- 1Medical Oncology, Department of Medicine, University of Verona, Verona, Italy
- 2Medical Oncology, San Bortolo Hospital, Vicenza, Italy
- 3Medical Oncology, IRCCS Sacro Cuore Don Calabria Hospital, Verona, Italy
- 4Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, Italy
- 5Medical Oncology 2, Istituto Oncologico Veneto IRCCS, Padua, Italy
- 6Thoracic Oncology, Lung Unit, P. Pederzoli Hospital, Peschiera del Garda, Italy
- 7Medical Oncology, Azienda Sanitaria dell’Alto Adige, Bolzano, Italy
- 8Medical Oncology, Santa Chiara Hospital, Trento, Italy
Small cell lung cancer (SCLC) represents about 13%–15% of all lung cancers. It has a particularly unfavorable prognosis and in about 70% of cases occurs in the advanced stage (extended disease). Three phase III studies tested the combination of immunotherapy (atezolizumab, durvalumab with or without tremelimumab, and pembrolizumab) with double platinum chemotherapy, with practice-changing results. However, despite the high tumor mutational load and the chronic pro-inflammatory state induced by prolonged exposure to cigarette smoke, the benefit observed with immunotherapy is very modest and most patients experience disease recurrence. Unfortunately, biological, clinical, or molecular factors that can predict this risk have not yet been identified. Thanks to these clinically meaningful steps forward, SCLC is no longer considered an “orphan” disease. Innovative treatment strategies and combinations are currently under investigation to further improve the expected prognosis of patients with SCLC. Following the recent therapeutic innovations, we have reviewed the available literature data about SCLC management, with a focus on current unmet needs and potential predictive factors. In detail, the role of radiotherapy; fragile populations, such as elderly or low-performance status patients (ECOG PS 2), usually excluded from randomized studies; predictive factors of response useful to optimize and guide therapeutic choices; and new molecular targets and future combinations have been explored and revised.
1 Introduction and the State of the Art
Small cell lung cancer (SCLC) accounts for about 13%–15% of all new lung cancer diagnoses. About 70% of SCLC are diagnosed at an advanced stage (1). Platinum-based chemotherapy is the standard of care for both limited disease (LD) and extensive disease (ED). Although this treatment favors survival and disease control, most patients relapse, and overall survival (OS) reaches a maximum of 2 years in 21% and 7% of LD and ED, respectively (2). However, the advent of ICIs (immune checkpoint inhibitors), including PD-1 (inhibitors of programmed cell death protein 1) and PD-L1 (programmed death-ligand 1), in the therapeutic landscape of this aggressive tumor started to change the outcome of patients with ED-SCLC.
The following review reports the state of the art, as well as recent data with immunotherapy in SCLC treatment, with a focus on unmet needs and potential predictive factors.
2 The Role of ICIs in SCLC Treatment
2.1 Biological Rationale
It has been hypothesized that genomic instability due to the expression of two defective tumor suppressor genes (TP53 and RB1), thus perpetuating the generation of tumor-associated antigens (3) and the long-term exposure to smoke, thus inducing smoking signatures (4), makes SCLC one of the tumors with the highest tumor mutational burden (TMB) but low immunogenicity (SCLC has low MHC I expression levels, and its mutation products is difficult to be recognized by CD8 T-cell receptor). Furthermore, chemotherapy may induce immunogenic cell death that results in prompt release of pro-inflammatory cytokines and tumor antigens in the tumor microenvironment (TME), thus enhancing tumor immunogenicity (5). Although SCLC appears morphologically homogeneous, the latest data from murine models and human tumors indicate the existence of SCLC subtypes, classified on differential expression of these transcription factors: ASCL1, NEUROD1, POU2F3, or YAP1 with different therapeutic vulnerabilities (6). Among them, the SCLC-inflamed tumor, characterized by overexpression of immune genes such as those of the STING pathway, showed better survival with chemoimmunotherapy than other subtypes (7). However, although a phenotype characterized by high immune cell infiltration in TME showed a prognostic value in SCLC (8), it was not associated with other well-known candidate immune-biomarkers such as PD-L1 or TMB or with tumor response in patients treated with immunotherapy (9). Thus, by trying to understand the immune microenvironment, we get to know better the immunobiology of SCLC. Identifying the predictive biomarkers of response to immunotherapy in patients with SCLC and determining the strategies to overcome resistance to ICIs are future challenges.
2.2 Update on Treatment Options for Limited-Stage Disease
Limited-stage SCLC (LS-SCLC), meaning a tumor limited in one hemithorax and feasible radiation field, accounts for about 40% of SCLC (<5% SCLC in early stages). The role of surgery is still controversial even in early-stage SCLC, where surgery may be considered within a multimodal approach in very selected patients (10). As reported in a Cochrane systematic review published in 2017, the randomized controlled trials (RCTs) did not demonstrate a clear benefit from surgical resection in SCLC stage I–III (11). Although multiple retrospective and observational studies demonstrated the advantage of surgery for local control in the early stage I–IIA of the disease (12), the indication of surgery plus chemotherapy remains controversial. Therefore, according to the ESMO guidelines, surgery should be taken as a treatment option in patients with clinical stages I and II (cT1-2N0) and in those suspected cases with mixed SCLC histology and non-small cell lung cancer (NSCLC) (10). Otherwise, the current standard of care in SCLC of limited stage (stage I–III) consists of thoracic radiation (45 Gy in 30 fractions twice daily) plus platinum-etoposide (PE) chemotherapy (13). The advantage of this treatment is that it can be applied at full dose in patients during treatment with concomitant chemoradiotherapy (CRT) with a favorable toxicity profile. If patients are not suitable for cisplatin, carboplatin-etoposide is another treatment choice (14).
An increasing median OS was observed across CRT trials in LD-SCLC, due to technological advances and dose fractionation of radiotherapy (13). More recent trials exploring higher radiation dose schemes report even better survival outcomes, with a median OS of 37–39 months (15, 16).
However, a 70% risk of recurrence at 5 years was reported in the best-case scenarios, and maintenance or consolidation therapy strategies did not achieve a significant survival benefit (17).
Although studies are still ongoing, beneficial effects are speculated from the introduction of immunotherapy plus chemoradiation in both therapy choices, either concurrent or consolidative. The advantage of combining immunotherapy with CRT has been shown in different preclinical studies; radiotherapy used for the treatment of a primary tumor may cause the release of tumor antigens followed by a tumor-specific immune response, which is intensified by immune-stimulating elements (18). According to the abscopal effect, while radiotherapy causes a local tumor response at a targeted site, it may also cause a tumor response in non-targeted sites (metastatic disease).
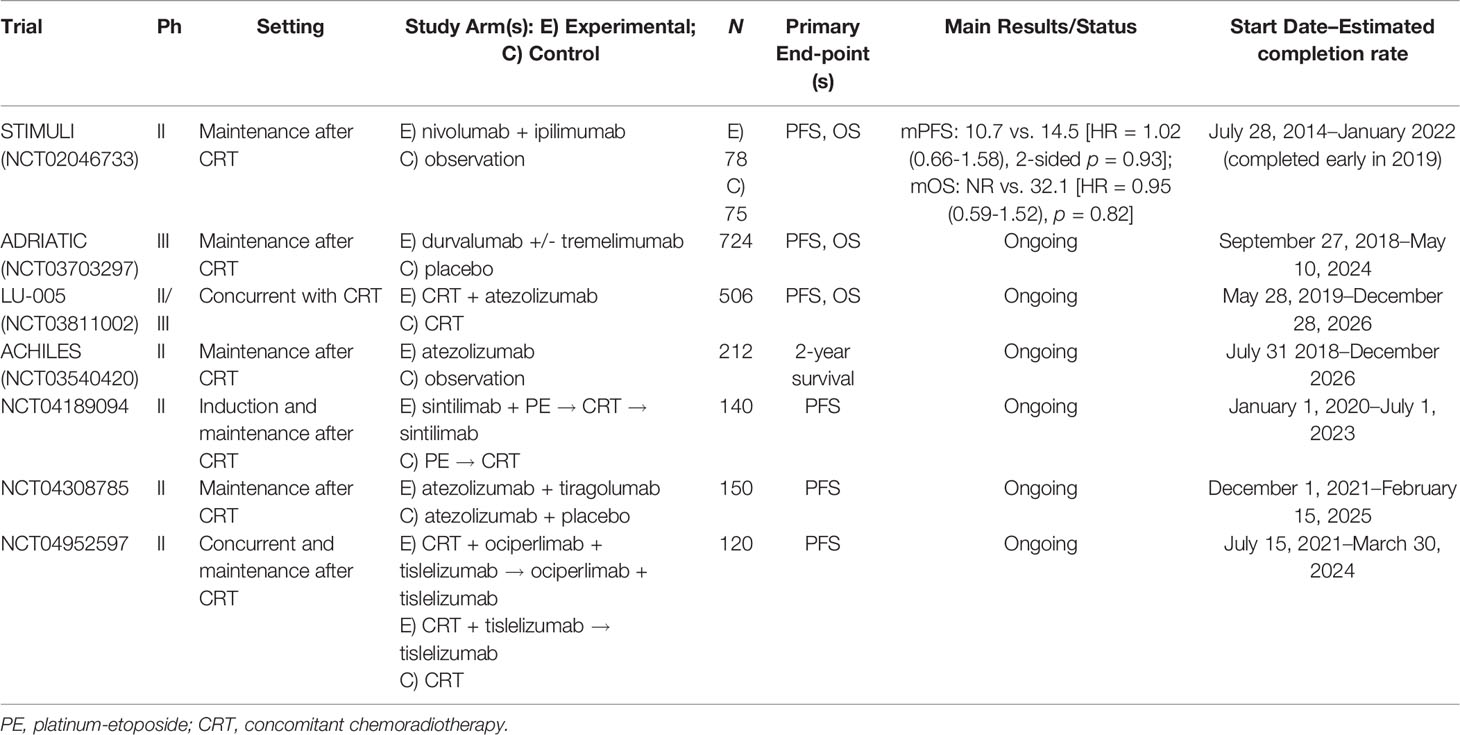
We have analyzed four randomized trials, studying the concomitant therapy: immunotherapy plus CRT in LS-SCLC (Table 1). STIMULI (NCT02046733) is a phase II trial that studied the efficacy and tolerability of consolidation of nivolumab and ipilimumab for four cycles followed by nivolumab for 1 year versus observation after chemoradiation therapy and prophylactic cranial irradiation (PCI) in LS-SCLC (19). The statistical analysis plan considered PFS as the only primary endpoint. In total, 153 patients were randomized, and after a follow-up of 22.4 months, the trial confirmed that there were no benefits in PFS or OS with the addition of nivolumab and ipilimumab (19). Furthermore, 50% of patients included in the experimental arm were unable to receive the full course of immunotherapy due to its toxicity. However, it was outlined how biobanking will be used to investigate hematological profiles and other biomarkers to define a group of patients that may benefit from the addition of immunotherapy to standard CRT. This study began in July 2014 but was terminated early in 2019 due to slow accrual. ADRIATIC (NCT03703297) is a phase III study that evaluates the efficacy of durvalumab or durvalumab plus tremelimumab compared to placebo for consolidation in patients with LS-SCLC who have not progressed after concomitant CRT (20). PFS and OS are the primary endpoints. The study started in September 2018 and will end in May 2024. LU-005 (NCT03811002) is a phase II/III trial that studies CRT compared to atezolizumab plus CRT (21). PFS is the primary endpoint of phase II and OS is the primary endpoint of phase III. Atezolizumab is administered every 3 weeks in association with radiotherapy up to 12 months in total. The stratification variables are performance status (PS 0/1 vs. 2), sex, use of chemotherapy (cisplatin vs. carboplatin), and radiation fractionation (twice daily at 45 Gy vs. once daily at 66 Gy). PCI (25 Gy in 10 fractions) is recommended in patients with a complete or almost complete response to therapy. This study opened to accrual in May 2019 and will end in December 2026. Moreover, ACHILES (NCT03540420), a phase II randomized trial comparing atezolizumab vs. observation after concurrent CRT (primary end-point is a 2-year OS rate) (22), and the NCT04189094, a phase II trial evaluating the role of adding sintilimab, an antiPD1 antibody, to chemoradiotherapy in LD-SCLC, are still ongoing. Interestingly, current ongoing trials are evaluating the combination of anti-PD1/anti-PDL1 and anti-TIGIT. In this light, the NCT04308785 represents a phase II study concentrated on the efficacy and safety of atezolizumab associated or not with tiragolumab (anti-TIGIT) as consolidation therapy in LD-SCLC patients who have not progressed during/after CRT (23), while NCT04952597 phase II trial examines the combination of ociperlimab plus tislelizumab plus concomitant CRT.
2.3 Novel Treatment Options for Extended Disease
Although current SCLC treatment remains “one size fit all”, promising results were reported in the recent phase III studies including immunotherapy, which led to regulatory drug agency approval of immuno-including regimens in the first-line setting.
2.3.1 First-Line Treatment
Before the arrival of ICIs, chemotherapy with PE was considered the frontline SoC regimen for ED-SCLC for almost 30 years (24). With this regimen, ORR reached 60%–80% but responses were transient (PFS 3–6 months) and the median OS was limited (8–10 months). Recently, three phase III trials have tested the combination of ICIs (atezolizumab, durvalumab +/- tremelimumab, and pembrolizumab) with chemotherapy as first-line setting. Overall efficacy and toxicity were comparable across the studies, whereas the percentages of included patients with brain metastases or treated with PCI were different. In general, ICI introduction in the treatment landscape of SCLC represents an important and well-accepted step forward in the therapeutic strategy of ED-SCLC (Table 2).
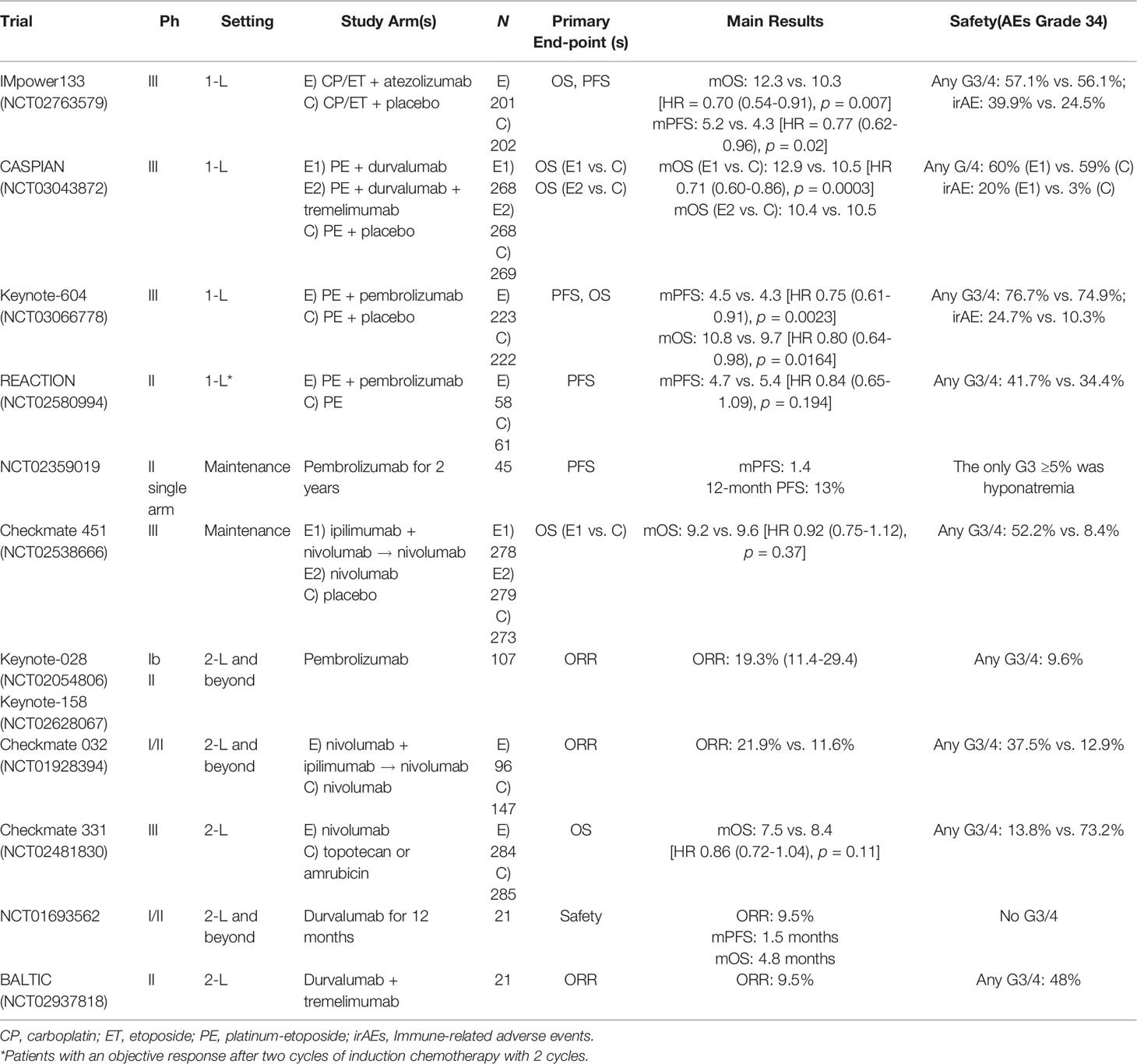
The IMpower133 trial evaluated the efficacy of adding the PD-L1 inhibitor atezolizumab to the standard carboplatin-etoposide in 403 naive patients with ED-SCLC, considering as stratifying factors sex, ECOG PS, and the presence of brain metastases (25, 26). After four cycles of treatment, PCI was included during the atezolizumab/placebo maintenance period; meanwhile, consolidation thoracic radiation was not considered. Primary endpoints were reached, with an important reduction of 30% and 23% risk of death and progression, respectively, in patients treated with atezolizumab. Median OS was 12.3 months and 10.3 months, respectively, for experimental and placebo arm (HR: 0.70; 95% CI: 0.54–0.91, p = 0.007); PFS was 5.2 months and 4.3 months, respectively, for atezolizumab and control arm (HR: 0.77; 95% CI: 0.62–0.96, p = 0.02) (26). One-year OS rate was 51.7% and 38.2% in patients undergoing chemotherapy plus atezolizumab vs. chemotherapy alone, respectively, regardless of PD-L1 expression and blood tumor molecular burden. According to these results, the combination of carboplatin, etoposide, and atezolizumab is considered as the new standard treatment for ED-SCLC in the first-line setting.
The CASPIAN trial tested the efficacy of the PD-L1 inhibitor durvalumab +/- CTLA-4 inhibitor tremelimumab, in combination with standard PE in 805 ED-SCLC naïve patients (27, 28). The control group was represented by PE alone for up to six cycles. In this trial, PCI was allowed in the control arm following chemotherapy at the investigator’s discretion, but it was not allowed in the immunotherapy groups before discontinuation of all study treatments. The co-primary endpoint was OS for durvalumab platinum-etoposide compared to chemotherapy, and for durvalumab/tremelimumab plus platinum-etoposide compared to chemotherapy. At the updated median follow-up after >3 years, combining durvalumab with platinum-etoposide significantly improved OS over only chemotherapy (12.9 vs. 10.5 months; HR: 0.71; 95% CI: 0.60–0.86; p = 0.0003) (27). Although the combination of durvalumab/tremelimumab plus PE numerically improved OS vs. PE, it was not statistically significant. In consideration of these results, durvalumab plus cisplatin or carboplatin etoposide has been approved by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) as first-line treatment in patients with ED-SCLC.
The KEYNOTE 604 trial investigated the efficacy of the PD-1 inhibitor pembrolizumab plus PE vs. chemotherapy alone in 453 ED-SCLC naive patients (29). One of its primary endpoints showed an important PFS improvement by adding pembrolizumab to chemotherapy (HR: 0.75; 95% CI: 0.61–0.91), also a prolonged OS (10.8 vs. 9.7 months); the pre-specified significance threshold was not reached (HR: 0.80; 95% CI: 0.64–0.98; p = 0.0164).
More recently, the REACTION trial randomized patients, with a response after two cycles of chemotherapy, to be treated with pembrolizumab in combination with chemotherapy or chemotherapy alone (30). The primary PFS endpoint was not reached (4.7 vs. 5.4 months, HR: 0.84; 80% CI: 0.65–1.09, p = 0.194). However, a statistically significant OS improvement (12.3 vs. 10.4 months, HR 0.73; 80% CI: 0.54–1.00) was reported.
Overall, a grade 3 or higher toxicity rate was observed during immune-chemotherapy combinations compared to chemotherapy alone, although an expected increase in immune-related AEs was reported in the experimental arms. However, data on long-term or deterioration in quality of life are limited.
2.3.2 Maintenance Therapy With ICIs
The efficacy of ICIs as a maintenance strategy in ED-SCLC is still controversial. In a phase two study, 8 weeks after the last cycle of PE chemotherapy, pembrolizumab was started as maintenance therapy for up to 2 years (31). Although both median PFS and OS were not significantly improved by pembrolizumab, the 1-year PFS and OS rate were 13% and 37%, respectively, showing that a subgroup of patients could have a clinical benefit. However, none of the biomarkers analyzed, including PD-L1, were predictive of a better response to immunotherapy.
As expected by previous studies with anti-CTLA4 ipilimumab (32) in combination with chemotherapy in ED-SCLC, no significant benefit was reported in a phase III study of immunotherapy doublet with the anti-PD-L1, nivolumab, and the anti-CTLA4, ipilimumab, as maintenance therapy for ED-SCLC (33). A total of 834 patients enrolled in the Checkmate 451 trial did not progress after receiving four cycles of platinum-based chemotherapy. These patients were randomized to receive immunotherapy with nivolumab and ipilimumab, nivolumab alone, or placebo for 2 years. The OS did not improve with nivolumab plus ipilimumab vs. placebo (HR: 0.92; 95% CI: 0.75–1.12; p = 0.3693) or with nivolumab vs. placebo (HR: 0.84; 95% CI: 0.69–1.02), but still there was a modest improvement in PFS with ipilimumab plus nivolumab (HR: 0.72; 95% CI: 0.60–0.87) and nivolumab (HR: 0.67; 95% CI: 0.56–0.81) compared to placebo.
Finally, the addition of an anti-PD-L1 therapy (atezolizumab or durvalumab) to the standard platinum-etoposide chemotherapy, and then keeping immunotherapy as maintenance, improved both PFS and OS (25, 28). On the other hand, the use of an anti-PD1 therapy (pembrolizumab) for the same purpose showed a similar benefit that was instead statistically significant only for PFS (29). According to previous studies in other tumors (32), although the overall benefit is only about 2 months in the extension of median survival, there are potential advantages for long-term survivors, looking at the tail of survival curves. In fact, the 2-year survival rate increased from 11% to 22%, suggesting that some patients with SCLC have a significant benefit with immunotherapy, but useful biomarkers for their a priori identification are still lacking.
2.3.3 Second-Line Treatment and Beyond
Unfortunately, most patients relapse within 6 months after first-line chemotherapy. The second-line treatment response rates depend on the treatment-free interval (TFI) and are approximately 20%–30% in platinum-sensitive patients (TFI ≥3 months) and 15% in platinum-resistant patients (TFI <3 months). According to clinical guidelines, the two possible second-line options for patients with ED-SCLC who progressed after platinum-based first-line chemotherapy are the topoisomerase I inhibitor topotecan and anthracycline-based regimes, including cyclophosphamide plus doxorubicin and vincristine (CAV) (10). The latest option was commonly used before a randomized trial with topotecan vs. CAV, which showed similar outcomes in both treatment arms, but intravenous topotecan showed better tolerability (34), resulting in the preferred standard of care nowadays. However, in platinum-sensitive patients, a rechallenge with PE should be also considered as a reasonable second-line option. In fact, a phase III trial recently showed that carboplatin plus etoposide had a significant improvement in PFS compared to topotecan (4.7 vs. 2.7 months, HR: 0.57; 95% CI 0.41–0.73; p = 0.0041), with a similar safety profile (35). In the ESMO therapeutic algorithm, lurbinectidin (selective inhibitor of RNA polymerase II) was also introduced as an alternative option for recurrent SCLC (10). This drug was recently approved by the FDA, according to the results of a phase II single-arm trial (NCT02454972), in which the single-agent lurbinectedin showed significant activity as second-line therapy. Overall, patients reported an ORR equal to 35.2% (22.2% in platinum-resistant and 45% in platinum-sensitive patients), a median duration of response up to 5.3 months (36), and a median OS of 9.3 months, with a manageable safety profile (37). Meanwhile, the combination of lurbinectedin plus doxorubicin explored in the phase III trial ATLANTIS (NCT02566993) vs. investigator’s treatment choice (topotecan or CAV: cyclophosphamide, doxorubicin, vincristine) did not improve the prespecified endpoint of OS (38).
Promising preliminary antitumor activity and a good safety profile were shown with ICIs in patients progressed after standard first-line chemotherapy. The administration of pembrolizumab 200 mg every 3 weeks, as the standard dose, was tested in a phase Ib (Keynote-028) and a phase II (Keynote-158) trial in different tumor types, including SCLC. Overall, an ORR (primary endpoint) of 19.3% was reported regardless of PD-L1 expression. On this basis, in June 2019, the FDA accelerated the approval of pembrolizumab for the treatment of metastatic SCLC patients with disease progression after receiving platinum-based chemotherapy and at least one other prior line of therapy.
The use of nivolumab in SCLC pretreated patients has been evaluated in the phase I/II Checkmate 032 (39) and in the phase III Checkmate 331 (40) clinical trials. The first one is a basket trial that studied the activity of nivolumab alone and nivolumab plus ipilimumab in different tumors including metastatic SCLC. Overall, an objective response of 10% was observed in patients treated with nivolumab alone, 23% in those treated with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, and 19% in those treated with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg. Like pembrolizumab studies, the response rate was not related to PD-L1 status. Further analysis after 18 months of follow-up showed an ORR of 11% in patients treated with nivolumab alone and of 25% in patients treated with nivolumab plus ipilimumab (41). These early results, in August 2018, led the FDA to accelerate the approval of nivolumab for pretreated SCLC patients. Recently, results of the expansion cohort of patients randomized to nivolumab vs. nivolumab plus ipilimumab were published, reporting an ORR of 11.6% in the group with nivolumab alone, and 21.9% in the combination group that experienced more frequent G3–G4 adverse events (12.9% and 37.5% in the nivolumab and nivolumab + ipilimumab group, respectively) and four deaths due to toxicity (autoimmune-related hepatitis, pneumonitis and encephalitis, and autoimmune colitis).
The second study, Checkmate 331, compared nivolumab vs. chemotherapy with topotecan or amrubicin as second-line treatment (42). Patients were grouped as platinum responders and non-responders. Although this trial did not reach its primary endpoint [median OS was 7.5 vs. 8.4 months in the nivolumab and chemotherapy arm (HR: 0.86; CI: 95%: 0.72–1.04)], the HR for OS in patients who did not respond to cisplatin was 0.71 (95% CI: 0.54–0.94). Additionally, the nivolumab group reported 55% of all grade AE vs. 90% in the chemotherapy arm (40).
Durvalumab was reported to have similar results, received every 2 weeks at a dose of 10 mg/kg, in a phase I/II study that included 21 patients with pretreated ES-SCLC disease (43). Patients were treated for up to 1 year and reported a median OS of 4.8 months, PFS of 1.5 months, and a 1-year OS rate of 27.6%. In addition, an ORR of 9.5% was recently observed with durvalumab + tremelimumab in preliminary analysis of the phase II BALTIC study (NCT02937818) (44).
The anti-PD-L1 atezolizumab as single therapy did not show significant results in pretreated patients vs. topotecan (up to six cycles) or re-induction chemotherapy in the randomized phase II IFCT-1603 study, which included 73 patients with ES-SCLC disease after failure of first-line PE-basing chemotherapy (45).
Overall, the potential use of ICIs in the second-line setting still requires further evidence. Furthermore, considering that immunotherapy is currently included in the first-line standard of care approach, it must be taken into consideration the lack of data on the role of ICIs rechallenge in patients whose disease has progressed after first-line immune-based treatment.
3 Open Issues
3.1 Radiotherapy: The Role of Consolidation Treatment and Prophylactic Cranial Irradiation
Thoracic radiotherapy (TRT) combined with chemotherapy is the standard treatment in patients with limited disease. In ED-SCLC, the importance of consolidation of TRT in patients with a good response to first-line treatment has become increasingly recognized (46). The ASTRO guidelines conditionally recommend thoracic radiotherapy to 30 Gy in 10 fractions within 6 to 8 weeks of chemotherapy completion and before maintenance immunotherapy, in patients with ED-SCLC who respond to chemotherapy and immunotherapy, and in case of residual disease in the thorax. At the ASCO 2021, SBRT was suggested to be applied more frequently in early-stage SCLC patients not eligible for resection, or who refuse surgery. Additionally, retrospective data suggest that this strategy is likely safe and effective. Therefore, ASTRO guidelines have recently incorporated SBRT as an acceptable treatment option for early-stage, node-negative, and medically inoperable SCLC.
Prophylactic cranial irradiation (PCI) is still controversial, following the publication of a Japanese randomized phase III trial that found that magnetic resonance imaging (MRI) surveillance could replace PCI for extensive-stage disease (47). In this trial, patients with ED-SCLC who responded to platinum-based doublet chemotherapy and with no brain metastases on MRI were randomly assigned (1:1) to receive PCI (25 Gy in 10 daily fractions of 2.5 Gy) or observation. The primary endpoint was OS. All patients underwent brain MRI every 3 months in a 12-month period followed by another brain MRI at 18 and 24 months after enrolment. The study showed that there was no improvement in OS with PCI therapy compared to observation (11.6 vs. 13.7 months, HR = 1.27, 95% CI 0.96–1.68, p = 0.094), concluding that PCI is not essential for ED-SCLC responders to initial chemotherapy, without evidence of brain metastases (48).
However, due to the incidence of brain metastases at diagnosis (about 18% of cases of ED-SCLC, which increase to 80% at 2 years), PCI is still recommended in patients who respond to treatment in both LD and ED-SCLC (49). However, active surveillance with a brain MRI every 12 weeks seems to be an acceptable option, especially to preserve patients’ quality of life (48).
Although consistent data are not available for SCLC in the immunotherapy era, the safety and efficacy data obtained in NSCLC about the integration of ICIs and radiotherapy may support the feasibility of this approach. In this light, it will be crucial to better define the potential (positive and negative) synergy between local and systemic therapy in both LD and ED-SCLC, similar to what is recognized in stage III NSCLC and in the oligometastatic/oligoprogressive setting. The patient’s condition, stage, and characteristics of the disease, response to therapy, dosage and schedule of TRT/PCI, as well as the future availability of new drugs or combinations may influence the decision-making process in SCLC. In conclusion, the integration of thoracic radiotherapy in patients with ED-SCLC who after chemotherapy have persistent intrathoracic disease, as well as the role of PCI and immunotherapy in the metastatic setting, remains an important unanswered question, prioritizing the need for ad hoc trials.
3.2 Frail Population
Although etoposide plus carboplatin was accepted as a tolerable and equivalent regimen in terms of efficacy compared to etoposide and cisplatin, a review of the Alberta Cancer Registry showed that 32% of elderly patients (age 75+) were not treated with chemotherapy (50). Moreover, the randomized phase III trials with ICIs enrolled patients with a median age ≤70 years; they included only patients with good performance status (PS, 0-1) (23, 25, 27). In contrast, in real life, there are more and more cases of elderly patients with median age ≥70 years (51). Overall, 52% of the patients treated with chemotherapy completed all cycles and 34% of them underwent at least one dose reduction. Patients who completed all cycles with a dose reduction had a lower risk of death of 1.02 (95% CI: 0.57–1.82) compared to a risk of death of 2.72 (95% CI: 1.52–4.87) for patients who did not complete therapy. Furthermore, phase II studies aimed to point out that carboplatin and etoposide dose modifications in the elderly reported similar survival benefits versus standard doses (52). Therefore, elderly patients should receive standard treatment, but they may also require dose modifications.
Recently, NSCLC studies showed that immunosenescence, defined as the gradual deterioration of the immune system caused by natural advances in age, seems to be related to decreased efficacy of ICIs, regardless of the age (53). However, survival data from phase III clinical trials with ICIs are controversial in SCLC elderly population. In the KEYNOTE 604, similar magnitudes of survival benefit were reported with the use of pembrolizumab despite the patient’s age (29). In contrast, in the CASPIAN trial, durvalumab was significantly effective in patients aged <65 years [HR 0.72, (95% CI: 0.56–0.92)] but not in patients aged ≥65 years [HR 0.84 (95% CI: 0.62-1.12)] (27), whereas the anti-PD-L1, atezolizumab, in the IMpower133 trial seemed to be more effective in patients aged ≥65 years [HR 0.53 (95% CI: 0.36–0.77)] than patients aged <65 years [HR 0.92 (95%: 0.64–1.32)]
In real-world data, up to 60% of elderly patients have a PS equal to 2, resulting in worse survival (54). Furthermore, they were generally also affected by at least two chronic comorbidities, followed by a higher probability of exposure to polypharmacy, which can affect the efficacy of ICIs (55). In this scenario, the REACTION trial hypothesized an interesting strategy. In this phase II trial, there were randomized patients with complete or partial response after two cycles of PE induction. In this study, 5% of the patients enrolled had an ECOG PS 2, but those patients who upgraded to PS 1 or 0 with treatment benefit were eligible for the immune-chemotherapy strategy (30). Finally, a large sample of PS 2 patients will be enrolled in the ongoing phase II SPACE trial (NCT04221529) (56) and in the phase III MAURIS trial (NCT04028050) (23), which may help to clarify whether these patients benefit or not from the addition of ICI to chemotherapy.
In terms of brain radiotherapy, the role of PCI in elderly patients is controversial. Indeed, even if PCI improved the OS in patients aged ≥70 years, it was not significantly effective among patients aged ≥80 years with SCLC (57). This finding suggests that in this group of patients, a shared decision process is necessary rather than proposing an overtreatment. Less is known in patients with ECOG-PS = 2 and those with a history of neurological conditions, such as stroke or epilepsy. Retrospective analyses have shown that PCI improves survival compared to no PCI, but its correlation with increased neurocognitive dysfunction has limited its use (57–59). In fact, a comparison of the results of cognitive tests in two RTOG trials that evaluated PCI in patients with LS-SCLC showed higher rates of cognitive decline with advanced age (60). Modern radiation techniques, hippocampal sparing, and memantine may minimize the occurrence of cognitive decline.
Geriatric Assessment Tools Geriatric oncology addresses the right approach to the care of this category of patients through the development of geriatric assessment tools to help define risks and benefits. Investigators and treating physicians are encouraged to include these tools in their clinical trials and daily practice (46). In general, clinical trials and trials that address the unique needs of the elderly are strongly recommended.
3.3 Potential Predictive Factors
SCLC is a highly aggressive tumor with still very poor prognosis marked by a very high proliferative rate and an early spread of metastasis. Moreover, despite the promising results, the magnitude of benefit with ICIs in SCLC is different from what was reported in NSCLC. Although SCLC has a high TMB, its immunosuppressive pattern in the stroma, the lack of antigen presentation, and the low expression of PD-L1 suggest a less immunogenic T-cell profile in SCLC compared to NSCLC (36, 39, 61). Similarly, a multiplexed quantitative immunofluorescence analysis in SCLC samples showed significantly lower levels of all TIL markers, MHC class II expression, and CD8+ T cells compared to NSCLC (62). However, high immune activity was reported in patients with SCLC and paraneoplastic syndromes, resulting in a better prognosis compared to patients without these syndromes (63).
Moreover, a clear therapeutic algorithm and consolidated data in special populations, like the elderly or patients with an ECOG PS ≥ 2, are still unavailable. Therefore, the identification of potential predictive factors of response to better guide the physician’s choice is awaited.
3.3.1 Molecular Factors
3.3.1.1 Gene Expression Profile
The genomic profile of SCLC shows extensive chromosomal rearrangements and a high TMB. Moreover, the dual inactivation of the tumor suppressors TP53 and RB1 is found in most cases with SCLC (3). Sequencing analysis on both DNA and RNA of larger cohorts of primary tumors as well as CTC-derived xenograft models confirmed this result. Furthermore, the amplification of genes from the MYC family (MYC, MYCL, and MYCN), FGFR1 (encoding fibroblast growth factor receptor 1), and GNAS (encoding the α-subunit of the heterotrimeric G protein Gs) was also well described (64). Moreover, alterations in the PTEN pathway and overexpression of BCL-2 could interfere with the promotion of cell growth, proliferation, and survival in SCLC. Relapsed tumors are more frequently characterized by WNT pathway alterations, thus supposing a role for WNT signaling in chemo-resistant SCLC (65), and the heterogeneity of SCLC tumors may explain an important mechanism by which SCLC tumors evade treatment; additionally, heterogeneity itself is increased in response to treatment (66). The lineage plasticity of SCLC cells could be explained by the high levels of the stem cell transcription factor SOX2 downstream of p53 and RB loss, or as a consequence of genomic amplification. Moreover, mutations in chromatin modifiers are frequent in SCLC, suggesting that alterations in epigenetic regulation may also contribute to cell fate changes. However, better understanding the TME and the molecular mechanisms underlying SCLC tumorigenesis, progression, metastasis, and response to treatment is still a challenge. Recently, some researchers have developed the first comprehensive framework to classify SCLC into four subtypes based on gene expression (6). This classification depends on the relative expression of dominant transcriptional regulators and on the substantial intra-tumoral heterogeneity that could explain the main aspects of tumor evolution, metastasis, and acquired therapeutic resistance, as well as potential targeted therapeutic strategies (64).
The first three groups are characterized by activation of the ASCL1 (SCLC-A), NEUROD1 (SCLC-N), and POU2F3 (SCLC-P) genes, while the SCLC-I subtype is characterized by an inflamed gene signature with high expression of multiple immune genes, including significantly higher levels of genes indicating the presence of CD8-positive cytotoxic T cells (7). The research team first identified the four groups by applying non-negative matrix factorization to 81 SCLC patients with surgically resected tumors. The data from 276 SCLC patients enrolled in the phase III IMpower133 clinical trial were then analyzed to validate the four subtypes in the advanced stage. This study showed that SCLC-I was the most sensitive to immune checkpoint blockade, SCLC-A was the most sensitive to BCL2 inhibitors, SCLC-N was the most sensitive to Aurora kinase inhibitors (overall, more effective in those SCLC with increased MYCL expression) (67), and SCLC-P was the most sensitive to PARP inhibitors, thus suggesting different classes of drugs for different specific subtypes. This study described the subtype “switching” to resistance in a series of patient-derived SCLC models. Data from a mouse model also suggest that SCLC-A tends to switch to SCLC-I after being treated with chemotherapy, which could be correlated with resistance to treatment (7). Since SCLC is about 15 years behind NSCLC, in terms of developments in the field of biomarkers and personalized therapies, this emerging molecular classification represents the first step in a better understanding of the molecular pathway involved in SCLC and the choice of the best drugs for each patient, thus moving towards personalized approaches for the cure of the rare and aggressive SCLC tumor.
3.3.1.2 Liquid Biopsy
A pressing issue in the SCLC field has been the small quantity of material to be used for histological diagnosis and subsequent research. Therefore, isolating circulating tumor cells (CTCs) from the blood of SCLC patients could overcome this problem (68). However, we are still far from adequate clinical trials that concentrate on tumor material collection to identify key genetic drivers of SCLC, and while liquid biopsies may represent an important factor for exploring ICI-resistance mechanisms in SCLC, this technique itself needs more evaluation.
3.3.2 Immunological Factors
Exploratory biomarker analysis of principal phase III clinical trials showed that PD-L1 expression is not correlated to immunotherapy benefit in SCLC patients. The importance of TMB is more controversial, which seems to be predictive of nivolumab-ipilimumab benefit as the Checkmate-032 analysis suggests (69) but not predictive of atezolizumab benefit in the IMPOWER133 blood-based analysis (25). Similarly, in the CASPIAN trial, durvalumab plus chemotherapy resulted in improved OS compared to chemotherapy alone regardless of PD-L1 and TMB expression (28). Also, in the KEYNOTE 604 trial, both PFS and OS improved with the addition of pembrolizumab to chemotherapy, regardless of the combined positive PD-L1 expression score (29). Therefore, we cannot consider PD-L1 or TMB to be good predictive factors of response to immunotherapy in SCLC, at least so far.
3.3.3 Clinical Factors
According to their different microenvironments, brain metastasis and liver metastasis deserve to be mentioned as potential predictive factors of response to immune-based chemotherapy.
Indeed, the brain metastases showed an active immune microenvironment with a PD-L1 expression of 75% in SCLC samples. However, the percentage of patients with baseline brain metastases included in phase II/III clinical trials ranged from 9% to 14.2% in the immunochemotherapy arms (25, 27, 29, 30). Moreover, all trials, except the CASPIAN trial, included only asymptomatic and treated brain metastases. Thus, the limited sample size and the limited benefit in survival by adding immunotherapy to chemotherapy do not allow conclusive results. The presence of liver metastases should be considered a negative predictive factor. In particular, in the three phase III trials, anti-PD-L1 addiction to chemotherapy did not improve survival results compared to chemotherapy alone. Accordingly, in NSCLC, the occurrence of liver metastases was associated with an immune-suppressive phenotype characterized by fewer infiltrating CD8+ T-cell densities at the invasive margin in distant tumors (66) and limited immunotherapy efficacy by macrophage-mediated elimination of T cells (70). These data support the hypothesis that there is a lack of a synergistic effect of immunochemotherapy in SCLC patients affected by liver metastases.
4 New Targets and Future Perspectives
Despite the high potential immunogenicity of SCLC, the magnitude of benefit with ICIs in SCLC is not the same as that reported in NSCLC patients. Different immunophenotypes, as well as the TMEs of SCLC compared with NSCLC, may explain the different efficacy of ICIs in these two diseases (61).
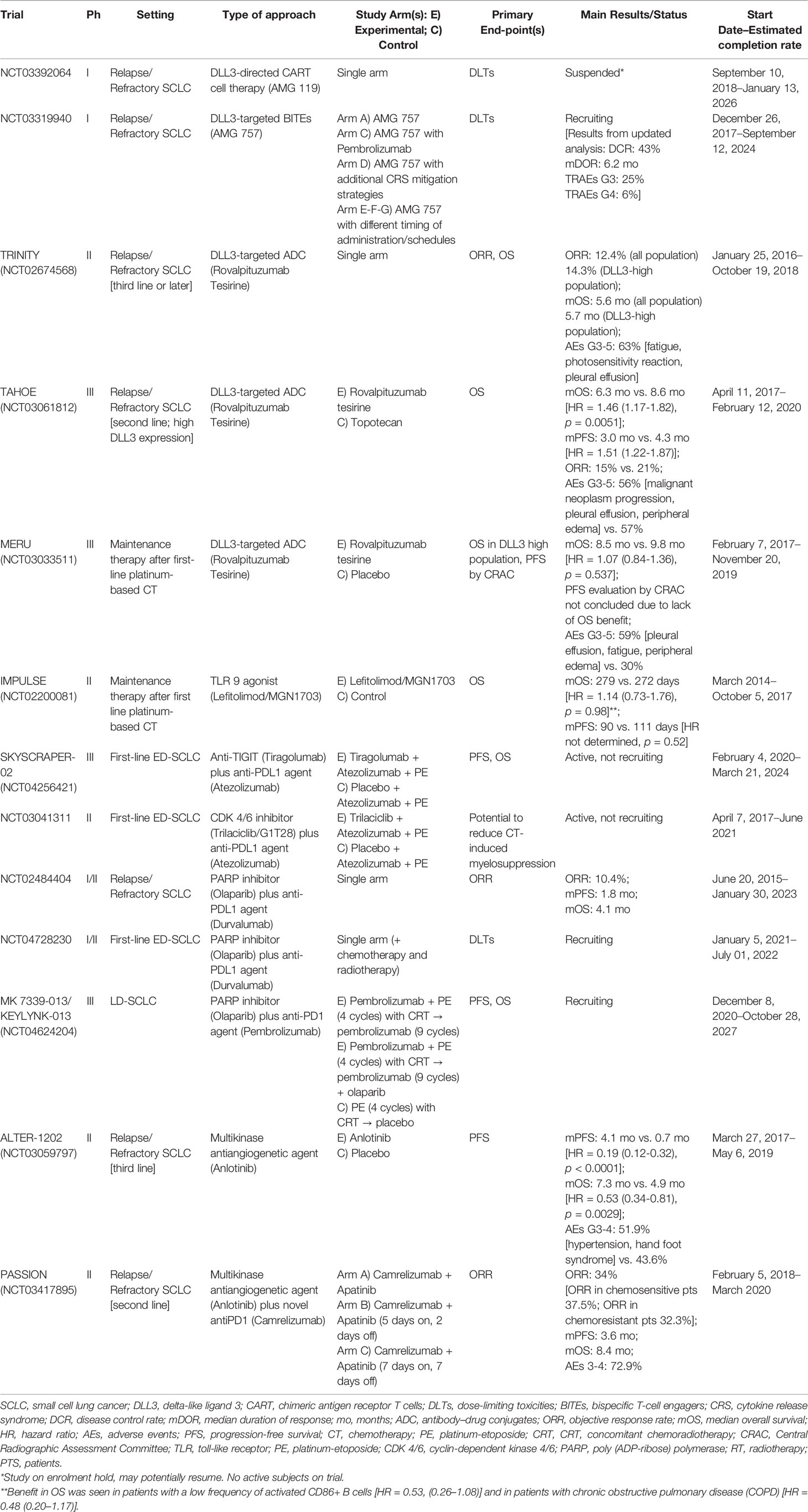
Recently, other immunotherapeutic approaches, used alone or in combination with ICIs, are being explored to improve the immune response in SCLC patients. These include chimeric antigen receptor (CAR) T-cell therapy, bispecific T-cell engagers (BiTEs), antibody–drug conjugates (ADC), and immunomodulators. Multiple cell surface molecules, including CD56, CD47, and delta-like ligand 3 (DLL3), have an important expression in SCLC, thus emerging as potential therapeutic targets of CART therapy (71–73) (Table 3).
T cell-based therapy is an MHC-independent therapeutic option, where chimeric antigen receptors are recombinant receptors for tumor-specific antigens, engineered into T cells to allow expression, expansion, and antitumor specificity (74).
AMG 119, a DLL3-directed CART cell therapy, showed a potent antitumor response in preclinical models (75) and is being studied in an ongoing phase I trial that includes patients with advanced SCLC in progression after receiving at least one platinum-based regimen (NCT03392064). Unlike CART, BiTEs are recombinant bispecific proteins that simultaneously target a T-cell surface molecule (such as CD3) and a tumor-specific surface antigen, facilitating both T-cell adherence and antitumor response independent of MHC (76).
Preclinical studies showed that the DLL3-targeted BITEs AMG 757 demonstrated a potent and specific killing activity in SCLC cell lines as well as orthotopic and patient-derived xenograft (PDX) mouse models with DLL3 expression, by inducing T-cell activation and its redirection against tumor cells (77). AMG 757 is currently being evaluated alone or in combination with pembrolizumab in a phase I trial (NCT03319940) (78). In the updated analysis of 10 cohorts including 64 patients, AMG757 at doses up to 100 mg reported promising results in terms of response rate (43% of the disease control rate, with 13% of PR) and median response duration (6.2 months), with a relative safety profile (grade ≥3 and 4); treatment-related adverse events (AEs) occurred in 25% and 6% of cases, respectively. Cytokine release syndrome occurred in 42% of patients, mainly as mild grade toxicity (79).
Rovalpituzumab tesirine (Rova-T), a DLL3-targeted ADC, has been largely investigated in different settings of SCLC, first of all in the third-line (phase II single-arm TRINITY) (80), then in the second-line (phase III TAHOE) (81), and later as first-line maintenance therapy after platinum-based chemotherapy (phase III MERU) (82). Unfortunately, it does not show the expected activity, thus failing to improve the landscape of SCLC treatment.
Vaccines, such as fucosyl GM-1, GD3 ganglioside, polysialic acid, and dendritic cell-based p53, are also a potentially promising strategy in the management of SCLC but remaining under investigation (83).
Lefitolimod, a toll-like receptor (TLR) 9 agonist, is an immunomodulator drug studied as maintenance therapy after first-line chemotherapy in the phase II trial IMPULSE (84). Although this trial did not demonstrate an OS benefit in the intention-to-treat population, a subgroup analysis of patients with a low frequency of activated CD86+ B cells resulted in a potential OS benefit.
Promising activity in SCLC is being shown with the combination of ICIs and anti-LAG-3 (78) as well as with anti-TIM-3 agents (85), which are both correlated with the development of resistance to PD-1 blockade (80). Similarly, SKYSCRAPER-02 (NCT04256421) is a phase III randomized, double-blind, placebo-controlled trial investigating the addition of another ICI, tiragolumab (anti-TIGIT agent), to first-line atezolizumab, carboplatin, plus etoposide in patients with ES-SCLC.
Given the high expression levels of DNA damage response (DDR) proteins, such as PARP, ATR, CHK1, and WEE1 in SCLC, many DDR pathway inhibitors are under development.
Indeed, combining ICIs with small molecules, such as cyclin-dependent kinases (CDK) 4/6 inhibitors and poly(ADP-ribose) polymerase (PARP) inhibitors, is an emerging strategy. Trilaciclib, a CDK4/6 inhibitor, is being evaluated within the first-line atezolizumab, carboplatin, and etoposide in a phase II placebo-controlled trial (NCT03041311).
The PARP inhibitor, olaparib, is under investigation in phase II trials, in combination with durvalumab for relapsed SCLC. Although the first phase II study did not meet its primary endpoint (86), this combination of ICI and PARP inhibition is currently being explored (87). Furthermore, the phase III MK 7339-013/KEYLYNK-013 (NCT04624204) is currently ongoing to evaluate the combination of pembrolizumab with concurrent CRT followed by pembrolizumab with or without olaparib in LD-SCLC.
Finally, preliminary results with other targets such as Aurora A kinase inhibitor, CDK7 inhibitors, and epigenetic inhibitors showed modest further benefit in preclinical and clinical models.
The multikinase antiangiogenic anlotinib was also tested in pretreated SCLC and showed a slightly better response compared to placebo (ORR 4.9% vs. 2.6%; DCR 71.6% vs. 13.2%) (88). A higher percentage of responses was reported with the combination of the anti-VEGFR2 apatinib and camrelizumab in the phase II trial PASSION, including both chemosensitive and chemoresistant ED-SCLC (89).
5 Conclusions
SCLC is still considered the most aggressive form of lung cancer. However, the advent of immunotherapy has changed the treatment paradigm as well as the outcome of a subgroup of patients affected with extensive SCLC. In this scenario, many open issues remain. Despite the benefit from the combination of ICIs and chemotherapy reported in the recent studies, a significant percentage of patients shows disease progression within 2 years. Moreover, some categories of patients like the elderly or those with an ECOG PS of 2, largely represented in real-world settings, were not studied enough in clinical trials. Therefore, the identification of the predictive factors of the response could be very important in achieving better patient selection in daily clinical practice. Based on recent data from gene profiling and classification in four molecular subtypes of SCLC, as well as the correlation between these molecular subtypes and response to treatment, a strong effort is currently ongoing to personalize cancer care in SCLC tumors, moving this scenario to the new concept of one-size-does-not-fit-all. However, we are still far from this concept, and profound knowledge of SCLC cell biology is necessary to improve the survival of these patients.
Besides ICIs combinations, several new treatment strategies, as well as novel molecules to overcome potential mechanisms of resistance, are under investigation with promising results. Thus, is it possible to talk about an effective therapeutic algorithm in SCLC treatment in the near future? Further studies with confirmatory results and a deeper understanding of SCLC biology could be the way to answer this question and expand therapeutic opportunities in this aggressive tumor.
Author Contributions
LB, JM, and SP conceived the original idea of the article, drafting, and writing the paper. LC, AI, GP, ER, EV and AV revised the scientific content of specific sections of the manuscript and participated in drafting specific section of the paper. LC, AI, GP, ER, EV, AV and JM participated in the critical revision of the paper. LB, JM, and SP participated in the critical revision and editing of the manuscript. LB, LC, AI, GP, ER, EV, AV, JM and SP conceived the original idea and provided critical revision of the manuscript as well as the final approval of the version to publish. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Medical writing support in the preparation of this article was provided by Michela Roberto, MD, on behalf of Edra S.p.A., with an unconditioned contribution by Roche S.p.A.
References
1. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing Epidemiology of Small-Cell Lung Cancer in the United States Over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J Clin Oncol (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859
2. Amarasena IU, Chatterjee S, Walters JAE, Wood-Baker R, Fong KM. Platinum Versus Non-Platinum Chemotherapy Regimens for Small Cell Lung Cancer. Cochrane Database Syst Rev (2015) 2015(8):CD006849. doi: 10.1002/14651858.CD006849.pub3
3. George J, Lim JS, Jang SJ, Cun Y, Ozretia L, Kong G, et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature (2015) 524(7563):47–53. doi: 10.1038/nature14664
4. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of Mutational Processes in Human Cancer. Nature (2013) 502(7470):258. doi: 10.1038/nature12477
5. De Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence. Clin Cancer Res (2014) 20(21):5384–91. doi: 10.1158/1078-0432.CCR-14-1298
6. Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Author Correction: Molecular Subtypes of Small Cell Lung Cancer: A Synthesis of Human and Mouse Model Data. Nat Rev Cancer (2019) 19(5):289–97. doi: 10.1038/s41568-019-0164-2
7. Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of Transcription Factor Programs and Immune Pathway Activation Define Four Major Subtypes of SCLC With Distinct Therapeutic Vulnerabilities. Cancer Cell (2021) 39(3):346–60. doi: 10.1016/j.ccell.2020.12.014
8. Muppa P, Parrilha Terra SBS, Sharma A, Mansfield AS, Aubry MC, Bhinge K, et al. Immune Cell Infiltration May Be a Key Determinant of Long-Term Survival in Small Cell Lung Cancer. J Thorac Oncol (2019) 14(7):1286–95. doi: 10.1016/j.jtho.2019.03.028
9. Schwendenwein A, Megyesfalvi Z, Barany N, Valko Z, Bugyik E, Lang C, et al. Molecular Profiles of Small Cell Lung Cancer Subtypes: Therapeutic Implications. Mol Ther - Oncol (2021) 20:470–83. doi: 10.1016/j.omto.2021.02.004
10. Dingemans A-MC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann Oncol (2021) 32:839–53. doi: 10.1016/J.ANNONC.2021.03.207
11. Barnes H, See K, Barnett S, Manser R. Surgery for Limited-Stage Small-Cell Lung Cancer. Cochrane Database Syst Rev (2017) 4:CD011917. doi: 10.1002/14651858.CD011917.pub2
12. Gergen AK, Scott CD, Mitchell JD. Surgery for Limited Stage Small Cell Lung Cancer. J Thorac Dis (2020) 12(10):6291–7. doi: 10.21037/jtd.2020.03.79
13. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent Once-Daily Versus Twice-Daily Chemoradiotherapy in Patients With Limited-Stage Small-Cell Lung Cancer (CONVERT): An Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol (2017) 18(8):1116–25. doi: 10.1016/S1470-2045(17)30318-2
14. Karam I, Jiang SY, Khaira M, Lee CW, Schellenberg D. Outcomes of Small Cell Lung Cancer Patients Treated With Cisplatin-Etoposide Versus Carboplatin-Etoposide. Am J Clin Oncol Cancer Clin Trials (2015) 38(1):51–4. doi: 10.1097/COC.0b013e31828aab2a
15. Grønberg BH, Killingberg KT, Fløtten Ø, Brustugun OT, Hornslien K, Madebo T, et al. High-Dose Versus Standard-Dose Twice-Daily Thoracic Radiotherapy for Patients With Limited Stage Small-Cell Lung Cancer: An Open-Label, Randomised, Phase 2 Trial. Lancet Oncol (2021) 22:321–31. doi: 10.1016/S1470-2045(20)30742-7
16. Qiu B, Li QW, Liu JL, Huang Y, Pang QS, Zhu ZF, et al. Moderately Hypofractionated Once-Daily Compared With Twice-Daily Thoracic Radiation Therapy Concurrently With Etoposide and Cisplatin in Limited-Stage Small Cell Lung Cancer: A Multicenter, Phase II, Randomized Trial. Int J Radiat Oncol Biol Phys (2021) 111:424–35. doi: 10.1016/J.IJROBP.2021.05.003
17. Noronha V, Sekhar A, Patil VM, Menon N, Joshi A, Kapoor A, et al. Systemic Therapy for Limited Stage Small Cell Lung Carcinoma. J Thorac Dis (2020) 12(10):6275–90. doi: 10.21037/jtd-2019-sclc-11
18. Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining Radiation and Immunotherapy: A New Systemic Therapy for Solid Tumors? Cancer Immunol Res (2014) 2(9):831–8. doi: 10.1158/2326-6066.CIR-14-0069
19. Peters S, Pujol J-L, Dafni U, Dómine M, Popat S, Reck M, et al. Consolidation Nivolumab and Ipilimumab Versus Observation in Limited-Disease Small-Cell Lung Cancer After Chemo-Radiotherapy - Results From the Randomised Phase II ETOP/IFCT 4-12 STIMULI Trial. Ann Oncol Off J Eur Soc Med Oncol (2021) 33(1):67–79. doi: 10.1016/j.annonc.2021.09.011
20. Study of Durvalumab + Tremelimumab, Durvalumab, and Placebo in Limited Stage Small-Cell Lung Cancer in Patients Who Have Not Progressed Following Concurrent Chemoradiation Therapy. Available at: https://clinicaltrials.gov/ct2/show/NCT03703297 (Accessed September 9, 2021).
21. Chemoradiation With or Without Atezolizumab in Treating Patients With Limited Stage Small Cell Lung Cancer - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03811002 (Accessed September 9, 2021).
22. Atezolizumab After Concurrent Chemo-Radiotherapy Versus Chemo-Radiotherapy Alone in Limited Disease Small-Cell Lung Cancer - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03540420 (Accessed September 9, 2021).
23. A Study of Atezolizumab in Combination With Carboplatin Plus Etoposide to Investigate Safety and Efficacy in Patients With Untreated Extensive-Stage Small Cell Lung Cancer - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04028050 (Accessed September 9, 2021).
24. Lazzari C, Mirabile A, Bulotta A, Viganó MG, Ogliari FR, Ippati S, et al. History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature. Cancers (Basel) (2021) 13(5):998. doi: 10.3390/cancers13050998
25. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMOA1809064
26. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (Impower133). J Clin Oncol (2021) 39:619–30. doi: 10.1200/JCO.20.01055
27. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, With or Without Tremelimumab, Plus Platinum–Etoposide Versus Platinum–Etoposide Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): Updated Results From a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8
28. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab Plus Platinum–Etoposide Versus Platinum–Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
29. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
30. Besse B, Menis J, Bironzo P, Gervais R, Greillier L, Monnet I, et al. LBA85 REACTION: A Phase II Study of Etoposide and Cis/Carboplatin With or Without Pembrolizumab in Untreated Extensive Small Cell Lung Cancer. Ann Oncol (2020) 31:S1211–2. doi: 10.1016/j.annonc.2020.08.2327
31. Gadgeel SM, Pennell NA, Fidler MJ, Halmos B, Bonomi P, Stevenson J, et al. Phase II Study of Maintenance Pembrolizumab in Patients With Extensive-Stage Small Cell Lung Cancer (SCLC). J Thorac Oncol (2018) 13(9):1393–9. doi: 10.1016/j.jtho.2018.05.002
32. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol (2016) 34(31):3740–8. doi: 10.1200/JCO.2016.67.6601
33. Owonikoko TK, Kim HR, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab (Nivo) Plus Ipilimumab (Ipi), Nivo, or Placebo (Pbo) as Maintenance Therapy in Patients (Pts) With Extensive Disease Small Cell Lung Cancer (ED-SCLC) After First-Line (1l) Platinum-Based Chemotherapy (Chemo): Results From the Double-Blind, Randomized Phase III CheckMate 451 Study. Ann Oncol (2019) 30:20019 S2. doi: 10.1093/annonc/mdz094
34. von P J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan Versus Cyclophosphamide, Doxorubicin, and Vincristine for the Treatment of Recurrent Small-Cell Lung Cancer. J Clin Oncol (1999) 17:658–67. doi: 10.1200/JCO.1999.17.2.658
35. Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, et al. Carboplatin Plus Etoposide Versus Topotecan as Second-Line Treatment for Patients With Sensitive Relapsed Small-Cell Lung Cancer: An Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Oncol (2020) 21(9):1224–33. doi: 10.1016/S1470-2045(20)30461-7
36. Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol (2020) 15(4):618–27. doi: 10.1016/j.jtho.2019.12.109
37. Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, et al. Lurbinectedin as Second-Line Treatment for Patients With Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol (2020) 21(5):645–54. doi: 10.1016/S1470-2045(20)30068-1
38. Farago AF, Drapkin BJ, Lopez-Vilarino De Ramos JA, Galmarini CM, Núñez R, Kahatt C, et al. ATLANTIS: A Phase III Study of Lurbinectedin/Doxorubicin Versus Topotecan or Cyclophosphamide/Doxorubicin/Vincristine in Patients With Small-Cell Lung Cancer Who Have Failed One Prior Platinum-Containing Line. Futur Oncol (2019) 15(3):231–9. doi: 10.2217/fon-2018-0597
39. Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol (2020) 15(3):426–35. doi: 10.1016/j.jtho.2019.10.004
40. Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-Line Nivolumab in Relapsed Small-Cell Lung Cancer: CheckMate 331☆. Ann Oncol Off J Eur Soc Med Oncol (2021) 32:631–41. doi: 10.1016/j.annonc.2021.01.071
41. Efficacy and Safety of Nivolumab (Nivo) Monotherapy Versus Chemotherapy (Chemo) in Recurrent Small Cell Lung Cancer (SCLC): Results From CheckMate 331 | OncologyPRO. Available at: https://oncologypro.esmo.org/meeting-resources/esmo-immuno-oncology-congress-2018/Efficacy-and-safety-of-nivolumab-nivo-monotherapy-versus-chemotherapy-chemo-in-recurrent-small-cell-lung-cancer-SCLC-Results-from-CheckMate-331 (Accessed September 9, 2021).
42. Reck M, Vicente D, Ciuleanu T, Gettinger S, Peters S, Horn L, et al. Efficacy and Safety of Nivolumab (Nivo) Monotherapy Versus Chemotherapy (Chemo) in Recurrent Small Cell Lung Cancer (SCLC): Results From CheckMate 331. Ann Oncol (2018) 29:x43. doi: 10.1093/ANNONC/MDY511.004
43. Goldman JW, Dowlati A, Antonia SJ, Nemunaitis JJ, Butler MO, Segal NH, et al. Safety and Antitumor Activity of Durvalumab Monotherapy in Patients With Pretreated Extensive Disease Small-Cell Lung Cancer (ED-SCLC). J Clin Oncol (2018) 36:8518–8. doi: 10.1200/JCO.2018.36.15_SUPPL.8518
44. Bondarenko I, Juan-Vidal O, Pajkos G, Kryzhanivska A, Székely ZP, Vicente D, et al. Preliminary Efficacy of Durvalumab Plus Tremelimumab in Platinum-Refractory/Resistant ED-SCLC From Arm A of the Phase II BALTIC Study. Ann Oncol (2018) 29:viii596. doi: 10.1093/ANNONC/MDY298.001
45. Pujol J-L, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients With Small Cell Lung Cancer: Results From the IFCT-1603 Trial. J Thorac Oncol (2019) 14:903–13. doi: 10.1016/J.JTHO.2019.01.008
46. Daly ME, Ismaila N, Decker RH, Higgins K, Owen D, Saxena A, et al. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline. J Clin Oncol (2021) 39:931–9. doi: 10.1200/JCO.20.03364
47. PRophylactic Cerebral Irradiation or Active MAgnetic Resonance Imaging Surveillance in Small-Cell Lung Cancer Patients (PRIMALung Study) - Full Text View -ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04790253 (Accessed October 27, 2021).
48. Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic Cranial Irradiation Versus Observation in Patients With Extensive-Disease Small-Cell Lung Cancer: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18:663–71. doi: 10.1016/S1470-2045(17)30230-9
49. Rodriguez de Dios N, Calvo P, Rico M, Martín M, Couñago F, Sotoca A, et al. Recent Developments in Radiotherapy for Small-Cell Lung Cancer: A Review by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol (2017) 19:1183–92. doi: 10.1007/S12094-017-1667-5
50. Alberta Cancer Registry. Alberta Health Services. Available at: https://www.albertahealthservices.ca/cancer/Page17367.aspx (Accessed September 9, 2021).
51. Abdel-Rahman O. Smoking and EGFR Status may Predict Outcomes of Advanced NSCLC Treated With PD-(L)1 Inhibitors Beyond First Line: A Meta-Analysis. Clin Respir J (2018) 12:1809–19. doi: 10.1111/crj.12742
52. Fisher S, Al-Fayea TM, Winget M, Gao H, Butts C. Uptake and Tolerance of Chemotherapy in Elderly Patients With Small Cell Lung Cancer and Impact on Survival. J Cancer Epidemiol (2012) 2012:708936. doi: 10.1155/2012/708936
53. Ferrara R, Naigeon M, Auclin E, Duchemann B, Cassard L, Jouniaux J-M, et al. Circulating T-Cell Immunosenescence in Patients With Advanced Non-Small Cell Lung Cancer Treated With Single-Agent PD-1/PD-L1 Inhibitors or Platinum-Based Chemotherapy. Clin Cancer Res (2021) 27:492–503. doi: 10.1158/1078-0432.CCR-20-1420
54. Montella TC, Vasco MM, Silva ALM, Rossi MS, Sena CVS, Oliveira LN, et al. Evaluation of Elderly Patients With Extended Disease Small-Cell Lung Cancer. J Clin Oncol (2013) 31:S15. doi: 10.1200/jco.2013.31.15_suppl.e18515
55. Bjoernhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. 1333p The Influence of Polypharmacy on Outcome in Real Life Non-Small Cell Lung Cancer (NSCLC) Patients Treated With Immunotherapy. Ann Oncol (2020) 31:S858. doi: 10.1016/J.ANNONC.2020.08.1647
56. Patients With ES-SCLC and ECOG PS=2 Receiving Atezolizumab-Carboplatin-Etoposide - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04221529 (Accessed September 9, 2021).
57. Eaton BR, Kim S, Marcus DM, Prabhu R, Chen Z, Ramalingam SS, et al. Effect of Prophylactic Cranial Irradiation on Survival in Elderly Patients With Limited-Stage Small Cell Lung Cancer. Cancer (2013) 119(21):3753–60. doi: 10.1002/cncr.28267
58. Damhuis RAM, Senan S, Belderbos JS. Usage of Prophylactic Cranial Irradiation in Elderly Patients With Small-Cell Lung Cancer. Clin Lung Cancer (2018) 19:e263–7. doi: 10.1016/j.cllc.2017.11.005
59. Rule WG, Foster NR, Meyers JP, Ashman JB, Vora SA, Kozelsky TF, et al. Prophylactic Cranial Irradiation in Elderly Patients With Small Cell Lung Cancer: Findings From a North Central Cancer Treatment Group Pooled Analysis. J Geriatr Oncol (2015) 6:119–26. doi: 10.1016/j.jgo.2014.11.002
60. Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in Tested and Self-Reported Cognitive Functioning After Prophylactic Cranial Irradiation for Lung Cancer: Pooled Secondary Analysis of Radiation Therapy Oncology Group Randomized Trials 0212 and 0214. Int J Radiat Oncol Biol Phys (2013) 86:656–64. doi: 10.1016/j.ijrobp.2013.02.033
61. Remon J, Aldea M, Besse B, Planchard D, Reck M, Giaccone G, et al. Small Cell Lung Cancer: A Slightly Less Orphan Disease After Immunotherapy. Ann Oncol (2021) 32:698–709. doi: 10.1016/J.ANNONC.2021.02.025
62. Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, et al. Expression and Clinical Significance of PD-L1, B7-H3, B7-H4 and TILs in Human Small Cell Lung Cancer (SCLC). J Immunother Cancer (2019) 7(1):65. doi: 10.1186/s40425-019-0540-1
63. Iams WT, Shiuan E, Meador CB, Roth M, Bordeaux J, Vaupel C, et al. Improved Prognosis and Increased Tumor-Infiltrating Lymphocytes in Patients Who Have SCLC With Neurologic Paraneoplastic Syndromes. J Thorac Oncol (2019) 14(11):1970–81. doi: 10.1016/j.jtho.2019.05.042
64. Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-Cell Lung Cancer. Nat Rev Dis Prim (2021) 7:1–20. doi: 10.1038/s41572-020-00235-0
65. Wagner AH, Devarakonda S, Skidmore ZL, Krysiak K, Ramu A, Trani L, et al. Recurrent WNT Pathway Alterations are Frequent in Relapsed Small Cell Lung Cancer. Nat Commun (2018) 9:1–11. doi: 10.1038/s41467-018-06162-9
66. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver Metastasis and Treatment Outcome With Anti-PD-1 Monoclonal Antibody in Patients With Melanoma and NSCLC. Cancer Immunol Res (2017) 5:417–24. doi: 10.1158/2326-6066.CIR-16-0325
67. Mollaoglu G, Guthrie MR, Böhm S, Brägelmann J, Can I, Ballieu PM, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype With Vulnerability to Aurora Kinase Inhibition. Cancer Cell (2017) 31(2):270–85. doi: 10.1016/j.ccell.2016.12.005
68. Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, et al. Tumorigenicity and Genetic Profiling of Circulating Tumor Cells in Small-Cell Lung Cancer. Nat Med (2014) 20(8):897–903. doi: 10.1038/nm.3600
69. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination With Ipilimumab in Small-Cell Lung Cancer. Cancer Cell (2019) 35(2):329. doi: 10.1016/j.ccell.2019.01.011
70. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver Metastasis Restrains Immunotherapy Efficacy via Macrophage-Mediated T Cell Elimination. Nat Med 2021 271 (2021) 27:152–64. doi: 10.1038/s41591-020-1131-x
71. Crossland DL, Denning WL, Ang S, Olivares S, Mi T, Switzer K, et al. Antitumor Activity of CD56-Chimeric Antigen Receptor T Cells in Neuroblastoma and SCLC Models. Oncogene (2018) 37:3686–97. doi: 10.1038/s41388-018-0187-2
72. Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. CD47-Blocking Immunotherapies Stimulate Macrophage-Mediated Destruction of Small-Cell Lung Cancer. J Clin Invest (2016) 126:2610–20. doi: 10.1172/JCI81603
73. Owen DH, Giffin MJ, Bailis JM, Smit M-AD, Carbone DP, He K. DLL3: An Emerging Target in Small Cell Lung Cancer. J Hematol Oncol (2019) 12:61. doi: 10.1186/s13045-019-0745-2
74. Sadelain M, Brentjens R, Rivière I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548
75. Giffin M, Cooke K, Lobenhofer E, Friedrich M, Raum T, Coxon A. P3.12-03 Targeting DLL3 With AMG 757, a BiTE® Antibody Construct, and AMG 119, a CAR-T, for the Treatment of SCLC. J Thorac Oncol (2018) 13:S971. doi: 10.1016/J.JTHO.2018.08.1826
76. Slaney CY, Wang P, Darcy PK, Kershaw MH. CARs Versus BiTEs: A Comparison Between T Cell-Redirection Strategies for Cancer Treatment. Cancer Discov (2018) 8(8):924–34. doi: 10.1158/2159-8290.CD-18-0297
77. Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res (2021) 27:1526–37. doi: 10.1158/1078-0432.CCR-20-2845
78. Uboha NV, Milhem MM, Kovacs C, Amin A, Magley A, Das Purkayastha D, et al. Phase II Study of Spartalizumab (PDR001) and LAG525 in Advanced Solid Tumors and Hematologic Malignancies. J Clin Oncol (2019) 37:2553–3. doi: 10.1200/JCO.2019.37.15_SUPPL.2553
79. Owonikoko TK, Champiat S, Johnson ML, Govindan R, Izumi H, Lai WVV, et al. Updated Results From a Phase 1 Study of AMG 757, a Half-Life Extended Bispecific T-Cell Engager (BiTE) Immuno-Oncology Therapy Against Delta-Like Ligand 3 (DLL3), in Small Cell Lung Cancer (SCLC). J Clin Oncol (2021) 39:8510–0. doi: 10.1200/JCO.2021.39.15_SUPPL.8510.
80. Sun J-Y, Zhang D, Wu S, Xu M, Zhou X, Lu X-J, et al. Resistance to PD-1/PD-L1 Blockade Cancer Immunotherapy: Mechanisms, Predictive Factors, and Future Perspectives. Biomark Res (2020) 8:1–10. doi: 10.1186/S40364-020-00212-5
81. Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J Thorac Oncol (2021) 16:1547–58. doi: 10.1016/j.jtho.2021.02.009
82. Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J Thorac Oncol (2021) 16:1570–81. doi: 10.1016/j.jtho.2021.03.012
83. Wong SK, Iams WT. Front Line Applications and Future Directions of Immunotherapy in Small-Cell Lung Cancer. Cancers (Basel) (2021) 13:1–15. doi: 10.3390/CANCERS13030506
84. Thomas M, Ponce-Aix S, Navarro A, Riera-Knorrenschild J, Schmidt M, Wiegert E, et al. Immunotherapeutic Maintenance Treatment With Toll-Like Receptor 9 Agonist Lefitolimod in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Exploratory, Controlled, Randomized, International Phase II IMPULSE Study. Ann Oncol Off J Eur Soc Med Oncol (2018) 29:2076–84. doi: 10.1093/annonc/mdy326
85. Friedlaender A, Addeo A, Banna G. New Emerging Targets in Cancer Immunotherapy: The Role of TIM3. ESMO Open (2019) 4:e000497. doi: 10.1136/esmoopen-2019-000497
86. Thomas A, Vilimas R, Trindade C, Erwin-Cohen R, Roper N, Xi L, et al. Durvalumab in Combination With Olaparib in Patients With Relapsed SCLC: Results From a Phase II Study. J Thorac Oncol (2019) 14:1447–57. doi: 10.1016/j.jtho.2019.04.026
87. Barayan R, Ran X, Lok BH. PARP Inhibitors for Small Cell Lung Cancer and Their Potential for Integration Into Current Treatment Approaches. J Thorac Dis (2020) 12:6240–52. doi: 10.21037/JTD.2020.03.89
88. Shi J, Cheng Y, Wang Q, Li K, Wu L, Han B, et al. Effect of Anlotinib in Advanced Small Cell Lung Cancer (SCLC) Patients Relapsed Within Three Months After Second-Line Treatment: A Subgroup Analysis From a Randomized, Double-Blind Phase II Trial (ALTER 1202). J Clin Oncol (2020) 38:9063–3. doi: 10.1200/JCO.2020.38.15_SUPPL.9063
Keywords: small cell lung cancer (SCLC), immune checkpoint inhibitors, immunotherapy, fragile patients, predictive factor
Citation: Belluomini L, Calvetti L, Inno A, Pasello G, Roca E, Vattemi E, Veccia A, Menis J and Pilotto S (2022) SCLC Treatment in the Immuno-Oncology Era: Current Evidence and Unmet Needs. Front. Oncol. 12:840783. doi: 10.3389/fonc.2022.840783
Received: 21 December 2021; Accepted: 21 March 2022;
Published: 14 April 2022.
Edited by:
Idris Bahce, Academic Medical Center, NetherlandsCopyright © 2022 Belluomini, Calvetti, Inno, Pasello, Roca, Vattemi, Veccia, Menis and Pilotto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Pilotto, c2FyYS5waWxvdHRvQHVuaXZyLml0
†These authors have contributed equally to this work and share last authorship
 Lorenzo Belluomini
Lorenzo Belluomini Lorenzo Calvetti2
Lorenzo Calvetti2 Alessandro Inno
Alessandro Inno Giulia Pasello
Giulia Pasello Elisa Roca
Elisa Roca Emanuela Vattemi
Emanuela Vattemi Antonello Veccia
Antonello Veccia Jessica Menis
Jessica Menis Sara Pilotto
Sara Pilotto