
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 July 2022
Sec. Pediatric Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.848877
This article is part of the Research TopicHot Topics in PediatricsView all 50 articles
 Changjuan Zeng1,2,3,4†
Changjuan Zeng1,2,3,4† Minglei Han2,3†
Minglei Han2,3† Jiayan Fan2,3†
Jiayan Fan2,3† Xiaoyu He2,3
Xiaoyu He2,3 Renbing Jia2,3
Renbing Jia2,3 Li Li4,5*
Li Li4,5* Xuyang Wen2,3*
Xuyang Wen2,3* Xuefei Song2,3*
Xuefei Song2,3* Lili Hou4*
Lili Hou4*Background: Retinoblastoma (Rb) is a common ocular malignant tumor in children. Intra-arterial chemotherapy (IAC) has been widely used in children with Rb and has achieved an ideal therapeutic effect. However, IAC has side effects, including anemia and bone marrow suppression, for which explicit evidence on the risk factors is lacking. This study aimed to evaluate the covariates that may affect the occurrence of anemia and bone marrow suppression in children with Rb after IAC.
Methods: Children with Rb admitted between May 2019 and January 2021 were included into the study. The differences in the number of children with anemia and bone marrow suppression before and after IAC according to different covariates were recorded. All potential impact factors were included into the univariate and multivariate regression models to identify the related covariates of post-IAC anemia and bone marrow suppression.
Results: Data of 282 children with Rb were retrospectively collected. After IAC, children with Rb had increased severities of anemia (p <0.0001, chi-square test) and bone marrow suppression (p = 0.001, chi-square test). Moreover, the number of children with Rb who had an increased cross-level change in the severity of anemia and degree of bone marrow suppression was 80 (41.24%) and 64 (32.49%), respectively. The univariate regression analysis showed that numerous factors (such as pre-IAC intravenous chemotherapy, results of pre-IAC routine blood tests, and some serological indicators for liver and kidney function) affected the anemia severity and degree of bone marrow suppression in children with Rb after IAC. Additionally, the predictive model of the multivariate regression could predict anemia and bone marrow suppression.
Conclusion: Children with Rb may have an increased risk of anemia and bone marrow suppression after IAC, but this is temporary and can be influenced by several factors. Therefore, IAC should be maintained as the standard of care. We generated predictive equations for predicting anemia severity and degree of bone marrow suppression, which can guide the prediction and timely control of anemia and bone marrow suppression after IAC.
Retinoblastoma (Rb) is a common ocular malignant tumor in children. Rb occurs in 1 out of every 15,000–20,000 newborns and accounts for 4% of all malignant tumors in children (1). If the tumor is confined within the eye, it can be controlled by enucleation (2). If the tumor spreads to the entire eyeball or body, the survival rate is greatly reduced (3, 4). The 1-, 3-, and 5-year overall survival rates of patients with retinoblastoma are 95%, 86%, and 83%, respectively (5).
Rb treatment has gradually changed from enucleation and radiotherapy to chemoreduction and local treatment (6, 7). Chemotherapy can effectively reduce the tumor volume and create favorable conditions for local surgical treatment and is currently one of the primary treatment methods for Rb in children (8). It is divided into systemic intravenous chemotherapy and intra-arterial chemotherapy (IAC) (9, 10). Intravenous chemotherapy (IVC) is a primary treatment method for bilateral cases and is the only available treatment in low-income countries. As a postoperative adjuvant treatment, IVC can largely prevent distant metastasis in patients with high-risk pathological factors after eyeball enucleation (9, 10). However, the blood–retinal barrier can limit the effect of drugs on the tumor site, resulting in unsatisfactory chemotherapy effects and an increased risk of systemic side effects. Unlike in intravenous chemotherapy, in IAC, drugs are delivered through the arterial system under digital subtraction angiography and are delivered directly into the tumor via the ophthalmic artery (11). This increases the drug concentration in the tumor tissue, while reducing it in normal tissues, thereby reducing systemic side effects (12).
Previous studies have shown that patients with Rb have varying severities of anemia and degrees of myelosuppression after IAC, but there is no explicit evidence of their risk factors (13, 14). Therefore, it is difficult to intervene before IAC in anticipation of IAC-related complications. Our study aimed to comprehensively evaluate the covariates and risk factors of anemia and bone marrow suppression in children with Rb after IAC.
All the patients in this study were from the Department of Ophthalmology, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The patients’ treatment information, including surgical records, was collected from the electronic medical record system. The doctors who performed interventional treatments on these children had extensive clinical experience. The patients’ medical information, including medication status, intervention process, and serological examination reports of patients, were stored in an encrypted public network disk and was accessible only to the clinician who processed the data. This study was approved by the Ninth people’s hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2019-T291-3), and the need for informed consent was waived, owing to the retrospective nature of the study.
We performed a retrospective study and used a multivariate regression analysis to summarize the clinical data and assess the risk of anemia and bone marrow suppression in children with Rb after IAC.
Children with Rb treated with IAC between May 2019 and January 2021 were enrolled in this study.
Inclusion criteria:
1) Clinical diagnosis of Rb.
2) Children who met the indications for IAC and were treated accordingly.
3) No history of central system diseases, such as brain metastasis or epilepsy.
Exclusion criteria:
1) Children with severe systemic diseases.
2) Children with iodine contrast agent allergy.
3) Children suffering from systemic blood diseases.
4) Children with severe anemia [hemoglobin (Hb) level ≤60 g/L] or bone marrow suppression [white blood cell (WBC) count <2.0 × 109/L, Hb <80 g/L, and platelet (PLT) count <50 × 109/L] before IAC.
After anesthesia was administered, the surgical site was disinfected, and the femoral artery on the affected side was punctured using the Seldinger technique. We then inserted the vascular sheath and injected heparin. Under radiographic guidance, a Cobra catheter was inserted into the internal carotid artery on the affected side. Thereafter, the intensifier was turned to an angle of 90°, and the head image was captured in a lateral position. A contrast agent for digital subtraction angiography of the internal carotid artery was administered manually. After identifying the ophthalmic artery, the ev3 micro-guide wire was used to guide the 1.7F ev3 45° micro-catheter for ophthalmic artery intubation. After the angiography was performed, intra-ophthalmic arterial chemotherapy was administered. The IAC regimen was a 2- or 3-drug regimen (melphalan, topotecan, carboplatin, or a combination thereof); 3–4 cycles were administered. The dose of chemotherapeutic drugs increased with age and tumor size (melphalan, 3.5–7.5 mg; topotecan, 1 mg; and carboplatin, 20 mg) (15). When intubation of the ophthalmic artery was not feasible, the route of administration was the main primary ophthalmic artery (OA) pathway or the external carotid artery (ECA) pathway. The alternative ECA pathway does not affect IAC efficacy in Rb treatment (16). The sheath was removed postoperatively.
The data retrieved from the medical charts included sex, age, duration of Rb, affected eyes (left, right, or bilateral), International Intraocular Retinoblastoma Classification group at diagnosis, times of IAC, chemotherapy drugs (melphalan + topotecan, melphalan + carboplatin), duration of surgery, duration of heparin administration, height, weight, head circumference, chest circumference, operating doctors’ details, and whether chemotherapy (intravenous chemotherapy or arterial chemotherapy) was administered 6 months before this investigation
Children with Rb were divided into the no anemia (Hb, >120 g/L), mild anemia (Hb, 91–120 g/L), moderate anemia (Hb, 61–90 g/L), severe anemia (Hb, 31–60 g/L), and extreme anemia (Hb, <30 g/L) groups according to the Hb levels. The difference in anemia severity before and after IAC was compared.
The degree of bone marrow suppression was evaluated according to the grading standard of acute and subacute toxicity of anti-cancer drugs of the World Health Organization. According to the Hb, WBC, and PLT levels during routine blood tests, the degree of bone marrow suppression was divided into grade 0 (WBC >4.0 × 109/L, Hb >110 g/L, or PLT ≥100 × 109/L), grade I (3.0< WBC ≤4.0 × 109/L, 95< Hb ≤110 g/L, or 75< PLT ≤99 × 109/L), grade II (2.0< WBC ≤3.0 × 109/L, 80< Hb ≤95 g/L, or 50< PLT ≤75 × 109/L), grade III (1.0< WBC ≤2.0 × 109/L, 65< Hb ≤80 g/L, or 25< PLT ≤50 × 109/L), and grade IV (WBC ≤1.0 × 109/L, Hb ≤65 g/L, or PLT ≤25 × 109/L). We compared the changes in WBC, Hb, and PLT levels and graded the changes in bone marrow suppression before and after IAC.
To fully understand the factors that affect the occurrence of anemia and bone marrow suppression in children with Rb after IAC, we incorporated all covariates and other factors that may affect the results (such as routine blood tests, liver and kidney function, and arterial or intravenous chemotherapy before IAC) into the univariate regression model to identify the most relevant variables. After analyzing the results of the univariate regression model, variables with a significant effect on anemia and bone marrow suppression were included in the multivariate regression analysis for further exploration and verification.
According to the results of the multivariate regression analysis, we generated formulas to predict the possible status of anemia or bone marrow suppression after IAC. The generated equations were all based on the multivariate regression analysis results.
All statistical analyses were performed using R 4.0.2 software. The chi-square test was used to compare the distribution of categorical variables (such as sex, age group) between the groups. The t-test or Mann–Whitney test was used to compare differences in continuous variables (such as age and duration of surgery) according to the data distribution. Univariate and multivariate regression models were constructed to explore the association of demographic and clinicopathological characteristics with anemia and bone marrow suppression after IAC. The grade of anemia and bone marrow suppression were used as dependent variables, and covariates were used as independent variables. Variables with a two-tailed p-value <0.1 in the univariate analysis were analyzed using the multivariate regression model. A p-value <0.05 was considered statistically significant.
The number of patients who received melphalan, melphalan + topotecan, melphalan + carboplatin, and melphalan + topotecan + carboplatin as chemotherapy drugs was 3, 163, 119 and 1, respectively, accounting for a total of 286 patients. Since the number of patients who used melphalan and melphalan +topotecan +carboplatin as chemotherapy drugs was too small, we excluded the 4 patients to avoid increasing analysis bias. Therefore, the final number of patients enrolled into our study were 282.The data of 282 children with Rb treated with IAC were analyzed in this study. Among them, there were 114 (40.43%) and 181 (64.18%) children with anemia before and after IAC, respectively, and 61 (21.63%) and 97 (34.40%) children with bone marrow suppression before and after IAC (Figure 1), respectively. Table 1 summarizes the distribution of anemia and bone marrow suppression according to demographics or clinical data. There were no significant differences in these variables between the patients, except for the time of operation and heparin use in terms of possible covariates of bone marrow suppression.
The data of approximately 19.5% of the patients were missing; these patients were excluded. We found that there were 126 (52.50%), 112 (46.67%), and 2 (0.83%) children with Rb with no, mild, and moderate anemia, respectively. After IAC, the proportion of children with mild and moderate anemia increased significantly, with 170 children having mild anemia (74.89%) and 11 having moderate anemia (4.85%) (p <0.0001, chi-square test) (Figure 2A).
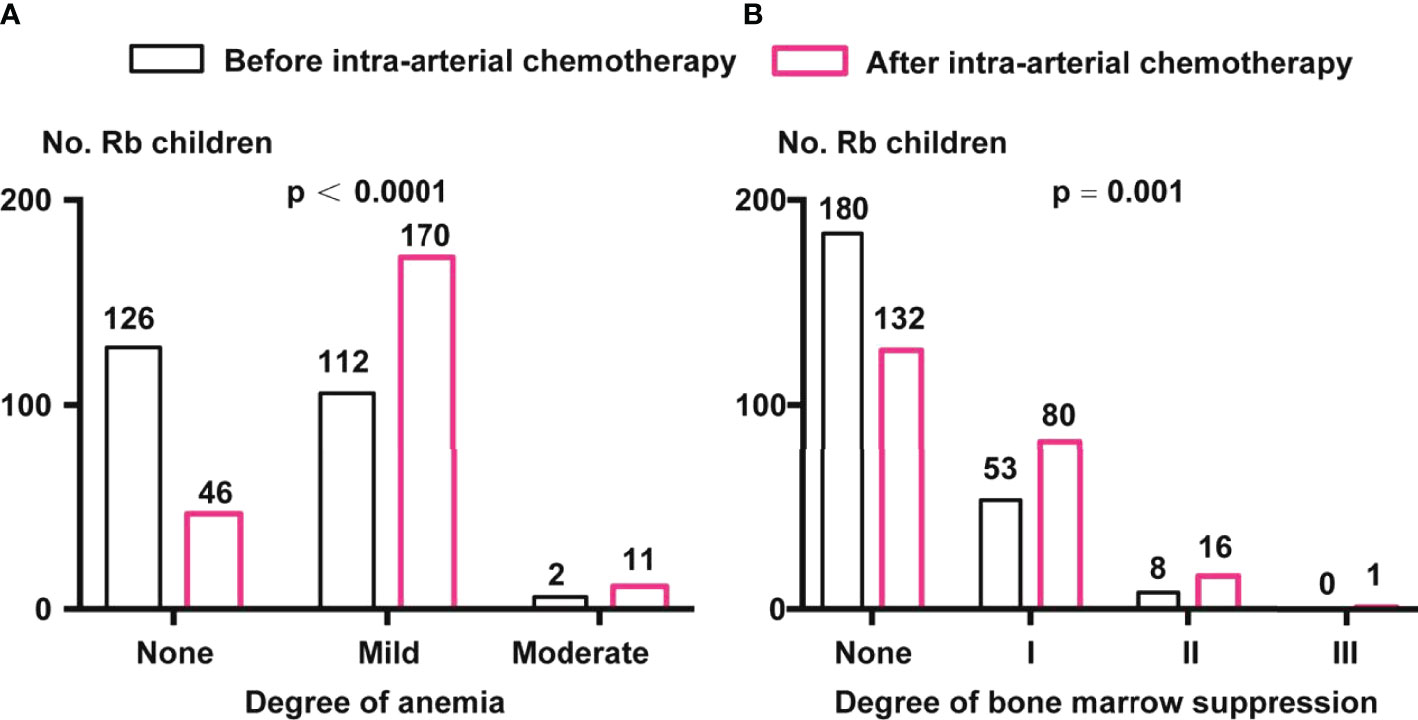
Figure 2 Comparison of anemia and bone marrow suppression before and after IAC. (A) Anemia classification before and after IAC. Percentage of children with Rb in mild and moderate anemia were enhanced after IAC (p < 0.0001 by chi-square test). (B) Degree of bone marrow suppression before and after IAC. Percentage of children with Rb in I, II, and III grade of bone marrow suppression were enhanced after IAC (p = 0.001 by chi-square test).
Before IAC, there were 180 (74.69%), 53 (21.99%), and 8 (3.32%) children with grades 0, I, and II bone marrow suppression, respectively. After IAC, the proportion of children with grades I (80, 34.93%), II (16, 6.99%), and III (1, 0.44%) (p = 0.001, chi-square test) (Figure 2B).
Regarding “anemia severity,” there were 93 (47.94%) children whose classification remained unchanged. After IAC, there were 73 (37.63%) and 7 (3.61%) children with Rb who had a cross-level change in anemia severity to one and two levels up, respectively (Figure 3A).
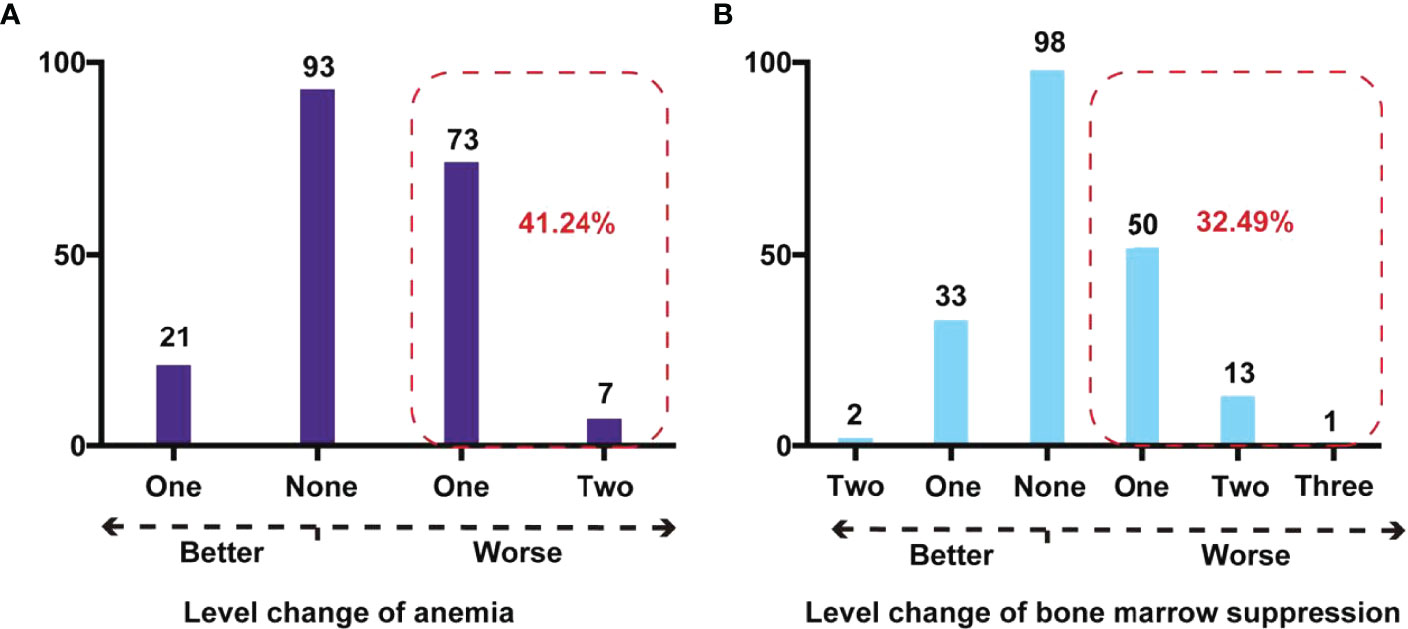
Figure 3 Cross-level impact on degree of anemia and bone marrow suppression after IAC in children with Rb. (A) Cross-level change of anemia in Rb children. The red dashed box showed the number and percentage of children with Rb whose anemia stage deteriorated after IAC. (B) Cross-level change of bone marrow suppression in children with Rb. The red dashed box showed the number and percentage of children with Rb whose bone marrow suppression stage deteriorated after IAC.
Regarding the “degree of bone marrow suppression,” 98 children (49.75%) did not show a change in the degree before and after IAC. However, there were 50 (25.38%), 13 (6.60%), and 1 (0.51%) children with Rb who had a cross-level increase in the degree of bone marrow suppression by one, two, and three grades, respectively (Figure 3B).After IAC, the proportion of anemia and bone marrow suppression in children with Rb who developed worse conditions were 41.24% and 32.49%.
To clarify possible reasons that affect the cross-level changes in anemia severity and bone marrow suppression degree, we collected and analyzed available information from the patients with detailed records (such as results of routine blood and liver and kidney function tests before and after IAC). After excluding the patients with incomplete data, 194 and 197 participants were enrolled in the univariate regression analysis of anemia and bone marrow suppression, respectively.
In the univariate regression model generated by taking the cross-level changes in anemia severity and degree of bone marrow suppression before and after IAC as the dependent variables, and covariates as the control variables, the factors that affected the change in the anemia severity and the degree of bone marrow suppression included anemia severity before IAC, degree of bone marrow suppression before IAC, intravenous and arterial chemotherapy within 1–2 months before IAC, WBC count, red blood cell count, Hb, and PLT count before IAC, mean erythrocyte Hb concentration, red cell volume distribution width, lymphocyte count (absolute), hematocrit, neutrophil count (absolute), alanine aminotransferase, aspartate aminotransferase, serum total bilirubin and serum direct bilirubin (Table 2). In addition, the covariates that did not show statistically significant effects on the cross-level changes in anemia severity and degree of bone marrow suppression are listed in the Supplementary file (S Table 1).
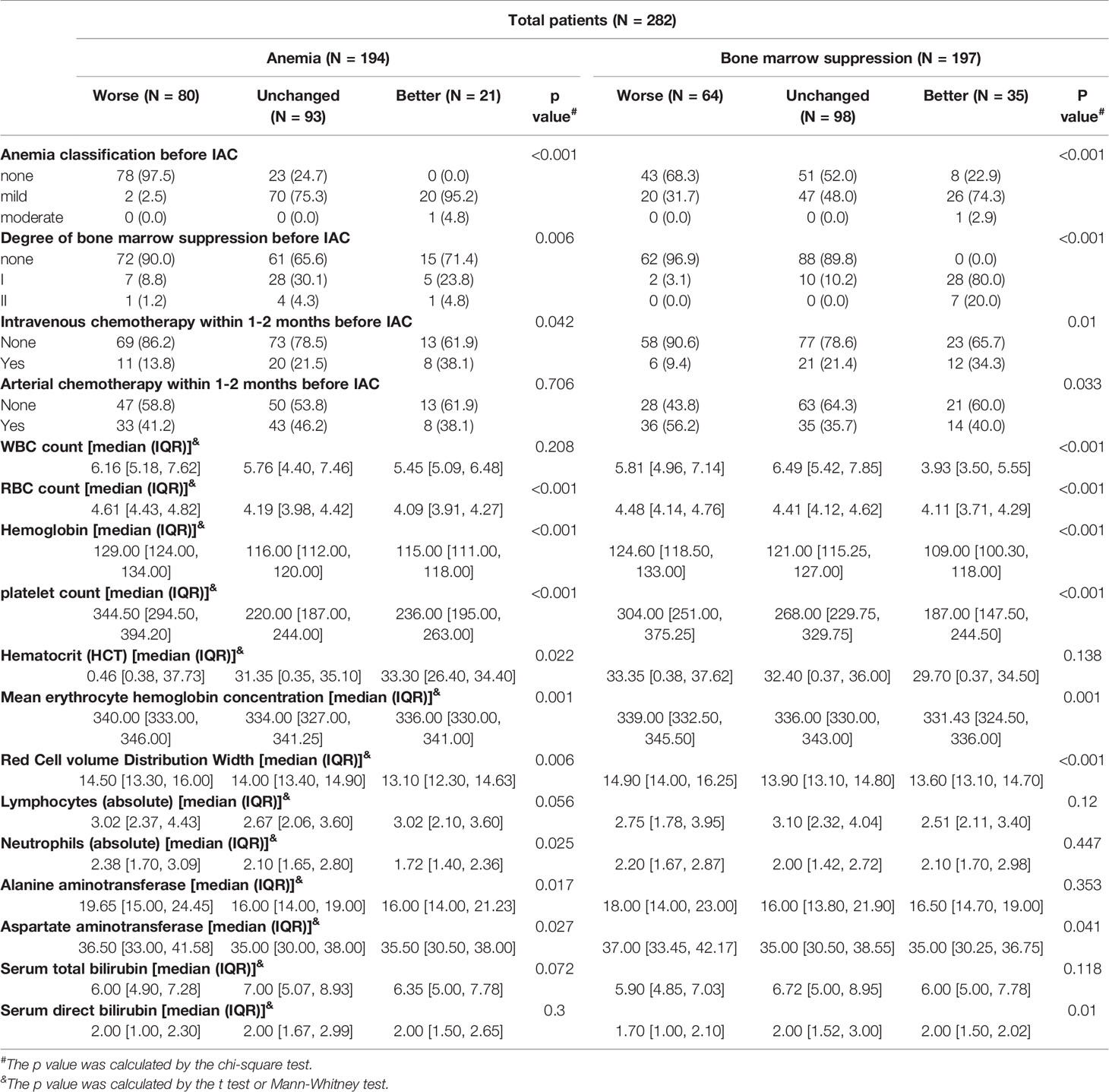
Table 2 Univariate regression analysis of IAC related to anemia severity and degree of bone marrow suppression.
After including all the variables that were identified the univariate regression analysis into the multivariate regression model, the factors affecting anemia severity in children with Rb after IAC were anemia severity and bone marrow suppression degree before IAC, red cell volume distribution width, PLT count, lymphocyte count (absolute), and neutrophil count (absolute). Meanwhile, the factors that affected the degree of bone marrow suppression were arterial chemotherapy within 1–2 months before IAC, red cell volume distribution width, and serum direct bilirubin (Table 3).

Table 3 Multivariate regression analysis of IAC on anemia severity and degree of bone marrow suppression.
Based on the results of the multivariate regression model, we generated an equation to predict anemia and bone marrow suppression before IAC. The equations for predicting anemia severity and degree of bone marrow suppression were based on Table 3. The equations are shown below.
Anemia severity after IAC = −13.230 + 1.356 × (anemia severity before IAC) + 1.398 × (degree of bone marrow suppression before IAC) + 0.688 × (red cell volume distribution width) + 0.012 × (PLT count) − 0.230 × (absolute lymphocyte count) + 0.565 × (absolute neutrophil count)
Degree of bone marrow suppression after IAC = −8.251 + 0.770 × (arterial chemotherapy within 1–2 months before IAC) + 0.576 × (red cell volume distribution width) − 0.463 × (serum direct bilirubin)
Although anemia and myelosuppression occur in most patients with cancer receiving chemotherapy, so far, no clinically useful tool for the accurate identification of patients at risk of anemia and myelosuppression has been developed. This study collected information on children with Rb before and after IAC and constructed multivariate regression models to initially explore the factors that may affect anemia and bone marrow suppression after IAC, evaluate the risk of anemia or bone marrow suppression for children with Rb before IAC, and provide clinical guidance for surgeons.
Rb is a devastating, blinding, and life-threatening disease in children; 36%, 77%, 95%, and 100% of patients with Rb who abandon treatment die within 12, 24, 36, and 48 months after diagnosis, respectively (17). Since IAC was first introduced in China in 2009, it has been used widely in some retinoblastoma treatment centers (18). IAC has become a very effective treatment option for Rb. By delivering chemotherapeutic drugs directly to the tumor, IAC can ensure that the drug concentration in the tumor is significantly increased while the peripheral blood chemotherapeutic drug concentration is low (19). Therefore, IAC can maximize the tumoricidal effect of chemotherapy drugs and reduce their adverse effects (20, 21). Thus far, IAC has a high rate of ocular salvage and a low incidence of serious complications in patients with refractory Rb (22). Melphalan-based IAC is an innovative treatment for patients with Rb because of its related high globe salvage rate. The overall globe salvagerate of patients receiving IAC as the main treatment is 79.6% (23), and the technical success rate is 98.5% (24). IAC has gradually been first-line treatment for unilateral retinoblastoma and very effective treatment for refractory retinoblastoma.
Although IAC has good clinical treatment effects in children with Rb, it still has adverse effects on the eyes and systemic effects (19). A systematic review of the application of IAC in refractory Rb showed that the commonest systemic complications were nausea/vomiting (20.5%), neutropenia (14.1%), fever (8.2%), and bronchospasm (6.2%) (22).The commonest ocular complications were retinopathy (32%) and eyelid edema (29.7%) (23). Ocular complications (ophthalmic artery obstruction, vitreous hemorrhage, and optic neuropathy) and systemic complications (transient pancytopenia, ototoxicity, and leukemia) have been associated with shield use (25). The local side effects of IAC are a concern for many scholars. Although the systemic side effects caused by IAC are not serious, they affect the quality of life of children and should not be ignored, especially anemia and bone marrow suppression caused by IAC. Although the human body tries to counteract the effects of anemia through various mechanisms, almost every organ system of the human body will eventually be affected. Patients experience a variety of symptoms, ranging from skin chills, heart failure, and palpitations to pulmonary edema, dizziness, depression, and severe cognitive impairment. Anemia seriously affects the quality of life of patients (26). Anemia was significantly associated with poor performance status (27). The incidence of bone marrow suppression after IAC in infants less than 3 months old with advanced Rb was 7.7% (18). The European Cancer Anaemia Survey evaluated anemia in 15,367 patients with cancer in Europe for 6 months and found that the incidence of anemia was 53.7% (Hb <10.0 g/dL) (27). Anemia and bone marrow suppression is very common in patients with cancer, and their pathogenesis is complex.
Our study found that children with Rb had varying anemia severities and bone marrow suppression degrees after IAC. This study investigated 282 retinoblastoma cases; the rates of post-IAC anemia and myelosuppression in children were 64.18% and 34.40%, respectively, which were significantly higher than 31.8% and 11.8% in adult patients with cancer (28). This difference may be due to the differences in tumor types, chemotherapeutic drugs, and the potential disease status of the study population. Another reason may be that 84.04% (237/282) of the children received intra-venous and or intra-arterial chemotherapy within 6 months before this investigation. For IVC, our institution adopts the standard protocol, i.e., intravenous vincristine, etoposide, and carboplatin for 6 cycles, once every 3 to 4 weeks. For IAC, the chemotherapy drugs are melphalan + topotecan and melphalan + carboplatin. For IAC, there was no strong evidence showing that chemotherapy drugs affect anemia or bone marrow suppression. However, compared to IVC, the occurrence of anemia and bone marrow suppression after IAC was greatly reduced, and anemia and bone marrow suppression were transient, causing less damage to children with Rb (29).
The causes of anemia and bone marrow suppression in many patients with cancer are unclear (30). In a retrospective study, malnutrition, >3 previous chemotherapies, and a combination of more than three drugs were significantly associated with severe bone marrow suppression (31). In the child model, lymphopenia was the strongest predictor of the risk model of severe anemia, followed by Hb levels and performance status (32). Variables identified as predictors of anemia were associated with patient characteristics: baseline Hb <13.5 g/dL, age >60 years, and body mass index <25 kg/m2 (33). In the univariate and multivariate regression analysis and predictive model generated by the multivariate regression model, we found that many factors can affect anemia and bone marrow suppression after IAC. The anemia severity and bone marrow suppression severity before IAC had a significant effect on anemia severity, and as the grade increased, its effect on post-IAC anemia was stronger. The reason for this situation may be that “bone marrow suppression” was comprehensively assessed by WBC, Hb, and PLT levels, which can objectively show the patient’s blood status before IAC. Regarding the “degree of bone marrow suppression,” variables such as IVC within 1–2 months before IAC, red cell volume distribution width, and serum direct bilirubin affect the degree of bone marrow suppression. Possible causes may need to be further explored.
This study had some limitations. First, this study is a retrospective study, and in the 282 children, the routine blood tests of some children were incomplete, and the rate of missing values of laboratory information reached 19.5%. Although we excluded patients with missing data in the univariate and multivariate regression analyses, further high-quality evidence is necessary to guide clinical practice. Second, the interval of routine blood examination before and after IAC was inconsistent. Some children with Rb who had a long time interval related to routine blood tests may have experienced factors with a probable effect on their results, such as an increased WBC level caused by infection. Finally, our research only recorded the results of routine blood tests after a single session of IAC and failed to reflect the dynamic changes in serological indicators caused by IAC. However, the large sample size can compensate for these limitations to a certain extent, and there was no statistically significant deviation in the conclusion that “IAC will increase the risk of anemia and bone marrow suppression in children with Rb.” In subsequent research, we will increase the frequency of routine blood tests in children with Rb in each period after IAC and include other serological indicators, such as liver and kidney function and coagulation function to determine the factors that guide the prediction of dynamic changes in serology and adverse reactions after IAC and improve the management of children with Rb.
Children with Rb have an increased risk of anemia and myelosuppression after IAC, but this is temporary; therefore, IAC should be considered as the standard of care. The univariate and multivariate regression model results showed several factors that may affect the occurrence of anemia and bone marrow suppression after IAC. Based on the model, we generated equations to calculate the anemia severity and degree of bone marrow suppression, which played a clinical guiding role in the prediction and timely control of anemia and bone marrow suppression after IAC. The model was developed using data of patients with retinoblastoma and needs to be validated in a larger population.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study has been approved by the Ninth people’s hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2019-T291-3). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
CZ wrote the manuscript as the first author. Professor LH, XS, XW, and LL conceived and designed the study. MH and JF contributed to the data analysis, data interpretation, and manuscript revision. XH and RJ were involved in the data collection. All authors read and approved the final manuscript.
This study was supported by the Gaoyuan Nursing Grant Support of Shanghai Municipal Education (Hlgy1808kyx); the Shanghai Jiao Tong University School of Medicine: Nursing Development Program; the Nursing Program of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYHL20193D05). The above-mentioned funding sponsor played no role in the study design; data collection, analysis, and interpretation; and manuscript writing. Innovative research team of high-level local universities in Shanghai (SHSMU- ZDCX20210902); Clinical Research Plan of SHDC (SHDC2020CR1009A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.848877/full#supplementary-material
1. Shields CL, Shields JA. Retinoblastoma Management: Advances in Enucleation, Intravenous Chemoreduction, and Intra-Arterial Chemotherapy. Curr Opin Ophthalmol (2010) 21:203–12. doi: 10.1097/ICU.0b013e328338676a
2. Rodriguez-Galindo C, Orbach DB, VanderVeen D. Retinoblastoma. Pediatr Clin North Am (2015) 62:201–23. doi: 10.1016/j.pcl.2014.09.014
3. Canturk S, Qaddoumi I, Khetan V, Ma Z, Furmanchuk A, Antoneli CB, et al. Survival of Retinoblastoma in Less-Developed Countries Impact of Socioeconomic and Health-Related Indicators. Br J Ophthalmol (2010) 94:1432–6. doi: 10.1136/bjo.2009.168062
4. MacCarthy A, Birch JM, Draper GJ, Hungerford JL, Kingston JE, Kroll ME, et al. Retinoblastoma: Treatment and Survival in Great Britain 1963 to 2002. Br J Ophthalmol (2009) 93:38–9. doi: 10.1136/bjo.2008.139626
5. Luo Y, Zhou C, He F, Fan J, Wen X, Ding Y, et al. Contemporary Update of Retinoblastoma in China: Three-Decade Changes in Epidemiology, Clinical Features, Treatments, and Outcomes. Am J Ophthalmol (2022) 236:193–203. doi: 10.1016/j.ajo.2021.09.026
6. Fabian ID, Onadim Z, Karaa E, Duncan C, Chowdhury T, Scheimberg I, et al. The Management of Retinoblastoma. Oncogene (2018) 37:1551–60. doi: 10.1038/s41388-017-0050-x
7. Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. LANCET (2012) 379:1436–46. doi: 10.1016/S0140-6736(11)61137-9
8. Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of Retinoblastoma in 2015: Agreement and Disagreement. JAMA Ophthalmol (2015) 133:1341–7. doi: 10.1001/jamaophthalmol.2015.3108
9. Shields CL, Kaliki S, Rojanaporn D, Al-Dahmash S, Bianciotto CG, Shields JA. Intravenous and Intra-Arterial Chemotherapy for Retinoblastoma: What Have We Learned? Curr Opin Ophthalmol (2012) 23:202–9. doi: 10.1097/ICU.0b013e3283524130
10. Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Xia J, et al. Evaluating Primary Intra-Arterial Chemotherapy Versus Intravenous Plus Intra-Arterial Chemotherapy for Advanced Intraocular Retinoblastoma. Cancer Chemother Pharmacol (2020) 85:723–30. doi: 10.1007/s00280-020-04036-w
11. Daniels AB, Patel SN, Milam RW, Kohanim S, Friedman DL, Koyama T. Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class-A Meta-Analysis. Cancers (Basel) (2021) 13:2216. doi: 10.3390/cancers13092216
12. Sweid A, Jabbour P. Intra-Arterial Chemotherapy for Retinoblastoma: Transradial and Transfemoral Approach. J neurointerv Surg (2020) 12:828. doi: 10.1136/neurintsurg-2019-015627
13. Ravindran K, Dalvin LA, Pulido JS, Brinjikji W. Intra-Arterial Chemotherapy for Retinoblastoma: An Updated Systematic Review and Meta-Analysis. J neurointerv Surg (2019) 11:1266–72. doi: 10.1136/neurintsurg-2019-014909
14. Oporto JI, Zuniga P, Ossandon D, Zanolli M, Perez V, Lopez JP, et al. Intra-Arterial Chemotherapy for Retinoblastoma Treatment in Chile: Experience and Results 2013-2020. Arch Soc Esp Oftalmol (Engl Ed) (2021) 96:288–92. doi: 10.1016/j.oftale.2020.10.003
15. Zhou M, Wen X, Jia S, Han Y, He X, Han M, et al. Risk Factors for Ophthalmic Artery Stenosis and Occlusion in Patients With Retinoblastoma Treated With Intra-Arterial Chemotherapy. Brit J Ophthalmol (2021). doi: 10.1136/bjophthalmol-2021-319118
16. Jia S, Wen X, Zhou M, He X, Han M, Fan J, et al. Comparison of Intra-Arterial Chemotherapy Efficacy Delivered Through the Ophthalmic Artery or External Carotid Artery in a Cohort of Retinoblastoma Patients. Front Med (2021) 8:658305. doi: 10.3389/fmed.2021.658305
17. Zhao J, Feng Z, Gallie BL. Natural History of Untreated Retinoblastoma. Cancers (Basel) (2021) 13:3646. doi: 10.3390/cancers13153646
18. Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Xia J, et al. Intra-Arterial Chemotherapy as Primary or Secondary Treatment for Infants Diagnosed With Advanced Retinoblastoma Before 3 Months of Age. BMC Cancer (2019) 19:693. doi: 10.1186/s12885-019-5844-5
19. Biewald EM, Bornfeld N, Metz KA, Schluter S, Kiefer T, Radbruch A, et al. Histopathology of Retinoblastoma Eyes Enucleated After Intra-Arterial Chemotherapy. Br J Ophthalmol (2020) 104:1171–5. doi: 10.1136/bjophthalmol-2019-315209
20. Yamane T, Kaneko A, Mohri M. The Technique of Ophthalmic Arterial Infusion Therapy for Patients With Intraocular Retinoblastoma. Int J Clin Oncol (2004) 9:69–73. doi: 10.1007/s10147-004-0392-6
21. Taich P, Requejo F, Asprea M, Sgroi M, Gobin P, Abramson DH, et al. Topotecan Delivery to the Optic Nerve After Ophthalmic Artery Chemosurgery. PLoS One (2016) 11:e0151343. doi: 10.1371/journal.pone.0151343
22. Runnels J, Acosta G, Rose A, Haynes M, Nikolaidis D, Wong A, et al. The Role for Intra-Arterial Chemotherapy for Refractory Retinoblastoma: A Systematic Review. Clin Transl Oncol (2021) 23:2066–77. doi: 10.1007/s12094-021-02610-z
23. Cao Y, Zhou M, Tian M, Lv H. The Safety and Effectiveness of Melphalan-Based Intra-Arterial Chemotherapy for Retinoblastoma: An Updated Single-Arm Systematic Review and Meta-Analysis. Evid-Based Compl Alt (2022) 2022:1–11. doi: 10.1155/2022/3156503
24. Chen M, Jiang H, Zhang J, Shen G, Jiang Y, Li H, et al. Outcome of Intra-Arterial Chemotherapy for Retinoblastoma and its Influencing Factors: A Retrospective Study. Acta Ophthalmol (2017) 95:613–8. doi: 10.1111/aos.13333
25. Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma Frontiers With Intravenous, Intra-Arterial, Periocular, and Intravitreal Chemotherapy. Eye (Lond) (2013) 27:253–64. doi: 10.1038/eye.2012.175
26. Ludwig H, Strasser K. Symptomatology of Anemia. Semin Oncol (2001) 28:7–14. doi: 10.1016/s0093-7754(01)90206-4
27. Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): A Large, Multinational, Prospective Survey Defining the Prevalence, Incidence, and Treatment of Anaemia in Cancer Patients. Eur J Cancer (2004) 40:2293–306. doi: 10.1016/j.ejca.2004.06.019
28. Sah SK, Karn A, Shah A, Paudel BD, Adhikari K, Acharya B, et al. Incidence and Attributes of Chemotherapy Induced Myelotoxicity, Anemia and Neutropenia in Adults With Cancer in Nepal: A Cross-Sectional Observational Study. J Oncol Pharm Pract (2019) 25:1823–30. doi: 10.1177/1078155218817815
29. Daniels AB, Froehler MT, Kaczmarek JV, Bogan CM, Santapuram PR, Pierce JM, et al. Efficacy, Toxicity, and Pharmacokinetics of Intra-Arterial Chemotherapy Versus Intravenous Chemotherapy for Retinoblastoma in Animal Models and Patients. Transl Vis Sci Technol (2021) 10:10. doi: 10.1167/tvst.10.11.10
30. Abdel-Razeq H, Hashem H. Recent Update in the Pathogenesis and Treatment of Chemotherapy and Cancer Induced Anemia. Crit Rev Oncology/Hematology (2020) 145:102837. doi: 10.1016/j.critrevonc.2019.102837
31. Ouyang Z, Peng D, Dhakal DP. Risk Factors for Hematological Toxicity of Chemotherapy for Bone and Soft Tissue Sarcoma. Oncol Lett (2013) 5:1736–40. doi: 10.3892/ol.2013.1234
32. Marec-Berard P, Blay JY, Schell M, Buclon M, Demaret C, Ray-Coquard I. Risk Model Predictive of Severe Anemia Requiring RBC Transfusion After Chemotherapy in Pediatric Solid Tumor Patients. J Clin Oncol (2003) 21:4235–8. doi: 10.1200/JCO.2003.09.121
Keywords: retinoblastoma, anemia, bone marrow suppression, intra-arterial chemotherapy, multivariate regression
Citation: Zeng C, Han M, Fan J, He X, Jia R, Li L, Wen X, Song X and Hou L (2022) Anemia and Bone Marrow Suppression After Intra-Arterial Chemotherapy in Children With Retinoblastoma: A Retrospective Analysis. Front. Oncol. 12:848877. doi: 10.3389/fonc.2022.848877
Received: 05 January 2022; Accepted: 27 May 2022;
Published: 25 July 2022.
Edited by:
Andrea Di Cataldo, University of Catania, ItalyReviewed by:
Vishal Raval, L V Prasad Eye Institute, IndiaCopyright © 2022 Zeng, Han, Fan, He, Jia, Li, Wen, Song and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, c3VuYm9va18yMDAxQDEyNi5jb20=; Xuyang Wen, d2luc3Rvbi1hQGhvdG1haWwuY29t; Xuefei Song, c29uZ3h1ZWZlaUBzaHNtdS5lZHUuY24=; Lili Hou, aG91bGlsaXRvdWdhb0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.