- 1Radiology and Diagnostic Imaging, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 2Precision Medicine in Breast Cancer Unit, Fondazione Policlinico Universitario A. Gemelli, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 3Division of Medical Oncology 2, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 4Division of Breast Surgery, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 5Human Neuroscience Department, Sapienza University of Rome, Rome, Italy
- 6Neuroimmunology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Rome, Italy
Introduction: In the past decade, a new technique derived from full-field digital mammography has been developed, named contrast-enhanced spectral mammography (CESM). The aim of this study was to define the association between CESM findings and usual prognostic factors, such as estrogen receptors, progesterone receptors, HER2, and Ki67, in order to offer an updated overview of the state of the art for the early differential diagnosis of breast cancer and following personalized treatments.
Materials and Methods: According to the PRISMA guidelines, two electronic databases (PubMed and Scopus) were investigated, using the following keywords: breast cancer AND (CESM OR contrast enhanced spectral mammography OR contrast enhanced dual energy mammography) AND (receptors OR prognostic factors OR HER2 OR progesterone OR estrogen OR Ki67). The search was concluded in August 2021. No restriction was applied to publication dates.
Results: We obtained 28 articles from the research in PubMed and 114 articles from Scopus. After the removal of six replicas that were counted only once, out of 136 articles, 37 articles were reviews. Eight articles alone have tackled the relation between CESM imaging and ER, PR, HER2, and Ki67. When comparing radiological characterization of the lesions obtained by either CESM or contrast-enhanced MRI, they have a similar association with the proliferation of tumoral cells, as expressed by Ki-67. In CESM-enhanced lesions, the expression was found to be 100% for ER and 77.4% for PR, while moderate or high HER2 positivity was found in lesions with non-mass enhancement and with mass closely associated with a non-mass enhancement component. Conversely, the non-enhancing breast cancer lesions were not associated with any prognostic factor, such as ER, PR, HER2, and Ki67, which may be associated with the probability of showing enhancement. Radiomics on CESM images has the potential for non-invasive characterization of potentially heterogeneous tumors with different hormone receptor status.
Conclusions: CESM enhancement is associated with the proliferation of tumoral cells, as well as to the expression of estrogen and progesterone receptors. As CESM is a relatively young imaging technique, a few related works were found; this may be due to the “off-label” modality. In the next few years, the role of CESM in breast cancer diagnostics will be more thoroughly investigated.
Introduction
Breast cancer is the first cause of death in the female population in western countries (1). Early diagnosis and treatment have led to an increase in survival rate and better clinical outcome of women affected by breast cancer. However, up to 50% of patients may experience the relapse. Therefore, early identification of women at high risk of recurrence or who may benefit from treatment adjuvant setting is needed (2). Prognostic factors are essential to estimating individual patient risk of developing clinically silent micro-metastatic diseases and to determining patient eligibility for postsurgical systemic adjuvant therapy (3). The immunohistochemical prognostic factors that are assessed in order to plan a surgical and medical treatment for breast cancer are estrogen receptors (ER), progesterone receptors (PR), and epidermal growth factor (HER-2) (4). These factors, assessed on biopsy or surgical specimens, have permitted a classification in subtypes of breast cancer and a fine personalization of the treatment, thus tailoring the treatment in single cases. In addition to the abovementioned factors, also nuclear protein Ki-67 may influence the prognosis of the disease (5). Lastly, the histological grade is assessed in the diagnostic process (6) and used in the prognosis evaluation.
In mammography, breast cancer may not be identified due to the low difference between tumoral and background tissue x-ray attenuation (7), and to overcome this limit, during the past years, several studies have aimed at providing aid to physicians in the imaging analysis process, resulting in automated software able to improve sensitivity and specificity of diagnostic performances (8–10). Moreover, artificial intelligence (AI) has been applied to mammography and other imaging methodologies in cancer diagnosis, characterization, prognosis, and prediction of therapy outcome (11).
A recent diagnostic tool, with an improved background subtraction procedure, is the contrast-enhanced spectral mammography (CESM), a new technique derived from full-field digital mammography. CESM includes the administration of an iodine-based contrast material and the performance of low- (28–32 kV) and high-energy (45–49 kV) consecutive exposures to reveal areas of increased blood supply within the breast. In post-processing, these exposures are mutually subtracted in order to create a contrast-enhanced image and detect tumor vascularity (7). An image is acquired before contrast injection, and two more images are acquired about 2 min after contrast injection, one at low and the other at high energy. Postinjection images are combined in a single image that minimizes the appearance of breast tissue and increases the signal of an iodinated contrast agent (enhancement) (12). Recently, CESM has been becoming a valuable tool in the diagnosis and staging of primary breast cancer. It improves the diagnostic accuracy of mammography, providing a more accurate tumor sizing and the identification of multifocal diseases (13). Indeed, CESM improves the sensitivity for breast cancer detection without decreasing specificity, since it provides higher contrast and better lesion delineation as well as a better evaluation of lesion size and detects more multifocal breast cancers, than mammography alone or combined with ultrasonography (14–17). Similarly to breast magnetic resonance imaging (MRI), which is considered the gold standard in the assessment of tumor, the findings obtained with CESM examination suggest that it should be considered a useful tool in the evaluation of disease extension. As a matter of fact, both CESM and MRI may also evaluate tumor response during neoadjuvant chemotherapy (NAC), which, reducing tumor volume and metastasis occurrence, increases the probability of a positive response to breast-conserving surgery, to be used instead of mastectomy, and of a high survival rate in advanced breast cancer (18).
The aim of this study was to define the association between CESM findings and prognostic factors, such as ER, PR, HER2, and Ki67, with the aim to offer an updated overview of state of the art for the early differential diagnosis of breast cancer and the following personalized treatments. In this framework, we performed a systematic review of the literature.
Materials and Methods
According to the PRISMA guidelines (19), two electronic databases (PubMed and Scopus) were used to perform the literature investigation, using the following keywords: breast cancer AND (CESM OR contrast enhanced spectral mammography OR contrast enhanced dual energy mammography) AND (receptors OR prognostic factors OR her2 OR progesterone OR estrogen OR Ki67). The search was concluded in August 2021. No restriction was applied to publication dates.
First, we identified all documents in both databases. After identifying existing studies, we cross-checked all the collected articles to avoid duplicates. Abstracts were examined carefully, and the following exclusion criteria were applied: not a research article (e.g., review, book chapter, conference report, case report, meta-analysis), articles written in languages other than English, and articles investigating diagnostic methodologies other than CESM or not investigating prognostic factors. The flowchart of article selection is shown in Figure 1.
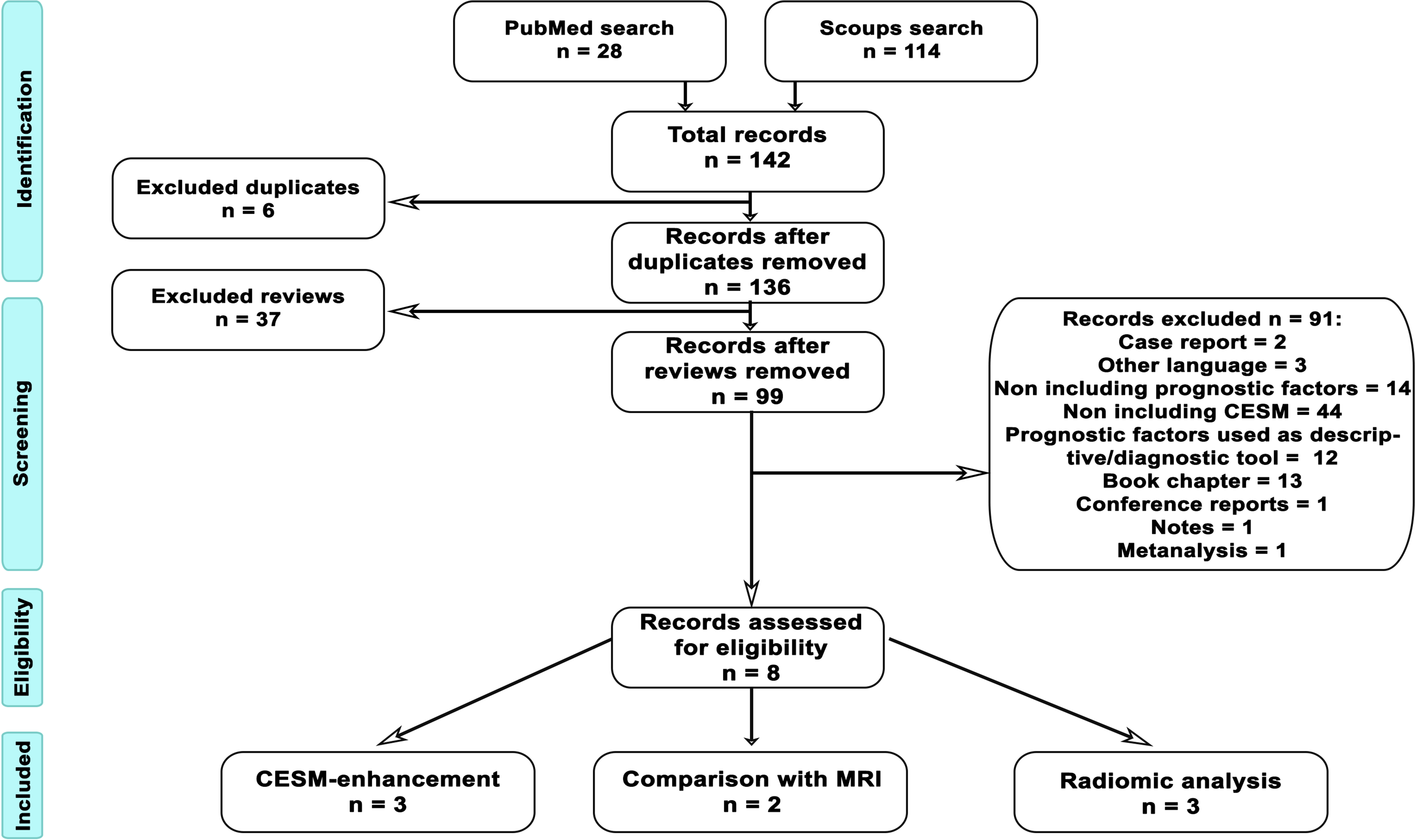
Figure 1 Flowchart of article selection. The procedure to identify suitable articles to be included in the systematic review was performed following PRISMA guidelines and schematized in the figure. From database search, we identified 28 articles in PubMed and 114 in Scopus, with a total of 142 records. Six of the retrieved articles were duplicated across the two databases; therefore, we further investigated 136 articles. Out of those, 37 articles were reviews and, after abstract examination, 99 more articl es were excluded, because they were case reports (2), were written in no English language (3), did not include prognostic factors (14), did not include CESM (44), used prognostic factors as diagnostic characterization (12), were book chapters (13), were conference reports (1), were notes (1), and were meta-analyses (1). Finally, eight records were assessed to be eligible. Specifically, three articles addressed CESM enhancement, two articles compared CESM and MRI, and three articles investigated radiomic analysis of CESM images.
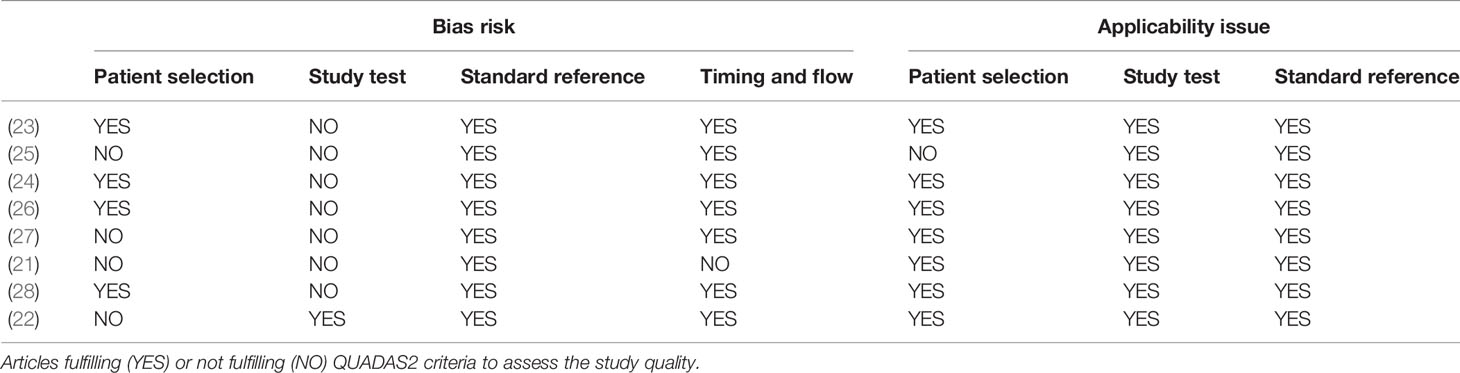
To assess the scientific quality of the studies included in our review and any possible source of bias, we prepared a checklist of questions in accordance with QUADAS guidelines (20). The overall procedure was carried out by two investigators (FV, ST).
Results
We obtained 28 articles from the research in PubMed and 114 articles from Scopus. After the removal of six replicas that were counted only once, out of 136 articles, 37 articles were reviews and were removed. The abstracts of the remaining 99 articles were inspected to verify conformity to exclusion criteria: 2 articles were case reports, 3 were written in a non-English language, 14 did not include prognostic factors, 44 did not include CESM, 12 used prognostic factors as diagnostic characterization, 13 were book chapters, 1 was a conference report, 1 was a note, and 1 was a meta-analysis. Finally, eight articles tackled the relation between CESM imaging and ER, PR, HER2, and Ki67. Among the eight articles admissible for the following analysis, the relation between CESM and prognostic factors was investigated with CESM-MRI comparison (two articles), with CESM enhancement (three articles), and with radiomic analysis of CESM enhancement (three articles).
Since CESM is a recent diagnostic technique, articles investigating how CESM may provide clinical information on biological prognostic factors date back to the last 2 years.
CESM MRI Comparison
CESM is often compared to MRI to test its utility in tumor diagnosis, and indeed, enhancement patterns were moderately in agreement between the two techniques (21). CESM may produce an enhancement intensity weaker in the ER-positive group than in the ER-negative group, as well as weaker in the PR-positive group than in the PR-negative group, and stronger in the HER-2-positive group than in the HER-2-negative group (21). Further, when comparing radiological characterization of the lesions obtained by either CESM or contrast-enhanced MRI, they have a similar association with the proliferation of tumoral cells, as expressed by Ki-67 (22). However, the authors do not describe if there are any differences between CESM and MRI in differentiating hormonal receptor status.
CESM Enhancement
In CESM-enhanced lesions, the expression was found to be 100% for ER and 77.4% for PR, while moderate or high HER2 positivity was found in lesions with non-mass enhancement and with mass closely associated with a non-mass enhancement component (23). Further, via CESM enhancement, neoplasms larger than 5 mm, with a high proliferative index and frequently HER2-positive, are recognized (24). Conversely, the non-enhancing breast cancer lesions were not associated with any prognostic factor, such as status of ER and/or PR, HER2 expression and/or amplification, and percentage of Ki67, which might be associated with the probability of showing enhancement (25).
Radiomic Analysis
Nowadays, one of the cutting-edge methods for image analysis is based on radiomics. For non-invasively assessing the hormone receptor status, other than tumor invasiveness and grade, radiomic features were derived from the first-order histogram of primary breast cancer lesions contoured on both CESM and MRI images and the two techniques resulted to be alternative in the assessment of hormone receptor status (26). Further, radiomics on CESM images showed the potential for the non-invasive characterization of heterogeneous tumors with different hormone receptor statuses (27). Lastly, radiomic features may predict histological outcomes and molecular subtypes via discriminating lesions with a positive or negative expression of hormonal receptors, and being associated with HER2. In particular, in an immunohistochemical study, the performances for discriminating positive versus negative expressions were 90.87% for HER2 positive versus HER2 negative, 83.79% for ER positive versus ER negative, and 84.80% for Ki67 positive versus Ki67 negative (28). The list of the final articles and their relationship with biologic prognostic factors is summarized in Table 1.
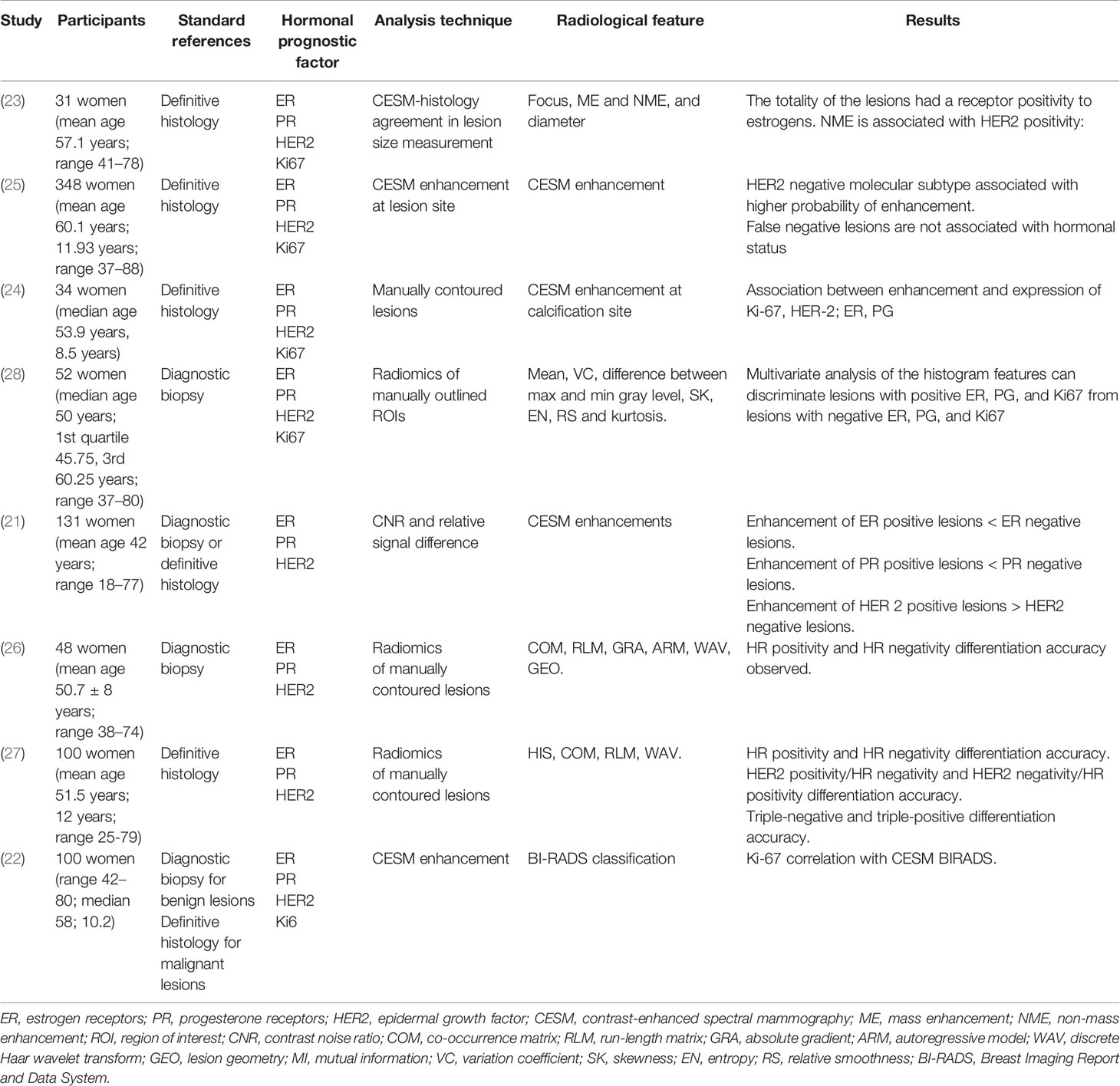
Table 1 Characteristics of articles investigating the relationship between dual-energy contrast enhanced spectral mammography (CESM) and biologic prognostic factors.
Quality Assessment
The Quadas-2 survey showed that the articles considered in the analysis were at risk of bias, especially for what concerns the study tests conducted in each research. Indeed, at the time of the radiological evaluation the investigators, i.e., the radiologists, were aware of the results of the histological test, in all studies with the exception of one study, who performed a blinded histological analysis (22). However, all articles referred to a proper reference test, i.e., definitive histology or diagnostic biopsy. Further, four articles were biased in patient selection, because they removed either patients with a tumor not easily identifiable, such as that with suspicious but not contrast-enhancing lesions (27), or patients with post-histology edema or not willing to undergo CESM (25), or because they did not clarify whether patients have mono- or multifocal diseases (21, 22). Lastly, one article alone was at risk of flow bias, because of using both definitive histology and diagnostic biopsy as standard reference (21). The Quadas-2 survey is shown in Table 2.
Discussion
CESM is a very young modality recently introduced in the breast imaging scenario; therefore, the eligible articles found on our research were not older than 2 years. CESM shows considerable promise as the primary imaging test in symptomatic patients, providing improved diagnostic and staging information at the first evaluation.
Prognostic Factors
Prognostic factors are correlated with patient prognosis and allow important information about the efficacy of antitumoral treatment. Literature demonstrated that proliferative activity indicator (Ki67), HER-2, and hormonal receptor, such as ER and PR, statuses are important in treatment choice and that they have prognostic value in predicting pathological response and clinical outcome (29). As a matter of fact, HER-2 status represents a solid prognostic factor that predicts the response to trastuzumab alone or associated with pertuzumab treatment in locally advanced or early disease therapy (30). Also, determination of ER and PR status is crucial as their expression on the tumor cellular surface is related to a good response to endocrine therapy in both neoadjuvant and adjuvant therapy (30).
Among the biomarkers used to define tumor aggressiveness, Ki67, HER-2, ER, and PR are quantitative values. On the contrary, grading, which is used as well to define tumor aggressiveness, is a qualitative biomarker; therefore, we rather avoided to include it as an investigated prognostic factor in this study.
Comparison Between CESM and MRI and the Association With Prognostic Factors
CESM is a recent tool for diagnostic imaging that, although it uses ionizing radiations thus presenting some limitations in terms of radioprotection (7), may overtake the use of MRI in breast cancer monitoring, since it is more accessible, cheaper, faster, and more tolerated by patients (31), while maintaining performance equivalent to MRI and improving specificity (7, 17). As a matter of fact, the promising results of diagnostic performance could suggest CESM to be a valid alternative for patients who are not eligible for MRI. As a matter of fact, CESM and breast MRI similarly detect physiological, benign background parenchymal enhancement, which may be significantly associated with menopausal status, radiation therapy, hormonal treatment, and breast density and that rarely causes diagnostic issues if showing a bilateral, symmetrical appearance (32, 33). At the same time, the background parenchymal enhancement on MRI is considered a biomarker for increased risk of breast malignancy, while it is not known if the same holds true for CESM (34, 35).
Indeed, CESM and MRI show similar enhancement patterns (21) and a similar association with the proliferation of tumoral cells (22). The equivalence of CESM and MRI might rise from tumor vascularization, which is a crucial feature observed by both diagnostic modalities and is influenced by Ki67.
A necessary step to include CESM in everyday clinical practice will be the standardization of diagnostic criteria. Given the similarity of the basic principles of lesion blood supply of the two modalities, MRI morphology descriptors have been already investigated and used to characterize lesions on CESM (36); however, more studies are needed to finalize the use of these descriptors in CESM image evaluation.
As in any imaging modality, patient motion may affect image quality. Due to the simultaneous acquisition of low-energy and high-energy images, the length of each exposure with CESM is longer than a standard full-field digital mammography, increasing the possibility of motion. However, the examination time of CESM is still shorter than the second-level examination MRI, reducing the risk of motion artifacts. Moreover, to instruct well the patient to hold as still as possible during the exposure is fundamental to reducing the possibility of motion (37).
CESM Enhancement and Prognostic Factors
CESM combines an iodinated contrast agent with the standard mammographic technique to improve lesion detectability. Since the growth of tumors is accompanied by angiogenesis, CESM permits to assess the enhancement related to the neovascularity of breast cancers, allowing a functional characterization in addition to the morphological features provided by structural images (16).
In literature, CESM-enhancing lesions have been associated with higher levels of prognostic factors, such as ER, PR, and HER2 (23, 24). On the other hand, non-enhancing lesions have been found not to relate to prognostic factors (25). Indeed, tumors have a higher enhancement compared to normal tissue due to the increase in vascularization, which in turn is associated with different tumor characteristics and therefore different expressions of prognostic factors.
CESM-Based Radiomic Analysis and Prognostic Factors
Feature extraction in radiomics is typically realized by means of pattern recognition algorithms and provides, as a result, a set of numbers, each one representing a quantitative description of a specific either geometric or physical property of the image portion under consideration. In oncological applications, examples of features are tumor size, shape, intensity, and texture, collectively providing a comprehensive tumor characterization, called the radiomics signature of the tumor (38). From an epistemological perspective, radiomics is based on the hypothesis that the extracted features reflect mechanisms occurring at genetic and molecular levels (39) and may reveal the relationship of tumor lesion surfaces with prognostic factor expression. The potential of radiomics applied on breast imaging has been investigated recently, and studies have already demonstrated the additive value of radiomics on MRI in breast cancer evaluation and prognosis (40, 41). Indeed, radiomics on CESM images might assess hormone receptor status (26) and characterize the related heterogeneous tumors (27), as well as predict histological outcomes and molecular subtypes associated with hormone receptors’ expression (28). As a matter of fact, radiomics arises from the analysis of cell morphology, which may be influenced by the expression of the different receptors on the cell surface of the different tumors, thus permitting to differentiate the receptor status starting from imaging.
Radiomics could also contribute to differentiating benign from malignant enhancement in complicated cases, as in patients with high background parenchymal enhancement or low vascularized lesions, that may have a high risk for underestimation or even overestimation of the lesion (42), and to predicting response to NAC (43).
Literature
The articles included are all published in the last 2 years, and none of them was blinded, except that of Petrillo et al. (22). Nevertheless, this bias did not invalidate the articles, as the goal of these studies was to find a relationship between imaging features and prognostic factors, not just detecting a tumor. Indeed, knowing the histological subtypes was part of patients’ preliminary information needed to obtain a sample of patients with heterogeneous radiologic patterns. Conversely, homogeneous histologic analysis was crucial in order to obtain consistent results among patients, and in one article the authors did not grant this consistency.
Only eight articles investigated the association between CESM imaging and prognostic factors, suggesting that the use of this technique in cancer prognosis and monitoring is still to be deeply investigated. Indeed, the modality is relatively young and large data pools are required to get strong results on this topic.
Conclusion
CESM is a relatively young diagnostic tool, and our review showed its potential on finding a precise imaging semeiotic, thanks to its association with prognostic factors, in order to provide patients with the most accurate pre-therapy and surgery evaluation. In this review, CESM enhancement showed an association with the proliferation of tumoral cells, as well as the expression of estrogen and progesterone receptors, although there is not a certain correlation between specific patterns of enhancement and prognostic factor outlines. Future studies might investigate CESM’s ability in identifying ER/PR positivity and HER2 positivity/amplification, as, so far, they have not been investigated. Moreover, even if recent studies have investigated the radiomic application on CESM (26, 28), more results are requested to enforce these promising applications.
As CESM is a relatively young imaging technique, literature shows a few related works, often suffering from bias risk, and this is certainly due to the “off-label” use in clinical practice. The role of CESM in breast cancer diagnostics will be further investigated, and radiomics studies will provide further predictive and prognostic information on the clinical impact of this technique.
Author Contributions
Study concept and design, analysis and interpretation of data: FV and ST; statistical analysis: MB and CB; drafting of the manuscript: AF, FF, and AV; critical revision and final approval of the manuscript: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Funds Ricerca Corrente 2022 from Italian Ministry of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Pisano ED, Baum JK, Bassett L. Diagnostic Performance of Digital Versus Film Mammography for Breast-Cancer Screening. N Engl J Med (2005) 353:1773–83. doi: 10.1056/NEJMoa052911
3. Cianfrocca M, Goldstein LJ. Prognostic and Predictive Factors in Early-Stage Breast Cancer. Oncologist (2004) 9:606–16. doi: 10.1634/theoncologist.9-6-606
4. Kwa M, Makris A, Esteva FJ. Clinical Utility of Gene-Expression Signatures in Early Stage Breast Cancer. Nat Rev Clin Oncol (2017) 14:595–610. doi: 10.1038/nrclinonc.2017.74
5. Penault-Llorca F, Radosevic-Robin N. Ki67 Assessment in Breast Cancer: An Update. Pathology (2017) 49:166–71. doi: 10.1016/j.pathol.2016.11.006
6. Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, et al. Breast Cancer Prognostic Classification in the Molecular Era: The Role of Histological Grade. Breast Cancer Res (2010) 12:207. doi: 10.1186/bcr2607
7. Sogani J, Mango VL, Keating D, Sung JS, Jochelson MS. Contrast-Enhanced Mammography: Past, Present, and Future. Clin Imaging (2021) 69:269–79. doi: 10.1016/j.clinimag.2020.09.003
8. De Mitri I, MAGIC-5 Collaboration. The MAGIC-5 Project: Medical Applications on a Grid Infrastructure Connection. Stud Health Technol Inform (2005) 112:157–66.
9. Salama WM, Aly MH. Deep Learning in Mammography Images Segmentation and Classification: Automated CNN Approach. Alexandria Eng J (2021) 60:4701–9. doi: 10.1016/j.aej.2021.03.048
10. Massafra R, Bove S, Lorusso V, Biafora A, Comes MC, Didonna V, et al. Radiomic Feature Reduction Approach to Predict Breast Cancer by Contrast-Enhanced Spectral Mammography Images. Diagnostics (Basel) (2021) 11:684. doi: 10.3390/diagnostics11040684
11. Avanzo M, Porzio M, Lorenzon L, Milan L, Sghedoni R, Russo G, et al. Artificial Intelligence Applications in Medical Imaging: A Review of the Medical Physics Research in Italy. Phys Med (2021) 83:221–41. doi: 10.1016/j.ejmp.2021.04.010
12. Patel BK, Lobbes MBI, Lewin J. Contrast Enhanced Spectral Mammography: A Review. Semin Ultrasound CT MRI (2018) 39:70–9. doi: 10.1053/j.sult.2017.08.005
13. Helal MH, Mansour SM, Zaglol M, Salaleldin LA, Nada OM, Haggag MA. Staging of Breast Cancer and the Advanced Applications of Digital Mammogram: What the Physician Needs to Know? BJR (2017) 90:20160717. doi: 10.1259/bjr.20160717
14. Dromain C, Balleyguier C, Muller S, Mathieu M-C, Rochard F, Opolon P, et al. Evaluation of Tumor Angiogenesis of Breast Carcinoma Using Contrast-Enhanced Digital Mammography. Am J Roentgenology (2006) 187:W528–37. doi: 10.2214/AJR.05.1944
15. Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-Energy Contrast-Enhanced Digital Mammography: Initial Clinical Results. Eur Radiol (2011) 21:565–74. doi: 10.1007/s00330-010-1944-y
16. Dromain C, Thibault F, Diekmann F, Fallenberg EM, Jong RA, Koomen M, et al. Dual-Energy Contrast-Enhanced Digital Mammography: Initial Clinical Results of a Multireader, Multicase Study. Breast Cancer Res (2012) 14:R94. doi: 10.1186/bcr3210
17. Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-Enhanced Spectral Mammography Versus MRI: Initial Results in the Detection of Breast Cancer and Assessment of Tumour Size. Eur Radiol (2014) 24:256–64. doi: 10.1007/s00330-013-3007-7
18. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16:310–20. doi: 10.6004/jnccn.2018.0012
19. Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ (2009) 339:b2535–5. doi: 10.1136/bmj.b2535
20. Whiting PF. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155:529. doi: 10.7326/0003-4819-155-8-201110180-00009
21. Liu Y, Zhao S, Huang J, Zhang X, Qin Y, Zhong H, et al. Quantitative Analysis of Enhancement Intensity and Patterns on Contrast-Enhanced Spectral Mammography. Sci Rep (2020) 10:9807. doi: 10.1038/s41598-020-66501-z
22. Petrillo A, Fusco R, Vallone P, Filice S, Granata V, Petrosino T, et al. Digital Breast Tomosynthesis and Contrast-Enhanced Dual-Energy Digital Mammography Alone and in Combination Compared to 2D Digital Synthetized Mammography and MR Imaging in Breast Cancer Detection and Classification. Breast J (2020) 26:860–72. doi: 10.1111/tbj.13739
23. Amato F, Bicchierai G, Cirone D, Depretto C, Di Naro F, Vanzi E, et al. Preoperative Loco-Regional Staging of Invasive Lobular Carcinoma With Contrast-Enhanced Digital Mammography (CEDM). Radiol Med (2019) 124:1229–37. doi: 10.1007/s11547-019-01116-7
24. Depretto C, Borelli A, Liguori A, Presti G, Vingiani A, Cartia F, et al. Contrast-Enhanced Mammography in the Evaluation of Breast Calcifications: Preliminary Experience. Tumori (2020) 106:491–6. doi: 10.1177/0300891620919170
25. Bicchierai G, Amato F, Vanzi B, De Benedetto D, Boeri C, Vanzi E, et al. Which Clinical, Radiological, Histological, and Molecular Parameters Are Associated With the Absence of Enhancement of Known Breast Cancers With Contrast Enhanced Digital Mammography (CEDM)? Breast (2020) 54:15–24. doi: 10.1016/j.breast.2020.08.009
26. Marino MA, Leithner D, Sung J, Avendano D, Morris EA, Pinker K, et al. Radiomics for Tumor Characterization in Breast Cancer Patients: A Feasibility Study Comparing Contrast-Enhanced Mammography and Magnetic Resonance Imaging. Diagnostics (2020) 10:492. doi: 10.3390/diagnostics10070492
27. Marino MA, Pinker K, Leithner D, Sung J, Avendano D, Morris EA, et al. Contrast-Enhanced Mammography and Radiomics Analysis for Noninvasive Breast Cancer Characterization: Initial Results. Mol Imaging Biol (2020) 22:780–7. doi: 10.1007/s11307-019-01423-5
28. La Forgia D, Fanizzi A, Campobasso F, Bellotti R, Didonna V, Lorusso V, et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics (2020) 10:708. doi: 10.3390/diagnostics10090708
29. Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-Analysis of Breast Cancer Outcomes in Adjuvant Trials of Aromatase Inhibitors Versus Tamoxifen. JCO (2010) 28:509–18. doi: 10.1200/JCO.2009.23.1274
30. Placido SD, Laurentiis MD, Carlomagno C, Gallo C, Perrone F, Pepe S, et al. Twenty-Year Results of the Naples GUN Randomized Trial: Predictive Factors of Adjuvant Tamoxifen Efficacy in Early Breast Cancer. Clin Cancer Res (2003) 9(3):1039–46.
31. Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-Enhanced Spectral Mammography (CESM) and Contrast Enhanced MRI (CEMRI): Patient Preferences and Tolerance: CESM and CEMRI Preferences and Tolerance. J Med Imaging Radiat Oncol (2015) 59:300–5. doi: 10.1111/1754-9485.12296
32. Sogani J, Morris EA, Kaplan JB, D’Alessio D, Goldman D, Moskowitz CS, et al. Comparison of Background Parenchymal Enhancement at Contrast-Enhanced Spectral Mammography and Breast MR Imaging. Radiology (2017) 282:63–73. doi: 10.1148/radiol.2016160284
33. Savaridas SL, Taylor DB, Gunawardana D, Phillips M. Could Parenchymal Enhancement on Contrast-Enhanced Spectral Mammography (CESM) Represent a New Breast Cancer Risk Factor? Correlation With Known Radiology Risk Factors. Clin Radiol (2017) 72:1085.e1–1085.e9. doi: 10.1016/j.crad.2017.07.017
34. Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR, et al. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated With Breast Cancer Risk? Radiology (2015) 276:371–80. doi: 10.1148/radiol.2015142304
35. Sorin V, Yagil Y, Shalmon A, Gotlieb M, Faermann R, Halshtok-Neiman O, et al. Background Parenchymal Enhancement at Contrast-Enhanced Spectral Mammography (CESM) as a Breast Cancer Risk Factor. Acad Radiol (2020) 27:1234–40. doi: 10.1016/j.acra.2019.10.034
36. Kamal RM, Helal MH, Mansour SM, Haggag MA, Nada OM, Farahat IG, et al. Can We Apply the MRI BI-RADS Lexicon Morphology Descriptors on Contrast-Enhanced Spectral Mammography? BJR (2016) 89:20160157. doi: 10.1259/bjr.20160157
37. Bhimani C, Li L, Liao L, Roth RG, Tinney E, Germaine P. Contrast-Enhanced Spectral Mammography: Modality-Specific Artifacts and Other Factors Which May Interfere With Image Quality. Acad Radiol (2017) 24:89–94. doi: 10.1016/j.acra.2016.08.024
38. Crivelli P, Ledda RE, Parascandolo N, Fara A, Soro D, Conti M. A New Challenge for Radiologists: Radiomics in Breast Cancer. BioMed Res Int (2018) 2018:1–10. doi: 10.1155/2018/6120703
39. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: The Bridge Between Medical Imaging and Personalized Medicine. Nat Rev Clin Oncol (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141
40. Parekh VS, Jacobs MA. Integrated Radiomic Framework for Breast Cancer and Tumor Biology Using Advanced Machine Learning and Multiparametric MRI. NPJ Breast Cancer (2017) 3:43. doi: 10.1038/s41523-017-0045-3
41. Whitney HM, Taylor NS, Drukker K, Edwards AV, Papaioannou J, Schacht D, et al. Additive Benefit of Radiomics Over Size Alone in the Distinction Between Benign Lesions and Luminal A Cancers on a Large Clinical Breast MRI Dataset. Acad Radiol (2019) 26:202–9. doi: 10.1016/j.acra.2018.04.019
42. Boca (Bene) I, Ciurea AI, Ciortea CA, Ştefan PA, Lisencu LA, Dudea SM. Differentiating Breast Tumors From Background Parenchymal Enhancement at Contrast-Enhanced Mammography: The Role of Radiomics—A Pilot Reader Study. Diagnostics (2021) 11:1248. doi: 10.3390/diagnostics11071248
Keywords: breast cancer: mammography, contrast-enhanced spectral mammography, HER2, progesterone, estrogen, Ki67
Citation: Vasselli F, Fabi A, Ferranti FR, Barba M, Botti C, Vidiri A and Tommasin S (2022) How Dual-Energy Contrast-Enhanced Spectral Mammography Can Provide Useful Clinical Information About Prognostic Factors in Breast Cancer Patients: A Systematic Review of Literature. Front. Oncol. 12:859838. doi: 10.3389/fonc.2022.859838
Received: 21 January 2022; Accepted: 27 May 2022;
Published: 22 July 2022.
Edited by:
Xiaosong Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Guolin Ma, China-Japan Friendship Hospital, ChinaRaffaella Massafra, National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Vasselli, Fabi, Ferranti, Barba, Botti, Vidiri and Tommasin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonello Vidiri, YW50b25lbGxvLnZpZGlyaUBpZm8uaXQ=
 Federica Vasselli
Federica Vasselli Alessandra Fabi
Alessandra Fabi Francesca Romana Ferranti1
Francesca Romana Ferranti1 Antonello Vidiri
Antonello Vidiri Silvia Tommasin
Silvia Tommasin