- 1Department of Radiation Oncology, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China
- 2Department of Oncology, Affiliated Wu-Ming Hospital of Guangxi Medical University, Nanning, China
Purpose: The role of concurrent chemoradiotherapy (CCRT) in stage II nasopharyngeal carcinoma (NPC) is still controversial. Our objective is to evaluate the value of concurrent chemotherapy in stage II NPC receiving radiotherapy (RT).
Methods: We searched the PubMed, Embase, and Scopus databases for studies comparing CCRT versus RT alone in stage II NPC with survival outcomes and toxicities, including locoregional recurrence-free survival (LRFS), metastasis-free survival (DMFS), progression-free survival (PFS), overall survival (OS), and grade 3–4 acute toxicities. The hazard ratios (HRs) of survival outcomes and risk ratios (RRs) of toxicities were extracted for meta-analysis. Subgroup analysis for stage N1 patients was performed to further explore whether these populations can earn benefits from concurrent chemotherapy.
Results: Nine eligible studies with a total of 4,092 patients were included. CCRT was associated with a better OS (HR = 0.61, 95% CI 0.44–0.82), LRFS (HR = 0.62, 95% CI 0.50–0.78), and PFS (HR = 0.65, 95% CI 0.54–0.79), but with similar DMFS (HR = 0.81, 95% CI = 0.46–1.45) compared with two-dimensional RT (2DRT) alone. However, CCRT showed no survival benefit in terms of OS (HR = 0.84, 95% CI 0.62–1.15), LRFS (HR = 0.85, 95% CI 0.54–1.34), DMFS (HR = 0.96, 95% CI 0.60–1.54), and PFS (HR = 0.96, 95% CI 0.66–1.37) compared with intensity-modulated RT (IMRT) alone. Subgroup analyses indicated that CCRT had similar OS (HR = 1.04, 95% CI 0.37–2.96), LRFS (HR = 0.70, 95% CI 0.34–1.45), DMFS (HR = 1.03, 95% CI 0.53–2.00), and PFS (HR = 1.04, 95% CI 0.58–1.88) in the stage N1 populations. Meanwhile, compared to RT alone, CCRT significantly increased the incidence of grade 3–4 leukopenia (RR = 4.00, 95% CI 2.29–6.97), mucositis (RR = 1.43, 95% CI 1.16–1.77), and gastrointestinal reactions (RR = 8.76, 95% CI 2.63–29.12). No significant differences of grade 3–4 toxicity in thrombocytopenia (RR = 3.45, 95% CI 0.85–13.94) was found between the two groups.
Conclusion: For unselected patients with stage II NPC, CCRT was superior to 2DRT alone with better LRFS, PFS, and OS, while adding concurrent chemotherapy to IMRT did not significantly improve survival but exacerbated acute toxicities.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022318253.
Background
Nasopharyngeal carcinoma (NPC) is one of the major cancers within Southeastern Asia (1), with an annual incidence rate of 10 to 30 per 100,000 among these prevalence regions (2). Over 20% of patients present with stage II NPC at initial diagnosis (3). Radiotherapy (RT) is the main radical treatment for NPC and has brought outstanding disease control (4). Studies have shown that chemotherapy played a significant role in stage III–IVA patients (5, 6), while stage I patients cannot earn benefits from concurrent chemotherapy (7). However, the role of concurrent chemotherapy in stage II NPC remains controversial.
There are two small-sample prospective studies (8, 9) comparing concurrent chemoradiation (CCRT) with RT alone in stage II NPC patients. Among these two studies, the study (9) using two-dimensional radiotherapy (2DRT) technology reached positive results with better 10-year metastasis-free survival (DMFS), progression-free survival (PFS), overall survival (OS), and cancer-specific survival (CSS), in the CCRT group, while the other study (8) using IMRT technology obtained negative results with no survival benefit but higher hematological toxicity. However, multiple retrospective studies that compared CCRT with 2DRT alone or IMRT alone showed opposite results. Xu et al. (10) found that, compared with 2DRT, CCRT had no role in improving OS, DMFS, and PFS in stage II NPC patients, but it increased the incidence of acute adverse events. Ahmed et al. (11) reported that CCRT was superior to IMRT alone with significant benefits in OS. A systematic review (12) on treatment patterns for stage II NPC indicated that IMRT alone may be sufficient, but more aggressive treatment interventions may be needed for the T2N1M0 subgroup which has poorer survival outcomes than those in the T1N1M0 or T2N0M0 subgroup. In addition, there are three meta-analyses (13–15) evaluating the role of chemotherapy adding to RT alone for stage II NPC. Regrettably, patients with stage I/III or receiving CCRT combined with induction chemotherapy (IC) or adjuvant chemotherapy (AC) were included. The actual value of concurrent chemotherapy adding to RT is still uncertain. Therefore, we performed this meta-analysis to evaluate the benefit of concurrent chemotherapy on stage II NPC patients receiving RT.
We present the following article in accordance with the PRISMA reporting checklist (16) (eTable 1 in the Supplement).
Methods
Search Strategy
A systematic electronic search of PubMed, Embase, and Scopus databases was performed for literature published from January 1, 1990, to December 20, 2021. The detailed search strategy is presented in eTable 2 in the Supplement. Furthermore, we also searched relevant studies registered on ClinicalTrials.gov. A manual search of reference lists from all available reviews was conducted to identify the ultimate selection. Two investigators (Y-CX and Z-GL) independently carried out the literature retrieval.
Selection Criteria
Studies that met the following preset specific criteria were included: (a) original English articles published in peer-reviewed journals; (b) studies that compared CCRT versus radiotherapy alone in stage II NPC patients; and (c) studies must contain time-to-event data such as locoregional recurrence-free survival (LRFS), PFS, DMFS, or OS, which could be obtained directly from the article or extracted indirectly through the method introduced by Tierney et al. (17). The LRFS was the time from the date of diagnosis to the date of first local and/or regional failure. The DMFS was considered as the interval from the date of the diagnosis to the date of distant metastasis. The PFS was defined as the interval from the date of the diagnosis to disease progression. The OS was defined as the duration from the date of diagnosis to the date of death for any reason. The exclusion criteria were as follows: (a) conference abstracts, case reports, and reviews and (b) studies involving patients who received IC and AC.
Data Extraction and Literature Quality Assessment
Two investigators (Y-CX and Z-GL) evaluated the relevant articles according to the eligible criteria independently then extracted OS, DMFS, LRRFS, PFS, and grade 3–4 acute toxicity (leukopenia, thrombocytopenia, mucositis, gastrointestinal reactions) data from the included article, evaluated the quality of the included literature, and cross-checked the extracted data. Disagreements were resolved through discussion among the two investigators or consulting a third researcher (K-HC) to reach an agreement.
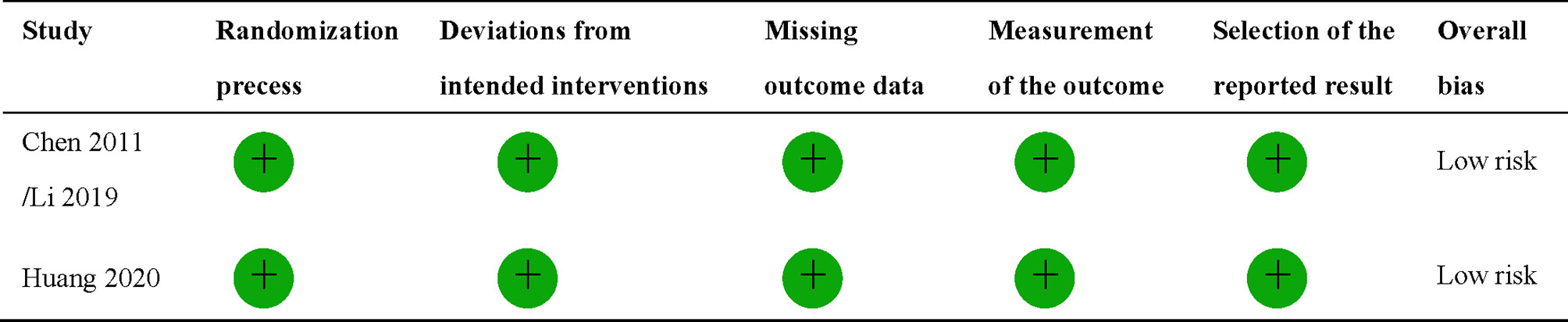
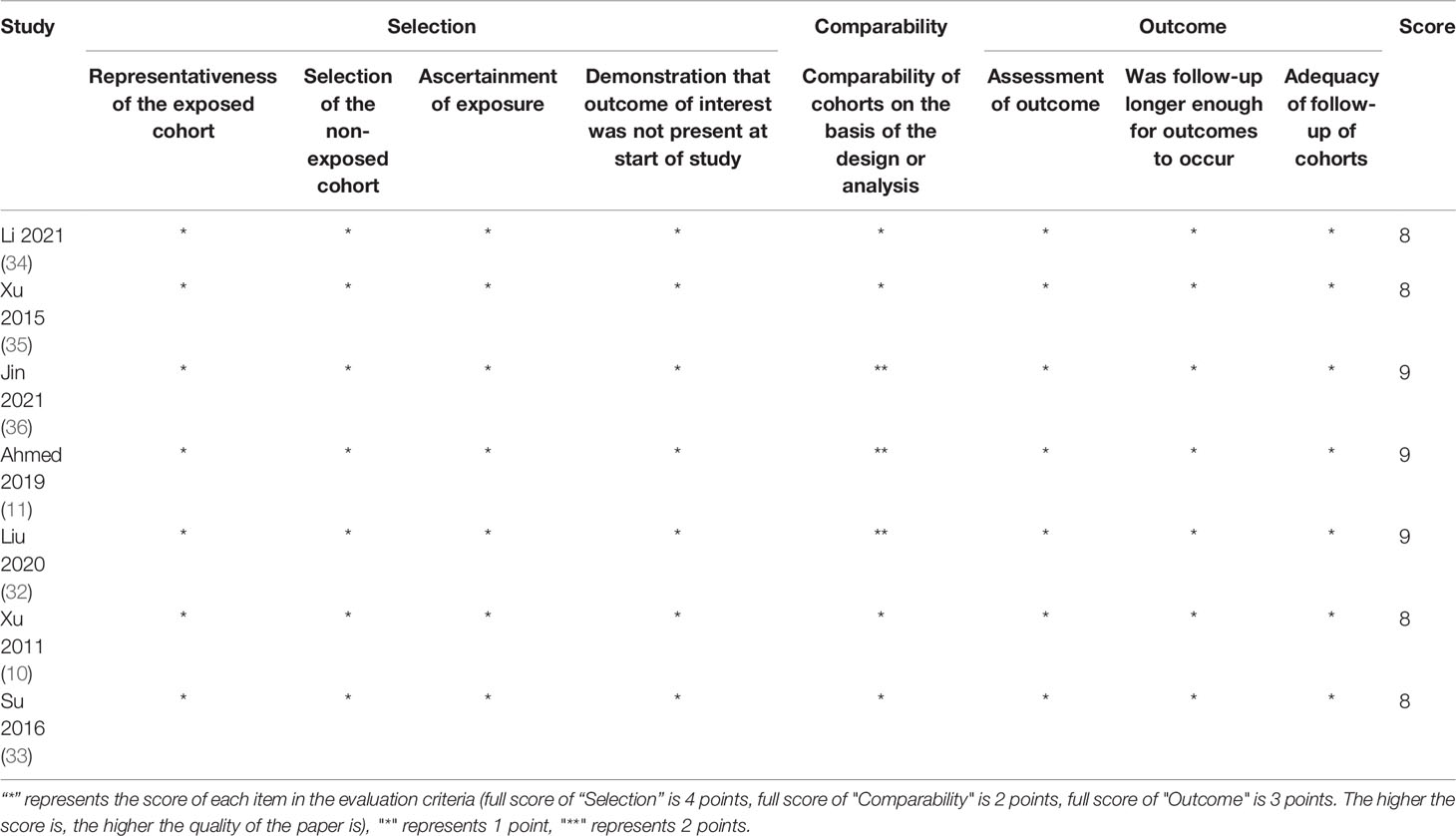
The quality of randomized controlled trial (RCT) was evaluated using the revised Cochrane risk-of-bias tool for randomized trials (RoB2) (18). The tool evaluates the risk of bias in individual RCT based on six domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Overall bias will be considered as low risk of bias, some concerns, or high risk of bias. Any domain-level judgement reaching a high risk of bias will result in overall high risk of bias. Some concerns for any individual domain will eventually contribute to the overall evaluation of the paper being identified as some concerns or high risk of bias. The quality of retrospective studies was assessed by the modified Newcastle–Ottawa scale assessment criteria, which comprises eight items: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, a demonstration that outcome of interest was not present at the start of the study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, if follow-up was longer enough for outcomes to occur, and adequacy of follow-up of cohorts.
Statistical Analysis
This meta-analysis was performed with Review Manager 5.3 software. To assess survival outcomes (OS, DMFS, LRRFS, PFS) and grade 3–4 acute toxicities (leukopenia, thrombocytopenia, mucositis, gastrointestinal reactions) between CCRT and RT alone, the HRs and relative ratios (RRs) with 95% CIs were pooled, respectively. Heterogeneity between included studies was assessed with the χ² heterogeneity test. I2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. The fixed-effect model was employed for meta-analysis if the heterogeneity test revealed no important heterogeneity between studies (P > 0.10, I2 < 50%); otherwise, the random-effect model was applied. When the HR or RR was less than 1, it indicated a better survival outcome or safety in the CCRT group. If the 95% CI did not contain the value 1, it suggested that there was a significant difference in the statistics. Sensitivity analysis was applied to assess the stability of the survival results.
According to the Cochrane Handbook for Systematic Reviews of Interventions, we did not assess publication bias because only nine studies were included in the meta-analysis, and it was not possible to assess publication bias employing a funnel plot.
Results
Characteristics and Quality of Included Studies
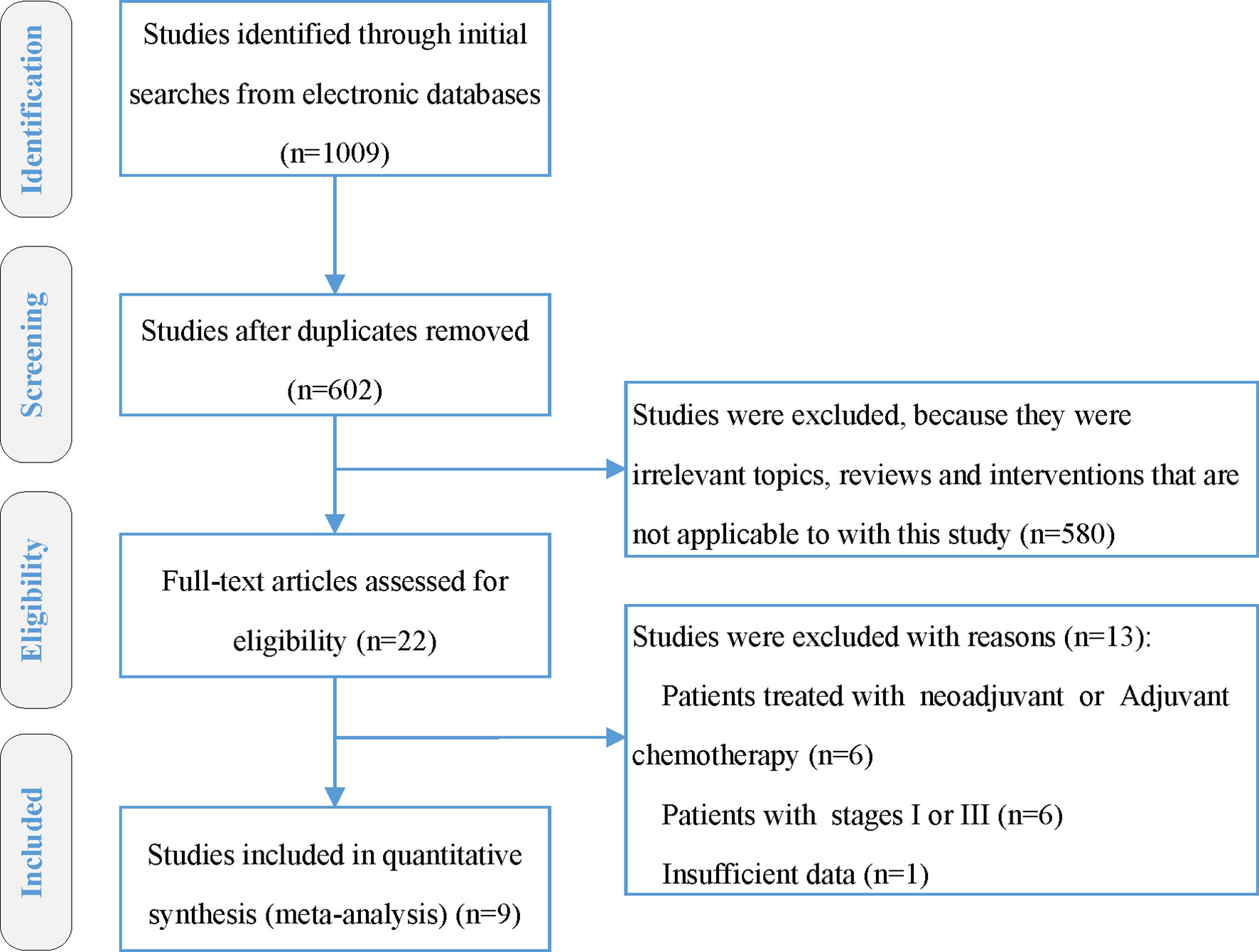
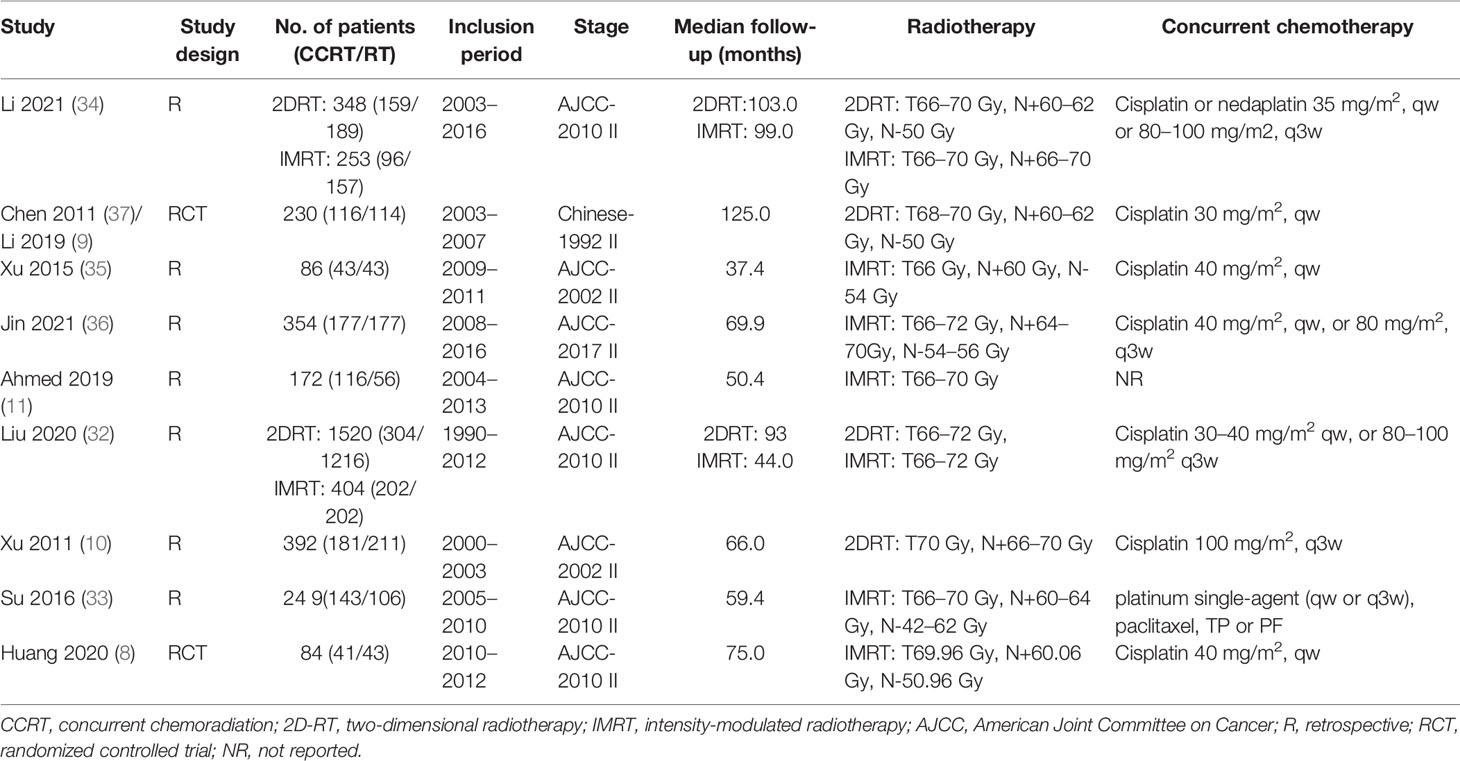
Totally 1,009 items, including 435 from PubMed, 287 from Embase, and 287 from Scopus, were obtained after the initial search. After duplication removal, 602 studies were retrieved. Only 22 studies remained after the titles and abstracts were assessed. Among the remaining 22 studies, six studies (19–24) involving patients with adjuvant or neoadjuvant chemotherapy were excluded, another six studies (25–30) involving patients with stage I or III were eliminated, and one study (31) with insufficient data was also eliminated (Figure 1). Nine studies were finally included, two of which were RCTs (8, 9), and the rest were retrospective studies (10, 11, 32–36). A total of 4,092 patients were enrolled, 2,462 received CCRT, and 1,632 received RT alone. There are four studies (9, 10, 32, 34) with a total of 2,490 patients that investigated 2DRT combined with concurrent chemotherapy, and 7 studies (8, 11, 32–36) with 1,602 patients that explored IMRT plus concurrent chemotherapy (Table 1). According to RoB2 assessment criteria, the overall risk of bias was low for the two included RCTs (Figure 2). According to the Newcastle–Ottawa Scale assessment criteria, four retrospective studies received eight stars, and the other three got nine stars (Table 2).
Survival Outcomes
Six studies directly provided HR values and 95%CI of time-to-event data, and the other three studies (8, 10, 33) did not provide HR values but provided survival curves. The method recommended by Tierney was used to extract HR and 95% CI from survival curves. OS data were available in all included studies.
Based on different radiotherapy techniques, the included studies were separated into two categories and meta-analyses were performed respectively. It revealed that, for stage II NPC patients undergoing 2DRT, concurrent chemotherapy could significantly prolong OS (HR = 0.61, 95% CI 0.44–0.82) (heterogeneity P = 0.08, I2 = 55%), LRFS (HR = 0.62, 95% CI 0.50–0.78) (heterogeneity P = 0.82, I2 = 0.00%), and PFS (HR = 0.65, 95% CI 0.54–0.79) (heterogeneity P = 0.67, I2 = 0.00%), except DMFS (HR = 0.81, 95% CI = 0.46–1.45) (heterogeneity P = 0.04, I2 = 65%) (Figure 3). Nevertheless, with IMRT, no remarkable difference between the CCRT group and the IMRT-alone group was observed in terms of OS (HR = 0.84, 95% CI 0.62–1.15) (heterogeneity P = 0.11, I2 = 43%), LRFS (HR=0.85, 95% CI 0.54–1.34) (heterogeneity P = 0.86, I2 = 0.00%), DMFS (HR = 0.96, 95% CI 0.60–1.54) (heterogeneity P = 0.87, I2 = 0.00%), and PFS (HR = 0.96, 95% CI 0.66–1.37) (heterogeneity P = 0.97, I2 = 0.00%) (Figure 4). Moreover, to explore the potential beneficiaries of concurrent chemotherapy for stage II NPC in the IMRT era, we conducted a subgroup analysis of stage T1-2N1 patients treated with IMRT. Unfortunately, it was found that additional concurrent chemotherapy did not improve OS (HR = 1.04, 95% CI 0.37–2.96) (heterogeneity P = 0.44, I2 = 0.00%), LRFS (HR = 0.70, 95% CI 0.34–1.45) (heterogeneity P = 0.85, I2 = 0.00%), DMFS (HR = 1.03, 95% CI 0.53–2.00) (heterogeneity P = 0.60, I2 = 0.00%), and PFS (HR = 1.04, 95% CI 0.58–1.88) (heterogeneity P = 0.81, I2 = 0.00%) in this population (Figure 5).
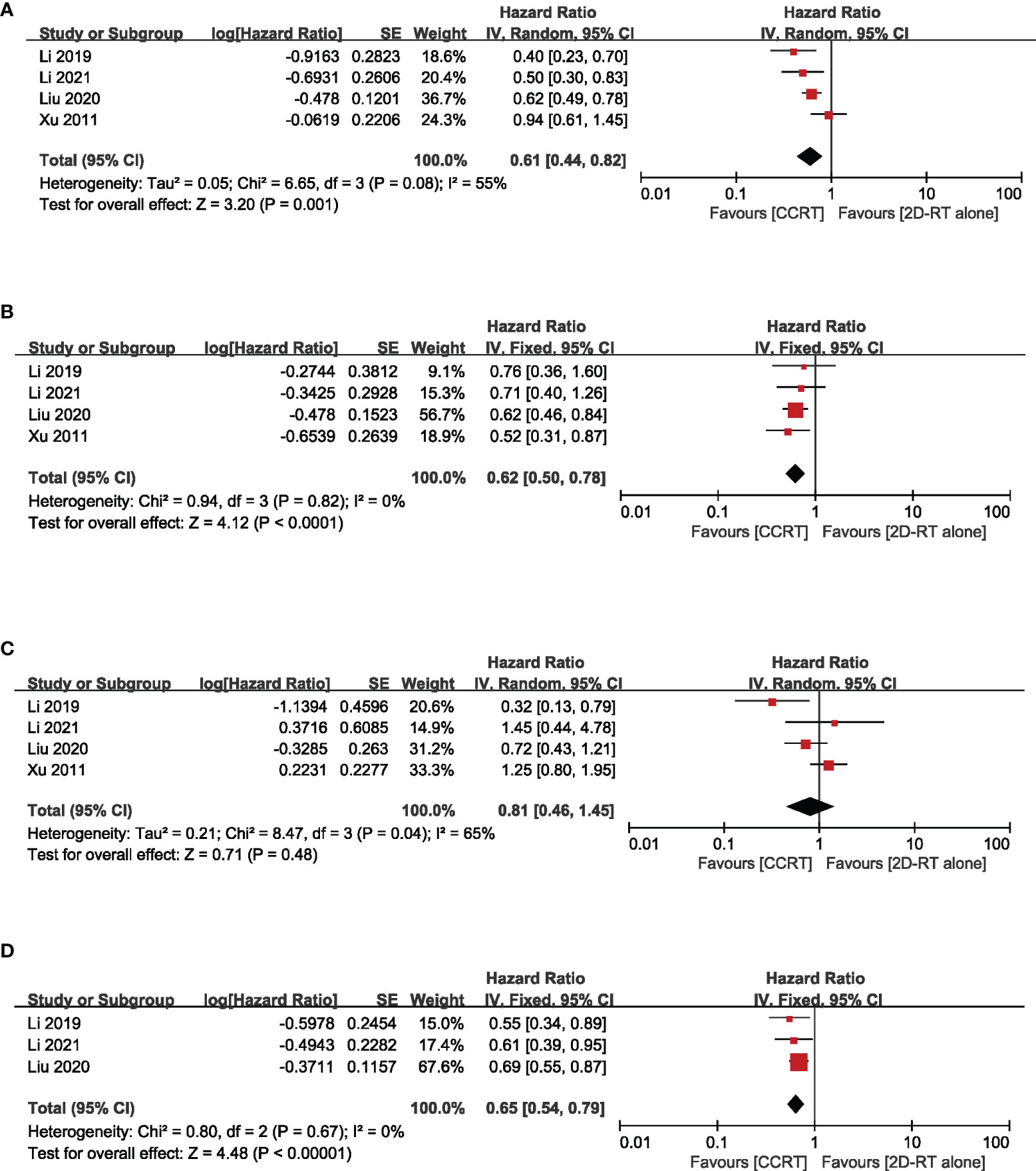
Figure 3 Forest plot of the meta-analysis regarding OS (A), LRFS (B), DMFS (C), and PFS (D) with CCRT vs. 2DRT alone. OS, overall survival; LRFS, locoregional recurrence-free survival; DMFS, distant metastasis-free survival; PFS, progression-free survival; CCRT, concurrent chemoradiation; 2D-RT, two-dimensional radiotherapy.
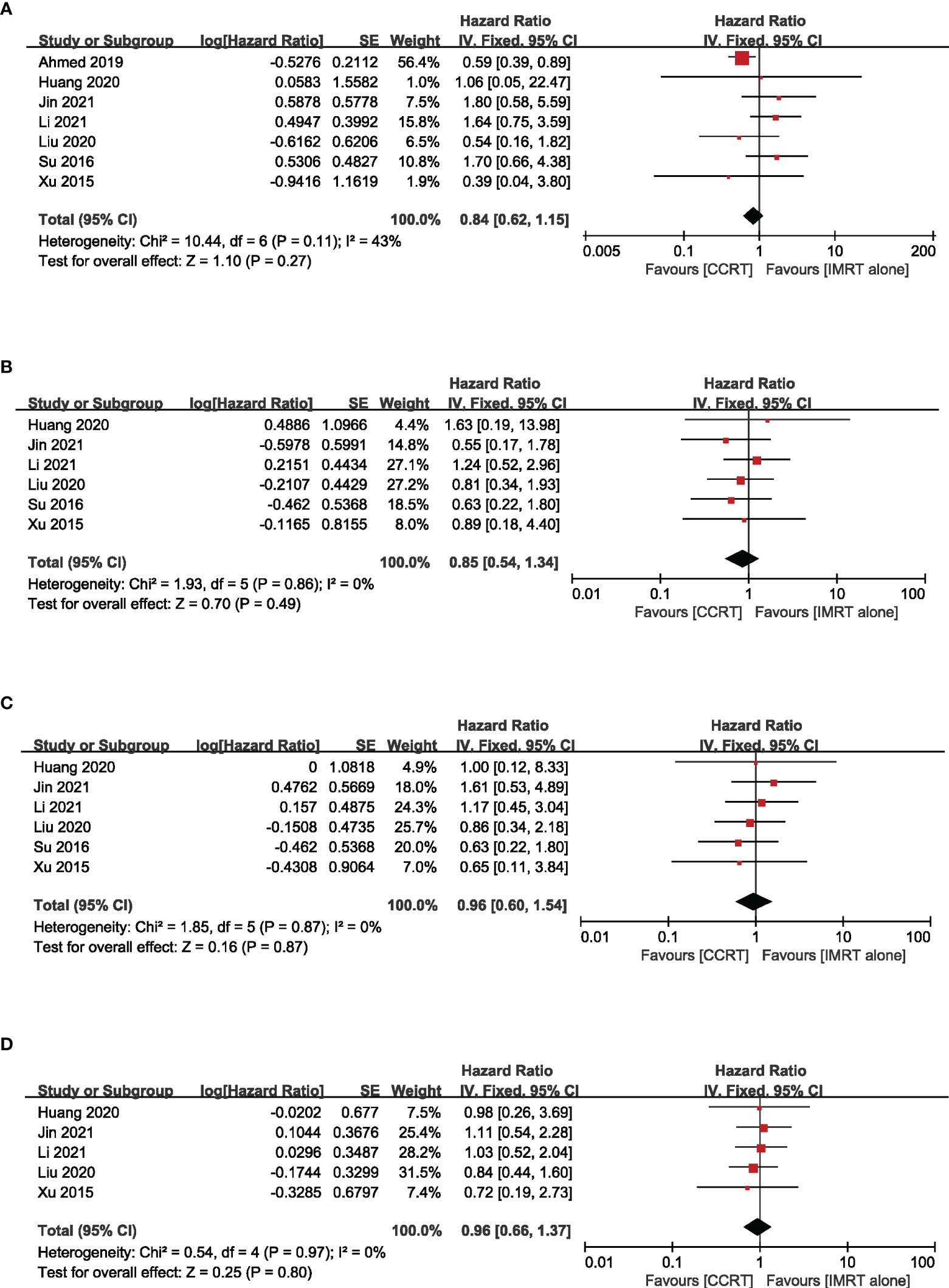
Figure 4 Forest plot of the meta-analysis regarding OS (A), LRFS (B), DMFS (C), and PFS (D) with CCRT vs. IMRT alone. IMRT, intensity-modulated radiotherapy.

Figure 5 Forest plot of the meta-analysis regarding OS (A), LRFS (B), DMFS (C), and PFS (D) with CCRT vs. IMRT alone in the N1 subgroup.
A Sensitivity Analysis
The stability of the results was evaluated by removing some studies according to different standards (Table 3). First of all, the sensitivity analysis was conducted in IMRT studies by separately eliminating two studies (8, 35) with a sample size of less than 100 patients, four studies (11, 32, 33, 35) with a median follow-up time of fewer than 60 months, and three studies (11, 33, 34) that included concurrent chemotherapy regimens other than cisplatin, respectively. It suggested that OS, LRFS, DMFS, and PFS were similar between the CCRT group and IMRT alone group, which was consistent with that before sensitivity analysis. Then, the sensitivity analysis was carried out in 2DRT studies by excluding one study that included concurrent chemotherapy with nedaplatin. There was no statistically significant change in survival outcomes. We did not perform sensitivity analyses for sample size and follow-up time because all 2DRT studies had a sample size of more than 100 and were followed up for more than 60 months. In summary, the survival results of the meta-analysis were robust and reliable.
Acute Toxicity
The incidence of grade 3–4 acute toxicity was reported in five studies with a total of 741 patients. The results of the meta-analysis suggested that the incidence of grade 3–4 leukopenia (RR = 4.00, 95% CI 2.29~6.97) (heterogeneity P = 0.14, I2 = 41%), mucositis (RR = 1.43, 95% CI 1.16–1.77) (heterogeneity P = 0.20, I2 = 38%), and gastrointestinal reaction (RR = 8.76, 95% CI 2.63~29.12) (heterogeneity P = 0.66, I2 = 0%) in the CCRT group were significantly higher than those in the IMRT-alone group. The incidence of grade 3–4 thrombocytopenia (RR = 3.45, 95% CI 0.85–13.94) (heterogeneity P = 0.97, I2 = 0%) was similar in the two groups (Figure 6).
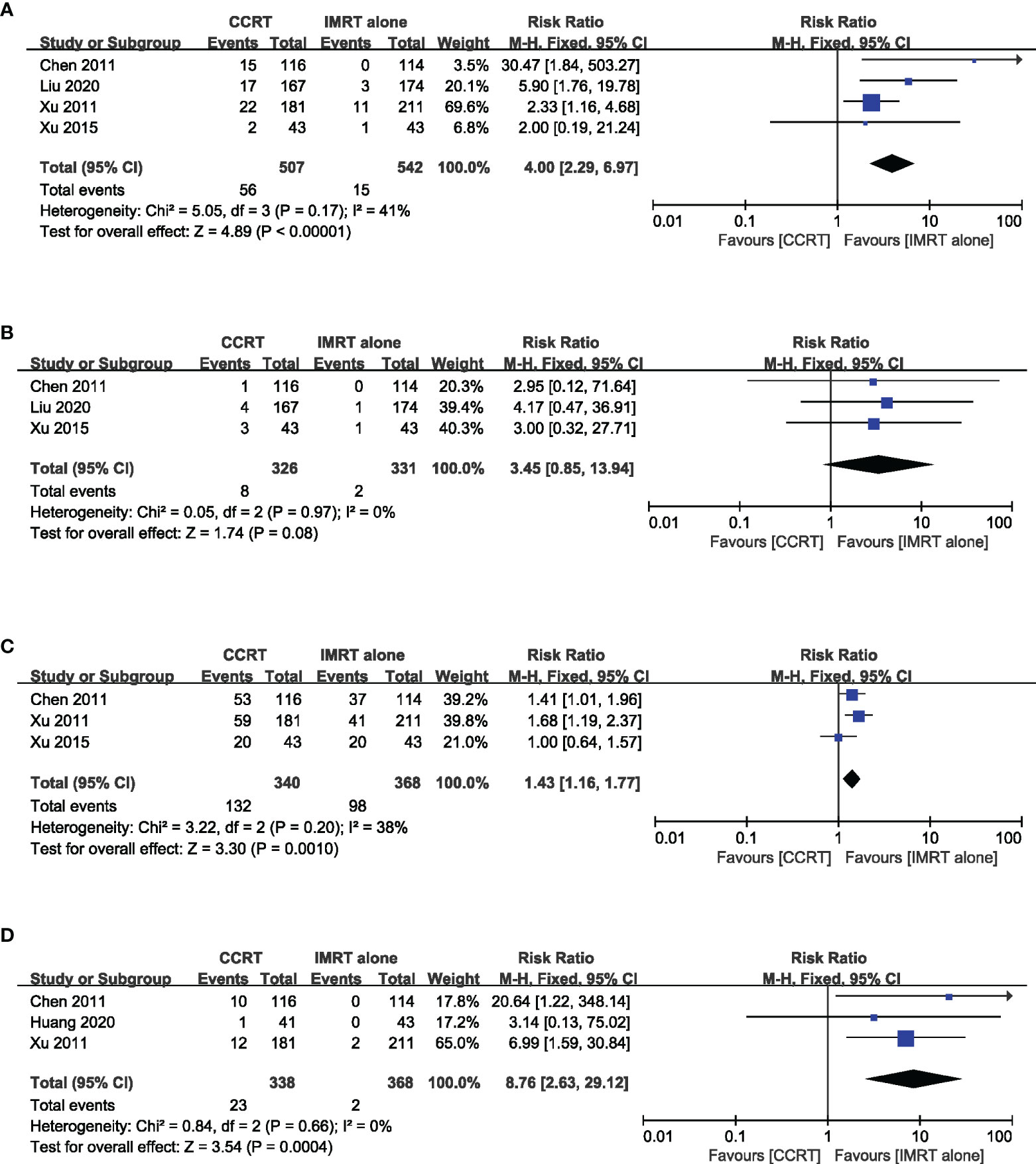
Figure 6 Forest plot of the meta-analysis regarding grade 3–4 leukopenia (A), thrombocytopenia (B), mucositis (C), and gastrointestinal reactions (D) with CCRT vs. IMRT alone.
Discussion
Radiotherapy is the main treatment for NPC. Stage III–IVA NPC patients receiving CCRT can further earn survival benefits from induction chemotherapy (5), but so far, whether chemotherapy can bring survival benefits to stage II patients is still controversial. The ASCO and CSCO Guideline recommends that it is not necessary for stage II NPC to routinely receive chemotherapy unless there are high-risk factors, such as high pretreatment EBV-DNA level, bulky tumor volumes, or extranodal extension (38). We assessed the therapeutic effect and toxicity of CCRT compared with 2DRT alone or IMRT alone for stage II NPC patients by conducting a meta-analysis.
Our study suggested that, compared with 2DRT alone, CCRT was associated with improved OS, LRFS, and PFS in stage II NPC patients. In the 2DRT era, a retrospective study conducted by Cheng and colleagues (23) revealed that stage II NPC patients receiving CCRT had similar PFS and LRFS compared with stage I patients receiving 2DRT alone. Another large retrospective study (39) included 1,790 patients and exhibited that the N1 subgroup of stage II NPC patients is more prone to distant metastasis, leading to a poorer prognosis. Furthermore, a combined subgroup analysis from two RCTs showed the survival benefit obtained from two or three cycles of cisplatin-based induction chemotherapy in stage II NPC (40). Hence, for stage II IPC patients treated with 2DRT, concurrent chemotherapy is highly crucial, particularly for the T1-2N1 population.
IMRT has become a daily choice for NPC. Several studies (8, 11, 32–36) have investigated whether concurrent chemotherapy can further improve the efficacy of stage II NPC patients receiving IMRT. However, the results of these studies are not completely consistent. Our meta-analysis revealed that concurrent chemotherapy had no therapeutic effect but increased toxicity in patients with stage II NPC receiving IMRT. Multiple possible explanations could account for the negative result in survival outcomes. First of all, as a high-precision radiotherapy therapy, IMRT can not only accurately irradiate the irregular tumor target with a higher dose but also protect the adjacent critical structures to the greatest extent. Several studies (41, 42) have consistently found that IMRT can significantly reduce radiation-induced toxicity and improve local control and long-term survival outcomes versus 2DRT, particularly for T1-2 patients (26, 41, 43). A prospective randomized study (41) comparing 2DRT with IMRT suggested that, with 2DRT, the 5-year OS and local control rates of stage II NPC were 67.1% and 84.7%, respectively, while with IMRT, they can be increased to 79.6% and 90.5%, respectively. Lai and colleagues (44) performed a retrospective study and found that IMRT significantly prolonged 5-year LRFS for patients with stage II NPC (92.7% vs. 86.8%) compared with 2DRT. The 5-year LRFS of stage T1 patients even reached 100% in the IMRT group versus 94.4% in the 2DRT group. Interestingly, a study (26) directly comparing IMRT alone with 2DRT plus concurrent chemotherapy indicated that the two groups were similar in terms of 4-year OS, LRFS, and DMFS (97.4% vs. 97.4%; 93.8% vs. 95.7%; 96.5% vs. 97.3%, respectively). Thanks to the progress of radiation therapy technology, the 5-year OS and local control rates of stage II NPC have improved substantially in the IMRT era. Concurrent chemotherapy might not bring survival benefits to this population. Secondly, an update result from the only phase 3 RCT (9) for stage II NPC revealed that CCRT significantly improved the 10-year OS (83.6% vs. 65.8%) and PFS (76.7% vs. 64.0%) compared to RT alone. However, the enrolled patients were evaluated by the Chinese 1992 staging system, and 31 (13%) of them were reclassified as stage III/N2 based on the AJCC TNM Staging System (7th ed., 2017). The survival benefit from stage N2 patients may lead to an overestimation of the role of concurrent chemotherapy in this study. Thirdly, stage II NPC is composed of three subsets (T2N0, T1N1, and T2N1), with obvious heterogeneity. Each subgroup has a different prognosis, and N1 patients are more likely to develop distant metastases (39). Hence, we conducted an N1 subgroup analysis for stage II patients. Unfortunately, it was found that additional concurrent chemotherapy did not improve survival outcomes.
Studies have found that baseline characteristics, such as plasma EBV-DNA level (45), lymph node size (46), and extranodal extension (47, 48), were independent unfavorable factors of NPC. Growing evidence indicated that the plasma EBV-DNA level was highly associated with tumor burden and elevated pretreatment plasma EBV-DNA was related to worse clinical outcomes (49, 50). EBV DNA-positive stage II patients had similar overall survival to stage III patients (51). This is the first study to demonstrate that pretreatment plasma EBV-DNA can be used to distinguish high-risk subgroups in early-stage patients. Results from real-world research (52) indicated that high pretreatment plasma EBV-DNA levels (≥4,000 copies/ml) was an adverse independent factor in LA-NPC. Patients with high EBV-DNA levels had a comparable survival outcome to T4 or N2–3 patients, with a 5-year PFS of 69%. Another large cohort study (53) of 1,357 patients with LA-NPC revealed that, for patients with high EBV-DNA levels (>4,000 copies/ml), IMRT with concurrent chemotherapy improved OS, DFS, and DMFS compared with IMRT alone. However, there was no observed benefit with the addition of concurrent chemotherapy in patients with low EBV-DNA levels. Pretreatment EBV-DNA has been widely accepted as a useful prognostic biomarker and plays an important role in tailoring treatment strategies in the clinic (54). Therefore, stage II NPC patients with high pretreatment EBV-DNA levels might be ideal candidates for concurrent chemotherapy. However, two issues need to be addressed before EBV-DNA was widely used in clinical practice for risk stratification. Firstly, the harmonization and standardization of the quantitative plasma EBV-DNA measurement between laboratories have not been established, resulting in poor inter-laboratory concordance. Secondly, although the EBV-DNA cutoff values have been set at 2,000 or 4,000 copies/ml in most studies, there is still no consensus on the optimal thresholds for risk discretization. A retrospective study showed that the tumor volume was a significant independent predictor of increasing risk of recurrence (33). Another study (55) from Hong Kong reported no role of using concurrent chemotherapy in stage II NPC, except for lymph nodes >2 cm. However, these two studies are small-sample retrospective studies, and the value of tumor volume and lymph node size needs to be further studied. Studies demonstrated that extranodal extension played an important role in predicting distant metastasis in stage II NPC patients with N1 category (56–58). Patients with high-grade extranodal extension (including coalescent nodes and metastatic node infiltrating into adjacent structures) had a significantly higher risk of distant metastasis and death than those without (including metastatic nodes infiltrating into surrounding fat and without extranodal extension) and were suggested to be classified as cN3. However, patients with metastatic nodes infiltrating into surrounding fat (low-grade extranodal extension) had a similar outcome to those without extranodal extension. Hence, stage II nasopharyngeal carcinoma patients with high-grade extranodal invasion are likely candidates for concurrent chemotherapy. Although the risk stratification factors mentioned above might have the potential to identify candidates for concurrent chemotherapy, we were unable to conduct further subgroup analyses for these factors because they were not reported in the included literature. The tumor volume, size of metastatic lymph nodes, extranodal extension, and EBV-DNA levels were not disaggregated in the N1 subgroup analysis, which may have a significant impact on the results. Therefore, the negative results of the N1 subgroup analysis in this study should be interpreted with caution. Future studies should focus on these high-risk groups who are most likely to benefit from chemotherapy.
Stage II NPC has a good prognosis, with 5-year OS 97.8%, so it is particularly significant to relieve toxicity and improve quality of life (59). Studies in terms of anti-EGFR antibodies, such as cetuximab, nimotuzumab, and Endostar, combined with RT in patients with LA-NPC have been launched. Xu and colleagues carried out a comparative study between concurrent cisplatin-chemoradiotherapy (CRT) and cetuximab-radiotherapy (ERT) (60). ERT was not superior to CRT, while it was more prone to result in acute adverse events. Similar results were obtained in another retrospective study (61); cetuximab/nimotuzumab combined concurrently with IMRT suggested equivalence to the standard CCRT in terms of DFS, LRRFS, DMFS, and OS. Skin reaction and mucositis are more common in the cetuximab/nimotuzumab group. A phase II study enrolling 23 stage III–IV NPC patients found that, compared to CCRT, radiotherapy combined with Endostar had similar efficacy, but lighter acute adverse reactions, which improved quality of life (62). In conclusion, Endostar has the potential to serve as a concurrent treatment option for the high-risk subgroup of stage II patients and deserves further study. Anti-PD1 checkpoint inhibitors, such as nivolumab (63), pembrolizumab (64), camrelizumab (65, 66), toripalimab (67), and tislelizumab (68), had a clinically meaningful antitumor activity with a manageable safety profile. Two phase 3 trials demonstrated that, as first-line treatment for recurrent/metastatic NPC, camrelizumab or toripalimab in combination with gemcitabine and cisplatin prolonged PFS as compared to gemcitabine and cisplatin (median PFS 9.7 vs. 6.9 months, 11.7 vs. 8.0 months, respectively) (65, 67). Several phase II–III trials (NCT05305131, NCT03700476, NCT03267498, NCT04782765, NCT04227509, NCT03427827, NCT04557020, NCT04453826, NCT05211232) are in progress to clarify the efficacy and safety of PD-1 in combination with CCRT for high-risk LA-NPC (except for T3N0–1 and T4N0). Of particular concern is a phase II trial (NCT05229315) evaluating the safety and efficacy of toripalimab combined with IMRT in the treatment of stage II NPC. Nevertheless, risk stratification factors, such as EBV-DNA, lymph node size, and extranodal extension, were not evaluated as a part of eligibility criteria in most studies, with the exceptions of NCT04453826 (enrolled patients were required to have EBV-DNA >0 copies/ml after 3 cycles of induction chemotherapy) and NCT05229315 (enrolled patients were required to have EBV-DNA <4,000 copies/ml).
In terms of acute toxicities, this meta-analysis found that grade 3–4 leukopenia, mucositis, and gastrointestinal reactions were more frequent in patients receiving CCRT versus IMRT alone. A previous study (69) suggested that CCRT is related to higher incidences of treatment-related mortality (1.7% vs. 0.8%) as compared with radiotherapy alone. Leukopenia is the most common cause of death. Because of higher acute toxicity and treatment-related death, the application of concurrent chemotherapy in stage II NPC should be considered prudently. Currently, four RCTs (NCT02116231, NCT02633202, NCT02610010, NCT03068936) that evaluate the role of concurrent chemotherapy for stage II patients are ongoing in China, and the eventual results are expected to be released in the near future.
The present meta-analysis has multiple limitations. First of all, both RCTs and retrospective studies were enrolled, which may influence the level of evidence to some extent. Second, because staging systems vary in some included studies, it may contribute to heterogeneity in this meta-analysis. Third, several studies with relatively small sample sizes or median follow-up of less than 5 years were included. Finally, survival data of three studies (8, 10, 33) were obtained from survival curves by Tierney’s methods, which may lead to potential bias.
Conclusion
In summary, for patients with stage II NPC, current evidence suggested that CCRT was superior to 2DRT alone with significantly better LRFS, PFS, and OS. However, IMRT alone was comparable to CCRT with similar efficacy but lower acute toxicities. Consequently, routine use of concurrent chemotherapy in unselected patients should not be encouraged in the IMRT era. There is an urgent need to identify subgroups of stage II patients who might derive clinical benefits from concurrent chemotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Study design: X-DZ, K-HC; data acquisition: Y-CX, Z-GL; quality control of data: Y-CX, Z-GL, and K-HC; data analysis and interpretation: Y-CX, Z-GL; statistical analysis: Y-CX, Z-GL; manuscript preparation: Y-CX, Z-GL, and K-HC; manuscript review: X-DZ. All authors read and X-DZ approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.843675/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Wee JT, Ha TC, Loong SL, Qian CN. Is Nasopharyngeal Cancer Really a "Cantonese Cancer"? Chin J Cancer (2010) 29(5):517–26. doi: 10.5732/cjc.009.10329
3. Yang XL, Wang Y, Liang SB, He SS, Chen DM, Chen HY, et al. Comparison of the Seventh and Eighth Editions of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma: Analysis of 1317 Patients Treated With Intensity-Modulated Radiotherapy at Two Centers. BMC Cancer (2018) 18(1):606. doi: 10.1186/s12885-018-4419-1
4. Lin YH, Huang TL, Chien CY, Chen HC, Hsu HC, Huang EY, et al. Pretreatment Prognostic Factors of Survival and Late Toxicities for Patients With Nasopharyngeal Carcinoma Treated by Simultaneous Integrated Boost Intensity-Modulated Radiotherapy. Radiat Oncol (2018) 13(1):45. doi: 10.1186/s13014-018-0990-5
5. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med (2019) 381(12):1124–35. doi: 10.1056/NEJMoa1905287
6. Bongiovanni A, Vagheggini A, Fausti V, Mercatali L, Calpona S, Di Menna G, et al. Induction Chemotherapy Plus Concomitant Chemoradiotherapy in Nasopharyngeal Carcinoma: An Updated Network Meta-Analysis. Crit Rev Oncol Hematol (2021) 160:103244. doi: 10.1016/j.critrevonc.2021.103244
7. Verma V, Ryckman JM, Simone CB 2nd, Lin C. Patterns of Care and Outcomes With the Addition of Chemotherapy to Radiation Therapy for Stage I Nasopharyngeal Cancer. Acta Oncol (2018) 57(2):257–61. doi: 10.1080/0284186x.2017.1351039
8. Huang X, Chen X, Zhao C, Wang J, Wang K, Wang L, et al. Adding Concurrent Chemotherapy to Intensity-Modulated Radiotherapy Does Not Improve Treatment Outcomes for Stage II Nasopharyngeal Carcinoma: A Phase 2 Multicenter Clinical Trial. Front Oncol (2020) 10:1314. doi: 10.3389/fonc.2020.01314
9. Li XY, Chen QY, Sun XS, Liu SL, Yan JJ, Guo SS, et al. Ten-Year Outcomes of Survival and Toxicity for a Phase III Randomised Trial of Concurrent Chemoradiotherapy Versus Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma. Eur J Cancer (2019) 110:24–31. doi: 10.1016/j.ejca.2018.10.020
10. Xu T, Hu C, Wang X, Shen C. Role of Chemoradiotherapy in Intermediate Prognosis Nasopharyngeal Carcinoma. Oral Oncol (2011) 47(5):408–13. doi: 10.1016/j.oraloncology.2011.03.008
11. Ahmed Z, Kujtan L, Kennedy K, Wood V, Schomas D, Subramanian J. The Role of Chemotherapy in the Treatment of Stage II Nasopharyngeal Carcinoma: Retrospective Analysis of the National Cancer Database. Cancer Med (2019) 8(4):1500–7. doi: 10.1002/cam4.2033
12. Wu P, Zhao Y, Xiang L, Yang L. Management of Chemotherapy for Stage II Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era: A Review. Cancer Manag Res (2020) 12:957–63. doi: 10.2147/cmar.S239729
13. Xu C, Zhang LH, Chen YP, Liu X, Zhou GQ, Lin AH, et al. Chemoradiotherapy Versus Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: A Systemic Review and Meta-Analysis of 2138 Patients. J Cancer (2017) 8(2):287–97. doi: 10.7150/jca.17317
14. Wang S, Li S, Shen L. Combined Chemoradiation vs Radiation Therapy Alone in Stage-II Nasopharyngeal Carcinoma: A Meta-Analysis of the Published Literature. Curr Probl Cancer (2018) 42(3):302–18. doi: 10.1016/j.currproblcancer.2018.03.004
15. Liu F, Jin T, Liu L, Xiang Z, Yan R, Yang H. The Role of Concurrent Chemotherapy for Stage II Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era: A Systematic Review and Meta-Analysis. PloS One (2018) 13(3):e0194733. doi: 10.1371/journal.pone.0194733
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
18. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Guo Q, Lu T, Lin S, Zong J, Chen Z, Cui X, et al. Long-Term Survival of Nasopharyngeal Carcinoma Patients With Stage II in Intensity-Modulated Radiation Therapy Era. Jpn J Clin Oncol (2016) 46(3):241–7. doi: 10.1093/jjco/hyv192
20. Wang L, Miao J, Huang H, Chen B, Xiao X, Zhu M, et al. Long-Term Survivals, Toxicities and the Role of Chemotherapy in Early-Stage Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiation Therapy: A Retrospective Study With 15-Year Follow-Up. Cancer Res Treat (2021) 54(1):118–29. doi: 10.4143/crt.2021.101
21. Kang MK, Oh D, Cho KH, Moon SH, Wu HG, Heo DS, et al. Role of Chemotherapy in Stage II Nasopharyngeal Carcinoma Treated With Curative Radiotherapy. Cancer Res Treat (2015) 47(4):871–8. doi: 10.4143/crt.2014.141
22. Pan XB, Huang ST, Chen KH, Zhu XD. Chemotherapy Use and Survival in Stage II Nasopharyngeal Carcinoma. Oncotarget (2017) 8(60):102573–80. doi: 10.18632/oncotarget.21751
23. Cheng SH, Tsai SY, Yen KL, Jian JJ, Chu NM, Chan KY, et al. Concomitant Radiotherapy and Chemotherapy for Early-Stage Nasopharyngeal Carcinoma. J Clin Oncol (2000) 18(10):2040–5. doi: 10.1200/jco.2000.18.10.2040
24. Ding X-C, Fan P-P, Xie P, Fan B-J, Yang J, Jiang L-Y, et al. Ten-Year Outcomes Of Intensity-Modulated Radiotherapy (IMRT) Combine With Chemotherapy Versus IMRT Alone For Stage II Nasopharyngeal Carcinoma In The Real-World Study (RWD). Cancer Manag Res (2019) 11:8893–903. doi: 10.2147/CMAR.S218842
25. Zhang F, Zhang Y, Li WF, Liu X, Guo R, Sun Y, et al. Efficacy of Concurrent Chemotherapy for Intermediate Risk NPC in the Intensity-Modulated Radiotherapy Era: A Propensity-Matched Analysis. Sci Rep (2015) 5:17378. doi: 10.1038/srep17378
26. Zhang LN, Gao YH, Lan XW, Tang J, Su Z, Ma J, et al. Propensity Score Matching Analysis of Cisplatin-Based Concurrent Chemotherapy in Low Risk Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era. Oncotarget (2015) 6(41):44019–29. doi: 10.18632/oncotarget.5806
27. Aftab O, Liao S, Zhang R, Tang N, Luo M, Zhang B, et al. Efficacy and Safety of Intensity-Modulated Radiotherapy Alone Versus Intensity-Modulated Radiotherapy Plus Chemotherapy for Treatment of Intermediate-Risk Nasopharyngeal Carcinoma. Radiat Oncol (2020) 15(1):66. doi: 10.1186/s13014-020-01508-4
28. Luo S, Zhao L, Wang J, Xu M, Li J, Zhou B, et al. Clinical Outcomes for Early-Stage Nasopharyngeal Carcinoma With Predominantly WHO II Histology Treated by Intensity-Modulated Radiation Therapy With or Without Chemotherapy in Nonendemic Region of China. Head Neck (2014) 36(6):841–7. doi: 10.1002/hed.23386
29. Katano A, Takahashi W, Yamashita H, Yamamoto K, Ando M, Yoshida M, et al. Radiotherapy Alone and With Concurrent Chemotherapy for Nasopharyngeal Carcinoma: A Retrospective Study. Medicine (Baltimore) (2018) 97(18):e0502. doi: 10.1097/md.0000000000010502
30. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-Term Outcomes of Intensity-Modulated Radiotherapy for 868 Patients With Nasopharyngeal Carcinoma: An Analysis of Survival and Treatment Toxicities. Radiother Oncol (2014) 110(3):398–403. doi: 10.1016/j.radonc.2013.10.020
31. Pan X-B, Li L, Qu S, Chen L, Liang S-X, Zhu X-D. The Efficacy of Chemotherapy in Survival of Stage II Nasopharyngeal Carcinoma. Oral Oncol (2020) 101. doi: 10.1016/j.oraloncology.2019.104520
32. Liu DH, Zhou XY, Pan YG, Chen S, Ye ZH, Chen GD. Survival of Stage II Nasopharyngeal Carcinoma Patients With or Without Concurrent Chemotherapy: A Propensity Score Matching Study. Cancer Med (2020) 9(4):1287–97. doi: 10.1002/cam4.2785
33. Su Z, Mao YP, Tang J, Lan XW, OuYang PY, Xie FY. Long-Term Outcomes of Concurrent Chemoradiotherapy Versus Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma Treated With IMRT: A Retrospective Study. Tumour Biol (2016) 37(4):4429–38. doi: 10.1007/s13277-015-4266-5
34. Li PJ, Lai YL, He F, Chen YY, Gu ZS, Luo W, et al. Explore the Usefulness of Concurrent Chemotherapy in Stage II Nasopharyngeal Carcinoma: A Retrospective Study. Front Pharmacol (2021) 12:688528. doi: 10.3389/fphar.2021.688528
35. Xu T, Shen C, Zhu G, Hu C. Omission of Chemotherapy in Early Stage Nasopharyngeal Carcinoma Treated With IMRT: A Paired Cohort Study. Medicine (Baltimore) (2015) 94(39):e1457. doi: 10.1097/md.0000000000001457
36. Jin YN, Tang QN, Yao JJ, Xu XW, He WZ, Wang L, et al. The Effect of Adding Concurrent Chemotherapy to Radiotherapy for Stage II Nasopharyngeal Carcinoma With Undetectable Pretreatment Epstein-Barr Virus DNA: Retrospective Analysis With a Large Institutional-Based Cohort. Transl Oncol (2021) 14(2):100990. doi: 10.1016/j.tranon.2020.100990
37. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent Chemoradiotherapy vs Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: Phase III Randomized Trial. J Natl Cancer Inst (2011) 103(23)::1761–70. doi: 10.1093/jnci/djr432
38. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J Clin Oncol (2021) 39(7):840–59. doi: 10.1200/jco.20.03237
39. Tang LL, Chen YP, Mao YP, Wang ZX, Guo R, Chen L, et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J Natl Compr Canc Netw (2017) 15(7):913–9. doi: 10.6004/jnccn.2017.0121
40. Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Improvement of Survival After Addition of Induction Chemotherapy to Radiotherapy in Patients With Early-Stage Nasopharyngeal Carcinoma: Subgroup Analysis of Two Phase III Trials. Int J Radiat Oncol Biol Phys (2006) 65(5):1300–6. doi: 10.1016/j.ijrobp.2006.02.016
41. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A Prospective, Randomized Study Comparing Outcomes and Toxicities of Intensity-Modulated Radiotherapy vs. Conventional Two-Dimensional Radiotherapy for the Treatment of Nasopharyngeal Carcinoma. Radiother Oncol (2012) 104(3):286–93. doi: 10.1016/j.radonc.2012.08.013
42. Du T, Xiao J, Qiu Z, Wu K. The Effectiveness of Intensity-Modulated Radiation Therapy Versus 2D-RT for the Treatment of Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. PloS One (2019) 14(7):e0219611. doi: 10.1371/journal.pone.0219611
43. Lin S, Pan J, Han L, Guo Q, Hu C, Zong J, et al. Update Report of Nasopharyngeal Carcinoma Treated With Reduced-Volume Intensity-Modulated Radiation Therapy and Hypothesis of the Optimal Margin. Radiother Oncol (2014) 110(3):385–9. doi: 10.1016/j.radonc.2014.01.011
44. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How Does Intensity-Modulated Radiotherapy Versus Conventional Two-Dimensional Radiotherapy Influence the Treatment Results in Nasopharyngeal Carcinoma Patients? Int J Radiat Oncol Biol Phys (2011) 80(3):661–8. doi: 10.1016/j.ijrobp.2010.03.024
45. Lee VH, Kwong DL, Leung TW, Choi CW, O'Sullivan B, Lam KO, et al. The Addition of Pretreatment Plasma Epstein-Barr Virus DNA Into the Eighth Edition of Nasopharyngeal Cancer TNM Stage Classification. Int J Cancer (2019) 144(7):1713–22. doi: 10.1002/ijc.31856
46. Toya R, Murakami R, Saito T, Murakami D, Matsuyama T, Baba Y, et al. Radiation Therapy for Nasopharyngeal Carcinoma: The Predictive Value of Interim Survival Assessment. J Radiat Res (2016) 57(5):541–7. doi: 10.1093/jrr/rrw038
47. Tsai TY, Chou YC, Lu YA, Kang CJ, Huang SF, Liao CT, et al. The Prognostic Value of Radiologic Extranodal Extension in Nasopharyngeal Carcinoma: Systematic Review and Meta-Analysis. Oral Oncol (2021) 122:105518. doi: 10.1016/j.oraloncology.2021.105518
48. Mao Y, Wang S, Lydiatt W, Shah JP, Colevas AD, Lee AWM, et al. Unambiguous Advanced Radiologic Extranodal Extension Determined by MRI Predicts Worse Outcomes in Nasopharyngeal Carcinoma: Potential Improvement for Future Editions of N Category Systems. Radiother Oncol (2021) 157:114–21. doi: 10.1016/j.radonc.2021.01.015
49. Zhang W, Chen Y, Chen L, Guo R, Zhou G, Tang L, et al. The Clinical Utility of Plasma Epstein-Barr Virus DNA Assays in Nasopharyngeal Carcinoma: The Dawn of a New Era?: A Systematic Review and Meta-Analysis of 7836 Cases. Medicine (Baltimore) (2015) 94(20):e845. doi: 10.1097/md.0000000000000845
50. Kim KY, Le QT, Yom SS, Ng RHW, Chan KCA, Bratman SV, et al. Clinical Utility of Epstein-Barr Virus DNA Testing in the Treatment of Nasopharyngeal Carcinoma Patients. Int J Radiat Oncol Biol Phys (2017) 98(5):996–1001. doi: 10.1016/j.ijrobp.2017.03.018
51. Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, et al. Plasma Epstein-Barr Virus DNA and Residual Disease After Radiotherapy for Undifferentiated Nasopharyngeal Carcinoma. J Natl Cancer Inst (2002) 94(21):1614–9. doi: 10.1093/jnci/94.21.1614
52. Tang SQ, Chen L, Li WF, Chan ATC, Huang SH, Chua MLK, et al. Identifying Optimal Clinical Trial Candidates for Locoregionally Advanced Nasopharyngeal Carcinoma: Analysis of 9468 Real-World Cases and Validation by Two Phase 3 Multicentre, Randomised Controlled Trial. Radiother Oncol (2021) 167:179–86. doi: 10.1016/j.radonc.2021.12.029
53. Liang H, Lv X, Wang L, Wu YS, Sun R, Ye YF, et al. The Plasma Epstein-Barr Virus DNA Level Guides Precision Treatment for Nasopharyngeal Carcinoma in the Intensity-Modulated Radiotherapy Era: A Large Population-Based Cohort Study From an Endemic Area. Ther Adv Med Oncol (2018) 10:1758835918782331. doi: 10.1177/1758835918782331
54. Lee AWM, Lee VHF, Ng WT, Strojan P, Saba NF, Rinaldo A, et al. A Systematic Review and Recommendations on the Use of Plasma EBV DNA for Nasopharyngeal Carcinoma. Eur J Cancer (2021) 153:109–22. doi: 10.1016/j.ejca.2021.05.022
55. Ng AWY, Tung SY, Cheung AKW, Chan PC. No Role of Using Chemoradiation in T2N0 and T1N1 With Small Lymph Node Size Stage II Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2015) 93(3):E306–7. doi: 10.1016/j.ijrobp.2015.07.1329
56. Hu Y, Lu T, Huang SH, Lin S, Chen Y, Fang Y, et al. High-Grade Radiologic Extra-Nodal Extension Predicts Distant Metastasis in Stage II Nasopharyngeal Carcinoma. Head Neck (2019) 41(9):3317–27. doi: 10.1002/hed.25842
57. Lu T, Hu Y, Xiao Y, Guo Q, Huang SH, O'Sullivan B, et al. Prognostic Value of Radiologic Extranodal Extension and its Potential Role in Future N Classification for Nasopharyngeal Carcinoma. Oral Oncol (2019) 99:104438. doi: 10.1016/j.oraloncology.2019.09.030
58. Chin O, Yu E, O'Sullivan B, Su J, Tellier A, Siu L, et al. Prognostic Importance of Radiologic Extranodal Extension in Nasopharyngeal Carcinoma Treated in a Canadian Cohort. Radiother Oncol (2021) 165:94–102. doi: 10.1016/j.radonc.2021.10.018
59. Li WZ, Wu HJ, Lv SH, Hu XF, Liang H, Liu GY, et al. Assessment of Survival Model Performance Following Inclusion of Epstein-Barr Virus DNA Status in Conventional TNM Staging Groups in Epstein-Barr Virus-Related Nasopharyngeal Carcinoma. JAMA Netw Open (2021) 4(9):e2124721. doi: 10.1001/jamanetworkopen.2021.24721
60. Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly Cetuximab Concurrent With IMRT Aggravated Radiation-Induced Oral Mucositis in Locally Advanced Nasopharyngeal Carcinoma: Results of a Randomized Phase II Study. Oral Oncol (2015) 51(9):875–9. doi: 10.1016/j.oraloncology.2015.06.008
61. You R, Sun R, Hua YJ, Li CF, Li JB, Zou X, et al. Cetuximab or Nimotuzumab Plus Intensity-Modulated Radiotherapy Versus Cisplatin Plus Intensity-Modulated Radiotherapy for Stage II-IVb Nasopharyngeal Carcinoma. Int J Cancer (2017) 141(6):1265–76. doi: 10.1002/ijc.30819
62. Kang M, Wang F, Liao X, Zhou P, Wang R. Intensity-Modulated Radiotherapy Combined With Endostar has Similar Efficacy But Weaker Acute Adverse Reactions Than IMRT Combined With Chemotherapy in the Treatment of Locally Advanced Nasopharyngeal Carcinoma. Medicine (Baltimore) (2018) 97(25):e11118. doi: 10.1097/md.0000000000011118
63. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–8. doi: 10.1200/jco.2017.77.0388
64. Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol (2017) 35(36):4050–6. doi: 10.1200/jco.2017.73.3675
65. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab Versus Placebo in Combination With Gemcitabine and Cisplatin as First-Line Treatment for Recurrent or Metastatic Nasopharyngeal Carcinoma (CAPTAIN-1st): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2021) 22(8):1162–74. doi: 10.1016/s1470-2045(21)00302-8
66. Yang Y, Zhou T, Chen X, Li J, Pan J, He X, et al. Efficacy, Safety, and Biomarker Analysis of Camrelizumab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma (CAPTAIN Study). J Immunother Cancer (2021) 9(12):e003790. doi: 10.1136/jitc-2021-003790
67. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or Placebo Plus Chemotherapy as First-Line Treatment in Advanced Nasopharyngeal Carcinoma: A Multicenter Randomized Phase 3 Trial. Nat Med (2021) 27(9):1536–43. doi: 10.1038/s41591-021-01444-0
68. Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, et al. Tislelizumab in Chinese Patients With Advanced Solid Tumors: An Open-Label, non-Comparative, Phase 1/2 Study. J Immunother Cancer (2020) 8(1):e000437. doi: 10.1136/jitc-2019-000437
Keywords: stage II, nasopharyngeal carcinoma, chemotherapy, radiotherapy, meta-analysis
Citation: Xu Y-C, Chen K-H, Liang Z-G and Zhu X-D (2022) A Systematic Review and Meta-Analysis of Studies Comparing Concurrent Chemoradiotherapy With Radiotherapy Alone in the Treatment of Stage II Nasopharyngeal Carcinoma. Front. Oncol. 12:843675. doi: 10.3389/fonc.2022.843675
Received: 26 December 2021; Accepted: 31 May 2022;
Published: 12 July 2022.
Edited by:
Shan Shan Guo, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Maria Grazia Ghi, Veneto Institute of Oncology (IRCCS), ItalyFeng Mei, Sichuan Cancer Hospital, China
Copyright © 2022 Xu, Chen, Liang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Zhu, emh1eGRvbmdneG11QDEyNi5jb20=
 Yao-Can Xu
Yao-Can Xu Kai-Hua Chen1
Kai-Hua Chen1 Xiao-Dong Zhu
Xiao-Dong Zhu