
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 27 April 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.852611
In many distinct forms of malignancies, there is a close relationship between lymph node (LN) metastases and further dissemination to distant organs, and this is a critical prognostic factor. At the beginning of the process, the original tumor secretes soluble substances or releases extracellular vesicles (EVs) that are carried through lymphatic channels to draining (sentinel) LN. The tumor-derived factors then drive LN remodeling. These significant alterations occur prior to the emergence of the first metastatic cell, bringing about the development of a pre-metastatic niche that allows metastatic cells to survive and thrive. In this review, we discuss current information available about the regulation of lymph node pre-metastatic niche in head and neck squamous cell carcinoma (HNSCC), and the role of EVs in forming the pre-metastatic niche.
Head and neck squamous cell carcinoma (HNSCC) is the most common head and neck cancer, and arises from the mucosal epithelium of the oral cavity, pharynx, and larynx (1). Patients with HNSCC are at risk of cervical lymph node (LN) metastases. Cervical LN involvement is a well-known prognostic marker for HNSCC, and the presence of LN metastases is thought to be a predictor of poor patient outcomes (2, 3). Specifically, high levels of lymphangiogenic growth factors and high lymphatic vessel (LV) density in individuals with cancer indicate that LN metastases are more likely to occur, and the prognosis is usually poor (4, 5).
To facilitate the development of new LVs (lymphangiogenesis), tumor cells produce growth hormones, RNA, and cytoplasmic proteins, and growth factors. These tumor-derived factors can create microenvironment around the organs in which metastases might consequently occur. This advance preparation of the target organ microenvironment is thought to facilitate the tumor cells’ survival and multiplication at the distant site.
This review outlines recent achievements of the regulation of lymph node pre-metastatic niche formation in HNSCC, and the role of EVs in forming the pre-metastatic niche was also discussed.
According to the early studies of Isaiah Josh Fidler et al. (6, 7), metastasis was determined by the structure of the arterial and lymphatic pathways that drain the primary tumor, although tumor cells reached the vasculature of all organs, metastases selectively formed in certain organs but not others. Later related researches (8, 9) have showed the interplay among the microenvironment of the primary and metastatic organs: in order for tumor cells to engraft (metastatic niche) and flourish in secondary locations, a suitable microenvironment (premetastatic niche) must form. These niches are formed by tumor-secreted substances and can be either freshly generated or modifications of preexisting physiological niches. Psaila and Lyden have proposed the notion of a pre-metastatic niche (10), their groundbreaking research showed that tumor cells shed or secrete substances that create a circumstance-related metastasis. These growth factors and chemokines/cytokines produce a distinct milieu that promotes metastatic progression: pre-metastatic niche formation, and LN metastasis (4, 11–13).
Lymph node metastases (LNM) are common over the course of many cancers, and their presence often indicates a bad prognosis. The lymphatic system’s pre-metastatic conditioning of the microenvironment in lymph nodes (so-called lymph node pre-metastatic niche), which makes them receptive and supportive metastatic habitats for disseminated tumor cells, is aided by the discharge of tumor-derived substances such as antigens, growth factors, cytokines, and exosomes (12). HNSCCs, like many other cancers, spread through the lymphatic system (14–18). Indeed, sentinel LNs are the first draining LNs where metastases occur, and they are thought to be a predictor of poor patient outcomes (19). In several types of solid tumors, the level of lymphangiogenesis and the density of LVs are related to LN metastasis and the prognosis of patients (20, 21). For HNSCC, this link has been verified numerous times (22–24). These results show that lymphangiogenesis occurs before tumor cells arrive in the metastasis locations of patients with HNSCC. Tumor-derived signals travel from the lymphatics to the draining LN, where they stimulate the formation of localized LVs. As a result, the enlarged lymphatic network in tumor-free lymph nodes is a very early pre-metastatic indicator. Family members of vascular endothelial growth factors (VEGF) and other non-VEGF-mediated molecular are able to induce lymphangiogenesis in tumor (25), and their specific mechanisms in regulating lymphangiogenesis in HNSCC are discussed in the following sections (Table 1).
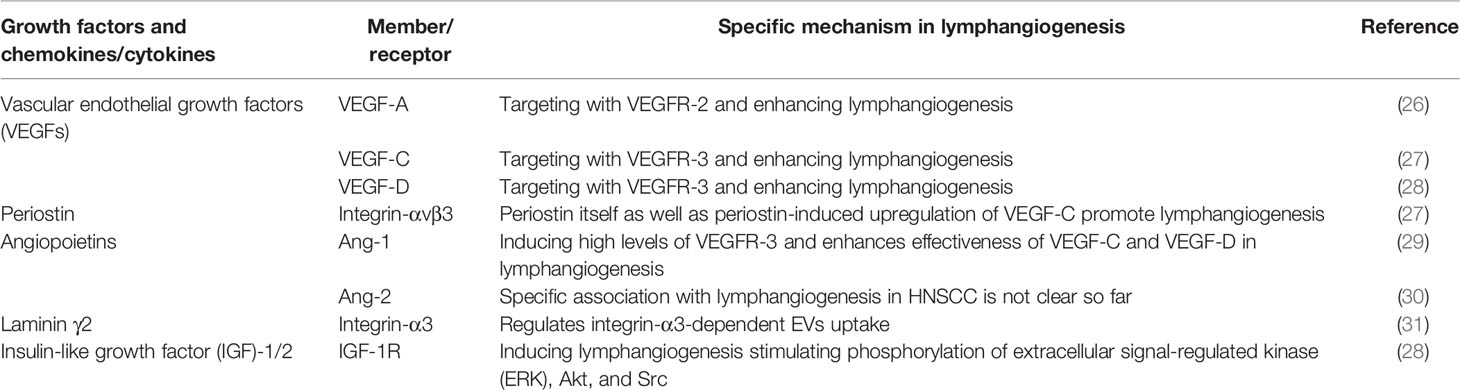
Table 1 The role of different molecular in inducing lymphangiogenesis in head and neck squamous cell carcinoma (HNSCC).
Secreted growth factors have been shown to be major regulators of lymphangiogenesis, regardless of their source. Angiogenesis is the process by which new blood vessels develop from primary vessels, and vascular endothelial growth factors (VEGFs) are considered essential elements in angiogenesis (32). The role of VEGF family proteins in angiogenesis and tumor formation is well understood (33, 34).
VEGF-C and VEGF-D have angiogenic capabilities in lymphangiogenesis, and they are the most widely researched components to date (35). These two molecules promote lymphangiogenesis by linking with the receptor VEGFR-3, which is mostly expressed in lymphatic endothelium cells (LECs) and monocytic hematopoietic cells in adults (36). When VEGFR-3 is stimulated in LECs, a cascade of signals causes the cells to expand and migrate, protecting them against apoptosis (37–39). Furthermore, VEGF-A can cause LV growth by activating the receptor VEGFR-2: it was found that original tumors with high levels of VEGF-A induced lymphangiogenesis in sentinel lymph node (SLN) before LN metastases (40). Higher levels of these three VEGF have been linked to the density of LVs and LN metastases and poor prognoses in patients with HNSCC (41, 42), and there are some molecular can induce lymphangiogenesis in HNSCC by regulating the expression of VEGF (Figure 1).
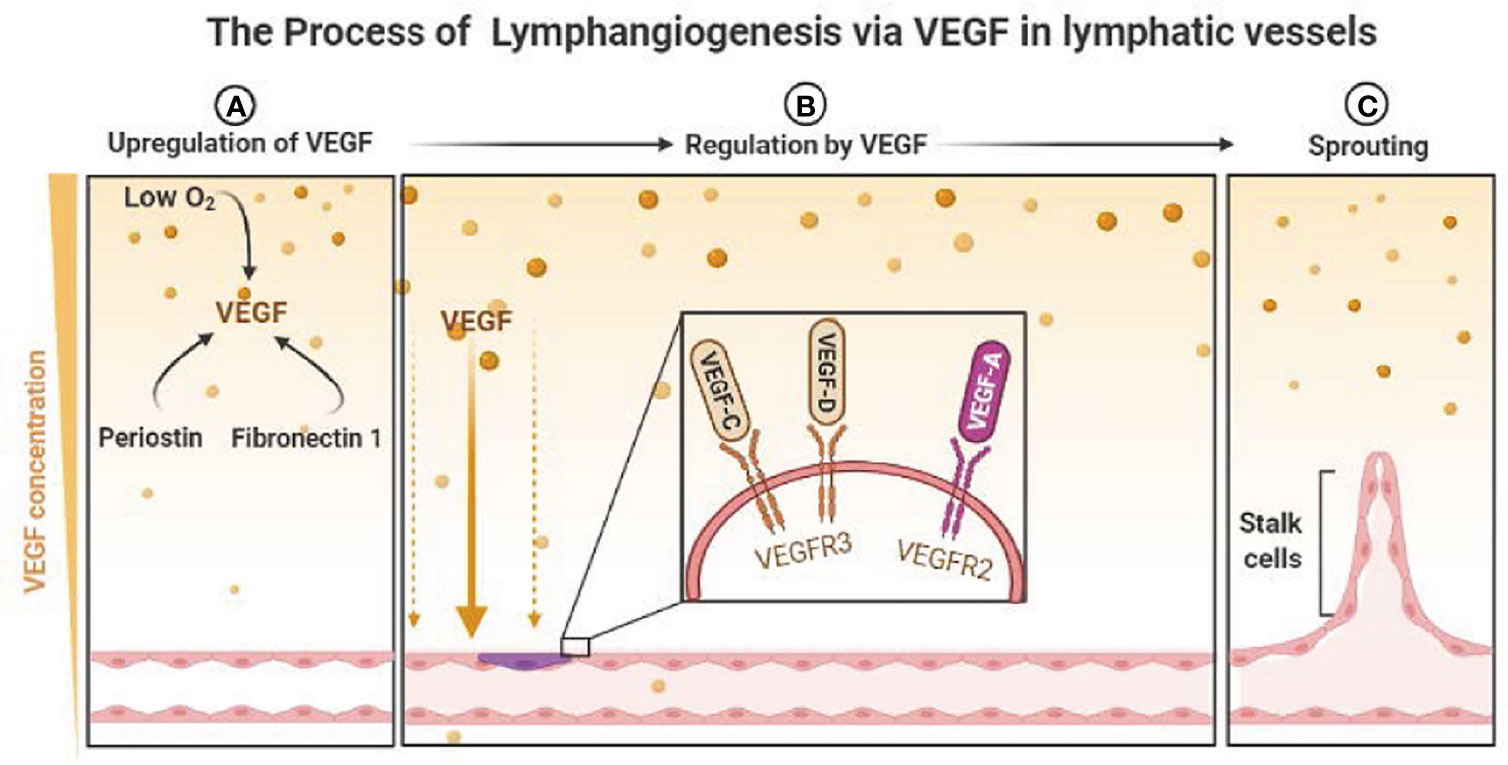
Figure 1 The Process of Lymphangiogenesis via VEGF in lymphatic vessels. (A) Low O2 and some molecular induce the upregulation of VEGF. (B) The regulation of lymphangiogenesis by the link of VEGFs and VEGFRs. (C) The sprouting of lymphatic vessels. (P.S. Created with https://BioRender.com).
In a study by Kudo et al. (27), periostin enhanced lymphangiogenesis by increasing the tumor secretion of VEGF-C and diffusion and tube creation in LECs via Src and Akt activation. Importantly, periostin itself can directly promote lymphangiogenesis by binding integrin-αvβ3. And according to their result, serum periostin levels in patients with HNSCC were found to be strongly linked to VEGF-C levels and malignant characteristics, such as advanced tumors and LN metastases. Ching-Chia Lin et al. (43) have demonstrated that WNT1-inducible signaling pathway protein-1(WISP-1) can promotes VEGF-C expression in OSCC cells through the ILK/Akt pathway and WISP-1 induces VEGF-C production by inhibiting miR-300 expression, which lead to VEGF-C-dependent lymphangiogenesis. Moreover, research of Yoshihiro Morita (44) demonstrates that elevated expression of cellular Fibronectin 1 (FN1) and following activation of focal adhesion kinase (FAK) lead to increased VEGF-C expression, lymphangiogenesis, LNM and promoted epithelial-mesenchymal transition (EMT) in OSCC cells and suggest that FN1-phosphorylated FAK signaling cascade is a potential therapeutic target in the treatment of LNM in OSCC (44).
Furthermore, research of Hu An et al. (45) showed that sirtuin 2 (SIRT2) inhibits hypoxia-induced VEGF-D synthesis in head and neck cancer (HNC) cells, and expression of SIRT2 was significantly linked to VEGF-D expression and lymphangiogenesis in HNC tissue, where a substantial fraction of SIRT2 protein was produced at a lower level. They have investigated SIRT2 mediated regulation of VEGF-D expression and lymphangiogenesis by deacetylating endothelial PAS domain protein 1 (EPAS1). However, in individuals with HNC, increased SIRT2 levels are associated with a worse prognosis.
In addition, VEGF-A has been shown to induce lymphangiogenesis and SLN metastasis of OSCC cells in vitro, and 3-O-acetyloleanolic acid (3AOA) has been shown to inhibit tumor growth, tumor-induced lymphangiogenesis, and SLN metastasis in a VEGF-A-induced oral cancer sentinel lymph node (OCSLN) animal model created with high expression of VEGF-A in squamous cell carcinoma (SCCVII) cells (26). The anti-lymphangiogenic effects of 3AOA are mediated via suppression of VEGF-A/VEGFR-1 and VEGFR-2 signaling.
Despite the fact that lymphatic spread is an important event in the progression of HNSCC, the levels of VEGF-C, VEGF-D, and VEGFR-3 are not related to the clinicopathological characteristics reported in other studies, suggesting that lymphangiogenesis in HNSCC is mediated by other signaling pathways (46).
To identify non-VEGF-mediated molecular mechanisms that lead to tumor lymphangiogenesis and LN metastasis, a plethora of other growth factors have been researched (28) (Table 1).
In one study, VEGFR-3 expression increased in LECs after treatment with angiopoietin-1 (Ang-1), implying that Ang-1 promotes lymphangiogenesis by making lymphatic capillaries more sensitive to VEGF-C or VEGF-D (29). In another study, overexpression of Ang-1 and Ang-2 was linked to a worse prognosis in OSCC (30). However, studies on the potential role of Ang-2 in the lymphangiogenesis of HNSCC are lacking.
Wang et al. discovered that the secreted EVs in patients with OSCC with LN metastases was considerably elevated (31). Low expression of laminin-332 in LN1-1 cells reduce EV-mediated LEC migration, lymphangiogenesis, and LN metastasis. The study showed that knocking down integrin-3 resulted in a decrease in the role of laminin-γ2-enriched EVs, implying that integrin is required for EV uptake by LECs.
Furthermore, insulin-like growth factor (IGF)-1 and IGF-2 can promote a signaling pathway that is distinct from that induced by the VEGF-C/VEGF-D–VEGFR-3 system, showing that these two growth factors have direct lymphangiogenic activity (28). Both substances promote LEC proliferation and migration by phosphorylating intracellular signaling components (47), which can lead to lymphatic dissemination, metastasis, and tumor recurrence. Enhanced IGF-1 receptor (IGF-1R) expression has been observed in initial undifferentiated oropharyngeal and nasopharyngeal cancers, as well as in LN metastases (48), and is related to high metastasis and recurrence rates (49).
Additionally, Vyomesh Patel et al. (50) show that the activation of mTOR is a critical event which induces lymphangiogenesis in HNSCC. Furthermore, the prolonged treatment with rapamycin and rapalogRAD001 diminished the dissemination of HNSCC cancer cells to the cervical lymph nodes in a newly developed orthotopic HNSCC model, thereby prolonging animal survival. Thus, the use of mTOR inhibitors may represent a novel molecular-targeted approach for metastasis prevention in patients with HNSCC. In the study of SATOMI ARIMOTO et al. (51), their results clearly showed that podoplanin and lymphatic vessel endothelial hyaluronan receptor 1(LYVE-1) were expressed in most of the OSCC cases and were strongly associated with lymphangiogenesis. Podoplanin and LYVE-1 may be used for predicting lymphatic status in OSCC in the future, while there is no consensus, yet. Moreover, Jiajia Li et al. (52) have discovered that 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) was correlated with lymphangiogenesis in OSCC, PFKFB3 may promote LNM by regulating the expression of podoplanin (PDPN). However, the real role of PFKFB3 in lymphangiogenesis remains need further researches (52).
EVs are lipid bilayer particles with dimensions ranging from 30 nanometers to several micrometers (53), and they comprise a heterogenous population, with differences in biogenesis, size and contents between different subpopulations. Based on these parameters, EVs are generally classified as large (large extracellular vesicles (lEVs)), including apoptotic bodies, large oncosomes and microvesicles; small (small extracellular vesicles (sEVs)), including exosomes; or extracellular particles (EPs), including exomeres and chromatimeres (54). The term “extracellular vesicle” or “EV” has now been agreed on by the international community as the consensus generic term for lipid bilayer-delimited particles released from the cell (55, 56). EVs contain proteins, messenger RNA (mRNA), and noncoding microRNA (miRNA) (57, 58), the composition of an EV depends on the cell type and the physiological/pathological setting, the pathological setting can also influence the cytokine profile associated with EVs (59, 60). EVs were once assumed to be a route for cells to dispose of unwanted materials; however, recent research has shown that EVs are crucial for strictly regulated bidirectional communication (61, 62). EVs have recently attracted considerable attention because their molecular/genetic profiles have been found to be similar to those of the original cells (63, 64). Moreover, due to the selective sorting of cargo into EVs, the inherent features of EVs can differ from those of their cells of origin (65). EVs are secreted by nearly every cell, and their form, source, and molecular composition vary (66). Hence, EVs have potential as circulating biomarkers that carry information about the tissue-bound parent cells’ molecular composition and activity (67). For instance, if a cancer cell produces EVs, it may be possible to detect these cancer-derived EVs in the plasma and use them as an inspection index in the diagnosis of cancer (68, 69).
Organotropism is a condition that describes how circulating tumor cells homing to certain organs as a result of complicated tumor–stroma interactions, however, the exact potential mechanism is not clear yet (70). According to other researches, sEVs may play a critical role in organotropism. sEVs not only recruit bone marrow-derived cells, endothelial progenitor cells, and mesenchymal cells to generate an appropriate niche environment, but they also cause the overexpression of proinflammatory chemicals and facilitate vascular leakiness (10, 71). Prior to the entrance of cancer cells, these changes in distant organs have already happened. Interestingly, melanoma sEVs tend to move to sentinel lymph nodes, breast cancer cells sEVs to the lung, and pancreatic cancer cells sEVs to the liver, according to multiple studies (72–74). Thus, the questions arise of why and how sEVs are directed to specific sites to enable organotropic metastasis may explain the role of EVs in the lymph node pre-metastatic niche formation.
It has been demonstrated that tumor-secreted EVs can communicate with neighboring non-tumor cells (75, 76), even they can transport oncogenic molecules to normal cells which lead to development of tumor (60). Ferdinando Pucci et al. (77) have showed that endogenous tumor-secreted EVs efficiently disseminate via lymphatics in mice and humans. And tumor-secreted EVs induce vascular leakiness and facilitate circulating tumor cell arrival to distant sites, accumulating researches confirm that vascular leakiness is considered a hallmark of pre-metastatic niche formation (10, 60, 78). EVs have been extensively explored and well documented in the literature in terms of how they regulate pre-metastatic niche development in various cancers (73). For instance, it has been found that sEVs derived from metastatic melanoma cell lines are rich in nerve growth factor receptor, and can enhance lymphangiogenesis, tumor cell adhesion, pre-metastatic niche formation, and LN metastasis (79); Noelle Leary et al. identify EVs-mediated melanoma—LN LEC communication as a new pathway involved in tumor progression and tumor immune inhibition (80). And the study of Li et al. showed that exosomal CXC chemokine recepter-4 from Hca-F cells promoted LECs proliferative rate and lymphatic tube formation ability (81). The lymphatic network remodeling may guide tumor metastasis in SLNs, and sun et al. found that CT26 cell exosomes promote the proliferation of lymphatic endothelial cells and the formation of lymphatic network in SLN, facilitating the SLN metastasis of colorectal cancer, which demonstrates tumor-derived exosomes can modify the microenvironment in adjacent organs and initiate a premetastatic niche (82). Moreover, Zhou et al. identify that cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting vasohibin-1-secreted exosomal miR-221-3p transfers into lymphatic endothelial cells to promote lymphangiogenesis and lymphatic metastasis via downregulation of VASH1 (83).
Nowadays, HNSCC-derived EVs have received more and more attention (84). Cancer cells release EVs containing immunoregulatory factors, affecting the tumor microenvironment by mediating immune escape and playing a crucial role in the formation of the premetastatic niche (85, 86). And HNSCC is one of the most immunosuppressive human tumors, LNM is the most important prognostic determinant of HNSCC tumors in the survival rate of patients (87), and tumor-derived EVs and communication with the tumor microenvironment are critical factors in tumor metastasis (88). Apart from lymphangiogenesis, tumor-derived EVs can mediate the formation of pre-metastatic niche by other mechanisms in HNSCC. Chan et al. reported that EVs derived from nasopharyngeal carcinoma cells could markedly enhance the tubulogenesis, migration and invasion of human umbilical vein endothelial cells (89). Recent studies have shown that EVs rich in PFKFB3, MMP-13, intercellular cell adhesion molecule-1 or thrombospondin-1 can enhance the release of VEGF-A, IL-8 and then downregulate junction-related proteins, which promote tumor angiogenesis and vascular permeability and become a potential channel system for distant metastasis of tumor cells (89–91). The incidence of HPV (+) HNSCC has risen sharply in recent decades (92), while HPV (+) HNSCC responded better to treatment and had a significantly better prognosis than HPV (-) HNSCC (93). HPV (+) HNSCC EVs stimulated dendritic cells maturation and HPV (-) HNSCC suppressed it instead, which is critical for the good prognosis of HPV (+) HNSCC (94–96). Furthermore, it was found that the most abundant miRNA in HPV (+) EVs was miRNA-363-3p (97). Notably, in OSCC cells expressing miRNA-363-5p, cell proliferation decreased by 40–50% (98). These results suggest that intercellular communication mediated by HPV (+) EVs might play a dominant role in antitumor immune responses and inhibit tumor proliferation, which may provide a new treatment for HPV (+) HNSCC. According to the study of T. Whiteside et al., levels of PD-L1 carried by exosomes correlated with the lymph node status, and blocking of PD-L1+ exosome signaling to PD-1+ T cells attenuated immune suppression (99). Body fluids of patients with HNSCC are enriched in exosomes that reflect properties of the tumor, recent research of T. Whiteside et al. found that the purine metabolite levels in exosomes decreased in patients with advanced cancer and nodal involvement, their report provides the first evidence that HNSCC cells shuttle purine metabolites in exosomes, with immunosuppressive adenosine being the most prominent purine (100). Furthermore, related articles highlight the role of tumor-derived EVs in HNSCC: EVs mediate immune suppression and tumor progression by reducing the proliferation of CD8+ T cells and promoting the expansion, suppressive activity, and resistance of apoptosis of regulatory T cells (101).
In summary, EVs work in a coordinated and planned manner to enhance tumor survival, re-educate immune cells, and generate pre-metastatic microenvironment (60). As a natural nanoscale vesicle, EVs can pass through the interstitial matrix entering the lymphatic circulation (102), which makes EVs ideal carriers for message transport between the lymphatic system and tumor cells, then prepare a lymph node pre-metastatic niche for HNSCC metastasis (Figure 2). Future research is needed to better understand the features and mechanisms driving EV production, trafficking, and uptake that are particular to HNSCC to fully comprehend their impact on disease development and progression. To completely correlate cause and function, an understanding of the presence of EV subpopulations is also required. For example, a recent study identified a new type of EV, the supermere, that has potential as a circulating biomarker and therapeutic target for a variety of diseases in the future (103).
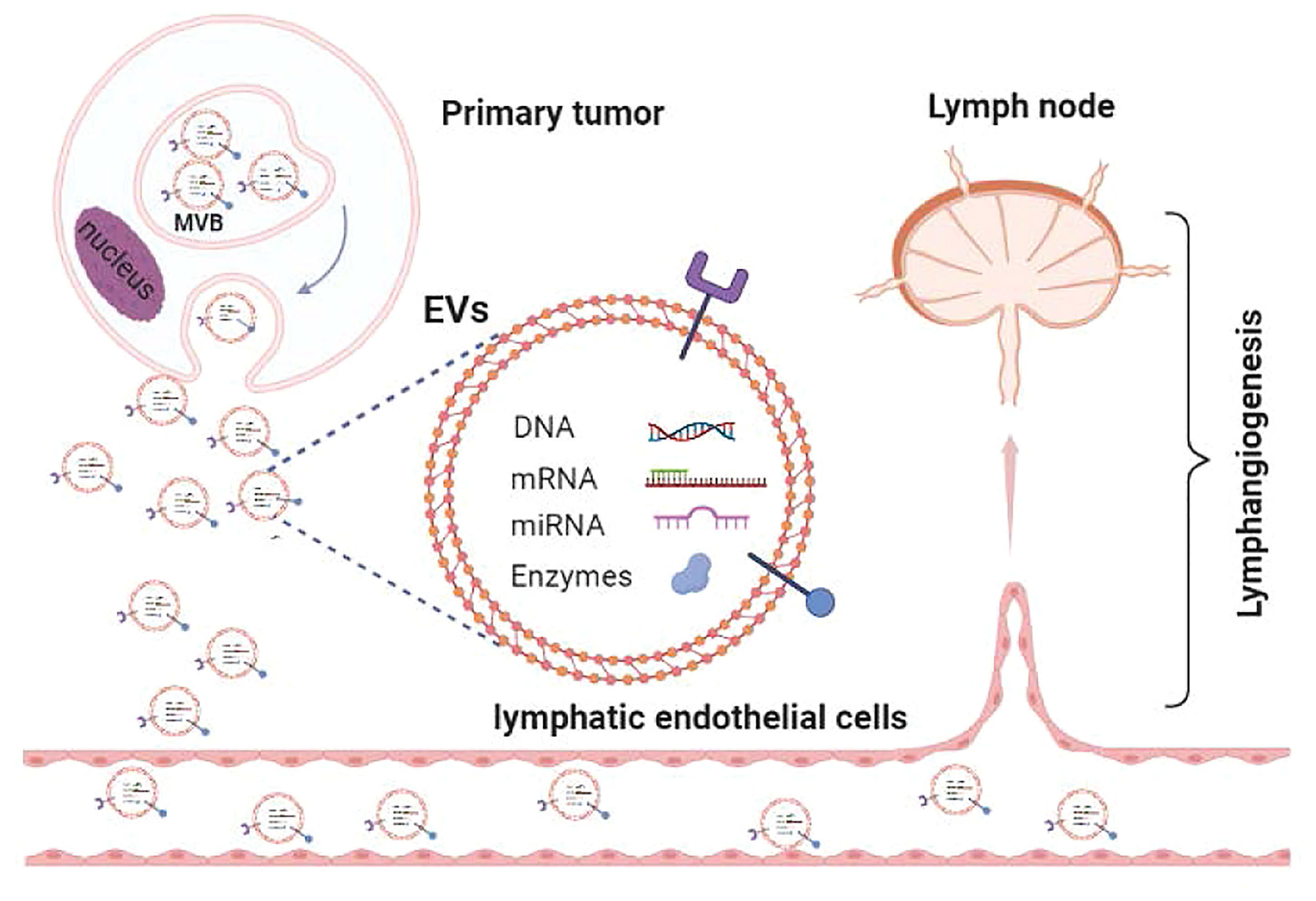
Figure 2 The role of EVs in regulating lymph node pre-metastatic niche formation in HNSCC. (P.S. Created with BioRender.com).
Given that lymphangiogenesis plays a critical role in LN metastasis, it may be viable to treat cancers using an anti-lymphangiogenesis strategy (104). Some receptor tyrosine kinase inhibitors may exert negative effects on tumor-related lymphangiogenesis (and LN and distant organ metastases) by targeting the VEGF-C–VEGFR3 signaling pathway (105, 106). Many tyrosine kinase receptors are targeted by receptor tyrosine kinase inhibitors, yet it is difficult to determine which elements and pathways are involved in lymphangiogenesis.
A related study demonstrated that adipose stem cells treated with VEGF-C secreted exosomes, and this more efficient lymphangiogenic response revealed a potential therapeutic modality for increasing the efficiency of anti-lymphangiogenesis (107). Furthermore, it has been found that LECs with laminin-γ2-enriched EVs can improve lymphangiogenesis in vitro, and that the lymphangiogenesis resulting from the activity of EV-mediated LECs can be reduced by lowering the laminin-γ2 level (31). This unique approach to anti-lymphangiogenesis therapy via EV targeting requires additional research and validation. Furthermore, the angiostatic N-terminal 16 kDa fragment of human prolactin has been shown to induce apoptosis and inhibit lymphangiogenesis in microvascular dermal LECs (108). After treatment with the angiostatic fragment of prolactin, the density of the LVs of the primary tumor and the LNs was considerably reduced in a melanoma model. That study was the first to demonstrate that prolactin plays a critical role in lymphangiogenesis, and this finding could have implications for general therapeutic strategies (108). Related research demonstrated that a selective histone deacetylase (HDAC) 1/2 inhibitor (B390) not only restrains tumor growth by inducing apoptosis of tumor cells but also inhibits lymphangiogenesis and LV invasion in vivo (109). Liu et al. found that overexpression of miR-320b is closely linked to peritumoral lymphangiogenesis and LN metastasis, and it is also worth mentioning that the miR-320b–PDCD4 axis activates the Akt pathway independent of VEGF-C (110). The anti-lymphangiogenesis strategy applying in the clinical treatment of HNSCC, however, not common so far.
On a more positive note, because EVs can traverse membranes in the tumor microenvironment by fusion and/or endocytosis (111), they have the potential to be used as biological drug delivery vehicles (112). For instance, tumor-derived EVs mediate the delivery of miRNA-9 to inhibit angiogenesis by targeting midkine gene and regulating the PDK/AKT pathway nasopharyngeal carcinoma. Additionally, the miRNA-9 levels in EVs were positively associated with overall survival, while midkine gene overexpression was positively correlated with poor prognosis in nasopharyngeal carcinoma patients. Thus, we can conclude that miRNA-9 can inhibit tumor angiogenesis, providing a new direction for anticancer treatment (113). In the future, EVs may play a significant role in cancer treatment of HNSCC.
The abovementioned studies show limitations of the anti-lymphangiogenic therapy in HNSCC based on the lack of studies that evidence its potential efficacy in this tumor type. While with further researches, anti-lymphangiogenesis may become a treatment option for HNSCC in the future.
The pre-metastatic niche is gradually being recognized as a tumor-induced circumstance that facilitates tumor cell dissemination and metastasis production. The development of a pre-metastatic niche is a complicated process, yet it is an essential step in the metastatic cascade, occurring prior to tumor cell colonization. Recent research conducted with numerous tumor models has identified many critical elements that play important roles in pre-metastatic niche formation. The present study has focused on LN pre-metastatic niche creation in HNSCC, which is dependent on lymphangiogenesis. However, paying attention to a single biological mechanism, such as lymphangiogenesis, or a single molecular pathway is likely to lead to failure in terms of therapeutic development.
Many questions remain unanswered: What are the stages of pre-metastatic development in LNs? Which significant element could be utilized for tumor diagnosis and/or to assess the effect that tumors have on the development of LN and distant metastases? It is also essential to identify the optimal therapeutic molecular target(s) and the factors that are critical for cross-talk between tumors and LNs.
Although accumulating studies have prompted the understanding of LN pre-metastatic niche formation in HNSCC, many problems need to be further elucidated. Further research is needed to elucidate the basic mechanisms/characteristics of anti-lymphangiogenesis in HNSCC. Due to the key role of lymphangiogenesis in pre-metastatic niche formation, more researches are needed in this field to explore the potential of anti-lymphangiogenesis in HNSCC treatment, which can support new strategy for the patients.
CH: Conceptualization, Writing - original draft, Writing - review and editing. QH: Conceptualization, Writing - original draft, Writing - review and editing. QS: Conceptualization, Resources, Supervision, Writing - review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LN, lymph node; LNM, Lymph node metastasis; EVs, extracellular vesicles; HNSCC, head and neck squamous cell carcinoma; LV, lymphatic vessel; SLN, sentinel lymph node; VEGFs, vascular endothelial growth factors; LECs, lymphatic endothelium cells; FN1, Fibronectin 1; SIRT2, sirtuin 2; HNC, head and neck cancer; Ang-1, angiopoietin-1; OSCC, oral squamous cell carcinoma; IGF, insulin-like growth factor; sEVs, small extracellular vesicles.
1. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR, et al. Head and Neck Squamous Cell Carcinoma. Nat Rev Dis Primers (2020) 6(1):92.
2. Xing Y, Zhang J, Lin H, Gold KA, Sturgis EM, Garden AS, et al. Relation Between the Level of Lymph Node Metastasis and Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma. Cancer (2016) 122(4):534–45.
3. Zhang Q, Xiong Y, Lin L, Yuan K. Analysis of Related Factors of Surgical Treatment Effect on 215 Patients With Laryngeal Cancer. Exp Ther Med (2018) 15(3):2786–91.
4. Padera TP, Meijer EF, Munn LL. The Lymphatic System in Disease Processes and Cancer Progression. Annu Rev Biomed Eng (2016) 18:125–58.
5. Petrova TV, Koh GY. Biological Functions of Lymphatic Vessels. Sci (New York NY) (2020) 369(6500):eaax4063.
6. Hart IR, Fidler IJ. Role of Organ Selectivity in the Determination of Metastatic Patterns of B16 Melanoma. Cancer Res (1980) 40(7):2281–7. doi: 10.1016/S0006-3495(96)79598-X
7. Fidler IJ, Kripke ML. Metastasis Results From Preexisting Variant Cells Within a Malignant Tumor. Sci (New York NY) (1977) 197(4306):893–5.
9. Psaila B, Kaplan RN, Port ER, Lyden D. Priming the 'Soil' for Breast Cancer Metastasis: The Pre-Metastatic Niche. Breast Dis (2006) 26:65–74.
10. Psaila B, Lyden D. The Metastatic Niche: Adapting the Foreign Soil. Nat Rev Cancer (2009) 9(4):285–93.
11. Parayath NN, Padmakumar S, Amiji MM. Extracellular Vesicle-Mediated Nucleic Acid Transfer and Reprogramming in the Tumor Microenvironment. Cancer Lett (2020) 482:33–43.
12. Sleeman JP. The Lymph Node Pre-Metastatic Niche. J Mol Med (Berlin Germany) (2015) 93(11):1173–84.
13. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat Rev Cancer (2017) 17(5):302–17.
14. Chatterjee G, Pai T, Hardiman T, Avery-Kiejda K, Scott RJ, Spencer J, et al. Molecular Patterns of Cancer Colonisation in Lymph Nodes of Breast Cancer Patients. Breast Cancer Res (2018) 20(1):143.
15. Balsat C, Blacher S, Herfs M, Van de Velde M, Signolle N, Sauthier P, et al. A Specific Immune and Lymphatic Profile Characterizes the Pre-Metastatic State of the Sentinel Lymph Node in Patients With Early Cervical Cancer. Oncoimmunology (2017) 6(2):e1265718.
16. Wakisaka N, Hasegawa Y, Yoshimoto S, Miura K, Shiotani A, Yokoyama J, et al. Primary Tumor-Secreted Lymphangiogenic Factors Induce Pre-Metastatic Lymphvascular Niche Formation at Sentinel Lymph Nodes in Oral Squamous Cell Carcinoma. PloS One (2015) 10(12):e0144056.
17. Tammela T, Alitalo K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell (2010) 140(4):460–76.
18. Maus RLG, Jakub JW, Hieken TJ, Nevala WK, Christensen TA, Sutor SL, et al. Identification of Novel, Immune-Mediating Extracellular Vesicles in Human Lymphatic Effluent Draining Primary Cutaneous Melanoma. Oncoimmunology (2019) 8(12):e1667742.
19. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG, et al. Lymphangiogenesis and Lymphatic Vessel Remodelling in Cancer. Nat Rev Cancer (2014) 14(3):159–72.
20. Lee SK, Cho EY, Kim WW, Kim SH, Hur SM, Kim S, et al. The Prediction of Lymph Node Metastasis in Ductal Carcinoma In Situ With Microinvasion by Assessing Lymphangiogenesis. J Surg Oncol (2010) 102(3):225–9.
21. Coşkun U, Akyürek N, Dursun A, Yamac D. Peritumoral Lymphatic Microvessel Density Associated With Tumor Progression and Poor Prognosis in Gastric Carcinoma. J Surg Res (2010) 164(1):110–5.
22. Garcia-Carracedo D, Rodrigo JP, Astudillo A, Nieto CS, Gonzalez MV. Prognostic Significance of Lymphangiogenesis in Pharyngolaryngeal Carcinoma Patients. BMC Cancer (2010) 10:416.
23. Audet N, Beasley NJ, Macmillan C, Jackson DG, Gullane PJ, Kamel-Reid S, et al. Lymphatic Vessel Density, Nodal Metastases, and Prognosis in Patients With Head and Neck Cancer. Arch Otolaryngol Head Neck Surg (2005) 131(12):1065–70.
24. Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor Lymphangiogenesis Correlates With Lymph Node Metastasis and Clinicopathologic Parameters in Oral Squamous Cell Carcinoma. Cancer (2007) 110(6):1287–94.
25. Wang CA, Tsai SJ. Regulation of Lymphangiogenesis by Extracellular Vesicles in Cancer Metastasis. Exp Biol Med (Maywood) (2021) 246(19):2048–56.
26. Hwang-Bo J, Bae MG, Park JH, Chung IS. 3-O-Acetyloleanolic Acid Inhibits VEGF-A-Induced Lymphangiogenesis and Lymph Node Metastasis in an Oral Cancer Sentinel Lymph Node Animal Model. BMC Cancer (2018) 18(1):714.
27. Kudo Y, Iizuka S, Yoshida M, Nguyen PT, Siriwardena SB, Tsunematsu T, et al. Periostin Directly and Indirectly Promotes Tumor Lymphangiogenesis of Head and Neck Cancer. PloS One (2012) 7(8):e44488.
28. Karatzanis AD, Koudounarakis E, Papadakis I, Velegrakis G. Molecular Pathways of Lymphangiogenesis and Lymph Node Metastasis in Head and Neck Cancer. Eur Arch Otorhinolaryngol (2012) 269(3):731–7.
29. Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, et al. Angiopoietin-1 Promotes Lymphatic Sprouting and Hyperplasia. Blood (2005) 105(12):4642–8.
30. Chien CY, Su CY, Chuang HC, Fang FM, Huang HY, Chen CM, et al. Angiopoietin-1 and -2 Expression in Recurrent Squamous Cell Carcinoma of the Oral Cavity. J Surg Oncol (2008) 97(3):273–7.
31. Wang SH, Liou GG, Liu SH, Chang JS, Hsiao JR, Yen YC, et al. Laminin γ2-Enriched Extracellular Vesicles of Oral Squamous Cell Carcinoma Cells Enhance In Vitro Lymphangiogenesis via Integrin α3-Dependent Uptake by Lymphatic Endothelial Cells. Int J Cancer (2019) 144(11):2795–810.
32. Roy H, Bhardwaj S, YLä-Herttuala S. Biology of Vascular Endothelial Growth Factors. FEBS Lett (2006) 580(12):2879–87.
33. Yan S, Wang H, Chen X, Liang C, Shang W, Wang L, et al. MiR-182-5p Inhibits Colon Cancer Tumorigenesis, Angiogenesis, and Lymphangiogenesis by Directly Downregulating VEGF-C. Cancer Lett (2020) 488:18–26.
34. Ferrara N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr Rev (2004) 25(4):581–611.
35. Li S, Li Q. Cancer Stem Cells, Lymphangiogenesis, and Lymphatic Metastasis. Cancer Lett (2015) 357(2):438–47.
36. Nogues L, Benito-Martin A, Hergueta-Redondo M, Peinado H. The Influence of Tumour-Derived Extracellular Vesicles on Local and Distal Metastatic Dissemination. Mol Asp Med (2018) 60:15–26.
37. Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of Tumor Lymphangiogenesis by Vegf-C Promotes Breast Cancer Metastasis. Nat Med (2001) 7(2):192–8.
38. Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular Endothelial Growth Factor C Promotes Tumor Lymphangiogenesis and Intralymphatic Tumor Growth. Cancer Res (2001) 61(5):1786–90. doi: 10.1046/j.1523-5394.2001.009002104.x
39. Thelen A, Scholz A, Benckert C, von Marschall Z, Schröder M, Wiedenmann B, et al. VEGF-D Promotes Tumor Growth and Lymphatic Spread in a Mouse Model of Hepatocellular Carcinoma. Int J Cancer (2008) 122(11):2471–81.
40. Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M, et al. VEGF-A Induces Tumor and Sentinel Lymph Node Lymphangiogenesis and Promotes Lymphatic Metastasis. J Exp Med (2005) 201(7):1089–99.
41. Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H, et al. Expression of Vascular Endothelial Growth Factor A, B, C, and D in Oral Squamous Cell Carcinoma. Oral Oncol (2004) 40(1):13–20.
42. Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, et al. VEGF-C and VEGF-D Expression Is Correlated With Lymphatic Vessel Density and Lymph Node Metastasis in Oral Squamous Cell Carcinoma: Implications for Use as a Prognostic Marker. Int J Oncol (2009) 34(3):673–80.
43. Lin CC, Chen PC, Lein MY, Tsao CW, Huang CC, Wang SW, et al. WISP-1 Promotes VEGF-C-Dependent Lymphangiogenesis by Inhibiting miR-300 in Human Oral Squamous Cell Carcinoma Cells. Oncotarget (2016) 7(9):9993–10005.
44. Morita Y, Hata K, Nakanishi M, Omata T, Morita N, Yura Y, et al. Cellular Fibronectin 1 Promotes VEGF-C Expression, Lymphangiogenesis and Lymph Node Metastasis Associated With Human Oral Squamous Cell Carcinoma. Clin Exp Metastasis (2015) 32(7):739–53.
45. Hu A, Yang LY, Liang J, Lu D, Zhang JL, Cao FF, et al. SIRT2 Modulates VEGFD-Associated Lymphangiogenesis by Deacetylating EPAS1 in Human Head and Neck Cancer. Mol Carcinog (2020) 59(11):1280–91.
46. De Sousa EA, Lourenço SV, De Moraes FP, Vartanian JG, Gonçalves-Filho J, Kowalski LP, et al. Head and Neck Squamous Cell Carcinoma Lymphatic Spread and Survival: Relevance of Vascular Endothelial Growth Factor Family for Tumor Evaluation. Head Neck (2015) 37(10):1410–6.
47. Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, et al. Insulin-Like Growth Factors 1 and 2 Induce Lymphangiogenesis In Vivo. Proc Natl Acad Sci USA (2005) 102(43):15593–8.
48. Friedrich RE, Hagel C, Bartel-Friedrich S. Insulin-Like Growth Factor-1 Receptor (IGF-1R) in Primary and Metastatic Undifferentiated Carcinoma of the Head and Neck: A Possible Target of Immunotherapy. Anticancer Res (2010) 30(5):1641–3. doi: 10.1245/s10434-009-0891-9
49. Yuan Y, Zhou X, Song J, Qiu X, Li J, Ye L, et al. Expression and Clinical Significance of Epidermal Growth Factor Receptor and Type 1 Insulin-Like Growth Factor Receptor in Nasopharyngeal Carcinoma. Ann Otol Rhinol Laryngol (2008) 117(3):192–200.
50. Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, et al. Decreased Lymphangiogenesis and Lymph Node Metastasis by mTOR Inhibition in Head and Neck Cancer. Cancer Res (2011) 71(22):7103–12.
51. Arimoto S, Hasegawa T, Takeda D, Saito I, Amano R, Akashi M, et al. Lymphangiogenesis and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Anticancer Res (2018) 38(11):6157–62.
52. Li J, Tang X. Increased Expression of PFKFB3 in Oral Squamous Cell Carcinoma and Its Association With Lymphangiogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol (2021) 132(1):57–65.
53. Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, Biogenesis and Function. Nat Rev Immunol (2002) 2(8):569–79.
54. Malkin EZ, Bratman SV. Bioactive DNA From Extracellular Vesicles and Particles. Cell Death Dis (2020) 11(7):584.
55. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles (2018) 7(1):1535750.
56. Witwer KW, Théry C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J Extracell Vesicles (2019) 8(1):1648167.
57. Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal Stem Cell Exosomes: A Two-Edged Sword in Cancer Therapy. Int J Nanomed (2019) 14:2847–59.
58. Huang Q, Yang J, Zheng J, Hsueh C, Guo Y, Zhou L, et al. Characterization of Selective Exosomal microRNA Expression Profile Derived From Laryngeal Squamous Cell Carcinoma Detected by Next Generation Sequencing. Oncol Rep (2018) 40(5):2584–94.
59. Colombo M, Raposo G, Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol (2014) 30:255–89.
60. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D, et al. Extracellular Vesicles in Cancer: Cell-To-Cell Mediators of Metastasis. Cancer Cell (2016) 30(6):836–48.
61. Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular Vesicles-Mediated Intercellular Communication: Roles in the Tumor Microenvironment and Anti-Cancer Drug Resistance. Mol Cancer (2019) 18(1):55.
62. Pretti MAM, Bernardes SS, Da Cruz JGV, Boroni M, Possik PA. Extracellular Vesicle-Mediated Crosstalk Between Melanoma and the Immune System: Impact on Tumor Progression and Therapy Response. J Leukoc Biol (2020) 108(4):1101–15.
63. Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol (2016) 36(3):301–12.
64. Huang Q, Hsueh CY, Guo Y, Wu XF, Li JY, Zhou L, et al. Lack of miR-1246 in Small Extracellular Vesicle Blunts Tumorigenesis of Laryngeal Carcinoma Cells by Regulating Cyclin G2. IUBMB Life (2020) 72(7):1491–503.
65. Lara P, Chan AB, Cruz LJ, Quest AFG, Kogan MJ.. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery Towards Specific Cells and Tissues. Pharmaceutics (2020) 12(11):1022.
66. Cocucci E, Meldolesi J. Ectosomes and Exosomes: Shedding the Confusion Between Extracellular Vesicles. Trends Cell Biol (2015) 25(6):364–72.
67. Broggi MAS, Maillat L, Clement CC, Bordry N, Corthésy P, Auger A, et al. Tumor-Associated Factors are Enriched in Lymphatic Exudate Compared to Plasma in Metastatic Melanoma Patients. J Exp Med (2019) 216(5):1091–107.
68. Cao J, Zhang M, Xie F, Lou J, Zhou X, Zhang L, et al. Exosomes in Head and Neck Cancer: Roles, Mechanisms and Applications. Cancer Lett (2020) 494:7–16.
69. Huang Q, Hsueh CY, Shen YJ, Guo Y, Huang JM, Zhang YF, et al. Small Extracellular Vesicle-Packaged Tgfβ1 Promotes the Reprogramming of Normal Fibroblasts Into Cancer-Associated Fibroblasts by Regulating Fibronectin in Head and Neck Squamous Cell Carcinoma. Cancer Lett (2021) 517:1–13.
70. Seibold T, Waldenmaier M, Seufferlein T, Eiseler T. Small Extracellular Vesicles and Metastasis-Blame the Messenger. Cancers (2021) 13(17):4380.
71. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells Toward a Pro-Metastatic Phenotype Through MET. Nat Med (2012) 18(6):883–91.
72. Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM, et al. Imaging Exosome Transfer From Breast Cancer Cells to Stroma at Metastatic Sites in Orthotopic Nude-Mouse Models. Adv Drug Deliv Rev (2013) 65(3):383–90.
73. Hood JL, San RS, Wickline SA. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res (2011) 71(11):3792–801.
74. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat Cell Biol (2015) 17(6):816–26.
75. Cho JA, Park H, Lim EH, Lee KW.. Exosomes From Breast Cancer Cells can Convert Adipose Tissue-Derived Mesenchymal Stem Cells Into Myofibroblast-Like Cells. Int J Oncol (2012) 40(1):130–8. doi: 10.3892/ijo.2011.1193
76. Srinivasan S, Vannberg FO, Dixon JB. Lymphatic Transport of Exosomes as a Rapid Route of Information Dissemination to the Lymph Node. Sci Rep (2016) 6:24436.
77. Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. SCS Macrophages Suppress Melanoma by Restricting Tumor-Derived Vesicle-B Cell Interactions. Sci (New York NY) (2016) 352(6282):242–6.
78. Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary Vascular Destabilization in the Premetastatic Phase Facilitates Lung Metastasis. Cancer Res (2009) 69(19):7529–37.
79. García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V, et al. Melanoma-Derived Small Extracellular Vesicles Induce Lymphangiogenesis and Metastasis Through an NGFR-Dependent Mechanism. Nat Cancer (2021) 2(12):1387–405.
80. Leary N, Walser S, He Y, Cousin N, Pereira P, Gallo A, et al. Melanoma-Derived Extracellular Vesicles Mediate Lymphatic Remodelling and Impair Tumour Immunity in Draining Lymph Nodes. J Extracell Vesicles (2022) 11(2):e12197.
81. Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, et al. Horizontal Transfer of Exosomal CXCR4 Promotes Murine Hepatocarcinoma Cell Migration, Invasion and Lymphangiogenesis. Gene (2018) 676:101–9.
82. Sun B, Zhou Y, Fang Y, Li Z, Gu X, Xiang J, et al. Colorectal Cancer Exosomes Induce Lymphatic Network Remodeling in Lymph Nodes. Int J Cancer (2019) 145(6):1648–59.
83. Zhou CF, Ma J, Huang L, Yi HY, Zhang YM, Wu XG, et al. Cervical Squamous Cell Carcinoma-Secreted Exosomal miR-221-3p Promotes Lymphangiogenesis and Lymphatic Metastasis by Targeting VASH1. Oncogene (2019) 38(8):1256–68.
84. Wang X, Guo J, Yu P, Guo L, Mao X, Wang J, et al. The Roles of Extracellular Vesicles in the Development, Microenvironment, Anticancer Drug Resistance, and Therapy of Head and Neck Squamous Cell Carcinoma. J Exp Clin Cancer Res CR (2021) 40(1):35.
85. Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-Cancer-Secreted miR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat Cell Biol (2015) 17(2):183–94.
86. Atay S, Godwin AK. Tumor-Derived Exosomes: A Message Delivery System for Tumor Progression. Commun Integr Biol (2014) 7(1):e28231.
87. Bosetti C, Scelo G, Chuang SC, Tonita JM, Tamaro S, Jonasson JG, et al. High Constant Incidence Rates of Second Primary Cancers of the Head and Neck: A Pooled Analysis of 13 Cancer Registries. Int J Cancer (2011) 129(1):173–9.
88. Alfieri S, Carenzo A, Platini F, Serafini MS, Perrone F, Galbiati D, et al. Tumor Biomarkers for the Prediction of Distant Metastasis in Head and Neck Squamous Cell Carcinoma. Cancers (2020) 12(4):922.
89. Chan YK, Zhang H, Liu P, Tsao SW, Lung ML, Mak NK, et al. Proteomic Analysis of Exosomes From Nasopharyngeal Carcinoma Cell Identifies Intercellular Transfer of Angiogenic Proteins. Int J Cancer (2015) 137(8):1830–41.
90. You Y, Shan Y, Chen J, Yue H, You B, Shi S, et al. Matrix Metalloproteinase 13-Containing Exosomes Promote Nasopharyngeal Carcinoma Metastasis. Cancer Sci (2015) 106(12):1669–77.
91. Gu M, Li L, Zhang Z, Chen J, Zhang W, Zhang J, et al. PFKFB3 Promotes Proliferation, Migration and Angiogenesis in Nasopharyngeal Carcinoma. J Cancer (2017) 8(18):3887–96.
92. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J Clin Oncol (2011) 29(32):4294–301.
93. Nulton TJ, Olex AL, Dozmorov M, Morgan IM, Windle B. Analysis of The Cancer Genome Atlas Sequencing Data Reveals Novel Properties of the Human Papillomavirus 16 Genome in Head and Neck Squamous Cell Carcinoma. Oncotarget (2017) 8(11):17684–99.
94. Shen KY, Liu HY, Yan WL, Wu CC, Lee MH, Leng CH, et al. Liposomal TLR9 Agonist Combined With TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment Through Dendritic Cells. Cancers (2020) 12(4):810.
95. Welters MJP, Ma W, Santegoets S, Goedemans R, Ehsan I, Jordanova ES, et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin Cancer Res (2018) 24(3):634–47.
96. Ludwig S, Sharma P, Theodoraki MN, Pietrowska M, Yerneni SS, Lang S, et al. Molecular and Functional Profiles of Exosomes From HPV(+) and HPV(-) Head and Neck Cancer Cell Lines. Front Oncol (2018) 8(445).
97. Peacock B, Rigby A, Bradford J, Pink R, Hunter K, Lambert D, et al. Extracellular Vesicle microRNA Cargo Is Correlated With HPV Status in Oropharyngeal Carcinoma. J Oral Pathol Med (2018) 47(10):954–63.
98. Khuu C, Jevnaker AM, Bryne M, Osmundsen H. An Investigation Into Anti-Proliferative Effects of microRNAs Encoded by the miR-106a-363 Cluster on Human Carcinoma Cells and Keratinocytes Using Microarray Profiling of miRNA Transcriptomes. Front Genet (2014) 5(246).
99. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL, et al. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin Cancer Res (2018) 24(4):896–905.
100. Ludwig N, Gillespie DG, Reichert TE, Jackson EK, Whiteside TL, et al. Purine Metabolites in Tumor-Derived Exosomes May Facilitate Immune Escape of Head and Neck Squamous Cell Carcinoma. Cancers (2020) 12(6):1602.
101. Hofmann L, Ludwig S, Vahl JM, Brunner C, Hoffmann TK, Theodoraki MN, et al. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int J Mol Sci (2020) 21(11):4072.
102. Park RJ, Hong YJ, Wu Y, Kim PM, Hong YK. Exosomes as a Communication Tool Between the Lymphatic System and Bladder Cancer. Int Neurourol J (2018) 22(3):220–4.
103. Zhang Q, Jeppesen DK, Higginbotham JN, Graves-Deal R, Trinh VQ, Ramirez MA, et al. Supermeres Are Functional Extracellular Nanoparticles Replete With Disease Biomarkers and Therapeutic Targets. Nat Cell Biol (2021) 23(12):1240–54.
104. Wang L, Li L, Zhu G. Role of Extracellular Vesicles on Cancer Lymphangiogenesis and Lymph Node Metastasis. Front Oncol (2021) 11:721785.
105. Qin T, Liu Z, Wang J, Xia J, Liu S, Jia Y, et al. Anlotinib Suppresses Lymphangiogenesis and Lymphatic Metastasis in Lung Adenocarcinoma Through a Process Potentially Involving VEGFR-3 Signaling. Cancer Biol Med (2020) 17(3):753–67.
106. Zhang Y, Yang X, Liu H, Cai M, Shentu Y. Inhibition of Tumor Lymphangiogenesis Is an Important Part That EGFR-TKIs Play in the Treatment of NSCLC. J Cancer (2020) 11(1):241–50.
107. Wang X, Wang H, Cao J, Ye C. Exosomes From Adipose-Derived Stem Cells Promotes VEGF-C-Dependent Lymphangiogenesis by Regulating miRNA-132/TGF-β Pathway. Cell Physiol Biochem (2018) 49(1):160–71.
108. Kinet V, Castermans K, Herkenne S, Maillard C, Blacher S, Lion M, et al. The Angiostatic Protein 16K Human Prolactin Significantly Prevents Tumor-Induced Lymphangiogenesis by Affecting Lymphatic Endothelial Cells. Endocrinology (2011) 152(11):4062–71.
109. Wang CA, Li CF, Huang RC, Li YH, Liou JP, Tsai SJ, et al. Suppression of Extracellular Vesicle VEGF-C-Mediated Lymphangiogenesis and Pancreatic Cancer Early Dissemination By a Selective HDAC1/2 Inhibitor. Mol Cancer Ther (2021) 20(9):1550–60.
110. Liu T, Li P, Li J, Qi Q, Sun Z, Shi S, et al. Exosomal and Intracellular miR-320b Promotes Lymphatic Metastasis in Esophageal Squamous Cell Carcinoma. Mol Ther Oncolytics (2021) 23:163–80.
111. Joshi BS, De Beer MA, Giepmans BNG, Zuhorn IS, et al. Endocytosis of Extracellular Vesicles and Release of Their Cargo From Endosomes. ACS Nano (2020) 14(4):4444–55.
112. Chinnappan M, Srivastava A, Amreddy N, Razaq M, Pareek V, Ahmed R, et al. Exosomes as Drug Delivery Vehicle and Contributor of Resistance to Anticancer Drugs. Cancer Lett (2020) 486:18–28.
Keywords: lymphangiogenesis, extracellular vesicles, pre-metastatic niche, head and neck squamous cell carcinoma, prognosis
Citation: Hu C, Huang Q and Sun Q (2022) The Regulation of Lymph Node Pre-Metastatic Niche Formation in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 12:852611. doi: 10.3389/fonc.2022.852611
Received: 11 January 2022; Accepted: 28 March 2022;
Published: 27 April 2022.
Edited by:
Moran Amit, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jadwiga Jablonska, Essen University Hospital, GermanyCopyright © 2022 Hu, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Sun, MTM2MTE5NzYxNTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.