- 1Department of Medical Oncology, Hangzhou Cancer Hospital, Hangzhou, China
- 2Department of Oncology and Hematology, Hangzhou Red Cross Hospital, Hangzhou, China
- 3Department of Medical Oncology, Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: In patients with metastatic colorectal cancer (mCRC) with an asymptomatic primary tumor, there is no consensus on the indication for resection of the primary tumor.
Methods: The PubMed, Embase and the Cochrane Library databases were searched from inception to November 30,2021. A meta-analysis was performed using RevMan (version 5.3.3; The Cochrane Collaboration) on the outcome of mCRC patients with or without resection of the primary tumor in 8 selected studies.
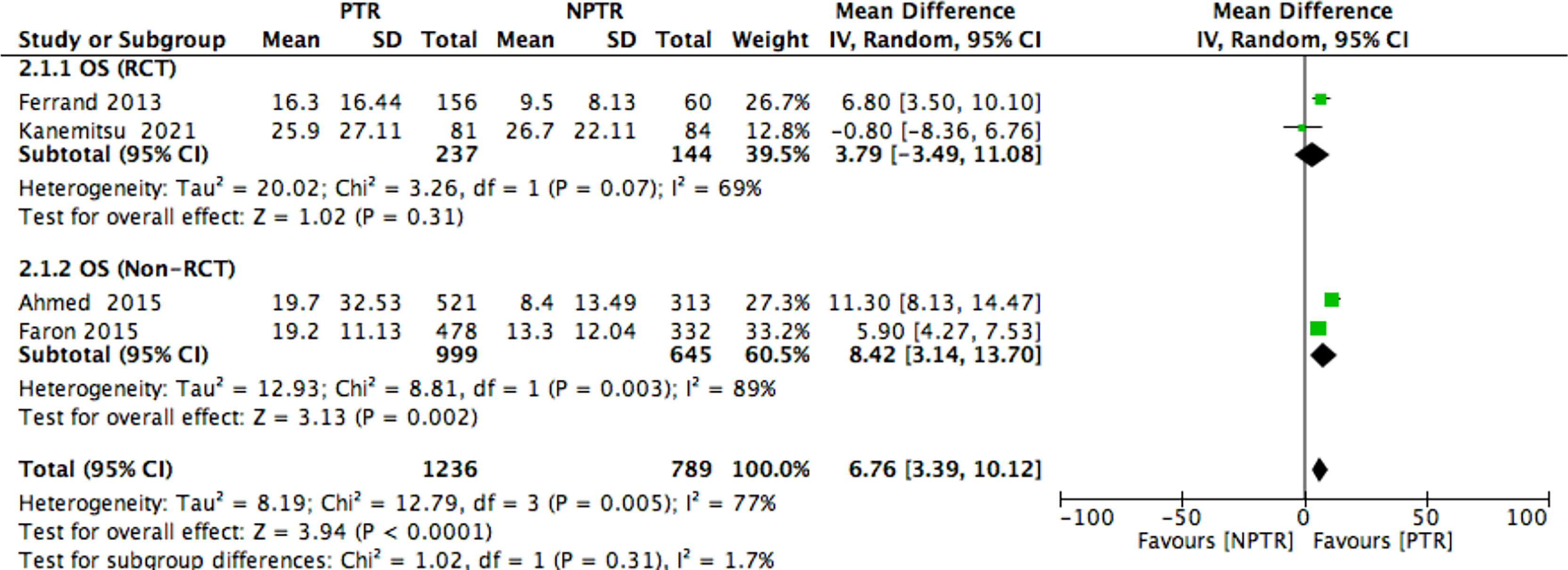
Results: This meta-analysis included 2805 colorectal cancer patients with an asymptomatic primary tumor from 8 selected studies. Primary tumor resection (PTR) patients had longer overall survival (OS: MD =6.76 [3.39, 10.12], I2 = 77%, P < 0.0001), compared with non-primary tumor resection (NPTR) patients. In the subgroup, the randomized controlled trials (RCT) PTR group didn’t have longer overall survival (OS: MD =3.79 [-3.49, 11.08], I2 = 69%, P= 0.31); the Non-RCT PTR group had longer overall survival (OS: MD =8.42 [3.14, 13.70], I2 = 89%, P= 0.002). In the meanwhile, compared with NPTR group, the 2-year overall survival rate, the 3-year overall survival rate, 5-year overall survival rate in the PTR group is higher (OR=2.35 [1.74, 3.18], I2 = 0%, P < 0.00001; OR=3.61 [2.35, 5.54], I2 = 0%, P < 0.00001; OR=3.02 [1.72, 5.33], I2 = 48%, P= 0.0001, respectively).
Conclusions: Our results from studies demonstrate that the resection of primary tumor is a prognostic factor for survival in mCRC patients. However, 2 RCTs showed the resection of primary tumor was not related with a significant survival benefit in subgroup. Therefore, a larger RCT in the era of modern chemotherapy and liver resection techniques would be helpful.
Introduction
Clinically, synchronous liver metastasis of colorectal cancer can be divided into four types according to whether the primary tumor has clinical symptoms and whether the liver metastasis can be resected: (1) the primary tumor has no symptoms and the liver metastasis can be resected, (2) The primary lesion is asymptomatic and liver metastases are unresectable, (3) The primary lesion was symptomatic and the liver metastases can be resected, (4) The primary lesion is symptomatic and the liver metastases are unresectable. There is no dispute about the treatment principle of the latter two situations. The primary lesion should be treated first (or liver metastasis should be treated at the same time). In the first situation, the treatment involving preoperative chemotherapy, simultaneous or staged resection will be not discussed here. For the second situation, should we first convert to chemotherapy, or should we first remove the primary tumor?
At present, most studies suggest that primary resection is better than chemotherapy alone in asymptomatic colorectal cancer patients with liver metastasis. In a population-based cohort research, palliative primary tumor resection was related to improved overall and cancer-specific survival (1). The benefit that resection of the primary tumor in asymptomatic patients with metastatic colorectal cancer (mCRC) improved survival independent of other prognostic variables was more pronounced in stage IVA patient (2). Another study also indicated that the resection of primary tumor was a prognostic factor for survival in stage IV CRC patients (3). Compared to patients who received chemotherapy as the first treatment, mCRC patients with synchronous unresectable metastases who underwent primary tumor resection (PTR) followed by chemotherapy had significantly longer survival times (4).
However, the efficacy of the PTR for unresectable mCRC has been controversial (5). A study demonstrated that compared to patients receiving chemotherapy alone the overall risk of death was significantly higher after elective surgery in palliative treatment of asymptomatic mCRC. In other words, there is no substantial difference between these treatments (6).Moreover, PTR in patients with asymptomatic primary tumor and unresectable metastases was not associated with an improvement in overall survival (OS) after propensity score matching (7). PTR is not recommended for the reason that PTR was not associated with improved survival compared with systemic chemotherapy among patients with unresectable metastatic colon cancer (8). In a conclusion, PTR should no longer be considered a standard therapy for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases (9).
Patients with asymptomatic mCRC represent a significant heterogeneous group. The aim of our study was to analyze the studies, which compared the clinical outcomes of patients with asymptomatic mCRC and un-resectable metastases who had PTR with those without resection. There is no consensus about the solution. In this paper, meta-analysis was performed on the retrospective study and randomized controlled study on the benefit of PTR.
Search Strategy
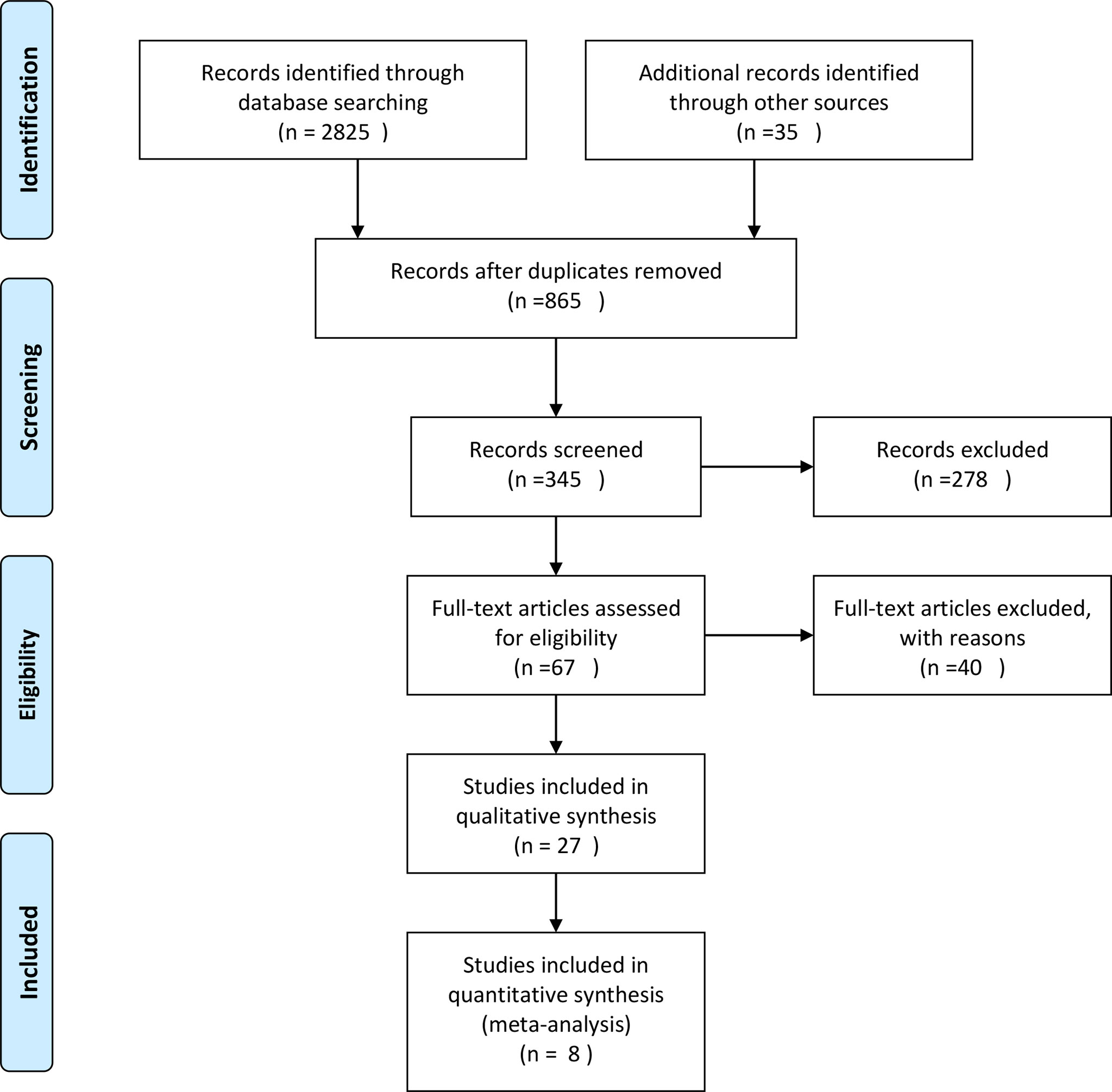
The PubMed, Embase and the Cochrane Library databases were searched from inception to November 30,2021 for relevant studies using specified eligibility criteria. Only human-related studies published from 2013 to 2021were included. The search terms included two combinations: combination 1 (Primary Asymptomatic Tumor Resection) AND (colorectal cancer); and combination 2 (colorectal cancer) AND (unresectable OR stage IV) AND (Resection); with the search filter: clinical trial, abstract, title-abstract. Titles and abstracts were screened for all studies, and full text was obtained for those meeting the inclusion criteria. Disagreements were resolved by consensus. PRISMA Flow Diagram is shown in Figure 1.
Inclusion Criteria
Inclusion criteria were: enrolled adults (age ≥18); comparative study, patients with mCRC, primary PTR as one intervention and chemotherapy alone as the other. Only article in English were selected. Finally, 8 studies were determined eligible for meta-analysis.
Exclusion Criteria
Researches are excluded if they were reviews, letters, case reports, case series, comments, editorials, or observational studies, as well as non-English studies. Studies including less than 40 patients were excluded.
Outcomes
The primary outcome measures for the studies included was OS. Secondary outcomes were 2-year OS rate, 3year OS rate, and 5-year OS rate. Two authors determined the eligibility of all retrieved studies independently, and discrepancies were resolved through consultation with a third reviewer.
Assessment of Risk of Bias and Quality of Evidence
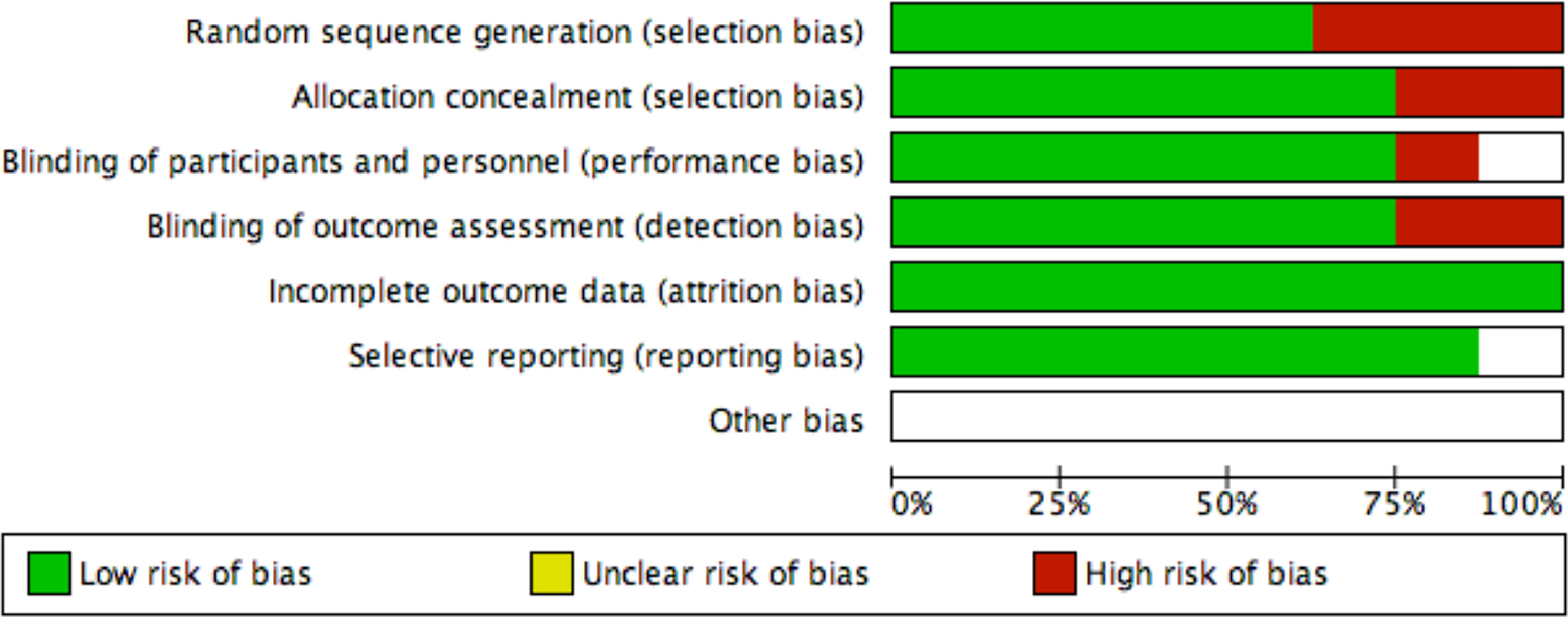
Yefei Shu and Ling Xu independently assessed the quality of all included trials by using the Cochrane Collaboration risk of bias tool. We also examined the quality of evidence for outcomes using the grading of recommendations assessment and evaluation (GRADE) approach (Figure 2).
Data Synthesis
Statistical analyses were performed using RevMan (version 5.3.3; The Cochrane Collaboration). Analyses for all outcomes were conducted on an intention to treat basis. We used odds ratios and their associated 95% confidence intervals to assess outcomes, and considered a P value less than 0.05 to be statistically significant. We assessed heterogeneity using the I2 test. If significant heterogeneity was not present (I2<50%), we used fixed effects models to pool outcomes; we used random effects models when significant heterogeneity was present (I2≥50%). The possibility of small study effects was assessed qualitatively by visual estimate of the funnel plot and quantitatively by calculation of the Egger test.
Subgroup Analyses
A subgroup analysis was performed to test interactions according to study design (RCT and Non-RCT).
Sensitivity Analyses
Sensitivity analyses were conducted by excluding trials with high or unknown risk of bias; excluding trials with high risk or unknown risk of bias of the different domains; excluding trials with a follow-up of less than one year; using random effect models.
Results
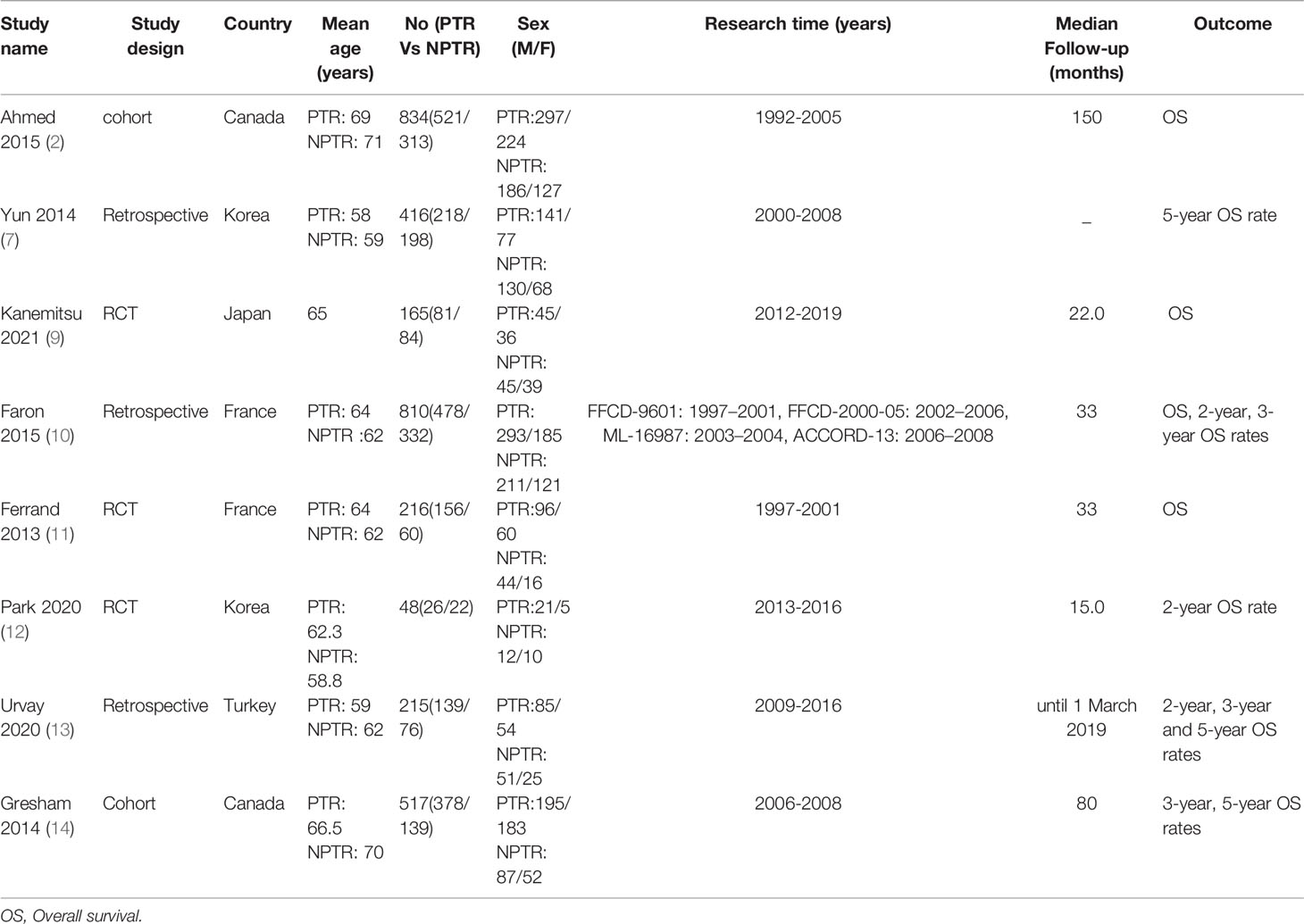
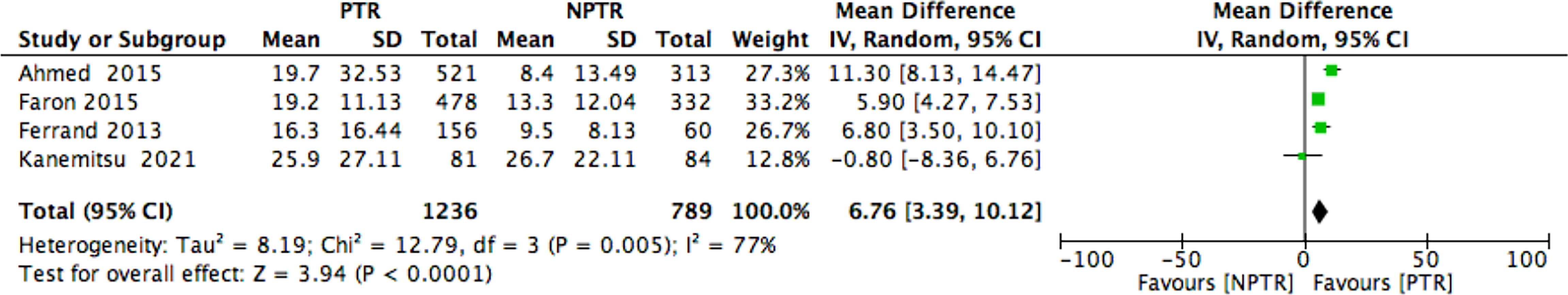
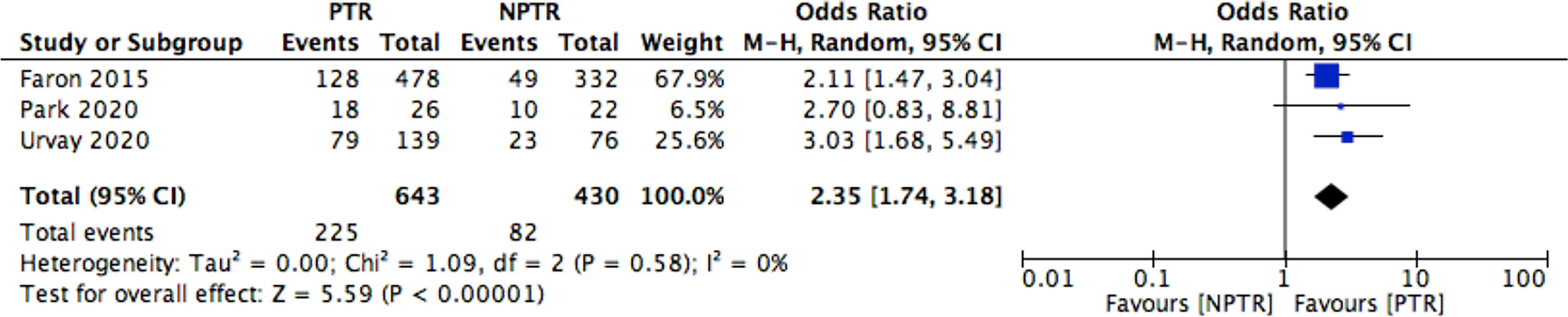
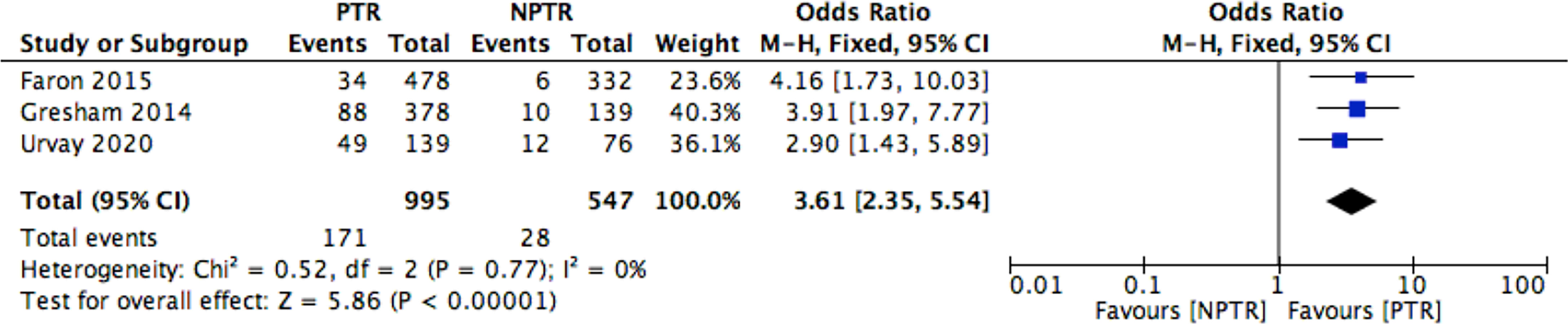
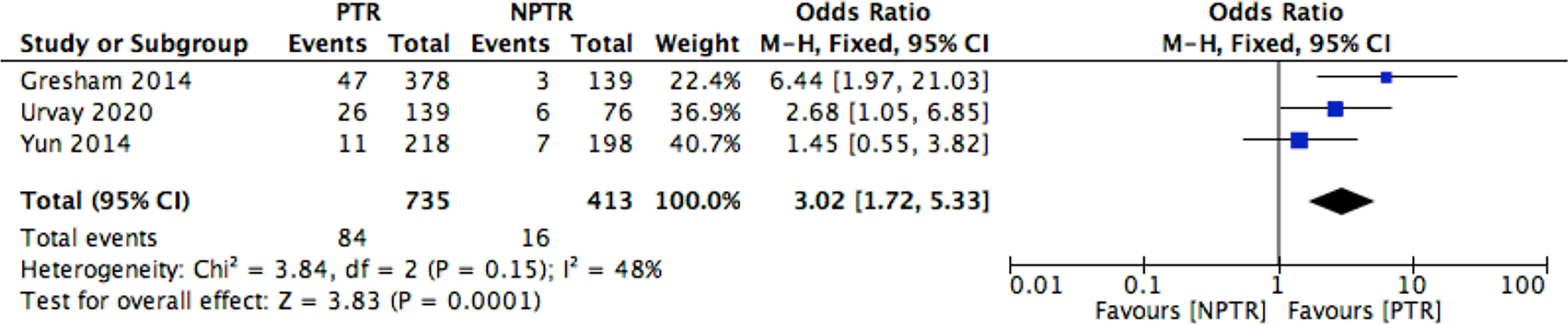
This meta-analysis included 2805 colorectal cancer patients with an asymptomatic primary tumor from 8 studies. Characteristics of included studies and outcome events are shown in Table 1. Compared with non-primary tumor resection (NPTR) patients, PTR patients had longer overall survival (2, 9–11) (OS: MD =6.76 [3.39, 10.12], I2 = 77%, P < 0.0001) (Figure 3). In the meanwhile, compared with NPTR group, the 2-year overall survival rate (10, 12, 13), the 3-year overall survival rate (10, 13, 14), 5-year overall survival rate (7, 13, 14)in the PTR group is higher (OR=2.35 [1.74, 3.18], I2 = 0%, P < 0.00001; OR=3.61 [2.35, 5.54], I2 = 0%, P < 0.00001; OR=3.02 [1.72, 5.33], I2 = 48%, P= 0.0001, respectively) (Figures 4–6).
Subgroup analyses found that the randomized controlled trials (RCT) PTR group didn’t have longer overall survival (9, 11) (OS: MD =3.79 [-3.49, 11.08], I2 = 69%, P= 0.31); the Non-RCT PTR group had longer overall survival (2, 10) (OS: MD =8.42 [3.14, 13.70], I2 = 89%, P= 0.002) (Figure 7).
Discussion
There is no consensus on the indication for PTR in patients with mCRC with asymptomatic primary tumor. However, PTR remains common practice for patients with synchronous mCRC. In addition, treatment disparities are also associated with socioeconomic as well as clinicopathologic factors (15). PTR in conjunction with postoperative chemotherapy among stage IV colon cancer patients with unresectable metastases was associated with a longer survival benefit compared with other therapy strategies (16). In the majority of the previous reports, PTR was correlated to improved survival and to the possibility for a better response to postoperative chemotherapy (17). Both PTR and up front chemotherapy appear appropriate initial management strategies. However, a trend towards an overall survival advantage with PTR, for the reason that the procedure has a low post-operative mortality, and that most complications are transient and minor (18). PTR in patients with synchronous metastasized CRC is controversial, although data suggested that resection might be a positive prognostic factor for survival (19). In multivariate survival analysis, PTR was associated with a significant survival benefit (HR 0.59, p < 0.001) (20).
However, this conclusion has been widely criticized for the following reasons: (1) the proportion of patients with tumor stage IV B is low, the tumor load is relatively small, and the proportion of colon cancer is higher (2) The patients who received surgery had better general condition, lower performance status score and more chances to receive multi line chemotherapy and targeted therapy (3)Some studies included patients who underwent radical resection of primary lesions and liver metastases (4) all studies included are non RCT studies. Therefore, the evidence level of this conclusion is not high.
Korean scholar Yun JA et al. (7) Retrospectively analyzed 259 cases of asymptomatic unresectable synchronous colorectal cancer with liver metastasis. Multivariate analysis found that tumor located in the rectum and tumor diameter greater than 5cm were independent risk factors for intervention. Nitzkorski Jr (21), an American scholar, summarized the data of 143 patients with asymptomatic primary stage IV colorectal cancer who first received systemic chemotherapy in our center. Multivariate analysis showed that there was no independent risk factor for primary symptoms. Overall, in the era of modern chemotherapy and targeted therapy, the probability of primary tumor symptoms requiring surgical intervention has a downward trend.
In order to reduce the bias of potential case selection, MD Anderson scholar alawadi Z et al. (8). Used experimental variable analysis and landmark method (patients with survival longer than 12 months were included in the statistical analysis). Results a total of 15154 patients were included in the study, of which 8641 patients (57%) underwent primary tumor resection. Cox regression analysis and propensity score analysis showed that the overall survival of primary tumor resection group was significantly prolonged, However, after adjustment by experimental variable analysis and landmark method, the survival advantage of primary tumor resection group disappeared.
Recently, some scholars have proposed that patients with unresectable left colon liver metastasis are prone to acute complications such as obstruction, bleeding and perforation, which need surgical or endoscopic treatment; In addition, the biological behavior of patients with left colon is better than that of patients with right colon, and the proportion of RAS mutation is low, so they have more opportunities to receive anti EGFR treatment; Therefore, patients with unresectable liver metastasis from left colon cancer may benefit more from primary resection than patients with right colon cancer. Zhang RX (22)of the cancer center of Sun Yat sen University in Guangzhou analyzed the clinicopathological data of 194 patients with asymptomatic primary tumor and unresectable synchronous colorectal cancer with liver metastasis from 2007 to 2013. Among them, 125 patients received palliative primary tumor resection and 69 patients only received systemic chemotherapy, Multivariate analysis showed that the location of primary tumor was the only risk factor for survival (RR 0.569, P = 0.007). Further subgroup analysis showed that for the right colon group, the only independent risk factor affecting the prognosis was histological type; For the left colon group, the independent prognostic factors included histological type, multiple liver metastases and palliative resection. The authors suggest that primary resection of the left colon with unresectable mCRC can significantly prolong survival, but it needs to be further confirmed by prospective RCT studies.
In the course of modern chemotherapy and targeted treatment, the incidence of primary symptoms in asymptomatic colorectal cancer patients with non resectable liver metastasis is lower. Although retrospective studies suggest that palliative resection of primary focus can prolong the survival of patients, especially in the left hemicolon, there is a significant bias in these studies. Therefore, this conclusion needs to be treated carefully and more prospective RCT studies are needed to confirm.
In 2008, Eisenberger, A reviewed the medical literature from 1996–2006 using the search terms metastatic colorectal cancer and primary resection to find non-curative resection of asymptomatic colorectal primary tumors may prolong survival (23). In 2015, Faron, M. et al. made a pooled analysis of individual data from four randomised trials and fonud that primary tumour resection was independently associated to a better OS in patients with CRC and unresectable synchronous metastases (10). Currently, aggressive surgical treatments should be integrated with all the available non-surgical options to maximize disease control and patient survival (24). Furthermore, multidisciplinary team approaches may be helpful in finding the suitable therapy. In case of surgical resection, minimally invasive surgery is recommended (25). In a selected population of patients with colon cancer and unresectable synchronous distant metastases, immediate colectomy followed by chemotherapy in association with targeted therapy was associated with longer OS. This strategy appears to be the most appropriate, especially for those with good performance status, well-differentiated tumors, and synchronous liver metastases only (26). In mCRC patients with unresectable metastases receiving chemotherapy, up-front PTR was independently associated with prolonged OS. Patients eligible for secondary metastases resection and/or bevacizumab may benefit the most from PTR (27). On the contrary, resection of the primary tumour in asymptomatic patients with unresectable mCRC who are managed with chemo/radiotherapy is not associated with a consistent improvement in overall survival. In addition, resection does not significantly reduce the risk of complications from the primary tumour (i.e. obstruction, perforation or bleeding) (28). The rate of complications related to the non-resected colorectal tumor is very low using oxaliplatin as first line chemotherapy. Non-operative management of asymptomatic CRC with un-resectable liver metastases is a safe approach (29). Some people hold the view that asymptomatic patients with mCRC do not routinely need to undergo resection of the primary tumor. Although several retrospective analyses suggest that patients who undergo resection of the primary tumor live longer, most of these reviewed data prior to the advent of modern polychemotherapy and are subject to considerable bias, as patients who were considered able to undergo surgery likely had better overall prognoses than those who were not (30).
Our results from studies indicate that resection of the primary tumor is a prognostic factor for survival in mCRC patients. However, 2 RCTs showed resection of the primary tumor was not associated with a significant survival benefit in subgroup. The resection of the primary tumor was a prognostic factor for longer survival in non-randomized trial but was not associated with better survival in randomized trial. This finding may suggest that a bias has likely occurred in the former, as patients in better conditions (and consequently better prognosis) are suitable to primary tumor resection.
Limitations
Selection bias and potential confounders were limitations of this study. Because there are not enough clinical data, we did not analyze the subgroup on the left and right colorectal cancer, staging, differentiation degree or whether only liver metastasis. Moreover, more RCT studies are needed to further verify this conclusion.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
Data extraction was conducted independently by YS and LX, and discrepancies were resolved by WY and XX before the final analysis. SZ revised the work critically for important intellectual content.
Conflict of Interest
The authors declare no potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, et al. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann Surg (2015) 262(1):112–20. doi: 10.1097/SLA.0000000000000860
2. Ahmed S, Fields A, Pahwa P, Chandra-Kanthan S, Zaidi A, Le D, et al. Surgical Resection of Primary Tumor in Asymptomatic or Minimally Symptomatic Patients With Stage IV Colorectal Cancer: A Canadian Province Experience. Clin Colorectal Cancer (2015) 14(4):e41–47. doi: 10.1016/j.clcc.2015.05.008
3. Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, et al. Prognostic Value of Resection of Primary Tumor in Patients With Stage IV Colorectal Cancer: Retrospective Analysis of Two Randomized Studies and a Review of the Literature. Ann Surg Oncol (2011) 18(12):3252–60. doi: 10.1245/s10434-011-1951-5
4. Kim MS, Chung M, Ahn JB, Kim CW, Cho MS, Shin SJ, et al. Clinical Significance of Primary Tumor Resection in Colorectal Cancer Patients With Synchronous Unresectable Metastasis. J Surg Oncol (2014) 110(2):214–21. doi: 10.1002/jso.23607
5. Yeom SS, Lee SY, Kwak HD, Kim CH, Kim YJ, Kim HR. The Outcome of Primary Tumor Resection in the Unresectable Stage IV Colorectal Cancer Patients Who Received the Bevacizumab-Containing Chemotherapy. Med (Baltimore) (2020) 99(7):e19258. doi: 10.1097/MD.0000000000019258
6. Boselli C, Renzi C, Gemini A, Castellani E, Trastulli S, Desiderio J, et al. Surgery in Asymptomatic Patients With Colorectal Cancer and Unresectable Liver Metastases: The Authors’ Experience. Onco Targets Ther (2013) 6:267–72. doi: 10.2147/OTT.S39448
7. Yun JA, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. The Role of Palliative Resection for Asymptomatic Primary Tumor in Patients With Unresectable Stage IV Colorectal Cancer. Dis Colon Rectum (2014) 57(9):1049–58. doi: 10.1097/DCR.0000000000000193
8. Alawadi Z, Phatak UR, Hu CY, Bailey CE, You YN, Kao LS, et al. Comparative Effectiveness of Primary Tumor Resection in Patients With Stage IV Colon Cancer. Cancer (2017) 123(7):1124–33. doi: 10.1002/cncr.30230
9. Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; Ipacs): A Randomized Clinical Trial. J Clin Oncol (2021) 39(10):1098–107. doi: 10.1200/JCO.20.02447
10. Faron M, Pignon JP, Malka D, Bourredjem A, Douillard JY, Adenis A, et al. Is Primary Tumour Resection Associated With Survival Improvement in Patients With Colorectal Cancer and Unresectable Synchronous Metastases? A Pooled Analysis of Individual Data From Four Randomised Trials. Eur J Cancer (2015) 51(2):166–76. doi: 10.1016/j.ejca.2014.10.023
11. Ferrand F, Malka D, Bourredjem A, Allonier C, Bouche O, Louafi S, et al. Impact of Primary Tumour Resection on Survival of Patients With Colorectal Cancer and Synchronous Metastases Treated by Chemotherapy: Results From the Multicenter, Randomised Trial Federation Francophone De Cancerologie Digestive 9601. Eur J Cancer (2013) 49(1):90–7. doi: 10.1016/j.ejca.2012.07.006
12. Park EJ, Baek JH, Choi GS, Park WC, Yu CS, Kang SB, et al. The Role of Primary Tumor Resection in Colorectal Cancer Patients With Asymptomatic, Synchronous, Unresectable Metastasis: A Multicenter Randomized Controlled Trial. Cancers (Basel) (2020) 12(8):2306. doi: 10.3390/cancers12082306
13. Urvay S, Eren T, Civelek B, Kilickap S, Yetiysigit T, Ozaslan E. The Role of Primary Tumor Resection in Patients With Stage IV Colorectal Cancer With Unresectable Metastases. J BUON (2020) 25(2):939–44.
14. Gresham G, Renouf DJ, Chan M, Kennecke HF, Lim HJ, Brown C, et al. Association Between Palliative Resection of the Primary Tumor and Overall Survival in a Population-Based Cohort of Metastatic Colorectal Cancer Patients. Ann Surg Oncol (2014) 21(12):3917–23. doi: 10.1245/s10434-014-3797-0
15. Shapiro M, Rashid NU, Whang EE, Boosalis VA, Huang Q, Yoon C, et al. Trends and Predictors of Resection of the Primary Tumor for Patients With Stage IV Colorectal Cancer. J Surg Oncol (2015) 111(7):911–6. doi: 10.1002/jso.23906
16. Xu Z, Becerra AZ, Fleming FJ, Aquina CT, Dolan JG, Monson JR, et al. Treatments for Stage IV Colon Cancer and Overall Survival. J Surg Res (2019) 242:47–54. doi: 10.1016/j.jss.2019.04.034
17. Sterpetti AV, Costi U, D’Ermo G. National Statistics About Resection of the Primary Tumor in Asymptomatic Patients With Stage IV Colorectal Cancer and Unresectable Metastases. Need for Improvement in Data Collection. A Systematic Review With Meta-Analysis. Surg Oncol (2020) 33:11–8. doi: 10.1016/j.suronc.2019.12.004
18. Wilkinson KJ, Chua W, Ng W, Roohullah A. Management of Asymptomatic Primary Tumours in Stage IV Colorectal Cancer: Review of Outcomes. World J Gastrointest Oncol (2015) 7(12):513–23. doi: 10.4251/wjgo.v7.i12.513
19. Verhoef C, de Wilt JH, Burger JW, Verheul HM, Koopman M. Surgery of the Primary in Stage IV Colorectal Cancer With Unresectable Metastases. Eur J Cancer (2011) 47 Suppl 3:S61–66. doi: 10.1016/S0959-8049(11)70148-4
20. Birkett RT, O’Donnell MMT, Epstein AJ, Saur NM, Bleier JIS, Paulson EC. Elective Colon Resection Without Curative Intent in Stage IV Colon Cancer. Surg Oncol (2019) 28:110–5. doi: 10.1016/j.suronc.2018.11.010
21. Nitzkorski JR, Farma JM, Watson JC, Siripurapu V, Zhu F, Matteotti RS, et al. Outcome and Natural History of Patients With Stage IV Colorectal Cancer Receiving Chemotherapy Without Primary Tumor Resection. Ann Surg Oncol (2012) 19(2):379–83. doi: 10.1245/s10434-011-2028-1
22. Zhang RX, Ma WJ, Gu YT, Zhang TQ, Huang ZM, Lu ZH, et al. Primary Tumor Location as a Predictor of the Benefit of Palliative Resection for Colorectal Cancer With Unresectable Metastasis. World J Surg Oncol (2017) 15(1):138. doi: 10.1186/s12957-017-1198-0
23. Eisenberger A, Whelan RL, Neugut AI. Survival and Symptomatic Benefit From Palliative Primary Tumor Resection in Patients With Metastatic Colorectal Cancer: A Review. Int J Colorectal Dis (2008) 23(6):559–68. doi: 10.1007/s00384-008-0456-6
24. Elias D, Vigano L, Orsi F, Scorsetti M, Comito T, Lerut J, et al. New Perspectives in the Treatment of Colorectal Metastases. Liver Cancer (2016) 6(1):90–8. doi: 10.1159/000449492
25. Kim YW, Kim IY. The Role of Surgery for Asymptomatic Primary Tumors in Unresectable Stage IV Colorectal Cancer. Ann Coloproctol (2013) 29(2):44–54. doi: 10.3393/ac.2013.29.2.44
26. Karoui M, Roudot-Thoraval F, Mesli F, Mitry E, Aparicio T, Des Guetz G, et al. Primary Colectomy in Patients With Stage IV Colon Cancer and Unresectable Distant Metastases Improves Overall Survival: Results of a Multicentric Study. Dis Colon Rectum (2011) 54(8):930–8. doi: 10.1097/DCR.0b013e31821cced0
27. de Mestier L, Manceau G, Neuzillet C, Bachet JB, Spano JP, Kianmanesh R, et al. Primary Tumor Resection in Colorectal Cancer With Unresectable Synchronous Metastases: A Review. World J Gastrointest Oncol (2014) 6(6):156–69. doi: 10.4251/wjgo.v6.i6.156
28. Cirocchi R, Trastulli S, Abraha I, Vettoretto N, Boselli C, Montedori A, et al. Non-Resection Versus Resection for an Asymptomatic Primary Tumour in Patients With Unresectable Stage IV Colorectal Cancer. Cochrane Database Syst Rev (2012) 8):CD008997. doi: 10.1002/14651858.CD008997.pub2
29. Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, et al. Asymptomatic Colorectal Cancer With Un-Resectable Liver Metastases: Immediate Colorectal Resection or Up-Front Systemic Chemotherapy? Ann Surg Oncol (2007) 14(2):766–70. doi: 10.1245/s10434-006-9146-1
Keywords: colorectal cancer, asymptomatic, primary tumor, resection, meta-analysis
Citation: Shu Y, Xu L, Yang W, Xu X and Zheng S (2022) Asymptomatic Primary Tumor Resection in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:836404. doi: 10.3389/fonc.2022.836404
Received: 15 December 2021; Accepted: 07 March 2022;
Published: 29 March 2022.
Edited by:
Bjørn Steinar Nedrebø, Haukeland University Hospital, NorwayReviewed by:
Satvinder Singh Mudan, Imperial College London, United KingdomDario Baratti, Fondazione IRCCS Istituto Nazionale Tumori (IRCCS), Italy
Copyright © 2022 Shu, Xu, Yang, Xu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yefei Shu, c2h1eWVmZWkzNjA0MjNAMTYzLmNvbQ==; Song Zheng, dHp0cmVlQDEyNi5jb20=
 Yefei Shu
Yefei Shu Ling Xu2
Ling Xu2 Wei Yang
Wei Yang