
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 04 March 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.856452
Background: Lynch syndrome (LS), an autosomal dominant disorder, is characterized by germline pathogenic variants in DNA mismatch repair (MMR) genes like MSH2. EPCAM deletions cause a minority (3%) of LS cases. However, there are only a few reports of LS-associated endometrial cancer (LS-EC) induced by the inactivation of the MSH2 gene due to EPCAM deletions.
Case Presentation: We present the case of a 45-years old woman diagnosed with endometrial cancer (EC). Definitive surgery revealed meso-differentiated endometrioid adenocarcinoma, stage IA without lymph-vascular space invasion. Four months later, she received radiation therapy (125I radioactive seeds implantation), and platinum-containing regimen combined chemotherapy because of vaginal stump metastasis of EC. After five years, we performed immunohistochemistry (IHC) on pelvic mass because of presacral metastatic lymph node. IHC showed the absence of MSH2 and MSH6 protein expression in the pelvic mass tissue. Peripheral blood was used for genetic testing based on her cancer diagnosis and family history of cancer in close relatives. Genetic testing revealed deletions of exon 8 and 9 in EPCAM and deletions of exon 1 and 8 in MSH2; thus, we diagnosed the presence of LS. The patient underwent interstitial brachytherapy (BT) of the presacral metastatic lymph node.
Conclusion: This case highlights that patients with LS-EC who are carriers of combined EPCAM-MSH2 deletion might experience better oncologic outcomes even with early recurrence.
Lynch syndrome (LS) is caused by germline pathogenic variants (PVs) in one of the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, or PMS2) or by deletions in the epithelial cell adhesion molecule gene (EPCAM), which increases susceptibility to colorectal and endometrial cancers and other tumors (1–3). There is evidence that cancer risk depends on the affected gene (4, 5). MLH1 and MSH2 are most commonly associated with a higher risk of colorectal cancer. And female MSH6 carriers may have the highest risk of endometrial cancer (EC). Up to 3% of LS cases are due to variants involving the 3’ end of the EPCAM gene (immediately adjacent to MSH2), which result in hypermethylation of the MSH2 promoter or partial deletion of MSH2 (6, 7). EPCAM deletions cause LS by causing transcriptional readthrough into the neighboring gene, MSH2, leading to its epigenetic silencing. The difference in tumor occurrence or spectrum resulting from MSH2 mutation carriers versus EPCAM deletion carriers may be related to the mosaic pattern of MSH2 inactivation displayed by EPCAM deletion carriers (8, 9). Unlike colorectal cancer where EPCAM deletions are an independent risk factor, EPCAM deletions will increase the risk of EC only if deletions extend near the MSH2 promoter (10). The cumulative risk by the age of 70 years of EC in women with EPCAM deletions was 12% and that of colorectal cancer in EPCAM deletion carriers was 75%. The risk of developing colorectal cancer was not significantly different between EPCAM deletion carriers and EPCAM-MSH2 deletion (p=0·8609) or MSH2 mutation (p=0·5892) carriers. However, the risk of developing EC was lower in EPCAM deletion carriers than in EPCAM-MSH2 deletion (p<0·0001) or MSH2 mutation (p=0·0006) carriers (10).
This study shows the presentation and outcomes of a patient with LS-EC with combined EPCAM-MSH2 deletion.
The patient is a 45-years-old Chinese woman who visited Yunnan Cancer Hospital and complained of prolonged and increased menstruation. Her past medical history was unremarkable. After an EC diagnosis, the gynecologist performed a total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection under general anesthesia. Histopathological examination of surgically obtained samples led to a meso-differentiated endometrioid adenocarcinoma diagnosis. Pathological staging of surgery identified the disease as stage IA with no lymph-vascular space invasion. Four months after the operation, colonoscopy and computed tomography (CT) scan showed rectal occupancy. She underwent exploratory laparotomy and was diagnosed with vaginal stump metastasis of EC. As the patient refused to have an enterostomy, the radiation oncologist performed radiation therapy (125I radioactive seeds implantation), followed by six cycles of chemotherapy (paclitaxel combined with carboplatin). Re-examination of imaging data showed that the lesion was in complete remission. During the 5-year follow-up period, there were no reports of the recurrence of gynecological tumors.
Five years later, she visited our hospital with a pelvic mass during follow-up (Figure 1). Figure 2 shows hematoxylin-eosin (HE) staining and IHC staining of pelvic mass tissues. IHC staining results: CK7(+), CK20 (-), CEA (+), Vim (-), ER (-), PR (+, 60%), P16(+), Ki67 (+, 50%), P53 (+, 40%), PMS2 (+), MLH1 (+), MSH6(-), MSH2 (-). The patient had two first-degree relatives with endometrial and colorectal cancer and two second-degree relatives with gallbladder and stomach cancer. We provided her with genetic counseling based on her cancer diagnosis and family history of multiple cancers. After obtaining consent, we carried out the genetic analysis of her peripheral blood. Results were as follows: deletions of exon 8 and 9 in EPCAM and deletions of exon 1 and 8 in MSH2 (Figure 3); thus, we diagnosed the presence of LS. At the same time, the radiation oncologist of our hospital performed interstitial BT of presacral metastatic lymph node, and the prescription dose was 2700cGy. Figure 4 shows three-dimensional conformal dose assessment for interstitial BT.
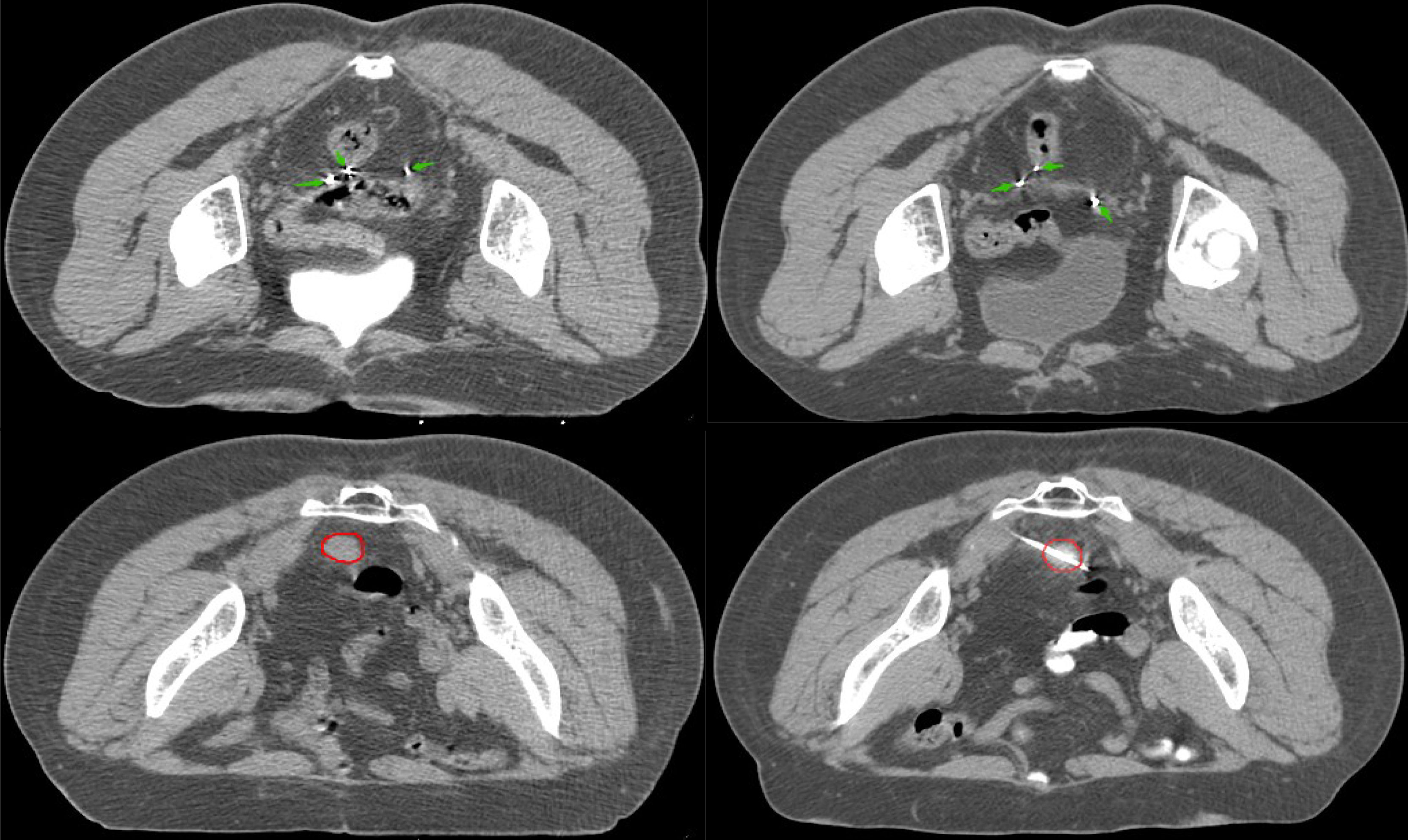
Figure 1 There are twenty radioactive seeds in CT images of pelvic recurrence. The green arrow represents 125I radioactive seed; the red circle represents lesions.
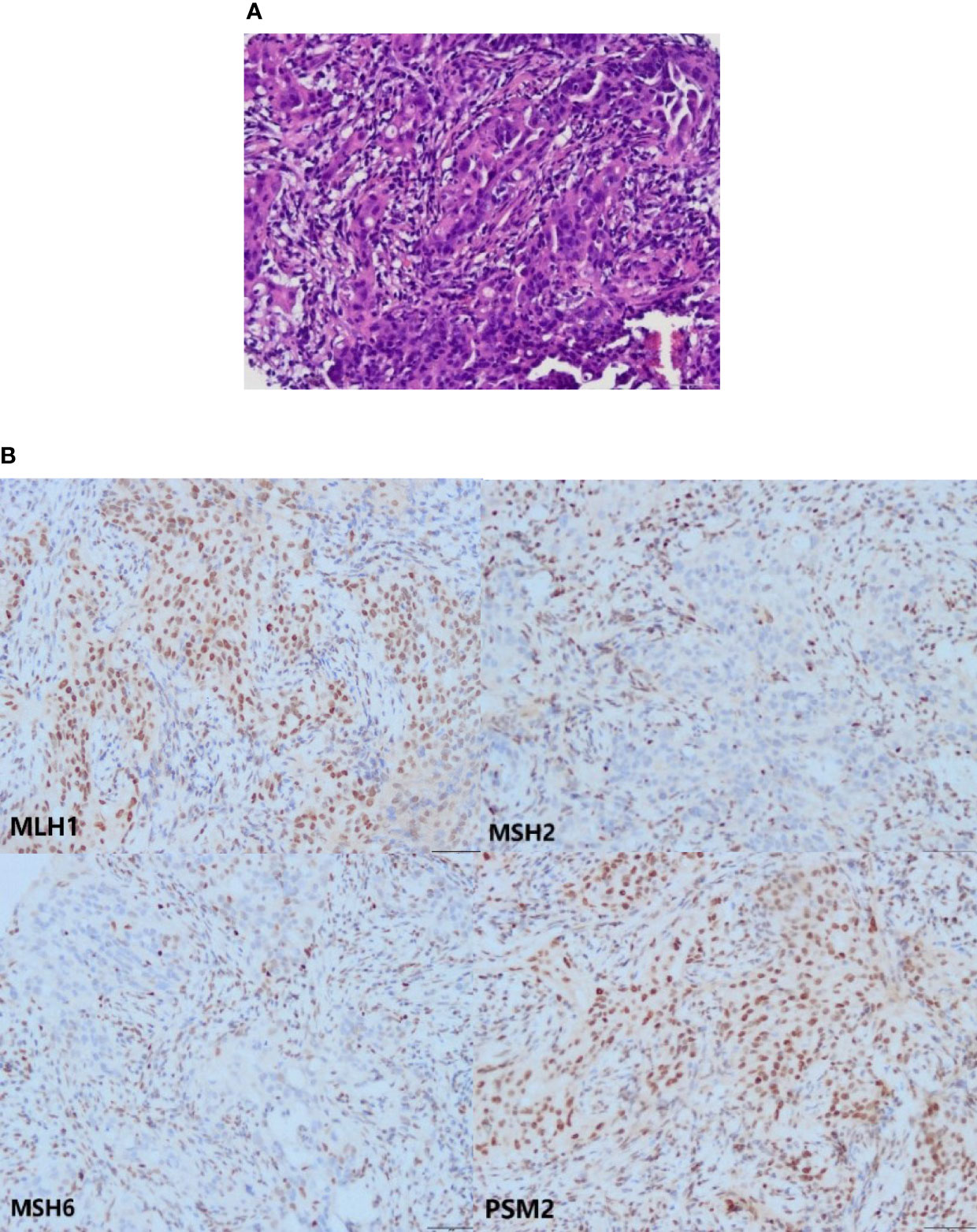
Figure 2 The HE and IHC staining. (A) The HE staining of pelvic mass tissues (the microscope magnifying×200). (B) Loss of MSH2 and MSH6 proteins in the tumor cells. Expression of MLH1 and PSM2 proteins in the tumor cells (the microscope magnifying×200).
Approximately 3% of endometrial cancers are causally related to PVs in one of the DNA MMR genes (MLH1, MSH2, MSH6, and PMS2) (11). Among first cancer detected in each patient from the first prospective lynch syndrome database, the EC cumulative incidences at 70 years by gene were 34%, 51%, 49% and 24% for MLH1, MSH2, MSH6 and PMS2 mutation carriers, respectively (5). EPCAM is not an MMR gene, but structural alterations in EPCAM may lead to LS as it is adjacent to the MSH2 gene (9). In LS, the deletion of heterozygote sequences at the 3’ end of EPCAM can lead to the inactivation of MSH2 in tissues expressing EPCAM due to its promoter hypermethylation (12). And what’s more, the EPCAM 3’ end deletion might extend to the first MSH2 exon, which includes the promoter region, resulting in the suppression of both EPCAM protein and MSH2 protein expressions without MSH2 hypermethylation (7). Therefore, MSH2 negative patients need to be tested for EPCAM deletions. This patient’s IHC showed the absence of MSH2 and MSH6 protein expression in the tumor cells. According to the recommendation of the Manchester International Consensus Group, patients with MSH2, MSH6 or PMS2 protein deletion require germline testing related to LS (13). This report is a kind of MSH2 germline mutation EC with deletions of EPCAM, a relatively rare condition.
A related study showed that the risk of EC in EPCAM deletion carriers might depend on the location and size of the deletion. This cumulative risk of developing EC in women with EPCAM deletions at age 70 (12%) was significantly lower than in EPCAM-MSH2 deletion (51%) or MSH2 mutation (55%) carriers (p<0·0001, p=0·0006, respectively) (10). Ring et al. found mutations associated with LS in 5.8% of 381 EC cases. For mutations in MLH1, MSH2, and EPCAM-MSH2, 80% of patients were diagnosed with EC at <50 years of age, and most patients had a family history of LS-associated cancer (14). The patient’s age and family history in this report are consistent with the characteristics of EPCAM-MSH2 mutation.
Molecular tumor screening for LS in EC was supported by the Society of Gynecologic Oncology clinical practice statement in 2017. However, the prognostic value of MMR status has not been fully established in EC outside the LS diagnosis. Data from studies show that MMR deficiency is associated with lower recurrence rates, better progression-free, and overall survival in advanced EC treated with adjuvant therapy (15, 16). Contrastingly, MMR deficiency in early-stage EC is associated with poor prognostic factors and worse progression-free survival (17, 18). Moreover, some studies have shown that MMR deficiency in LS-EC could be targeted for immunotherapy (19, 20). Pembrolizumab and nivolumab have been recommended by the NCCN guidelines for advanced or recurrent EC patients with MMR deficiency (21). The patient with LS-EC in stage IA, carrier of combined EPCAM-MSH2 deletion, soon developed a local pelvic recurrence and could not achieve satisfactory surgical resection. However, local 125I radioactive seeds implantation and platinum-containing regimen combined chemotherapy led to a five-year disease-free survival period.
After five years, the presacral metastatic lymph node was identified as endometrioid adenocarcinoma by biopsy and IHC. Even though the recurrent lesions were localized and small, gynecological oncologists did not consider surgical treatment. Based on the patient’s genetic phenotype, we recommend immunotherapy with or without radiotherapy according to the guidelines. Re-irradiation needs are individualized according to the extent of disease, the time elapsed from the previous treatment, and prior radiation fields. In addition, recurrences less than 2-4 cm and recurrences with longer intervals of disease-free time tend to improve the prognosis. The most frequent re-irradiation method is intracavitary or interstitial BT (22). The patient refused immunotherapy because of possible side effects, and we only performed interstitial BT for the presacral metastatic lymph node based on the above treatment modalities and the previous permanent seed implant. We will be evaluating the therapeutic effect and long-term side effects over time.
The present case report shows disease manifestations and outcomes of LS-EC. It indicates that EC-LS patients, carriers with combined EPCAM-MSH2 deficiency might experience better oncologic outcomes even with early recurrence. Further molecular/genetic studies are needed to evaluate prognostic factors in patients with combined EPCAM-MSH2 deletion LS-EC.
Acquisition of data: RH, DL, QW, and ZZ. Manuscript writing: RH, DL and XD. Critical review of the manuscript: All authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA (2011) 305:2304–10. doi: 10.1001/jama.2011.743
2. Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch Syndrome Among Patients With Colorectal Cancer. JAMA (2012) 308:1555–65. doi: 10.1001/jama.2012.13088
3. Pathak SJ, Mueller JL, Okamoto K, Das B, Hertecant J, Greenhalgh L, et al. “EPCAM Mutation Update: Variants Associated With Congenital Tufting Enteropathy and Lynch Syndrome.” Hum Mutat (2019) 40:142–61. doi: 10.1002/humu.23688
4. Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol (2014) 109:1159–79. doi: 10.1038/ajg.2014.186
5. Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer Incidence and Survival in Lynch Syndrome Patients Receiving Colonoscopic and Gynaecological Surveillance: First Report From the Prospective Lynch Syndrome Database. Gut (2017) 66:464–72. doi: 10.1136/gutjnl-2015-309675
6. Tiwari AK, Roy HK, Lynch HT. Lynch Syndrome in the 21st Century: Clinical Perspectives. QJM (2016) 109:151–8. doi: 10.1093/qjmed/hcv137
7. Perez-Cabornero L, Infante SM, Velasco SE, Lastra AE, Acedo BA, Miner PC, et al. Frequency of Rearrangements in Lynch Syndrome Cases Associated With MSH2: Characterization of a New Deletion Involving Both EPCAM and the 5’ Part of MSH2. Cancer Prev Res (Phila) (2011) 4:1556–62. doi: 10.1158/1940-6207
8. Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, Hoogerbrugge N. EPCAM Deletion Carriers Constitute a Unique Subgroup of Lynch Syndrome Patients. Fam Cancer (2013) 12:169–74. doi: 10.1007/s10689-012-9591-x
9. Ligtenberg MJ, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable Somatic Methylation and Inactivation of MSH2 in Families With Lynch Syndrome Due to Deletion of the 3’ Exons of TACSTD1. Nat Genet (2009) 41:112–7. doi: 10.1038/ng.283
10. Kempers MJ, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, et al. Risk of Colorectal and Endometrial Cancers in EPCAM Deletion-Positive Lynch Syndrome: A Cohort Study. Lancet Oncol (2011) 12:49–55. doi: 10.1016/S1470-2045(10)70265-5
11. Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The Proportion of Endometrial Cancers Associated With Lynch Syndrome: A Systematic Review of the Literature and Meta-Analysis. Genet Med (2019) 21:2167–80. doi: 10.1038/s41436-019-0536-8
12. Kastrinos F, Stoffel EM. History, Genetics, and Strategies for Cancer Prevention in Lynch Syndrome. Clin Gastroenterol Hepatol (2014) 12:715–e43. doi: 10.1016/j.cgh.2013.06.031
13. Crosbie EJ, Ryan NAJ, Arends MJ, Bosse T, Burn J, Cornes JM, et al. The Manchester International Consensus Group Recommendations for the Management of Gynecological Cancers in Lynch Syndrome. Genet Med (2019) 21:2390–400. doi: 10.1038/s41436-019-0489-y
14. Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, et al. Germline Multi-Gene Hereditary Cancer Panel Testing in an Unselected Endometrial Cancer Cohort. Mod Pathol (2016) 29:1381–9. doi: 10.1038/modpathol.2016.135
15. Kim SR, Pina A, Albert A, McAlpine J, Wolber R, Blake Gilks C, et al. Does MMR Status in Endometrial Cancer Influence Response to Adjuvant Therapy? Gynecol Oncol (2018) 151:76–81. doi: 10.1016/j.ygyno.2018.08.020
16. McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol (2016) 34:3062–8. doi: 10.1200/JCO.2016.67.8722
17. Kim SR, Pina A, Albert A, McAlpine JN, Wolber R, Gilks B, et al. Mismatch Repair Deficiency and Prognostic Significance in Patients With Low-Risk Endometrioid Endometrial Cancers. Int J Gynecol Cancer (2020) 30:783–8. doi: 10.1136/ijgc-2019-000910
18. Carr C, Son J, Yao M, Priyadarshini A, Marquard J, Vargas R, et al. Clinicopathologic Characteristics and Outcomes of Endometrial Cancer Patients With Mismatch Repair Deficiency in the Era of Universal Lynch Syndrome Screening. Gynecol Oncol (2020) 159:712–20. doi: 10.1016/j.ygyno.2020.09.039
19. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol (2020) 38:1–10. doi: 10.1200/JCO.19.02105
20. Azad NS, Gray RJ, Overman MJ, Schoenfeld JD, Mitchell EP, Zwiebel JA, et al. Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study. J Clin Oncol (2020) 38:214–22. doi: 10.1200/JCO.19.00818
21. National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Uterine Neoplasms, V Ersion 2 (2021). Available at: https://www.nccn.org/guidelines/category_1.
Keywords: lynch syndrome, endometrial carcinoma, MSH2, EPCAM, case
Citation: Huang R, Deng X, Zhang Z, Wen Q and Li D (2022) Lynch Syndrome-Associated Endometrial Cancer With Combined EPCAM-MSH2 Deletion: A Case Report. Front. Oncol. 12:856452. doi: 10.3389/fonc.2022.856452
Received: 17 January 2022; Accepted: 09 February 2022;
Published: 04 March 2022.
Edited by:
Inge Bernstein, Aalborg University Hospital, DenmarkReviewed by:
D. Gareth Evans, The University of Manchester, United KingdomCopyright © 2022 Huang, Deng, Zhang, Wen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Li, bGlkYW4wOTE4QDE2My5jb20=; Qinglian Wen, d3FsNzMxMTVAaG90bWFpbC5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.