
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 March 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.845549
This article is part of the Research Topic Differential Diagnoses of Thyroid Neoplasms: Molecular and Histological Features and the Impact on Follow-Up View all 10 articles
 Vito Cantisani1*
Vito Cantisani1* Annalisa De Silvestri2
Annalisa De Silvestri2 Valeria Scotti2
Valeria Scotti2 Daniele Fresilli1*
Daniele Fresilli1* Maria Grazia Tarsitano3
Maria Grazia Tarsitano3 Giorgia Polti1
Giorgia Polti1 Olga Guiban1
Olga Guiban1 Eleonora Polito1
Eleonora Polito1 Patrizia Pacini1
Patrizia Pacini1 Cosimo Durante4
Cosimo Durante4 Giorgio Grani4
Giorgio Grani4 Andrea M. Isidori5
Andrea M. Isidori5 Elisa Giannetta5
Elisa Giannetta5 Salvatore Sorrenti6
Salvatore Sorrenti6 Pierpaolo Trimboli7
Pierpaolo Trimboli7 Carlo Catalano1
Carlo Catalano1 Roberto Cirocchi8
Roberto Cirocchi8 Augusto Lauro6
Augusto Lauro6 Vito D’Andrea6
Vito D’Andrea6Background: Thyroid nodules are frequent in adult population and thyroid cancer incidence has increased dramatically over the past three decades. The aim of this systematic review and meta-analysis was to evaluate the US-Elastosonography (USE) diagnostic performance in assessing the thyroid nodules malignancy risk.
Methods: PubMed and Embase databases were searched from January 2011 to July 2021. We extracted data from selected studies and calculated the overall diagnostic accuracy of qualitative USE, semi-quantitative USE and quantitative USE. Summary receiver operating characteristic (ROC) curve was elaborated to show the results. All statistical tests were performed using Metadisc and Medcal software package.
Results: Finally 72 studies with 13,505 patients and 14,015 thyroid nodules (33% malignant) undergoing elastography were included. The pooled sensitivity, specificity and AUC were 84%, 81%, and 0.89 respectively for qualitative USE; 83%, 80%, and 0.93 for semi-quantitative USE and 78%, 81% and 0.87, for quantitative USE. The qualitative and semiquantitative USE present very similar diagnostic accuracy values and both better than the quantitative USE.
Conclusions: USE is a useful imaging tool for thyroid nodule characterization. In accordance with recent guidelines and meta-analyses, the USE could be used daily in thyroid nodule malignancy risk stratification.
Systematic Review Registration: PROSPERO: CRD42021279257.
Thyroid nodules are frequent in adult population up to 60%, with a prevalence of cancer as 5% (1, 2). Since the incidence of thyroid cancer has mostly increased in the last decade (3, 4) the initial assessment of these patients is a hot topic and ultrasound (US) represents the first line imaging modality in this context. In fact, the US features such as micro- or macrocalcifications, marked hypo echogenicity, taller than wide shape, and thick irregular or lobulated margins are recognized as associated with malignancy (5), but they are not highly predictive: US sensitivity and specificity have high variability ranging between 52 and 97% and 26.6 and 83%, respectively. In addition, low reproducibility and operator-depending performance might reduce US diagnostic value. Thus, the only US images are suboptimal to actually diagnose a thyroid cancer.
To reduce or delete these limitations, several Thyroid Imaging Reporting and Data Systems (TIRADS) (6–9) have recently been proposed as a tool for uniform reporting and consistent evaluation.
This risk stratification should guide the indication for fine needle aspiration biopsy (FNAB) that is required when a suspicious nodule is identified, with normal thyroid stimulating hormone. FNAB presents a specificity of 60 to 98% and sensitivities from 54 to 90% (10, 11), so it is not such an accurate exam. In fact, non-diagnostic and indeterminate responses are common (12–15). Consequentially, on one hand, a significant number of patients have to repeat the procedure with incremented costs and on the other hand, some patients could receive unnecessary thyroid surgery, more for diagnostic than for therapeutic purposes. Considering these points and the known risks of thyroid surgery, improving the techniques for thyroid nodules diagnosis is mandatory. Among the different techniques, in the present paper we will address the role of Ultrasound elastography (USE) for thyroid characterization. Based on the fact that a suspicious nodule is at palpatory firm or hard in consistency, stiffness was adopted as indicator of malignancy for elastography (16, 17).
In this way, USE was utilized, and by the beginning encouraged literature data were obtained and as a consequence it was suggested very soon as an additional tool for thyroid nodule differentiation, in combination with conventional US and FNAB (18, 19).
Consequently, USE methods have been incorporated into international guidelines published by the WFUMB (World Federation for Ultrasound in Medicine and Biology) (20) and the EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) (21); in the above-mentioned Guidelines, technical details, advantages and limitations for strain elastography (SE) and quantitative 2 D ultrasound shear wave elastography (SWE) have extensively been reported.
However, technology improvements, and open issues already reported by guidelines were reported to be addressed. To the best of our knowledge, presently, few studies have investigated the diagnostic performance of various thyroid Ultrasound elastography (UE) methods as applied in the clinical context and shown variable results.
Hence, this present, updated systematic review (registered in the international prospective register of systematic reviews PROSPERO: CRD42021279257) and meta-analysis assesses and summarizes current evidence on the diagnostic performance of various thyroid USE software in differentiating benign and malignant thyroid nodules.
The following electronic databases were searched: PubMed and EMBASE.
The search strategy was based on the PICOS framework to identify search key words relating to the population, intervention, and outcomes in the different databases. The search concepts were: 1. Thyroid nodule AND 2. ultrasound 3. elastography OR elastosonography 4. SWE OR Shear wave elastography, 5. Strain 6. ARFI OR acoustic radiation force, and their related terms as MeSH terms, keywords and/or EmTree terms.
The search was conducted between January 2011 and July 2021 and only in English language.
From all retrieved references, duplicates were eliminated and the remaining records were screened.
All references identified were independently assessed by two authors, first by means of title and abstract, then by the review of the complete paper.
All the studies analyzed had to meet the following criteria: 1. The study involved only human subjects; 2. The study investigated the diagnostic performance of USE techniques as Strain and Shear wave for differentiation of benign and malignant thyroid nodules in a clinical setting; 3. Use of an appropriate reference standard (FNAC or histopathology); and 4. Diagnostic performance outcomes of interest were reported in terms of sensitivity, specificity, negative predictive values (NPV), positive predictive values (PPV), diagnostic accuracy, and/or area under receiver operator characteristic curve ROC curve (AUROC).
Exclusion criteria were: 1. Case reports, editorial letters, or commentaries; 2. Studies that included less of 50 thyroid nodules 3. Non-English; and 4. Insufficient diagnostic accuracy outcomes and studies without values of sensitivity, specificity, NPV and PPV; 5. paper related to specific categories such as Indeterminate nodules at Cytology.
Two independent readers extracted the data in a pre-specified form. For each article, the following data were extracted: bibliographic data, type of study, type of setting, number of patients, demographic/clinical data (age, type of lesions, percentage of men and women), and number of nodules and prevalence of malignant nodules. Furthermore, for each USE techniques true (TP) and false positive (FP), true (TN) and false negative (FN) were retrieved or calculated from sensitivity/specificity.
The quality of the studies included in the meta-analysis was assessed with a checklist based on the Quality Assessment for Studies of Diagnostic Accuracy (QUADAS 2) tool (22). Two investigators performed a quality assessment of the included studies independently, and disagreements were resolved by discussion.
The statistical pooling of test accuracy studies presents an added level of complexity as accuracy is usually quantified by two related statistics (sensitivity and specificity) rather than one, and meta-analysis must allow for the trade-off between the two. Positive and negative likelihood ratios (LRs) (that allow for this trade-off) were pooled with weighted averages applied, in which the weight of each study was its sample size. For each pooled estimate, a 95% confidence interval (CI) was calculated using random effects model. Positive and negative LRs (representing likelihood of malignancy in case of positive or negative results of index USE technique) could be interpreted as in Table 1 (23).
A symmetric summary receiver operating characteristic (ROC) curve, as described by Moses et al. (24) was constructed to summarize the results; the area under this curve (AUC) was calculated.
Study heterogeneity was assessed by the I2 index, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. A value of greater than 50% may be considered indicative of significant heterogeneity.
AUCs were compared with a z-test of the ratio between difference of AUC and square root of the variance of the difference (25).
Furthermore, a sub-analysis regarding prospective and retrospective papers was carried out.
All statistical tests were performed using Metadisc (26) and Medcal (27) software package.
We retrieved 437 records (113 in PubMed and 324 in Embase) that were 353 after removing the duplicates; of them 72 full-text were carefully examined and all of them, from whom TP, FP FN and TN were retrievable for single USE techniques, were included in meta-analysis. Quality of studies was generally high. Mean age of the 13,505 patients was 46 years; mean percentage of men was 24%.
The total thyroid nodules included in our study was 14,015.
A high malignancy rate (33%) was observed compared to the general population and with a pooled malignancy of 32% for qualitative USE, 29% for semi-quantitative USE and 33% for quantitative USE. The pooled sensitivity, specificity and AUC were 84% (95% confidence interval (CI), 0.83–0.85), 81% (95% CI, 0.80–0.82) and 0.89 (95% CI, 0.87–0.91) respectively for qualitative USE; 83% (95% CI, 0.81–0.84), 80% (95% CI, 0.79–0.82) and 0.93 (95% CI, 0.91–0.95) respectively for semi-quantitative USE and 78% (95% CI, 0.76–0.79), 81% (95% CI, 0.80–0.82) and 0.87 (95% CI, 0.86–0.88), respectively for quantitative USE. The positive likelihood ratios (PLR) and negative likelihood ratios (NLR) were 4.7 (95% CI, 3.5–6.3) and 0.24 (95% CI, 0.17–0.34) for qualitative USE; 6.5 (95% CI, 4.2–10.1) and 0.19 (95% CI, 0.13–0.27) for semi-quantitative USE; 4.4 (95% CI, 3.6–5.5) and 0.28 (95% CI, 0.24–0.33) for quantitative USE.
The results are synthesized in Tables 2–4 and Figures 1–3.
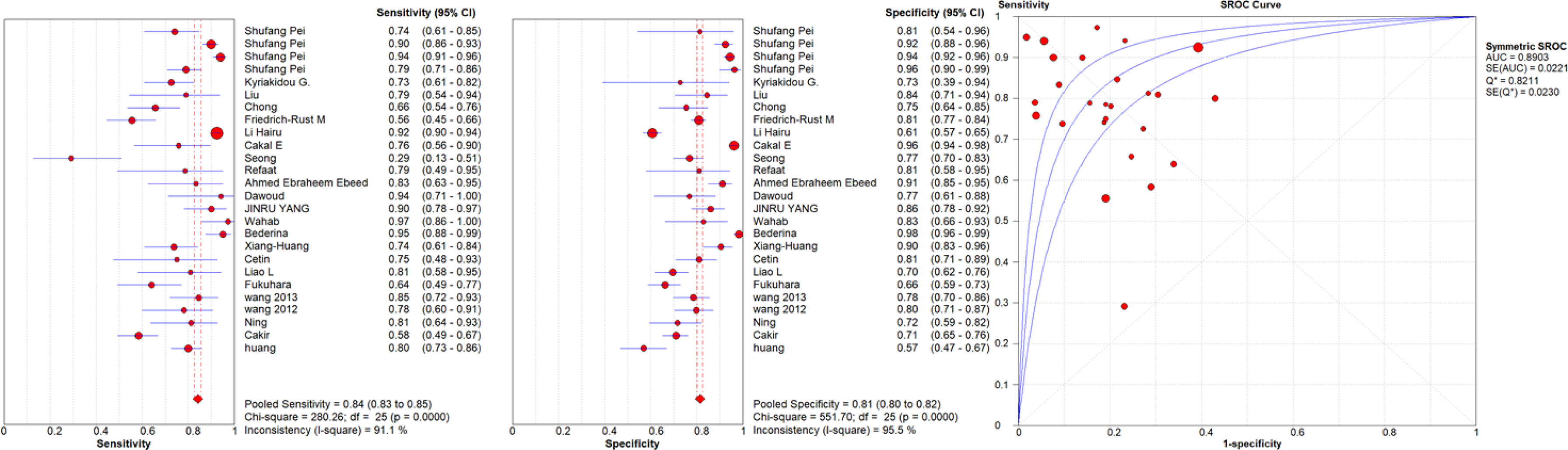
Figure 1 Sensitivity and specificity forest plots and SROC curve for the qualitative USE. The circle size represents the nodule population of the selected articles; the line represents the confidence interval of the selected articles.
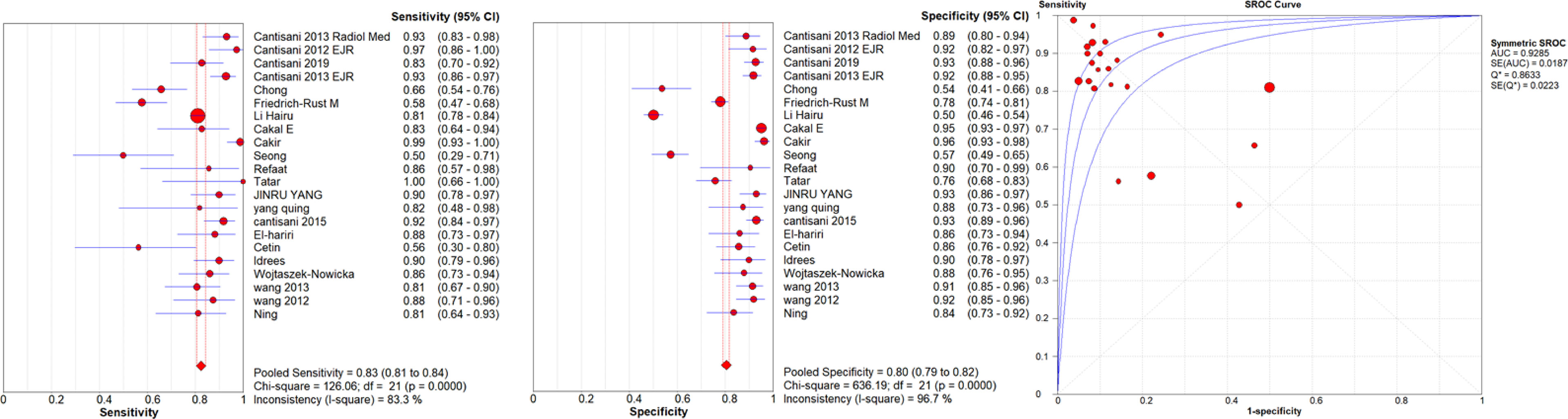
Figure 2 Sensitivity and specificity forest plots and SROC curve for the semiquantitative USE. The circle size represents the nodule population of the selected articles; the line represents the confidence interval of the selected articles.
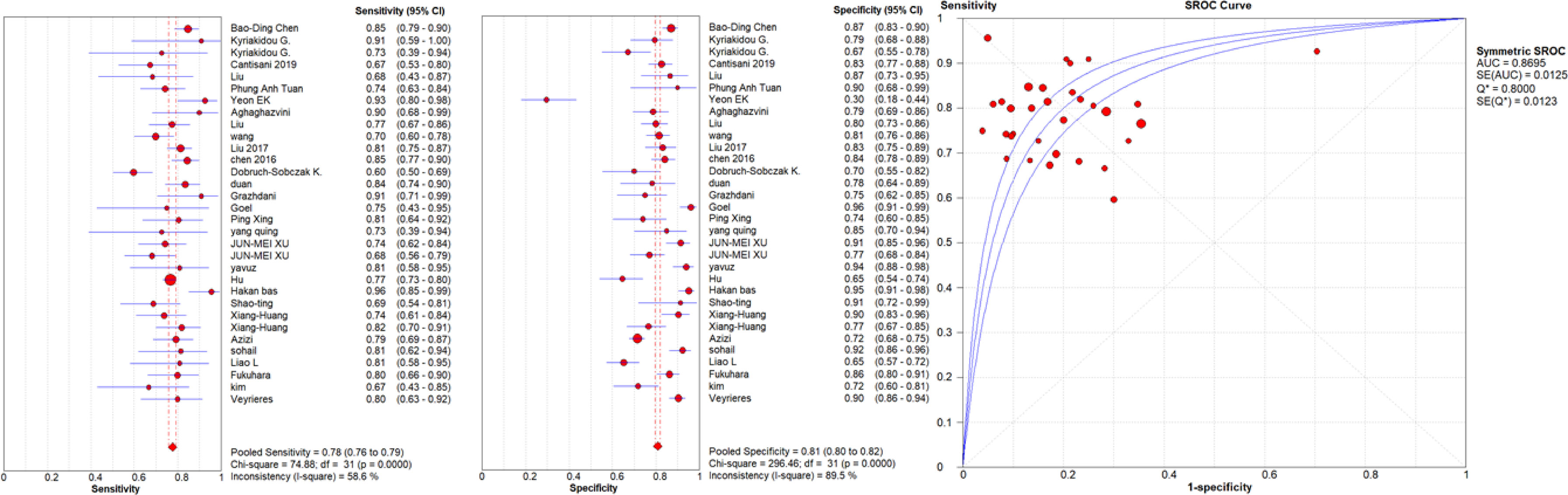
Figure 3 Sensitivity and specificity forest plots and SROC curve for the quantitative USE. The circle size represents the nodule population of the selected articles; the line represents the confidence interval of the selected articles.
Furthermore, a sub-analysis regarding prospective and retrospective works was carried out (Tables 5, 6) showing followings results:
o from the prospective studies analysis resulted a pooled sensitivity, specificity and AUC of 74.4%, 82.3%, and 0.87 respectively for qualitative USE; 83.9%, 87.8%, and 0.94 respectively for semi-quantitative USE and finally, 78.0%, 80.9%, and 0.88 respectively for quantitative USE;
o from the retrospective studies analysis resulted a pooled sensitivity, specificity and AUC of 89.0%, 79.7%, and 0.92 respectively for qualitative USE and 78.7%, 81.8%, and 0.87 respectively for quantitative USE.
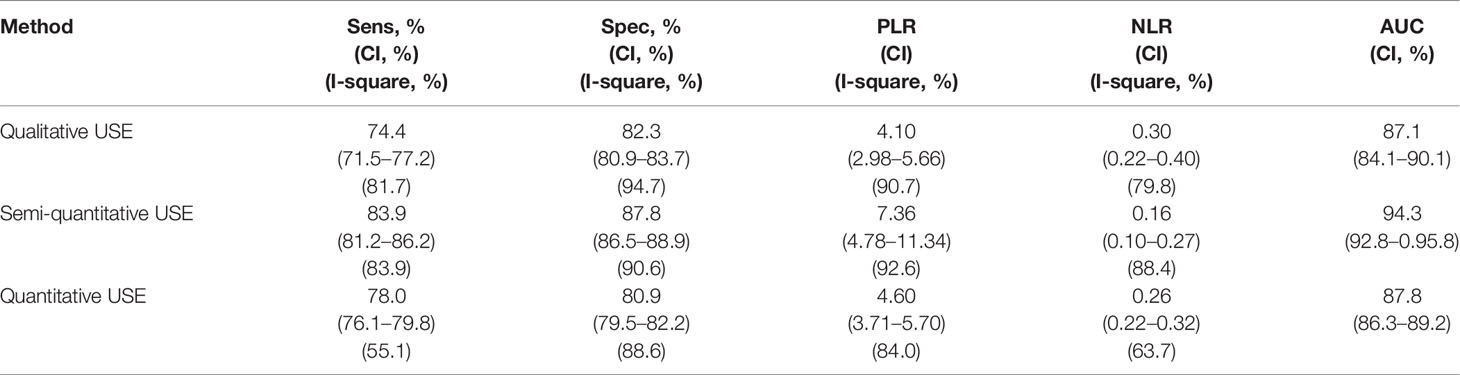
Table 5 Pooled diagnostic performance of qualitative USE using only prospective papers. USE, Ultrasound Elastography.
The retrospective papers analysis on semi-quantitative USE was not carried out due to the few papers.
The area under the SROC curve was higher than 90% only for semi-quantitative USE (p = 0.19 for semi-quantitative USE vs qualitative USE; p = 0.41 for quantitative USE vs qualitative USE; p = 0.01 quantitative USE vs semi-quantitative USE).
The USE techniques with higher PLR (according to Table 1 classification could be judged as useful) and lower NLR (according to Table 1 classification could be judged as useful) was the semi-quantitative USE. Regarding the single dimensions of accuracy, the pooled specificity is equal among USE techniques while sensitivity is lower in quantitative USE than in strain elastography.
The qualitative and semiquantitative USE present very similar diagnostic accuracy values but both better than the quantitative USE. In particular, semi-quantitative USE AUC was statistically higher than quantitative USE one (p-value <0.05).
In addition to the clinical-laboratory evaluations, the clinical–therapeutic management of thyroid nodule is based on the ultrasound examination, which is the preferred thyroid imaging modality due to its non-invasiveness, wide availability and low cost. Several ultrasound features are used to classify thyroid nodules, each of them carrying a more or less high risk of malignancy (84). Trying to standardize the ultrasound estimate of thyroid nodules malignancy risk, it was introduce a risk-score called TIRADS (8, 19, 85, 86). The TIRADS lexicon is based on echo structure (solid, mixed or cystic), echogenicity (hyper, iso, hypoechoic or markedly hypoechoic), margins (regular, microlobulated; irregular/spiculate), internal components (micro or macro calcifications; cystic areas), and the shape [oval; taller than wide (87)] on ultrasound evaluation.
The main advantage of the routine TIRADS use is to identify with a great accuracy suspected thyroid nodules worthy of cytological investigation (88) and to exclude those not deserving at that time, thus reducing the total number and costs of FNA procedures (88, 89). However, TIRADS have limitations: there are many and different TIRADS with similar but non-overlapping classifications, accuracy is far less than 100% they are rarely used in real-life practice [in about 27.2% of the Italian reports (90)]. Therefore, fine-needle aspiration cytology (FNAC) still represents the gold-standard technique for classification of thyroid nodules, due to its high specificity (60–98%) to identify malignant thyroid nodules, but with variable sensitivity (54–90%) (10–14, 91).
In the last decades, many studies and meta-analyzes have demonstrated the effectiveness of new ultrasound techniques such as CEUS (contrast ultrasound) and, above all, USE (US-Elastography) to improve the B-mode assessment of the thyroid nodule (38, 55, 92–100).
Recently USE was introduced in the last guidelines as an additional tool for stratifying the thyroid nodules malignancy risk, in combination with conventional US and FNA. In particular, the EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) guidelines assert that Strain Ratio Elastography (SRE) should be part of the thyroid work-up due to its high diagnostic accuracy (92, 93).
The WFUMB guidelines (World Federation for Ultrasound in Medicine and Biology) state that both qualitative and semi-quantitative USE can be used for the evaluation of thyroid nodules and in particular qualitative USE which improves the B-mode ultrasound specificity but semi-quantitative USE is more easily learned (20). Furthermore, they state that SWE also improves the conventional US specificity, particularly in subcentimeter thyroid nodules (20).
Already several papers and meta-analyses assert that US-elastography is superior or similar to conventional ultrasound, in particular the following studies:
– in 2015, Nell et al. published a meta-analysis with 20 articles and 3,908 thyroid nodules assessed by qualitative USE using Asteria elastography (ES) classification. They showed a sensitivity and specificity of 85 and 80% respectively, using an elasticity score threshold between 2 and 3, and a sensitivity and specificity of 99 and 14% respectively, using an elasticity score threshold between 1 and 2. In conclusion they affirm that qualitative elastography can detect benign nodules with a high accuracy (101);
– in 2014, Ghajarzadeh et al. published a metanalysis with 12 articles and 1,180 thyroid nodules assessed by qualitative US-elastography. They showed a sensitivity and specificity of 86 and 66.7% respectively, using an elasticity score threshold between 2 and 3, and a sensitivity and specificity of 98.3 and 19.6% respectively, using a elasticity score threshold between 1 and 2. In conclusion they affirm that USE could be used as thyroid nodule screening tool (102).
Almost in parallel, articles began to be published comparing qualitative and semi-quantitative USE and in particular:
– in 2016, the metanalysis of Tian showed the better SRE accuracy than qualitative USE, with a sensitivity and specificity of 86.5% vs. 81.8% and 86.6% vs. 81.7% respectively (103);
– in 2014, Sun et al. published a metanalysis with 31 papers and 6,544 thyroid nodules assessed by real-time ultrasound elastography. They showed the better SRE accuracy than qualitative USE with a sensitivity and specificity of 85% vs 79% and 80% vs 77% respectively. In conclusion they affirm that the SRE and qualitative USE accuracy are similar although SRE diagnostic value is slightly higher than elasticity score (104);
– in 2013, Razavi et al. published a metanalysis with 24 papers and 3,531 thyroid nodules (2,604 benign and 927 malignant) assessed by qualitative and semiquantitative USE. They showed the better accuracy of SRE assessment than elasticity score evaluation with a sensitivity of 89% vs. 82%, respectively, but with same specificity (82%) (105).
After the introduction of new elastosonographic techniques based on shear wave speeds, new studies and various meta-analyses were published to evaluate the SWE diagnostic performance compared to gold-standards, in particular:
- in 2015, Zhan et al. published a meta-analysis with 16 papers and 2,436 thyroid nodules (1,691 benign and 745 malignant) assessed by ARFI (acoustic radiation force impulse) imaging. They showed a sensitivity and specificity of 80% and 85% respectively, affirming SWE could help identify which patients should be treated surgically (106);
- in 2018, Chang et al. published a meta-analysis with 20 papers and 3397 thyroid nodules, assessed by quantitative SWE. They showed a sensitivity and specificity of 68 and 85% concluding that SWE is very accurate in distinguishing malignant and benign nodules (107);
- in 2020, Filho et al. published a meta-analysis with 17 papers and 3,806 thyroid nodules (2,428 benign and 1,378 malignant) assessed by 2D–SWE elastosonography by various manufacturers. They showed a sensitivity and specificity of 77 and 76% respectively for T–SWE (Toshiba shear-wave elastography); a sensitivity and specificity of 72 and 81% respectively for VTIQ (Virtual Touch tissue imaging and Quantification); and a sensitivity and specificity of 63 and 81% respectively for S-SWE (SuperSonic shear-wave elastography). In conclusion they affirm that 2D–SWE could rule out the malignant thyroid nature (108).
Furthermore, some meta-analyses which compare these two different elastosonographic techniques were published:
- in 2017, Hu et al. published a meta-analysis with 22 original articles and 2,661 thyroid nodules (2,063 benign and 598 malignant) assessed by SE (Strain Elastography) and SWE. They showed a sensitivity and specificity of 84 and 90% respectively for SE and a sensitivity and specificity of 79 and 87% respectively for SWE. In conclusion, they state that SE has a better sensitivity than SWE (0.84 vs. 0.79) with p-value <0.05 and above all a statistically better specificity than SWE (0.90 vs. 0.87 with p <0.05) (109);
- in 2016, Tian et al. published a meta-analysis with 54 papers with 10,001 thyroid nodules (7,380 benign and 2,621 malignant) assessed by SE (Strain Elastography) and SWE. They showed a sensitivity and specificity of 82.9 and 82.8% respectively for SE, and sensitivity and specificity of 78.4 and 82.4% for SWE. In conclusion they affirm that the SE sensitivity is better than SWE one (0.829 vs. 0.784) but with similar specificity (103).
The USE role is not limited to the thyroid cancer diagnosis but it is also useful in the detection of cervical lymph node metastases and to guide interventistic procedures (110). In fact, the EFSUMB guidelines state that USE can identify the most suspicious lymph nodes and the most suspicious internal areas worthy of cyto-histological investigation (92).
Our meta-analysis is the first meta-analysis since 2016 that individually takes into consideration the diagnostic performance of different USE types in the characterization of the thyroid nodule however using studies with at least 50 thyroid nodules because the smaller ones may have low precision (wide confidence interval of the estimates), may be of low quality and may increase heterogeneity.
In particular we examined qualitative USE, semiquantitative USE and quantitative USE and demonstrate that all of them are useful in the thyroid nodule characterization with high accuracy values and especially the same specificity. However, the semiquantitative and qualitative elastosonography showed the best diagnostic performance compared to SWE with the following sensitivity and specificity values 84 and 81% for qualitative USE, 83 and 80% for semiquantitative USE and 78 and 81% for quantitative USE.
Our results about strain-based USE techniques are similar with no significant statistical difference (p-value >0.05).
By contrast, the AUCs evaluation slightly favors the SRE over others (semiquantitative USE AUC: 0.93; qualitative USE: 0.89; quantitative USE: 0.87) with statistically significant values between semiquantitative USE and quantitative USE (p-value <0.05).
Our metanalysis results are quite in line with this recent meta-analyses and guidelines that indicate SRE the most accurate USE method in the malignancy risk stratification of the thyroid nodules (10).
Although in 2017 the Hu et al. meta-analysis showed the better qualitative USE sensitivity and specificity than SWE ones (0.84 vs. 0.79 with p >0.05 and 0.90 vs. 0.87 with p <0.05, respectively), the semiquantitative USE was poorly represented in their paper and not distinguished from qualitative USE in the statistical analysis (109).
In 2016 the meta-analysis of Tian concluded asserting that the SE (Strain Elastography) diagnostic performance (both qualitative and semiquantitative USE) was better than SWE with a p <0.05 and among the SE techniques the SRE (strain ratio elastography) accuracy was better than SE with elasticity score with sensitivity and specificity values of 86.5% vs 81.8% and 86.6% vs 81.7% (103).
These differences could be explained because the main qualitative USE limitation is the operator-dependence related to the subjective diagnostic evaluation based on different eye-type scales without agreement about the score to be used (55). In literature, several qualitative USE color pattern involving five, four, or two color score are used, but showing different diagnostic performances without having a better one (55).
SRE improves the subjective assessment of the nodule stiffness, in some cases it is not feasible due to the presence of micro-macrocalcifications, pathological changes in the surrounding parenchyma such as in autoimmune thyroiditis or when the nodule is so large as to replace the entire gland without healthy parenchyma to compare. Furthermore, there is no agreement on the SRE cut-off to choose, and therefore without having a real standardization of this method.
SWE is the quantitative USE technique based on the Shear-wave speeds measurement and so less affected by a subjective interpretation. But to date, the current and recent papers showed a worse SWE diagnostic accuracy than SE one. SWE can evaluate thyroid nodule also in presence of autoimmune thyroiditis (111) and so when SE is unfeasible for the pathological changes of peri nodular surrounding thyroid parenchyma.
I2 quantify the effect of heterogeneity, describing the percentage of total variation across studies that is due to heterogeneity rather than chance. In our results it is very high for all parameters (>80%) except for the sensitivity of the SWE which is instead about 55.1%. This could lead to think that it is a technique less influenced by interobservational variability but nevertheless its I2 is too high (>50%) and not low enough to affirm a good homogeneity between the different studies. One explanation could be that the qualitative and semi-quantitative USE techniques have been used for a long time and therefore the resulting studies are older and more heterogeneous. Other reasons should explain it such as a possible more homogeneous population or settings. The interobservational variability of the different USE techniques is beyond our purposes but to date it has been evaluated by few studies. Therefore, further studies are needed, especially prospective and with a large population.
In our study there are some limitations: at first, calcified and/or cystic nodules are not included by some studies for possible artifacts generation; secondly, the heterogeneity of the articles included may represent a source of bias as no consensus about the optimal elastosonographic methodology as the preferential use of carotid or freehand pulsation in the strain elastography; the non-univocal qualitative USE score to use (score 1–2; score 1–4 or score 1–5) and different Strain Ratio cut-off values; thirdly, the possible selection bias. In fact our thyroid nodules population presents a high pooled malignancy rate (33%) deriving from the studies published by various research institutes considered as a reference center for thyroid pathology and so with many patients with already suspected thyroid nodule. All this might have contributed to have misleading results.
In addition, we have to mention that we did not evaluate the inter-observational variability between the different USE techniques and secondly specific papers on indeterminate nodules at FNAC have not been.
Noteworthy, although FNAC is the gold standard for the thyroid nodule classification, it can show cellular atypia of undetermined significance (TIR3 category) in the 5–20% of cases (112). Therefore a fairly large number of patients undergo thyroidectomy for diagnostic rather than therapeutic purposes, with increased costs and possible complications. Therefore, in recent years, efforts have been made to better evaluate the cytologically indeterminate nodule and reduce the number of these thyroidectomies as only up to 30% of these patients harbor indeed thyroid cancer.
In this regard, MPUS tries to better characterize indeterminate thyroid nodules and with encouraging results (96, 106, 107, 113).
In conclusion, this comprehensive meta-analysis shows that all USE methods (quantitative, semi-quantitative and quantitative USE) have a good sensitivity and specificity in differentiating malignancy from benignancy, with a slight better performance by means of qualitative USE.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conceptualization, VC, VDA, and CC Methodology, VC, DF, VS, ADS, CD, and GG Investigation, ADS, VS, DF, PP, EP, GP, and OG Resources, SS, PT, CD, and MT Data curation, DF, VC, CD, GG, and PT Writing—original draft preparation, DF, CD, VC, AL, RC, OG, and PT Writing—review and editing, VC, AMI, CD, GG, and EG Supervision, VC, CC, VDA, PT, and CD All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Author VC reports a lecturer fee from Bracco, Samsung and Toshiba.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Durante C, Grani G, Lamartina L Filetti S, Mandel SJ, Cooper DS. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA (2018) 319:914–24. doi: 10.1001/jama.2018.0898
2. Ulisse S, Baldini E, Lauro A, Pironi D, Tripodi D, Lori E, et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers (2021) 13(21):5567. doi: 10.3390/cancers13215567
3. Brito JP, Morris JC, Montori VM. Thyroid Cancer: Zealous Imaging has Increased Detection and Treatment of Low Risk Tumours. BMJ (2013) 347:f4706. doi: 10.1136/bmj.f4706
4. Hoang JK, Nguyen XV, Davies L. Overdiagnosis of Thyroid Cancer. Acad Radiol (2015) 22:1024–9. doi: 10.1016/j.acra.2015.01.019
5. Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive Predictive Values of Sonographic Features of Solid Thyroid Nodule. Clin Imaging (2010) 34:127–33. doi: 10.1016/j.clinimag.2008.10.034
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Asso-Ciation Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
7. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol (2016) 17:370–95. doi: 10.3348/kjr.2016
8. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology (2011) 260:892–9. doi: 10.1148/radiol.11110206
9. Ulisse S, Bosco D, Nardi F, Nesca A, D'Armiento E, Guglielmino V, et al. Thyroid Imaging Reporting and Data System Score Combined With the New Italian Classification for Thyroid Cytology Improves the Clinical Management of Indeterminate Nodules. Int J Endocrinol (2017) 2017:9692304. doi: 10.1155/2017/9692304
10. Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-Needle Aspiration may Miss a Third of All Malignancy in Palpable Thyroid Nodules: A Comprehensive Literature Review. Ann Surg (2007) 246:714–20. doi: 10.1097/SLA.0b013e3180f61adc
11. Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of Repeated Fine Needle Aspirations of the Thyroid: An Analysis of Over Ten Thousand FNAs. Thyroid (2007) 17(11):1061–6. doi: 10.1089/thy.2007.0159
12. Grani G, Calvanese A, Carbotta G, D'Alessandri M, Nesca A, Bianchini M, et al. Intrinsic Factors Affecting Adequacy of Thyroid Nodule Fine-Needle Aspiration Cytology. Clin Endocrinol (2013) 78:141–4. doi: 10.1111/j.1365-2265.2012.04507.x
13. Grani G, Lamartina L, Ascoli V, Bosco D, Nardi F, D'Ambrosio F, et al. Ultrasonography Scoring Systems Can Rule Out Malignancy in Cytologically Indeterminate Thyroid Nodules. Endocrine (2017) 57:256–61. doi: 10.1007/s12020-016-1148-6
14. He YP, Xu HX, Zhao CK, Sun LP, Li XL, Yue WW, et al. Cytologically Indeterminate Thyroid Nodules: Increased Diagnostic Performance With Combination of US TI-RADS and a New Scoring System. Sci Rep (2017) 7(1):6906. doi: 10.1038/s41598-017-07353-y
15. Horvath E, Silva CF, Majlis S, Rodriguez I, Skoknic V, Castro A, et al. Prospective Validation of the Ultrasound Based TIRADS (Thyroid Imaging Reporting And Data System) Classification: Results in Surgically Resected Thyroid Nodules. Eur Radiol (2017) 27(6):2619–28. doi: 10.1007/s00330-016-4605-y
16. Papale F, Cafiero G, Grimaldi A, Marino G, Rosso F, Mian C, et al. Galectin-3 Expression in Thyroid Fine Needle Cytology (T-FNAC) Uncertain Cases: Validation of Molecular Markers and Technology Innovation. J Cell Physiol (2013) 228(5):968–74. doi: 10.1002/jcp.24242
17. Cantisani V, Ulisse S, Guaitoli E, De Vito C, Caruso R, Mocini R, et al. Q-Elastography in the Presurgical Diagnosis of Thyroid Nodules With Indeterminate Cytology. PloS One (2012) 7(11):e50725. doi: 10.1371/journal.pone.0050725
18. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. Aace/Ace/Ame Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Association Medici Endocrinology Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules–2016 Update. Endocr Pract (2016) 22:622–39. doi: 10.4158/EP161208.GL
19. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J (2017) 6(5):225–37. doi: 10.1159/000478927
20. Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 4. Thyroid. Ultrasound Med Biol (2017) 43(1):4–26. doi: 10.1016/j.ultrasmedbio.2016.06.022
21. Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 1: Basic Principles and Technology. Ultraschall Med (2013) 34(2):169–84. doi: 10.1055/s-0033-1335205
22. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
23. Henderson M, Tierney LM, Smetana GW. The Patient History. 2nd ed. New York, NY: McGraw-Hill (2012).
24. Moses LE, Shapiro D, Littenberg B. Combining Independent Studies of a Diagnostic Test Into a Summary ROC Curve: Data-Analytic Approaches and Some Additional Considerations. Stat Med (1993) 12:1293– 1316. doi: 10.1002/sim.4780121403
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the Areas Under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics (1988) 44:837–45. doi: 10.2307/2531595
26. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: A Software for Meta-Analysis of Test Accuracy Data. BMC Med Res Methodol (2006) 6:31. doi: 10.1186/1471-2288-6-31
27. MedCalc® Statistical Software Version 20.010. Ostend, Belgium:MedCalc Software Ltd (2021). Available at: https://www.medcalc.org/manual/howtociteMedCalc.php.
28. Chong Y, Shin JH, Ko ES, Han BK. Ultrasonographic Elastography of Thyroid Nodules: Is Adding Strain Ratio to Colour Mapping Better? Clin Radiol (2013) 68:1241–6. doi: 10.1016/j.crad.2013.06.023
29. Cantisani V, Grazhdani H, Ricci P, Mortele K, Di Segni M, D'Andrea V, et al. Q-Elastosonography of Solid Thyroid Nodules: Assessment of Diagnostic Efficacy and Interobserver Variability in a Large Patient Cohort. Eur Radiol (2014) 24:143–50. doi: 10.1007/s00330-013-2991-y
30. Refaat R, Kamel A, Elganzory M, Awad NM. Can Real-Time Ultrasound Elastography Using the Color Score and Strain Ratio Differentiate Between Benign and Malignant Solitary Thyroid Nodules? Egypt J Radiol Nucl Med (2014) 45:75–87. doi: 10.1016/j.ejrnm.2013.12.005
31. Tatar IG, Kurt A, Yilmaz KB, Doğan M, Hekimoglu B, Hucumenoglu S. The Role of Elastosonography, Gray-Scale and Colour Flow Doppler Sonography in Prediction of Malignancy in Thyroid Nodules. Radiol Oncol (2014) 48:348–53. doi: 10.2478/raon-2014-0007
32. Grazhdani H, Cantisani V, Lodise P, Di Rocco G, Proietto MC, Fioravanti E, et al. Prospective Evaluation of Acoustic Radiation Force Impulse Technology in the Differentiation of Thyroid Nodules: Accuracy and Interobserver Variability Assessment. J Ultrasound (2014) 17:13–20. doi: 10.1007/s40477-013-0062-5
33. El-Hariri M, Ali T, Abueldahab M, Magid A, Elshiekh A. The Clinical Value of Ultrasound Elastography in Predicting Malignant Thyroid Nodules. Egypt J Radiol Nucl Med (2014) 45:353–9. doi: 10.1016/j.ejrnm.2014.03.006
34. Xu JM, Xu HX, Xu XH, Liu C, Zhang YF, Guo LH, et al. Solid Hypo-Echoic Thyroid Nodules on Ultrasound: The Diagnostic Value of Acoustic Radiation Force Impulse Elastography. Ultrasound Med Biol (2014) 40:2020–30. doi: 10.1016/j.ultrasmedbio.2014.04.012
35. Cakir B, Ersoy R, Cuhaci FN, Aydin C, Polat B, Kılıc M, et al. Elastosonographic Strain Index in Thyroid Nodules With Atypia of Undetermined Significance. J Endocrinol Invest (2014) 37:127–33. doi: 10.1007/s40618-013-0005-1
36. Bederina EL, Orlinskaya NY, Konovalov VA, Zubeev PS. Diagnostic Value Sonoelastography in Differential Diagnosis of Thyroid Nodules. Sovrem Tekhnologii Med (2014) 6:43–5.
37. Çakal E, Şahin M, Ünsal İÖ, Güngüneş A, Akkaymak E, Özkaya EÇ, et al. Elastography in the Differential Diagnosis of Thyroid Nodules. Ultrasonic Imaging (2015) 37:251–7. doi: 10.1177/0161734614547542
38. Cantisani V, Maceroni P, D'Andrea V, Patrizi G, Di Segni M, De Vito C, et al. Strain Ratio Ultrasound Elastography Increases the Accuracy of Colour-Doppler Ultrasound in the Evaluation of Thy-3 Nodules. A Bi-Centre University Experience. Eur Radiol (2016) 26(5):1441–9. doi: 10.1007/s00330-015-3956-0
39. Huang X, Guo LH, Xu HX, Gong XH, Liu BJ, Xu JM, et al. Acoustic Radiation Force Impulse Induced Strain Elastography and Point Shear Wave Elastography for Evaluation of Thyroid Nodules. Int J Clin Exp Med (2015) 15:10956–63.
40. Azizi G, Keller JM, Mayo ML, Piper K, Puett D, Earp KM, et al. Thyroid Nodules and Shear Wave Elastography: A New Tool in Thyroid Cancer Detection. Ultrasound Med Biol (2015) 41:2855–65. doi: 10.1016/j.ultrasmedbio.2015.06.021
41. Cetin N, Yücel C, Göçün PU, Kurt SA, Taneri F, Oktar S, et al. The Efficiency of Ultrasound Elastography in the Differential Diagnosis of Thyroid Nodules. JBR-BTR (2015) 98:20–6. doi: 10.5334/jbr-btr.747
42. Dobruch-Sobczak K, Zalewska EB, Gumińska A, Słapa RZ, Mlosek K, Wareluk P, et al. Diagnostic Performance of Shear Wave Elastography Parameters Alone and in Combination With Conventional B-Mode Ultrasound Parameters for the Characterization of Thyroid Nodules: A Prospective, Dual-Center Study. Ultrasound Med Biol (2016) 42:2803–11. doi: 10.1016/j.ultrasmedbio.2016.07.010
43. Duan SB, Yu J, Li X, Han ZY, Zhai HY, Liang P. Diagnostic Value of Two-Dimensional Shear Wave Elastography in Papillary Thyroid Microcarcinoma. Onc Targets Ther (2016) 9:1311–7. doi: 10.2147/OTT.S98583
44. Friedrich-Rust M, Vorlaender C, Dietrich CF, Kratzer W, Blank W, Schuler A, et al. Evaluation of Strain Elastography for Differentiation of Thyroid Nodules: Results of a Prospective DEGUM Multicenter Study. Ultraschall Med (2016) 37:262–70. doi: 10.1055/s-0042-104647
45. Xing P, Chen Q, Yang ZW, Liu CB, Wu CJ. Combination of Conventional Ultrasound and Tissue Quantification Using Acoustic Radiation Force Impulse Technology for Differential Diagnosis of Small Thyroid Nodules. Int J Clin Exp Med (2016) 9:8288–95.
46. Seong M, Shin JH, Hahn SY. Ultrasound Strain Elastography for Circumscribed Solid Thyroid Nodules Without Malignant Features Categorized as Indeterminate by B-Mode Ultrasound. Ultrasound Med Biol (2016) 42:2383–90. doi: 10.1016/j.ultrasmedbio.2016.06.011
47. Chen BD, Xu HX, Zhang YF, Liu BJ, Guo LH, Li DD, et al. Calcification of Thyroid Nodules Increases Shear-Wave Speed (SWS) Measurement: Using Multiple Calcification-Specific SWS Cutoff Values Outperforms a Single Uniform Cutoff Value in Diagnosing Malignant Thyroid Nodules. Oncotarget (2016) 7:66149–59. doi: 10.18632/oncotarget.11710
48. Ebeed AE, Romeih MA, Refat MM, Salah NM. Role of Ultrasound, Color Doppler, Elastography and Micropure Imaging in Differentiation Between Benign and Malignant Thyroid Nodules. Egypt J Radiol Nucl Med (2017) 48(3):603–10. doi: 10.1016/j.ejrnm.2017.03.012
49. Dawoud M, Dawoud R. Added Value of Strain Elastosonography in Prediction of Malignancy in Solitary Thyroid Nodule. Egypt J Radiol Nucl Med (2017) 48(4):905–12. doi: 10.1016/j.ejrnm.2017.06.011
50. Liu BJ, Zhao CK, Xu HX, Li D, Bo X, Li X. Quality Measurement on Shear Wave Speed Imaging: Diagnostic Value in Differentiation of Thyroid Malignancy and the Associated Factors. Oncotarget (2017) 8:4848–959. doi: 10.18632/oncotarget.13996
51. Liu Z, Jing H, Han X, Shao H, Sun YX, Wang QC, et al. Shear Wave Elastography Combined With the Thyroid Imaging Reporting and Data System for Malignancy Risk Stratification in Thyroid Nodules. Oncotarget (2017) 8:43406–16. doi: 10.18632/oncotarget.15018
52. Wang D, He YP, Zhang YF, Liu BJ, Zhao CK, Fu HJ, et al. The Diagnostic Performance of Shear Wave Speed (SWS) Imaging for Thyroid Nodules With Elasticity Modulus and SWS Measurement. Oncotarget (2017) 8:13387–99. doi: 10.18632/oncotarget.14534
53. Kyriakido G, Friedrich-Rust M, Bon D, Sircar I, Schrecker C, Bogdanou D, et al. Comparison of Strain Elastography, Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging and 2D-Shear Wave Elastography for the Differentiation of Thyroid Nodules. PloS One (2018) 13(9):e0204095. doi: 10.1371/journal.pone.0204095
54. Wahab S, Ahmad I. The Impact of Thyroid Sonoelastography in Preventing Irrational Needle Biopsies in Evaluation of Benign Thyroid Nodules. Hong Kong J Radiol (2018) 21:107–13. doi: 10.12809/hkjr1816829
55. Cantisani V, David E, Grazhdani H, Rubini A, Radzina M, Dietrich CF, et al. Prospective Evaluation of Semiquantitative Strain Ratio and Quantitative 2d Ultrasound Shear Wave Elastography (SWE) in Association With TIRADS Classification for Thyroid Nodule Characterization. Ultraschall Med (2019) 40:495–503. doi: 10.1055/a-0853-1821
56. Huang Y, Zhou H, Zhang C, Hong Y, Ye Q, Huang P. Diagnostic Performance of Ultrasound Strain Elastography in Transverse and Longitudinal Views in Predicting Malignant Thyroid Nodules. Ultrasound Med Biol (2019) 45:2289–97. doi: 10.1016/j.ultrasmedbio.2019.05.018
57. Yang Q, Zhou W, Li J, Wu G, Ding F, Tian X. Comparative Analysis of Diagnostic Value for Shear Wave Elastography and Real-Time Elastographic Imaging for Thyroid Nodules. J Med Imaging Health Infor (2019) 9:334–8. doi: 10.1166/jmihi.2019.2594
58. Aghaghazvini L, Maheronnaghsh R, Soltani A, Rouzrokh P, Chavoshi M. Diagnostic Value of Shear Wave Sonoelastography in Differentiation of Benign From Malignant Thyroid Nodules. Eur J Radiol (2020) 126:108926. doi: 10.1016/j.ejrad.2020.108926
59. Hairu L, Yulan P, Yan W, Hong A, Xiaodong Z, Lichun Y, et al. Elastography for the Diagnosis of High-Suspicion Thyroid Nodules Based on the 2015 American Thyroid Association Guidelines: A Multicenter Study. BMC Endocr Disord (2020) 20:43. doi: 10.1186/s12902-020-0520-y
60. Huang ST, Zhang B, Yin HL, Li B, Liao JT, Wang YB. Incremental Diagnostic Value of Shear Wave Elastography Combined With Contrast-Enhanced Ultrasound in TI-RADS Category 4a and 4b Nodules. J Med Ultrason (2020) 47:453–62. doi: 10.1007/s10396-020-01016-8
61. Goel S, Malhotra A, Agarwal A, Chandak S, Kumar A, Khan A. Comparative Efficacy of Ultrasonography and Acoustic Radiation Force Impulse (ARFI) Elastography in Prediction of Malignancy in Thyroid Nodules. J Diagn Med Sonogr (2020) 36:433–43. doi: 10.1177/8756479320931354
62. Pei S, Zhang B, Cong S, Liu J, Wu S, Dong Y, et al. Ultrasound Real-Time Tissue Elastography Improves the Diagnostic Performance of the ACR Thyroid Imaging Reporting and Data System in Differentiating Malignant From Benign Thyroid Nodules: A Summary of 1525 Thyroid Nodules. Int J Endocrinol (2020) 14:1749351. doi: 10.1155/2020/1749351
63. Yavuz A, Akbudak I, Üçler R, Özgökçe M, Arslan H, Batur A. Comparison of Efficiencies Between Shear Wave Elastography, Fine-Needle Aspiration Biopsy and American College of Radiology Thyroid Imaging Reporting and Data System Scoring System in Determining the Malignity Potential of Solid Thyroid Nodules. Ultrasound Q (2020) 37:155–60. doi: 10.1097/RUQ.0000000000000531
64. Yang J, Song Y, Wei W, Ruan L, Ai H. Comparison of the Effectiveness of Ultrasound Elastography With That of Conventional Ultrasound for Differential Diagnosis of Thyroid Lesions With Suspicious Ultrasound Features. Oncol Lett (2017) 14:3515–21. doi: 10.3892/ol.2017.6644
65. Yeon EK, Sohn YM, Seo M, Kim EJ, Eun YG, Park WS, et al. Diagnostic Performance of a Combination of Shear Wave Elastography and B-Mode Ultrasonography in Differentiating Benign From Malignant Thyroid Nodules. Clin Exp Otorhinolaryngol (2020) 13:86–193. doi: 10.21053/ceo.2019.01235
66. Tuan PA, Duc NM, An M, Vien MV, Giang BV. The Role of Shear Wave Elastography in the Discrimination Between Malignant and Benign Thyroid Nodules. Act Inform Med (2020) 28:248–53. doi: 10.5455/aim.2020.28.248-253
67. Hu L, Liu X, Pei C, Xie L, He N. Assessment of Perinodular Stiffness in Differentiating Malignant From Benign Thyroid Nodules. Endocr Connect (2021) 10:492–50. doi: 10.1530/EC-21-0034
68. Cantisani V, D'Andrea V, Biancari F, Medvedyeva O, Di Segni M, Olive M, et al. Prospective Evaluation of Multiparametric Ultrasound and Quantitative Elastosonography in the Differential Diagnosis of Benign and Malignant Thyroid Nodules: Preliminary Experience. Eur J Radiol (2012) 81:2678–83. doi: 10.1016/j.ejrad.2011.11.056
69. Cantisani V, D'Andrea V, Mancuso E, Maggini E, Di Segni M, Olive M, et al. Prospective Evaluation in 123 Patients of Strain Ratio as Provided by Quantitative Elastosonography and Multiparametric Ultrasound Evaluation (Ultrasound Score) for the Characterisation of Thyroid Nodules. Radiol Med (2013) 118:1011–21. doi: 10.1007/s11547-013-0950-y
70. Sohail S, Kaliq U, Zaman S. Diagnostic Value of Mean Elasticity Index as a Quantitative Shear Wave Elastography Parameter for Prediction of Malignancy in Small Suspicious Solid Thyroid Nodules. J Coll Physicians Surg Pak (2020) 30:683–7. doi: 10.29271/jcpsp.2020.07.683
71. Idrees A, Shahzad R, Fatim I, Shahid A. Strain Elastography for Differentiation Between Benign and Malignant Thyroid Nodules. J Coll Physicians Surg Pak (2020) 30:369–72. doi: 10.29271/jcpsp.2020.04.369
72. Liao LJ, Chen HW, Hsu WL, Chen YS. Comparison of Strain Elastography, Shear Wave Elastography, and Conventional Ultrasound in Diagnosing Thyroid Nodules. J Med Ultrasound (2019) 27:26–32. doi: 10.4103/JMU.JMU4618
73. Fukuhara T, Matsuda E, Donishi R, Koyama S, Miyake N, Fujiwara K, et al. Clinical Efficacy of Novel Elastography Using Acoustic Radiation Force Impulse (ARFI) for Diagnosis of Malignant Thyroid Nodules. Laryngoscope Investig Otolaryngol (2018) 3:319–25. doi: 10.1002/lio2.165
74. Wojtaszek-Nowicka M, Słowińska-Klencka D, Sporny S, Popowicz B, Kuzdak K, Pomorski L, et al. The Efficiency of Elastography in the Diagnostics of Follicular Lesions and Nodules With an Unequivocal FNA Result. Endokrynol Pol (2017) 68:610–22. doi: 10.5603/EP.a2017.0050
75. Kim H, Kim JA, Son EJ, Youk JH. Quantitative Assessment of Shear-Wave Ultrasound Elastography in Thyroid Nodules: Diagnostic Performance for Predicting Malignancy. Eur Radiol (2013) 23:2532–7. doi: 10.1007/s00330-013-2847-5
76. Wang H, Brylka D, Sun LN, Lin YQ, Sui GQ, Gao J. Comparison of Strain Ratio With Elastography Score System in Differentiating Malignant From Benign Thyroid Nodules. Clin Imaging (2013) 37:50–5. doi: 10.1016/j.clinimag.2012.04.003
77. Wang HL, Zhang S, Xin XJ, Zhao LH, Li X, Mu JL, et al. Application of Real-Time Ultrasound Elastography in Diagnosing Benign and Malignant Thyroid Solid Nodules. Cancer Biol Med (2012) 9:124–7. doi: 10.3969/j.issn.2095-3941.2012.02.008
78. Veyrieres JB, Albarel F, Lombard JV, Berbis J, Sebag F, Oliver C, et al. A Threshold Value in Shear Wave Elastography to Rule Out Malignant Thyroid Nodules: A Reality? Eur J Radiol (2012) 81:3965–72. doi: 10.1016/j.ejrad.2012.09.002
79. Ning CP, Jiang SQ, Zhang T, Sun LT, Liu YJ, Tian JW. The Value of Strain Ratio in Differential Diagnosis of Thyroid Solid Nodules. Eur J Radiol (2012) 81:286–91. doi: 10.1016/j.ejrad.2010.12.010
80. Cakir B, Aydin C, Korukluoğlu B, Ozdemir D, Sisman IC, Tüzün D, et al. Diagnostic Value of Elastosonographically Determined Strain Index in the Differential Diagnosis of Benign and Malignant Thyroid Nodules. Endocrine (2011) 39:89–98. doi: 10.1007/s12020-010-9416-3
81. Baş H, Üstüner E, Kula S, Konca C, Demirer S, Elhan AH. Elastography and Doppler May Bring a New Perspective to TIRADS, Altering Conventional Ultrasonography Dominance. Acad Radiol (2021) 29(3):e25–38. doi: 10.1016/j.acra.2021.02.011
82. Chen M, Zhang KQ, Xu YF, Zhang SM, Cao Y, Sun WQ. Shear Wave Elastography and Contrast-Enhanced Ultrasonography in the Diagnosis of Thyroid Malignant Nodules. Mol Clin Oncol (2016) 5:724–30. doi: 10.3892/mco.2016.1053
83. Liu BX, Xie XY, Liang JY, Zheng YL, Huang GL, Zhou LY, et al. Shear Wave Elastography Versus Real-Time Elastography on Evaluation Thyroid Nodules: A Preliminary Study. Eur J Radiol (2014) 83:1135–43. doi: 10.1016/j.ejrad.2014.02.024
84. Rago T, Vitti P. Potential Value of Elastosonography in the Diagnosis of Malignancy in Thyroid Nodules. Q J Nucl Med Mol Imaging (2009) 53:455–64.
85. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
86. Castellana M, Grani G, Radzina M, Guerra V, Giovanella L, Deandrea M, et al. Performance of EU-TIRADS in Malignancy Risk Stratification of Thyroid Nodules: A Meta-Analysis. Eur J Endocrinol (2020) 183:255–64. doi: 10.1530/EJE-20-0204
87. Grani G, Lamartina L, Ramundo V, Falcone R, Lomonaco C, Ciotti L, et al. A New Definition Improves the Specificity of TIRADS Systems. Eur Thyroid J (2020) 9:85–91. doi: 10.1159/000504219
88. Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J Clin Endocrinol Metab (2019) 104:95–102. doi: 10.1210/jc.2018-01674
89. Li W, Wang Y, Wen J, Zhang L, Sun Y. Diagnostic Performance of American College of Radiology TI-RADS: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol (2021) 216:38–47. doi: 10.2214/AJR.19.22691
90. Negro R, Attanasio R, Grimaldi F, Frasoldati A, Guglielmi R, Papini E. A 2016 Italian Survey About Guidelines and Clinical Management of Thyroid Nodules. Eur Thyroid J (2016) 6:75–81. doi: 10.1159/000453032
91. Dighe M, Barr R, Bojunga J, Cantisani V, Chammas MC, Cosgrove D, et al. Thyroid Ultrasound: State of the Art. Part 2—Focal Thyroid Lesions. Med Ultrason (2017) 19:195–210. doi: 10.11152/mu-999
92. Sidhu P, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med (2018) 39:e2–e44. doi: 10.1055/a-0586-1107
93. Saftoiu A, Gilja OH, Sidhu P, Dietrich CF, Cantisani V, Amy D, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update. Ultraschall Med (2019) 40:425–53. doi: 10.1055/s-0044-101254
94. Fresilli D, David E, Pacini P, Del Gaudio G, Dolcetti V, Lucarelli GT, et al. Thyroid Nodule Characterization: How to Assess the Malignancy Risk. Update of the Literature. Diagnostics (2021) 11:1374. doi: 10.3390/diagnostics11081374
95. Sorrenti S, Dolcetti V, Fresilli D, Del Gaudio G, Pacini P, Huang P, et al. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J Clin Med (2021) 10(19):4559. doi: 10.3390/jcm10194559
96. Celletti I, Fresilli D, De Vito C, Bononi M, Cardaccio S, Cozzolino A, et al. TIRADS, SRE and SWE in INDETERMINATE Thyroid Nodule Characterization: Which has Better Diagnostic Performance? Radiol Med (2021) 126:1189–200. doi: 10.1007/s11547-021-01349-5
97. Fresilli D, Grani G, De Pascali ML, Alagna G, Tassone E, Ramundo V, et al. Computer-Aided Diagnostic System for Thyroid Nodule Sonographic Evaluation Outperforms the Specificity of Less Experienced Examiners. J Ultrasound (2020) 23:169–74. doi: 10.1007/s40477-020-00453-y
98. Trimboli P, Castellana M, Virili C, Havre RF, Bini F, Marinozzi F, et al. Performance of Contrast-Enhanced Ultrasound (CEUS) in Assessing Thyroid Nodules: A Systematic Review and Meta-Analysis Using Histo-Logical Standard of Reference. Radiol Med (2020) 125:406–15. doi: 10.1007/s11547-019-01129-2
99. Trimboli P, Giovanella L, Valabrega S, Andrioli M, Baldelli R, Cremonini N, et al. Ultrasound Features of Medullary Thyroid Carcinoma Correlate With Cancer Aggressi-Veness: A Retrospective Multicenter Study. J Exp Clin Cancer Res (2014) 33:87. doi: 10.1186/s13046-014-0087-4
100. Sorrenti S, Carbotta G, Di Matteo FM, Catania A, Pironi D, Tartaglia F, et al. Evaluation of Clinicopathological and Molecular Parameters on Disease Recurrence of Papillary Thyroid Cancer Patient: A Retrospective Observational Study. Cancers (Basel) (2020) 12(12):3637. doi: 10.3390/cancers12123637
101. Nell S, Kist JW, Debray TP, De Keizer B, Van Oostenbrugge TJ, Borel Rinkes IH, et al. Qualitative Elastography can Replace Thyroid Nodule Fine-Needle Aspiration in Patients With Soft Thyroid Nodules. A Systematic Review and Meta-Analysis. Eur J Radiol (2015) 84(4):652–61. doi: 10.1016/j.ejrad.2015.01.003
102. Ghajarzadeh M, Sodagari F, Shakiba M. Diagnostic Accuracy of Sonoelastography in Detecting Malignant Thyroid Nodules: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol (2014) 202(4):W379–89. doi: 10.2214/AJR.12.9785
103. Tian W, Hao S, Gao B, Jiang Y, Zhang S, Guo L, et al. Comparison of Diagnostic Accuracy of Real-Time Elastography and Shear Wave Elastography in Differentiation Malignant From Benign Thyroid Nodules. Med (Baltimore) (2015) 94(52):e2312. doi: 10.1097/MD.0000000000002312. Erratum in: Medicine (Baltimore). 2016 Feb;95(8):e86b6. Gu, Lingji [Corrected to Guo, Lingji]. Erratum in: Medicine (Baltimore). 2016 Mar 03;95(8):e86b6.
104. Sun J, Cai J, Wang X. Real-Time Ultrasound Elastography for Differentiation of Benign and Malignant Thyroid Nodules: A Meta-Analysis. J Ultrasound Med (2014) 33(3):495–502. doi: 10.7863/ultra.33.3.495
105. Razavi SA, Hadduck TA, Sadigh G, Dwamena BA. Comparative Effectiveness of Elastographic and B-Mode Ultrasound Criteria for Diagnostic Discrimination of Thyroid Nodules: A Meta-Analysis. AJR Am J Roentgenol (2013) 200(6):1317–26. doi: 10.2214/AJR.12.9215
106. Zhan J, Jin JM, Diao XH, Chen Y. Acoustic Radiation Force Impulse Imaging (ARFI) for Differentiation of Benign and Malignant Thyroid Nodules–A Meta-Analysis. Eur J Radiol (2015) 84(11):2181–6. doi: 10.1016/j.ejrad.2015.07.015
107. Chang N, Zhang X, Wan W, Zhang C, Zhang X. The Preciseness in Diagnosing Thyroid Malignant Nodules Using Shear-Wave Elastography. Med Sci Monit (2018) 24:671–7. doi: 10.12659/msm.904703
108. Filho RHC, Pereira FL, Iared W. Diagnostic Accuracy Evaluation of Two-Dimensional Shear Wave Elastography in the Differentiation Between Benign and Malignant Thyroid Nodules: Systematic Review and Meta-Analysis. J Ultrasound Med (2020) 39(9):1729–41. doi: 10.1002/jum.15271
109. Hu X, Liu Y, Qian L. Diagnostic Potential of Real-Time Elastography (RTE) and Shear Wave Elastography (SWE) to Differentiate Benign and Malignant Thyroid Nodules: A Systematic Review and Meta-Analysis. Med (Baltimore) (2017) 96(43):e8282. doi: 10.1097/MD.0000000000008282
110. Radzina M, Cantisani V, Rauda M, Nielsen MB, Ewertsen C, D'Ambrosio F, et al. Update on the Role of Ultrasound Guided Radiofrequency Ablation for Thyroid Nodule Treatment. Int J Surg (2017) 41(Suppl 1):S82–93. doi: 10.1016/j.ijsu.2017.02.010
111. Shuzhen C. Comparison Analysis Between Conventional Ultrasonography and Ultrasound Elastography of Thyroid Nodules. Eur J Radiol (2012) 81(8):1806–11. doi: 10.1016/j.ejrad.2011.02.070
112. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis. Acta Cytol (2012) 56(4):333–9. doi: 10.1159/000339959
Keywords: thyroid, USE, shear wave elastography, strain elastography, meta-analysis, real-time elastography
Citation: Cantisani V, De Silvestri A, Scotti V, Fresilli D, Tarsitano MG, Polti G, Guiban O, Polito E, Pacini P, Durante C, Grani G, Isidori AM, Giannetta E, Sorrenti S, Trimboli P, Catalano C, Cirocchi R, Lauro A and D’Andrea V (2022) US-Elastography With Different Techniques for Thyroid Nodule Characterization: Systematic Review and Meta-analysis. Front. Oncol. 12:845549. doi: 10.3389/fonc.2022.845549
Received: 29 December 2021; Accepted: 16 February 2022;
Published: 16 March 2022.
Edited by:
Po-Hsiang Tsui, Chang Gung University, TaiwanReviewed by:
Marek Ruchala, Poznan University of Medical Sciences, PolandCopyright © 2022 Cantisani, De Silvestri, Scotti, Fresilli, Tarsitano, Polti, Guiban, Polito, Pacini, Durante, Grani, Isidori, Giannetta, Sorrenti, Trimboli, Catalano, Cirocchi, Lauro and D’Andrea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vito Cantisani, dml0by5jYW50aXNhbmlAdW5pcm9tYTEuaXQ=; Daniele Fresilli, ZGFuaWVsZS5mcmVzaWxsaUBob3RtYWlsLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.