
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 04 April 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.854997
We describe a case of reactive nodular fibrous pseudotumor (RNFP) misdiagnosed as lymph node metastasis after gastric cancer surgery. Additionally, we summarize the clinical and imaging characteristics of RNFP, combined with the literature, to improve the understanding of preoperative diagnosis. Radiological features of RNFP are a homogenous, isodense, solid mass with gradually mild enhancement on multiphasic abdominal computed tomography (CT), and slight 18F-FDG uptake by positron emission tomography/computed tomography (PET/CT). To the best of our knowledge, this is the first report in the English literature of a case of reactive nodular fibrous pseudotumor associated with gastric cancer and its appearance on PET/CT images.
Reactive nodular fibrous pseudotumor (RNFP), which is described as a rare benign tumor−like lesion, was first reported by Yantiss et al. in 2003 (1). RNFP is related to a history of abdominal surgery, injury, or inflammation, and lesions occurring after surgery for abdominal malignancy are easily misdiagnosed as tumor recurrence/metastasis. We analyzed a case of RNFP misdiagnosed as lymph node metastasis after surgery for gastric cancer and reviewed the relevant literature, with the aim of summarizing its clinical and imaging features and improving the understanding of the lesion.
In July 2018, a 54-year-old male patient with latent abdominal pain, and confirmed gastric malignant tumors by endoscopic biopsy, underwent laparoscopic radical distal gastrectomy (Billroth II gastrointestinal reconstruction) in our hospital. The postoperative pathological diagnosis was early gastric cancer with moderately to poorly differentiated adenocarcinoma in the gastric antrum, superficial bulge type (type IIa). The tumor was limited to the lamina propria.
One year later, a CT scan of the abdomen revealed a round-like mass with a diameter of 5.0 cm near the anastomosis in this patient, with well-defined isodensity and no necrosis. The lesion showed slight enhancement, and the CT values of the non-contrast, arterial, and venous phases were 32HU, 43HU, and 49HU, respectively (Figures 1A–C). Moreover, 18F-FDG PET/CT (Siemens Biograph 64) was performed 60 min after intravenous injection of 310.8 MBq (8.4 mCi) of 18F-FDG. 18F-FDG PET/CT images revealed a minimally increased FDG uptake with SUVmax of 3.6 for a solid mass in the operative area of gastric cancer with a relatively clear boundary (Figure 2). Gastrofiberscopy showed residual gastritis and anastomotic stomatitis, with no sign of a tumor. Based on laboratory tests, the carcinoembryonic antigen level of the patient was slightly increased (5.1 ng/ml, normal range 0–5).
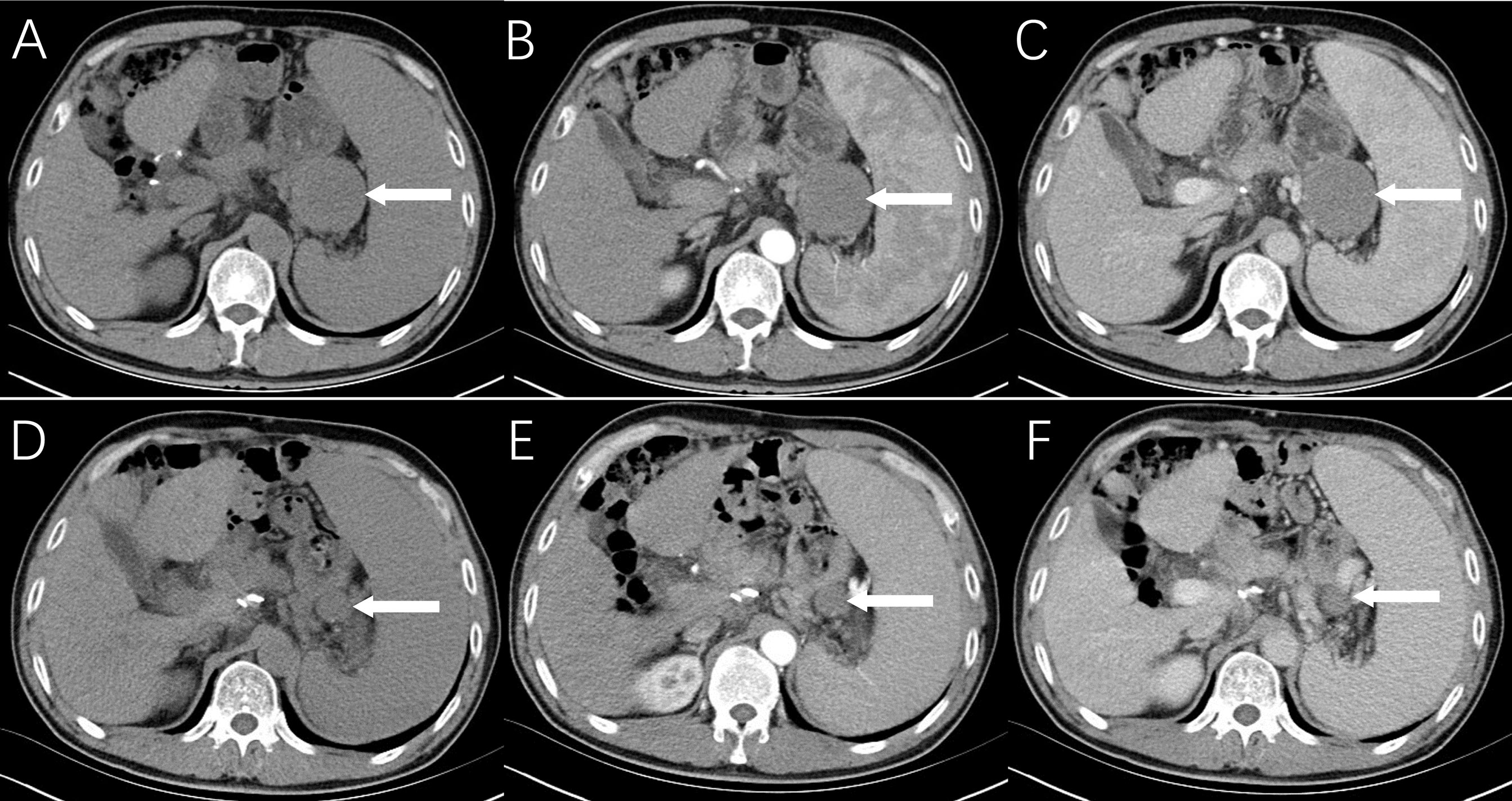
Figure 1 Radiological findings of reactive nodular fibrous pseudotumor. Multiphasic abdominal CT shows a solid mass measuring 5.0 cm × 5.2 cm in the operative area of gastric cancer with a relatively clear boundary, isodensity, and no necrosis (A), mild enhancement in the arterial phase (B), and increased contrast enhancement on portal venous phase (C). On abdominal CT images (D–F) at 8 months ago, only a nodule with a maximum diameter of 1.0 cm was observed.
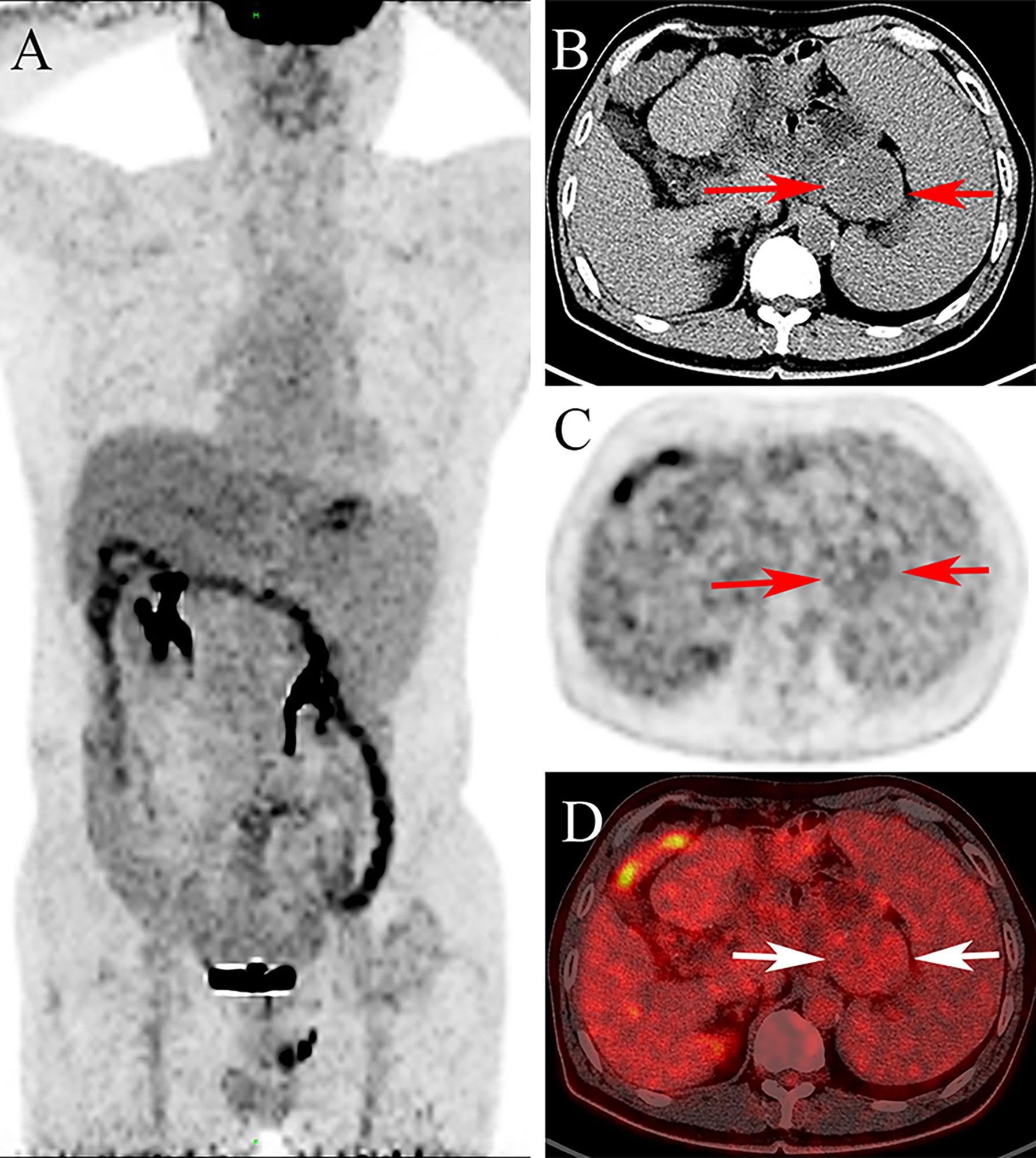
Figure 2 The 18F-FDG PET/CT images [(A): maximum intensity projection image, (B): the transverse CT, (C): PET, (D): fusion] show minimally increased FDG uptake with SUVmax of 3.6 in the lesion (arrow).
A retrospective review of previous postsurgical scans revealed a nodule with a diameter of approximately 1.0 cm (Figures 1D–F). Given the apparent growth of the lesion and the history of gastric cancer, lymph node metastasis was suspected, and laparoscopic splenectomy and splenic hilar lymph node dissection were performed. During the surgery, a 5.0 cm × 5.5 cm light-red mass with medium texture and clear boundaries was detected in the splenogastric space near the tail of the pancreas by laparoscopy (Figure 3A). The tumor was closely adhered to the spleen artery and back wall of the gastrointestinal anastomosis. Lesions on the cut section were grayish white. According to microscopic pathology, the proliferative spindle cells were disorderly arranged, with a small amount of interstitial lymphocyte infiltration. An expression of vimentin and smooth muscle actin (SMA) in most spindle cells was observed by immunohistochemical analysis, but there was no staining for S100 proteins, CD117 (c-kit), DOG1, P63, CK(Pan), or anaplastic lymphoma kinase (ALK-1 A4). The final diagnosis was reactive nodular fibrous pseudotumor (Figure 3B). Surgical excision was complete, and no evidence of disease was found 28 months later.
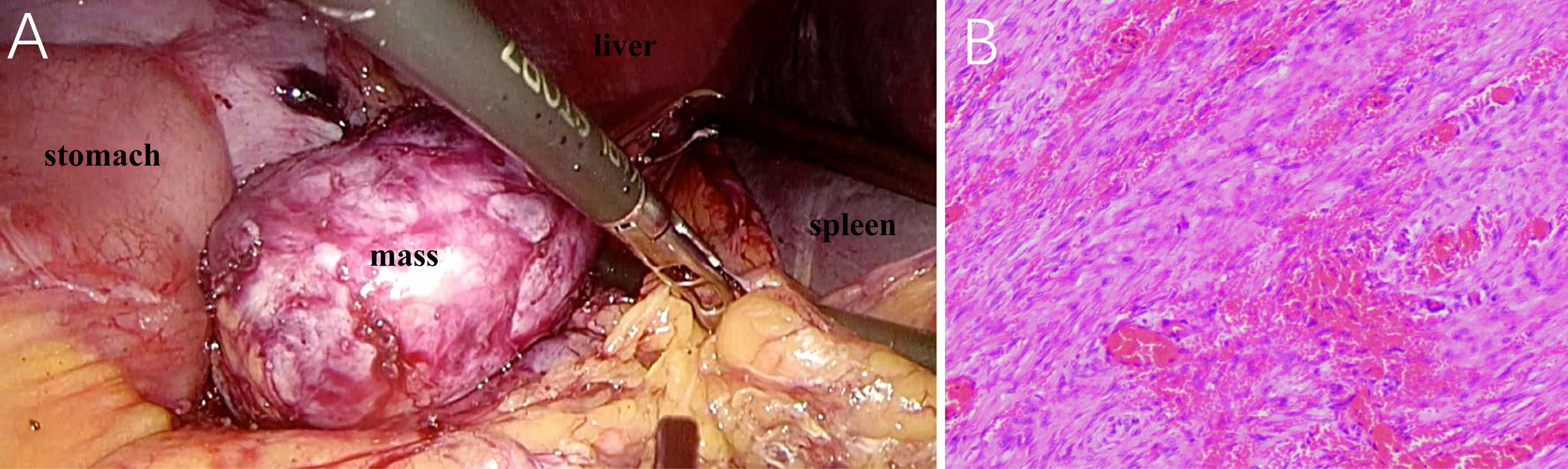
Figure 3 Laparoscopy presents a light-red mass with medium texture and complete capsule between the anastomotic stoma and spleen hilum (A). Photomicrograph (hematoxylin–eosin stain, original magnification ×100) demonstrates the proliferation of spindle cells in the stroma of extensive collagen degeneration (B). Most of the spindle cells are long fusiform, long, and deeply stained, with rare mitotic figures.
RNFP is considered to be a reactive benign lesion associated with previous surgical procedures or inflammatory disorders. Since 2013, twenty-seven cases of RNFP have been reported (Table 1) (1–16), involving 16 males and 11 females, with ages ranging from 1 day to 72 years of age; the mean age was 47 years of age. Eight of these patients had a history of abdominal surgery for abdominal trauma, acute abdomen, cholecystitis, gastric stromal tumor, or colon cancer, although no lesions following a diagnosis of gastric cancer have been reported. Of the 27 cases, 12 have single lesions, and the other 15 cases involved multiple lesions. Locations were as follows: the colon or appendix (8 cases), the small bowel (7 cases), the mesentery and omentum (7 cases), the gastric wall (2 cases), and the hepatic capsule, ovary, or peripancreas (1 case each).
Microscopic examination of RNFP reveals proliferation of stellate or spindle cells with a keloid-like appearance in a dense collagenous background, accompanied by infiltration of lymphocytes and plasma cells. Immunohistochemistry shows positive staining for vimentin and smooth muscle actin and negative staining for CD34 and S-100. The immunohistochemical characteristics of the present case are consistent with previous results (10).
Of the cases reported thus far, only 4 had CT and/or MR images. Combined with our case and the literature (6, 11, 12, 16), the imaging features are as follows. On plain CT, the lesion is isodense, with progressively mild enhancement observed on postcontrast CT. MR manifestations are related to the content of fibroblasts in the RNFP. In general, the lesion shows homogenous hypointensity on T1WI and a mixed high-signal shadow with a slightly hypointensity area on T2WI. Enhancement mode results are the same as those by CT. If the lesion is rich in fibroblastic components, the appearance of a markedly low signal on T2WI has certain characteristics. There is no PET/CT description of RNFP in the literature. In the present case, PET/CT images showed a round-like mass with slightly increased FDG metabolism. The features of isodensity and progressively mild enhancement on CT images corresponded with the literature.
RNFP should be differentiated from lymph node metastasis related to gastric cancer and extragastrointestinal stromal tumors (EGIST). Enlarged lymph nodes are irregular in shape, with marginal lobulation or spiny processes. Uneven high enhancement with central necrosis and ring enhancement when the diameter of the node is more than 10 mm are typical; PET/CT FDG metabolism is significantly increased. Another neoplastic entity to consider in differential diagnosis is an EGIST, which is commonly a unique large mass with a round or irregular shape and uneven density. Cystoid degeneration and necrosis are common. EGIST exhibits significant enhancement during the arterial phase due to the hypervasculature present, in contrast to the mild enhancement of RNFP.
Surgical resection is currently an effective treatment for RNFP. No cases of RNFP recurrence or metastasis have been reported to date.
RNFP is rare and may be related to postoperative fibroinflammation. We report for the first time a reactive nodular fibrous pseudotumor associated with surgery for gastric cancer and its appearance on PET/CT images. Awareness of the imaging features of RNFP is important for its diagnosis. For patients who have been treated with surgery for abdominal malignant tumor, to avoid misdiagnosis and overtreatment, the possibility of RNFP should be considered if the mass is found to have the above imaging findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College. The patients/participants provided their written informed consent to participate in this study.
YZ and LS performed the image acquisition. ZQ collected the clinical and pathological data. JC and LF performed the image analysis and wrote the manuscript. AC and LF revised the final manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LGC22H180002, GC20H180003 and Y21H160246) and Zhejiang Medical and Health Science and Technology Project (Nos. 2020KY406, 2021KY508).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yantiss RK, Nielsen GP, Lauwers GY, Rosenberg AE. Reactive Nodular Fibrous Pseudotumor of the Gastrointestinal Tract and Mesentery - A Clinicopathologic Study of Five Cases. Am J Surg Pathol (2003) 27(4):532–40. doi: 10.1097/00000478-200304000-00015
2. Chatelain D, Manaouil D, Levy P, Joly JP, Sevestre H, Regimbeau JM. Reactive Nodular Fibrous Pseudotumor of the Gastrointestinal Tract and Mesentery. Am J Surg Pathol (2004) 28(3):416. doi: 10.1097/00000478-200403000-00019
3. Zardawi IM, Catterall N, Cox SA. Reactive Nodular Fibrous Pseudotumor of the Gastrointestinal Tract and Mesentery. Am J Surg Pathol (2004) 28(2):276–7. doi: 10.1097/00000478-200402000-00022
4. Daum O, Vanecek T, Sima R, Curik R, Zamecnik M, Yamanaka S, et al. Reactive Nodular Fibrous Pseudotumors of the Gastrointestinal Tract: Report of 8 Cases. Int J Surg Pathol (2004) 12(4):365–74. doi: 10.1177/106689690401200409
5. Saglam EA, Usubutun A, Kart C, Ayhan A, Kucukali T. Reactive Nodular Fibrous Pseudotumor Involving the Pelvic and Abdominal Cavity: A Case Report and Review of Literature. Virchows Archiv (2005) 447(5):879–82. doi: 10.1007/s00428-005-0027-y
6. Gauchotte G, Bressenot A, Serradori T, Boissel P, Plenat F, Montagne K. Reactive Nodular Fibrous Pseudotumor: A First Report of Gastric Localization and Clinicopathologic Review. Gastroenterol Clin Et Biol (2009) 33(12):1076–81. doi: 10.1016/j.gcb.2009.04.012
7. Yin SS, Zhang LD, Cziffer-Paul A, Divino CM, Chin E. Reactive Nodular Fibrous Pseudotumor Presenting as a Small Bowel Obstruction. Am Surg (2011) 77(6):790–1. doi: 10.1177/000313481107700639
8. Virgilio E, Pucci E, Pilozzi E, Mongelli S, Cavallini M, Ferri M. Reactive Nodular Fibrous Pseudotumor of the Gastrointestinal Tract and Mesentery Giving Multiple Hepatic Deposits and Associated With Colon Cancer. Am Surgeon (2012) 78(5):E262–E4. doi: 10.1177/000313481207800508
9. Tam HM, Lee KF, To KF, Wong J, Lai PBS. Reactive Nodular Fibrous Pseudotumor After Laparoscopic Cholecystectomy. Surg Pract (2012) 16(3):116–7. doi: 10.1111/j.1744-1633.2012.00602.x
10. McAteer J, Huaco JC, Deutsch GH, Gow KW. Torsed Reactive Nodular Fibrous Pseudotumor in an Adolescent: Case Report and Review of the Literature. J Pediatr Surg (2012) 47(4):795–8. doi: 10.1016/j.jpedsurg.2012.01.001
11. Salihi R, Moerman P, Timmerman D, Van Schoubroeck D, Op de Beeck K, Vergote I. Reactive Nodular Fibrous Pseudotumor: Case Report and Review of the Literature. Case Rep Obstet Gynecol (2014) 2014:421234. doi: 10.1155/2014/421234
12. Yi X-J, Chen C-Q, Li Y, Ma J-P, Li Z-X, Cai S-R, et al. Rare Case of an Abdominal Mass: Reactive Nodular Fibrous Pseudotumor of the Stomach Encroaching on Multiple Abdominal Organs. World J Clin Cases (2014) 2(4):111–9. doi: 10.12998/wjcc.v2.i4.111
13. Yan F, Ma YL, Sun JH, Zhu PC. Reactive Nodular Fibrous Pseudotumor Involving the Gastrointestinal Tract and Mesentery: A Case Report and Review of the Literature. Oncol Lett (2015) 9(3):1343–6. doi: 10.3892/ol.2015.2882
14. Ciftci B, Vardar E, Tasli F, Yakan S, Top E, Yildirim M. Reactive Nodular Fibrous Pseudotumor Presenting as a Huge Intra Abdominal Mass After Abdominal Surgery: A Case Report. Iranian J Pathol (2015) 10(2):149–54.
15. Moodley J, Cutz JC, Schell M. Reactive Nodular Fibrous Pseudotumor Mimicking Metastatic Gastrointestinal Stromal Tumor to Perigastric Lymph Node: A Case Report and Review of the Literature. Int J Surg Pathol (2018) 26(7):664–70. doi: 10.1177/1066896918771192
Keywords: reactive nodular fibrous pseudotumor, gastric cancer, tomography, X-ray computed, FDG, PET/CT
Citation: Chen J, Zhang Y, Shi L, Qian Z, Cheng A and Fu L (2022) Reactive Nodular Fibrous Pseudotumor Mimicking Metastatic Tumor After Gastric Cancer Operation: A Case Report and Literature Review. Front. Oncol. 12:854997. doi: 10.3389/fonc.2022.854997
Received: 14 January 2022; Accepted: 07 March 2022;
Published: 04 April 2022.
Edited by:
Gabriele Anania, University of Ferrara, ItalyReviewed by:
Ali Coskun, Izmir Bozyaka Training and Research Hospital, TurkeyCopyright © 2022 Chen, Zhang, Shi, Qian, Cheng and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Fu, ZnVsaXBpbmdoekAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.