- 1Department of Gastrointestinal Surgery IV, Key Laboratory of Carcinogenesis and Translational Research, Peking University Cancer Hospital and Institute, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research, Department of Cancer Epidemiology, Peking University Cancer Hospital and Institute, Beijing, China
Background: Some high-quality clinical trials have proven the efficacy and safety of perioperative and postoperative S-1 with oxaliplatin (peri-SOX and post-SOX) for patients with locally advanced gastric cancer (LAGC) undergoing D2 gastrectomy. However, little is known about how health-related quality of life (HRQOL) changes over time in patients receiving peri-SOX or post-SOX chemotherapy.
Methods: A prospective observational cohort (NCT04408859) identified 151 eligible patients with LAGC who underwent D2 gastrectomy with at least six cycles of peri-SOX or post-SOX chemotherapy from 2018 to 2020. HRQOL was assessed using the EROTC QLQ-C30 and its gastric module, QLQ-STO22, at indicated measurements, including the baseline, 1st, 3rd, 6th and 12th month after initiation of therapy. Baseline characteristics, therapeutic effects, and longitudinal HRQOL were compared between the peri-SOX and post-SOX groups after propensity score matching. HRQOL changes over time and the risk factors for scales with severe deterioration were further analyzed.
Results: No statistically significant differences in longitudinal HRQOL were observed between patients in the peri-SOX and post-SOX groups, with comparable surgical outcomes and adverse chemotherapy events. Scales of social functioning, abnormal taste, and anxiety improved earlier in the peri-SOX group than in the post-SOX group. Score changes in both groups indicated that general deterioration and slower recovery usually occurred in the scales of physical, social, and role functioning, as well as symptoms of fatigue, reflux, diarrhea, and anxiety.
Conclusion: Peri-SOX showed a longitudinal HRQOL comparable to post-SOX in patients with LAGC who underwent D2 gastrectomy. The peri-SOX group had better performance in social functioning, abnormal taste, and anxiety at some measurements.
Introduction
Gastric cancer (GC) is the fourth and third leading cause of cancer-related deaths globally and in China, respectively (1, 2). The 5-year overall survival rate for patients with GC has improved after years of effort; however, it is still not more than 35% in China, which is partly attributable to nearly 80% of patients being diagnosed at advanced stages at initial hospitalization (3). Radical gastrectomy with adequate lymphadenectomy constitutes the mainstay of GC treatment, while recurrence and metastasis after complete resection illustrate that surgery alone cannot achieve a cure for gastric malignancy (4–6).
Adjuvant chemotherapy is now an essential treatment for Asian patients with locally advanced gastric cancer (LAGC) after surgery, since its encouraging therapeutic efficacy has been confirmed by the ACTS-GC and CLASSIC trials (7, 8). The MAGIC, FNCLCC, and FLOT4 trials conducted in Europe recommended perioperative chemotherapy, namely, neoadjuvant chemotherapy, followed by surgery and adjuvant chemotherapy as standard treatment for European patients with LAGC (9–11). Despite ethnic differences, the inconsistencies in chemotherapy regimens and administration durations between the East and West provide clues for healthcare providers to explore a more optimized chemotherapy strategy.
In East Asia, fluoropyrimidine-based chemotherapies are the preferred treatment for GC, with capecitabine plus oxaliplatin (CapOx) and S-1 with oxaliplatin (SOX) regimens as Grade I recommendations for postoperative adjuvant chemotherapy, and SOX for neoadjuvant chemotherapy in China (12–14). With an increasing number of perioperative chemotherapy-related studies in Asia, RESOLVE and PRODIGY have been devoted to comparing different sequences and combinations of perioperative chemotherapy with postoperative chemotherapy (15, 16). Results from the RESOLVE trial demonstrated a superior 3-year disease-free survival rate of perioperative SOX (59.4%) over adjuvant CapOx (51.1%) and a comparable efficacy between adjuvant SOX (56.5%) and adjuvant CapOx for T4 stage LAGC patients (15). Xue et al. also reported a comparable 5-year overall survival between perioperative SOX (peri-SOX) and postoperative SOX (post-SOX) after D2 resection (17). The gradually improved survival rate due to innovations in medical treatment has led to an increased number of long-term GC survivors, whose health-related quality of life (HRQOL) has attracted more attention.
As an important indicator of psychosocial burden emphasized by oncologists and psychologists, HRQOL has become an essential endpoint for evaluating the efficacy and impact of cancer treatment (18). Since gastrectomy may lead to nutritional and functional problems, while chemotherapy may result in nausea, vomiting, or fatigue, majority of studies related to HRQOL mainly focus on the effects of different surgical approaches or reconstruction methods in patients with resectable GC and the impact of palliative therapy on patients with unresectable or recurrent GC (19–32). The impact of the resection scope as well as the application of minimally invasive methods on patients’ HRQOL has greatly attracted the attention of surgeons. The recovery of patients receiving distal or proximal gastrectomy has been proven to be superior to total gastrectomy, as less functional and symptomatic problems will occur after subtotal resection (19–22). The potential HRQOL benefit of the laparoscopic approach compared to open surgery has also been reported for long-term follow-up (23, 24). Roux-en-Y reconstruction may lead to less severe gastrointestinal symptoms than Billroth I and II methods, although controversies still exist in this area (25–27). For patients with unresectable or recurrent GC, HRQOL is an important indicator for evaluating the therapeutic efficacy of different palliative chemotherapy regimens (28, 29). With the emergence of immunotherapy and targeted therapy, maintaining HRQOL for a longer time is important in palliative therapy using new drugs (30–32). Studies comparing the effects of different chemotherapy modalities on the HRQOL of patients with resectable upper alimentary tract carcinoma have mainly focused on esophageal or junctional cancers (33, 34). The ARTIST 2 trial recently reported a similar HRQOL in patients with LAGC undergoing D2 resection with adjuvant S-1, SOX, or SOX plus chemoradiotherapy (35). Studies evaluating HRQOL in patients with LAGC on pre- or perioperative chemotherapy are limited. Satake et al. assessed the neurotoxicity-related QOL in patients with LAGC receiving neoadjuvant SOX chemotherapy in a phase I study (36); however, additional data on HRQOL were not reported in its subsequent phase II trial (37). Studies comparing the impact of peri-SOX and post-SOX on the HRQOL of patients with LAGC undergoing D2 resection could hardly be found.
Therefore, the HRQOL changes in patients with LAGC receiving peri-SOX or post-SOX over time were evaluated in detail. Moreover, surgical outcomes, adverse chemotherapy events, changes in between-group and within-group longitudinal HRQOL in the peri-SOX and post-SOX groups, and risk factors for seriously deteriorated HRQOL scales with slow recovery were comprehensively analyzed in the present study.
Methods
Study Design and Patients
An observational cohort study focusing on HRQOL in patients with LAGC receiving perioperative versus postoperative chemotherapy with D2 gastrectomy (NCT04408859) was launched at the Department of Gastrointestinal Surgery IV of Peking University Cancer Hospital in 2018. From April 2018 to March 2020, 330 patients with LAGC undergoing D2 resection with SOX chemotherapy were administered self-reported questionnaires. This study was approved by the Research Ethics Committee of Peking University Cancer Hospital and Institute, and written informed consent was obtained from each participant.
The inclusion criteria were as follows: ① 18–80 years old; ② histologically confirmed gastric adenocarcinoma; ③ preoperatively diagnosed as clinical tumor stage II–III (T2-3N+M0 and T4NanyM0) according to the eighth edition of the American Joint Committee on Cancer (38); ④ patients receiving either peri-SOX or post-SOX chemotherapy, with two or three cycles of neoadjuvant SOX administered 3–6 weeks before surgery and the remaining cycles delivered 4–8 weeks after surgery for the peri-SOX group, or at least 6 cycles of adjuvant SOX started no later than 2 months after surgery for the post-SOX group. To identify the net effect of peri-SOX and post-SOX modalities on patients’ HRQOL, participants were excluded if ① the completed SOX chemotherapy was less than 6 cycles in total; ② questionnaires’ responses were less than twice in all measurements; ③ patients received postoperative chemoradiation with SOX; and ④ adjuvant SOX was commenced more than 3 months after surgery.
HRQOL Measurements
Questionnaires collecting patient-reported outcomes were distributed and returned online by a dedicated research nurse through the “Wenjuanxing” platform, a professional and widely accepted platform in China for questionnaire surveys. HRQOL surveys were administered to patients during the first hospitalization before treatment and in 1st, 3rd, 6th, and 12th month after the initiation of therapy. Further follow-up phone calls were to be given if the completed questionnaire was not sent back within 2 weeks.
HRQOL of LAGC patients was assessed using the Chinese versions of the previously validated European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) and EORTC QLQ-Gastric Cancer Module QLQ-STO22 (39–41). As a structured questionnaire for cancer self-management, the QLQ-C30 is generally suitable for all cancer patients, with widely accepted reliability and effectiveness (42). As a supplement to QLQ-C30, QLQ-STO22 further measures the HRQOL of GC patients at all pathological stages who underwent surgical resection, palliative intervention, endoscopic remission, or palliative chemotherapy (43, 44). Raw HRQOL questionnaire scores were linearly transformed from 0 to 100, with a higher score representing a better level of functional or global health status, but indicating a worse level of the symptom scales or items (45).
Statistical Analysis
To minimize possible selection bias in this observational study, between-treatment differences were adjusted using the propensity score matching (PSM) approach, with age, sex, body mass index (BMI), ASA score, comorbidities, clinical T stage, and clinical N stage as covariates, in consideration of clinical relevance (46). LAGC patients receiving peri-SOX or post-SOX were matched through a 1:1 nearest-neighbor algorithm using a caliper width setting as 0.2 of a standard deviation of the logit of the propensity score (47). Absolute standardized differences (ASD) were calculated to assess covariate balance in the matched sample (48).
The comparison of baseline characteristics and clinical outcomes between the peri-SOX and post-SOX groups was performed using Student’s t-test or Mann–Whitney U test for continuous variables after assessing normality, and chi-squared analysis or Fisher’s exact test for categorical variables.
Baseline HRQOL scores between the two groups were compared using Student’s t-test. As a set of longitudinal data with repeated measures obtained from the same population, a linear mixed model was applied to explore any differential effect on HRQOL between peri-SOX and post-SOX groups over time and to examine differences between both groups at each follow-up measurement (the 1st, 3rd, 6th, and 12th month after the initiation of therapy) (49). If treatment effects over time were comparable between the peri-SOX and post-SOX groups, the HRQOL scores of both groups were combined to analyze the longitudinal effects on each scale of the questionnaire. Due to the allowance of the linear mixed model that analyzed a longitudinal dataset with missing-at-random data (50–53), all QOL scores of eligible patients were included in this analysis, regardless of any incomplete single item or drop-out during follow-up. After standardization of both predictor and outcome variables, the treatment effect was estimated using a linear mixed model that included the treatment arm, time, and treatment-by-time interaction as fixed effect factors and a random effect on patients, adjusted for baseline HRQOL with an autoregressive covariance structure of the first order. The beta estimates in the mixed modeling procedure demonstrated standardized differential effects between treatment groups over time or longitudinal effects, which were represented as Cohen’s d (CD) effect sizes for clinical relevance after standardized comparison (33). CD values of 0.2, 0.5, and 0.8 were cutoff points indicating small, medium, and considerable clinical significance, respectively (54). To correct for multiple testing, Bonferroni correction was applied according to the number of comparisons performed. Sensitivity analyses were performed to examine attrition bias by comparing the baseline characteristics and surgical outcomes between those who completed all study visits and those who did not. Moreover, the longitudinal effects on HRQOL between the two groups were examined using data before and after multiple imputation.
To present the alterations in HRQOL over time more clearly, scores in each domain were classified as “improved”, “stable”, or “deteriorated” with clinical relevance at each follow-up measurement, defined as at least 10-point score changes from baseline (55, 56). For the purpose of exploring the associations of baseline and treatment factors with several HRQOL domains, which represented severe deterioration and slow recovery at the 12th month after the beginning of therapy, Mantel-Haenszel chi-squared tests were applied to screen out single variables potentially influencing HRQOL changes in the univariate analysis. Data before PSM were used because the matched data might not represent its original source owing to the selectively reduced sample size. Factors with p < 0.20 in the univariate analysis were selected and further entered into the multivariate analysis by using ordinal logistic regression models after validating proportional odds assumptions by the test of parallel lines (57). The significance level was set at p < 0.05, except for multiple comparisons, and all analyses were conducted using the Statistical Package for the Social Sciences software (version 25.0; SPSS, Chicago, IL, USA).
Results
Characteristics of Study Population
This observational cohort included 151 eligible patients from April 2018 to March 2020, with 51 in the peri-SOX group and 100 in the post-SOX group, respectively. As shown in the STROBE flow diagram (Figure 1), 33 patients (35.5%) in the peri-SOX group and 114 patients (48.1%) in the post-SOX group could not complete at least six cycles of SOX chemotherapy (chi-squared test, p = 0.038). Seven patients in the peri-SOX and eighteen patients in the post-SOX group were unwilling to respond to the questionnaires at least twice, even if they had completed the indicated cycles of chemotherapy. The baseline characteristics of these two arms were comparable except for BMI (p = 0.032) and clinical nodal stage (p = 0.025), which were reported to affect the HRQOL of cancer patients, and acted as potential confounders in this study (58–61). After PSM, baseline characteristics were evenly distributed between the peri-SOX and post-SOX groups, with 45 patients in each group (Table 1). The ASD in the matched sample further proved a good balance after PSM (Figure 2).
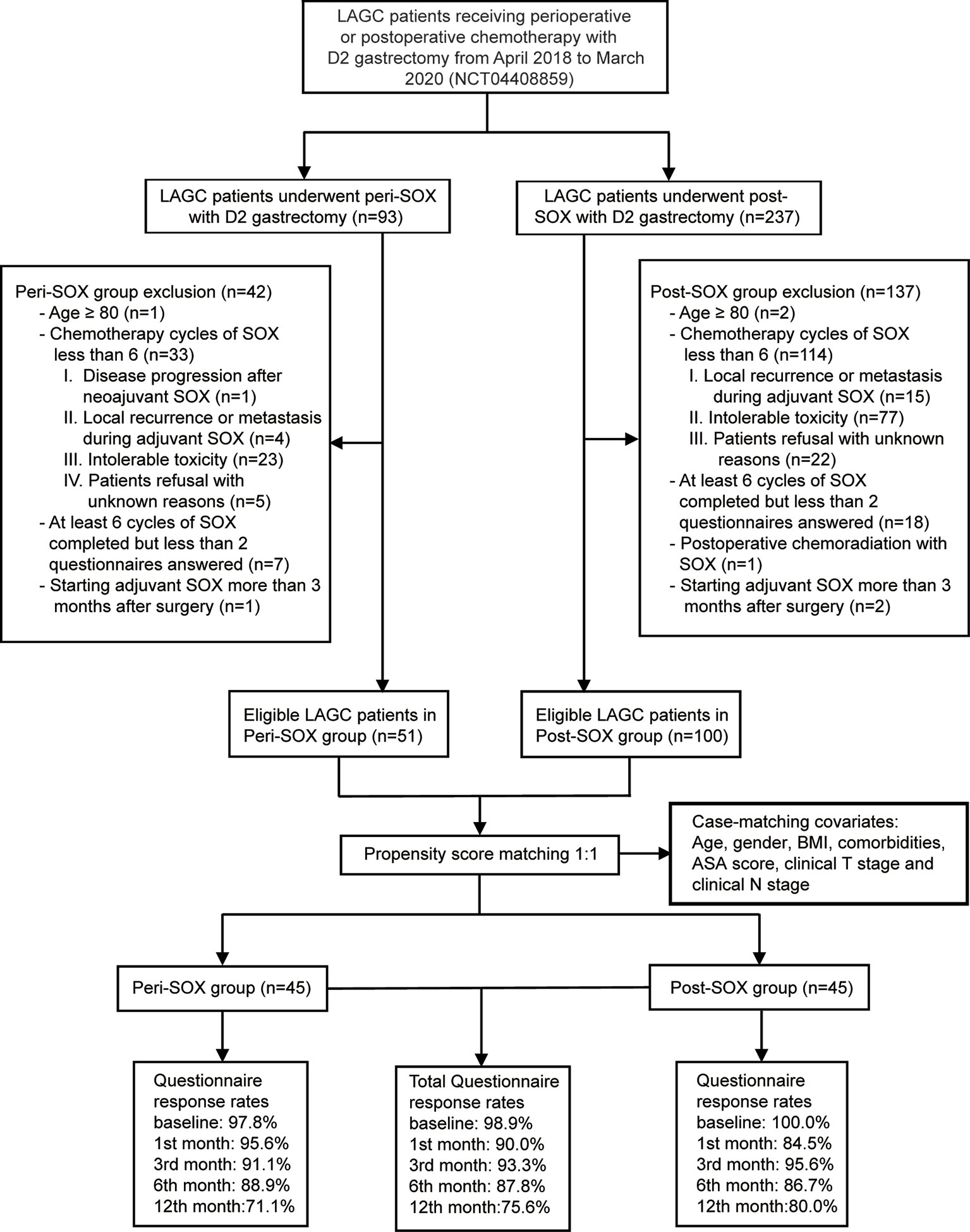
Figure 1 STROBE flow diagram illustrating the eligibility screening of LAGC patients receiving D2 gastrectomy with peri-SOX or post-SOX chemotherapy from April 2018 to March 2020, in a propensity score-matched observational cohort study.
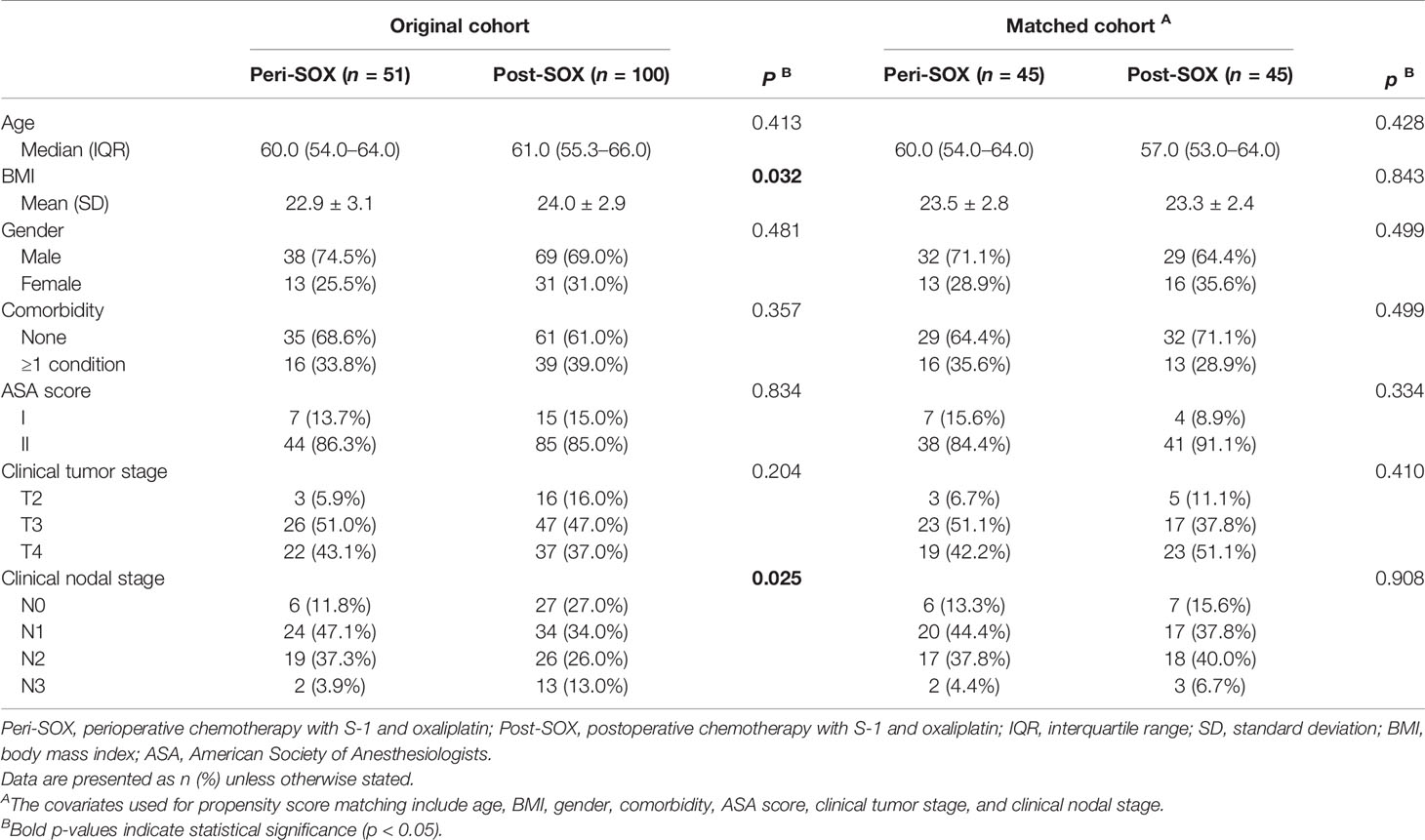
Table 1 Baseline characteristics of eligible LAGC patients in peri-SOX and post-SOX groups before and after PSM.
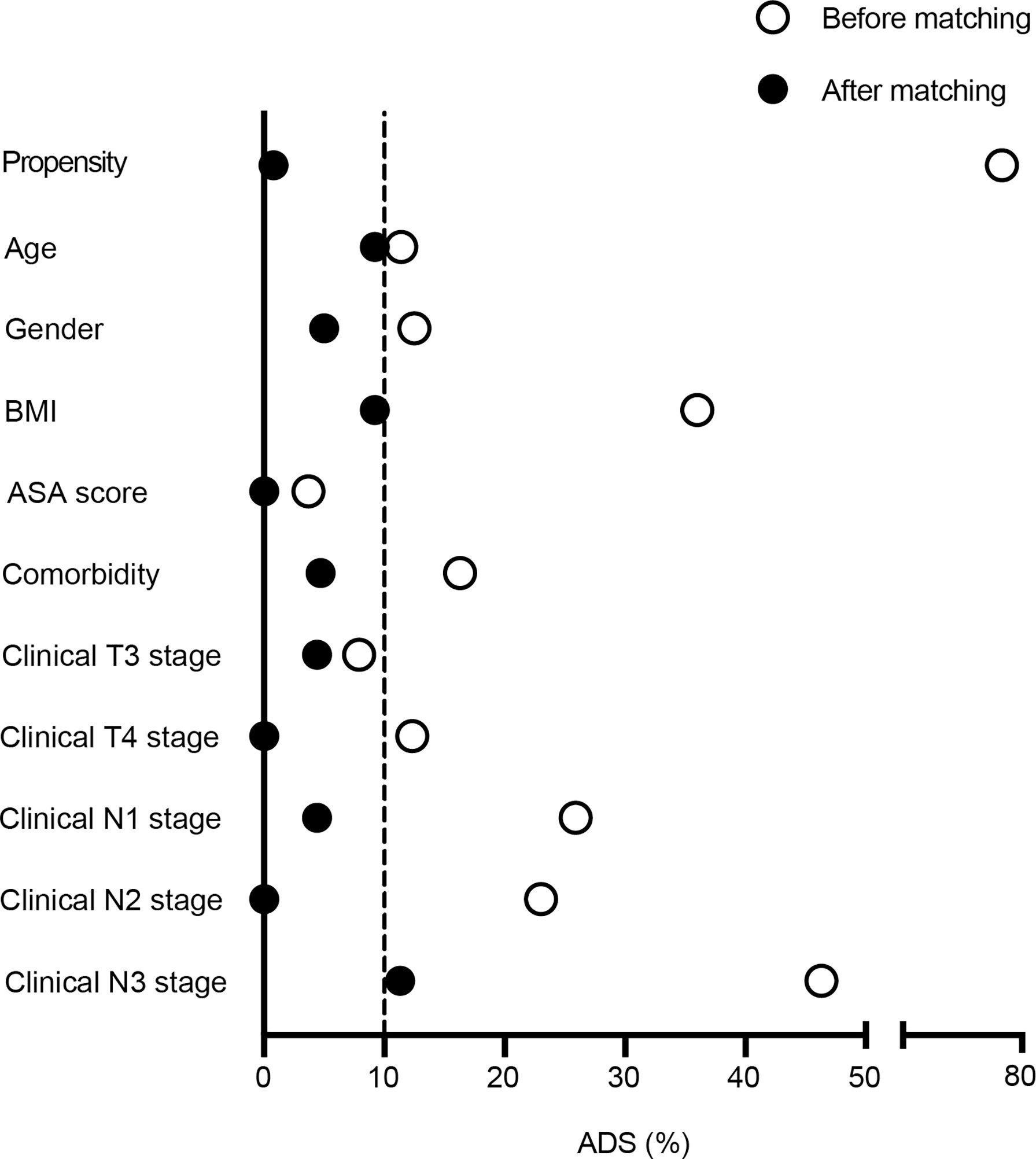
Figure 2 Absolute standardized differences were calculated for baseline variables before and after 1:1 propensity score matching. Labels in y-axis were the baseline characteristics of eligible LAGC patients, and the scatterplot represented absolute standardized differences of propensity scores before and after PSM.
Surgical Outcomes and Adverse Events of Chemotherapy
As reported in Table 2, intraoperative outcomes and postoperative complication rates were comparable between the peri-SOX and post-SOX arms after PSM. No severe complications or mortality was reported within 30 days of surgery. Adverse events of chemotherapy were similar between the two groups, suggesting a comparable impact of the perioperative or postoperative chemotherapy modality. Nearly 90% of patients with LAGC who completed at least six cycles of SOX had side effects, although adverse events of grades 4–5 were not observed in either group. The number of patients with grade 1–2 adverse events was similar between the two arms. Grade 3 adverse events occurred in 35.6% of patients in the peri-SOX group and 37.8% of patients in the post-SOX group, with neutropenia being the most common symptom, followed by leukopenia (Table S1).
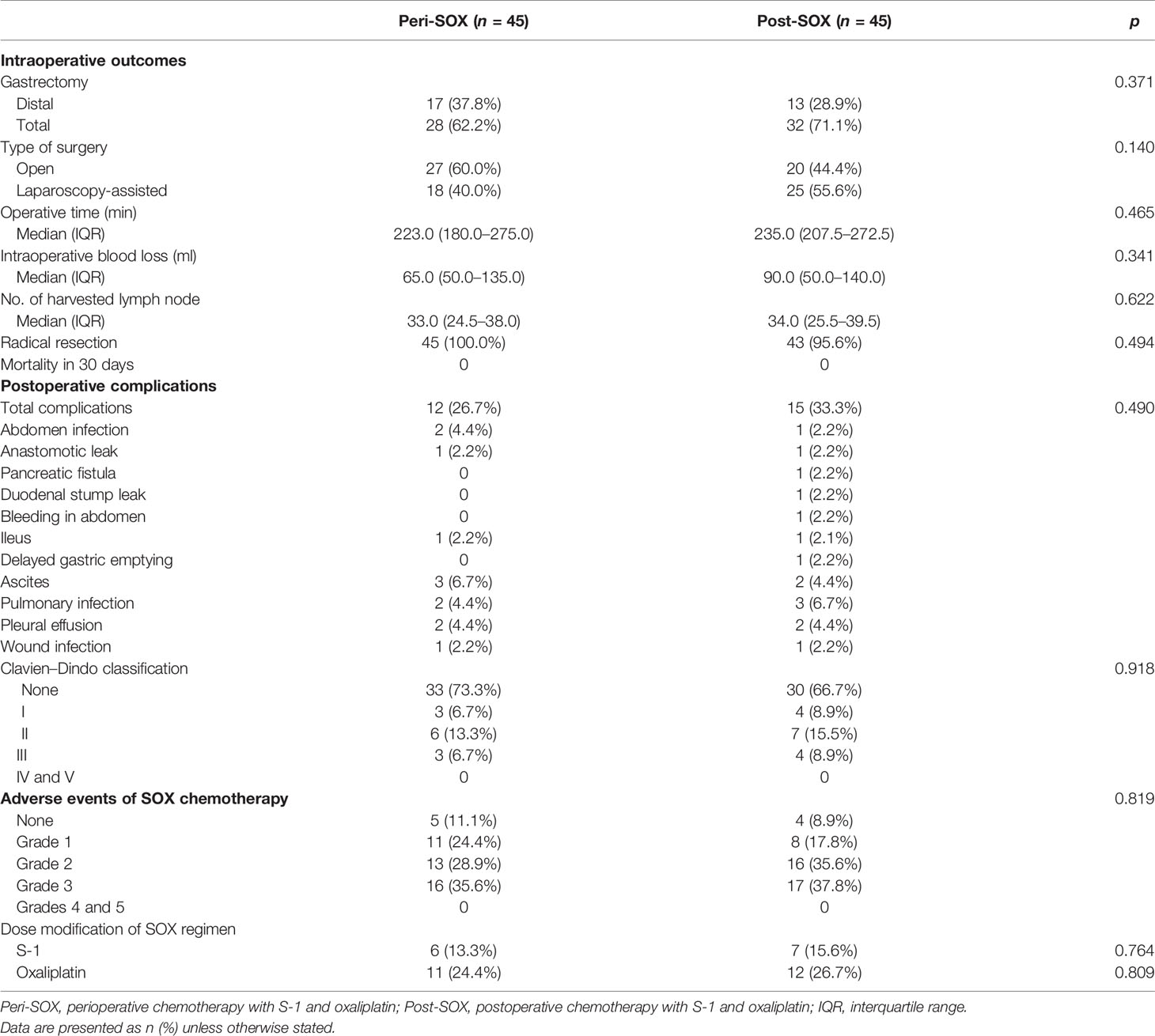
Table 2 Intraoperative outcomes, postoperative complications, and adverse events of chemotherapy between peri-SOX and post-SOX groups after PSM.
Changes of HRQOL in Peri-SOX and Post-SOX Groups
The overall questionnaire response rates throughout this 1-year follow-up period decreased slightly from 98.9% at baseline, to 75.6% in the 12th month after the initiation of therapy, even under rigorous follow-up by the research nurse. Similar response rates were observed in both populations who had completed sufficient chemotherapy cycles with good compliance (Figure 1).
The changing trajectories of mean HRQOL scores in each scale or item of the EORTC QLQ-C30 and STO22 at the indicated measurement points throughout the longitudinal study are presented in Figures 3, 4 for the peri-SOX and post-SOX groups. No statistically significant differences were observed in any scale of the questionnaires between the two groups over time. Similar baseline features or surgical outcomes were observed between those who completed all study visits and those who did not, indicating that the lost data were missing at random (Tables S2, S3). Results from linear mixed modeling before and after multiple imputations showed comparable overall trends in HRQOL trajectories between the groups (Table S4), suggesting that no attrition bias were observed in this study.
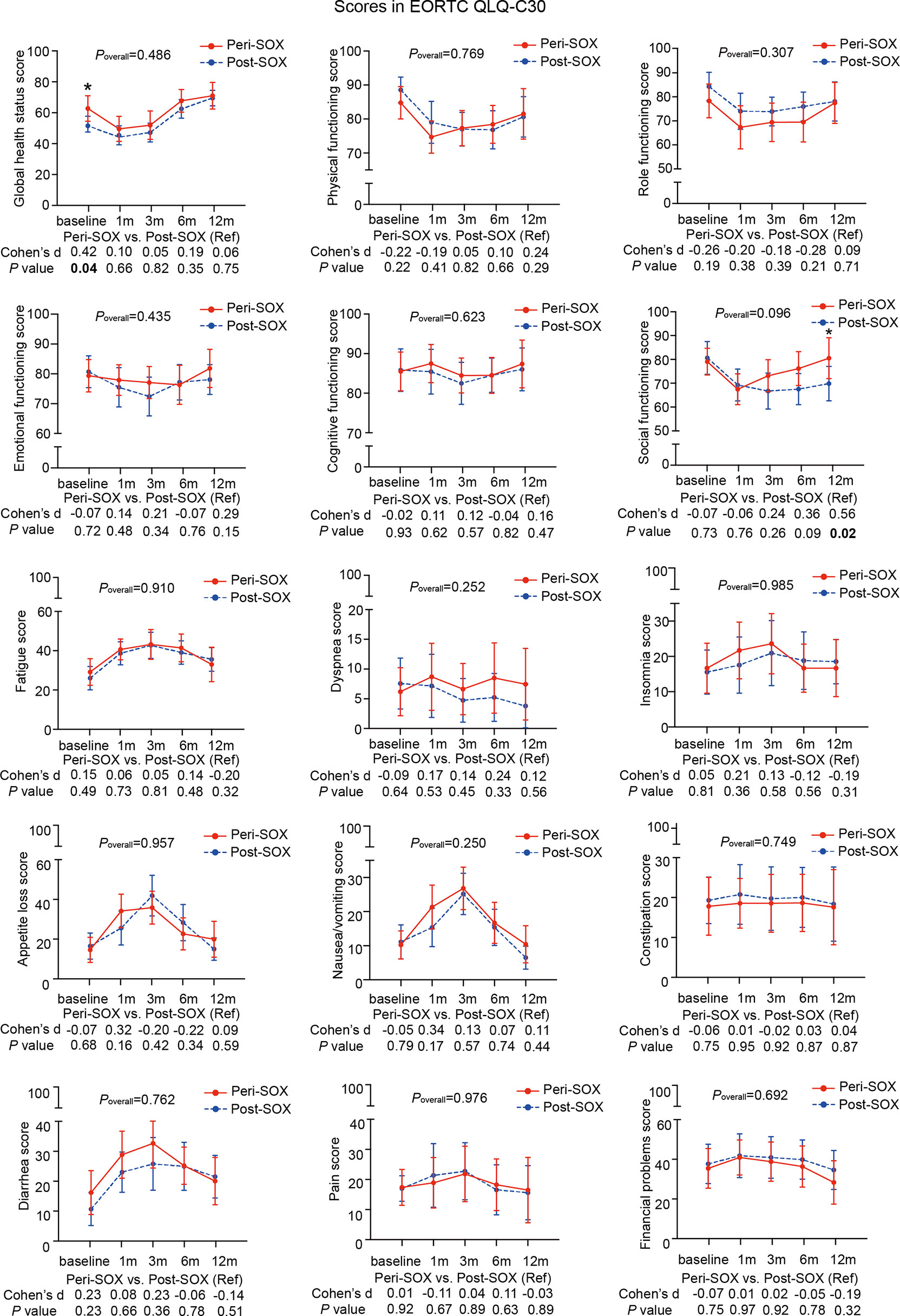
Figure 3 Mean scores with 95% confidence interval (CI) over time of each scale or item in the HRQOL questionnaire, EORTC QLQ-C30, according to the treatment group. Poverall represented statistical values of the longitudinal comparison between the peri-SOX and post-SOX groups. Between-group differences of HRQOL scores at baseline and at each follow-up measurement were also analyzed by the linear mixed model; Cohen’s d (CD) effect size and p-values were listed correspondingly under the line graph. Bold font and * indicated statistically significant difference with p < 0.05.
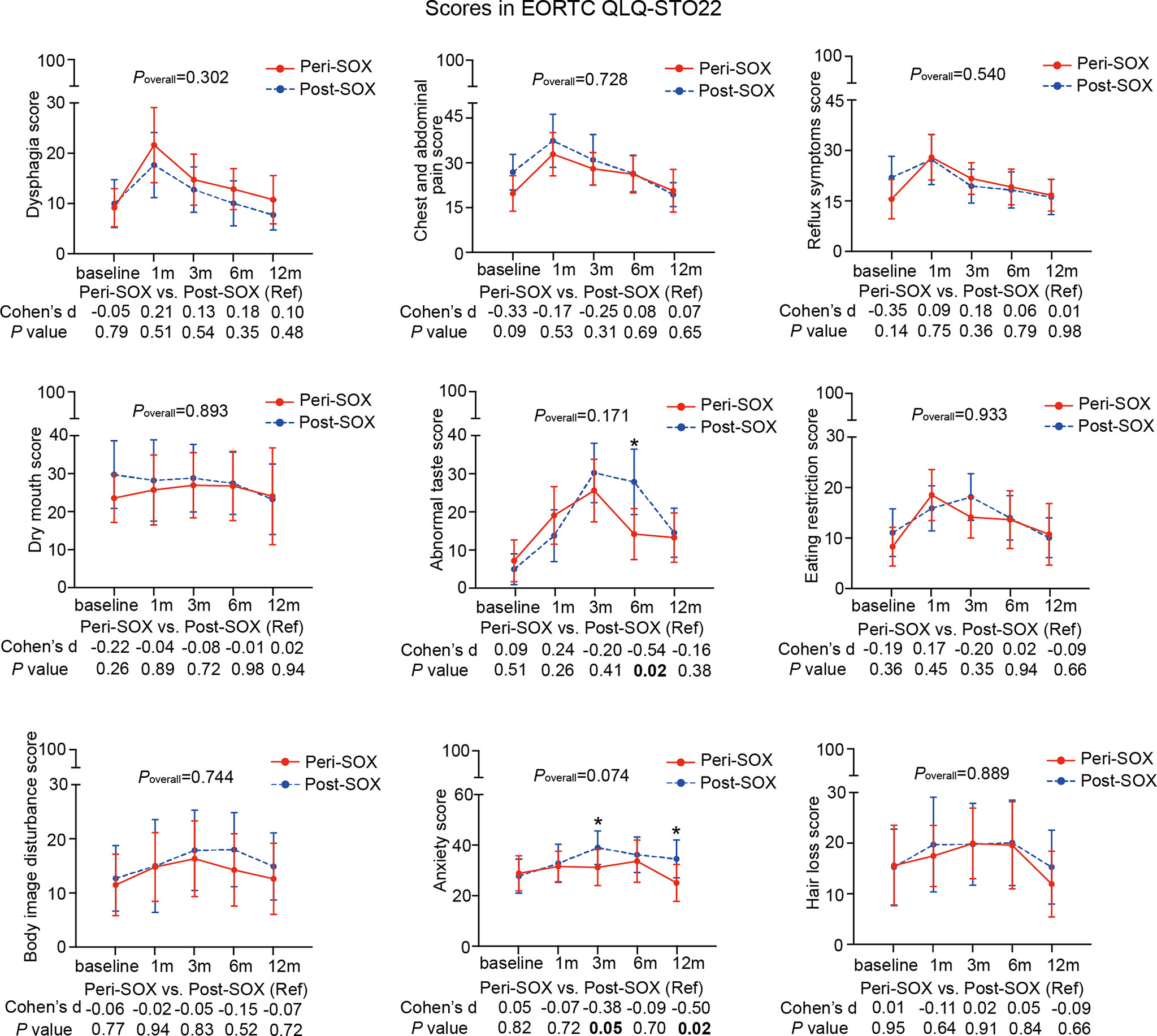
Figure 4 Mean scores with 95% confidence interval (CI) over time of each scale or item in the HRQOL questionnaire, EORTC QLQ-STO22, according to the treatment group. Poverall represented statistical values of the longitudinal comparison between the peri-SOX and post-SOX groups. Between-group differences of HRQOL scores at baseline and at each follow-up time point were also analyzed by the linear mixed model; Cohen’s d (CD) effect size and p-values were listed correspondingly under the line graph. Bold font and * indicated statistically significant difference with p < 0.05.
The baseline HRQOL scores were similar in all scales and items between the peri-SOX and post-SOX groups, except for global health status, which was significantly better in the peri-SOX group, with more than a ten-point difference. The changing trajectories were similar with the passage of time between the peri-SOX and post-SOX groups in the majority of HRQOL scales, with some exceptions (Figures 3, 4). Social functioning deteriorated and reached the lowest point 1 month after baseline and gradually recovered afterwards in both groups, while improvement in social functioning in the peri-SOX group was more obvious than in the post-SOX group, with a remarkable difference in the 12th month (CD, 0.56; p = 0.02). The symptom scores for abnormal taste were much lower in the peri-SOX arm than in the post-SOX arm in the 6th month, indicating a milder tasting problem in the peri-SOX group (CD, −0.54; p = 0.02). The anxiety scores in the peri-SOX group remained stable during treatment and follow-up; meanwhile, in the post-SOX group, this symptom deteriorated continuously and reached the worst level by the 3rd month, with a slow recovery thereafter. Patients in the peri-SOX group were remarkably less anxious than those in the post-SOX group by the 3rd (CD, −0.38; p = 0.05) and 12th month (CD, −0.50; p = 0.02) after the commencement of therapy.
Overall Trends of HRQOL Along With Time
The longitudinal changes in HRQOL scores in each scale or item between baseline and follow-up measurements were calculated with data from both groups using linear mixed models, as changes in HRQOL over time could not be affected by the sequence of chemotherapy. As shown in Table 3, the overall trends in 10 out of 24 domains remained stable over time, compared to their baseline levels, including cognitive functioning; symptoms of dyspnea, insomnia, constipation, pain, and financial problems in the QLQ-C30 questionnaire; and dry mouth, body image, anxiety, and hair loss in the QLQ-STO22 questionnaire.
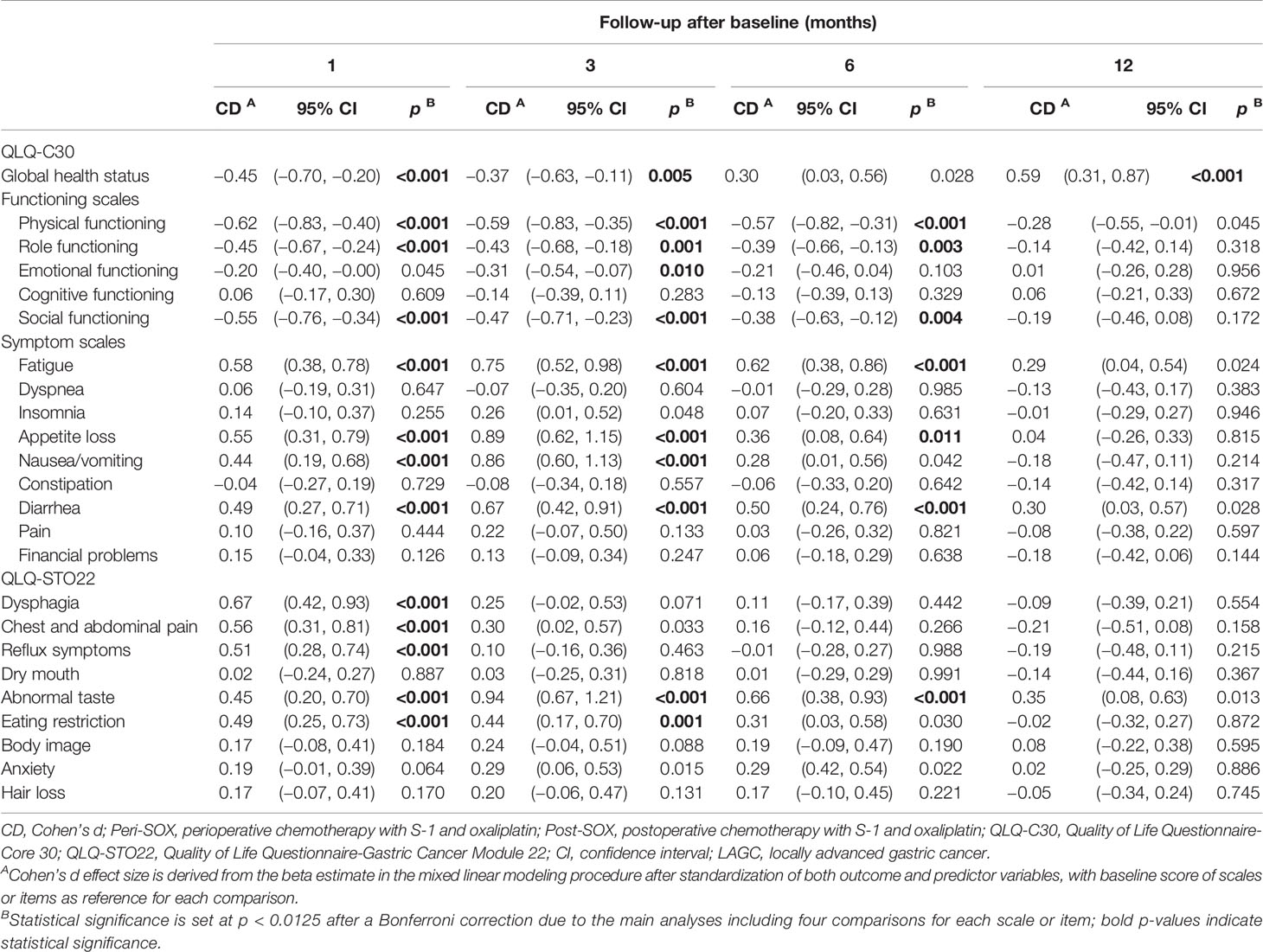
Table 3 Longitudinal effects of both peri-SOX and post-SOX treatments on scales or items in EORTC QLQ-C30 and QLQ-STO22 questionnaires of LAGC patients with D2 gastrectomy over time.
The global health status, physical functioning, role functioning, and social functioning in the QLQ-C30 questionnaire, and symptoms of dysphagia, chest and abdominal pain, reflux, and eating restriction in the QLQ-STO22 questionnaire deteriorated to the worst level in the first month after baseline, and their recovery trajectories were different. Symptoms of dysphagia, chest and abdominal pain, and reflux improved by the 3rd month, and remained stable thereafter. Global health status and eating restriction returned to a comparable level as the baseline till the 6th month, and the global health status became even significantly better than the pre-treatment level by the 12th month. Physical, role, and social functioning recovered slowly, and the significant differences compared to baseline values disappeared by the 12th month. Fatigue, appetite loss, nausea, and vomiting, in addition to diarrhea in the QLQ-C30 questionnaire and abnormal taste in the QLQ-STO22 questionnaire deteriorated significantly 1 month after the initiation of therapy and reached the worst level by the 3rd month. Symptoms of nausea and vomiting were relieved by the 6th month, while fatigue, appetite loss, diarrhea, and abnormal taste were still problematic and only returned to baseline levels by the 12th month (Table 3).
To clearly describe the dynamic changes of patients’ conditions over time from the clinical point of view, each HRQOL scale was classified into “improved”, “stable”, or “deteriorated” with corresponding ratios according to the clinically meaningful difference of HRQOL (55, 56). More than 40% of patients presented “deteriorated” in scales of physical, social, and role functioning, as well as symptoms of fatigue, reflux, diarrhea, and anxiety in the 1st, 3rd, and 6th month after baseline, and at least 30% patients remained troublesome even by the 12th month among these scales, with fatigue ranking with the highest deterioration rate at each follow-up measurement (Figure 5).

Figure 5 Proportions of patients with clinically significant statuses classified as “improved”, “stable”, and “deteriorated” in the scales or items of the QLQ-C30 and QLQ-STO22 questionnaires at each follow-up measurement, including (A) 1 month, (B) 3 months, (C) 6 months, and (D) 12 months after baseline. Status with clinical significance was defined as at least 10-point changes relative to the baseline. Titles of scales or items were colored in red if more than 40% of patients showed deteriorated status by the 1st, 3rd, and 6th month after initiation of therapy, and more than 30% of patients still had worrisome symptoms by the 12th month.
Further analyses were conducted to explore risk factors associated with the seven scales exhibiting general deterioration and slower recovery in this longitudinal study. Twelve clinically relevant factors were initially involved in finding single variables related to HRQOL change in the concerned scales, and independent risk factors were further confirmed using subsequent ordinal logistic regression (Table 4 and Tables S5–S10). The results indicated that after completion of gastrectomy and chemotherapy, baseline characteristics and therapeutic factors affected the HRQOL of patients. As presented in Table 4, a higher BMI can induce more severe fatigue. As we set the scores of fatigue in patients with BMI < 25 as a reference, the odds ratio (OR) for relief of fatigue in patients with BMI ≥ 25 was 0.81-fold than that in the reference group. Similar results were observed in the other scales. Higher BMI and comorbidity ≥1 were negatively correlated with the alleviation of anxiety (Table S5). Recovery of physical functioning was poorer in patients with adverse events of chemotherapy (Table S6). In addition, the scope of gastrectomy also correlated with recovery from diarrhea, with better improvement in patients undergoing distal gastrectomy than in those receiving total gastrectomy (Table S7). The involved 12 variables were not associated with score changes in role functioning, social functioning, and reflux symptoms (Tables S8–10), indicating their weakened roles in these scales 1 year later.
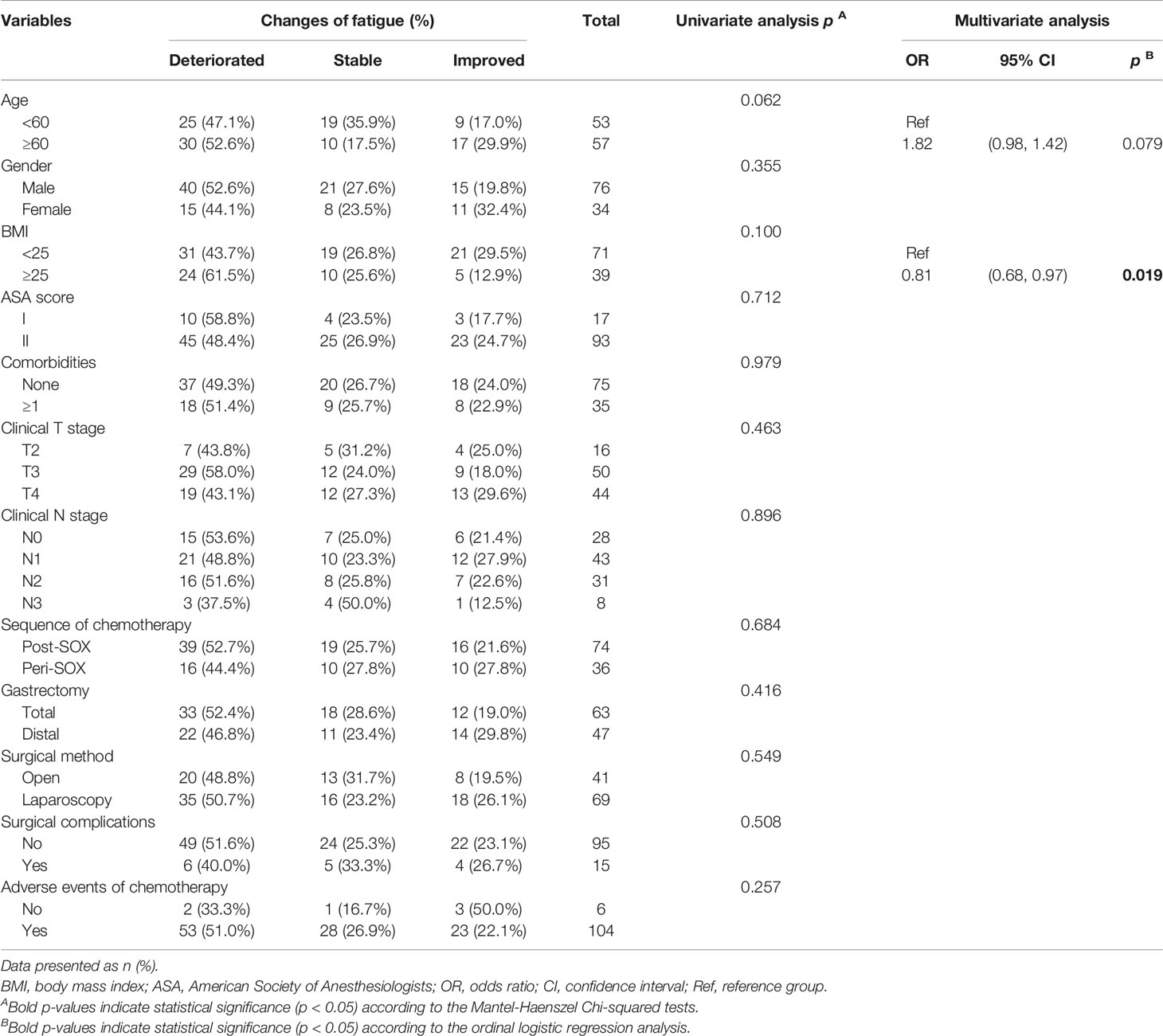
Table 4 Univariate and multivariate analyses of associations between clinically relevant factors and changes of fatigue of LAGC patients before PSM in the 12th month after the initiation of therapy.
Discussion
With good application prospects of the SOX regimen in either neoadjuvant or adjuvant chemotherapy, several high-quality clinical trials are being conducted to evaluate the surgical safety, efficacy, and survival benefit of D2 resection plus SOX with different sequences or cycles in patients with LAGC (15, 37, 62, 63). In addition to surgical and oncological endpoints, the HRQOL is another instrument used to evaluate the effect of cancer treatment on patients’ lives. In this longitudinal study, peri-SOX treatment had a comparable impact on HRQOL over time as the post-SOX group in LAGC patients with sufficient chemotherapy cycles. At some follow-up points, the scores of HRQOL in social functioning, abnormal taste, and anxiety were much better in the peri-SOX group than in the post-SOX group, indicating that the peri-SOX modality has a positive influence on few HRQOL scales.
Since a small sample size would lead to reduced statistical power, CD values with relatively small or moderate clinical effect sizes might not have statistical significance in this observational study (64). To describe the HRQOL changes in detail, clinical effect size rather than simply p-value was considered. Despite comparable comorbidity and ASA scores at baseline between these two groups, significantly superior general health statuses were reported by patients in the peri-SOX group than in the post-SOX group. Milder symptoms of chest and abdominal pain, reflux, and dry mouth were also observed in the peri-SOX arm, with only slightly worse physical and role functioning before treatment. This phenomenon is in accordance with real-world clinical practice. With proven therapeutic effects of neoadjuvant and adjuvant SOX chemotherapy with D2 resection, both are standard treatments for stage II–III LAGC (including both EGJ and non-EGJ cancers), except for a slight difference in the recommended level (13). Neoadjuvant chemotherapy could effectively degrade the tumor stage but destroy the anatomical dissection plane due to tissue fibrotic changes, which might lead to increased surgical difficulties. Therefore, the sequence of chemotherapy administration is a comprehensive decision considering both tumor degradation demand and surgical risks. In patients with similar general conditions, doctors usually tend to recommend that individuals with better self-feelings and milder gastric symptoms receive neoadjuvant chemotherapy before surgery.
In the first month after the initiation of therapy, symptoms of insomnia, appetite loss, nausea and vomiting, dysphagia, and abnormal taste, as well as role functioning deteriorated slightly in the peri-SOX group compared to the post-SOX group, which may be due to the administration of neoadjuvant chemotherapy in the peri-SOX group at this time point. Although with some skipping of CD values, scales of emotional functioning, social functioning, symptoms of appetite loss, abnormal taste, and anxiety improved considerably in the peri-SOX group than in the post-SOX group, with small clinical significance for at least two measurements. By the 12th month, scales of social functioning, physical functioning, emotional functioning, and symptoms of anxiety and fatigue showed small or moderate improvements in the peri-SOX group compared to the control group, which indicated a better recovery of patients in the peri-SOX group (Figures 3, 4).
The global health status in both the peri-SOX and post-SOX groups improved gradually, from significantly worse than the baseline level to remarkably better than the baseline level, indicating the curative effectiveness of gastrectomy together with chemotherapy in patients with LAGC (Table 3). Most scales of HRQOL presented a significant deterioration in the first and third month after baseline, as treatments were given in this period for both groups. Hereafter, the deteriorated domains of HRQOL were generally relieved. Up until the 12th month, no HRQOL score was significantly lower than the baseline level (Table 3). However, some scales still showed more than 30% deterioration at this time point, which continued to worsen from the beginning of therapy and returned to the baseline very slowly (Figure 5). Independent risk factors for HRQOL domains with general deterioration and slower recovery were further analyzed. We found that some baseline and clinically relevant characteristics could contribute to the deterioration of the HRQOL scales. Elevated BMI at baseline was a high-risk factor that led to worsened HRQOL in anxiety and fatigue, as previously reported (58–60). Total gastrectomy as a clinically relevant factor was associated with unrelieved diarrhea, compared to distal gastrectomy, while adverse events of chemotherapy might prolong the recovery time of patients (Table 4 and Tables S5–S10). As some symptoms are unavoidable due to therapeutic strategies, healthcare providers should pay much attention during treatment. Relevant problems should be fully informed to patients with LAGC before treatment. Furthermore, to minimize discomfort during this period, simultaneous supportive care should be provided if necessary.
This study had several limitations. First, we only included patients with LAGC who received at least six cycles of SOX in total. In a pilot study, we found that questionnaire response rates decreased significantly in patients unable to complete chemotherapy and led to a significant increase in missing data, which might influence the stability of the linear mixed model (53). In addition, the survival benefit was comparable between patients receiving no fewer than six cycles of SOX, but not in patients with fewer cycles (65, 66). Moreover, chemotherapy cycles affect the HRQOL of cancer patients (67, 68). Therefore, participants receiving insufficient cycles of SOX were excluded to explore the net effect of chemotherapy sequence on the HRQOL of patients with LAGC. However, approximately 30%–50% of patients with LAGC in China cannot complete sufficient cycles of chemotherapy (15, 63). Hence, the estimation of HRQOL in patients with less than six cycles of SOX should be done with caution because of the limited external validity of this study. Second, the long-term HRQOL of the peri-SOX and post-SOX groups could not be observed due to the 1-year follow-up. Third, although the reliability of this observational cohort study was increased by applying PSM, an underpowered risk of statistical analysis may have occurred because of the reduced sample size.
Conclusion
In conclusion, peri-SOX or post-SOX chemotherapy modalities have little effect on the HRQOL of LAGC patients who receive D2 gastrectomy over time. The scales of social functioning, abnormal taste, and anxiety improve earlier in the peri-SOX group than in the post-SOX group at some follow-up measurements. General deterioration and slower recovery usually occur in scales of physical functioning, social functioning, role functioning, and symptoms of fatigue, reflux, diarrhea, and anxiety; thus, patients with LAGC with higher risk factors should be fully informed before commencement of either peri-SOX or post-SOX treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Peking University Cancer Hospital & Institute. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JY and ZW drafted the protocol, extracted the data, performed the statistical analyses, and drafted the article. ZL performed the statistical analyses and directed statistical analyses. YL delivered and collected the questionnaire. YF, JD, MC, JX, CZ, HY, ZY, NZ, LC, ML, KX, FT, and PG drafted the protocol, participated in patient enrollment, and revised the manuscript. XS and ZW directed the writing, review, and revision of the article. JY, ZW, ZL, and YL have contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Capital’s Funds for Health Improvement and Research (CFH 2018-2-2153), the National Natural Science Foundation of China (nos. 82073357, 82171720, 81872022, and 81672439), and the Beijing Natural Science Foundation (no. 7162039).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to all the researchers at the Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital and Institute.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.853337/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics 2020. Chin Med J (Engl) (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
3. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing Cancer Survival in China During 2003-15: A Pooled Analysis of 17 Population-Based Cancer Registries. Lancet Glob Health (2018) 6:e555–67. doi: 10.1016/S2214-109X(18)30127-X
4. McConville SR, Crookes PF. The History of Gastric Surgery: The Contribution of the Belfast School. Ulster Med J (2007) 76:31–6.
5. Cardoso R, Coburn NG, Seevaratnam R, Mahar A, Helyer L, Law C, et al. A Systematic Review of Patient Surveillance After Curative Gastrectomy for Gastric Cancer: A Brief Review. Gastric Cancer (2012) 15 Suppl 1:S164–7. doi: 10.1007/s10120-012-0142-9
6. Kim JH, Lee HH, Seo HS, Jung YJ, Park CH. Borrmann Type 1 Cancer Is Associated With a High Recurrence Rate in Locally Advanced Gastric Cancer. Ann Surg Oncol (2018) 25:2044–52. doi: 10.1245/s10434-018-6509-3
7. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant Chemotherapy for Gastric Cancer With S-1, an Oral Fluoropyrimidine. N Engl J Med (2007) 357:1810–20. doi: 10.1056/NEJMoa072252
8. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant Capecitabine and Oxaliplatin for Gastric Cancer After D2 Gastrectomy (CLASSIC): A Phase 3 Open-Label, Randomised Controlled Trial. Lancet (2012) 379:315–21. doi: 10.1016/S0140-6736(11)61873-4
9. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van VCJ, Nicolson M, et al. Perioperative Chemotherapy Versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med (2006) 355:11–20. doi: 10.1056/NEJMoa055531
10. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J Clin Oncol (2011) 29:1715–21. doi: 10.1200/JCO.2010.33.0597
11. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative Chemotherapy With Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel Versus Fluorouracil or Capecitabine Plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet (2019) 393:1948–57. doi: 10.1016/S0140-6736(18)32557-1
12. Japanese Gastric Cancer A. Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer (2021) 24:1–21. doi: 10.1007/s10120-020-01042-y
13. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer, 2021. Cancer Commun (Lond) (2021) 41:747–95. doi: 10.1002/cac2.12193
14. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2016) 14:1286–312. doi: 10.6004/jnccn.2016.0137
15. Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or Postoperative Adjuvant Oxaliplatin With S-1 Versus Adjuvant Oxaliplatin With Capecitabine in Patients With Locally Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma Undergoing D2 Gastrectomy (RESOLVE): An Open-Label, Superiority and Non-Inferiority, Phase 3 Randomised Controlled Trial. Lancet Oncol (2021) 22:1081–92. doi: 10.1016/S1470-2045(21)00297-7
16. Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol (2021) 39:2903–13. doi: 10.1200/JCO.20.02914
17. Xue K, Ying X, Bu Z, Wu A, Li Z, Tang L, et al. Oxaliplatin Plus S-1 or Capecitabine as Neoadjuvant or Adjuvant Chemotherapy for Locally Advanced Gastric Cancer With D2 Lymphadenectomy: 5-Year Follow-Up Results of a Phase II-III Randomized Trial. Chin J Cancer Res (2018) 30:516–25. doi: 10.21147/j.issn.1000-9604.2018.05.05
18. Cascinu S, Labianca R, Daniele B, Beretta G, Salvagni S. Survival and Quality of Life in Gastrointestinal Tumors: Two Different End Points? Ann Oncol (2001) 12 Suppl 3:S31–6. doi: 10.1093/annonc/12.suppl_3.s31
19. Kim AR, Cho J, Hsu Y-J, Choi MG, Noh JH, Sohn TS, et al. Changes of Quality of Life in Gastric Cancer Patients After Curative Resection: A Longitudinal Cohort Study in Korea. Ann Surg (2012) 256:1008–13. doi: 10.1097/SLA.0b013e31827661c9
20. Brenkman HJF, Tegels JJW, Ruurda JP, Luyer MDP, Kouwenhoven EA, Draaisma WA, et al. Factors Influencing Health-Related Quality of Life After Gastrectomy for Cancer. Gastric Cancer (2018) 21:524–32. doi: 10.1007/s10120-017-0771-0
21. Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, et al. Superiority of Laparoscopic Proximal Gastrectomy With Hand-Sewn Esophagogastrostomy Over Total Gastrectomy in Improving Postoperative Body Weight Loss and Quality of Life. Surg Endoscopy (2017) 31:3664–72. doi: 10.1007/s00464-016-5403-y
22. Park JY, Park KB, Kwon OK, Yu W. Comparison of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction and Laparoscopic Total Gastrectomy in Terms of Nutritional Status or Quality of Life in Early Gastric Cancer Patients. Eur J Surg Oncol (2018) 44:1963–70. doi: 10.1016/j.ejso.2018.08.014
23. Misawa K, Fujiwara M, Ando M, Ito S, Mochizuki Y, Ito Y, et al. Long-Term Quality of Life After Laparoscopic Distal Gastrectomy for Early Gastric Cancer: Results of a Prospective Multi-Institutional Comparative Trial. Gastric Cancer (2015) 18:417–25. doi: 10.1007/s10120-014-0374-y
24. Kim Y-W, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved Quality of Life Outcomes After Laparoscopy-Assisted Distal Gastrectomy for Early Gastric Cancer: Results of a Prospective Randomized Clinical Trial. Ann Surg (2008) 248:721–7. doi: 10.1097/SLA.0b013e318185e62e
25. Yang K, Zhang W-H, Liu K, Chen X-Z, Zhou Z-G, Hu J-K. Comparison of Quality of Life Between Billroth-І and Roux-En-Y Anastomosis After Distal Gastrectomy for Gastric Cancer: A Randomized Controlled Trial. Sci Rep (2017) 7:11245. doi: 10.1038/s41598-017-09676-2
26. Du N, Chen M, Shen Z, Li S, Chen P, Khadaroo PA, et al. Comparison of Quality of Life and Nutritional Status of Between Roux-En-Y and Billroth-I Reconstruction After Distal Gastrectomy: A Systematic Review and Meta-Analysis. Nutr Cancer (2020) 72:849–57. doi: 10.1080/01635581.2019.1656262
27. Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, et al. A Comparison of Postoperative Quality of Life and Dysfunction After Billroth I and Roux-En-Y Reconstruction Following Distal Gastrectomy for Gastric Cancer: Results From a Multi-Institutional RCT. Gastric Cancer (2012) 15:198–205. doi: 10.1007/s10120-011-0098-1
28. Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of Life With Docetaxel Plus Cisplatin and Fluorouracil Compared With Cisplatin and Fluorouracil From a Phase III Trial for Advanced Gastric or Gastroesophageal Adenocarcinoma: The V-325 Study Group. J Clin Oncol (2007) 25:3210–6. doi: 10.1200/JCO.2006.08.3956
29. Curran D, Pozzo C, Zaluski J, Dank M, Barone C, Valvere V, et al. Quality of Life of Palliative Chemotherapy Naive Patients With Advanced Adenocarcinoma of the Stomach or Esophagogastric Junction Treated With Irinotecan Combined With 5-Fluorouracil and Folinic Acid: Results of a Randomised Phase III Trial. Qual Life Res (2009) 18:853–61. doi: 10.1007/s11136-009-9493-z
30. Van CE, Valderrama A, Bang YJ, Fuchs CS, Shitara K, Janjigian YY, et al. Quality of Life With First-Line Pembrolizumab for PD-L1-Positive Advanced Gastric/Gastroesophageal Junction Adenocarcinoma: Results From the Randomised Phase III KEYNOTE-062 Study. ESMO Open (2021) 6:100189. doi: 10.1016/j.esmoop.2021.100189
31. Van CE, Amonkar M, Fuchs CS, Alsina M, Özgüroğlu M, Bang Y-J, et al. Health-Related Quality of Life in Advanced Gastric/Gastroesophageal Junction Cancer With Second-Line Pembrolizumab in KEYNOTE-061. Gastric Cancer (2021) 24:1330–40. doi: 10.1007/s10120-021-01200-w
32. Al-Batran SE, Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. Quality-Of-Life and Performance Status Results From the Phase III RAINBOW Study of Ramucirumab Plus Paclitaxel Versus Placebo Plus Paclitaxel in Patients With Previously Treated Gastric or Gastroesophageal Junction Adenocarcinoma. Ann Oncol (2016) 27:673–9. doi: 10.1093/annonc/mdv625
33. Noordman BJ, Verdam MGE, Lagarde SM, Hulshof M, Hagen P, Berge HMI, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol (2018) 36:268–75. doi: 10.1200/JCO.2017.73.7718
34. Richard MEY, Michael SS, Debra L, Andrew W, A. Rashid D, Brian PY, et al. The Quality of Life in Neoadjuvant Versus Adjuvant Therapy of Esophageal Cancer Treatment Trial (QUINTETT). J Clin Oncol (2019) 37:4052–2. doi: 10.1200/JCO.2019.37.15_suppl.4052
35. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A Randomized Phase III Trial Comparing Adjuvant Single-Agent S1, S-1 With Oxaliplatin, and Postoperative Chemoradiation With S-1 and Oxaliplatin in Patients With Node-Positive Gastric Cancer After D2 Resection: The ARTIST 2 Trial(). Ann Oncol (2021) 32(3):368–74. doi: 10.1016/j.annonc.2020.11.017
36. Satake H, Miki A, Kondo M, Kotake T, Okita Y, Hatachi Y, et al. Phase I Study of Neoadjuvant Chemotherapy With S-1 and Oxaliplatin for Locally Advanced Gastric Cancer (Neo G-SOX Pi). ESMO Open (2017) 2:e000130. doi: 10.1136/esmoopen-2016-000130
37. Masato KHS, Motoko M, Akira M, Takanori W, Norimitsu T, Kenro H, et al. Multicenter Phase II Study of Neoadjuvant Chemotherapy With S-1 and Oxaliplatin for Locally Advanced Gastric Cancer (Neo G-SOX PII). J Clin Oncol (2020) 38:399–9. doi: 10.1200/JCO.2020.38.4_suppl.399
38. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More "Personalized" Approach to Cancer Staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
39. Wan C, Meng Q, Yang Z, Tu X, Feng C, Tang X, et al. Validation of the Simplified Chinese Version of EORTC QLQ-C30 From the Measurements of Five Types of Inpatients With Cancer. Ann Oncol (2008) 19:2053–60. doi: 10.1093/annonc/mdn417
40. Zhao H, Kanda K. Translation and Validation of the Standard Chinese Version of the EORTC QLQ-C30. Qual Life Res (2000) 9:129–37. doi: 10.1023/a:1008981520920
41. Huang CC, Lien HH, Sung YC, Liu HT, Chie WC. Quality of Life of Patients With Gastric Cancer in Taiwan: Validation and Clinical Application of the Taiwan Chinese Version of the EORTC QLQ-C30 and EORTC QLQ-Sto22. Psychooncology (2007) 16:945–9. doi: 10.1002/pon.1158
42. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
43. Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and Psychometric Validation of a Questionnaire Module, the EORTC QLQ-STO 22, to Assess Quality of Life in Patients With Gastric Cancer. Eur J Cancer (2004) 40:2260–8. doi: 10.1016/j.ejca.2004.05.023
44. Straatman J, Wielen N, Joosten PJ, Terwee CB, Cuesta MA, Jansma EP, et al. Assessment of Patient-Reported Outcome Measures in the Surgical Treatment of Patients With Gastric Cancer. Surg Endosc (2016) 30:1920–9. doi: 10.1007/s00464-015-4415-3
45. Neil WS, Neil KA, Andrew B, Alexander G, Mogens G, Chad G, et al. The EORTC QLQ-C30 Scoring Manual. 3rd Ed. Brussels: European Organisation for Research and Treatment of Cancer (2001).
46. D'Agostino RB Jr. Propensity Score Methods for Bias Reduction in the Comparison of a Treatment to a Non-Randomized Control Group. Stat Med (1998) 17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
47. Austin PC. Optimal Caliper Widths for Propensity-Score Matching When Estimating Differences in Means and Differences in Proportions in Observational Studies. Pharm Stat (2011) 10:150–61. doi: 10.1002/pst.433
48. Morgan CJ. Reducing Bias Using Propensity Score Matching. J Nucl Cardiol (2018) 25:404–6. doi: 10.1007/s12350-017-1012-y
49. Cnaan A, Laird NM, Slasor P. Using the General Linear Mixed Model to Analyse Unbalanced Repeated Measures and Longitudinal Data. Stat Med (1997) 16:2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e
50. Bonnetain F, Fiteni F, Efficace F, Anota A. Statistical Challenges in the Analysis of Health-Related Quality of Life in Cancer Clinical Trials. J Clin Oncol (2016) 34:1953–6. doi: 10.1200/JCO.2014.56.7974
51. Twisk J, de Vente W. Attrition in Longitudinal Studies How to Deal With Missing Data. J Clin Epidemiol (2002) 55:329–37. doi: 10.1016/s0895-4356(01)00476-0
53. Ronald H, Heck SLT, Lynn N. Tabata. Multilevel and Longitudinal Modeling With IBM SPSS. 2nd ed. New York: Routledge (2013).
54. Norman GR, Sloan JA, Wyrwich KW. Interpretation of Changes in Health-Related Quality of Life: The Remarkable Universality of Half a Standard Deviation. Med Care (2003) 41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C
55. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the Significance of Changes in Health-Related Quality-of-Life Scores. J Clin Oncol (1998) 16:139–44. doi: 10.1200/JCO.1998.16.1.139
56. Cocks K, King MT, Velikova G, Castro G Jr, Martyn SM, Fayers PM, et al. Evidence-Based Guidelines for Interpreting Change Scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer (2012) 48:1713–21. doi: 10.1016/j.ejca.2012.02.059
57. Ananth CV, Kleinbaum DG. Regression Models for Ordinal Responses: A Review of Methods and Applications. Int J Epidemiol (1997) 26:1323–33. doi: 10.1093/ije/26.6.1323
58. Park KB, Yu B, Park JY, Kwon OK, Yu W. Impact of Body Mass Index on Quality of Life After Distal Gastrectomy for Gastric Cancer. Ann Surg Treat Res (2019) 96:250–8. doi: 10.4174/astr.2019.96.5.250
59. Lin LL, Brown JC, Segal S, Schmitz KH. Quality of Life, Body Mass Index, and Physical Activity Among Uterine Cancer Patients. Int J Gynecol Cancer (2014) 24:1027–32. doi: 10.1097/IGC.0000000000000166
60. Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, et al. Quality of Life After Curative Gastrectomy for Gastric Cancer in a Randomised Controlled Trial. Br J Cancer (2008) 98:54–9. doi: 10.1038/sj.bjc.6604097
61. Rausei S, Mangano A, Galli F, Rovera F, Boni L, Dionigi G, et al. Quality of Life After Gastrectomy for Cancer Evaluated Via the EORTC QLQ-C30 and QLQ-STO22 Questionnaires: Surgical Considerations From the Analysis of 103 Patients. Int J Surg (2013) 11 Suppl 1:S104–9. doi: 10.1016/S1743-9191(13)60028-X
62. Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, et al. The Protocol of a Prospective, Multicenter, Randomized, Controlled Phase III Study Evaluating Different Cycles of Oxaliplatin Combined With S-1 (SOX) as Neoadjuvant Chemotherapy for Patients With Locally Advanced Gastric Cancer: RESONANCE-II Trial. BMC Cancer (2021) 21:20. doi: 10.1186/s12885-020-07764-7
63. Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, et al. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg (2019) 154:1093–101. doi: 10.1001/jamasurg.2019.3473
64. Serdar CC, Cihan M, Yucel D, Serdar MA. Sample Size, Power and Effect Size Revisited: Simplified and Practical Approaches in Pre-Clinical, Clinical and Laboratory Studies. Biochem Med (Zagreb) (2021) 31:10502. doi: 10.11613/BM.2021.010502
65. Yu Y, Zhang Z, Meng Q, Ma Y, Fan X, Sun J, et al. Comparison of the Efficacy of S-1 Plus Oxaliplatin or Capecitabine Plus Oxaliplatin for Six and Eight Chemotherapy Cycles as Adjuvant Chemotherapy in Patients With Stage II-III Gastric Cancer After D2 Resection. Front Oncol (2021) 11:684627. doi: 10.3389/fonc.2021.684627
66. Chen L, Zhang C, Yao Z, Cui M, Xing J, Yang H, et al. Adjuvant Chemotherapy is an Additional Option for Locally Advanced Gastric Cancer After Radical Gastrectomy With D2 Lymphadenectomy: A Retrospective Control Study. BMC Cancer (2021) 21:974. doi: 10.1186/s12885-021-08717-4
67. Heydarnejad MS, Hassanpour DA, Solati DK. Factors Affecting Quality of Life in Cancer Patients Undergoing Chemotherapy. Afr Health Sci (2011) 11:266–70.
Keywords: HRQOL, quality of life, gastric cancer, perioperative chemotherapy, SOX (S-1 + oxaliplatin)
Citation: Yu J, Wang Z, Li Z, Liu Y, Fan Y, Di J, Cui M, Xing J, Zhang C, Yang H, Yao Z, Zhang N, Chen L, Liu M, Xu K, Tan F, Gao P and Su X (2022) Health-Related Quality of Life in Patients With Locally Advanced Gastric Cancer Undergoing Perioperative or Postoperative Adjuvant S-1 Plus Oxaliplatin With D2 Gastrectomy: A Propensity Score-Matched Cohort Study. Front. Oncol. 12:853337. doi: 10.3389/fonc.2022.853337
Received: 12 January 2022; Accepted: 09 March 2022;
Published: 04 April 2022.
Edited by:
Jinqiu Jacky Yuan, Sun Yat-sen University, ChinaReviewed by:
Ningning Mi, First Hospital of Lanzhou University, ChinaQiangsheng He, Sun Yat-sen University, China
Copyright © 2022 Yu, Wang, Li, Liu, Fan, Di, Cui, Xing, Zhang, Yang, Yao, Zhang, Chen, Liu, Xu, Tan, Gao and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangqian Su, c3V4aWFuZ3FpYW5AYmptdS5lZHUuY24=; Zaozao Wang, emFvemFvODM2MzBAYmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Jianhong Yu
Jianhong Yu Zaozao Wang1*†
Zaozao Wang1*† Xiangqian Su
Xiangqian Su