
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 15 February 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.842072
 Xueqin Ruan1,2
Xueqin Ruan1,2 Rong Zhang3
Rong Zhang3 Ruijuan Li1,2
Ruijuan Li1,2 Hongkai Zhu1,2
Hongkai Zhu1,2 Zhihua Wang1,2
Zhihua Wang1,2 Canfei Wang1,2
Canfei Wang1,2 Zhao Cheng1,2*
Zhao Cheng1,2* Hongling Peng1,2,4*
Hongling Peng1,2,4*Tumour necrosis factor receptor-associated factor 4 (TRAF4) is a member of the TRAF protein family, a cytoplasmic bridging molecule closely associated with various immune functions. The physiological processes of TRAF4 are mainly involved in embryonic development, cell polarity, cell proliferation, apoptosis, regulation of reactive oxygen species production. TRAF4 is overexpressed in a variety of tumors and regulates the formation and development of a variety of tumors. In this review, we summarize the physiological and pathological regulatory functions of TRAF4 and focus on understanding the biological processes involved in this gene, to provide a reference for further studies on the role of this gene in tumorigenesis and development.
Tumour necrosis factor receptor (TNFR)-associated factor (TRAF) is a critical linker molecule in the tumor necrosis factor superfamily (TNFSF) and Toll-like/interleukin-1 receptor (TLR/ILR) superfamily that plays a regulatory role in cell proliferation, differentiation, apoptosis and survival, and immune response. TRAF family members all have TRAF homologous structural domains at the carboxyl terminus, zinc-finger of the amino terminus, and ring finger structural domains. TRAF is a cytoplasmic bridging protein class that binds to other TRAF proteins after connecting to the receptor’s cytoplasmic domain. Six classical members (TRAF1-TRAF6) and one non-classical member (TRAF7) are known in mammals. TRAF2-6 has ring and zinc-finger motifs that are important for the downstream regulation of signaling (1). Structural features of TRAF proteins suggest that these proteins function as cytoplasmic junctions that promote intracellular signaling through their ability to bind to receptors and enhance the recruitment of proteins to signaling complexes (2).
In terms of structure, the TRAF-N region of the TRAF family is typically composed of more than ten 7-peptide repeats. In contrast, this region of TRAF4 contains only three 7-peptide repeats and the relatively short coiled helix structural domain of TRAF4 results in weak binding of the TRAF4 protein to other TRAF family proteins. Although the TRAF domain of TRAF4 has a typical TRAF domain fold, its high-resolution structure reveals similarities and differences between this structure and other TRAF family members, and these structural differences lead to functional variability (3). TRAF4 is the only TRAF family member with three CART structural domains, for which TRAF4 was once named CART1. Only TRAF4 in the TRAF family contains a unique N-terminal RING-finger and a nuclear localization signal (NLS), and TRAF4 may belong to a subfamily of TRAFs that function in the nucleus (4).
In terms of origin, the TRAF1, 2, 3 and 5 genes arose from the duplication of recent independent genes and had a common ancestor gene. Correlative evolutionary analyses show that TRAF1, 2, 3 and 5 emerged during vertebrate evolution. The TNFR family was formed at this same stage, suggesting that the functions of these four TRAF family proteins are related to those of the TNFR family proteins. Furthermore, TRAF1, 2, 3, 5 and 6 have been shown to interact directly or indirectly with the TNFR superfamily (5, 6). TRAF4 emerged earlier in evolution, homologous analogues of TRAF4 have been identified in lower coelenterates, and TRAF4 homologues play similar roles during embryonic development in both invertebrates and vertebrates, leading to the inference that TRAF4 may be one of the older members of the TRAF family and that it exerts a function that is TNFR non-dependent (7).
In terms of function, unlike other members of the TRAF protein family, TRAF4 migrates in the cell membrane, cell plasma and nucleus mainly through indirect action or formation of complexes, thus participating in several signalling pathways such as NF-κB and JNK to exert physiopathological regulatory functions. However, TRAF4 is less capable of activating signalling pathways alone and must be combined with other interacting proteins before it can effectively activate signalling pathways (8, 9).
Tumour necrosis factor (TNF) receptor-associated factor 4 (TRAF4) is an E3 ubiquitin ligase and a member of the tumor necrosis factor receptor-associated factor (TRAF) family, which is a crucial molecule in individual development. TRAF4 contains a nuclear localization signal (NLS), a unique TRAF family (4). The TRAF4 amino-terminal ring finger (RING) domain has E3 ubiquitin ligase activity, which promotes the ubiquitination of related proteins, regulates downstream signaling (10), and mediates activation of TAK1 and AKT1 target proteins and ubiquitination of K63 linkages (11). TRAF4 is involved in the transduction of multiple signaling pathways, and most of the biological effects of TRAF4 accompany the activation of signaling pathways activation (Table 1).
TRAF4 is required during embryogenesis and is mainly involved in neurogenesis, cell polarity, cell proliferation, immunity, inflammation-related functions, apoptosis, oxidation, osteogenesis metabolism, and repair (12) (Figure 1).
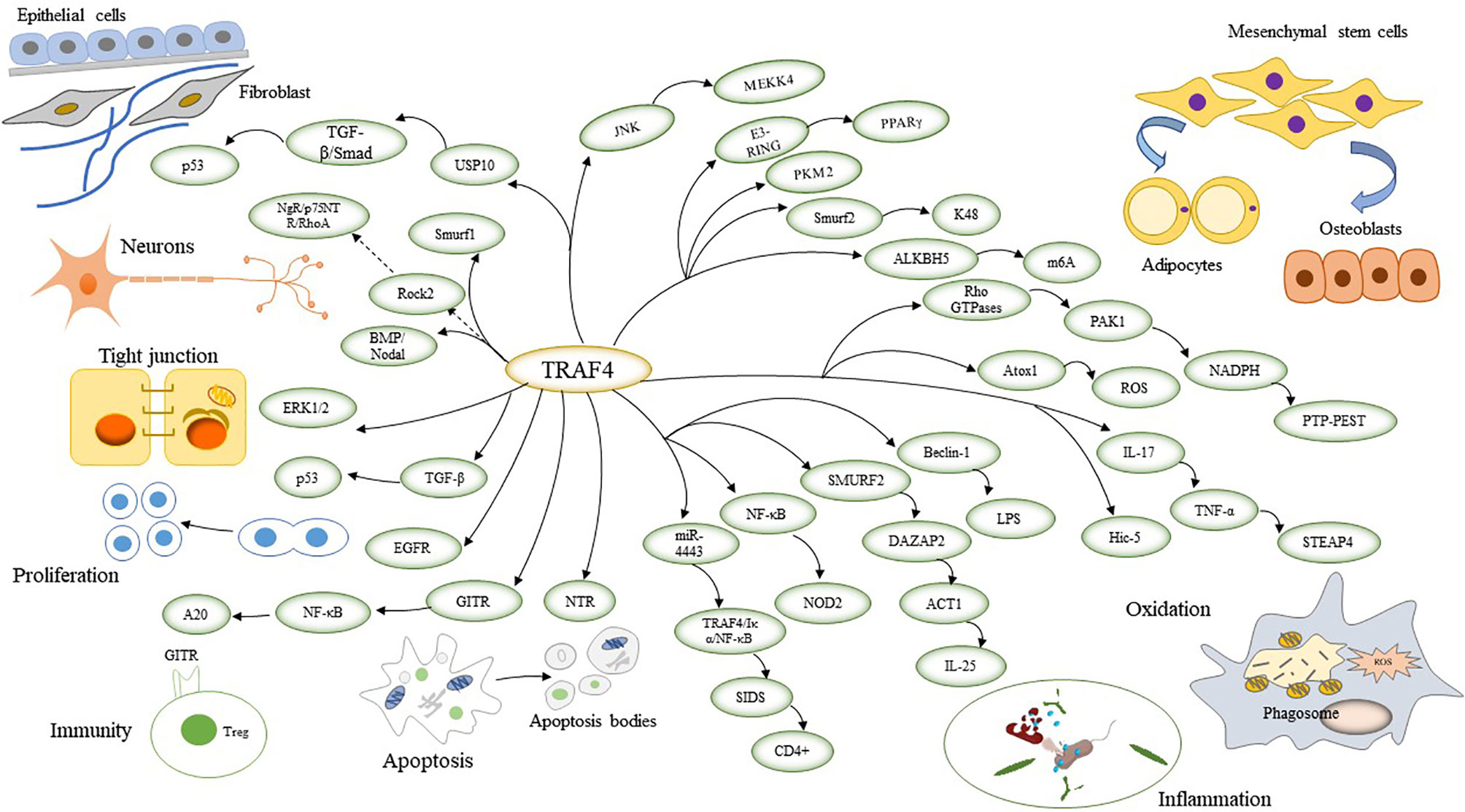
Figure 1 TRAF4 involves signalling pathways that link physiological functions by connecting to reciprocal genes. TRAF4 regulates neuronal cell formation and affects cell polarity by regulating tight junctions. TRAF4 also promotes cell proliferation and cell inflammation and inhibits apoptosis. TRAF4 affects cellular oxidation by inducing ROS. TRAF4 regulates the differentiation of mesenchymal stem cells into adipocytes and osteoblasts, thereby impacting adipose bone production and metabolism. TRAF4 promotes cellular repair by affecting fibroblasts.
In neurogenesis, in the absence of TRAF4, increased phosphorylation of Rock2 (a downstream target of RhoA) leads to activation of the NgR/p75NTR/RhoA signaling pathway induces actin cytoskeletal rearrangement and facilitates axonal regeneration inhibition and neuronal apoptosis (13). TRAF4 is identified as a novel target of Smurf1 that polyubiquitinates TRAF4 to trigger its proteasomal destruction. TRAF4 enhances bone morphogenetic proteins BMP and Nodal signaling. TRAF4 functions as a novel Smurf1 regulatory mediator of BMP and Nodal signaling, essential for neural crest development and neural plate morphogenesis (14).
In cell polarity, TRAF4 is highly mobile and shuttles between the tight cell junction (TJ) and the cytoplasm. Intracellular TRAF4 enhances ERK1/2 phosphorylation in proliferating HeLa cells, an epithelial cell line that lacks TJ. TRAF4 is involved in an emerging TJ-dependent signaling pathway that affects cell polarity by regulating cell proliferation/differentiation homeostasis and subsequent epithelial homeostasis (15). TRAF4 contributes to TGF-β-induced epithelial-mesenchymal transition (EMT), metastasis, and p53 instability (16).
In cell proliferation, activation of the epidermal growth factor receptor (EGFR) induces changes in cellular functions such as proliferation, migration, and differentiation. TRAF4 binding induces conformational rearrangements in the JM region to promote EGFR dimerization, and TRAF4 overexpression promotes EGF-induced EGFR autophosphorylation and downstream signaling. In contrast, deletion of TRAF4 binding sites and specific point mutations significantly attenuated EGFR activation and EGF-driven cell proliferation (17).
In immunity, TRAF4 is involved in immune function by promoting immune cell migration (18). TRAF4 enhances glucocorticoid-induced TNFR (GITR), a receptor expressed on T cells, B cells, and macrophages, thereby triggering activation of NF-kappaB. TRAF4-mediated activation of NF-kappaB downstream of GITR depends on the TRAF binding site in the previously mapped receptor cytoplasmic domain and is inhibited by cytoplasmic protein A20. TRAF4 is thought to inhibit the suppressive function of regulatory T cells (Treg cells) and promote T cell activation. TRAF4 elaborates on GITR signaling and has been shown to regulate the suppressive function of Treg cells (19). TRAF4 binding motifs are involved in the termination of innate immune signaling responses (20).
In terms of inflammation-related functions, miR-4443 overexpression inhibited the TRAF4/Iκα/NF-κB signaling pathway to activate anti-inflammatory cytokine expression in monocytes. miR-4443 increased expression-induced monocyte dysfunction by targeting TRAF4, which may be a key mediator of SIDS (21). MiR-4443 directly inhibited TRAF 4 expression, increasing CD4+ T cell cytokine secretion and proliferation via the NF-κB pathway. Increased miR-4443 expression induces CD4+ T cell dysfunction by targeting TRAF4, leading to GD (22). TRAF4 is a critical negative regulator of NOD2 signaling. TRAF4 may be involved in regulating and coordinating the immune response to the intracellular pattern recognition receptor NOD2, which negatively regulates NOD2 through two consecutive glutamate residues of NOD2, leading to NF-κB pathway activation. TRAF4 inhibits NOD2-induced NF-κB activation and binds directly to NOD2 to inhibit NOD2-induced bacterial killing. TRAF4 phosphorylation and subsequent inhibition of NOD2 signaling require binding to the Crohn’s disease susceptibility protein NOD2 (23).
TRAF4 deficiency leads to tracheal malformations, resulting in altered airflow and increased lung inflammation (24). TRAF4 recruits the E3-ligase SMURF2 to degrade the IL-25R inhibitory molecule DAZAP2. TRAF4-SMURF2-mediated degradation of DAZAP2 is a crucial component of ACT1 recruitment to IL-25R to initiate IL-25 signaling and a critical step in airway inflammation (25). Increased TRAF4 expression in ankylosing spondylitis mesenchymal stem cells (MSCs) may impair lipopolysaccharide (LPS)-induced apoptotic autophagy by inhibiting Beclin-1 phosphorylation (26).
In terms of apoptosis, TRAF4 interacts with the dimeric neurotrophic factor receptor p75(NTR), and TRAF4 overexpression reduces its ability to dimerize to prevent the induction of apoptosis mediated by monomeric p75(NTR). TRAF4 also inhibits the NF activation response (27). TRAF4 is localized in the cytoplasm and appears to be retained in the cytoplasm after DNA damage Overexpression of TRAF4 induces apoptosis and inhibits colony formation (28).
In terms of oxidation, IL-17 and TNF-α induce upregulation of STEAP4 largely dependent on changes in TRAF4. TRAF4 is resistant to copper-mediated oxidative damage. The IL-17/TNF-α-TRAF4-STEAP4 axis can be used to regulate copper homeostasis (29). In TNF-α-stimulated endothelial cells (ECs), the binding of copper-dependent antioxidant-1 (Atox1) to TRAF4 leads to Atox1 nuclear translocation and induces a reactive oxygen species (ROS)-dependent inflammatory response (30). TRAF4 is directly associated with focal contact scaffolding (Hic-5), and knockdown of the protein, disruption of the complex, or oxidant scavenging prevents cell migration. Active mutants of TRAF4 activate Rho GTPases and p21-activated kinase 1 (PAK1) downstream of NADPH oxidase and oxidatively modify focal contact phosphatase (PTP-PEST). TRAF4 initiates robust membrane puckering via Rac1, PAK1, and oxidase, whereas knockdown of PTP-PEST increases membrane puckering while being unrelated to oxidase activation unrelated. TRAF4 specifies the molecular address in the focal complex targeting oxidative modifications during cell migration (31).
In terms of osteogenic metabolism, TRAF4 negatively regulates adipogenesis in MSCs through activation of PKM2 kinase activity, and downregulation of TRAF4 during adipogenesis is regulated by ALKBH5-mediated N6-methyladenosine RNA demethylation. This may serve as a checkpoint for fine-tuning the balance of adipose-osteogenic differentiation and suggests that TRAF4 may be a novel target for clinical MSCs and may also elucidate potential mechanisms of bone metabolic diseases (32). TRAF4 positively regulates osteogenesis in MSCs both in vitro and in vivo. TRAF4 acts as an E3 ubiquitin ligase that degrades Smurf2 to regulate the osteogenic differentiation of MSCs positively. TRAF4 plays a crucial role in bone formation to elucidate the pathogenesis of bone metabolism disorders and improve the practical clinical application of MSCs (33). TRAF4 acts as an E3-RING ubiquitin ligase, promotes the degradation of the adipocyte differentiation regulator PPARγ through the ubiquitin-proteasome pathway, thereby inhibiting adipogenesis.
In terms of repair, TRAF4 acts in a TNF receptor- and JNK-independent manner to fine-tune the assembly of adhesion junctions in invaginated mesangial cells (34). TRAF4 stimulates MAPK/ERK kinase 4 (MEKK4) activity by promoting MEKK4 oligomerization, and JNK activation can be facilitated by chemically inducing MEKK4 dimerization. MEKK4 is a TRAF4-regulated JNK pathway of MAPK kinase (9). TRAF4 inhibits the p53 pathway independent of its E3 ubiquitin ligase activity. TRAF4 interacts with the deubiquitinating enzyme USP10 and blocks p53 from entering USP10, resulting in p53 instability. TRAF4 proliferation regulation in skin repair is dependent on p53 (35). UPS is involved in skin repair by regulating the transforming growth factor (TGF)- β/Smad signaling pathway in the development and progression of hypertrophic scars. TRAF4 binding to the deubiquitinating enzyme USP10 induces p53 instability and promotes scar tissue-derived fibroblasts and skin repair (36).
TRAF4 expression is suppressed in the nucleus of breast cancer cells and is associated with the invasive ability of breast cancer (37). TRAF4, known initially as CART1, is specifically expressed in breast cancer and localized in the nucleus in such tissues. Breast cancer cell proliferation is dependent mainly on TRAF4, and overexpression of TRAF4 can contribute to breast cancer progression by destabilizing the tight junctions (TJs) between cells, thereby promoting cell migration (38). TRAF4 recruits the TJs and prevents their formation and stabilization, facilitating cell migration that allows cancer progression. TRAF4 is dynamically localized to the TJs and is overexpressed in cancers. TRAF4 is a protein dynamically localized to the TJ and overexpressed in cancer and plays multiple functions in breast cancer progression (39). Girdin is mainly expressed in the cytoplasm of breast cancer cells, and TRAF4 facilitates its translocation to the nucleus. Cytoplasmic expression of TRAF4 may be a new potential marker of breast cancer cell migration (40).
TRAF4 upregulates PRMT5 expression in the nucleus, and PRMT5 forms a specific zinc finger structure with TRAF4, which plays an essential role in activating the NF-κB signaling pathway and promotes breast cancer cell proliferation (41). Overexpression of TRAF2 enhances the cytoplasmic expression of TRAF4, which promotes cell proliferation and suppresses NF-κB nuclear transcription through activation of apoptosis (42). Reducing TRAF4 inhibits proliferation, invasion, and metastasis of breast cancer cells by downregulating the AKT signaling pathway, inactivating the NF-κB path, and engaging the interaction of RSK4 (43). TRAF4 promotes membrane recruitment of the cell survival kinase AKT. The overexpression of activated AKT reverses cell growth arrest in TRAF4-silenced cells, which plays an essential role in breast carcinogenesis by activating and interacting with AKT. Overexpression of TRAF4 enhances IGF1-induced IGFR-IRS-1 interaction, IRS-1 tyrosine phosphorylation, and activation of AKT and ERK effector proteins downstream of IGF-1, while mutation of the IRS-1 ubiquitination site eliminates these effects (44).
Reducing TRAF4 increases the proportion of early to mid-stage apoptotic cells in MCF-7 cells, growing G1-phase cells, and decreasing S-phase cells detected. TRAF4 has an anti-apoptotic effect on TNF-α-induced apoptosis in MCF-7 cells (45). By promoting the activation of p70s6k signaling through upregulation of cytoplasmic expression of TRAF4, the p70s6k/S6 signaling pathway plays an essential role in promoting cell proliferation TRAF4 (46). Expression of SRC-3 was inversely correlated with the expression of p53-regulated proapoptotic genes in breast cancer, and SRC-3 and TRAF4 overexpression reduced cytotoxic stress-induced tumor suppression factor p53 protein upregulation. Breast cancer cells overexpressing TRAF4 were more resistant to stress-induced death. TRAF4-mediated inhibition of herpesvirus-associated ubiquitin-specific protease (HAUSP) subsequently led to the loss of p53 deubiquitination and its stability in the cellular stress response. TRAF4 overexpression in breast cancer patients was significantly associated with poor prognosis (47). TRAF4 was identified as a p53 target gene, and TRAF4 overexpression inhibited colony formation (48). TRAF4 is essential for migrating normal breast epithelial cells and breast cancer cells. The ability of TRAF4 to promote cell migration also depends on Smurf1-mediated ubiquitination, which is associated with TRAF4 activation of Rac1 (49).
The β-catenin was identified as a TRAF4-binding protein, TRAF4 enhances β-catenin expression, and TRAF4 mediates the translocation of β-catenin from the cytoplasm the nucleus, thereby facilitating the Wnt signaling pathway in breast cancer (8). TRAF4 is a novel substrate for SIAH1 and prevents SIAH1-mediated β-catenin degradation, the protective effect of TRAF4 on β-catenin during cellular stress, and linking TRAF4 to tumor chemoresistance, TRAF4 reduces the growth inhibitory effects of chemotherapeutic agents such as etoposide by reducing the number of S-phase cells and inhibiting apoptosis (50). TRAF4 is a crucial component that mediates pro-oncogenic transforming growth factor-β (TGF-β)-induced SMAD and non-SMAD signaling. TRAF4 is required for effective TGF-β-induced migration, epithelial-mesenchymal transition, and breast cancer metastasis. In breast cancer patients, elevated TRAF4 expression is associated with increased phosphorylated SMAD2 and phosphorylated TAK1 and poor prognosis. TRAF4 regulates the TGF-β pathway, promotes TGF-β receptor signaling, and drives breast cancer metastasis (11).
Breast cancer is divided into four subtypes based on the biomarkers estrogen receptor (ER) and progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The four main subtypes of breast cancer include Luminal A (ER or PR positive, or both, HER2 negative, low proliferation), Luminal B (ER or PR positive, or both, HER2 negative, high proliferation), HER-2/neu type (HER2 positive and ER and PR negative), Basal cell-like type (HER2 negative, ER and PR negative; triple-negative breast cancer) (51).
TRAF4 nucleus staining was more pronounced in breast tumour specimens than normal breast tissue, and high TRAF4 nucleus expression was significantly associated with poorer overall survival in breast cancer patients. TRAF4 expression in ER-positive breast tumours was inversely correlated with the expression of p53-regulated pro-apoptotic genes, and SRC-3 combined with TRAF4 overexpression reduced the ability of p53 to induce apoptosis in ER-positive breast tumours (47). Tamoxifen significantly reduces the risk of cancer recurrence and metastasis in patients with estrogen receptor-positive breast cancer. Still, a proportion of patients treated with tamoxifen develop intrinsic or acquired resistance, and overexpression of TRAF4 leads to poor prognosis in patients with estrogen receptor-positive breast cancer treated with tamoxifen (52).
Patients with high TRAF4 expression have a poorer prognosis, and those with high TRAF4 expression combined with HER-2 positivity have an even worse prognosis (11). The co-expression of TRAF4 and Girdin proteins was significantly higher in HER-2 negative cases compared to HER-2 positive patients. Girdin is mainly expressed in the cytoplasm of breast cancer cells, but TRAF4 could facilitate its translocation from the cytoplasm to the nucleus (40).
There was a significant correlation between PRMT5 nucleus expression and HER-2-positive subtypes. TRAF4 is involved in the activation of the NF-κB signalling pathway by upregulating PRMT5 and promoting its nucleus expression, and together with HER-2 ectopic expression promoting NF-κB pathway activation, high PRMT5 combined with TRAF4 expression is a poor indicator of prognosis in breast cancer patients (41). TRAF4 is mainly expressed in the cytoplasm, and the cytoplasmic positivity rate is higher than the nucleus positivity rate. Nucleus expression of TRAF4 correlates with clinical stage, molecular/pathological staging and p53 status. Nucleus TRAF4 expression is higher in HER-2/Neu cells than in basal cell types, suggesting that TRAF4 in the nucleus may play an essential role in tumour growth and invasion and may help guide the choice of chemotherapy regimens. The expression of TRAF4 was higher in HER-2/Neu cells than in basal cells (53).
MDA-MB-231 is a breast cancer cell line from patients with triple-negative breast cancer (TNBC) that is highly aggressive. MCF-7 is an estrogen receptor-positive subtype with weakly aggressive cells. TRAF2 and TRAF4 proteins were co-localised in two breast cancer cell lines, MCF-7 and MDA-MB231. In estrogen receptor-positive breast cancer cells (MCF-7), TRAF4 expression was higher in the nucleus than in estrogen receptor-negative breast cancer cells (MDA-MB231), with the opposite expression in the cytoplasm (42). TRAF4 was localized in the cytoplasm and nucleus of MCF-7 cells, and its expression was more robust in the nucleus than in the cytoplasm. And TRAF4 expression was higher in estrogen receptor-positive breast cancer cell lines than in estrogen receptor-negative breast cancer cell lines (45).
TRAF4 and β-catenin co-localize in the cytoplasm of MCF-7 and MDA-MB-231. TRAF4 promotes the proliferation of breast cancer cells by enhancing β-catenin expression (8). TRAF4 interacts with p70s6k in MCF7 and MDA-MB-231, leading to the proliferation of breast cancer cells (46). Wogonside inhibits breast tumor growth and metastasis by suppressing TRAF2 and TRAF4 expression in situ models of MDA-MB-231 cells (54). MTO1 binds to TRAF4 as a competitive endogenous RNA (ceRNA) in MCF-7 and MDA-MB-231, leading to decreased Ki-67 expression, inhibiting tumor activity, and reversing chemotherapeutic drug resistance (55). In estrogen receptor-positive breast cancer cells, TRAF4 binding to IRS-1 stimulates proliferation of breast cancer cell lines and attenuates sensitivity to chemotherapy (44).
High grade invasive ductal carcinoma (IDC) had significantly higher copy numbers of HER2 gene and TRAF4 gene than low/medium grade IDC, suggesting that TRAF4 and HER2 are synchronized in the progression of breast cancer infiltration (56). High levels of TRAF4 amplification were more common in invasive carcinoma and ductal carcinoma in situ (DCIS) than in columnar cell lesions (CCL) (57). Luminal A and B types are most common in male breast cancers, and increased TRAF4 gene copy number is present in male breast cancer patients with both, suggesting an association between TRAF4 and the molecular staging of male breast cancer (58).
Mediator subunit 1 (MED1) is a crucial ERa co-activator that plays a role in HER2-mediated tamoxifen resistance. MED1 gene amplification at the 17q12 locus correlates with TRAF4 gene amplification sites. It is associated with ER and HER2 positivity in breast cancer, triggering poor prognosis in breast cancer patients and leading to tumour resistance (59). Multiplex ligation-dependent probe amplification (MLPA) assays for infiltrating secretory carcinomas show increased copies of TRAF4 and HER2 genes, and HER2 with genes in the GRB7 (HER2 amplicon) 17q12-21 regions are vital discriminators. HER2+ clusters mediate higher proliferative activity, and TRAF4 may be associated with HER2+, leading to more aggressive tumours (60).
TRAF4 nucleus expression was significantly higher in non-invasive ductal carcinoma than in invasive ductal carcinoma, and TRAF4 nuclear expression was negatively correlated with breast cancer invasiveness. TRAF4 may promote breast cancer progression by altering the expression of cell nucleus (37). In epithelial breast cancer cells, TRAF4 depletion impairs EMT, while in mesenchymal-like cells, TRAF4 deficiency leads to loss of cell mobility. TRAF4-deficient cells exhibit reduced cell mobility (61). By studying tissue samples from 80 breast cancer patients (invasive ductal carcinoma, clinical-stage II-III) diagnosed by puncture biopsy, breast cancer patients with low TRAF4 expression benefited most from chemotherapy (50).
TRAF4 is more highly expressed in hepatocellular carcinoma (HCC) cell lines and tissues than regular hepatocyte lines and adjacent non-cancerous tissues. Overexpression of TRAF4 in HCC tissues correlates with tumor number and vascular invasion. Induced by EMT through the PI3K/AKT signaling pathway activation, TRAF4 promotes HCC cell migration and invasion (62). High levels of TRAF4 promote intrahepatic cholangiocarcinoma (ICC) cell invasiveness by activating AKT signaling, and TRAF4 overexpression is associated with shorter overall survival and higher recurrence rates in ICC patients (63). The mRNA levels of TRAF4 were negatively correlated with miR-302c-3p expression. TRAF4 repair reverses the effect of miR-302c-3p on AKT-induced inhibition of EMT and HCC cell metastasis. MiR-302c-3p exerts tumor-suppressive effects in hepatocellular carcinoma by targeting TRAF4. Inhibition of the miR-302c-3p/TRAF4 axis may be a therapeutic target for hepatocellular carcinoma (64).
TRAF4 is a crucial molecule for AKT activation in lung cancer. TRAF4 reduces lung cancer glucose metabolism by inhibiting AKT pathway-mediated expression of Glut1 and HK2. TRAF4 is overexpressed in lung cancer and is essential for lung cancer cells to maintain tumorigenic properties such as glycolysis and xenograft tumor growth (10). TRAF4 is a target gene for miR- in small cell lung cancer (SCLC). ZFPM2-AS1, miR-3612, and TRAF4 constitute an SCLC competitive endogenous RNA (ceRNA) network. ZFPM2-AS1 is significantly upregulated in SCLC tissues and cells, and TRAF4 reverses ZFPM2-AS1 downregulation-mediated proliferation and invasion of SCLC cells in vitro and tumor function of growth (65). MiR-370 overexpression downregulated TRAF4 protein expression in non-small lung cancer (NSCLC) cells. Overexpression of TRAF4 reversed the growth inhibitory effect of miR-370 overexpression on NSCLC cells (66). TRAF4 stabilized the NOX complex by reducing NOX2 and NOX4-mediated lysosomal degradation. In turn, the NOX complex increases the level of endosomal ROS that can penetrate the cytoplasm, leading to NF-κB-mediated upregulation of ICAM1, which affects the tumor microenvironment and increases the invasiveness of cancer cells (67).
TRAF4 is upregulated in endometrial cancer (EC) tissues. TRAF4 overexpression decreases apoptosis and increases cell proliferation and migration. TRAF4 downregulation inhibits primary EC cells’ PI3K/AKT signaling pathway. Oct4 is a downstream factor of the PI3K/AKT pathway and positively regulates TRAF4. TRAF4 may increase cancer cell viability through the PI3K/AKT/Oct4 pathway to increase cancer cell viability. TRAF4 plays an oncogenic role in EC progression by regulating PI3K/AKT/Oct4 pathway (68). The decrease in TRAF4 decreased protein kinase B (AKT) phosphorylation, promoted DNA double-strand breaks, and reduced levels of DNA repair-related proteins, including phosphorylated DNA-dependent protein kinase (p-DNA-PK) and RAD51 recombinase (RAD51). The effect of TRAF4 on the sensitivity of endometrial cancer EC cells to oncologic agents such as olaparib was mainly mediated by AKT phosphorylation mediated. The sensitivity of EC to PARP1 inhibitors was enhanced by reducing TRAF4, and the combination of reduced TRAF4 expression and PARP1 inhibition reduced lethality in EC treatment (69).
TRAF4 is a target gene of miR-519d-3p, down-regulated in prostate cancer cells. miR-519d-3p overexpression significantly reduced the expression of TRAF4 and its downstream TGF-β signaling pathway proteins in prostate cancer cells, thereby inhibiting the proliferation of prostate cancer cells (70). TRAF4 is an E3 ubiquitin ligase highly expressed in metastatic prostate cancer. It is a crucial player in the regulation of RTK-mediated prostate cancer metastasis. TrkA (a neurotrophin RTK) was identified as a TRAF4-targeted ubiquitination substrate that promotes cancer cell invasion, and inhibition of TrkA activity abrogates TRAF4-dependent cell invasion. TRAF4 promotes ubiquitination of K27 and K29 junctions in the TrkA kinase domain and increases their kinase activity. Mutations in TRAF4-targeted ubiquitination sites abolished TrkA tyrosine autophosphorylation and its interactions with downstream proteins. Reducing TRAF4 also inhibits nerve growth factor (NGF) stimulation of TrkA downstream p38 MAPK activation and invasion-associated gene expression. Elevated TRAF4 levels correlated significantly with increased NGF-stimulated invasion-related gene expression in prostate cancer patients, suggesting that this signaling axis is activated substantially during tumorigenesis. The results reveal a post-translational modification mechanism leading to aberrant non-mutant RTK activation in cancer cells (71). The tumor suppressor microRNA in miR-29a is one of the regulators of TRAF4 expression in metastatic prostate cancer, and the TRAF4 mRNA and protein expression is inversely correlated with miR-29a (72).
ZFPM2-AS1 is an oncogene for esophageal cancer (ESCC) cell growth and is regulated through upregulation of TRAF4 and activation of the NF-κB pathway. ZFPM2-AS1 is significantly upregulated in ESCC cells, and lowering ZFPM2-AS1 inhibits cell proliferation, migration, and invasion and promotes apoptosis in ESCC (73). TRAF4 is predominantly expressed in the ESCC cancer cells described in the cytoplasm at high expression, and TRAF4 overexpression is an independent risk factor for the overall prognosis of patients (74).
TRAF4 was overexpressed in colon cancer tissues and cells, and TRAF4 knockdown inhibited cell proliferation, invasion, and tumorigenesis in vitro and in vivo and induced apoptosis in colon cancer cells. SiRNA-TRAF4 significantly inhibited the expression levels of β-catenin, cyclinD1, and c-myc proteins in colon cancer cells. TRAF4 promoted the growth and invasion of colon cancer cells by enhancing the Wnt/β catenin pathway to promote colon cancer cell growth and invasion (75). TRAF4 catalyzes ubiquitination of CHK1 in several colorectal cancer (CRC) cell lines. Ubiquitination of CHK1 by TRAF4 at K132 after DNA damage is required for ATR-mediated CHK1 phosphorylation and activation. TRAF4 is highly expressed in chemotherapy-resistant CRC specimens and positively correlates with phosphorylated CHK1. Depletion of TRAF4 impairs CHK1 activity and sensitizes CRC cells to fluorouracil and other chemotherapeutic agents in vitro and in vivo. Activation of ATR-TRAF4-CHK1 signaling may lead to CRC chemotherapeutic drug resistance (76).
TRAF4 is a direct target of miR-29a and a significant negative expression correlation between TRAF4 and miR-29a. MiR-29a is a crucial tumor suppressor in gliomagenesis by forming a negative feedback loop for TRAF4/AKT signaling, inhibits cell proliferation, migration, and invasion, and is an effective candidate for the treatment of glioma (77). MiR -29a/b/c induces apoptosis and inhibits glioma cell proliferation directly targeting TRAF4. MiR-29a/b/c promotes apoptosis in a p53-dependent manner through the TRAF4/AKT/MDM2 pathway, and miR-29a/b/c induces cell proliferation by blocking phosphorylation of AKT and GSK-3β and expression of cyclin D1. C-myc induces G1 arrest and inhibits tumor cell proliferation (78). Increased expression levels of DLEU1 and TRAF4 in glioblastoma multiforme (GBM) tissues promote GBM cell proliferation and regulate cell migration through the Hippo signaling pathway and Wnt signaling pathway. DLEU1 in GBM can act as a competitive endogenous RNA, thereby promoting GBM tumorigenesis (79).
TRAF4 protein levels are significantly higher in osteosarcoma tissues than in normal bone tissue, and elevated TRAF4 causes tumor cell proliferation, promotes cancer cell colony formation, enhances osteosarcoma cell proliferation and invasion, and increases Ki67 expression through the AKT pathway (80). Decreased TRAF4 causes cell cycle arrest in the G1 phase. It promotes apoptosis through the action of TNF-α and nuclear factor κB, thereby affecting osteosarcoma cell proliferation, cell cycle, and apoptosis, providing a candidate molecular target for osteosarcoma prevention and treatment (81).
DR6 enhances the migratory ability of ovarian cancer cells. Reduced expression of DR6 inhibits the expression of MMP2 and MMP9 and increases the expression of E-cadherin. DR6 enhances the mobile capability of ovarian cancer cells through mitogen-activated protein kinase/ER and PI3K/AKT pathways. DR6, TRAF4, and KIF11 are upregulated in ovarian cancer, and DR6 may exert a significant oncogenic effect in ovarian malignancies through interaction with TRAF4 and KIF11 (82).
Nuclear expression of TRAF4 was observed in normal oral epithelium and highly and moderately differentiated squamous cell carcinoma of the head and neck (SCCN). In contrast, cytoplasmic expression of TRAF4 was observed in poorly differentiated SCCN, and the localization of TRAF4 was associated with the differentiation of SCCHN cells. TRAF4 is a common target of p53 family members, and p63 positively correlates with TRAF4 expression. P63, p73, and p53 transduce TRAF4 and promote tumor cell proliferation (83).
TRAF4 identification as a new direct target of miR-29s reveals that higher TRAF4 levels increase CLL response to CD40 activation and downstream nuclear factor-κB (NF-κB) signaling. In CLL, BCR inhibition of miR-29 expression via MYC allows for TRAF4 upregulation and more robust CD40-NF-κB signaling. This regulatory circuit can be disrupted by BCR inhibitors [Bruton tyrosine kinase (BTK) inhibitor ibrutinib or phosphatidylinositol 3-kinase (PI3K) inhibitor]. Mirna-dependent mechanisms can activate CD40 signaling/T-cell interactions in the CLL microenvironment, resulting in novel miR-29 regulated by BCR activity-TRAF4-CD40 signaling axis (84).
Multiple myeloma (MM) cells express CD40, and CD40L inhibits the growth and increases the apoptotic activity of MM cells by binding to Gp39. Gp39 treatment decreases TRAF4 expression, suggesting that CD40-dependent growth inhibition is associated with altered levels of TRAF4, which acts as an inhibitor of apoptosis in MM (85). Indole-3-hydroxy (I3C) and 3,3’-diindolylmethane (DIM) in vegetables may have a chemotherapeutic effect on T-cell acute lymphoblastic leukaemia (T-ALL) cells. In T-ALL, TRAF4 acts as an inhibitor of apoptosis in tumour cells. Treatment with DIM significantly reduced the expression of a transcript associated with human apoptosis (TRAF4) (86). Glucocorticoid-mediated E4BP4 promotes apoptotic effects in CEM lymphocytic leukaemia cells through upregulation, whereas TRAF4 expression in contrast to E4BP4 inhibits apoptosis in lymphocytic leukaemia cells (87) (Table 2).
Thus, by summarising, we can clearly elucidate that TRAF4 affects the physiological and pathological functions of tumor cells in appealing tumors by participating in signaling pathways. Through the NF-κB signaling pathway, TRAF4 promotes cancer cell proliferation in breast cancer, oesophageal cancer, osteosarcoma, and chronic lymphocytic leukemia. Through AKT or PI3K/AKT signaling pathway, TRAF4 promotes cancer cell migration and invasion in breast, liver, lung, endometrial, osteosarcoma, glioma, and ovarian cancers. Through the Wnt signaling pathway, TRAF4 promotes cancer cell invasion in colorectal cancer and glioma. In addition, through TNF-α signaling, TRAF4 exerts anti-apoptotic effects on breast cancer and osteosarcoma. Through the β-catenin signaling pathway, TRAF4 increases resistance to chemotherapeutic agents in breast and colorectal cancers. Through the TGF-β signaling pathway, TRAF4 promotes cancer cell migration and invasion in breast and prostate cancers. In breast cancer, TRAF4 promotes cancer cell migration and invasion through the Rac1 signaling pathway. In prostate cancer, TRAF4 promotes cancer cell proliferation through the RTK signaling pathway. In glioma, TRAF4 promotes cancer cell proliferation and invasion via the Hippo signaling pathway.
TRAF4 plays an essential role in physiological and pathological processes, which involves signalling pathways such as JNK, PI3K/AKT, MAPK/ERK, Wnt/β-catenin, TGF-β/Samd, NF-κB, Hippo and others. Exploring the interconnections between signalling pathways with a focus on TRAF4 will help understand the role of TRAF4 as an interacting protein and provide a reference for the use of TRAF4 in disease (Figure 2).
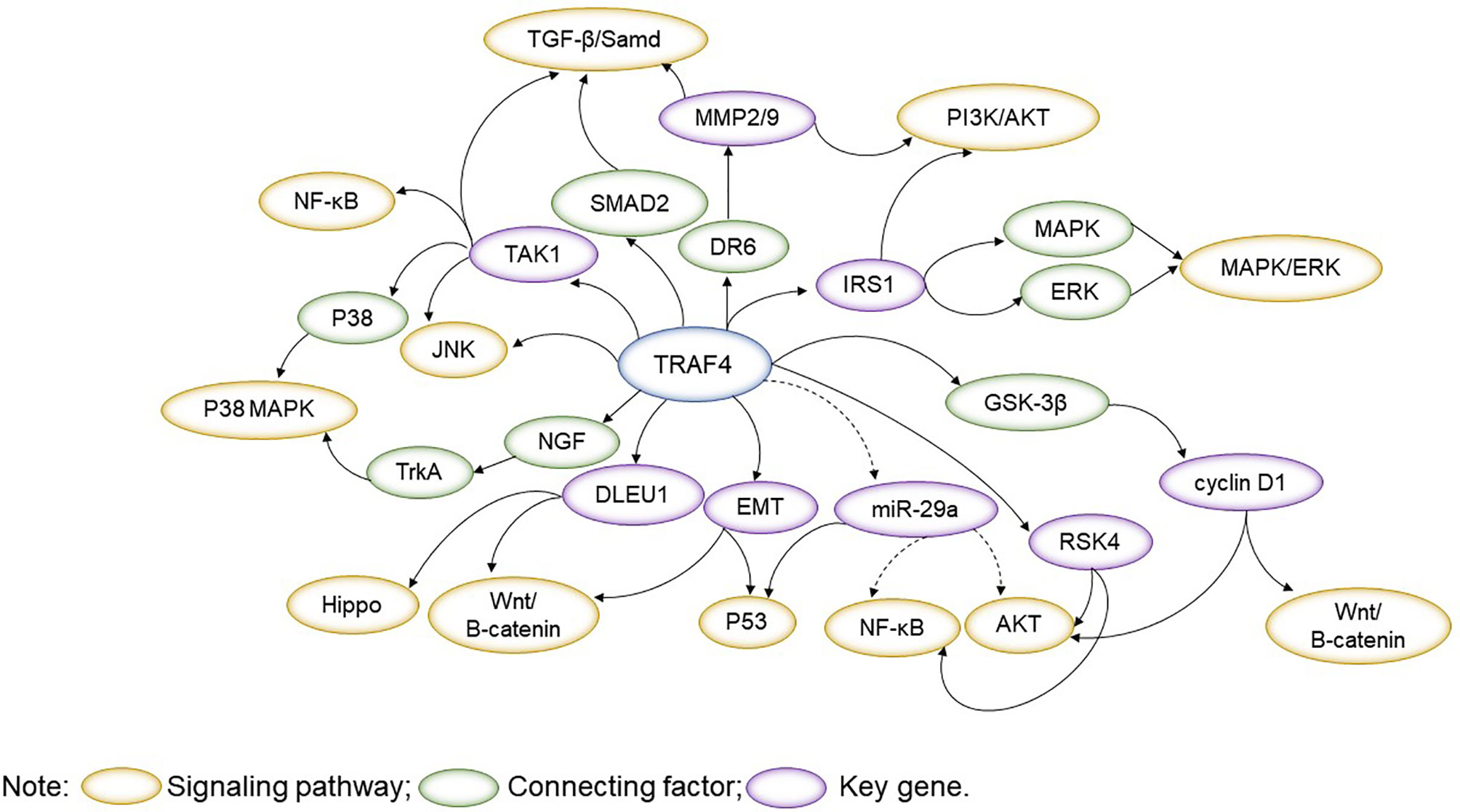
Figure 2 TRAF4 plays an essential role in physiological and pathological processes, which involves signalling pathways such as JNK, PI3K/AKT, MAPK/ERK, Wnt/β-catenin, TGF-β/Samd, NF-κB, Hippo. TRAF4-related signalling pathways are interlinked, with important regulators acting as bridges. By presenting the linkage of TRAF4-related signalling pathways throughout the text, it is easier to understand the regulatory role of TRAF4 in physiology and pathology.
TRAF4 promotes JNK activation by participating in the MAPK/ERK signaling pathway (9). TRAF4 binding to IRS-1 promotes its ubiquitination and phosphorylation, leading to activation of MAPK and PI3K/AKT signaling pathways. TRAF4 overexpression enhances IRS-1 tyrosine phosphorylation to encourage activation of ERK effector proteins (44). Increasing TRAF4 can promote NGF-stimulated activation of the p38 MAPK pathway downstream of TrkA and the expression of invasion-associated genes.
EMT induces TRAF4 to promote cancer cell migration and invasion by activating the Wnt/β catenin signaling pathway, and EMT induces p53 instability (16, 62). DR6 binding to TRAF4 enhances cancer cell mobility by enhancing MMP2 and MMP9 expression and participating in the PI3K/AKT pathway (82). TRAF4 involvement in the TGF-β/Samd signaling pathway can regulate MMP2, and MMP9 TRAF4 involvement in the TGF-β/Samd signaling pathway regulates the expression of MMP2 and MMP9 and induces P53 instability and tumorigenesis (16). Elevated TRAF4 expression regulates the TGF-β pathway and mediates an increase in phosphorylated SMAD2 and phosphorylated TAK1, triggering poor prognosis associated (11). In addition, TAK1 is involved in the NF-κB and JNK pathways and the p38 MAPK pathway (88).
Reducing TRAF4 can inhibit cancer cell proliferation, invasion and metastasis by downregulating the AKT signaling pathway, inhibiting the NF-κB pathway and engaging in RSK4 interactions (43). MiR-29a is an important tumor suppressor that promotes apoptosis and inhibits cell proliferation, migration and invasion by inhibiting the TRAF4/AKT/MDM2 pathway in a p53-dependent manner (77, 78). BCR promotes TRAF4 upregulation and activates the CD40-induced NF-κB signaling pathway through MYC inhibition of miR-29a expression (84). MiR-29a inhibits cell proliferation by blocking the phosphorylation of AKT and GSK-3β and cyclin D1 expression. High expression of TRAF4 significantly increased cyclinD1 and c-myc protein expression levels in cancer cells and activated the Wnt/β-catenin signaling pathway (75). DLEU1 can act as a competitive endogenous RNA that promotes elevated TRAF4 expression levels, promotes cancer cell proliferation through the Hippo signaling pathway and Wnt/β-catenin signaling pathway and regulate cell migration (79).
TRAF4 produces cell-specific and diverse biological effects in the regulation of cell differentiation, polarity, proliferation, and apoptosis, affecting embryonic development, rule of reactive oxygen species production, and mediating tumor formation and evolution. TRAF4 significantly enhances cancer development and progression in various malignancies, and its effects involve multiple signaling pathways such as AKT, NF-κB, Wnt, TGF-βand TNF-α. TRAF4 is a potential molecular target for cancer prevention and treatment, and the regulatory mechanism of TRAF4 needs to be further investigated. The study of TRAF4 can identify new targets suitable for tumor therapy and lay the foundation for developing new drugs and tumor drug resistance research.
XR wrote the review. RZ, RL, HZ, ZW, and CW discussed the review. All authors proofread the review. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (82070175), Natural Science Foundation of Hunan Province (2021JJ30937), Scientific program of Health Commission of Hunan Province (2022030442723), Changsha Municipal Natural Science Foundation (kq2014234).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bradley JR, Pober JS. Tumor Necrosis Factor Receptor-Associated Factors (TRAFs). Oncogene (2001) 20(44):6482–91. doi: 10.1038/sj.onc.1204788
2. Arch RH, Gedrich RW, Thompson CB. Tumor Necrosis Factor Receptor-Associated Factors (TRAFs)–A Family of Adapter Proteins That Regulates Life and Death. Genes Dev (1998) 12(18):2821–30. doi: 10.1101/gad.12.18.2821
3. Yoon JH, Cho YJ, Park HH. Structure of the TRAF4 TRAF Domain With a Coiled-Coil Domain and Its Implications for the TRAF4 Signalling Pathway. Acta Crystallographica Section D Biol Crystallogr (2014) 70(Pt 1):2–10. doi: 10.1107/s139900471302333x
4. Régnier CH, Tomasetto C, Moog-Lutz C, Chenard MP, Wendling C, Basset P, et al. Presence of a New Conserved Domain in CART1, a Novel Member of the Tumor Necrosis Factor Receptor-Associated Protein Family, Which Is Expressed in Breast Carcinoma. J Biol Chem (1995) 270(43):25715–21. doi: 10.1074/jbc.270.43.25715
5. Ha H, Han D, Choi Y. TRAF-Mediated TNFR-Family Signaling. Curr Protoc Immunol (2009) Chapter 11:Unit11.9D. doi: 10.1002/0471142735.im1109ds87
6. So T. The Immunological Significance of Tumor Necrosis Factor Receptor-Associated Factors (TRAFs). Int Immunol (2022) 34(1):7–20. doi: 10.1093/intimm/dxab058
7. Grech A, Quinn R, Srinivasan D, Badoux X, Brink R. Complete Structural Characterisation of the Mammalian and Drosophila TRAF Genes: Implications for TRAF Evolution and the Role of RING Finger Splice Variants. Mol Immunol (2000) 37(12-13):721–34. doi: 10.1016/s0161-5890(00)00098-5
8. Wang A, Wang J, Ren H, Yang F, Sun L, Diao K, et al. TRAF4 Participates in Wnt/β-Catenin Signaling in Breast Cancer by Upregulating β-Catenin and Mediating Its Translocation to the Nucleus. Mol Cell Biochem (2014) 395(1-2):211–9. doi: 10.1007/s11010-014-2127-y
9. Abell AN, Johnson GL. MEKK4 Is an Effector of the Embryonic TRAF4 for JNK Activation. J Biol Chem (2005) 280(43):35793–6. doi: 10.1074/jbc.C500260200
10. Li W, Peng C, Lee MH, Lim D, Zhu F, Fu Y, et al. TRAF4 Is a Critical Molecule for Akt Activation in Lung Cancer. Cancer Res (2013) 73(23):6938–50. doi: 10.1158/0008-5472.Can-13-0913
11. Zhang L, Zhou F, García de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, et al. TRAF4 Promotes TGF-β Receptor Signaling and Drives Breast Cancer Metastasis. Mol Cell (2013) 51(5):559–72. doi: 10.1016/j.molcel.2013.07.014
12. Régnier CH, Masson R, Kedinger V, Textoris J, Stoll I, Chenard MP, et al. Impaired Neural Tube Closure, Axial Skeleton Malformations, and Tracheal Ring Disruption in TRAF4-Deficient Mice. Proc Natl Acad Sci USA (2002) 99(8):5585–90. doi: 10.1073/pnas.052124799
13. Blaise S, Kneib M, Rousseau A, Gambino F, Chenard MP, Messadeq N, et al. In Vivo Evidence That TRAF4 Is Required for Central Nervous System Myelin Homeostasis. PloS One (2012) 7(2):e30917. doi: 10.1371/journal.pone.0030917
14. Kalkan T, Iwasaki Y, Park CY, Thomsen GH. Tumor Necrosis Factor-Receptor-Associated Factor-4 Is a Positive Regulator of Transforming Growth Factor-Beta Signaling That Affects Neural Crest Formation. Mol Biol Cell (2009) 20(14):3436–50. doi: 10.1091/mbc.e08-03-0325
15. Kédinger V, Alpy F, Baguet A, Polette M, Stoll I, Chenard MP, et al. Tumor Necrosis Factor Receptor-Associated Factor 4 Is a Dynamic Tight Junction-Related Shuttle Protein Involved in Epithelium Homeostasis. PloS One (2008) 3(10):e3518. doi: 10.1371/journal.pone.0003518
16. Rousseau A, Wilhelm LP, Tomasetto C, Alpy F. The Phosphoinositide-Binding Protein TRAF4 Modulates Tight Junction Stability and Migration of Cancer Cells. Tissue Barriers (2014) 2(4):e975597. doi: 10.4161/21688370.2014.975597
17. Cai G, Zhu L, Chen X, Sun K, Liu C, Sen GC, et al. TRAF4 Binds to the Juxtamembrane Region of EGFR Directly and Promotes Kinase Activation. Proc Natl Acad Sci USA (2018) 115(45):11531–6. doi: 10.1073/pnas.1809599115
18. Cherfils-Vicini J, Vingert B, Varin A, Tartour E, Fridman WH, Sautès-Fridman C, et al. Characterization of Immune Functions in TRAF4-Deficient Mice. Immunology (2008) 124(4):562–74. doi: 10.1111/j.1365-2567.2008.02810.x
19. Esparza EM, Arch RH. TRAF4 Functions as an Intermediate of GITR-Induced NF-kappaB Activation. Cell Mol Life Sci: CMLS (2004) 61(24):3087–92. doi: 10.1007/s00018-004-4417-0
20. Marinis JM, Homer CR, McDonald C, Abbott DW. A Novel Motif in the Crohn’s Disease Susceptibility Protein, NOD2, Allows TRAF4 to Down-Regulate Innate Immune Responses. J Biol Chem (2011) 286(3):1938–50. doi: 10.1074/jbc.M110.189308
21. Li S, Lu G, Wang D, He JL, Zuo L, Wang H, et al. MicroRNA-4443 Regulates Monocyte Activation by Targeting Tumor Necrosis Factor Receptor Associated Factor 4 in Stroke-Induced Immunosuppression. Eur J Neurol (2020) 27(8):1625–37. doi: 10.1111/ene.14282
22. Qi Y, Zhou Y, Chen X, Ye L, Zhang Q, Huang F, et al. MicroRNA-4443 Causes CD4+ T Cells Dysfunction by Targeting TNFR-Associated Factor 4 in Graves’ Disease. Front Immunol (2017) 8:1440. doi: 10.3389/fimmu.2017.01440
23. Marinis JM, Hutti JE, Homer CR, Cobb BA, Cantley LC, McDonald C, et al. Iκb Kinase α Phosphorylation of TRAF4 Downregulates Innate Immune Signaling. Mol Cell Biol (2012) 32(13):2479–89. doi: 10.1128/mcb.00106-12
24. Shiels H, Li X, Schumacker PT, Maltepe E, Padrid PA, Sperling A, et al. TRAF4 Deficiency Leads to Tracheal Malformation With Resulting Alterations in Air Flow to the Lungs. Am J Pathol (2000) 157(2):679–88. doi: 10.1016/s0002-9440(10)64578-6
25. Zepp JA, Wu L, Qian W, Ouyang W, Aronica M, Erzurum S, et al. TRAF4-SMURF2-Mediated DAZAP2 Degradation Is Critical for IL-25 Signaling and Allergic Airway Inflammation. J Immunol (Baltimore Md: 1950) (2015) 194(6):2826–37. doi: 10.4049/jimmunol.1402647
26. Li J, Wang P, Xie Z, Yang R, Li Y, Wu X, et al. Elevated TRAF4 Expression Impaired LPS-Induced Autophagy in Mesenchymal Stem Cells From Ankylosing Spondylitis Patients. Exp Mol Med (2017) 49(6):e343. doi: 10.1038/emm.2017.69
27. Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, et al. TRAF Family Proteins Interact With the Common Neurotrophin Receptor and Modulate Apoptosis Induction. J Biol Chem (1999) 274(42):30202–8. doi: 10.1074/jbc.274.42.30202
28. Sax JK, El-Deiry WS. Identification and Characterization of the Cytoplasmic Protein TRAF4 as a P53-Regulated Proapoptotic Gene. J Biol Chem (2003) 278(38):36435–44. doi: 10.1074/jbc.M303191200
29. Jiang C, Wu B, Xue M, Lin J, Hu Z, Nie X, et al. Inflammation Accelerates Copper-Mediated Cytotoxicity Through Induction of Six-Transmembrane Epithelial Antigens of Prostate 4 Expression. Immunol Cell Biol (2021) 99(4):392–402. doi: 10.1111/imcb.12427
30. Das A, Sudhahar V, Ushio-Fukai M, Fukai T. Novel Interaction of Antioxidant-1 With TRAF4: Role in Inflammatory Responses in Endothelial Cells. Am J Physiol Cell Physiol (2019) 317(6):C1161–c71. doi: 10.1152/ajpcell.00264.2019
31. Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA Jr, Terada LS. Subcellular Targeting of Oxidants During Endothelial Cell Migration. J Cell Biol (2005) 171(5):893–904. doi: 10.1083/jcb.200507004
32. Cen S, Li J, Cai Z, Pan Y, Sun Z, Li Z, et al. TRAF4 Acts as a Fate Checkpoint to Regulate the Adipogenic Differentiation of MSCs by Activating PKM2. EBioMedicine (2020) 54:102722. doi: 10.1016/j.ebiom.2020.102722
33. Li J, Wang P, Xie Z, Wang S, Cen S, Li M, et al. TRAF4 Positively Regulates the Osteogenic Differentiation of Mesenchymal Stem Cells by Acting as an E3 Ubiquitin Ligase to Degrade Smurf2. Cell Death Differ (2019) 26(12):2652–66. doi: 10.1038/s41418-019-0328-3
34. Mathew SJ, Rembold M, Leptin M. Role for Traf4 in Polarizing Adherens Junctions as a Prerequisite for Efficient Cell Shape Changes. Mol Cell Biol (2011) 31(24):4978–93. doi: 10.1128/mcb.05542-11
35. Deng CC, Zhu DH, Chen YJ, Huang TY, Peng Y, Liu SY, et al. TRAF4 Promotes Fibroblast Proliferation in Keloids by Destabilizing P53 via Interacting With the Deubiquitinase Usp10. J Invest Dermatol (2019) 139(9):1925–35.e5. doi: 10.1016/j.jid.2019.03.1136
36. Shen W, Zhang Z, Ma J, Lu D, Lyu L. The Ubiquitin Proteasome System and Skin Fibrosis. Mol Diagnosis Ther (2021) 25(1):29–40. doi: 10.1007/s40291-020-00509-z
37. Dai WB, Zheng YW, Mi XY, Liu N, Lin H, Yan J. Expression and Significance of TRAF4 Protein in Breast Carcinoma. Ai zheng = Aizheng = Chin J Cancer (2007) 26(10):1095–8.
38. Rousseau A, McEwen AG, Poussin-Courmontagne P, Rognan D, Nominé Y, Rio MC, et al. TRAF4 Is a Novel Phosphoinositide-Binding Protein Modulating Tight Junctions and Favoring Cell Migration. PloS Biol (2013) 11(12):e1001726. doi: 10.1371/journal.pbio.1001726
39. Rousseau A, Tomasetto C, Alpy F. TRAF4, A Multifaceted Protein Involved in Carcinoma Progression. Biologie Aujourd’hui (2014) 208(4):299–310. doi: 10.1051/jbio/2014026
40. Wang A, Wang J, Sun L, Jin J, Ren H, Yang F, et al. Expression of Tumor Necrosis Factor Receptor-Assicated Factor 4 Correlates With Expression of Girdin and Promotes Nuclear Translocation of Girdin in Breast Cancer. Mol Med Rep (2015) 11(5):3635–41. doi: 10.3892/mmr.2015.3211
41. Yang F, Wang J, Ren HY, Jin J, Wang AL, Sun LL, et al. Proliferative Role of TRAF4 in Breast Cancer by Upregulating PRMT5 Nuclear Expression. Tumour Biol: J Int Soc Oncodevelopmental Biol Med (2015) 36(8):5901–11. doi: 10.1007/s13277-015-3262-0
42. Zhang X, Wen Z, Sun L, Wang J, Song M, Wang E, et al. TRAF2 Regulates the Cytoplasmic/Nuclear Distribution of TRAF4 and Its Biological Function in Breast Cancer Cells. Biochem Biophys Res Commun (2013) 436(2):344–8. doi: 10.1016/j.bbrc.2013.05.107
43. Zhu L, Zhang S, Huan X, Mei Y, Yang H. Down-Regulation of TRAF4 Targeting RSK4 Inhibits Proliferation, Invasion and Metastasis in Breast Cancer Xenografts. Biochem Biophys Res Commun (2018) 500(3):810–6. doi: 10.1016/j.bbrc.2018.04.164
44. Yu W, Singh R, Wang Z, O’Malley BW, Yi P. The E3 Ligase TRAF4 Promotes IGF Signaling by Mediating Atypical Ubiquitination of IRS-1. J Biol Chem (2021) 296:100739. doi: 10.1016/j.jbc.2021.100739
45. Zhang X, Wen Z, Mi X. Expression and Anti-Apoptotic Function of TRAF4 in Human Breast Cancer MCF-7 Cells. Oncol Lett (2014) 7(2):411–4. doi: 10.3892/ol.2013.1703
46. Ren HY, Wang J, Yang F, Zhang XL, Wang AL, Sun LL, et al. Cytoplasmic TRAF4 Contributes to the Activation of P70s6k Signaling Pathway in Breast Cancer. Oncotarget (2015) 6(6):4080–96. doi: 10.18632/oncotarget.2977
47. Yi P, Xia W, Wu RC, Lonard DM, Hung MC, O’Malley BW. SRC-3 Coactivator Regulates Cell Resistance to Cytotoxic Stress via TRAF4-Mediated P53 Destabilization. Genes Dev (2013) 27(3):274–87. doi: 10.1101/gad.203760.112
48. Rozan LM, El-Deiry WS. Identification and Characterization of Proteins Interacting With Traf4, an Enigmatic P53 Target. Cancer Biol Ther (2006) 5(9):1228–35. doi: 10.4161/cbt.5.9.3295
49. Wang X, Jin C, Tang Y, Tang LY, Zhang YE. Ubiquitination of Tumor Necrosis Factor Receptor-Associated Factor 4 (TRAF4) by Smad Ubiquitination Regulatory Factor 1 (Smurf1) Regulates Motility of Breast Epithelial and Cancer Cells. J Biol Chem (2013) 288(30):21784–92. doi: 10.1074/jbc.M113.472704
50. Ren H, Mi X, Zhao P, Zhao X, Wei N, Huang H, et al. TRAF4, A New Substrate of SIAH1, Participates in Chemotherapy Resistance of Breast Cancer Cell by Counteracting SIAH1-Mediated Downregulation of β-Catenin. Breast Cancer Res Treat (2020) 183(2):275–89. doi: 10.1007/s10549-020-05789-x
51. Harbeck N, Gnant M. Breast Cancer. Lancet (London England) (2017) 389(10074):1134–50. doi: 10.1016/s0140-6736(16)31891-8
52. Zhou J, Li W, Ming J, Yang W, Lu L, Zhang Q, et al. High Expression of TRAF4 Predicts Poor Prognosis in Tamoxifen-Treated Breast Cancer and Promotes Tamoxifen Resistance. Anti Cancer Drugs (2020) 31(6):558–66. doi: 10.1097/cad.0000000000000943
53. Zhao ZJ, Ren HY, Yang F, Wang J, Wu GP, Mi XY. Expression, Correlation, and Prognostic Value of TRAF2 and TRAF4 Expression in Malignant Plural Effusion Cells in Human Breast Cancer. Diagn Cytopathol (2015) 43(11):897–903. doi: 10.1002/dc.23330
54. Yao Y, Zhao K, Yu Z, Ren H, Zhao L, Li Z, et al. Wogonoside Inhibits Invasion and Migration Through Suppressing TRAF2/4 Expression in Breast Cancer. J Exp Clin Cancer Res: CR (2017) 36(1):103. doi: 10.1186/s13046-017-0574-5
55. Liu Y, Dong Y, Zhao L, Su L, Luo J. Circular RNA−MTO1 Suppresses Breast Cancer Cell Viability and Reverses Monastrol Resistance Through Regulating the TRAF4/Eg5 Axis. Int J Oncol (2018) 53(4):1752–62. doi: 10.3892/ijo.2018.4485
56. Moelans CB, de Wegers RA, Monsuurs HN, Maess AH, van Diest PJ. Molecular Differences Between Ductal Carcinoma In Situ and Adjacent Invasive Breast Carcinoma: A Multiplex Ligation-Dependent Probe Amplification Study. Cell Oncol (Dordrecht) (2011) 34(5):475–82. doi: 10.1007/s13402-011-0043-7
57. Verschuur-Maes AH, Moelans CB, de Bruin PC, van Diest PJ. Analysis of Gene Copy Number Alterations by Multiplex Ligation-Dependent Probe Amplification in Columnar Cell Lesions of the Breast. Cell Oncol (Dordrecht) (2014) 37(2):147–54. doi: 10.1007/s13402-014-0170-z
58. Kornegoor R, Moelans CB, Verschuur-Maes AH, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Oncogene Amplification in Male Breast Cancer: Analysis by Multiplex Ligation-Dependent Probe Amplification. Breast Cancer Res Treat (2012) 135(1):49–58. doi: 10.1007/s10549-012-2051-3
59. Moelans CB, van der Groep P, Hoefnagel LDC, van de Vijver MJ, Wesseling P, Wesseling J, et al. Genomic Evolution From Primary Breast Carcinoma to Distant Metastasis: Few Copy Number Changes of Breast Cancer Related Genes. Cancer Lett (2014) 344(1):138–46. doi: 10.1016/j.scanlet.2013.10.025
60. Vranic S, Marchiò C, Castellano I, Botta C, Scalzo MS, Bender RP, et al. Immunohistochemical and Molecular Profiling of Histologically Defined Apocrine Carcinomas of the Breast. Hum Pathol (2015) 46(9):1350–9. doi: 10.1016/j.humpath.2015.05.017
61. Zhou F, Li F, Xie F, Zhang Z, Huang H, Zhang L. TRAF4 Mediates Activation of TGF-β Signaling and Is a Biomarker for Oncogenesis in Breast Cancer. Sci China Life Sci (2014) 57(12):1172–6. doi: 10.1007/s11427-014-4727-x
62. Liu K, Wu X, Zang X, Huang Z, Lin Z, Tan W, et al. Erratum. Oncol Res (2020) 28(5):559–60. doi: 10.3727/096504020x16032056440102
63. Kang Q, Zou H, Zhou L, Liu LX, Cai JB, Xie N, et al. Role of the Overexpression of TRAF4 in Predicting the Prognosis of Intrahepatic Cholangiocarcinoma. Int J Oncol (2018) 53(1):286–96. doi: 10.3892/ijo.2018.4383
64. Yang L, Guo Y, Liu X, Wang T, Tong X, Lei K, et al. The Tumor Suppressive miR-302c-3p Inhibits Migration and Invasion of Hepatocellular Carcinoma Cells by Targeting TRAF4. J Cancer (2018) 9(15):2693–701. doi: 10.7150/jca.25569
65. Yan Z, Yang Q, Xue M, Wang S, Hong W, Gao X. YY1-Induced lncRNA ZFPM2-AS1 Facilitates Cell Proliferation and Invasion in Small Cell Lung Cancer via Upregulating of TRAF4. Cancer Cell Int (2020) 20:108. doi: 10.1186/s12935-020-1157-7
66. Chen T, Gao F, Feng S, Yang T, Chen M. MicroRNA-370 Inhibits the Progression of Non-Small Cell Lung Cancer by Downregulating Oncogene TRAF4. Oncol Rep (2015) 34(1):461–8. doi: 10.3892/or.2015.3978
67. Kim E, Kim W, Lee S, Chun J, Kang J, Park G, et al. TRAF4 Promotes Lung Cancer Aggressiveness by Modulating Tumor Microenvironment in Normal Fibroblasts. Sci Rep (2017) 7(1):8923. doi: 10.1038/s41598-017-09447-z
68. Xie P, Wang X, Kong M, Bai X, Jiang T. TRAF4 Promotes Endometrial Cancer Cell Growth and Migration by Activation of PI3K/AKT/Oct4 Signaling. Exp Mol Pathol (2019) 108:9–16. doi: 10.1016/j.yexmp.2019.03.003
69. Tang L, Wang M, Jiang L, Zeng C. TRAF4 Knockdown Triggers Synergistic Lethality With Simultaneous PARP1 Inhibition in Endometrial Cancer. Hum Cell (2020) 33(3):801–9. doi: 10.1007/s13577-020-00363-5
70. Li X, Han X, Yang J, Sun J, Wei P. Overexpression of miR-519d-3p Inhibits the Proliferation of DU-145 Prostate Cancer Cells by Reducing TRAF4. Xi bao yu fen zi mian yi xue za zhi = Chin J Cell Mol Immunol (2018) 34(1):16–21. doi: 10.13423/j.cnki.cjcmi.008542
71. Singh R, Karri D, Shen H, Shao J, Dasgupta S, Huang S, et al. TRAF4-Mediated Ubiquitination of NGF Receptor TrkA Regulates Prostate Cancer Metastasis. J Clin Invest (2018) 128(7):3129–43. doi: 10.1172/jci96060
72. Ahmed F, Shiraishi T, Vessella RL, Kulkarni P. Tumor Necrosis Factor Receptor Associated Factor-4: An Adapter Protein Overexpressed in Metastatic Prostate Cancer Is Regulated by microRNA-29a. Oncol Rep (2013) 30(6):2963–8. doi: 10.3892/or.2013.2789
73. Sun G, Wu C. ZFPM2-AS1 Facilitates Cell Growth in Esophageal Squamous Cell Carcinoma via Up-Regulating TRAF4. Biosci Rep (2020) 40(4):BSR20194352. doi: 10.1042/bsr20194352
74. Li PC, Hu DD, Jia W, Hu B. Expression and Association of Tumor Necrosis Factor Receptor Associated Factor 4 (TRAF4) in Esophageal Squamous Cell Carcinoma. Med Sci Monit: Int Med J Exp Clin Res (2019) 25:2368–76. doi: 10.12659/msm.915474
75. Yang K, Wang F, Han JJ. TRAF4 Promotes the Growth and Invasion of Colon Cancer Through the Wnt/β-Catenin Pathway. Int J Clin Exp Pathol (2015) 8(2):1419–26. doi: 10.1029/JA081i019p03441
76. Yu X, Li W, Liu H, Deng Q, Wang X, Hu H, et al. Ubiquitination of the DNA-Damage Checkpoint Kinase CHK1 by TRAF4 Is Required for CHK1 Activation. J Hematol Oncol (2020) 13(1):40. doi: 10.1186/s13045-020-00869-3
77. Liu Y, Duan N, Duan S. MiR-29a Inhibits Glioma Tumorigenesis Through a Negative Feedback Loop of TRAF4/Akt Signaling. BioMed Res Int (2018) 2018:2461363. doi: 10.1155/2018/2461363
78. Shi C, Rao C, Sun C, Yu L, Zhou X, Hua D, et al. miR-29s Function as Tumor Suppressors in Gliomas by Targeting TRAF4 and Predict Patient Prognosis. Cell Death Dis (2018) 9(11):1078. doi: 10.1038/s41419-018-1092-x
79. Wang J, Quan X, Peng D, Hu G. Long Non−Coding RNA DLEU1 Promotes Cell Proliferation of Glioblastoma Multiforme. Mol Med Rep (2019) 20(2):1873–82. doi: 10.3892/mmr.2019.10428
80. Yao W, Wang X, Cai Q, Gao S, Wang J, Zhang P. TRAF4 Enhances Osteosarcoma Cell Proliferation and Invasion by Akt Signaling Pathway. Oncol Res (2014) 22(1):21–8. doi: 10.3727/096504014x14077751730351
81. Yao W, Wang X, Cai Q, Gao S, Wang J, Zhang P. Knockdown of TRAF4 Expression Suppresses Osteosarcoma Cell Growth In Vitro and In Vivo. Int J Mol Med (2014) 34(6):1655–60. doi: 10.3892/ijmm.2014.1948
82. Shi B, Bao J, Liu Y, Shi J. Death Receptor 6 Promotes Ovarian Cancer Cell Migration Through KIF11. FEBS Open Bio (2018) 8(9):1497–507. doi: 10.1002/2211-5463.12492
83. Gu X, Coates PJ, MacCallum SF, Boldrup L, Sjöström B, Nylander K. TRAF4 Is Potently Induced by TAp63 Isoforms and Localised According to Differentiation in SCCHN. Cancer Biol Ther (2007) 6(12):1986–90. doi: 10.4161/cbt.6.12.5002
84. Sharma S, Pavlasova GM, Seda V, Cerna KA, Vojackova E, Filip D, et al. miR-29 Modulates CD40 Signaling in Chronic Lymphocytic Leukemia by Targeting TRAF4: An Axis Affected by BCR Inhibitors. Blood (2021) 137(18):2481–94. doi: 10.1182/blood.2020005627
85. Tong AW, Seamour B, Chen J, Su D, Ordonez G, Frase L, et al. CD40 Ligand-Induced Apoptosis Is Fas-Independent in Human Multiple Myeloma Cells. Leukemia Lymphoma (2000) 36(5-6):543–58. doi: 10.3109/10428190009148403
86. Shorey LE, Hagman AM, Williams DE, Ho E, Dashwood RH, Benninghoff AD. 3,3’-Diindolylmethane Induces G1 Arrest and Apoptosis in Human Acute T-Cell Lymphoblastic Leukemia Cells. PloS One (2012) 7(4):e34975. doi: 10.1371/journal.pone.0034975
87. Beach JA, Nary LJ, Hovanessian R, Medh RD. Correlation of Glucocorticoid-Mediated E4BP4 Upregulation With Altered Expression of Pro- and Anti-Apoptotic Genes in CEM Human Lymphoblastic Leukemia Cells. Biochem Biophys Res Commun (2014) 451(3):382–8. doi: 10.1016/j.bbrc.2014.07.103
Keywords: TRAF4, carcinogenesis, apoptosis, proliferation, pathological
Citation: Ruan X, Zhang R, Li R, Zhu H, Wang Z, Wang C, Cheng Z and Peng H (2022) The Research Progress in Physiological and Pathological Functions of TRAF4. Front. Oncol. 12:842072. doi: 10.3389/fonc.2022.842072
Received: 23 December 2021; Accepted: 26 January 2022;
Published: 15 February 2022.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Srimoyee Mukherjee, Tufts University School of Medicine, United StatesCopyright © 2022 Ruan, Zhang, Li, Zhu, Wang, Wang, Cheng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongling Peng, cGVuZ2hvbmdsaW5nQGNzdS5lZHUuY24=; Zhao Cheng, ZWNob2N6QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.