- 1Department of Integrative Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy of Tianjin City, Tianjin, China
- 2Department of Esophageal Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy of Tianjin City, Tianjin, China
Studies that have attempted to validate the staging systems and the predictors of survival for patients with primary malignant melanoma of the esophagus (PMME) have been underpowered given their scarcity and small scale. We aimed to review a large number of PMME cases to know more about its clinicopathological features, TNM staging systems, and survival predictors of PMME. Case reports on PMME were extracted from PubMed/Medline through bibliography search and our center. A total of 287 PMME cases were identified. The majority of the patient population was male (72.08%). The most common location of PMME was the lower esophagus (50.62%) and middle esophagus (35.39%). Among the patients, 82.28% received surgical intervention. The median overall survival (OS) duration was 15 months (0.5–244). The American Joint Commission on Cancer staging classification (AJCC) for the mucosal melanoma of the upper aerodigestive tract with stage IVB and IVC integrated in stage IVA showed better distribution of OS than that for esophageal carcinoma. T stage, N stage, and surgery had significant impacts on OS duration in univariate analysis. However, only T stage and N stage were identified as independent factors for OS duration in the multivariate Cox models. PMME is an aggressive tumor with poor prognosis. The AJCC staging system for mucosal melanoma with stage IVB and IVC integrated in stage IVA may be a better option for staging PMME patients. T stage and N stage are independent factors for OS.
Introduction
Cutaneous melanoma accounts for 95% of all reported malignant melanoma cases (1). Extracutaneous melanoma is more aggressive than cutaneous melanoma with its poor prognosis (2, 3). Primary malignant melanoma of the esophagus (PMME) is a rare disease accounting for approximately 0.5% of all extracutaneous melanomas (4–7). Bisceglia (8) reported that only 337 cases of PMME have been published from 1906 to the end of October 2010. There are no standardized staging systems and treatment guidelines for PMME available now. The median survival duration of PMME patients ranges from 8 to 34.5 months (8–11). Several questions about PMME remain unanswered, including its clinicopathological characteristics, staging, and prognosis.
The rarity of PMME have underpowered the attempt to validate the staging systems and to identify predictors of survival for PMME, which in turn further hindered the prospective randomized trials. For the benefit of PMME patient, we aimed to review a large number of PMME cases to know more about its clinicopathological features, TNM staging systems, and survival predictors of PMME. There is no recognized staging system for PMME. In this article, two TNM staging systems were mentioned. TNMe stage represented the staging for esophageal carcinoma according to the 8th edition of the American Joint Commission on Cancer staging classification (AJCC) for esophageal carcinoma (11), while TNMm stage referred to mucosal melanoma staging according to 8th edition of the AJCC Cancer staging for mucosal melanoma of the upper aerodigestive tract (12).
In the mucosal melanoma classification, the T classification of T1 and T2 and TNM classification of Stages I and II are omitted. Stage T3 is defined as tumor limited within the submucosal layer, and T4a is the tumor invading deep soft tissue, cartilage, bone, or overlying skin. T4b is defined as tumor invading brain, dura, skull base, lower cranial nerve, masticator space, carotid artery, prevertebral space, mediastinal structure, cartilage, and skeletal muscle or bone. The details of mucosal melanoma classification and esophageal carcinoma are elaborated in Supplementary Table 1.
Materials and Methods
Patient Selection
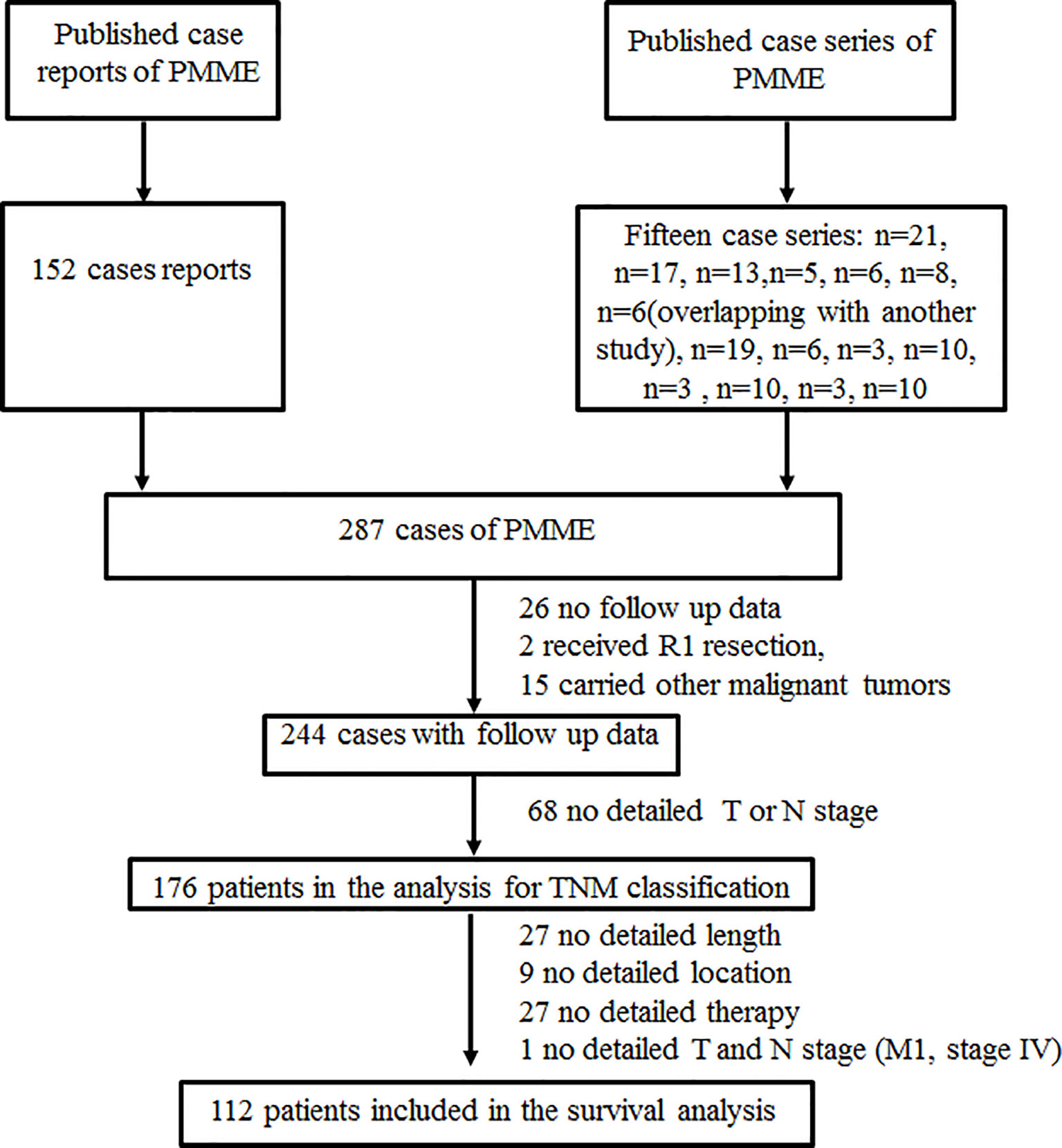
The PMME cases reviewed in this study included patients retrieved from the literatur. By conducting a PubMed/Medline bibliography search of the terms “melanoma” and “esophagus”, we retrieved 293 cases (Supplementary Table 2) of PMME reported from January 2007 to December 2021. After removing 6 cases (9) overlapped with the 17 cases from the same cancer center reported by Gao et al (12), a total of 287 PMME cases were identified in the study. The study was conducted in accordance with the guidelines approved by the Ethics Committee of the Tianjin Medical University Cancer Hospital and Institute.
The following clinicopathological data were retrieved from medical records in our center and extracted from published reports and studies: age, sex, race, tumor location, tumor number, tumor size, symptoms, histological differentiation, lymphovascular invasion, perineural invasion, soft tissue invasion, depth of the invasion of the primary tumor, N stage, metastasis, TNMe stage, TNMm stage, immunohistochemical features, mutational status, surgery, chemotherapy, radiotherapy, immunotherapy intervention, accompanied tumor, and survival data. Race/ethnicity was based on the countries of origin of the authors if unspecified. The details of mucosal melanoma classification are elaborated in Table 1. The exclusion criteria for survival analysis were as follows: did not receive R0 resection, carried other malignant tumors, had no complete data or follow-up data. Moreover, patients were only included in the analysis of the staging system when they had complete data, including T stage, nodal status, distant metastases, and follow-up data, allowing us to restage according to the 8th AJCC classifications for the esophageal and mucosal melanoma of the upper aerodigestive tract. Survival analysis was conducted based on gender, tumor location, tumor number, tumor size, TNM stage, surgery, chemotherapy, radiotherapy, and immunotherapy intervention. Consequently, 176 patients were included in the study in accordance with TNM classification, with only 112 of them included in the survival analysis. Selection of patients with esophageal melanoma was shown in Figure 1.
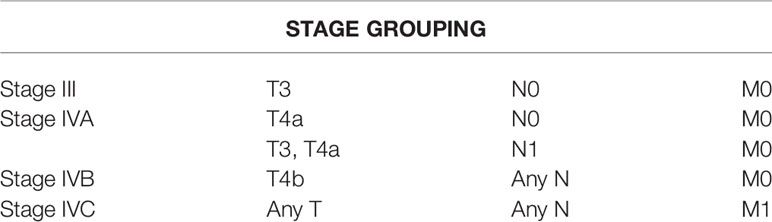
Table 1 American Joint Comission on Cancer staging for mucosal melanoma (upper aerodigestive) 8th edit.
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Numerical variables were expressed as the mean ± SD. TNM staging on survival analysis for the outcome measure was based on Kaplan–Meier methods. Univariate multivariate Cox proportional hazards regression analysis was performed to evaluate the two staging systems and other prognostic factors, including age, gender, tumor location, length, T stage, N stage, metastasis, and therapy intervention. P<0.05 was considered to indicate a statistically significant difference.
Results
Symptoms and Clinical Characteristics
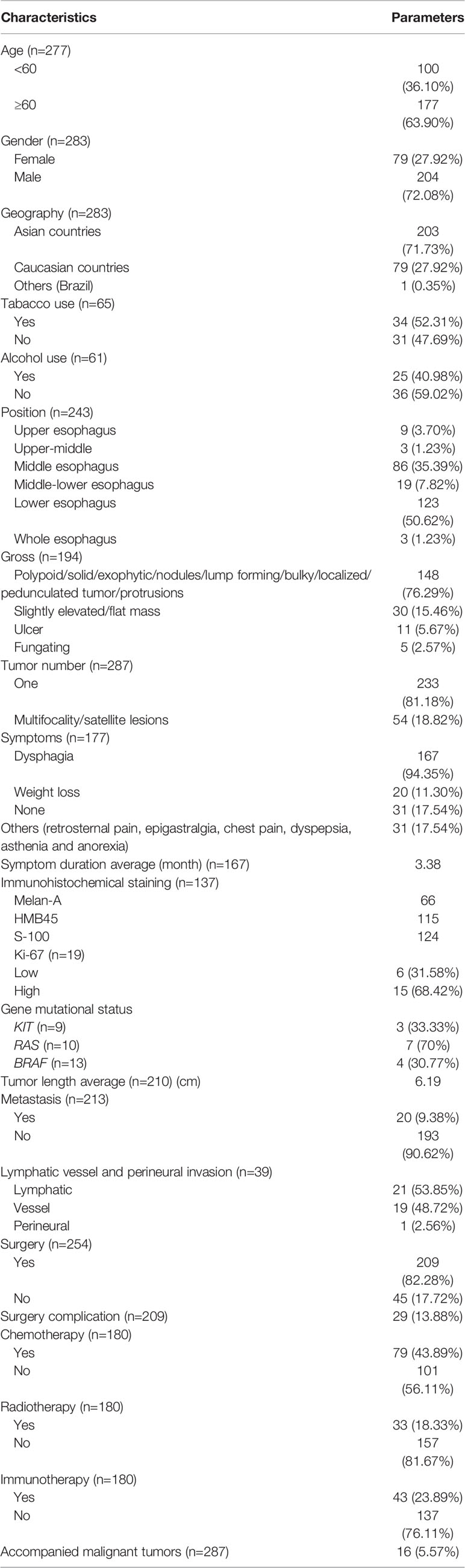
The clinicopathological characteristics of the patients are listed in Table 2. The median age was 63 (36–91) years and the majority of the patient population was male (204/283, (72.08%)) in term of gender and Asian (201/283, 71.73%) geographically. Twenty-nine patients were misdiagnosed, including 22 patients with esophageal squamous cell carcinoma, 6 with esophageal adenocarcinoma, and one with plasma cell myeloma. Histopathological examination revealed the presence of in situ melanoma in 11 cases. Fifty-four (18.82%) cases presented with multifocality or satellite lesions, with median tumor length of 5.25 cm (0.5 to 17).
Treatment and Survival
A total of 209 (82.28%) patients received surgical intervention. Among these cases, three underwent endoscopic submucosal dissection and three received R1 resection. Surgical complication occurred in 29 (13.88%) patients, and three died of surgery. The median number of dissected lymph nodes was 20 (1-105). 79 patients (43.89%) received chemotherapy, 33 patients (18.33%) went through radiotherapy, and 2 underwent thermoradiotherapy. Immunotherapy was recorded for 43 patients (23.89%).
Survival data are shown in Figure 2A. The median overall survival (OS) duration was 12.9 months (0.5-244 months, n = 226). The 1-, 2-, 3-, 4- and 5-year survival rates were 55.75%, 30.97%, 15.93%, 9.73%, and 5.31%, respectively.
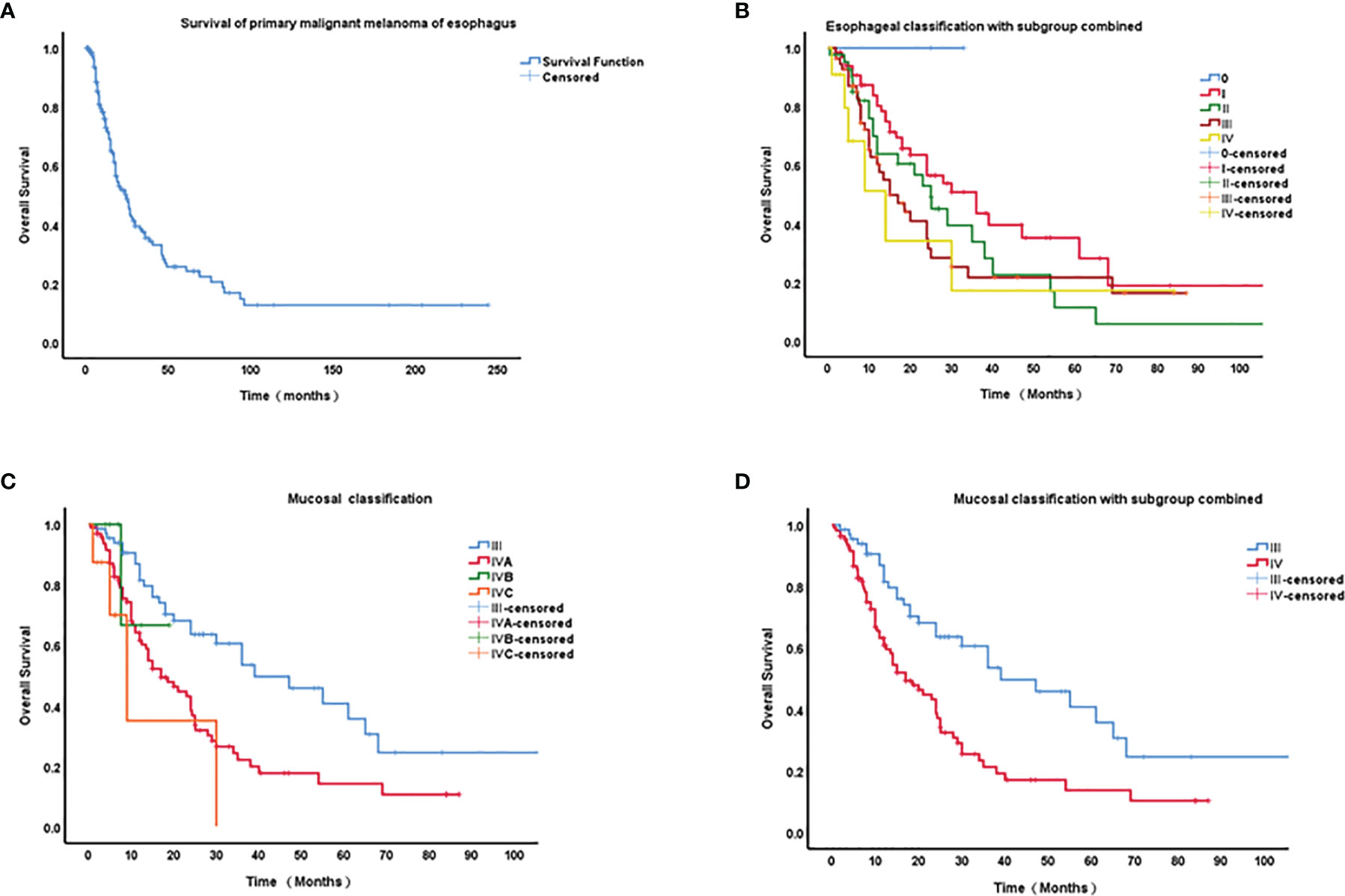
Figure 2 Kaplan–Meier curves of survival of primary malignant melanoma of esophagus (PMME) and different staging classifications for PMME patients. (A) survival of 182 cases with PMME; (B) The 8th AJCC classifications of esophageal carcinoma for PMMEs. (C) The 8th AJCC classifications of mucosal melanoma. (D) Mucosal classification with IVB and IVC integrated into IVA.
Correlation of Different Classification Systems With Survival
A final cohort of 176 patients with a known stage was included in the analysis of TNM classification for PMME. The Kaplan–Meier curves for OS based on AJCC staging systems for esophageal cancer and mucosal melanoma are plotted in Figures 2B–D. Univariate prognostic factor analysis by Cox proportional-hazards regression for OS was performed to identify the staging classifications of tumors on the basis of outcome (Table 3). Significant differences have been observed among the TNMe stages 0(n=2), I(n=66), II(n=41), III(n=56), and IV(n=11) (Figure 2B) as well as TNMm stages III(n=67), IVA(n=96), IVB(n=6) and IVC(n=8). Patients with TNMe stage II have a poorer survival compared with those patients having stage I (0.488 and 0.704, respectively in Table 3). However, there was overlap and intersection between the curves of stages II, III, and IV disease. The hazard ratio (HR) of stage II was comparable to that of stage III (0.704 and 0.816, respectively, shown in Table 3). A better distribution of OS for PMME with p<0.001 was observed when the staging system for mucosal melanoma of the upper aerodigestive tract was employed (Figure 2D). The HRs of stage IVA, IVB and IVC were 2.472, 7.122, 10.056 compared with stage III (Table 3), which meant patients with stage IVC had a greater relative risk for death than those with stage IVB and those with stage IVB had a greater relative risk for death than those with stage IVA. Notably, stage IVA and other stages were overlapped. When stage IVB and IVC were combined with stage IVA because of its rarity, the overlap disappeared and survival curves became well distinguishable between stages (Figure 2C). HRs were also observed with significant difference.
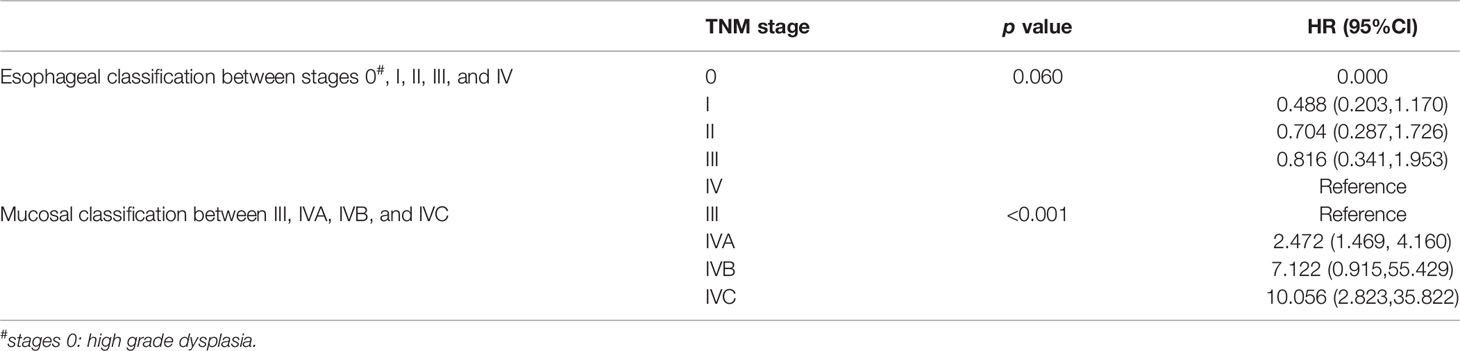
Table 3 Univariate Prognostic Factor Analysis for overall survival by Cox Proportional-hazards Regression.
Predictive Factors for OS
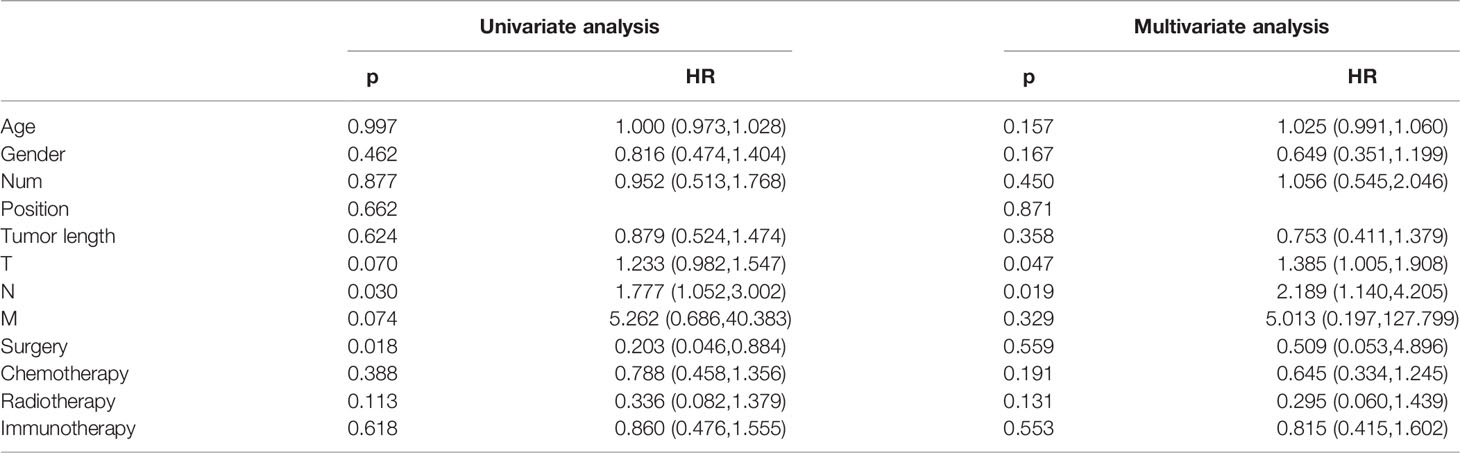
A total of 112 patients were included in the survival analysis, shown in Table 4, T stage, N stage, and surgery had significant impacts on OS time in univariate analysis. The predictive variables of metastasis and surgery were no longer statistically significant and only T stage and N stage were independent factors for OS in the multivariate Cox models.
Discussion
Given the extremely rare incidence of PMME, studies that involve high numbers of PMME cases are scarce. In this study, we used a large cohort to evaluate the validity of different staging classifications in predicting OS and to determine the predictive factors for the survival of PMME patients. We found that the mucosal staging system with stage IVB omitted and integrated into stage IVA is a valid staging system for PMME. T stage and N stage are two independent prognostic factors for OS.
PMME is an aggressive neoplasm with a five-year survival rate of only approximately 4% (9, 13). In this review, we found that the median survival duration is 15 months and that the 1-, 2-, and 5-year survival rates are 55.75%, 30.97% and 5.31%, respectively. Bishop (14) reported that the five-year survival rates of patients with mucosal melanoma are significantly lower than those with cutaneous melanoma. Gao (12) reported a median survival of 18.1 months and 1- and 5-year survival rates of 51% and 10%, respectively. Consistent with our present findings, Chalkiadaki (15) reported a mean survival duration of 13 months for 110 PMME patients. Although our findings showed that the surgical resection rate among patients with PMME reached 82.28% (209/254), the majority of the patients died after surgery from disseminated disease.
Currently, there is no standardized staging system for PMME available. Mucosal melanomas arise directly from resident melanocytes in the mucosa. Due to the biological aggressiveness of mucosal melanomas, the relatively advanced nature at diagnosis, and the molecular features different from those classically associated with cutaneous melanoma, the staging is generally not based on the cutaneous malignant melanoma staging. Mucosal melanoma is introduced in the 8th edition of AJCC cancer staging manual, for separate consideration from other mucosal-based lesions. The system omits TI and T2 categories, and even small superficial lesions have an overall poor prognosis. Stage IVB represents extensive local invasion for which treatment often is a nonsurgical approach for local palliation. Stage IVC denotes distant metastatic disease. Only a few authors (11, 14, 16) used mucosal melanoma staging classifications for PMME. In contrary, most reports utilized the staging system for esophageal carcinoma. The coexistence of these two parallel systems in practice has resulted in confusion for clinicians. A previous study conducted at our center suggested that the staging system for the mucosal melanoma of the upper aerodigestive tract might be a better option for staging patients with PMME than that for esophageal classification (16). In this study, we found that the difference of PMME patients between TNMe stages were not significant. The curves of stages II, III, and IV disease were overlapped. There was dramatic overlap among Stage IVB and IVBC disease and other stages in the mucosal staging system. These results revealed the shortcomings of the current staging classification. Therefore, changes of the current staging classification are urgently required. Given the rarity of stage IVB and IVC disease, we integrated stage IVB and IVC into stage IVA, resulting in disappearance of overlap and a worse survival from stages III to IV. These results together suggested that the staging classification for the mucosal melanoma of the upper aerodigestive tract with stage IV combination might be a better solution for staging patients with PMME.
Age, stage, the presence of lymph nodes metastasis and surgery are considered as main possible independent predictors. Cheung et al. (17) reviewed reports on primary gastrointestinal melanomas retrieved from the Surveillance, Epidemiology and End Reports database between 1973 and 2004. They found that increasing age, stage, and the presence of lymph nodes are independent predictors of lower OS, whereas surgical resection is an indicator of a significantly better outcome. The present study only revealed the T and N stages are independent factors for the OS of PMME patients. Although surgery was associated with a reduced risk of death in univariate analysis, multivariate analysis did not show survival benefits. This result may be related to the selection bias associated with the online database and the selective reports on early-stage disease (only two cases of metastasis were reported with complete data). Other authors (14, 18) reported that lymph node metastasis is an independent predictive factor for the postoperative survival of patients with PMME. Gao (12) and Harada (11) also found that patients with lymph node metastasis have poorer survival than those without metastasis even though there was no significant difference. In addition, Weiner (19) retrieved 56 case reports of PMME published between 2004 and 2011 from the National Cancer Database and showed that metastatic disease and regional disease are associated with worse survival.
The present study has several limitations despite its large cohort. First, the study is a retrospective analysis and all data were collected from PubMed/Medline bibliography. We did not list the cases included in the study of cancer registries that covered several decades, such as the cases reviewed by Weiner (19) Sekine (20) and Cote (21), which may overlap with the cases included the present study. Second, patients with missing data were excluded from the analysis, leading to selection bias. Third, given the fact that their IVB and IVC groups were so small, the conclusion that integrating IVA, IVB and IVC into a single mucosal melanoma staging IV may be overstated. Finally, we could not analyze patient comorbidities, performance status, histological differentiation, lymphovascular invasion, neural invasion, and soft tissue invasion or other potential prognostic factors for survival.
Conclusion
PMME is an extremely aggressive tumor with poor prognosis. The staging system for the mucosal melanoma of the upper aerodigestive tract with stage IVB and IVC integrated into stage IVA may be a better option for staging patients with PMME than current esophageal classification. T and N stages are independent factors for OS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
HS, NZ: design and initiation of the study, quality control of data, data analysis and interpretation, and manuscript preparation and editing. LG: data acquisition. LL: conduct the editing and critical revision. ZY: Participate in the writing of manuscripts or critically revise important intellectual content; ZP: Significant contributions to data analysis and interpretation. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Tianjin Health and Family Planning Commission program (No.2017166).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to all the teachers and classmates of the research group for their help.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.858145/full#supplementary-material
References
1. Herman J, Duda M, Lovecek M, Svach I. Primary Malignant Melanoma of the Esophagus Treated by Endoscopic Ablation and Interferon Therapy. Diseases of the Esophagus. Off J Int Soc Dis Esophagus (2001) 14(3-4):239–40. doi: 10.1046/j.1442-2050.2001.00192.x
2. McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of Noncutaneous Melanomas in the U.S. Cancer (2005) 103(5):1000–7. doi: 10.1002/cncr.20866
3. Mikkelsen LH, Larsen AC, von Buchwald C, Drzewiecki KT, Prause JU, Heegaard S. Mucosal Malignant Melanoma - a Clinical, Oncological, Pathological and Genetic Survey. APMIS Acta Pathologica Microbiologica Immunologica Scandinavica (2016) 124(6):475–86. doi: 10.1111/apm.12529
4. Archer HA, Owen WJ. Primary Malignant Melanoma of the Esophagus. Diseases of the Esophagus. Off J Int Soc Dis Esophagus (2000) 13(4):320–3. doi: 10.1046/j.1442-2050.2000.00140.x
5. Kido T, Morishima H, Nakahara M, Nakao K, Tanimura H, Nishimura R, et al. Early Stage Primary Malignant Melanoma of the Esophagus. Gastrointest Endoscopy (2000) 51(1):90–1. doi: 10.1016/S0016-5107(00)70397-X
6. Caldwell CB, Bains MS, Burt M. Unusual Malignant Neoplasms of the Esophagus. Oat Cell Carcinoma, Melanoma, and Sarcoma. J Thorac Cardiovasc Surg (1991) 101(1):100–7. doi: 10.1016/S0022-5223(19)36798-4
7. Mikami T, Fukuda S, Shimoyama T, Yamagata R, Nishiya D, Sasaki Y, et al. A Case of Early-Stage Primary Malignant Melanoma of the Esophagus. Gastrointest endoscopy. (2001) 53(3):365–7. doi: 10.1016/S0016-5107(01)70420-8
8. Bisceglia M, Perri F, Tucci A, Tardio M, Panniello G, Vita G, et al. Primary Malignant Melanoma of the Esophagus: A Clinicopathologic Study of a Case With Comprehensive Literature Review. Adv Anatomic Pathol (2011) 18(3):235–52. doi: 10.1097/PAP.0b013e318216b99b
9. Li B, Lei W, Shao K, Zhang C, Chen Z, Shi S, et al. Characteristics and Prognosis of Primary Malignant Melanoma of the Esophagus. Melanoma Res (2007) 17(4):239–42. doi: 10.1097/CMR.0b013e3281c4a079
10. Wang S, Tachimori Y, Hokamura N, Igaki H, Kishino T, Kushima R. Diagnosis and Surgical Outcomes for Primary Malignant Melanoma of the Esophagus: A Single-Center Experience. Ann Thorac Surg (2013) 96(3):1002–6. doi: 10.1016/j.athoracsur.2013.04.072
11. Harada K, Mine S, Yamada K, Shigaki H, Oya S, Baba H, et al. Long-Term Outcome of Esophagectomy for Primary Malignant Melanoma of the Esophagus: A Single-Institute Retrospective Analysis. Dis esophagus Off J Int Soc Dis Esophagus (2016) 29(4):314–9. doi: 10.1111/dote.12331
12. Gao S, Li J, Feng X, Shi S, He J. Characteristics and Surgical Outcomes for Primary Malignant Melanoma of the Esophagus. Sci Rep (2016) 6:23804. doi: 10.1038/srep23804
13. Wang M, Chen J, Sun K, Zhuang Y, Xu F, Xu B, et al. Primary Malignant Melanoma of the Esophagus Treated by Endoscopic Submucosal Dissection: A Case Report. Exp Ther Med (2016) 12(3):1319–22. doi: 10.3892/etm.2016.3482
14. Bishop KD, Olszewski AJ. Epidemiology and Survival Outcomes of Ocular and Mucosal Melanomas: A Population-Based Analysis. Int J Cancer (2014) 134(12):2961–71. doi: 10.1002/ijc.28625
15. Chalkiadakis G, Wihlm JM, Morand G, Weill-Bousson M, Witz JP. Primary Malignant Melanoma of the Esophagus. Ann Thorac Surg (1985) 39(5):472–75. doi: 10.1016/S0003-4975(10)61963-7
16. Sun H, Gong L, Zhao G, Zhan H, Meng B, Yu Z. Clinicopathological Characteristics, Staging Classification, and Survival Outcomes of Primary Malignant Melanoma of the Esophagus. J Surg Oncol (2017) 117(4):588–96. doi: 10.1002/jso.24905
17. Cheung MC, Perez EA, Molina MA, Jin X, Gutierrez JC, Franceschi D, et al. Defining the Role of Surgery for Primary Gastrointestinal Tract Melanoma. J Gastrointest Surg Off J Soc Surg Alimentary Tract (2008) 12(4):731–8. doi: 10.1007/s11605-007-0417-3
18. Ahn JY, Hwang HS, Park YS, Kim HR, Jung H-Y, Kim J-H, et al. Endoscopic and Pathologic Findings Associated With Clinical Outcomes of Melanoma in the Upper Gastrointestinal Tract. Ann Surg Oncol (2014) 21(8):2532–9. doi: 10.1245/s10434-014-3637-2
19. Weiner JP, Shao M, Schwartz D, Wong A, Schreiber D. Patterns of Care and Survival Outcomes in the Treatment of Esophageal Melanoma. Dis esophagus Off J Int Soc Dis Esophagus (2017) 30(2):1–6. doi: 10.1111/dote.12504
20. Sekine S, Nakanishi Y, Ogawa R, Kouda S, Kanai Y. Esophageal Melanomas Harbor Frequent NRAS Mutations Unlike Melanomas of Other Mucosal Sites. Virchows Archiv an Int J Pathol (2009) 454(5):513–7. doi: 10.1007/s00428-009-0762-6
Keywords: esophagus, melanoma, classification, survival analysis, predictor
Citation: Sun H, Zhu N, Gong L, Lan L, Yu Z and Pan Z (2022) Clinicopathological Features, Staging Classification, and Clinical Outcomes of Esophageal Melanoma: Evaluation of a Pooled Case Series. Front. Oncol. 12:858145. doi: 10.3389/fonc.2022.858145
Received: 14 March 2022; Accepted: 30 May 2022;
Published: 01 July 2022.
Edited by:
Vladimir Spiegelman, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Rocco Cappellesso, University Hospital of Padua, ItalyMauricio Ribeiro, University Health Network, Canada
Copyright © 2022 Sun, Zhu, Gong, Lan, Yu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanyu Pan, enBhbkB0bXUuZWR1LmNu; Zhentao Yu, eXp0YW8yMDE1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Haiyan Sun1†
Haiyan Sun1† Ningning Zhu
Ningning Zhu Lei Gong
Lei Gong Zhentao Yu
Zhentao Yu