- 1Equipe Labellisée Par La Ligue Contre Le Cancer, Université de Paris, Sorbonne Université, INSERM UMR1138, Centre de Recherche des Cordeliers, Institut Universitaire de France, Paris, France
- 2Metabolomics and Cell Biology Platforms, Gustave Roussy Cancer Campus, Université Paris Saclay, Villejuif, France
- 3Service d’Anesthésie Gustave Roussy Cancer Campus, Villejuif, France
- 4Pôle de Biologie, Hôpital Européen Georges Pompidou, AP-HP, Paris, France
Defective silencing of tumor suppressor genes through epigenetic alterations contributes to oncogenesis by perturbing cell cycle regulation, DNA repair or cell death mechanisms. Reversal of such epigenetic changes including DNA hypermethylation provides a promising anticancer strategy. Until now, the nucleoside derivatives 5-azacytidine and decitabine are the sole DNA methyltransferase (DNMT) inhibitors approved by the FDA for the treatment of specific hematological cancers. Nevertheless, due to their nucleoside structure, these inhibitors directly incorporate into DNA, which leads to severe side effects and compromises genomic stability. Much emphasis has been placed on the development of less toxic epigenetic modifiers. Recently, several preclinical studies demonstrated the potent epigenetic effects of local anesthetics, which are routinely used during primary tumor resection to relief surgical pain. These non-nucleoside molecules inhibit DNMT activity, affect the expression of micro-RNAs and repress histone acetylation, thus exerting cytotoxic effects on malignant cells. The in-depth mechanistic comprehension of these epigenetic effects might promote the use of local anesthetics as anticancer drugs.
Introduction
Epigenetic alterations and cancer
Epigenetic alterations are common molecular hallmarks of most cancers (1). In normal cells, epigenetic changes are fundamental for the control of gene expression, for the maintenance of cellular identities and for acquisition of an ever more differentiated and specialized phenotype (2). Epigenetic changes are highly regulated to maintain the stability of the epigenome and cellular homeostasis. However, aberrant patterns of DNA methylation, histone modifications (acetylation, methylation, phosphorylation, etc.) and dysregulation of non-coding RNAs correlate with the development of various kinds of cancers by inactivating tumor suppressor genes, by perturbing DNA repair and chromatin remodeling, or by promoting oncogenic pathways (2, 3). These modifications are under the control of interconnected regulators. For instance, many micro-RNAs (miRNAs) can stimulate cellular proliferation by directly interacting with cell-cycle components, as this has been reported for miR-17-92, miR-221/222, miR-663, miR-302 or miR-24, which target the transcription factor E2F1 or the cyclin dependent kinase (CDK) inhibitors p27Kip1, p21CIP1 and p16INK4a, respectively (4–8). The hypermethylation of DNA, which is associated with multiple pathologies, is characterized by the transfer of methyl groups to the position 5 of cytosine residues at CpG islands, which may be located in the promoter regions of tumor suppressive genes, thus inducing their inactivation (9). This reaction is catalyzed by a family of DNA methyltransferases encoded by four specific genes (DNMT1, DNMT2, DNMT3a and DNMT3b) that synergistically promote oncogenesis (9–11). Of note, hypermethylation of DNA is perfectly reversible, and silent genes can be reactivated by administration of hypomethylating agents. Two demethylating drugs were approved by the FDA for this purpose: 5-azacytidine and the cytidine analog 5-aza-2’-deoxycytidine also known as decitabine (sold under the brand name dacogen, DAC). After their incorporation into genomic DNA, both agents directly inhibit DNMTs. In the clinic, they are exclusively prescribed for the treatment of myelodysplasia and acute myeloid leukemia (12). However, despite promising preliminary preclinical data (such as the promotion of cancer cell apoptosis in vitro and the reduction of tumor growth in mouse models), 5-azacytidine and decitabine provoke considerable side-effects in patients (e.g. mutagenicity, thrombocytopenia and prolonged neutropenia), limiting their employment and motivating their continuous investigation in clinical trials (13). For this reason, the search for ever less toxic hypomethylating agents is ongoing.
Recently, local anesthetics (LA) such as bupivacaine, levobupivacaine, lidocaine, ropivacaine and procaine were described to act as non-nucleoside DNA demethylating agents responsible for upregulating transcriptionally silent genes (14–21), to interfere with the expression of several miRNAs and to impact on the level of histone acetylation (22). These LA are currently employed for their analgesic and anti-inflammatory properties, but also turned out to be endowed with potent anti-tumor effects (23–33).
Local anesthetics induce anticancer effects
LA are commonly used during oncological surgery to relief the acute pain generated by the surgical procedure. Several retrospective clinical trials reported a notable improvement of overall survival and a reduction in recurrence after primary tumor resection under local anesthesia compared to general anesthesia alone (23, 26, 34–36). This epidemiological evidence suggests that LA might have anticancer effects. Several pathways that may explain such antineoplastic effects have been described in the literature. Indeed, preclinical data indicate that LA influence the migration and the survival of cancer cells. At clinically relevant concentrations, LA inhibit the proliferation of cancer cells by provoking cell cycle arrest, by triggering mitochondrial dysfunction or by causing apoptotic cell death (28, 29, 37). Moreover, LA abrogate the migration of cancer cells after inducing intracellular Ca2+ changes that affect the cytoskeleton (24). LA also inhibit the secretion of matrix metalloproteinases necessary for the invasion of cancer cells into the extracellular matrix (38). The anti-inflammatory property of LA reduces the levels of procarcinogenic cytokine interleukin-6 (IL-6) detectable in the serum of patients during oncological surgery (25, 39). In vivo, LA elicit an anticancer immune response, thus causing tumor growth reduction in mice and extending the lifespan of animals with solid tumors (20, 40). When combined with chemotherapeutic agents such as 5-fluorouracil, paclitaxel or platinum salts, LA induce a synergistic antitumor effect, meaning that they sensitize cancer cells to the cytotoxicity of chemotherapy (14, 41). Taken together, the current state of the literature supports the contention that LA may directly kill cancer cells and also promote immune responses against neoplastic cells.
Hitherto, only few prospective trials investigated the role of local anesthetics on oncological prognosis (42). Most studies failed to support a direct impact on clinical outcome. However, the continued accumulation of irrefutable preclinical data demonstrating antitumor effects of local anesthetics encourages clinicians to further pursue investigations as illustrated by several randomized controlled trials recorded at www.clinicaltrials.gov and summarized in (43). Among the published scientific readouts, it can be suspected that at least some of these effects are secondary to LA effects on the tumor epigenome. Here, we summarize preclinical data highlighting the epigenetic mode of action through which LA could exert their antineoplastic activity.
Local anesthetics promote DNA demethylation and restore expression of tumor suppressor genes
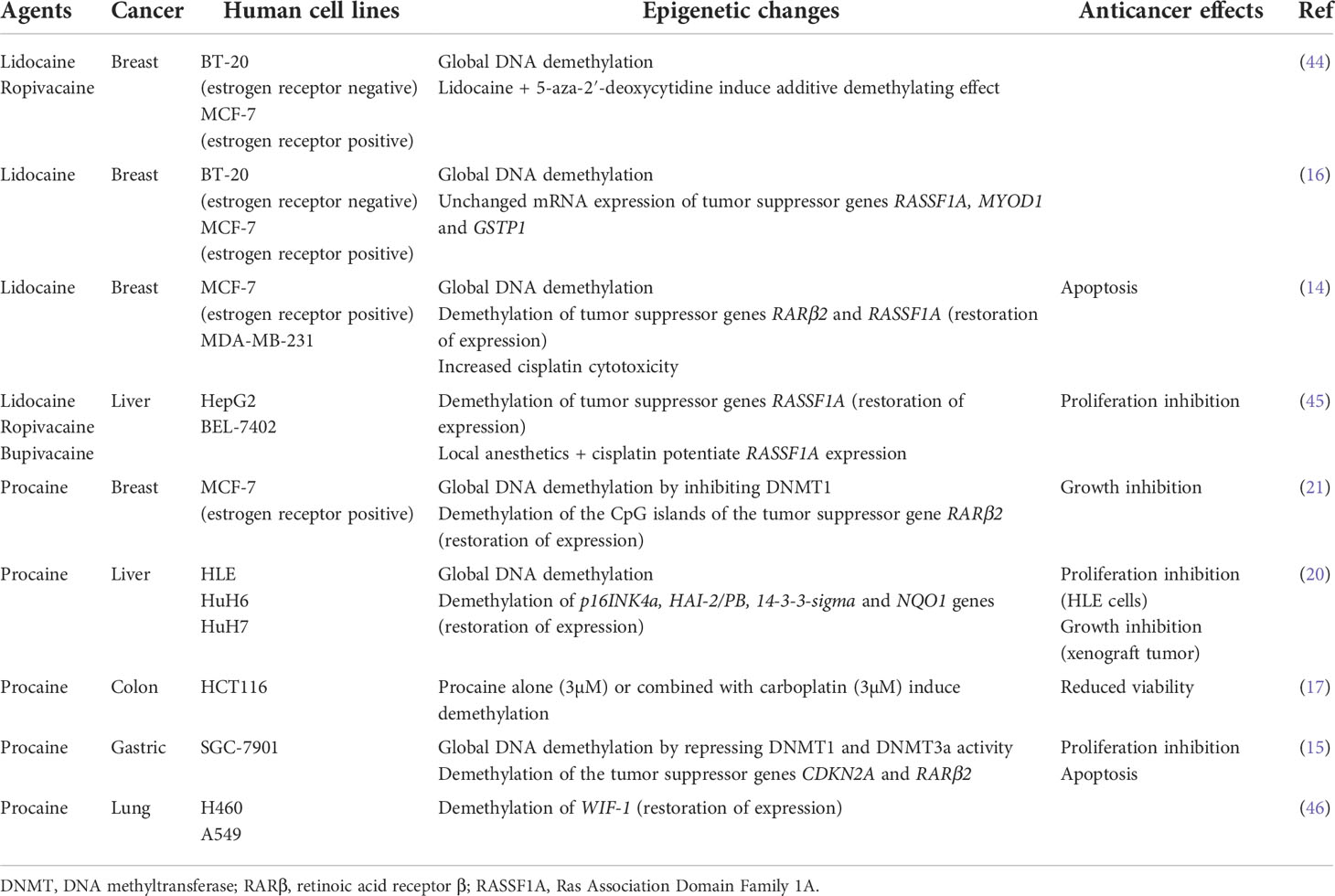
Several studies observed that aminoamide-type local anesthetics such as bupivacaine, lidocaine, ropivacaine and ester-type local anesthetic like procaine mediate antitumor effects as well as global DNA demethylation in many types of solid cancers in a time-and dose-dependent manner (Table 1). For instance, bupivacaine, lidocaine and ropivacaine turned out to be potent DNA-demethylating agents of RASSF1A, hampering the proliferation of human hepatocarcinoma HepG2 and BEL-7402 cells (45). Lidocaine triggered apoptosis of human breast cancer BT-20 and MCF-7 cells by inducing the expression of the tumor suppressive RARβ2 and RASSF1A genes (14). Procaine reduced global DNA methylation by 40% in breast cancer MCF-7 cells by inhibiting DNMT1 (21) and showed an outstanding ability to minimize the growth, the proliferation and the invasion of various human cancers both in vitro and in vivo (15, 17, 20, 21). Interestingly, LA can sterically inhibit DNMT binding to CpG islands or to DNA (15, 21, 47) (Figure 1). As a consequence, the epigenetic regulation by LA could represent a therapeutic option. Indeed, the cytotoxic effects of conventional chemotherapeutic agents such as cisplatin or carboplatin are significantly potentiated when they are combined with LA (14, 17, 45). The association of both lidocaine and cisplatin triggers a higher level of cancer cell apoptosis than lidocaine or cisplatin alone because of the re-expression of the RASSF1A and RARβ2 genes (14). Combined with 5-aza-2’-deoxycytidine, an interesting additive demethylating effect was observed for lidocaine (44).
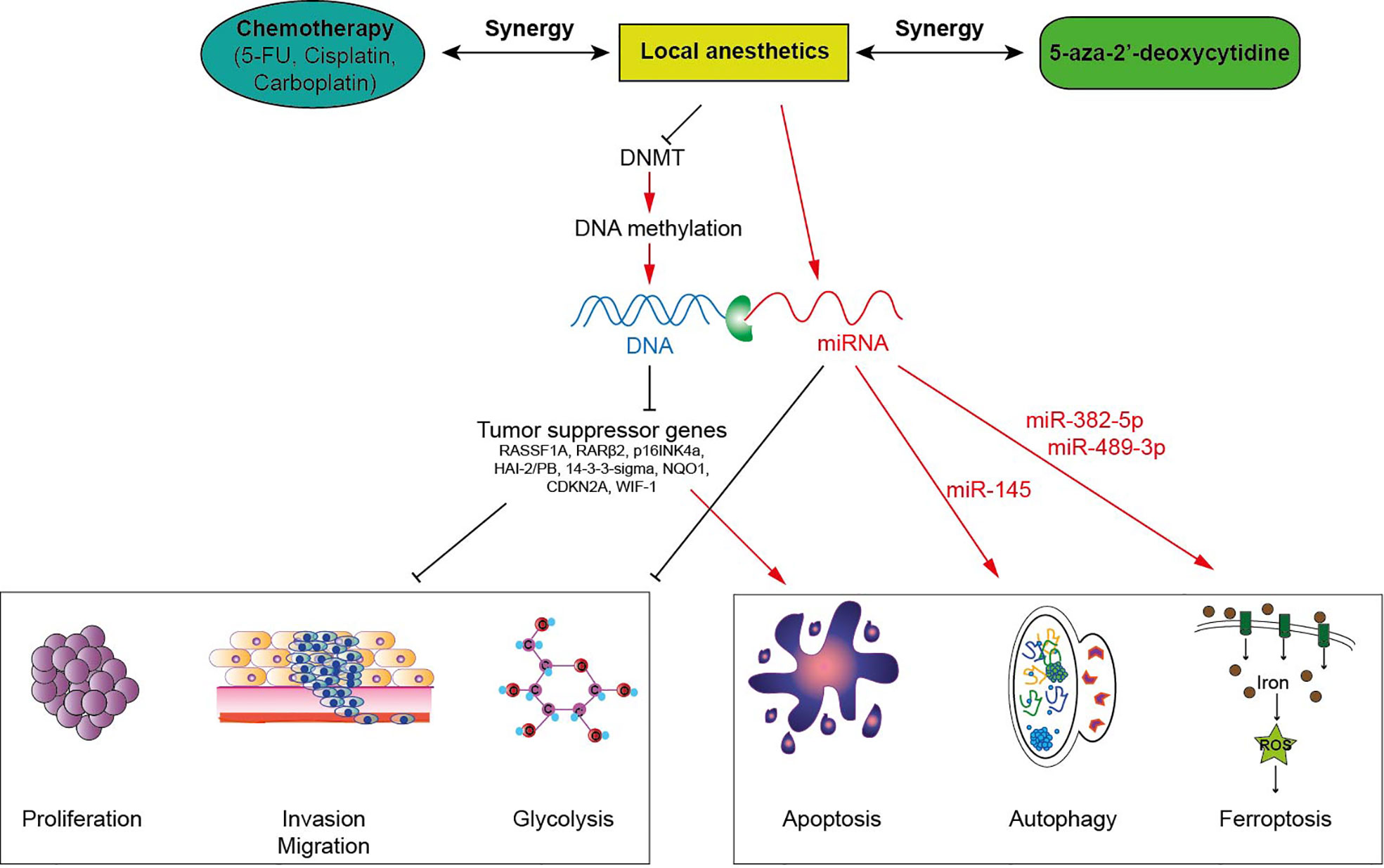
Figure 1 Local anesthetics induce anti-tumor effects via epigenetic modulation in cancer cells. Local anesthetics inhibit DNA methyltransferases (DNMT) decreasing the level of DNA methylation. This hypomethylation (or demethylation) restores the expression of various tumor suppressor genes impeding the proliferation, the invasion and the mitochondrial metabolism of tumor cells. This epigenetic effect of local anesthetics potentiates the cytotoxic activity of antineoplastic therapies.
The effects induced by LA-mediated epigenetic modulation are not limited to the restoration of tumor suppressor gene expression but also modulate the sensitivity to pain (48) and influence the response to corticoid stress during surgery (49, 50), altogether profoundly impinging on the activity of anti-tumor effectors (49, 51). Until now, opioids have been the most commonly used analgesics for controlling acute pain. However, preclinical data indicate that opioids mediate pro-tumorigenic effects via the activation of matrix metalloproteinases and oncogenes like c-Myc as well as via an increase in DNA methylation (52–54). Of note, DNA methylation leads to the expression of the mu opioid receptor and predicts the response to endogenous endorphins and opioid analgesics (55). Paradoxically, excessive administration of opioids increases the risk of hyperalgesia during the postoperative period. It is tempting to speculate that the epigenetic demethylating activity of LA could prevent the hyperalgesia induced by both hypermethylation and opioids and hence counteract the opioid-mediated protumoral effects as well. Thus, opioid-free anesthesia, in which opioids are replaced by a mix of local anesthetics and other analgesic agents, offers a possibility to relieve pain, and to alleviate surgical stress-induced epigenetic changes, thereby restoring the expression of tumor suppressor genes.
Local anesthetics regulate non-coding RNAs
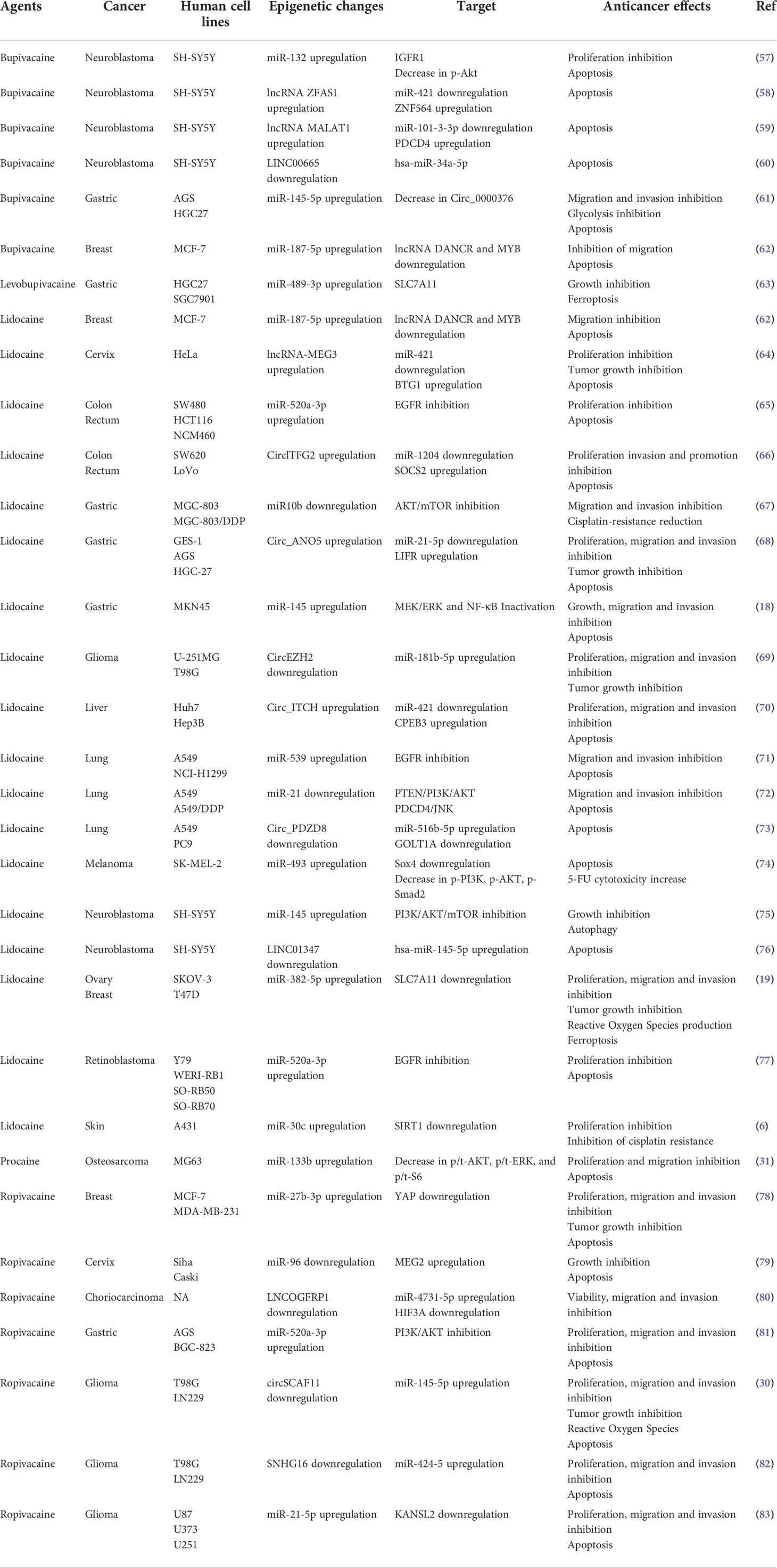
MiRNAs belong to the family of non-coding RNAs. Their main role is to control gene expression at different levels, and their dysregulation may trigger malignant transformation (56). LA are endowed with the capacity to enhance or suppress the expression of a variety of miRNAs, which differ according to the employed molecules and cancer cell lines (Table 2). The regulation of miRNAs by LA impacts several signaling pathways that mediate oncosuppression. Most of these pathways repress the downstream signaling pathway mediated by protein kinase B (PKB, best known as AKT) and mammalian target of rapamycin (mTOR), thus deeply affecting the proliferation, migration and invasion of cancer cells and inducing apoptosis (Figures 1, 2) (81). Interestingly, mTOR was described as a major regulator of energy metabolism by controlling oxidative phosphorylation (84). LA are known to induce mitochondrial dysfunction leading to the production of reactive oxygen species. Indeed, the antitumor activity of ropivacaine involves both the disruption of mitochondrial function and the inhibition of Akt and mTOR phosphorylation, highlighting a putative link between AKT/mTOR and mitochondrial activity in cancer (85). Moreover, the inhibition of the AKT-mTOR pathway by LA demonstrated a relevant impact in preclinical experiments. Indeed, lidocaine-promoted miRNA regulation reversed cisplatin-resistance in MGC-803/DDP gastric cells, minimized the cisplatin resistance in lung cancer cells A549/DDP and increased the cytotoxicity of 5-fluorouracil against SK-MEL-2 melanoma cells via upregulation of miR-493 (67, 72, 74). LA also exert antineoplastic properties by acting on the epithelial growth factor receptor (EGFR) axis. For instance, lidocaine inhibits the proliferation of lung cancer cells via upregulation of miR-539, which directly targets EGFR (71). Lidocaine also minimizes the progression of retinoblastoma both in vitro and in vivo by downregulating EGFR expression through the upregulation of miR-520a-3p (77).
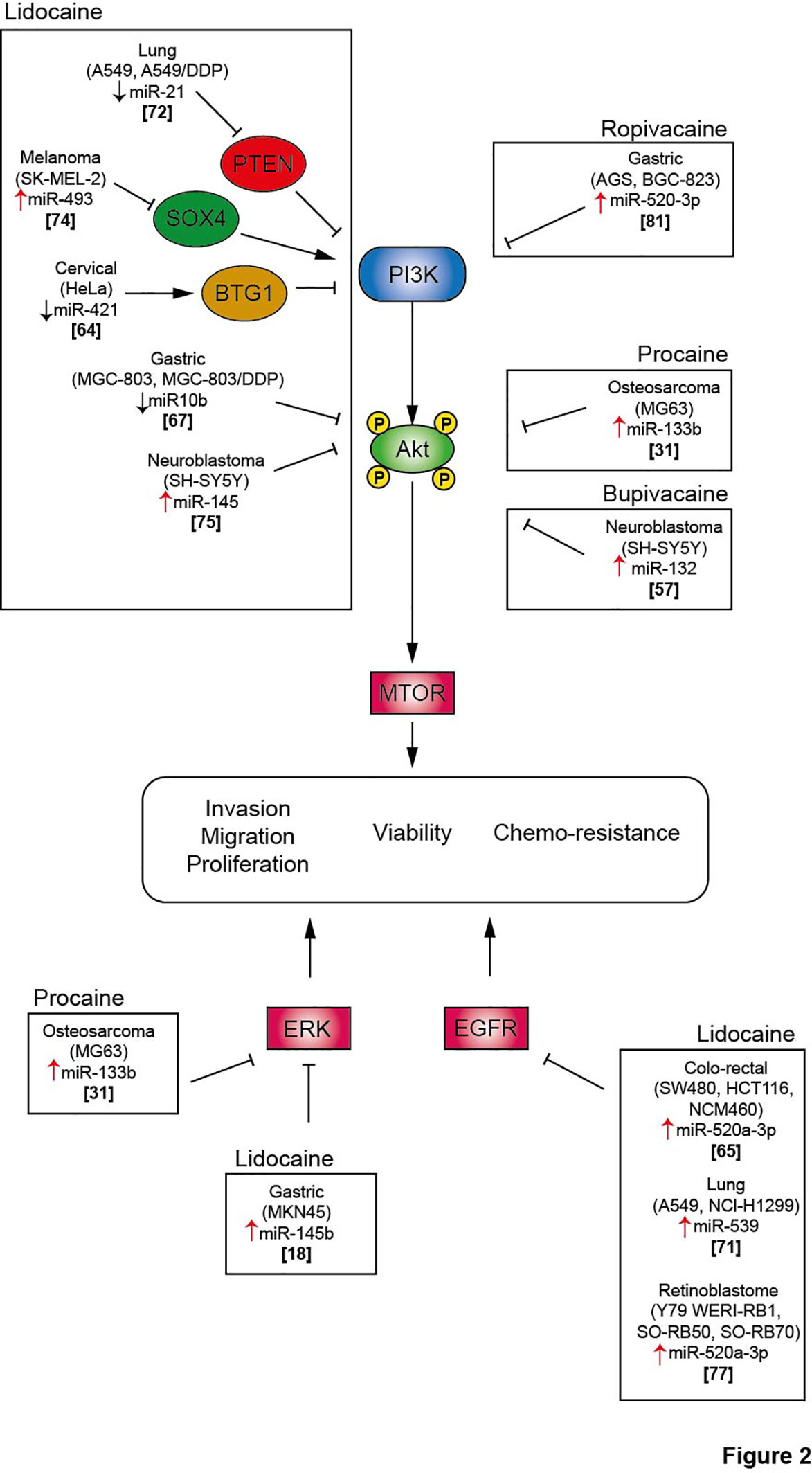
Figure 2 Local anesthetics inhibit cell proliferation, migration and invasion and promote cancer cell death via inhibition of several signaling pathway. Akt, protein kinase B; BTG1, B cell translocation gene 1; DDP, cisplatin; EGFR, Epithelial growth factor receptor; ERK, extracellular signal-regulated kinase; mTOR, mammalian Target of Rapamycin; PI3K, phosphoinositide-3 kinase; PTEN, Phosphatase and TENsin homolog; SOX4, SRY-Box Transcription Factor 4.
The extracellular signal-regulated kinases (ERK) signaling pathway is also impacted by the modulation of miRNA expression induced by LA. In a model of osteosarcoma, procaine significantly blocked the proliferation and migration of tumor cells and promoted apoptosis by upregulating miR-133b. In parallel, the level of p/t-ERK was profoundly decreased. The employment of miR-133b inhibitors reversed all the observed effects including the phosphorylation of ERK, revealing the interaction between this pathway and non-coding RNAs (31). Interestingly, the regulation of miRNAs by LA can target several pathways, thus inducing synergistic effect. Thus, lidocaine can upregulate the expression of miR-145b, which simultaneously inactivates both ERK and NF-κB pathways, potentiating the inhibition of proliferation, migration and invasion of malignant gastric cells (18).
Interestingly, different modalities of cell death triggered by epigenetic modulation were observed after LA treatment. The upregulation of miR-145 by lidocaine promoted autophagic flux in neuroblastoma SH-SY5Y cells (75). Lidocaine and levobupivacaine both induced ferroptosis by upregulating miR-382-5p and miR-489-3p, respectively (19, 63). The impact of LA on cellular stress and death pathways via the control of non-coding RNA emphasizes the possibility to use LA as novel antineoplastic therapeutics.
Finally, several reports suggest an intertwined regulation of multiple non-coding RNAs by LA. Indeed, lncRNAs and circular RNAs (circRNAs), a group of non-coding RNAs described to be involved in oncogenesis, may act as miRNA sponges. In a model of glioma, the treatment with ropivacaine suppressed tumor progression by upregulating the circRNA circSCAF11, while downregulating miR-145-5p (30). Inversely, bupivacaine decreased the expression of circ_0000376 while enhancing miR-145-5p in gastric cancer cells (61). Lidocaine hampered the proliferation of colorectal cancer cells by upregulating circlTFG2 and then decreasing miR-1204 (66). In a model of gastric cancer, lidocaine hindered tumor progression by modulating the miR-21-5p/LIFR axis via the overexpression of circ-ANO5 (68). Bupivacaine impeded neuroblastoma progression by modifying the expression of various long non-coding RNAs (ZFAS1, MALAT1, LINC00665, which sponged protumorigenic miR-421, miR-101-3-3p and miR-34a-5p, respectively) (58–60).
Local anesthetics repress histone acetylation in cancer cells
Previous publications reported that levobupivacaine, an amino amide LA widely used to control acute surgical pain, possesses the capacity to attenuate the oncological properties of several cancer types (86, 87). However, the mechanisms by which levobupivacaine exerts its anticancer activity remain poorly characterized. Lysine acetyltransferase 5 (KAT5) acetylates both non-histone and histone proteins and increases the invasiveness of cancer cells (88). Levobupivacaine inhibits the expression of KAT5 in osteosarcoma cells, thus inhibiting their proliferation and limiting their survival (22). This preclinical finding demonstrated the implication of LA in epigenetic changes on histones leading to anticancer properties. Interestingly, the inhibition of histone acetyltransferase activity decreases opioid-induced hyperalgesia in mice (89). Nevertheless, the impact of LA on histone modification as well as the oncological consequences remain unclear, calling for future exploration.
Discussion
The reversal of cancer-associated epigenetic dysregulations represents one possible antineoplastic strategy. Various demethylating molecules were characterized at the preclinical level (as exemplified by curcumin, (−)-epigallocatechin-3-gallate, N-phthalyl-tryptophan and zebularine) (90–94), and two agents (5-azacytidine and decitabine) have been approved by the FDA and EMA to treat patients with myelodysplastic syndrome or acute myeloid leukemia. These agents inhibit DNMT and hence reduce the global DNA methylation level in cancer cells. Despite their established anti-tumor activity, 5-azacytidine and decitabine induce severe myelosuppression, thus calling for the identification of novel epigenetic modulators.
Surprisingly, LA mediate significant antineoplastic activities by directly killing cancer cells and indirectly by eliciting anticancer immune responses (27, 32, 33, 37, 79, 95, 96). The detailed molecular comprehension of these effects may open a novel era in onco-anesthesia. Notably, the discovery of LA-promoted antitumor effects involving the induction of apoptosis secondary to the reduction of DNA methylation or the modulation of miRNAs has spurred much interest (18, 20, 30, 31, 67). Both amide and ester-type local anesthetics reduce global methylation levels in the promoter regions of tumor suppressor genes as a result of the inhibited interaction of DNMT with DNA. However, most preclinical studies have not yet investigated the effects of LA on the methylation of promoters of specific tumor suppressor genes as well as on the mRNA expression of such genes.
Beyond their effects on DNA methylation, LA also modulate (enhance or reduce) the expression of miRNAs in cancer cells, as summarized in a previous review (97). Compared to this published work, our review is the first one to critically evaluate all epigenetic changes induced by LA, including demethylating effects as well as miRNA regulation and histone acetylation, and to discuss their putative synergistic interaction with 5-azacytidine, decitabine and cytotoxicants. We surmise that the epigenetic effects of LA could be clinically relevant. Indeed, LA are well-known analgesics with a favorable toxicological profile that are commonly used during oncological intervention. A positive clinical impact of LA on cancer recurrence would provide a low-risk and low-cost benefit to oncological patients. However, before such a conclusion can be reached, further clinical and translational research must confirm the capacity of LA to improve the outcome of surgical procedures, especially if they are preceded or followed by (neo)adjuvant chemotherapy or immunotherapy. It will be particularly important to investigate the short-term (intra-operational) and long-term (post-operational) effects of LA on epigenetic signatures including DNA methylation patterns and the expression of non-coding RNAs in further translational studies.
Author contributions
LB, OK and GK wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
OK is supported by Institut National du Cancer (INCa) and the DIM Elicit of the Ile-de-France. LB received a research grant by Bristol Myers Squibb Foundation France. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; INCa; Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Acknowledgments
The authors are grateful to the support of Gustave Roussy Cancer Campus, Université Paris-Saclay.
Conflict of interest
OK is scientific co-founder of Samsara Therapeutics. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, Osasuna, PharmaMar, Samsara, Sanofi, Sotio, Vascage and Vasculox/Tioma. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Ca2+, calcium ion; DAC, dacogen (decitabine); DNMT, DNA methyltransferase; EGFR, Epithelial Growth Factor Receptor; EMA, European Medicines Agency; ERK, Extracellular signal-Regulated Kinases; FDA, Food and Drug Administration; 5-FU, 5 fluorouracil; IL, interleukin; LA, local anesthetics; mTOR, mammalian target of rapamycin; NK, natural killer cells; RARβ, retinoic acid receptor β; RASSF1A, Ras association domain family 1A.
References
1. Deltour S, Chopin V, Leprince D. Epigenetics and cancer. Medecine Sci M/S. (2005) 21(4):405–11. doi: 10.1051/medsci/2005214405
2. Esteller M. Relevance of DNA methylation in the management of cancer. Lancet Oncol (2003) 4(6):351–8. doi: 10.1016/s1470-2045(03)01115-x
3. Bezu L, Chuang AW, Liu P, Kroemer G, Kepp O. Immunological effects of epigenetic modifiers. Cancers (2019) 11(12):1–20. doi: 10.3390/cancers11121911
4. Liu X, Zhao S, Sui H, Liu H, Yao M, Su Y, et al. MicroRNAs/LncRNAs modulate MDSCs in tumor microenvironment. Front Oncol (2022) 12:772351. doi: 10.3389/fonc.2022.772351
5. Liu QW, He Y, Xu WW. Molecular functions and therapeutic applications of exosomal noncoding RNAs in cancer. Exp Mol Med (2022) 54(3):216–25. doi: 10.1038/s12276-022-00744-w
6. Liu T, Jiang F, Yu LY, Wu YY. Lidocaine represses proliferation and cisplatin resistance in cutaneous squamous cell carcinoma via miR-30c/SIRT1 regulation. Bioengineered. (2022) 13(3):6359–70. doi: 10.1080/21655979.2022.2031419
7. Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R Jr., Srikantan S, et al. p16(INK4a) translation suppressed by miR-24. PloS One (2008) 3(3):e1864. doi: 10.1371/journal.pone.0001864
8. He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. (2005) 435(7043):828–33. doi: 10.1038/nature03552
9. Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci (2006) 31(2):89–97. doi: 10.1016/j.tibs.2005.12.008
10. Okano M, Bell DW, Haber DA, Li E. DNA Methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. (1999) 99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6
11. Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem (2005) 74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721
12. Ruter B, Wijermans PW, Lubbert M. DNA Methylation as a therapeutic target in hematologic disorders: recent results in older patients with myelodysplasia and acute myeloid leukemia. Int J hematology. (2004) 80(2):128–35. doi: 10.1532/ijh97.04094
13. Jabbour E, Issa JP, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer. (2008) 112(11):2341–51. doi: 10.1002/cncr.23463
14. Li K, Yang J, Han X. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARbeta2 and RASSF1A demethylation. Int J Mol Sci (2014) 15(12):23519–36. doi: 10.3390/ijms151223519
15. Li YC, Wang Y, Li DD, Zhang Y, Zhao TC, Li CF. Procaine is a specific DNA methylation inhibitor with anti-tumor effect for human gastric cancer. J Cell Biochem (2018) 119(2):2440–9. doi: 10.1002/jcb.26407
16. Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J anaesthesia. (2012) 109(2):200–7. doi: 10.1093/bja/aes128
17. Sabit H, Samy MB, Said OA, El-Zawahri MM. Procaine induces epigenetic changes in HCT116 colon cancer cells. Genet Res Int (2016) 2016:8348450. doi: 10.1155/2016/8348450
18. Sui H, Lou A, Li Z, Yang J. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC cancer. (2019) 19(1):233. doi: 10.1186/s12885-019-5431-9
19. Sun D, Li YC, Zhang XY. Lidocaine promoted ferroptosis by targeting miR-382-5p /SLC7A11 axis in ovarian and breast cancer. Front Pharmacol (2021) 12:681223. doi: 10.3389/fphar.2021.681223
20. Tada M, Imazeki F, Fukai K, Sakamoto A, Arai M, Mikata R, et al. Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol Int (2007) 1(3):355–64. doi: 10.1007/s12072-007-9014-5
21. Villar-Garea A, Fraga MF, Espada J, Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res (2003) 63(16):4984–9.
22. Wang Z, Song Y, Zhang H, Yang Y, Zhang S, Wang W. Local anesthetic levobupivacaine inhibits stemness of osteosarcoma cells by epigenetically repressing MAFB though reducing KAT5 expression. Aging. (2022) 14(6):2793–804. doi: 10.18632/aging.203975
23. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. (2008) 109(2):180–7. doi: 10.1097/ALN.0b013e31817f5b73
24. D'Agostino G, Saporito A, Cecchinato V, Silvestri Y, Borgeat A, Anselmi L, et al. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br J anaesthesia. (2018) 121(4):962–8. doi: 10.1016/j.bja.2018.07.015
25. Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Regional Anesth Pain Med (2010) 35(6):490–5. doi: 10.1097/AAP.0b013e3181ef4d05
26. Hiller JG, Hacking MB, Link EK, Wessels KL, Riedel BJ. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta anaesthesiologica Scandinavica. (2014) 58(3):281–90. doi: 10.1111/aas.12255
27. Jiang Y, Gou H, Zhu J, Tian S, Yu L. Lidocaine inhibits the invasion and migration of TRPV6-expressing cancer cells by TRPV6 downregulation. Oncol letters. (2016) 12(2):1164–70. doi: 10.3892/ol.2016.4709
28. Lu J, Xu SY, Zhang QG, Xu R, Lei HY. Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol (2011) 657(1-3):51–8. doi: 10.1016/j.ejphar.2011.01.055
29. Wang W, Zhu M, Xu Z, Li W, Dong X, Chen Y, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res (2019) 52(1):36. doi: 10.1186/s40659-019-0242-7
30. Yin D, Liu L, Shi Z, Zhang L, Yang Y. Ropivacaine inhibits cell proliferation, migration and invasion, whereas induces oxidative stress and cell apoptosis by circSCAF11/miR-145-5p axis in glioma. Cancer Manage Res (2020) 12:11145–55. doi: 10.2147/CMAR.S274975
31. Ying B, Huang H, Li H, Song M, Wu S, Ying H. Procaine inhibits proliferation and migration and promotes cell apoptosis in osteosarcoma cells by upregulation of MicroRNA-133b. Oncol Res (2017) 25(9):1463–70. doi: 10.3727/096504017X14878518291077
32. Zhang Y, Peng X, Zheng Q. Ropivacaine inhibits the migration of esophageal cancer cells via sodium-channel-independent but prenylation-dependent inhibition of Rac1/JNK/paxillin/FAK. Biochem Biophys Res Commun (2018) 501(4):1074–9. doi: 10.1016/j.bbrc.2018.05.110
33. Zheng Q, Peng X, Zhang Y. Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of ras and RhoA signaling independent of sodium channel blockade. BMC anesthesiology. (2020) 20(1):43. doi: 10.1186/s12871-020-00957-4
34. Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. (2006) 105(4):660–4. doi: 10.1097/00000542-200610000-00008
35. Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res (2000) 10(2):165–9. doi: 10.1097/00008390-200004000-00009
36. Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. (2016) 7(12):15262–73. doi: 10.18632/oncotarget.7683
37. Chen J, Jiao Z, Wang A, Zhong W. Lidocaine inhibits melanoma cell proliferation by regulating ERK phosphorylation. J Cell Biochem (2019) 120(4):6402–8. doi: 10.1002/jcb.27927
38. Piegeler T, Schlapfer M, Dull RO, Schwartz DE, Borgeat A, Minshall RD, et al. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFalpha-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of akt and focal adhesion kinase. Br J anaesthesia. (2015) 115(5):784–91. doi: 10.1093/bja/aev341
39. Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J anaesthesia. (2006) 97(5):640–6. doi: 10.1093/bja/ael217
40. Xing W, Chen DT, Pan JH, Chen YH, Yan Y, Li Q, et al. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology. (2017) 126(5):868–81. doi: 10.1097/ALN.0000000000001528
41. Zhang X, Pang W, Liu H, Wang J. Lidocine potentiates the cytotoxicity of 5-fluorouracil to choriocarcinoma cells by downregulating ABC transport proteins expression. J Cell Biochem (2019) 120(10):16533–42. doi: 10.1002/jcb.28913
42. Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. (2019) 394(10211):1807–15. doi: 10.1016/S0140-6736(19)32313-X
43. Bezu L, Wu Chuang A, Sauvat A, Humeau J, Xie W, Cerrato G, et al. Local anesthetics elicit immune-dependent anticancer effects. J immunotherapy Cancer (2022) 10(4):1–17. doi: 10.1136/jitc-2021-004151
44. Lirk P, Hollmann MW, Fleischer M, Weber NC, Fiegl H. Lidocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in breast cancer cells in vitro. Br J anaesthesia (2014) 113 Suppl 1:i32–8. doi: 10.1093/bja/aeu201
45. Chen D, Yan Y, Xie J, Pan J, Chen Y, Li Q, et al. Amide-type local anesthetics may suppress tumor cell proliferation and sensitize human hepatocellular carcinoma cells to cisplatin via upregulation of RASSF1A expression and demethylation. J Cancer. (2020) 11(24):7312–9. doi: 10.7150/jca.46630
46. Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, et al. Procaine and procainamide inhibit the wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep (2009) 22(6):1479–84. doi: 10.3892/or_00000590
47. Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res (2006) 66(5):2794–800. doi: 10.1158/0008-5472.CAN-05-2821
48. Lessans S, Dorsey SG. The role for epigenetic modifications in pain and analgesia response. Nurs Res practice. (2013) 2013:961493. doi: 10.1155/2013/961493
49. Lirk P, Fiegl H, Weber NC, Hollmann MW. Epigenetics in the perioperative period. Br J Pharmacol (2015) 172(11):2748–55. doi: 10.1111/bph.12865
50. Caputi FF, Carboni L, Rullo L, Alessandrini I, Balzani E, Melotti RM, et al. An exploratory pilot study of changes in global DNA methylation in patients undergoing major breast surgery under opioid-based general anesthesia. Front Pharmacol (2021) 12:733577. doi: 10.3389/fphar.2021.733577
51. Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med (2019) 25(9):1428–41. doi: 10.1038/s41591-019-0566-4
52. Khabbazi S, Hassanshahi M, Hassanshahi A, Peymanfar Y, Su YW, Xian CJ. Opioids and matrix metalloproteinases: the influence of morphine on MMP-9 production and cancer progression. Naunyn-Schmiedeberg's Arch Pharmacol (2019) 392(2):123–33. doi: 10.1007/s00210-019-01613-6
53. Liu Z, Cheng S, Fu G, Ji F, Wang C, Cao M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci (2020) 251:117604. doi: 10.1016/j.lfs.2020.117604
54. Sandoval-Sierra JV, Salgado Garcia FI, Brooks JH, Derefinko KJ, Mozhui K. Effect of short-term prescription opioids on DNA methylation of the OPRM1 promoter. Clin epigenetics. (2020) 12(1):76. doi: 10.1186/s13148-020-00868-8
55. Chidambaran V, Zhang X, Martin LJ, Ding L, Weirauch MT, Geisler K, et al. DNA Methylation at the mu-1 opioid receptor gene (OPRM1) promoter predicts preoperative, acute, and chronic postsurgical pain after spine fusion. Pharmacogenomics personalized Med (2017) 10:157–68. doi: 10.2147/PGPM.S132691
56. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal transduction targeted Ther (2016) 1:15004. doi: 10.1038/sigtrans.2015.4
57. Zhang H, Lin J, Hu T, Ren Z, Wang W, He Q. Effect of miR-132 on bupivacaine-induced neurotoxicity in human neuroblastoma cell line. J Pharmacol Sci (2019) 139(3):186–92. doi: 10.1016/j.jphs.2019.01.014
58. Yuan L, Xu H, Guo R, Lu T, Li X. Long non-coding RNA ZFAS1 alleviates bupivacaine-induced neurotoxicity by regulating the miR-421/zinc finger protein564 (ZNF564) axis. Bioengineered. (2021) 12(1):5231–40. doi: 10.1080/21655979.2021.1960776
59. Zhao Y, Ai Y. Knockdown of lncRNA MALAT1 alleviates bupivacaine-induced neurotoxicity via the miR-101-3p/PDCD4 axis. Life Sci (2019) 232:116606. doi: 10.1016/j.lfs.2019.116606
60. Yang Z, Hu S, He Y, Ji L. LINC00665 rescues bupivacaine induced neurotoxicity in human neural cell of SH-SY5Y through has-miR-34a-5p. Brain Res bulletin. (2021) 177:210–6. doi: 10.1016/j.brainresbull.2021.10.004
61. Ju C, Zhou J, Miao H, Chen X, Zhang Q. Bupivacaine suppresses the progression of gastric cancer through regulating circ_0000376/miR-145-5p axis. BMC anesthesiology. (2020) 20(1):275. doi: 10.1186/s12871-020-01179-4
62. Lin CY, Tseng WT, Chang YY, Tsai MH, Chuang EY, Lu TP, et al. Lidocaine and bupivacaine downregulate MYB and DANCR lncRNA by upregulating miR-187-5p in MCF-7 cells. Front Med (2021) 8:732817. doi: 10.3389/fmed.2021.732817
63. Mao SH, Zhu CH, Nie Y, Yu J, Wang L. Levobupivacaine induces ferroptosis by miR-489-3p/SLC7A11 signaling in gastric cancer. Front Pharmacol (2021) 12:681338. doi: 10.3389/fphar.2021.681338
64. Zhu J, Han S. Lidocaine inhibits cervical cancer cell proliferation and induces cell apoptosis by modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am J Trans Res (2019) 11(9):5404–16.
65. Qu X, Yang L, Shi Q, Wang X, Wang D, Wu G. Lidocaine inhibits proliferation and induces apoptosis in colorectal cancer cells by upregulating mir-520a-3p and targeting EGFR. Pathology Res Pract (2018) 214(12):1974–9. doi: 10.1016/j.prp.2018.09.012
66. Wang H, Zhang X, Li Y, Li Y, Pang T. Lidocaine hampers colorectal cancer process via circITFG2/miR-1204/SOCS2 axis. Anti-cancer Drugs (2022) 33(3):235–44. doi: 10.1097/CAD.0000000000001091
67. Zhang X, Gu G, Li X, Zhang C. Lidocaine alleviates cisplatin resistance and inhibits migration of MGC-803/DDP cells through decreasing miR-10b. Cell Cycle (2020) 19(19):2530–7. doi: 10.1080/15384101.2020.1809914
68. Guan E, Liu H, Xu N. Lidocaine suppresses gastric cancer development through Circ_ANO5/miR-21-5p/LIFR axis. Digestive Dis Sci (2022) 67(6):2244–56. doi: 10.1007/s10620-021-07055-6
69. Wen J, Li X, Ding Y, Zheng S, Xiao Y. Lidocaine inhibits glioma cell proliferation, migration and invasion by modulating the circEZH2/miR-181b-5p pathway. Neuroreport. (2021) 32(1):52–60. doi: 10.1097/WNR.0000000000001560
70. Zhao L, Ma N, Liu G, Mao N, Chen F, Li J. Lidocaine inhibits hepatocellular carcinoma development by modulating circ_ITCH/miR-421/CPEB3 axis. Digestive Dis Sci (2021) 66(12):4384–97. doi: 10.1007/s10620-020-06787-1
71. Sun H, Sun Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artif cells nanomedicine Biotechnol (2019) 47(1):2866–74. doi: 10.1080/21691401.2019.1636807
72. Yang Q, Zhang Z, Xu H, Ma C. Lidocaine alleviates cytotoxicity-resistance in lung cancer A549/DDP cells via down-regulation of miR-21. Mol Cell Biochem (2019) 456(1-2):63–72. doi: 10.1007/s11010-018-3490-x
73. Zi H, Chen L, Ruan Q. Lidocaine represses the malignant behavior of lung carcinoma cells via the circ_PDZD8/miR-516b-5p/GOLT1A axis. Histol histopathology. (2022) 21:18423. doi: 10.14670/HH-18-423
74. Wang Y, Xie J, Liu W, Zhang R, Huang S, Xing Y. Lidocaine sensitizes the cytotoxicity of 5-fluorouacil in melanoma cells via upregulation of microRNA-493. Die Pharmazie. (2017) 72(11):663–9. doi: 10.1691/ph.2017.7616
75. Wang Z, Liu Q, Lu J, Cao J, Wang XY, Chen Y. Lidocaine promotes autophagy of SH-SY5Y cells through inhibiting PI3K/AKT/mTOR pathway by upregulating miR-145. Toxicol Res (2020) 9(4):467–73. doi: 10.1093/toxres/tfaa049
76. Zhang Y, Liu L, Xue P, Wang L. Long noncoding RNA LINC01347 modulated lidocaine-induced cytotoxicity in SH-SY5Y cells by interacting with hsa-miR-145-5p. Neurotoxicity Res (2021) 39(5):1440–8. doi: 10.1007/s12640-021-00363-9
77. Xia W, Wang L, Yu D, Mu X, Zhou X. Lidocaine inhibits the progression of retinoblastoma in vitro and in vivo by modulating the miR520a3p/EGFR axis. Mol Med Rep (2019) 20(2):1333–42. doi: 10.3892/mmr.2019.10363
78. Zhao L, Han S, Hou J, Shi W, Zhao Y, Chen Y. The local anesthetic ropivacaine suppresses progression of breast cancer by regulating miR-27b-3p/YAP axis. Aging. (2021) 13(12):16341–52. doi: 10.18632/aging.203160
79. Chen X, Liu W, Guo X, Huang S, Song X. Ropivacaine inhibits cervical cancer cell growth via suppression of the miR96/MEG2/pSTAT3 axis. Oncol Rep (2020) 43(5):1659–68. doi: 10.3892/or.2020.7521
80. Lu Y, Yang C, Zhang L, Ding J. Ropivacaine retards the viability, migration, and invasion of choriocarcinoma cells by regulating the long noncoding RNA OGFRP1/MicroRNA-4731-5p/HIF3A axis. Mol Biotechnol (2022) 64(5):499–09. doi: 10.1007/s12033-021-00429-1
81. Zhang N, Xing X, Gu F, Zhou G, Liu X, Li B. Ropivacaine inhibits the growth, migration and invasion of gastric cancer through attenuation of WEE1 and PI3K/AKT signaling via miR-520a-3p. OncoTargets Ther (2020) 13:5309–21. doi: 10.2147/OTT.S244550
82. Liu R, Wu M, Xu G, Ju L, Xiao J, Zhong W, et al. Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis. Open Life Sci (2020) 15(1):988–99. doi: 10.1515/biol-2020-0108
83. Deng Z, Jian Y, Cai H. Ropivacaine represses the proliferation, invasion, and migration of glioblastoma via modulating the microRNA-21-5p/KAT8 regulatory NSL complex subunit 2 axis. Bioengineered. (2022) 13(3):5975–86. doi: 10.1080/21655979.2022.2037955
84. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. (2007) 450(7170):736–40. doi: 10.1038/nature06322
85. Gong X, Dan J, Li F, Wang L. Suppression of mitochondrial respiration with local anesthetic ropivacaine targets breast cancer cells. J Thorac disease. (2018) 10(5):2804–12. doi: 10.21037/jtd.2018.05.21
86. Kwakye AK, Kampo S, Lv J, Ramzan MN, Richard SA, Falagan AA, et al. Levobupivacaine inhibits proliferation and promotes apoptosis of breast cancer cells by suppressing the PI3K/Akt/mTOR signalling pathway. BMC Res notes. (2020) 13(1):386. doi: 10.1186/s13104-020-05191-2
87. Li T, Chen L, Zhao H, Wu L, Masters J, Han C, et al. Both bupivacaine and levobupivacaine inhibit colon cancer cell growth but not melanoma cells in vitro. J anesthesia. (2019) 33(1):17–25. doi: 10.1007/s00540-018-2577-6
88. Kwan SY, Sheel A, Song CQ, Zhang XO, Jiang T, Dang H, et al. Depletion of TRRAP induces p53-independent senescence in liver cancer by down-regulating mitotic genes. Hepatology. (2020) 71(1):275–90. doi: 10.1002/hep.30807
89. Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J pain. (2013) 14(1):36–47. doi: 10.1016/j.jpain.2012.10.005
90. Cheng JC, Matsen CB, Gonzales FA, Ye W, Greer S, Marquez VE, et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Institute. (2003) 95(5):399–409. doi: 10.1093/jnci/95.5.399
91. Cheng JC, Weisenberger DJ, Gonzales FA, Liang G, Xu GL, Hu YG, et al. Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol Cell Biol (2004) 24(3):1270–8. doi: 10.1128/MCB.24.3.1270-1278.2004
92. Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res (2003) 63(22):7563–70.
93. Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, et al. Curcumin is a potent DNA hypomethylation agent. Bioorganic medicinal Chem letters. (2009) 19(3):706–9. doi: 10.1016/j.bmcl.2008.12.041
94. Schneeberger Y, Stenzig J, Hubner F, Schaefer A, Reichenspurner H, Eschenhagen T. Pharmacokinetics of the experimental non-nucleosidic DNA methyl transferase inhibitor n-Phthalyl-L-Tryptophan (RG 108) in rats. Basic Clin Pharmacol toxicology. (2016) 118(5):327–32. doi: 10.1111/bcpt.12514
95. Yoon JR, Whipple RA, Balzer EM, Cho EH, Matrone MA, Peckham M, et al. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res Treat (2011) 129(3):691–701. doi: 10.1007/s10549-010-1239-7
96. Dan J, Gong X, Li D, Zhu G, Wang L, Li F. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2018) 103:823–8. doi: 10.1016/j.biopha.2018.04.106
Keywords: local anesthetics, epigenetic, cancer, demethylation, miRNA
Citation: Bezu L, Kepp O and Kroemer G (2022) Impact of local anesthetics on epigenetics in cancer. Front. Oncol. 12:849895. doi: 10.3389/fonc.2022.849895
Received: 06 January 2022; Accepted: 01 August 2022;
Published: 30 August 2022.
Edited by:
Yinghong Shi, Fudan University, ChinaReviewed by:
Ashish Goyal, German Cancer Research Center (DKFZ), GermanyHao Fang, Fudan University, China
Copyright © 2022 Bezu, Kepp and Kroemer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucillia Bezu, bHVjaWxsaWFiZUBnbWFpbC5jb20=; Guido Kroemer, a3JvZW1lckBvcmFuZ2UuZnI=
 Lucillia Bezu
Lucillia Bezu Oliver Kepp
Oliver Kepp Guido Kroemer
Guido Kroemer