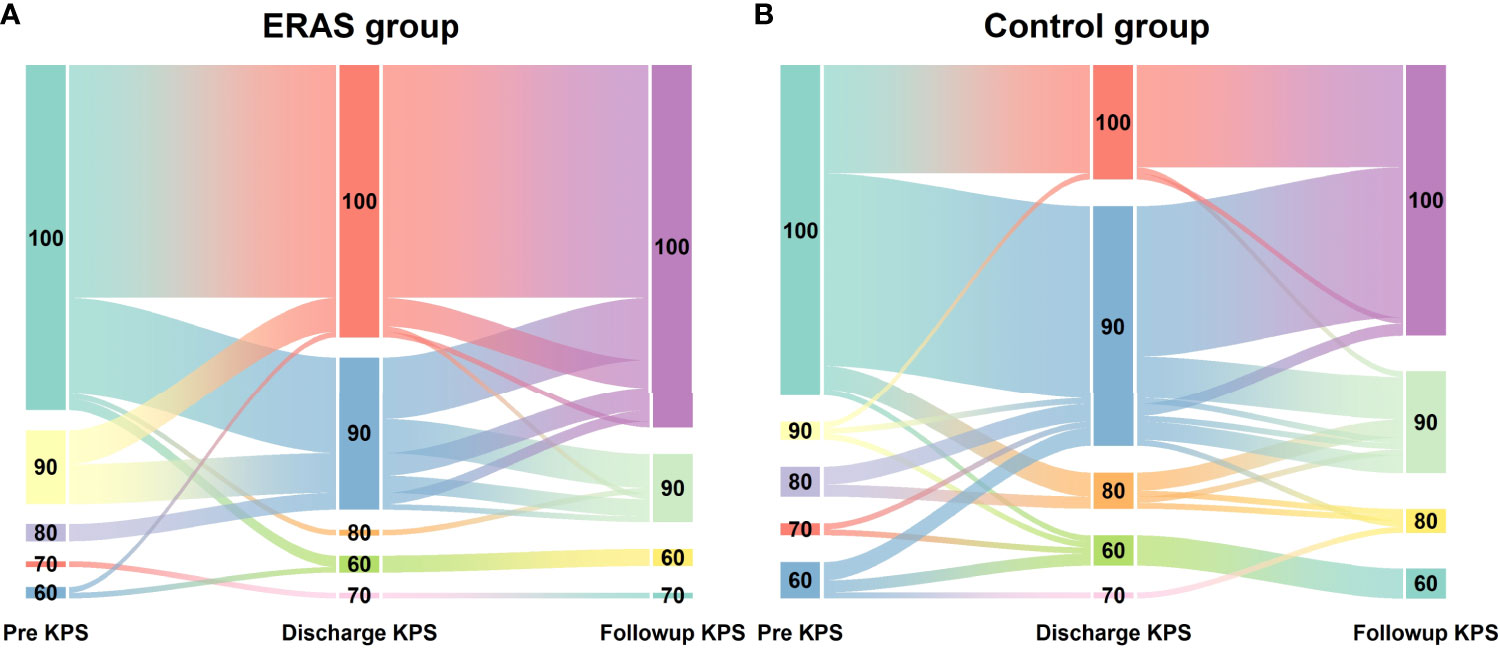
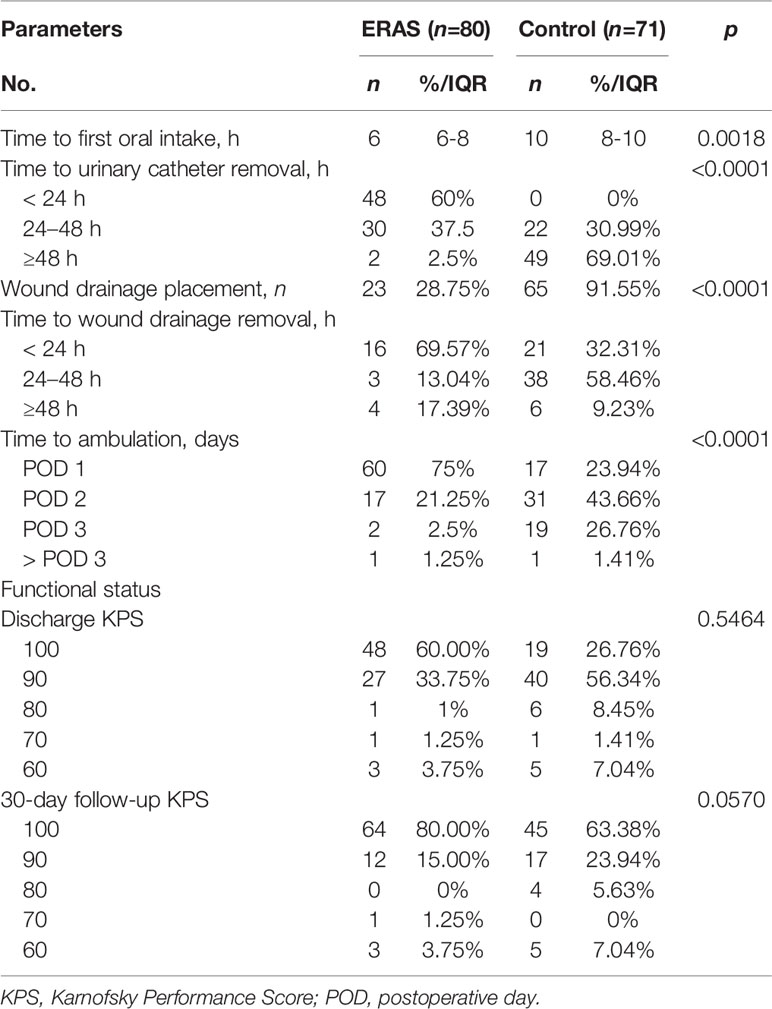
Figure 2 Dynamic changes of KPS scores in glioma patients receiving craniotomy with ERAS protocol (A) and conventional care (B) during hospitalization.
Primary Outcome Measures
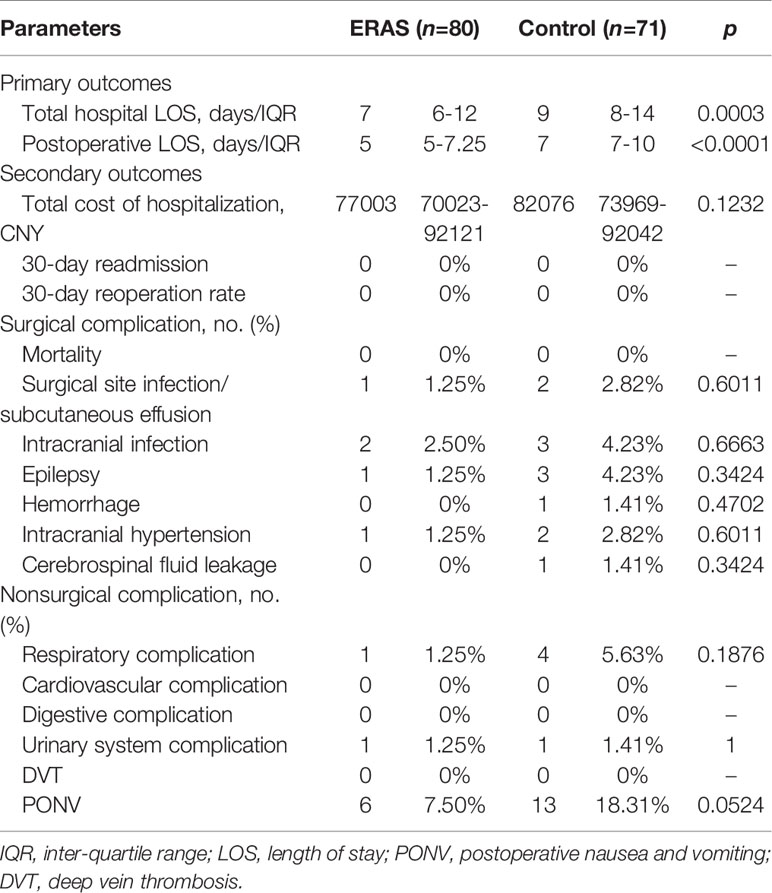
The total hospital LOS and postoperative LOS were significantly shorter in the ERAS group (median: 7 days (IQR: 6-12 days) and 5 days (IQR: 5-7.25 days), respectively) than in the control group (median: 9 days (IQR: 8-14 days) and 7 days (IQR: 7-10 days), respectively) (p<0.0001). However, the median value of total hospitalization cost was 82075.5 CNY (range: 74526-91352 CNY) in the control group and 76538 CNY (range: 69763-89220 CNY) in the ERAS group. However, no significant difference was found between the two groups (Table 4).
Secondary Outcomes Measures
No mortality or 30-day readmission was found in both groups. Postoperative complications are summarized in Table 4. No statistical difference was found in surgical-related complications between the two groups. However, three patients developed an incisional infection or subcutaneous effusion (one in the ERAS group and two in the control group), and five patients (two in the ERAS group and three in the control group) presented with intracranial infection. All these patients recovered following antibiotic treatment wound dressing replacement or lumbar drainage. Other non-surgical-related complications were similar between the two groups. PONV was the most common complication. None of the patients developed DVT.
Functional recovery was similar at hospital discharge in both groups (Table 5). The median discharge KPS score was 100 (range: 60-100) in the ERAS group and 90 (range: 60-100) in the control group. Although the median KPS score was 100 at 30-day follow-up, no significant difference was observed between groups. The trend of KPS change was also presented in Figure 3. Of note, a slow trend of KPS improvement was observed in the control group. The benefits of the ERAS protocol may account for this trend, but further studies with a large sample are needed to verify this hypothesis.
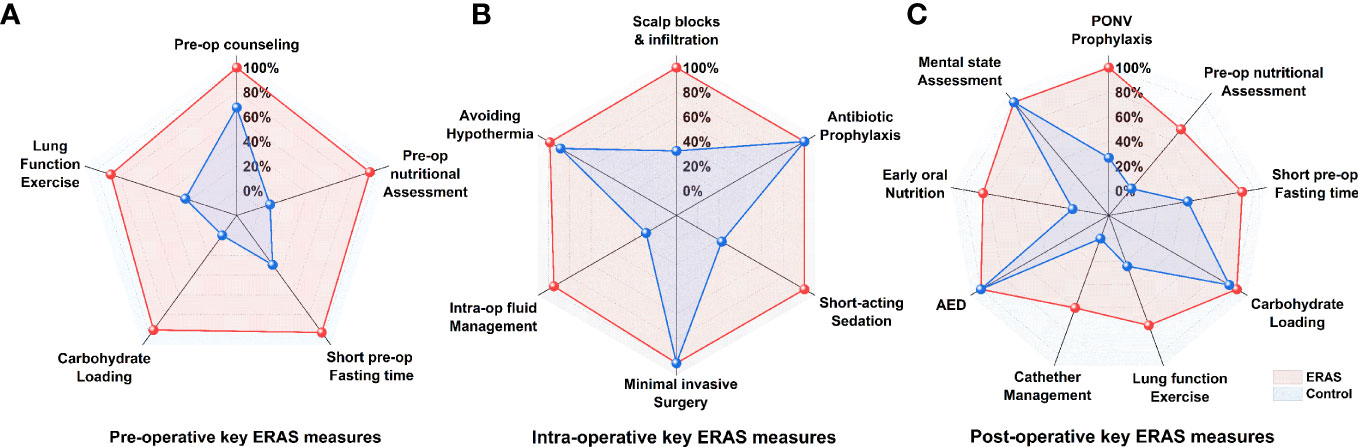
Figure 3 Percent compliance with the ERAS core elements between ERAS groups and control group, categorized by pre-operative (A), intra-operative (B) and post-operative (C) key measures.
Other major ERAS elements were summarized in Table 5. For postoperative dietary management, the time to first oral intake was significantly shorter in the ERAS group (6 h, IQR: 6-8 h) compared to the control group (10 h, IQR: 8-10 h; p = 0.0018). A significantly higher percentage of patients in the ERAS group had early urinary catheter removal (within 24 h) (p<0.0001). Postoperative wound drainage tubes were used in a few cases in the ERAS group (23/80, 28.75%) compared to the control group (65/71, 91.55%, p<0.0001). A significantly higher percentage of patients in the ERAS group had early ambulation on postoperative days (POD) 1 and 2 compared with the control group (60/80 vs. 17/71 on POD 1 and 17/80 vs. 31/71 on POD 2, all p<0.0001).
Mental Health Status
No significant differences in HADS-A scores were observed between the two groups (ERAS group: -2.54 ± 3.21 points; control group: -2.41 ± 3.20 points, p = 0.8892) (Table 6, Figure 4). The proportion of patients achieving a clinically relevant improvement (MCID) was also similar in both groups (ERAS group: 69% (55 cases); control group: 59% (47 cases), p = 0.7381).
Figure 4 Distribution of changes in HADS-anxiety (A) and HADS-depression (B) scores at hospital admission and discharge stratified by group allocation.
Similarly, no significant differences in HADS-D scores were observed between the two groups (ERAS group: -3.48 ± 3.73 points; control group: -3.61 ± 3.53 points, p = 0.5151) (Table 6, Figure 4). The proportion of patients achieving a clinically relevant reduction in HADS-D scores was comparable between groups (ERAS group: 83% (59 cases); control group: 76% (54 cases), p = 0.7445).
Surgical Start Time
Similar to the article by Sean et al. (8), we set the cut-off point as 2 PM. The demarcation of the 2 PM start time was not arbitrary, but based on the inherent shift changes that impact operating room staff, including nursing, surgical technologists, and anesthetists. In our current study, we did not observe the effect of surgical start time on total hospital cost. However, we noticed that the total LOS in ERAS group was significantly shorter than that in control group (P<0.0005) for the early cohort. There was no difference for the later cohort. As for the post-op LOS, our data demonstrated that the post-op LOS in ERAS group was also shorter than that in control group in both early cohort (p<0.0001) and later cohort (p=0.0048) (Figure 5 and Supplementary Tables 1–3).
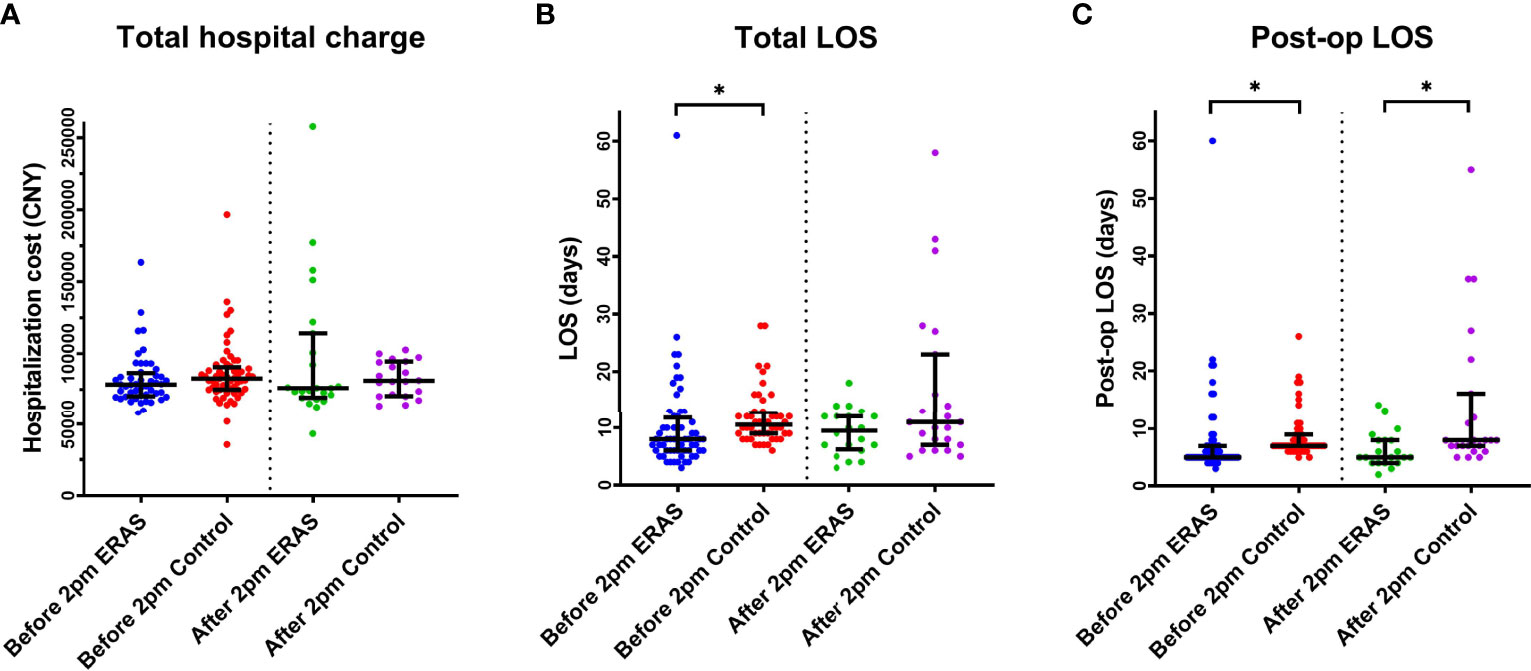
Figure 5 The effect of surgical start time (before or after 2 PM) on both groups on Total Hospital charge (A), Total LOS (B) and Post-op LOS (C). *p < 0.05. LOS, Length of stay.
Discussion
The present study has demonstrated a successful implementation of neurosurgical ERAS protocol for glioma patients. To the best of our knowledge, this is the first real-world study describing the neurosurgical ERAS protocol for glioma patients undergoing elective craniotomy. Our results confirmed that this protocol was particularly beneficial for this subgroup of patients, as it shortened postoperative LOS, accelerated rehabilitation recovery and reduced overall complications.
Interestingly, although the ERAS protocol is a quality improvement program, it also influenced the overall survival or progression-free survival, which was mainly determined by the disease features and treatment strategy. Since the initial application of neurosurgical ERAS protocol (4), our ERAS protocol has been continually refined based on the feedback from patients and medical personnel for constant quality improvement. In addition, new series of ERAS protocols for specific diseases subgroups have been developed based on the latest updates from related fields. In the current study, the ERAS protocol for glioma patients was amended to more closely reflect the characteristics of glioma patients, such as mental and neuropsychological changes.
Although the successful application of the ERAS protocol requires a high compliance rate (9, 10), previous studies on the neurosurgical ERAS protocol seldom describe the overall compliance status or partially report results of certain ERAS measures (2, 11). The current registry study tracked the completeness of the ERAS protocol for each patient and obtained 80% compliance for the 20 key measures of the ERAS protocol, including both patient-dependent and provider-dependent measures. Evidence has implied that improved compliance with the ERAS protocol was associated with the improved short-term benefits, such as fewer post-op complications and better functional recovery (12). It is noteworthy that the ERAS protocol was designed as a multidisciplinary clinical procedure, where a single measure cannot achieve cost-effective results without other coordination measures. For instance, early ambulation requires early urinary catheter removal, sufficient postoperative analgesic treatment, PONV prophylaxis, nutritional support and assistance and guidance of the medical staff. Therefore, a high compliance rate in the ERAS group was fundamental for the successful implementation of the ERAS protocol.
As one of the major preoperative management measures, a shorter fasting time with oral carbohydrate intake 2 h before surgery is recommended according to the American Society of Anesthesiologists (ASA) guidelines (13). Our previous study and other similar reports confirmed the benefits of shorter fasting time, including reduced insulin resistance and improved subjective feeling of hunger, thirst and fatigue after surgery (3, 4, 11). In our current study, no cases of aspiration or vomiting were reported during surgery. Similarly, early oral intake to resume gastrointestinal function was encouraged as another key ERAS measure. Together with rapid strategies of postoperative de-escalation of intravenous fluids, early restoration of a normal diet could accelerate perioperative rehabilitation (14, 15). Our current results confirmed this measure to be safe and effective for glioma patients. In addition, early mobilization can reduce the risk of pulmonary complications and DVT, as well as improve cardiopulmonary function (16, 17). In our case series, patients in the ERAS group were encouraged to ambulate on the first day after the operation, which was associated with shorter LOS and better rehabilitation.
Our results also indicated that postoperative LOS and total LOS were significantly reduced in the ERAS group compared to the control group, which is consistent with our previous study and other researches (2, 4). However, no significant differences were observed between the two groups regarding total hospital expenses, although the trend suggested potential economic benefits for the ERAS group. This could be due to the limited sample size. Moreover, no significant difference in postoperative complications (surgical and non-surgical) and KPS scores at discharge between the two groups, underscoring the safety of ERAS protocol.
Previous studies evaluated anxiety and depression in the context of the ERAS protocol in the perioperative period. A post-hoc analysis of a previous ERAS study found no effect on preoperative reduction of anxiety and depression measures (18). Another clinical study on total hip arthroplasty did not find any impact of anxiety or depression on functional outcome (19). Since the concept of ERAS involves early mobilization 2-3 h after surgery and early ambulation on POD 1, patients with a strong personalities showed higher pain levels and poorer functional outcomes. Another consideration could be preoperative patient communication and education, which had a positive impact on the patient’s psychological status, reducing the patient’s worries and fears before surgery, thereby resulting in lower admission anxiety and depression scores.
Unlike other previous studies, our results did not support the idea that the later surgical start time (later cohort) itself might associated with longer LOS or higher cost (8, 20). Specifically, our results showed similar total cost, total LOS or post-op LOS inside the same group regardless of surgical start time. However, our results highlighted that the implementation of ERAS might contribute to the reducing the total LOS and post-op LOS, especially for the early surgical start time cases. Considering the mixed factors influencing operation room conditions, such as staff shifting and anesthetic handoffs, we might suggest that the surgical start time might be an important indicator or co-variate to enlarge the effect of ERAS protocol for each case.
A major limitation of the current study is that it was not conducted as a randomized study. However, based on our previous experience, considering the nature of ERAS protocol as a medical care improvement strategy, blinding to study participants and medical care providers could only partially achieved and bias was inevitable. In addition, although our results support the efficacy and safety of the ERAS protocol for glioma patients, due to the differences in medical environment, healthcare provider system and socioeconomic status, multicenter studies with a larger sample size are needed to evaluate its generalizability to different healthcare systems and levels of patient care.
Conclusion
We have modified and implemented a neurosurgical ERAS protocol for glioma patients. Our results suggest that the application of the ERAS protocol has significant benefits over conventional neurosurgical care. The ERAS protocol accelerated postoperative recovery without additional perioperative risk. However, larger multicenter collaborative research is warranted to evaluate this protocol in relation to the patient’s prognosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Tangdu Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YuaW and Y-FX wrote the main manuscript text. YuaW, B-FZ, and LW designed the study. NW, P-GJ, S-CG, J-HL, YueW, Y-RX, and Y-FX helped to conduct the study, collected, and analyzed data. NW, S-CG, Y-LZ, and FC provide the service and technical support for this study. G-DG and YQ supervised this study. LW and YuaW prepared tables. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (81601100 and 81772661).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Special thanks to Mr. Kai Yao for their contributions in the management and coordination of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.860257/full#supplementary-material
Diagram 1 | Flow diagram of study design and process.
Supplementary Table 1 | Total hospital charge difference based on surgical start time.
Supplementary Table 2 | Hospital length of stay (LOS) based on surgical start time.
Supplementary Table 3 | Post-operative hospital length of stay (LOS) based on surgical start time.
References
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol (2021) 23(12 Suppl 2):iii1–105. doi: 10.1093/neuonc/noab200
2. Wang L, Cai H, Wang Y, Liu J, Chen T, Liu J, et al. Enhanced Recovery After Elective Craniotomy: A Randomized Controlled Trial. J Clin Anesth (2022) 76:110575. doi: 10.1016/j.jclinane.2021.110575
3. Liu B, Liu S, Wang Y, Lu D, Chen L, Zheng T, et al. Impact of Neurosurgical Enhanced Recovery After Surgery (ERAS) Program on Health-Related Quality of Life in Glioma Patients: A Secondary Analysis of a Randomized Controlled Trial. J Neurooncol (2020) 148:555–67. doi: 10.1007/s11060-020-03548-y
4. Wang Y, Liu B, Zhao T, Zhao B, Yu D, Jiang X, et al. Safety and Efficacy of a Novel Neurosurgical Enhanced Recovery After Surgery Protocol for Elective Craniotomy: A Prospective Randomized Controlled Trial. J Neurosurg (2018) 1:1–12. doi: 10.3171/2018.1.JNS171552
5. Elias KM, Stone AB, McGinigle K, Tankou JI, Scott MJ, Fawcett WJ, et al. The Reporting on ERAS Compliance, Outcomes, and Elements Research (RECOvER) Checklist: A Joint Statement by the ERAS[(R)] and ERAS[(R)] USA Societies. World J Surg (2019) 43:1–8. doi: 10.1007/s00268-018-4753-0
6. Annunziata MA, Muzzatti B, Bidoli E, Flaiban C, Bomben F, Piccinin M, et al. Hospital Anxiety and Depression Scale (HADS) Accuracy in Cancer Patients. Support Care Cancer (2020) 28:3921–6. doi: 10.1007/s00520-019-05244-8
7. Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the Minimal Clinically Important Difference for the Hospital Anxiety and Depression Scale in Patients With Cardiovascular Disease. J Cardiopulm Rehabil Prev (2019) 39:E6–E11. doi: 10.1097/HCR.0000000000000379
8. Neifert SN, Martini ML, Gal JS, Maron SZ, Rasouli JJ, Lamb CD, et al. Afternoon Surgical Start Time Is Associated With Higher Cost and Longer Length of Stay in Posterior Lumbar Fusion. World Neurosurg (2020) 144:e34–9. doi: 10.1016/j.wneu.2020.07.082
9. Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg (2017) 152:292–8. doi: 10.1001/jamasurg.2016.4952
10. Pedrazzani C, Conti C, Turri G, Lazzarini E, Tripepi M, Scotton G, et al. Impact of Age on Feasibility and Short-Term Outcomes of ERAS After Laparoscopic Colorectal Resection. World J Gastrointest Surg (2019) 11:395–406. doi: 10.4240/wjgs.v11.i10.395
11. Qu L, Liu B, Zhang H, Sankey EW, Chai W, Wang B, et al. Management of Postoperative Pain After Elective Craniotomy: A Prospective Randomized Controlled Trial of a Neurosurgical Enhanced Recovery After Surgery (ERAS) Program. Int J Med Sci (2020) 17:1541–9. doi: 10.7150/ijms.46403
12. Pisarska M, Torbicz G, Gajewska N, Rubinkiewicz M, Wierdak M, Major P, et al. Compliance With the ERAS Protocol and 3-Year Survival After Laparoscopic Surgery for Non-Metastatic Colorectal Cancer. World J Surg (2019) 43:2552–60. doi: 10.1007/s00268-019-05073-0
13. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology (2017) 126:376–93. doi: 10.1097/ALN.0000000000001452
14. Hagan KB, Bhavsar S, Raza SM, Arnold B, Arunkumar R, Dang A, et al. Enhanced Recovery After Surgery for Oncological Craniotomies. J Clin Neurosci (2016) 24:10–6. doi: 10.1016/j.jocn.2015.08.013
15. Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized Clinical Trial to Compare the Effects of Preoperative Oral Carbohydrate Versus Placebo on Insulin Resistance After Colorectal Surgery. Br J Surg (2010) 97:317–27. doi: 10.1002/bjs.6963
16. Yip VS, Dunne DF, Samuels S, Tan CY, Lacasia C, Tang J, et al. Adherence to Early Mobilisation: Key for Successful Enhanced Recovery After Liver Resection. Eur J Surg Oncol (2016) 42:1561–7. doi: 10.1016/j.ejso.2016.07.015
17. Feng S, Xie B, Li Z, Zhou X, Cheng Q, Liu Z, et al. Retrospective Study on the Application of Enhanced Recovery After Surgery Measures to Promote Postoperative Rehabilitation in 50 Patients With Brain Tumor Undergoing Craniotomy. Front Oncol (2021) 11:755378. doi: 10.3389/fonc.2021.755378
18. Taha A, Taha-Mehlitz S, Staartjes VE, Lunger F, Gloor S, Unger I, et al. Association of a Prehabilitation Program With Anxiety and Depression Before Colorectal Surgery: A Post Hoc Analysis of the pERACS Randomized Controlled Trial. Langenbecks Arch Surg (2021) 406:1553–61. doi: 10.1007/s00423-021-02158-0
19. Leiss F, Gotz JS, Maderbacher G, Meyer M, Reinhard J, Zeman F, et al. Excellent Functional Outcome and Quality of Life After Primary Cementless Total Hip Arthroplasty (THA) Using an Enhanced Recovery Setup. J Clin Med (2021) 10(4):621. doi: 10.3390/jcm10040621
Keywords: gliomas, enhanced recovery after surgery (ERAS), perioperative care, outcomes, craniotomy
Citation: Wang Y, Xue Y-F, Zhao B-F, Guo S-C, Ji P-G, Liu J-H, Wang N, Chen F, Zhai Y-L, Wang Y, Xue Y-R, Gao G-D, Qu Y and Wang L (2022) Real-World Implementation of Neurosurgical Enhanced Recovery After Surgery Protocol for Gliomas in Patients Undergoing Elective Craniotomy. Front. Oncol. 12:860257. doi: 10.3389/fonc.2022.860257
Received: 22 January 2022; Accepted: 11 April 2022;
Published: 24 May 2022.
Edited by:
David D. Eisenstat, Royal Children’s Hospital, AustraliaReviewed by:
Jefferson W. Chen, University of California, Irvine, United StatesChristopher Paul Cifarelli, West Virginia University Hospitals, United States
Copyright © 2022 Wang, Xue, Zhao, Guo, Ji, Liu, Wang, Chen, Zhai, Wang, Xue, Gao, Qu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wang, ZHJ3YW5nbGlhbmdAMTI2LmNvbQ==; Yan Qu, eWFucXUwMTIzQGZtbXUuZWR1LmNu
 Yuan Wang
Yuan Wang Ya-Fei Xue1
Ya-Fei Xue1 Shao-Chun Guo
Shao-Chun Guo Pei-Gang Ji
Pei-Gang Ji Jing-Hui Liu
Jing-Hui Liu Na Wang
Na Wang Fan Chen
Fan Chen Yu-Long Zhai
Yu-Long Zhai Yan-Rong Xue
Yan-Rong Xue Yan Qu
Yan Qu Liang Wang
Liang Wang