
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 04 March 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.851406
Myeloid sarcoma is a rare extramedullary tumor of immature myeloid cells. Certain known acute myeloid leukemia cytogenetic abnormalities, in particular t(8,21), has been associated with a higher incidence. Myeloid sarcoma, which rarely happens in acute promyelocytic leukemias, is more common in recurrent patients after the advent of all-trans retinoic acid (ATRA) and are rare in untreated acute promyelocytic leukemia. We described a case of, to our knowledge, de novo myeloid sarcoma of the spine confirmed as acute promyelocytic leukemia. Myeloid sarcoma is diagnosed by spinal tumor biopsy, and microscopic examination of a bone marrow smear and cytogenetic analysis led to a confirmed diagnosis of acute promyelocytic leukemia.
Myeloid sarcoma (MS), also known as granulocytic sarcoma or chloroma, is a rare extramedullary tumor of immature myeloid cells (1). MS can occur in patients with acute myeloid leukemia (AML), myelodysplastic syndrome, or chronic myelogenous leukemia. The incidence of MS in AML patients is 2%–10.4%, and most cases are associated with AML FAB M2, M4, and M5 (2, 3). Certain known acute myeloid leukemia (AML) cytogenetic abnormalities, in particular t(8,21), had been associated with a higher incidence of MS (4). The prevalence of myeloid sarcoma in any organ was 2.9% among all patients with acute and chronic myeloid leukemia. Furthermore, the prevalence of myeloid sarcoma of the spine was 1.0% among all patients with acute and chronic myeloid leukemia (5, 6). MS is more common in recurrent acute promyelocytic leukemia (APL) patients after the advent of all-trans retinoic acid (ATRA) but rare in untreated APL patients (7–9). Here, we describe a 50-year-old female patient that shows an MS involving the spine as the first manifestation of APL.
In March 2021, a 50-year-old woman presented with paresthesia in her right hand and difficulty walking for more than a week. On examination, there was no any visible or palpable para-vertebral soft tissue mass in cervico -thoracic region and no paraparesis with a power of 5/5 in both lower limbs. Magnetic resonance imaging (MRI) showed a space occupation lesion (0.7 × 2.0 × 2.9 cm3) in the cervical vertebra canal from C6 through C7. Lower intensity on T1-weighted imaging, equal intensity on T2-weighted imaging, and obvious enhancement were observed in the lumpy lesion of the left occipital area (Figure 1). Then, intraspinal tumor resection and spinal Galveston internal fixation was conducted. During the operation, the mass was seen around C6–7 and grew outward toward the right intervertebral foramen of C6–T1. Histopathological examination of the mass revealed the accumulation of immature lymphohematopoietic cells. Immunohistochemical staining showed tumor cells with MPO (+), TDT (+), CD56 (+), CD43 (+), and Ki67 (Li: about 60%). In situ hybridization detection of EBV showed EBER (−) (Figure 2).
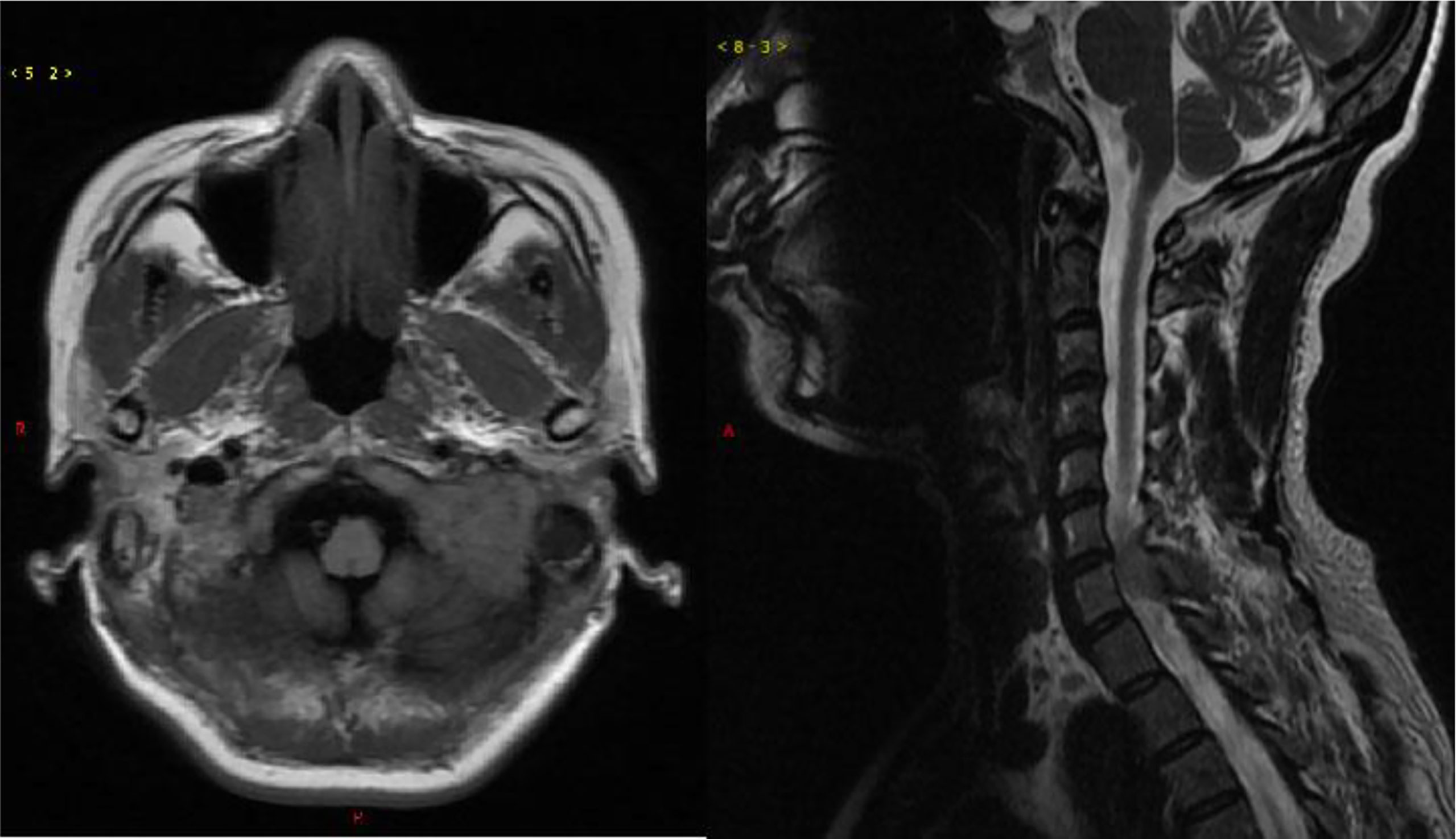
Figure 1 Magnetic resonance imaging of the brain and spine. Left, axial T1 weighted; right, sagittal T2 weighted.
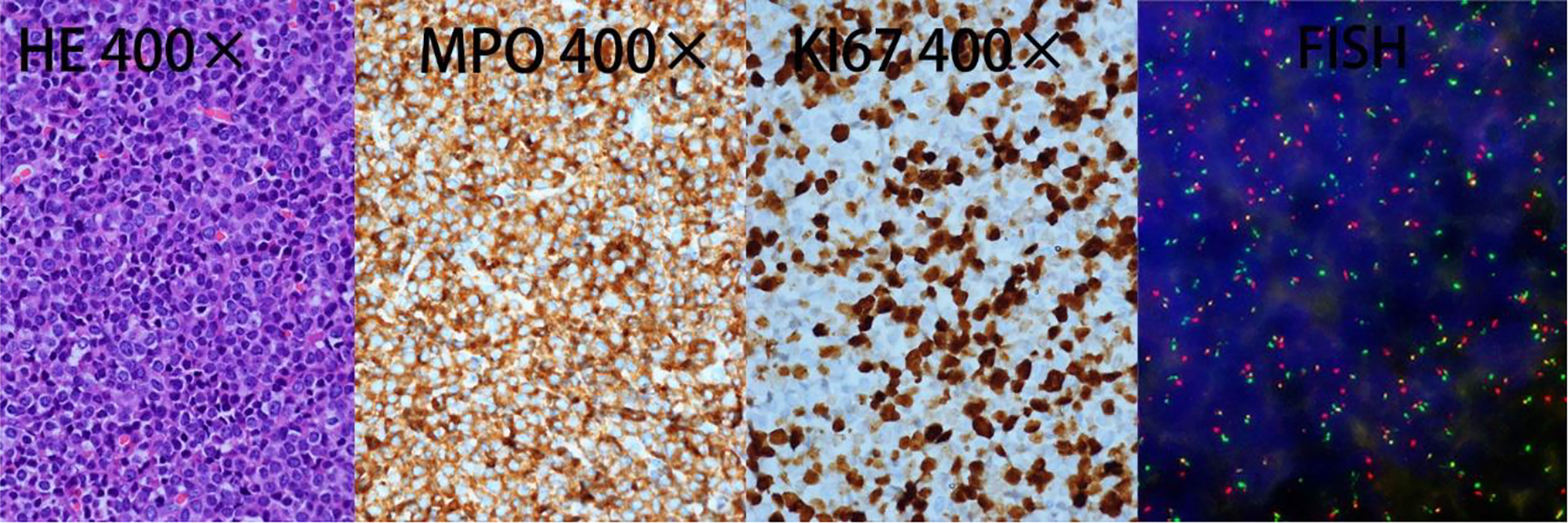
Figure 2 Histological analysis of the spine biopsy. HE, hematoxylin–eosin stain; MPO, myeloperoxidase positivity; KI67, proliferation index. FISH analysis performed on excised tumor demonstrated PML-RARα fusion.
Routine examination results in hospitalization showed abnormal blood coagulation with a decrease in fibrinogen (1.23 g/L). The peripheral blood (PB) showed decreased parameters of white blood cells (WBCs) of 1.48 × 109/L, neutrophils of 0.8 × 109/L, and normal parameters of hemoglobin (Hb) of 120 g/L and platelets (PLT) of 159 × 109/L. Liver and kidney functions and liver, spleen, and lymph node ultrasound showed no obvious abnormalities. Lung CT scan results revealed pulmonary infection and Insect-etch bone destruction in the posterior segment of the sixth costal axillary on the left.
After 14 days of surgery, peripheral blood (PB) film examination showed 13% promyelocytes, and bone marrow (BM) aspirate showed that granulocytes accounted for 70%, with 50% promyelocytes that varied in diameter and contained rich cytoplasm with abundant small azurophilic granules. However, these leukemic cells did not contain Auer rods (Figure 3). The immunophenotype profile of leukemic cells was consistent with APL (CD117+, MPO+, CD33+, CD38 part+, CD13part+, CD64 part+, CD123 part+, CD15dim+, CD34−, CD19−, CD7−, CD3−, HLA-DR−, cCD79a−, CD16−, and CD56-). In addition, positive PML-RARα gene rearrangement in bone marrow was confirmed by RT-PCR. Single nucleotide variation (SNV) and small fragment insertion/deletion (InDel) were detected in RUNX1, FLT3, and KMT2C genes, and no FLT3-ITD mutation was detected through high-throughput sequencing (NGS). Whole-exome sequencing in the bone marrow and the nails were conducted, but no obvious abnormal gene mutations were found. Retrospective fluorescence in situ hybridization (FISH) testing for the PML-RARα fusion gene was conducted on samples of excised tumor in March and showed positive results with t(15:17)(q24;q21) as well (Figure 2).
After the diagnosis was confirmed in the hematology department, treatment began with induction regimen composed of ATRA 10 mg three times a day and Arsenic trioxide (ATO) 10 mg daily, from day 1 until complete remission, combined with idarubicin 10 mg on days 2, 4, and 6. Bone marrow evaluation was performed after the induction therapy and showed complete remission with a reduction of PML–RARα/ABL1 to 0.29%. Intrathecal chemotherapy with methotrexate and dexamethasone was performed four times after complete remission. After 2 courses of consolidation chemotherapy with idarubicin and cytarabine, repeat cranial and cervical MRI analysis showed the lumpy lesion of left occipital area was complete regression. Then the maintenance therapy had started with the drugs of ATRA and Compound Huangdai tablets. After 10 months of follow-up, the patient still remained in complete hematologic and molecular remission.
As a special type of AML, APL is characterized by a block in differentiation where leukemic cells are halted at the promyelocyte stage, accounting for roughly 5%–8% of AML patients. The characteristic balanced translocation t(15;17) (q22;q12) is seen in 95% of cases, which results in the fusion transcript PML-RARα. We summarized the literature of APL with MS as the initial presentation published so far; a total of 21 articles with 22 cases are included (10–30). It was found that this type of leukemia affects patients at a wide age range, from 1 to 77 years old. The average age of onset is 36 years, mostly in the young and middle aged. In terms of gender, there is no significant difference between men and women. The most common sites of APL with MS are spine (five cases), skin (three cases) and tongue (two cases), followed by the testis, axilla, breast, cerebellum, colon, femur, tibia, mandible, oral cavity, ovary, pelvis, rectum, and thymus. In terms of myeloid sarcoma of the spine, spinal cord compression is the initial presentation, which is similar to this patient. Besides, most APL with MS cases did not present with characteristic hemorrhage of APL. Therefore, a small number of cases are first misdiagnosed as a lymphoma or Ewing sarcoma due to insufficient immunophenotyping of the tumor tissue. Proper histological, flow cytometric, and cytogenetic analyses are essential for the correct diagnosis.
Most APL with MS cases are associated with PML-RARα fusion and chromosomal abnormalities in the bone marrow or MS tissue. The fusion transcript PML-RARα is detected in most cases (17/22). Only one case for NPM1-RARα fusion, one case for NPM-RARα, and two cases for FLT3-ITD mutation were found. Aside from the expression of the characteristic PML-RARα fusion protein, other co-occurring mutations including FLT3, WT1, NRAS, KRAS and ARID gene have also been noted in primary and relapse APL with MS. FLT3, has been shown to be the most commonly mutated gene in primary APL. Additionally, other commonly known AML mutations such as NPM1 are rarely seen in APL (31). It is speculated that FLT3-ITD plays a significant role in relapse and central CNS involvement in patients with APL (32). Several analyses found that FLT3-ITD mutation is associated with high WBC count at diagnosis and poor prognosis in patients with APL (33–36). NPM-RARα arrests myeloid differentiation at the promyelocyte stage like PML-RARα. APL carrying NPM-RARα shows a good response to differentiation therapy with all-trans retinoic acid (12, 22, 37). In this case, single nucleotide variation and small fragment insertion/deletion of RUNX1, FLT3, and KMT2C genes are involved. Besides FLT3 gene, RUNX1 is important in maintaining normal hematopoiesis and preventing the development of malignancy (38). KMT2C gene is a putative tumor suppressor in several epithelia and in myeloid cells (39). Therefore, the two gene mutations can also lead to the development of tumors. But since only single nucleotide variation and small fragment insertion/deletion rather than gene mutation are detected in this case, it is still unclear whether such changes play an important role in the pathogenesis.
According to an updated recommendations from an Expert Panel of the European Leukemia Net, ATRA-ATO combination induction and consolidation therapy have become the standard chemotherapy for low- to intermediate-risk patient (WBC ≤ 10 × 109/L), which is associated with significantly less myelosuppression and fewer infections. Besides, in some countries, the classical combination of ATRA and chemotherapy is still an acceptable option, especially for high-risk patients with WBC>10 x 109/L (40). Of the 22 cases of APL with MS, most cases (14/22) were non-high-risk patients (WBC≤ 10 x 109/L) and the others (7/22) were high-risk patients. In high-risk patients, 3 patients received ATRA with chemotherapy, 2 received chemotherapy alone, 1 received ATRA with ATO and 1 received ATRA with ATO plus chemotherapy. Among them, 3 patients finally died from cerebral hemorrhage, CNS infiltration and hematological relapse, the others achieved complete or molecular remission. In non-high-risk patients, nine patients received ATRA with chemotherapy, four patients received ATRA with ATO plus chemotherapy, and one patient was given ATRA with ATO. Finally, two patients died from severe hemorrhagic episodes and sepsis, respectively, two cases eventually relapsed, and the others received complete molecular or hematological remission. It seemed that APL with MS were more frequently found in non-high-risk patients, and a poor prognosis was associated with high WBC counts. WBC count is lower than 10 × 109/L in our patient who received ATRA with ATO plus chemotherapy induction therapy, and following three courses of chemotherapy, complete remission had been attained and the lumpy lesion of left occipital area disappeared.
Patients with APL frequently manifest as an coagulation abnormalities, which always present with decreased PLT counts and low serum fibrinogen levels (41). In most APL with MS case reports, serum fibrinogen levels were not described. In patients of APL with MS, except for 1 case without description of PLT counts, 10 of 22 patients showed low PLT counts, 11 of 22 patients showed normal PLT counts. No significant hemorrhagic episodes were observed in all patients with normal PLT counts. In patients with low PLT counts, minor bleeding such as gingival, nose, and rectal bleeding were seen in four patients. Life threatening severe bleeding was seen in two patients. From these case reports, the risk of bleeding in APL presenting with MS patients were not high, and the prognosis is good, in general. But in five death cases, two of them died of bleeding; bleeding complications are still the common leading cause of death in these cases. In our patient, PLT counts were normal and fibrinogen declined lightly, and no obvious bleeding was observed during the whole treatment.
Five cases of APL presenting with a myeloid sarcoma in the spine were all non-high-risk patient, mostly occurring in male (4/5). The thoracic spine was involved in all cases, followed by the lumbar, which was consistent with our case. One responder died of sepsis while still in hematological remission, and the remaining four achieved consistent remission. Four of five patients were treated with radiotherapy; no significant radiotherapy effects were found. Radiotherapy alone seemed to be ineffective in some cases (18, 19, 21, 22, 24). One retrospective study demonstrated that majority of patients that presented with MS had not received radiotherapy because the mass regressed after induction chemotherapy. The majority of patients with MS were referred for radiotherapy when there was extramedullary progression, marrow relapse, or rapid symptom relief required (42). However, there are no specific studies on the efficacy of radiotherapy for myeloid sarcomas of the spine (42, 43).
In conclusion, the clinical manifestation of APL with MS is different from characteristic APL and first identified by tumor biopsy but easily misdiagnosed because of atypical morphology. Further microscopic examination of bone marrow smear and cytogenetic analysis plays an important role to determine the source of MS, which are also very vital for subsequent treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
XS, QW, and JL took care of the patient. XS and QW drafted the manuscript. XS, TG, and HY collected clinical data. QW and JL analyzed the review data and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of PR China (no. 81770219, for QW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bakst RL, Tallman MS, Douer D, Yahalom J. How I Treat Extramedullary Acute Myeloid Leukemia. Blood (2011) 118(14):3785–93. doi: 10.1182/blood-2011-04-347229
2. van der Weide M, Imandt LM, Langenhuijsen MM. Motility of Leukaemic Cells in Acute Leukaemia Related to Tumour Mass, Maturation and FAB Classification. Acta Haematol (1988) 80(1):34–9. doi: 10.1159/000205595
3. Shimizu H, Saitoh T, Hatsumi N, Takada S, Yokohama A, Handa H, et al. Clinical Significance of Granulocytic Sarcoma in Adult Patients With Acute Myeloid Leukemia. Cancer Sci (2012) 103(8):1513–7. doi: 10.1111/j.1349-7006.2012.02324.x
4. Tallman MS, Hakimian D, Shaw JM, Lissner GS, Russell EJ, Variakojis D. Granulocytic Sarcoma is Associated With the 8;21 Translocation in Acute Myeloid Leukemia. J Clin Oncol (1993) 11(4):690–7. doi: 10.1200/JCO.1993.11.4.690
5. Novick SL, Nicol TL, Fishman EK. Granulocytic Sarcoma (Chloroma) of the Sacrum: Initial Manifestation of Leukemia. Skeletal Radiol (1998) 27(2):112–4. doi: 10.1007/s002560050348
6. Seok JH, Park J, Kim SK, Choi JE, Kim CC. Granulocytic Sarcoma of the Spine: MRI and Clinical Review. AJR Am J Roentgenol (2010) 194(2):485–9. doi: 10.2214/AJR.09.3086
7. Pacilli L, Lo Coco F, Ramadan SM, Giannì L, Pingi A, Remotti D, et al. Promyelocytic Sarcoma of the Spine: A Case Report and Review of the Literature. Adv Hematol (2010) 2010:137608. doi: 10.1155/2010/137608
8. Yamashita T, Nishijima A, Noguchi Y, Narukawa K, Oshikawa G, Takano H. Acute Promyelocytic Leukemia Presenting as Recurrent Spinal Myeloid Sarcomas 3 Years Before Developing Leukemia: A Case Report With Review of Literature. Clin Case Rep (2019) 7(2):316–21. doi: 10.1002/ccr3.1991
9. He Z, Tao S, Deng Y, Chen Y, Song L, Ding B, et al. Extramedullary Relapse in Lumbar Spine of Patient With Acute Promyelocytic Leukemia After Remission for 16 Years: A Case Report and Literature Review. Int J Clin Exp Med (2015) 8(12):22430–4. doi: 10.3760CMA.J.ISSN.1671-7368.2016.01.018
10. Ajarim DS, Santhosh-Kumar CR, Higgy KE, el Saghir NS, Almomen AK, Shipkey FD. Granulocytic Sarcoma of the Thymus in Acute Promyelocytic Leukaemia. Clin Lab Haematol (1990) 12(1):97–9. doi: 10.1111/j.1365-2257.1990.tb01115.x
11. Gopal S, Marcussen S, Dobin SM, Koss W, Donner LR. Primary Myeloid Sarcoma of the Testicle With T (15,17). Cancer Genet Cytogenet (2005) 157(2):148–50. doi: 10.1016/j.cancergencyto.2004.06.010
12. Nicci C, Ottaviani E, Luatti S, Grafone T, Tonelli M, Motta MR, et al. Molecular and Cytogenetic Characterization of a New Case of T (5,17)(Q35;Q21) Variant Acute Promyelocytic Leukemia. Leukemia (2005) 19(3):470–2. doi: 10.1038/sj.leu.2403645
13. Fukushima S, Terasaki M, Tajima Y, Shigemori M. Granulocytic Sarcoma: An Unusual Complication of Acute Promyelocytic Leukemia Causing Cerebellar Hemorrhage. Case Report. J Neurosurg (2006) 105(6):912–5. doi: 10.3171/jns.2006.105.6.912
14. Mohamedbhai S, Pule M, Conn B, Hopper C, Ramsay A, Khwaja A. Acute Promyelocytic Leukaemia Presenting With a Myeloid Sarcoma of the Tongue. Br J Haematol (2008) 141(5):565. doi: 10.1111/j.1365-2141.2008.07080.x
15. Worch J, Ritter J, Frühwald MC. Presentation of Acute Promyelocytic Leukemia as Granulocytic Sarcoma. Pediatr Blood Cancer (2008) 50(3):657–60. doi: 10.1002/pbc.21190
16. Wang X, Liu H, Wu Z, Xu X, Chen X, Zhai Z, et al. A Case of Acute Promyelocytic Leukemia Presenting With a Nonleukemic Granulocytic Sarcoma of the Ovary, With Subsequent Development of Acute Myeloid Leukemia Associated With T (8,21). Leuk Res (2009) 33(4):580–2. doi: 10.1016/j.leukres.2008.08.008
17. Benjazia E, Khalifa M, Benabdelkader A, Laatiri A, Braham A, Letaief A, et al. Granulocytic Sarcoma of the Rectum: Report of One Case That Presented With Rectal Bleeding. World J Gastrointest Pathophysiol (2010) 1(4):144–6. doi: 10.4291/wjgp.v1.i4.144
18. Bittencourt H, Teixeira Junior AL, Glória AB, Ribeiro AF, Fagundes EM. Acute Promyelocytic Leukemia Presenting as an Extradural Mass. Rev Bras Hematol Hemoter (2011) 33(6):478–80. doi: 10.5581/1516-8484.20110126
19. Kyaw TZ, Maniam JA, Bee PC, Chin EF, Nadarajan VS, Shanmugam H, et al. Myeloid Sarcoma: An Unusual Presentation of Acute Promyelocytic Leukemia Causing Spinal Cord Compression. Turk J Haematol (2012) 29(3):278–82. doi: 10.5505/tjh.2012.94809
20. Yamashita Y, Isomura N, Hamasaki Y, Goto M. Case of Pediatric Acute Promyelocytic Leukemia Presenting as Extramedullary Tumor of the Mandible. Head Neck (2013) 35(10):E310–3. doi: 10.1002/hed.23163
21. Piñán MA, Ardanaz MT, Guinea JM, García-Ruiz JC. Myeloid Sarcoma Preceding an Acute Promyelocytic Leukaemia With Neuromeningeal Infiltration. Ann Hematol (2014) 93(2):339–40. doi: 10.1007/s00277-013-1795-0
22. Kikuma T, Nakamachi Y, Noguchi Y, Okazaki Y, Shimomura D, Yakushijin K, et al. A New Transcriptional Variant and Small Azurophilic Granules in an Acute Promyelocytic Leukemia Case With NPM1/RARA Fusion Gene. Int J Hematol (2015) 102(6):713–8. doi: 10.1007/s12185-015-1857-2
23. Li J, Tu C, Wang D, Huang C, Zhang X. [Myeloid Sarcoma With Acute Promyelocytic Leukemia:Two Cases Report]. Zhonghua Xue Ye Xue Za Zhi (2015) 36(5):438–40. doi: 10.3760/cma.j.issn.0253-2727.2015.05.020
24. Shah NN, Stonecypher M, Gopal P, Luger S, Bagg A, Perl A. Acute Promyelocytic Leukemia Presenting as a Paraspinal Mass. J Community Support Oncol (2016) 14(3):126–9. doi: 10.12788/jcso.0220
25. de Andrade BA, Farneze RB, Agostini M, Cortezzi EB, Abrahão AC, Cabral MG, et al. Myeloid Sarcoma of the Oral Cavity: A Case Report and Review of 89 Cases From the Literature. J Clin Exp Dent (2017) 9(9):e1167–71. doi: 10.4317/jced.53935
26. Collinge E, Tigaud I, Balme B, Gerland LM, Sujobert P, Carlioz V, et al. Case Report: Purulent Transformation of Granulocytic Sarcoma: An Unusual Pattern of Differentiation in Acute Promyelocytic Leukemia. Med (Baltimore) (2018) 97(8):e9657. doi: 10.1097/MD.0000000000009657
27. Oravcova I, Mikuskova E, Leitnerova M, Gyarfas J, Mlcakova A, Szepe P, et al. A Unique Clinical Presentation of De Novo Acute Promyelocytic Leukemia as a Myeloid Sarcoma of the Breast. Int J Hematol (2018) 108(5):550–3. doi: 10.1007/s12185-018-2479-2
28. Ignacio-Cconchoy FL, Benites-Zapata VA, Yanac-Avila RL, Vela-Velàsquez CT. Myeloid Sarcoma of the Tongue as a First Manifestation of Acute Promyelocytic Leukemia: A Case Report. Rep Pract Oncol Radiother (2020) 25(2):174–7. doi: 10.1016/j.rpor.2019.12.026
29. Kasinathan GA-O, Sathar J. Extramedullary Disease in Acute Promyelocytic Leukaemia: A Rare Presentation. SAGE Open Med Case Rep (2020) 8:2050313X20926076. doi: 10.1177_2050313X20926076
30. Wang L, Cai DL, Lin N. Myeloid Sarcoma of the Colon as Initial Presentation in Acute Promyelocytic Leukemia: A Case Report and Review of the Literature. World J Clin cases (2021) 9(21):6017–25. doi: 10.12998/wjcc.v9.i21.6017
31. Madan V, Shyamsunder P, Han L, Mayakonda A, Nagata Y, Sundaresan J, et al. Comprehensive Mutational Analysis of Primary and Relapse Acute Promyelocytic Leukemia. Leukemia (2016) 30(8):1672–81. doi: 10.1038/leu.2016.69
32. Tashiro H, Shirasaki R, Oka Y, Sugao T, Mizutani-Noguchi M, Yamamoto T, et al. FLT3 Internal Tandem Duplication is Associated With a High Relapse Rate and Central Nervous System Involvement in Acute Promyelocytic Leukemia Cases: Single Institutional Analysis. Eur J Haematol (2011) 86(3):272–3. doi: 10.1111/j.1600-0609.2010.01559.x
33. Lucena-Araujo AR, Kim HT, Jacomo RH, Melo RA, Bittencourt R, Pasquini R, et al. Internal Tandem Duplication of the FLT3 Gene Confers Poor Overall Survival in Patients With Acute Promyelocytic Leukemia Treated With All-Trans Retinoic Acid and Anthracycline-Based Chemotherapy: An International Consortium on Acute Promyelocytic Leukemia Study. Ann Hematol (2014) 93(12):2001–10. doi: 10.1007/s00277-014-2142-9
34. Beitinjaneh A, Jang S, Roukoz H, Majhail NS. Prognostic Significance of FLT3 Internal Tandem Duplication and Tyrosine Kinase Domain Mutations in Acute Promyelocytic Leukemia: A Systematic Review. Leuk Res (2010) 34(7):831–6. doi: 10.1016/j.leukres.2010.01.001
35. Picharski GL, Andrade DP, Fabro A, Lenzi L, Tonin FS, Ribeiro RC, et al. The Impact of Flt3 Gene Mutations in Acute Promyelocytic Leukemia: A Meta-Analysis. Cancers (Basel) (2019) 11(9):1311. doi: 10.3390/cancers11091311
36. Fan Y, Cao Y, Bai X, Zhuang W. The Clinical Significance of FLT3 ITD Mutation on the Prognosis of Adult Acute Promyelocytic Leukemia. Hematology (2018) 23(7):379–84. doi: 10.1080/10245332.2017.1415717
37. Falini B, Nicoletti I, Bolli N, Martelli MP, Liso A, Gorello P, et al. Translocations and Mutations Involving the Nucleophosmin (NPM1) Gene in Lymphomas and Leukemias. Haematologica (2007) 92(4):519–32. doi: 10.3324/haematol.11007
38. Sood R, Kamikubo Y, Liu P. Role of RUNX1 in Hematological Malignancies. Blood (2017) 129(15):2070–82. doi: 10.1182/blood-2016-10-687830
39. Liao C, Hu NX, Song H, Zhang JY, Shen DY, Xu XJ, et al. Pediatric Blastic Plasmacytoid Dendritic Cell Neoplasm: Report of Four Cases and Review of Literature. Int J Hematol (2021) 113(5):751–9. doi: 10.1007/s12185-020-03070-x
40. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of Acute Promyelocytic Leukemia: Updated Recommendations From an Expert Panel of the European LeukemiaNet. Blood (2019) 133(15):1630–43. doi: 10.1182/blood-2019-01-894980
41. David S, Mathews V. Mechanisms and Management of Coagulopathy in Acute Promyelocytic Leukemia. Thromb Res (2018) 164 Suppl 1:S82–S8. doi: 10.1016/j.thromres.2018.01.041
42. Bakst R, Wolden S, Yahalom J. Radiation Therapy for Chloroma (Granulocytic Sarcoma). Int J Radiat Oncol Biol Phys (2012) 82(5):1816–22. doi: 10.1016/j.ijrobp.2011.02.057
Keywords: acute promyelocytic leukemia (APL), myeloid sarcoma (MS), spine, diagnosis, chemotherapy
Citation: Shu X, Wu Q, Guo T, Yin H and Liu J (2022) Acute Promyelocytic Leukemia Presenting With a Myeloid Sarcoma of the Spine: A Case Report and Literature Review. Front. Oncol. 12:851406. doi: 10.3389/fonc.2022.851406
Received: 09 January 2022; Accepted: 07 February 2022;
Published: 04 March 2022.
Edited by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarReviewed by:
Patrizia Chiusolo, Università Cattolica del Sacro Cuore, ItalyCopyright © 2022 Shu, Wu, Guo, Yin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingdi Liu, bGl1amluZ2RpMDkxMEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.