- 1Regenerative Medicine Research Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Liver Surgery and Liver Transplantation Centre, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Anesthesiology, The Affiliated Hospital of Guizhou Medical University, Guizhou, China
- 4Department of Thoracic Surgery, The 3rd Affiliated Hospital of Chengdu Medical College, Pidu District People’s Hospital, Chengdu, China
- 5Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
Objectives: Robot-assisted thoracic surgery (RATS) and video-assisted thoracic surgery (VATS) are the two principal minimally invasive surgical approaches for patients with lung cancer. This study aimed at comparing the long-term and short-term outcomes of RATS and VATS for lung cancer.
Methods: A comprehensive search for studies that compared RATS versus VATS for lung cancer published until November 31, 2021, was conducted. Data on perioperative outcomes and oncologic outcomes were subjected to meta-analysis. PubMed, Web of Science, and EMBASE were searched based on a defined search strategy to identify eligible studies before November 2021.
Results: Twenty-six studies comparing 45,733 patients (14,271 and 31,462 patients who underwent RATS and VATS, respectively) were included. The present meta-analysis showed that there were no significant differences in operative time, any complications, tumor size, chest drain duration, R0 resection rate, lymph station, 5-year overall survival, and recurrence rate. However, compared with the VATS group, the RATS group had less blood loss, a lower conversion rate to open, a shorter length of hospital stay, more lymph node dissection, and better 5-year disease-free survival.
Conclusions: RATS is a safe and feasible alternative to VATS for patients with lung cancer.
Introduction
Lung cancer is still the most common malignancy worldwide (1, 2), and the main effective treatment is surgery for early-stage non-small-cell lung cancer (NSCLC) (3, 4). Emerging evidence has revealed that video-assisted thoracic surgery (VATS) is also a safe and effective treatment method for NSCLC compared with conventional thoracotomy (5–7). Additionally, it has been reported that VATS patients have less postoperative pain, shorter hospital length of stay, fewer complications, faster physical recovery, and better postoperative lung function outcomes than thoracotomy patients (5, 6, 8). Based on these advantages, VATS (including uniportal, two-port, three-port, or four-port) has been widely used to treat lung cancer. Although VATS is widely used in thoracic surgery based on its advantages, it also suffers from shortcomings, such as two-dimensional vision, difficult hand–eye coordination, amplification of hand tremor, steep learning curve, lack of flexibility, and limited ranges of instrument movement (8). Based on new emerging technologies and advances in medical knowledge, robot-assisted thoracic surgery (RATS) may be an alternative to VATS, as RATS can provide 3D high-definition visualization over the operative field, increase comfort for the surgeon, and improve the precision of manipulations with tremor filtration and instrument dexterity (9). Some studies have confirmed the safety and feasibility of RATS (7, 9) and emphasized its advantages in less bleeding, lower conversion rate, shorter hospital stay, more harvested lymph nodes and stations, lower overall complication rate, and lower recurrence rate (8) due to 3D high-definition visualization, tremor filtration, and instrument dexterity of robotic surgery systems. With several new studies about RATS and VATS for NSCLC published recently, a new updated meta-analysis is urgently needed to compare the perioperative and oncologic outcomes of RATS and VATS for NSCLC.
Methods
Data Sources and Search Strategy
This study was registered at PROSPERO under registration number CRD42021298987 and reported on the basis of the PRISMA guidelines (10). Studies investigating RATS versus VATS for NSCLC were systematically searched in PubMed, Web of Science, and EMBASE before November 31, 2021, by two independent investigators (JZ and QF). The search terms used were (“robotic surgery” OR “robot-assisted” OR robot OR robotic OR RATS) AND (“video-assisted surgery” OR “video-assisted” OR video OR thoracoscopic OR VATS) AND (“lung neoplasms” OR “lung cancer” OR “non-small-cell lung cancer” or NSCLC) and (“lung resection” OR “pulmonary resection” OR lobectomy OR segmentectomy), either individually or in combination. The “related articles” function was used to broaden the search, and all citations were considered for relevance. A manual search of the references of publication was adopted to prevent missing relevant studies.
Inclusion and Exclusion Criteria
Two investigators (JZ and QF) independently reviewed the currently available literature, screened all titles and abstracts, and identified eligible studies according to the following criteria.
The inclusion criteria were as follows (1): participants: all patients had lung cancer defined histologically (2); types of interventions: RATS and VATS (3); study type: randomized controlled trials (RCTs), propensity score matching studies, retrospective studies, cohort studies, and case–control studies comparing RATS and VATS with NSCLC patients; if repeated studies were published from the same center, we captured the latest data and PSM data for analysis (4); at least one outcome was reported in the literature, including operation time, intraoperative bleeding, tumor size, R0 rate, conversion rate, lymph node harvested, and spleen preservation rate; and (5) language restrictions: English.
The exclusion criteria were as follows (1): conference abstracts, editorials, letters, and case reports and (2) no comparative analysis between RATS and VATS.
Data Extraction and Quality Assessment
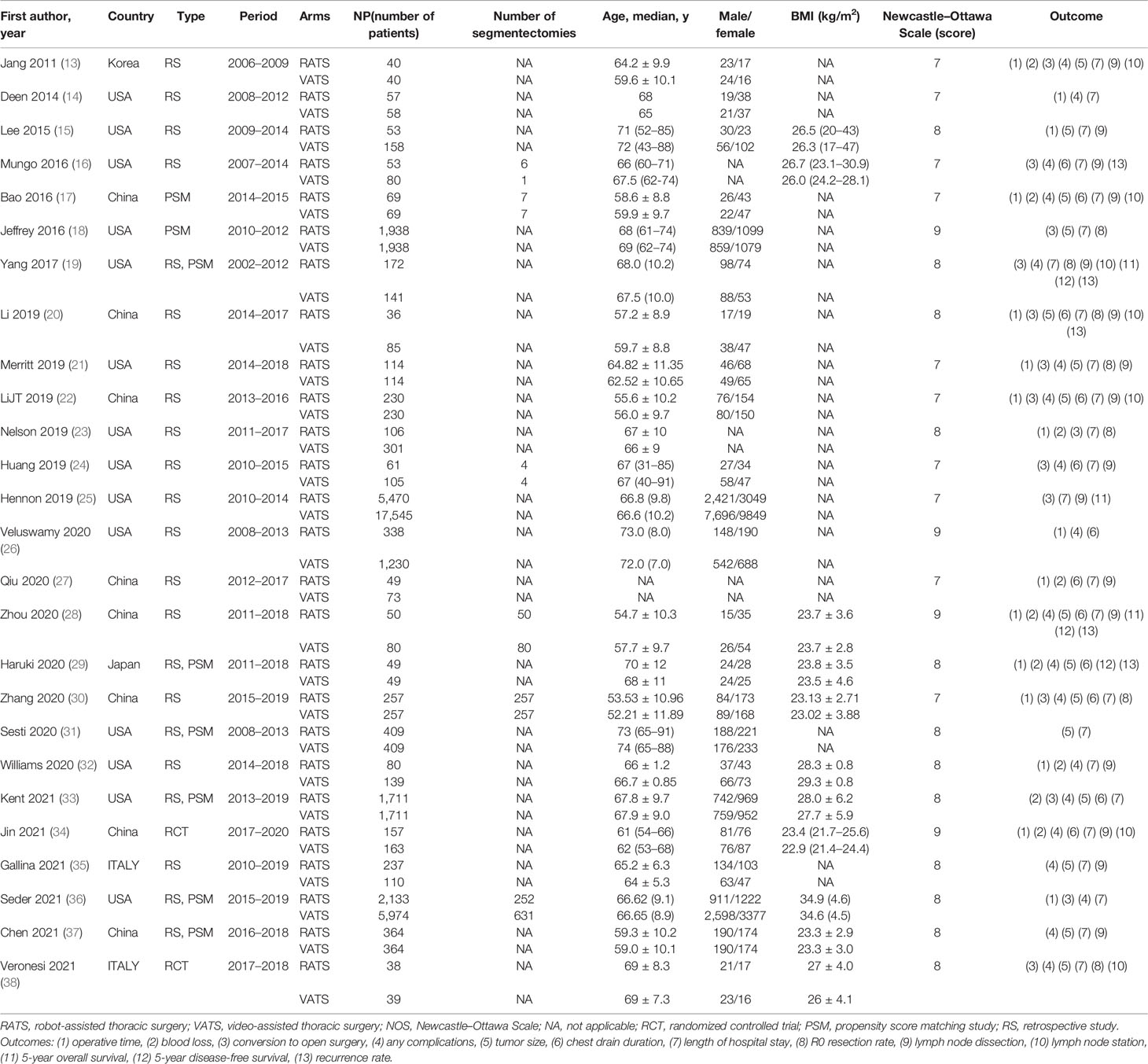
The original data from all candidate articles were independently assessed and extracted by two reviewers (JZ and QF) by using a unified datasheet, and any ambiguity was resolved by a third researcher (YH). The major data extraction includes the following: name of first author, publication year, study design, country, number of patients, mean age, sex, operative times, tumor size, bleeding, hospitalization, overall complication, overall complications, mortality, blood transfusion, and R0 rate. The quality of the eligible studies was assessed by the Newcastle–Ottawa Scale (NOS) by two different assessors (11). Every included study was independently evaluated by two authors (JZ and YH), and an NOS score>6 was considered high quality.
Statistical Analysis
Review Manager 5.3 software was used for statistical analyses. The 95% confidence interval (CI) and mean difference (MD) were used for continuous data, while categorical variables were used as odds ratios (ORs). The method originally described by Hozo et al. was used to convert medians with ranges into means with standard deviations (12). Potential publication bias was visually assessed by Begg’s funnel plot and Egger’s test. Statistical heterogeneity was quantified using the I2 value. A fixed-effect model (FEM) was adopted when heterogeneity was low or moderate (I2 <50%), while heterogeneity was high (I2 ≥50%). A random-effect model (REM) was used.
Results
Characteristics of the Included Studies
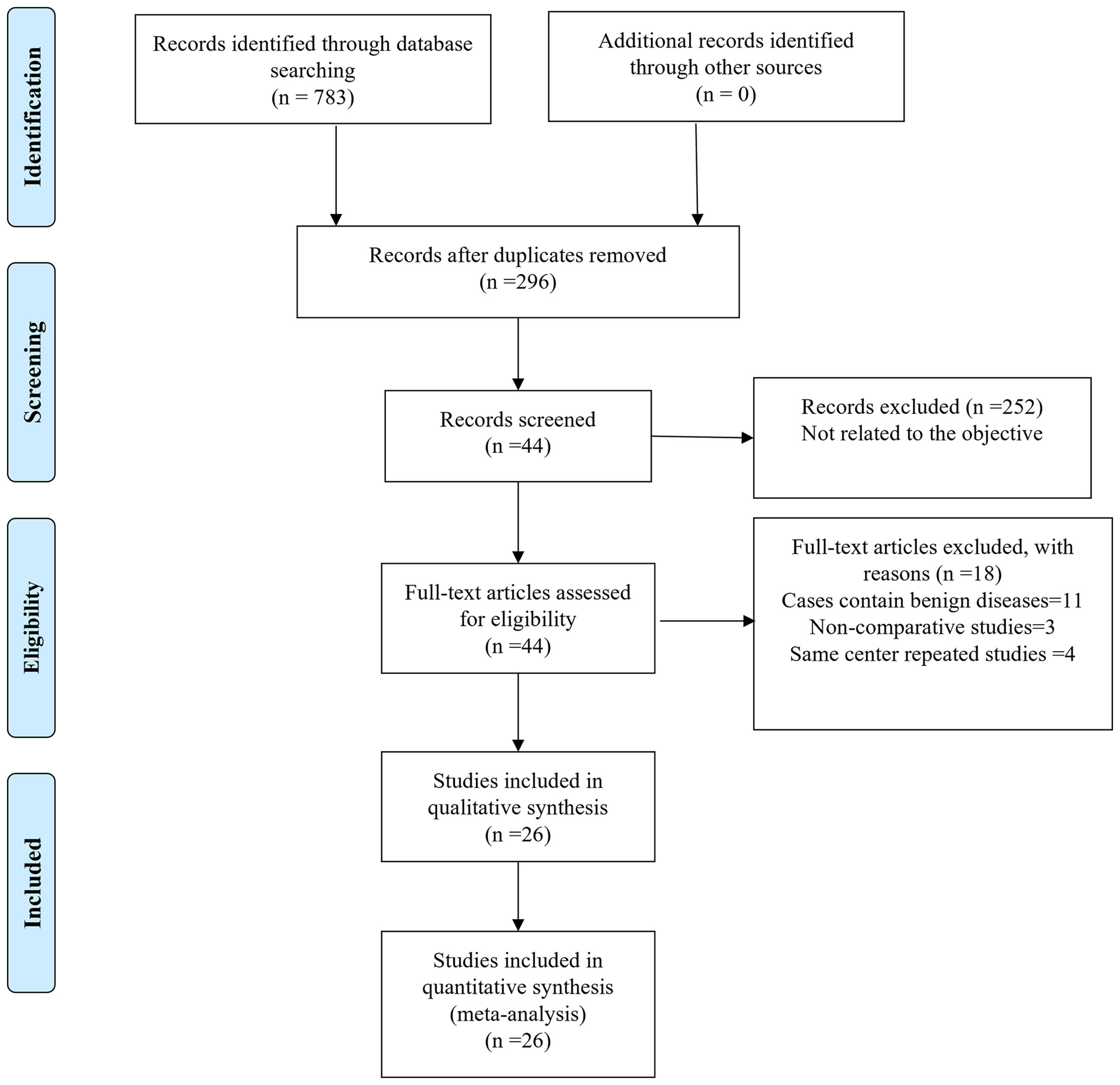
Finally, a total of 783 relevant English publications from the various electronic databases were identified. Finally, according to the inclusion criteria, 26 studies (13–38) comparing RATS and VATS in a total of 45,733 patients (14,271 and 31,462 underwent RATS and VATS, respectively) were included for further analysis. A flow diagram of our analysis protocol is shown in Figure 1. The general information and summary of NOS scores of all the included studies are given in Table 1.
Intraoperative Outcomes
To evaluate the intraoperative outcomes, we compared the operative time, blood loss, and conversion to open surgery in patients who underwent RATS and VATS. The heterogeneity of the operative time, blood loss, and conversion to open surgery was extremely high (I2 = 99%, 98%, 86%, respectively). Sixteen studies that encompassed 11,347 patients (3,518 and 7,859 underwent RATS and VATS, respectively) reported operative times. The meta-analysis showed no difference in operative time between the two groups (p value = 0.94; 95% CI -16.86 to 15.64). Nine studies recorded intraoperative blood loss, and fourteen studies provided data on conversion to open surgery. A meta-analysis of these data suggested that RATS was associated with less bleeding (p value = 0.003; 95% CI -65.99 to -14.08) and a lower conversion rate to open surgery (p value = 0.004; 95% CI 0.45 to 0.85) than VATS (shown in Figure 2).
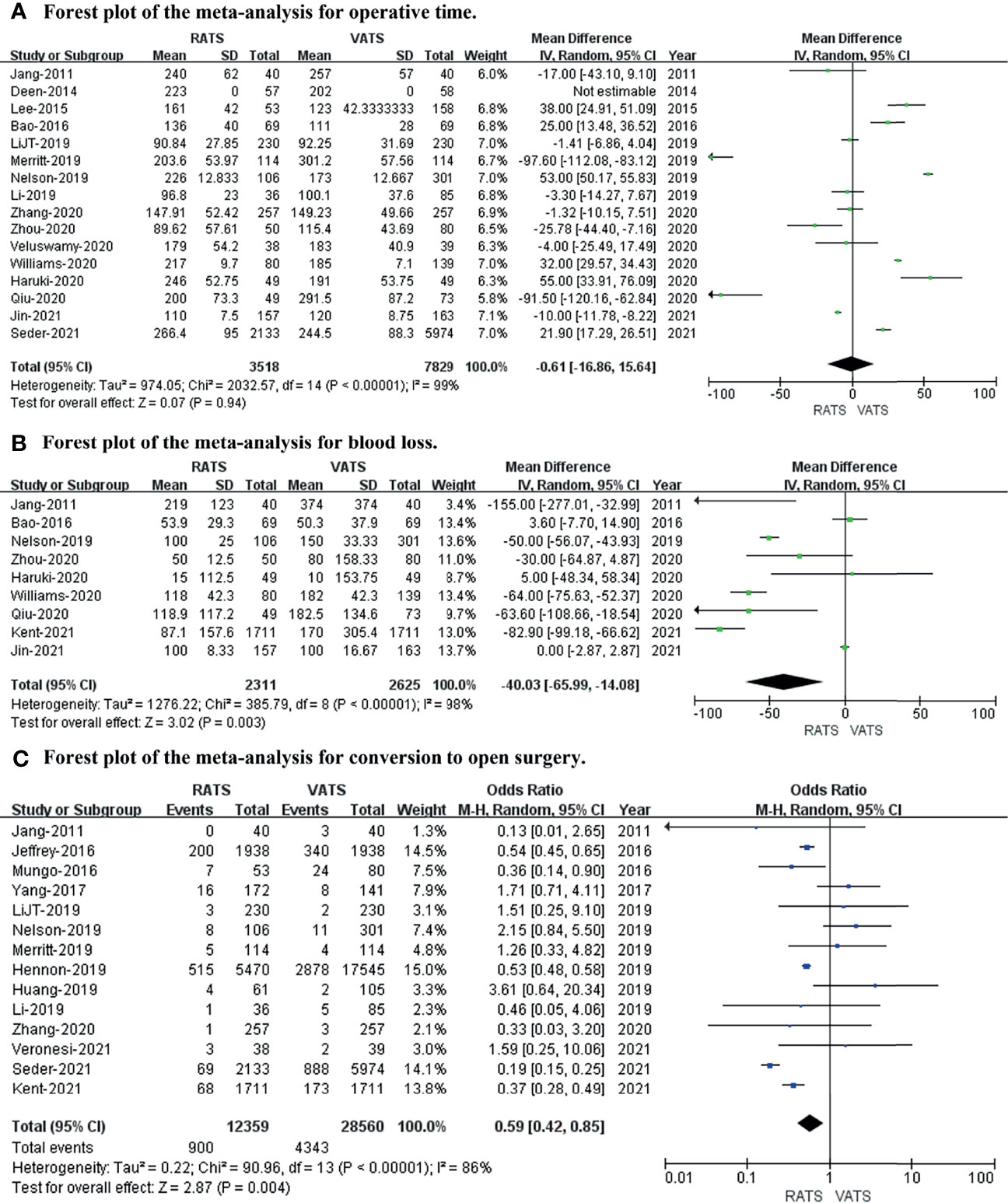
Figure 2 Forest plot of the meta-analysis for intraoperative outcomes. (A) Forest plot of the meta-analysis for operative time. (B) Forest plot of the meta-analysis for blood loss. (C) Forest plot of the meta-analysis for conversion to open surgery.
Postoperative Outcomes
We used any complications, tumor size, chest drain duration, and length of hospital stay to evaluate the postoperative outcome. After meta-analysis, the length of hospital stay (p value = 0.02; 95% CI -0.60 to -0.04; I2 = 99%) was slightly longer in the VATS group than in the RATS group, with no differences in any complications (p value = 0.15; 95% CI 0.88 to 1.02; I2 = 31%), tumor size (p value = 0.19; 95% CI -0.09 to 0.47; I2 = 98%), or chest drain duration (p value = 0.24; 95% CI -0.16 to 0.04; I2 = 63%) between the two approaches (shown in Figure 3).
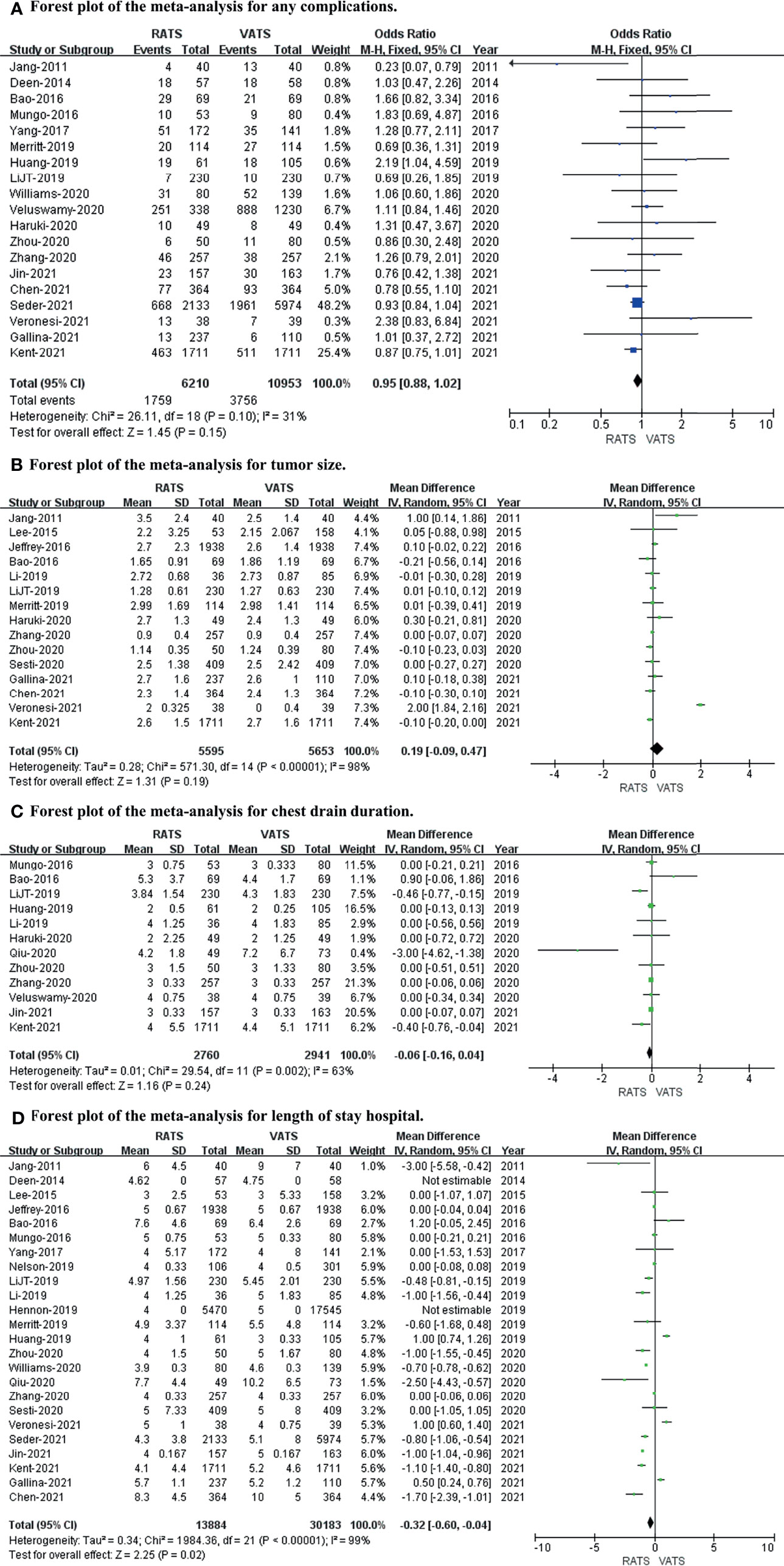
Figure 3 Forest plot of the meta-analysis for postoperative outcomes. (A) Forest plot of the meta-analysis for any complications. (B) Forest plot of the meta-analysis for tumor size. (C) Forest plot of the meta-analysis for chest drain duration. (D) Forest plot of the meta-analysis for length of hospital stay.
Short−Term Oncological Outcomes
To evaluate short-term oncological outcomes, the R0 resection rate, lymph node dissection, and lymph stations were included in the meta-analysis. The results revealed no difference in R0 resection rate (p value = 0.99; 95% CI 0.68 to 1.45; I2 = 0%) or lymph station (p value = 0.73; 95% CI -0.98 to 0.68; I2 = 99%), while the lymph node dissection (p value = 0.0006; 95% CI 0.51 to 1.86; I2 = 98%) was captured in RATS more than in VATS (shown in Figure 4).
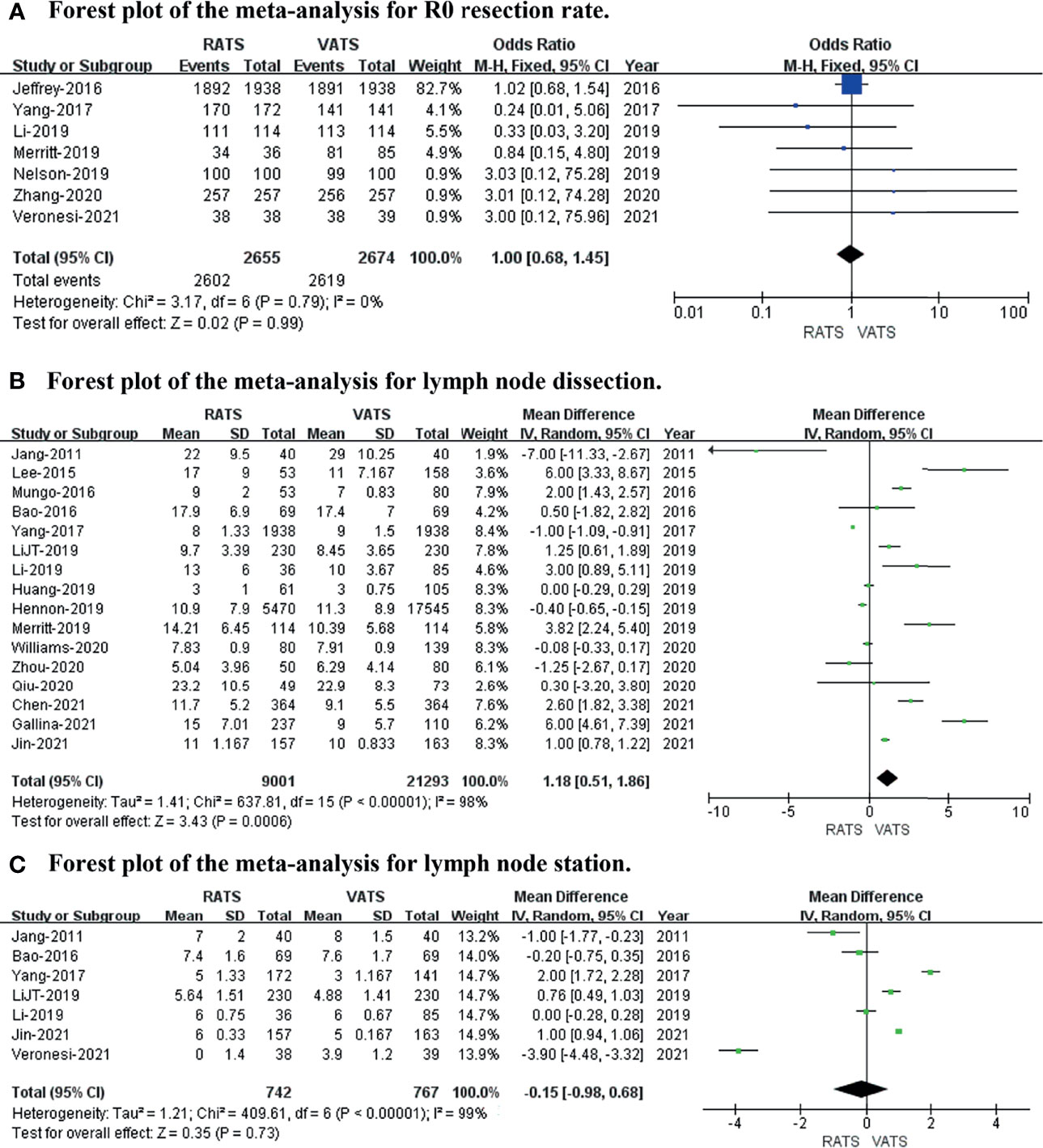
Figure 4 Forest plot of the meta-analysis for short−term oncological outcomes. (A) Forest plot of the meta-analysis for the R0 resection rate. (B) Forest plot of the meta-analysis for lymph node dissection. (C) Forest plot of the meta-analysis for lymph stations.
Long−Term Oncological Outcomes
The 5-year overall survival, 5-year disease-free survival, and recurrence rates were included to evaluate long-term oncological outcomes. The results revealed no difference in 5-year overall survival (p value = 0.22; 95% CI 0.90 to 1.02; I2 = 0%) or recurrence rate (p value = 0.08; 95% CI 0.47 to 1.04; I2 = 47%), and the 5-year disease-free survival (p value = 0.01; 95% CI 1.11 to 2.57; I2 = 23%) was slightly better in RATS than in VATS (shown in Figure 5).
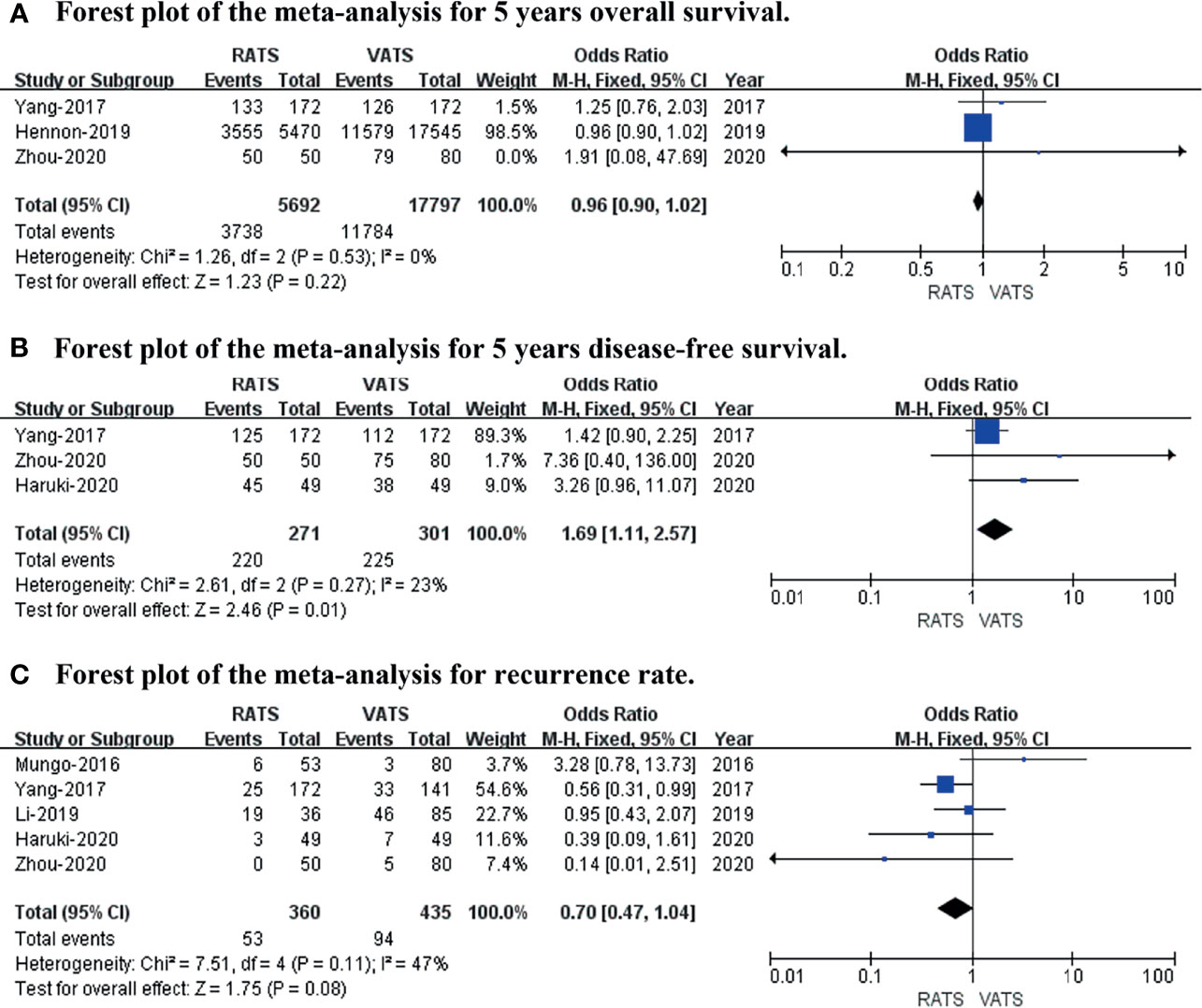
Figure 5 Forest plot of the meta-analysis for long−term oncological outcomes. (A) Forest plot of the meta-analysis for 5-year overall survival. (B) Forest plot of the meta-analysis for 5-year disease-free survival. (C) Forest plot of the meta-analysis for recurrence rate.
Publication Bias
A funnel plot for length of hospital stay and operative time was drawn to investigate the potential publication bias. The funnel plot shows obvious publication bias between studies (shown in Figure 6).
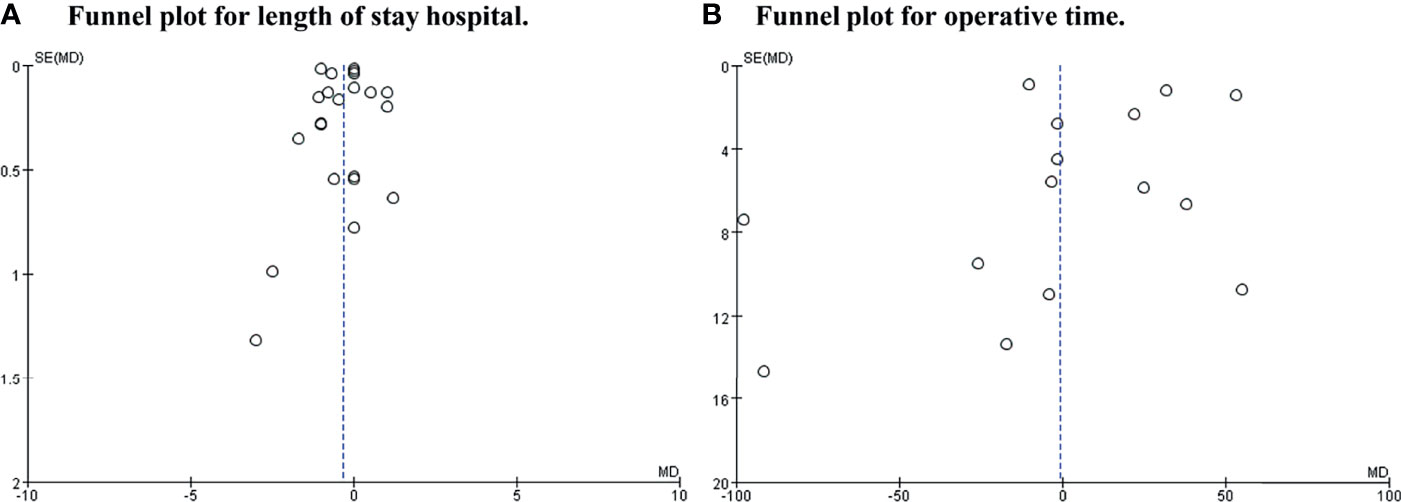
Figure 6 Funnel plot for length of hospital stay and operative time. (A) Was length of stay hospital. (B) Was operative time.
Discussion
Lobectomy with lymphadenectomy is regarded as the standard surgical treatment for patients with early-stage NSCLC. Due to the popularity of high-resolution CT for screening NSCLC, numerous small early-stage NSCLC (tumor diameter ≤2 cm) are captured (39). Recent reports have shown that sublobar resection or segmentectomy with lymph node sampling has similar outcomes compared with lobectomy for small early-stage NSCLC patients (39, 40). During the past decade, there is sufficient evidence that minimally invasive surgery (MIS) can be used as an alternative standard for NSCLC (5). MIS, including VATS and RATS, could strongly improve short-term outcomes and maintain equivalent long-term outcomes compared with traditional thoracotomy (7, 41). Currently, RATS has been increasingly used worldwide as a new surgical approach for NSCLC.
Although some meta-analyses have demonstrated that RATS is a feasible and safe alternative compared with VATS (7, 8, 42), the debate about the type of operation chosen for NSCLC continues to maintain people’s attention. We read two meta-analyses with great interest published recently by Wu et al. and Ma et al., who aimed to compare the short-term and long-term efficacy between RATS and VATS for NSCLC. Although the research by them was well conducted, unfortunately, we found some flaws in their studies. In the study by Ma et al. (8), we found that the included original articles contained benign lung diseases and metastatic lung cancer, and not all pathologies were NSCLC, which could lead to potential bias in the results. In another study by Wu et al. (7), one study was retracted because this research was fabricated and was included in the analysis of postoperative complications, which could lead to potential flaws in the research. Meanwhile, several new studies were published recently; 26 studies, including 2 randomized controlled trials, 8 propensity score matching studies, and other retrospective studies, were included based on strict inclusion criteria, and an updated meta-analysis was conducted to explore and compare the clinical efficacy of RATS and VATS for patients with NSCLC from 2011 to 2021. Our study included a total of 45,733 patients from 26 studies comparing RATS and VATS, and we focused on both the short-term and long-term oncological outcomes of RATS and VATS for NSCLC.
In short, this meta-analysis did not detect any statistically significant differences in operative time, any complications, tumor size, chest drain duration, R0 resection rate, lymph station, 5-year overall survival, or recurrence rate. However, less blood loss (p value = 0.003; 95% CI -65.99 to -14.08), a lower conversion rate to open (p value = 0.004; 95% CI 0.45 to 0.85), a shorter length of hospital stay (p value = 0.02; 95% CI -0.60 to -0.04; I2 = 99%), more lymph node dissection, and a better 5-year disease-free survival (p value = 0.01; 95% CI 1.11 to 2.57; I2 = 23%) were captured in RATS than VATS. The results of the meta-analysis revealed that the operative time, any complications, chest drain duration, and number of lymph stations were similar between the two groups, which was consistent with the results reported by Liang et al. (42). Regarding less blood loss and a lower conversion rate to open, the possible reason is that RATS has better visualization and reduces natural tremors. Thorough oncological surgical margins and lymph node dissections are two important malignancy prognosis factors in the surgical approach for NSCLC. There was no significant difference in terms of the R0 resection rate or recurrence rate between RATS and VATS, which showed that both RATS and VATS are feasible surgical techniques for NSCLC. However, more lymph node dissection and a better 5-year disease-free survival were captured in RATS compared with VATS, which might be related to the robot system being flexible operating instruments and having a greater advantage in obtaining lymph nodes, and more lymph node dissection could lead to a better 5-year DFS for patients with NSCLC. Moreover, the 5-year overall survival was similar in the two groups, while a better 5-year disease-free survival was found in RATS, which was similar to the results of a previous study (7).
To evaluate the safety and efficiency of RATS for NSCLC, this meta-analysis included 26 studies and showed that RATS was comparable to VATS. However, this review has some limitations that should be considered. First, there were only two RCTs out of all 26 included studies, which may have contributed to selection bias. Furthermore, of the 26 included studies, the long-term oncological outcomes with NSCLC have not been reported in most of the studies (including the two RCTs that did not report the long-term survival outcomes of NSCLC). Moreover, there was obvious publication bias with the included studies. Therefore, further studies, particularly large-scale prospective studies and RCTs, are expected to assess the effectiveness and safety of RATS for patients with NSCLC.
In conclusion, this systematic review and meta-analysis suggests that RATS is a technically and oncologically safe and feasible approach for NSCLC patients. Large randomized and controlled prospective studies are needed to confirm the superiority of RATS.
Author Contributions
JZ, QF, YH, and FL designed the project. JZ, QF, and YH extracted the data, made the figures and tables, and wrote the manuscript. LO and FL revised the manuscript. All authors reviewed manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82060390).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Nccn Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw (2021) 19(3):254–66. doi: 10.6004/jnccn.2021.0013
4. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of Stage I and Ii Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2013) 143(5 Suppl):e278S–313S. doi: 10.1378/chest.12-2359
5. Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative Pain and Quality of Life After Lobectomy Via Video-Assisted Thoracoscopic Surgery or Anterolateral Thoracotomy for Early Stage Lung Cancer: A Randomised Controlled Trial. Lancet Oncol (2016) 17(6):836–44. doi: 10.1016/S1470-2045(16)00173-X
6. Aiolfi A, Nosotti M, Micheletto G, Khor D, Bonitta G, Perali C, et al. Pulmonary Lobectomy for Cancer: Systematic Review and Network Meta-Analysis Comparing Open, Video-Assisted Thoracic Surgery, and Robotic Approach. Surgery (2021) 169(2):436–46. doi: 10.1016/j.surg.2020.09.010
7. Wu H, Jin R, Yang S, Park BJ, Li H. Long-Term and Short-Term Outcomes of Robot- Versus Video-Assisted Anatomic Lung Resection in Lung Cancer: A Systematic Review and Meta-Analysis. Eur J Cardiothorac Surg (2021) 59(4):732–40. doi: 10.1093/ejcts/ezaa426
8. Ma J, Li X, Zhao S, Wang J, Zhang W, Sun G. Robot-Assisted Thoracic Surgery Versus Video-Assisted Thoracic Surgery for Lung Lobectomy or Segmentectomy in Patients With Non-Small Cell Lung Cancer: A Meta-Analysis. BMC Cancer (2021) 21(1):498. doi: 10.1186/s12885-021-08241-5
9. Veronesi G, Novellis P, Voulaz E, Alloisio M. Robot-Assisted Surgery for Lung Cancer: State of the Art and Perspectives. Lung Cancer (2016) 101:28–34. doi: 10.1016/j.lungcan.2016.09.004
10. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
11. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing Reviewers' to Authors' Assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
12. Hozo SP, Djulbegovic B, Hozo I. Estimating the Mean and Variance From the Median, Range, and the Size of a Sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
13. Jang HJ, Lee HS, Park SY, Zo JI. Comparison of the Early Robot-Assisted Lobectomy Experience to Video-Assisted Thoracic Surgery Lobectomy for Lung Cancer: A Single-Institution Case Series Matching Study. Innovations (Phila) (2011) 6(5):305–10. doi: 10.1097/IMI.0b013e3182378b4c
14. Deen SA, Wilson JL, Wilshire CL, Vallieres E, Farivar AS, Aye RW, et al. Defining the Cost of Care for Lobectomy and Segmentectomy: A Comparison of Open, Video-Assisted Thoracoscopic, and Robotic Approaches. Ann Thorac Surg (2014) 97(3):1000–7. doi: 10.1016/j.athoracsur.2013.11.021
15. Lee BE, Shapiro M, Rutledge JR, Korst RJ. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann Thorac Surg (2015) 100(1):229–33. doi: 10.1016/j.athoracsur.2015.03.109
16. Mungo B, Hooker CM, Ho JS, Yang SC, Battafarano RJ, Brock MV, et al. Robotic Versus Thoracoscopic Resection for Lung Cancer: Early Results of a New Robotic Program. J Laparoendosc Adv Surg Tech A (2016) 26(4):243–8. doi: 10.1089/lap.2016.0049
17. Bao F, Zhang C, Yang Y, He Z, Wang L, Hu J. Comparison of Robotic and Video-Assisted Thoracic Surgery for Lung Cancer: A Propensity-Matched Analysis. J Thorac Dis (2016) 8(7):1798–803. doi: 10.21037/jtd.2016.05.99
18. Yang CF, Sun Z, Speicher PJ, Saud SM, Gulack BC, Hartwig MG, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg (2016) 101(3):1037–42. doi: 10.1016/j.athoracsur.2015.11.018
19. Yang HX, Woo KM, Sima CS, Bains MS, Adusumilli PS, Huang J, et al. Long-Term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-Assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg (2017) 265(2):431–7. doi: 10.1097/SLA.0000000000001708
20. Li C, Hu Y, Huang J, Li J, Jiang L, Lin H, et al. Comparison of Robotic-Assisted Lobectomy With Video-Assisted Thoracic Surgery for Stage IIB–IIIA Non-Small Cell Lung Cancer. Transl Lung Cancer Res (2019) 8(6):820–8. doi: 10.21037/tlcr.2019.10.15
21. Merritt RE, Kneuertz PJ, D'Souza DM. Successful Transition to Robotic-Assisted Lobectomy With Previous Proficiency in Thoracoscopic Lobectomy. Innovations (Phila) (2019) 14(3):263–71. doi: 10.1177/1556984519845672
22. Li JT, Liu PY, Huang J, Lu PJ, Lin H, Zhou QJ, et al. Perioperative Outcomes of Radical Lobectomies Using Robotic-Assisted Thoracoscopic Technique Vs. Video-Assisted Thoracoscopic Technique: Retrospective Study of 1,075 Consecutive P-Stage I Non-Small Cell Lung Cancer Cases. J Thorac Dis (2019) 11(3):882–91. doi: 10.21037/jtd.2019.01.78
23. Nelson DB, Mehran RJ, Mitchell KG, Rajaram R, Correa AM, Bassett RL Jr., et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann Thorac Surg (2019) 108(2):370–6. doi: 10.1016/j.athoracsur.2019.03.051
24. Huang L, Shen Y, Onaitis M. Comparative Study of Anatomic Lung Resection by Robotic Vs. Video-Assisted Thoracoscopic Surgery. J Thorac Dis (2019) 11(4):1243–50. doi: 10.21037/jtd.2019.03.104
25. Hennon MW, DeGraaff LH, Groman A, Demmy TL, Yendamuri S. The Association of Nodal Upstaging With Surgical Approach and Its Impact on Long-Term Survival After Resection of Non-Small-Cell Lung Cancer. Eur J Cardiothorac Surg (2020) 57(5):888–95. doi: 10.1093/ejcts/ezz320
26. Veluswamy RR, Whittaker Brown SA, Mhango G, Sigel K, Nicastri DG, Smith CB, et al. Comparative Effectiveness of Robotic-Assisted Surgery for Resectable Lung Cancer in Older Patients. Chest (2020) 157(5):1313–21. doi: 10.1016/j.chest.2019.09.017
27. Qiu T, Zhao Y, Xuan Y, Qin Y, Niu Z, Shen Y, et al. Robotic Sleeve Lobectomy for Centrally Located Non-Small Cell Lung Cancer: A Propensity Score-Weighted Comparison With Thoracoscopic and Open Surgery. J Thorac Cardiovasc Surg (2020) 160(3):838–46 e2. doi: 10.1016/j.jtcvs.2019.10.158
28. Zhou Q, Huang J, Pan F, Li J, Liu Y, Hou Y, et al. Operative Outcomes and Long-Term Survival of Robotic-Assisted Segmentectomy for Stage Ia Lung Cancer Compared With Video-Assisted Thoracoscopic Segmentectomy. Transl Lung Cancer Res (2020) 9(2):306–15. doi: 10.21037/tlcr-20-533
29. Haruki T, Kubouchi Y, Takagi Y, Kidokoro Y, Matsui S, Nakanishi A, et al. Comparison of Medium-Term Survival Outcomes Between Robot-Assisted Thoracoscopic Surgery and Video-Assisted Thoracoscopic Surgery in Treating Primary Lung Cancer. Gen Thorac Cardiovasc Surg (2020) 68(9):984–92. doi: 10.1007/s11748-020-01312-7
30. Zhang Y, Chen C, Hu J, Han Y, Huang M, Xiang J, et al. Early Outcomes of Robotic Versus Thoracoscopic Segmentectomy for Early-Stage Lung Cancer: A Multi-Institutional Propensity Score-Matched Analysis. J Thorac Cardiovasc Surg (2020) 160(5):1363–72. doi: 10.1016/j.jtcvs.2019.12.112
31. Sesti J, Langan RC, Bell J, Nguyen A, Turner AL, Hilden P, et al. A Comparative Analysis of Long-Term Survival of Robotic Versus Thoracoscopic Lobectomy. Ann Thorac Surg (2020) 110(4):1139–46. doi: 10.1016/j.athoracsur.2020.03.085
32. Williams AM, Zhao L, Grenda TR, Kathawate RG, Biesterveld BE, Bhatti UF, et al. Higher Long-Term Quality of Life Metrics After Video-Assisted Thoracoscopic Surgery Lobectomy Compared With Robotic-Assisted Lobectomy. Ann Thorac Surg (2020) S0003-4975(20)30971-1. doi: 10.1016/j.athoracsur.2020.05.033
33. Kent MS, Hartwig MG, Vallieres E, Abbas AE, Cerfolio RJ, Dylewski MR, et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (Portal) Study: An Analysis of 5,721 Cases. Ann Surg (2021). doi: 10.1097/SLA.0000000000005115
34. Jin R, Zheng Y, Yuan Y, Han D, Cao Y, Zhang Y, et al. Robotic-Assisted Versus Video-Assisted Thoracoscopic Lobectomy: Short-Term Results of a Randomized Clinical Trial (Rvlob Trial). Ann Surg (2021) 275(2):295–302. doi: 10.1097/SLA.0000000000004922
35. Gallina FT, Melis E, Forcella D, Mercadante E, Marinelli D, Ceddia S, et al. Nodal Upstaging Evaluation After Robotic-Assisted Lobectomy for Early-Stage Non-Small Cell Lung Cancer Compared to Video-Assisted Thoracic Surgery and Thoracotomy: A Retrospective Single Center Analysis. Front Surg (2021) 8:666158. doi: 10.3389/fsurg.2021.666158
36. Seder CW, Farrokhyar F, Nayak R, Baste JM, Patel Y, Agzarian J, et al. Robotic Vs. Thoracoscopic Anatomic Lung Resection in Obese Patients: A Propensity Adjusted Analysis. Ann Thorac Surg (2021) 10.1097. doi: 10.1016/j.athoracsur.2021.09.061
37. Chen D, Kang P, Tao S, Li Q, Wang R, Tan Q. Cost-Effectiveness Evaluation of Robotic-Assisted Thoracoscopic Surgery Versus Open Thoracotomy and Video-Assisted Thoracoscopic Surgery for Operable Non-Small Cell Lung Cancer. Lung Cancer (2021) 153:99–107. doi: 10.1016/j.lungcan.2020.12.033
38. Veronesi G, Abbas AE, Muriana P, Lembo R, Bottoni E, Perroni G, et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (Roman Study). Front Oncol (2021) 11:726408. doi: 10.3389/fonc.2021.726408
39. Moon MH, Moon YK, Moon SW. Segmentectomy Versus Lobectomy in Early Non-Small Cell Lung Cancer of 2 Cm or Less in Size: A Population-Based Study. Respirology (2018) 23(7):695–703. doi: 10.1111/resp.13277
40. Winckelmans T, Decaluwé H, De Leyn P, Van Raemdonck D. Segmentectomy or Lobectomy for Early-Stage Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Eur J Cardiothorac Surg (2020) 57(6):1051–60. doi: 10.1093/ejcts/ezz339
41. Louie BE, Wilson JL, Kim S, Cerfolio RJ, Park BJ, Farivar AS, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using the Society of Thoracic Surgeons Database. Ann Thorac Surg (2016) 102(3):917–24. doi: 10.1016/j.athoracsur.2016.03.032
Keywords: lung cancer, video-assisted thoracic surgery, robot-assisted thoracic surgery, meta-analysis, systematic review
Citation: Zhang J, Feng Q, Huang Y, Ouyang L and Luo F (2022) Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:853530. doi: 10.3389/fonc.2022.853530
Received: 12 January 2022; Accepted: 02 March 2022;
Published: 12 April 2022.
Edited by:
Marcello Migliore, University of Catania, ItalyReviewed by:
Subroto Paul, RWJBarnabas Health, United StatesBeatrice Aramini, University Hospital of Modena, Italy
Copyright © 2022 Zhang, Feng, Huang, Ouyang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengming Luo, ZmVuZ21pbmdsdW9Ab3V0bG9vay5jb20=
†These authors have contributed equally to this work
 Jianyong Zhang
Jianyong Zhang Qingbo Feng
Qingbo Feng Yanruo Huang3†
Yanruo Huang3† Lanwei Ouyang
Lanwei Ouyang Fengming Luo
Fengming Luo