
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 24 March 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.835956
This article is part of the Research TopicMetastatic Castration Resistant Prostate Cancer: Prognosis and TreatmentView all 7 articles
 Ting Bu1†
Ting Bu1† Lulu Zhang1†
Lulu Zhang1† Fei Yu1†
Fei Yu1† Xiaochen Yao1
Xiaochen Yao1 Wenyu Wu1
Wenyu Wu1 Pengjun Zhang1
Pengjun Zhang1 Liang Shi1
Liang Shi1 Shiming Zang1
Shiming Zang1 Qingle Meng1
Qingle Meng1 Yudan Ni1
Yudan Ni1 Guoqiang Shao1
Guoqiang Shao1 Xuefeng Qiu2
Xuefeng Qiu2 Shuyue Ai1
Shuyue Ai1 Ruipeng Jia3*
Ruipeng Jia3* Hongqian Guo2*
Hongqian Guo2* Feng Wang1*
Feng Wang1*Purpose: There is increasing evidence for convincing efficacy and safety of 177Lu-labled prostate-specific membrane antigen (PSMA)-targeted radioligand therapy (PRLT) for metastatic castration-resistant prostate cancer (mCRPC). However, data are not available regarding the feasibility of 177Lu-labled PSMA-targeted RLT in East Asians. The present study summarized the first experience with 177Lu-PSMA-I&T therapy for mCRPC in China.
Methods: Forty consecutive patients with mCRPC were enrolled from December 2019 to September 2021. Eligible patients received 177Lu-PSMA-I&T RLT at intervals of 8-12 weeks. Toxicity was assessed based on standardized physicians’ reports and the Common Toxicity Criteria for Adverse Events criteria. Response to PRLT was evaluated according to the changes of prostate specific antigen (PSA) response and imaging response. Quality of life (QOL), Karnofsky performance status (KPS) and pain (visual analogue scale, VAS) were also evaluated. The impacts of baseline parameters on the therapeutic effects were explored by univariate and multivariate logistic regression analyses.
Results: All patients underwent a total of 86 cycles of 177Lu-PSMA-I&T (range: 1-5 cycles) with dosages of 3.70-14.43GBq per cycle, with a median of 8 months followed up. Six patients (15%) developed mild reversible xerostomia during follow-up, and 28 patients (70%) experienced grade 1-4 bone marrow dysfunction. Changes in PSA were assessed after therapy, accompanied by the partial response (PR) in 25 patients (62.5%), the stable disease (SD) in 5 patients (12.5%), and the progressive disease (PD) in 10 patients (25%), respectively. QOL, KPS (%) and VAS scores were improved significantly due to treatment (P<0.05). Overweight and elevated AST, ALP, and LDH were associated with poor outcomes.
Conclusions: 177Lu-PSMA-I&T achieves the favourable response and well tolerance in mCRPC, which associates with not only PSA decline but also with tumor remission including lymphadenopathy and bone metastasis. We also find that patients with overweight and high AST, ALP, and LDH should be cautious to undergo the PRLT. Large-cohort studies are warranted to confirm the initial findings and elucidate the survival benefit of the treatment.
According to cancer statistics, prostate cancer (PCa) was the third most common cancer in the United States in 2020 (1). In contrast, the incidence of prostate cancer in Asia is lower compared with western world, however, the incidence of high-risk PCa is likely to be increased, and the mortality of metastatic castration prostate cancer has been increased in the last decade. Lack of screening, limited treatment strategies in developing countries and subsequent high metastatic disease rates (30.5% in China and 23.7% in India) usually lead to reduced survival (2, 3). Once the disease has progressed to metastatic CRPC (also known as mCRPC), which is the leading cause of the morality, treatment options are limited (4, 5). Hence, novel strategies are needed to improve outcomes for these patients.
Prostate-specific membrane antigen (PSMA) is overexpressed in prostate cancer and is further increased in mCRPC. However, the biological characteristics of this type II transmembrane protein with glutamate-carboxypeptidase activity makes it an ideal target for radionuclide therapy (6–8). We previously showed that 68Ga-PSMA positron emission tomography/computed tomography (PET/CT) had higher sensitivity for detecting lymphadenopathy and visceral metastasis compared with multi-parametric magnetic resonance imaging, in terms of describing the clinical characteristics of intra-prostatic primary lesions including tumour size, shape, and location (9–12).
PSMA-targeted radioligand therapy (RLT) has been validated as a potential novel therapeutic strategy for mCRPC (13–16). A meta-analysis of 10 studies involving 455 mCRPC patients showed a pooled 68.0% decline of PSA after PSMA RLT (17). The latest VISION Clinical Trials showed that RLT with 177Lu-PSMA-617 prolonged PFS and OS when added to standard care in patients with PSMA-positive mCRPC (18). Previous studys showed that 177Lu-PSMA-I&T was produced conveniently and efficiently using an automated module, and had higher affinity towards prostate cancer cells and xenografts with higher PSMA expression (19). However, 177Lu-PSMA RLT has not been reported to treat Asian patients with mCRPC. We conducted a single-centre study to evaluate the treatment efficacy and safety of 177Lu-PSMA RLT in East Asian populations, and assessed the factors that may affect therapeutic efficacy. 68Ga-PSMA PET and 177Lu-PSMA-I&T SPECT/CT were also used to predict tumor response.
A total of 40 consecutive patients (35 Chinese and 5 South Korean cases) with confirmed mCRPC were enrolled in this study from December 2019 to September 2021. All patients underwent 68Ga-PSMA-11 PET/CT in the outpatient clinic. At least three PSMA-avid lesions were detected by PET and the patients were transferred to the nuclear medicine ward. This prospective study was approved by the local ethics committee at Nanjing Medical University (KY20171208-03, Nanjing, China), and the trial was registered at clinicaltrials.gov (NCT04188587). All patients were fully informed of the precautions and possible adverse reactions and provided signed informed consent before treatment. The study flow chart is shown in Figure 1.
PSMA-11 was purchased from ABX (Germany) and 68Ga-PSMA-11 was synthesised using an automation module (ITG, Munich, Germany). PET/CT (uMI780, United Imaging, China) was performed 45–60 min after injection of 111-185 MBq 68Ga-PSMA-11 (3-5 mCi). CT images were used for attenuation correction and accurate localization. PET imaging was acquired immediately after CT scan (matrix 256) with a 15.5 cm field of view and 3 min acquisition for each bed position.
Images were interpreted by a radiologist and a nuclear medicine physician who were blinded to the clinical characteristics and pathology. Tumour metastasis was evaluated by visual and semiquantitative analyses. Except for organs with physiologic uptake of PSMA (e.g., salivary gland, kidney, liver and small intestine), other sites with visual uptake greater than the average of the surrounding normal tissues (soft tissue/normal vertebral body) were regarded as PSMA-positive, suggesting local recurrence or metastasis. For all suspected pathological lesions (n=2329), the mean, maximum, and peak standardised uptake values (SUVmax, SUVmean and SUVpeak) of metastatic lesions and the tumour uptake volume (PSMA-TV/MTV) were calculated by VIOS with isocontours set at 45% of the maximum uptake within the respective focus. The SUVmax of liver parenchyma was also measured (mean value of 2 layers of normal liver parenchyma excluding lesions) to calculate the target/background ratio (T/B), and lesion PSMA uptake (TL-PSMA) was obtained by multiplying PSMA-TV by SUVmean. Whole-body PSMA TV (wbPSMA-TV) was the total volume of all PSMA-postive lesions, and whole-body TL-PSMA (wbTL-PSMA) was the summation of PSMA uptake by all lesions.
High-purity lutetium chloride (177LuCl3) was obtained from ITG and PSMA-I&T was purchased from the Technical University of Munich (Germany) and was synthesized using an automation module (ITG). Only 177Lu-PSMA-I&T with more than 99% radiochemical purity was used in the clinical practice.
177Lu-PSMA-I&T was administered individually every 10 ( ± 2) weeks on average. Baseline haematological tests, liver and kidney function assays, dynamic salivary gland and renal imaging were performed, and the glomerular filtration rate was calculated prior to the initiation of treatment. Renal and salivary gland functions were therefore evaluated at baseline. 177Lu-PSMA-I&T was diluted to 20 ml with saline and infused intravenously within 20 min at a rate of 60 ml/h using a special infusion pump. To minimize treatment-related salivary gland damage, all patients received oral potassium perchlorate at 30 min and 4 and 12 h before intravenous 177Lu-PSMA-I&T to reduce salivary gland uptake. Patients also received vitamin C three times a day to protect salivary gland function, and folium sennae was used as a laxative to clear the intestines. In addition, patients continued standard treatment (hormone-deprivation treatment).
Whole-body scintigraphy and SPECT/CT were performed at 48 h post-injection of 177Lu-PSMA-I&T to evaluate tumour accumulation. SPECT images were displayed in three planes. A region of interest was drawn around the tumour and main organs and the ratio of tumour to liver was calculated to quantify tumour uptake. According to the regulations of the Chinese Office of Radiation Protection, all patients were discharged 48 h after treatment.
Toxicities were assessed following the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Vital signs such as heart rate and blood pressure, and side effects such as nausea, vomiting, dyspnoea, and fatigue were observed for 4h during and after 177Lu-PSMA RLT. Blood, liver, and kidney function indexes were obtained at the fourth week after therapy. In addition to blood tests, side effects were observed during treatment and follow-up. Patients were asked about any symptoms related to RLT during their stay and at follow-up, including dry mouth, dry eyes, dysgeusia, weight loss, anorexia, fatigue, constipation, and dyspepsia.
All patients were observed to evaluate their quality of life (QOL), Karnofsky performance status (KPS, %) and pain (visual analogue scale, VAS) within 2-4 days after RLT. Serum PSA is the most important marker for evaluating the biochemical response to treatment. PSA levels were obtained before therapy and every 4 weeks subsequently. A decrease of ≥30% from baseline was considered as a partial response (PR), >25% increase in PSA above the baseline was defined as progressive disease (PD), and the change between PR and PD (< −30% and < +25%) was considered as stable disease (SD). 68Ga-PSMA-PET/CT at the 12th week after each therapy was also used to evaluate tumour response to 177Lu-PSMA. Soft tissue progression was defined according to RECIST 1.1 and bone disease progression was defined according to PCWG3 Consensus.
All statistical analyses were carried out and graphs were drawn using R (version 3.6.1) and GraphPad Prism software, respectively. Quantitative data with a normal distribution were expressed as mean ± standard deviation and data with a non-normal distribution were expressed as median (interquartile range, IQR). Wilcoxon’s signed-rank test was used to analyse treatment-related changes in safety and efficacy parameters. Univariate and multivariate logistic regression analyses were applied to identify the factors influencing the therapeutic effect. Statistical significance was established as P < 0.05.
Forty patients treated with 177Lu-PSMA-I&T were included in the study, baseline clinical characteristics are summarized in Table 1. All patients identified with mCRPC failed to standard treatment, who accompanied by multiple metastases (Table 2). Overall, 86 cycles were applied with a median of two cycles per patient (range 1-5), including two, three, four, and five cycles in sixteen, six, two, and two patients, respectively. Among the patients with only one cycle’ treatment, 10 patients gave up continuing treatment for various reasons, four patients with progressive diseases, five with financial difficulties, and one for the strict control of coronavirus. Individualized dose administration varied in all 40 patients, with the dosage per cycle ranging from 3.70-14.43GBq based on ECOG performance status, the extent of disease, and haematological, liver, and kidney function parameters. The median follow-up duration was 8 months (range: 2-20 months).
Post-therapy 177Lu-PSMA-I&T SPECT showed higher focal uptake in primary and metastatic lesions and lower uptake in normal liver and spleen. Adequate hydration and oral potassium perchlorate decreased the physiological uptake in normal lacrimal and salivary glands, small intestine, and kidneys. Radioactivity was mainly excreted rapidly via the kidneys, with uptake in the main organs significantly reduced or absent after 24h (Figure 2). PSMA-avid lesions were detected on 177Lu-PSMA-I&T images, with a median (IQR) tumour to liver ratio of 3.38 (2.50, 5.72) in the PR group compared with 2.80 (1.56, 4.70) in the PD group.
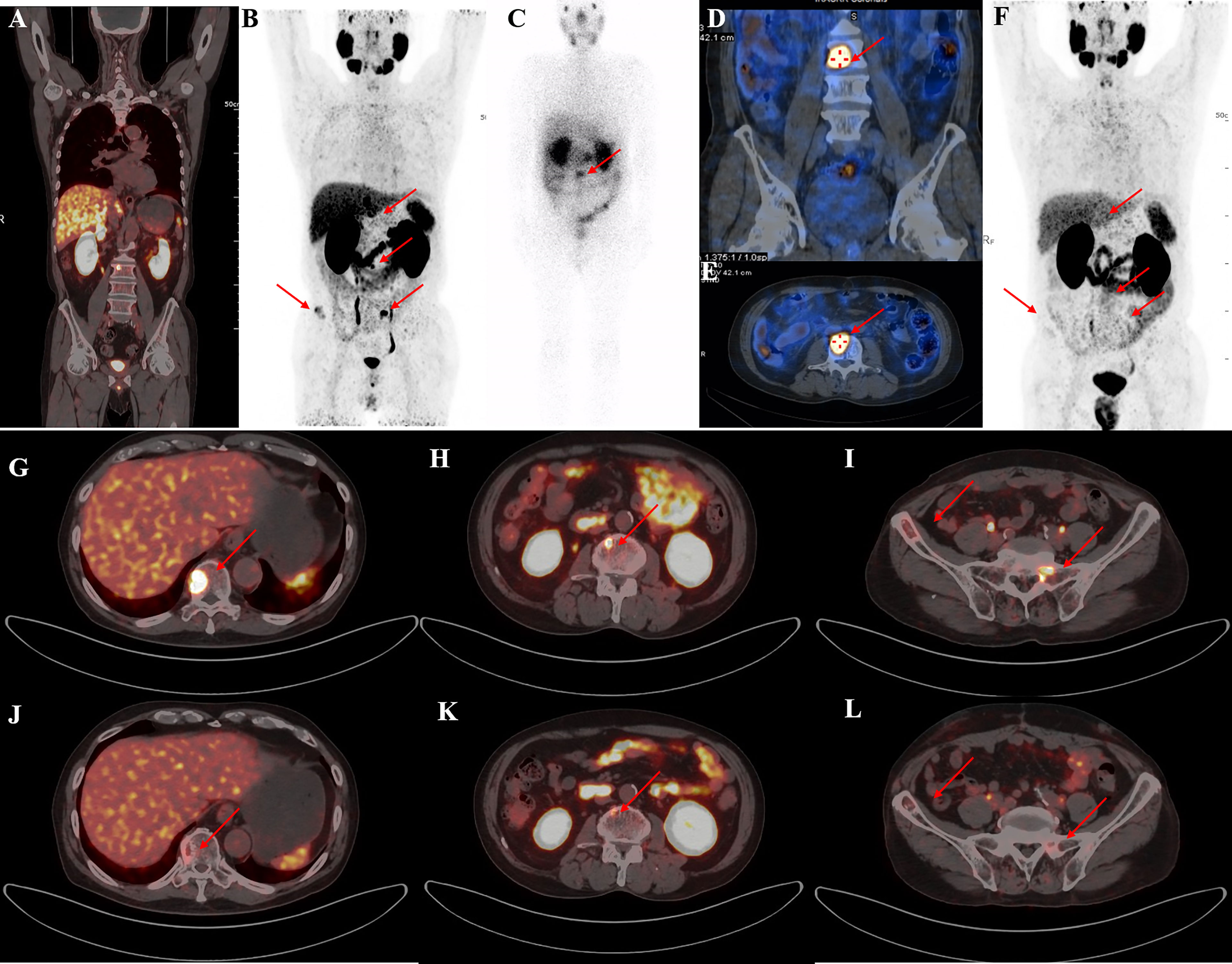
Figure 2 A representative 59-year-old patient with PSA decline ≥99% before and after Lu-PSMA-I&T therapy. (A, B) Baseline image and (F) image 3 months post-therapy. 177Lu-PSMA SPECT whole-body scan (C) showing higher contrast images 24 h post-injection with significant accumulation in the tenth thoracic vertebra sclerotic lesion. (D, E): 177Lu SPECT image at 48 h showing destroyed and uneven density of L3 vertebrae with PSMA-avid lesion. Fusion image (G-L) showing reduced metastases in T10, L3, the right iliac, and sacrum compared with before therapy.
There were no transfusion-related complications or treatment-related deaths within 24 h after 177Lu-PSMA-I&T treatment, and no significant changes was seen in heart rate, blood pressure, or body temperature. Chest tightness with panic and shortness of breath occurred in one patient within 1-2 months after PSMA RLT treatment. Grade 1-2 fatigue and drowsiness were seen in 3(7.5%) and 2(5%) patients, respectively, on the second day after therapy. About 6 patients (15%) had mild to moderate xerostomia during follow-up.
Grade 1-4 impairment of bone marrow dysfunction occurred in up to 28 patients (70%). Before RLT, 5(12.5%) and 2(5%) patients had grade 2 and 3 anaemia, respectively, 8(20%) had grade 1 leukopenia, and 1(2.5%) had grade 1 and grade 3 thrombocytopenia. After treatment, 5 patients (12.5%) reported grade 3-4 anaemia, 1 patient (2.5%) reported grade 3 leukopenia, and 5 patients (12.5%) had grade 3–4 thrombocytopenia. In all 86 cycles, there was a significant decrease in haemoglobin [pre-therapy 110.00 (96.00, 125.00), post-therapy 107.00 (97.00, 119.00), P<0.05], erythrocyte counts [pre-therapy 3.56 (3.15, 3.87), post-therapy 3.33 (3.13, 3.79), P<0.05], leukocyte counts [pre-therapy 5.01 (4.00, 6.87), post-therapy 4.42 (3.80, 5.80), P<0.05] and platelet counts [pre-therapy 207.00 (158.20, 243.00), post-therapy 158.50 (120.50, 226.00), P<0.05].
No significant hepatotoxicity or nephrotoxicity was reported in the study. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels remained relatively stable after each cycle, while 2 patients diagnosed with liver injury with elevated AST, 1 patient developed transient grade 1 renal impairment with a slight increase in serum creatinine after therapy. All adverse events are listed in Table 3.
At a median of 8 weeks after the first cycle of 177Lu-PSMA-I&T RLT, PSA was declined in 26 patients (65%) and PR was achieved in 18 patients (45%), indicating an improved efficacy for most patients. Furthermore, at the 8-week follow-up after the last cycle of therapy for all patients (including those who received only one cycle), the best PSA response showed that 28 patients (70%) had a PSA decline (P<0.05) (Figure 3). Overall, 10 patients (25%) had PD (>25% increase in serum PSA over baseline), 25 patients (62.5%) had PR (≥30% decrease in serum PSA). The number of patients achieving maximum PSA decline of >50% and >90% were 22, 4, respectively. At follow-up, 10 patients (25%) had developed clinical progression and 5 were dead (among them, 3 patients died of malignant tumor, the other 2 patients were because of heart and cerebral vascular disease).
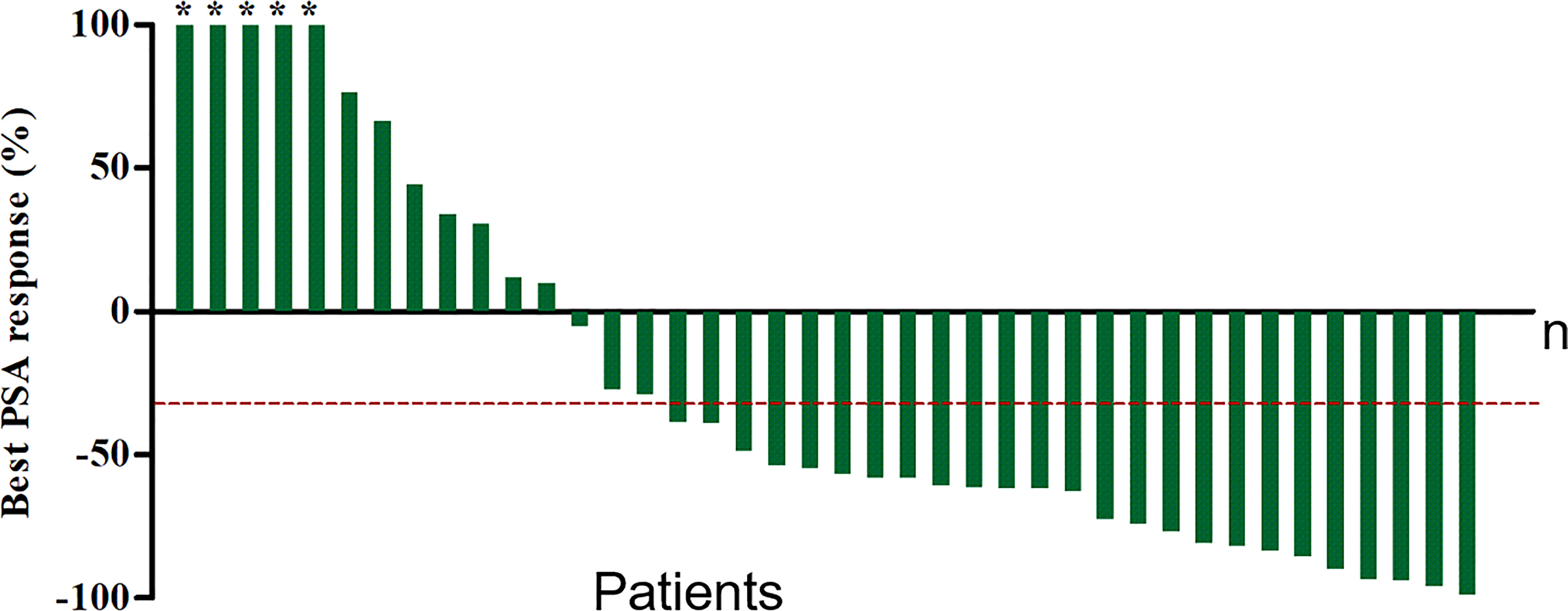
Figure 3 Waterfall plot of best PSA decline compared with baseline. Plots ordered from worst to best responder. Red dotted line represents 30% PSA decline. *The maximum PSA decline >90%.
`Of the 40 patients, 35 patients (87.5%) had pain at baseline and the VAS decreased compared with baseline after all cycles [median (IQR): baseline 3 (1, 4); after all cycles 1 (0, 2.25), P<0.05]. KPS, QOL were increased in 36 patients and were significantly improved after treatment [KPS: median (interquartile range): baseline 90 (77.5, 90); after all cycles 95 (80, 100), P<0.05; QOL: baseline 44 (40, 47.25); after all cycles 49 (46.75, 52.25), P<0.05].
68Ga-PSMA PET-CT was performed in 23 patients followed by two or more cycles of 177Lu-PSMA-I&T. The median (IQR) SUVmax values of the target lesion before and after therapy were 27.7 (11.5, 44.7) and 20.27 (7.35, 35.92), respectively. 68Ga-PSMA PET revealed PD in 4 patients (17.4%). Eleven patients (47.8%) had PR with a better response for the significant decreased tumour size, reduced SUVmax, and disappearance of bone and lymph node metastases (Figures 4–6). One patient from Korea with multiple bone metastases achieved a ≥98% PSA decline after one cycle of 177Lu-PSMA-I&T and an almost complete response (Figure 2).
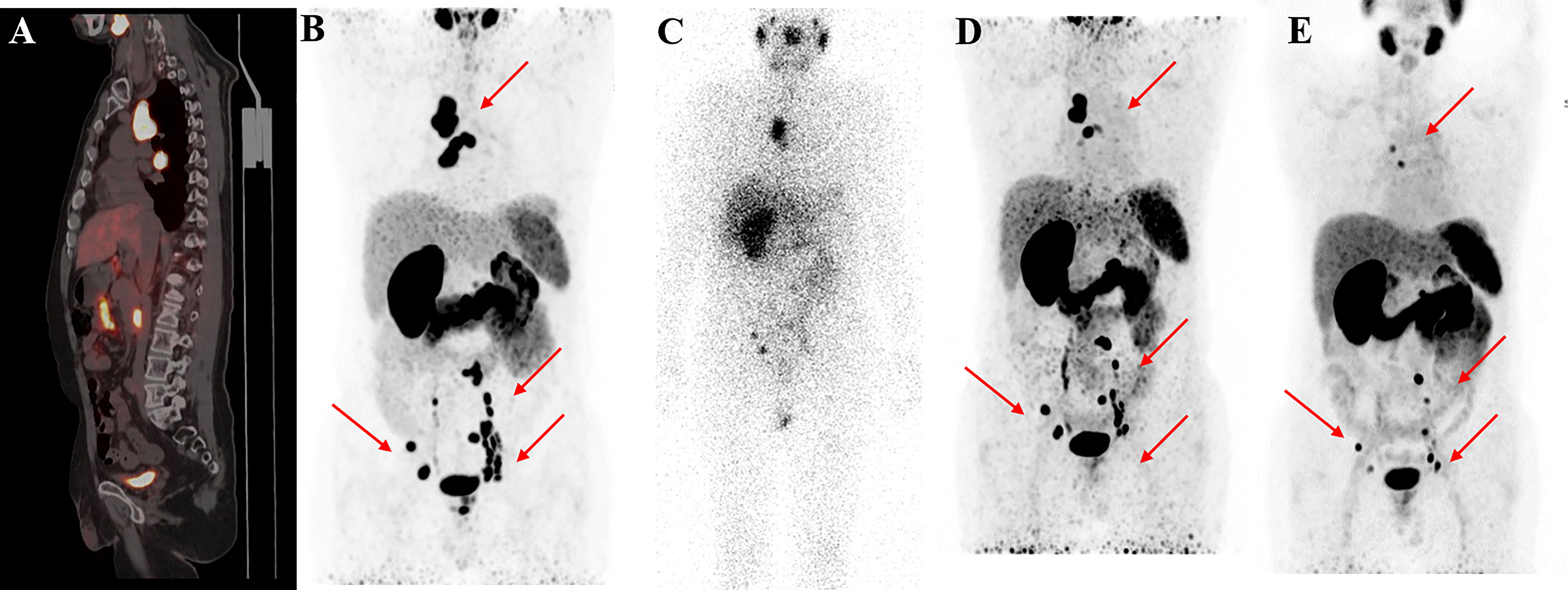
Figure 4 68Ga-PSMA-11 PET/CT in a 55-year-old patient who underwent four cycles of 177Lu-PSMA-I&T therapy. Baseline images (A, B) of multiple lymph node metastases in the right subclavian area, mediastinum, retroperitoneum, and pelvis. Post-therapy image (C) showing higher 177Lu-PSMA-I&T aggregation in primary and metastatic lesions. 68Ga-PSMA PET/CT after the first cycle (D) and after the third cycle (E) showing multiple decreased or disappeared metastases.
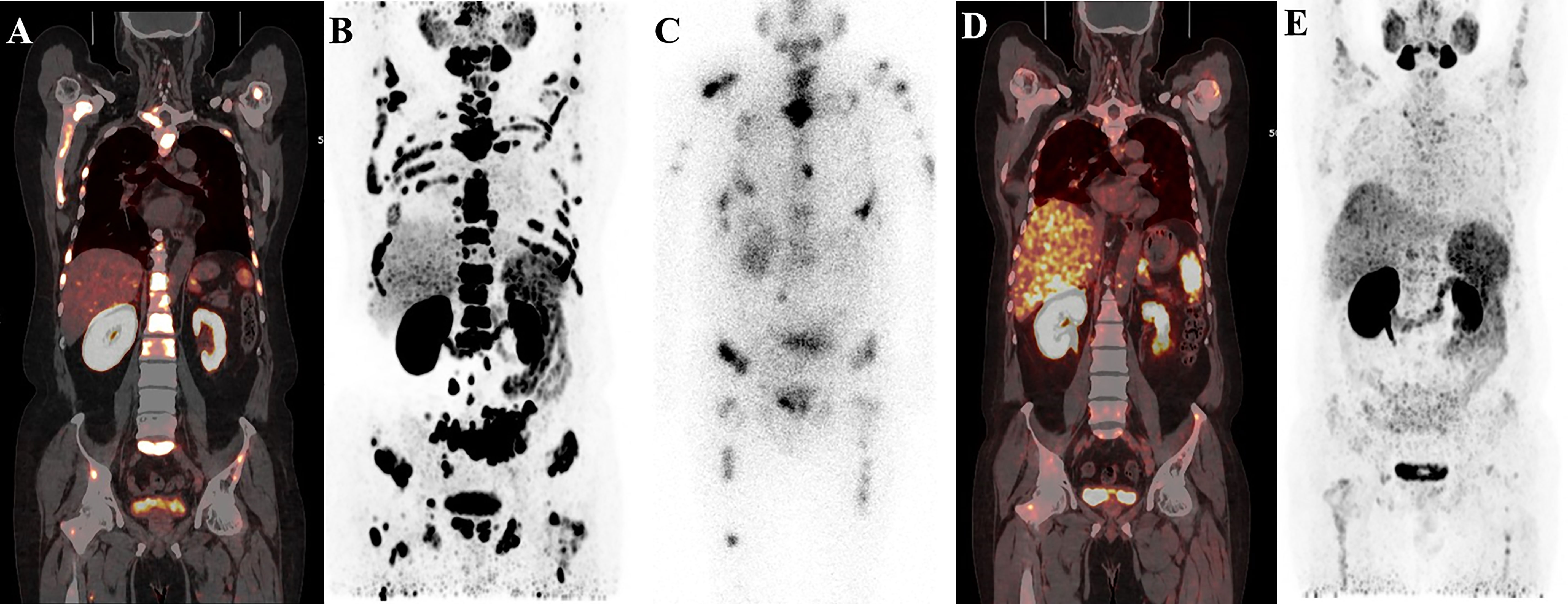
Figure 5 A 58-year-old patient with mCRPC with multiple systemic metastases. Baseline (A, B) 68Ga-PSMA-11 PET/CT image showing sclerotic bone metastases with higher PSMA expression. Post-therapy image (C) showing higher accumulation of 177Lu-PSMA-I&T in primary and metastatic lesions. Post-treatment image (D, E) showing extensive osteogenic bone metastases throughout the body, and reduced expression of PSMA compared with before therapy, but some weak remaining PSMA expression, suggesting that the treatment was effective.
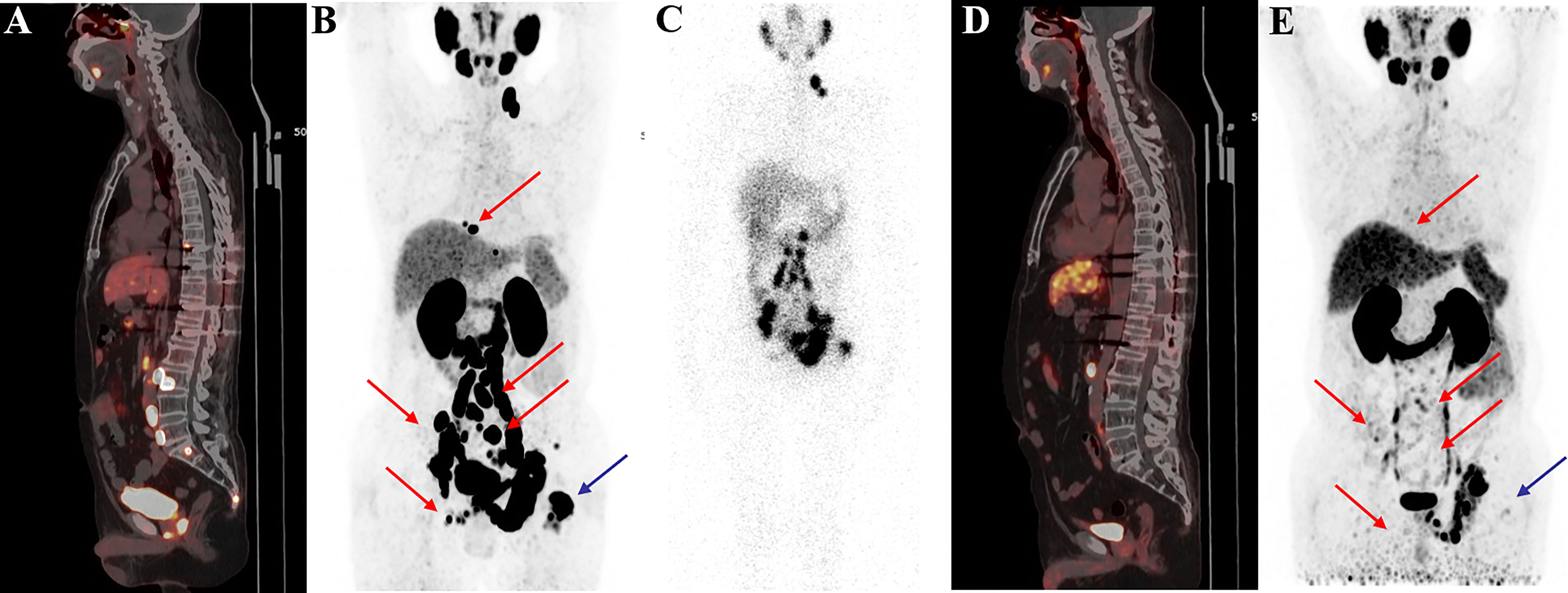
Figure 6 68Ga-PSMA-11 PET/CT in a 62-year-old patient with mCRPC. Baseline images (A, B) of multiple bone and lymph node metastases with increased PSMA expression. Post-therapy image (C) showing high 177Lu-PSMA-I&T aggregation in primary and metastatic lesions. Post-treatment image (D, E) showing multiple osteogenic metastases and reduced PSMA expression compared with before therapy. Metastatic foci in the left clavicle, abdominal aorta, and bilateral iliac paravascular lymph nodes were significantly reduced and PSMA expression was decreased, suggesting that the treatment was effective.
Patients were classified into a remission and non-remission, and progression and non-progression groups according to PR and PD. Univariate logistic regression analysis of pretherapeutic signs [age, body mass index (BMI), KPS, serum PSA] and prior treatments confirmed that higher BMI was related to clinical progression [odds ratio (OR) and 95% confidence intervals (CI) 6.91 (1.43, 51.56)]. We also found that higher baseline serum AST, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were associated with poorer outcomes (high levels of the above three biomarkers were negatively related to remission [OR (95%CI) 0.17 (0.03, 0.68) for AST, 0.20 (0.05, 0.79) for ALP, and 0.20 (0.05, 0.79) for LDH] and positively related to progression [11.77 (1.87, 231.47), 6.00 (1.24, 44.58), and 6.00 (1.24, 44.58), respectively)]. A higher BMI was also associated with clinical progression (OR (95%CI) 6.91 (1.43, 51.56) (Table 4). In addition, baseline imaging parameters including post-therapy SPECT/CT (T/BSPECT/CT), pre-therapeutic 68Ga-PSMA-11 PET-CT (e.g., PSMA-TV, TL-PSMA, SUVmax, SUVpeak, T/BPET, number of metastatic lesions, and presence of visceral metastasis) showed no significant correlation with response (Table 5).
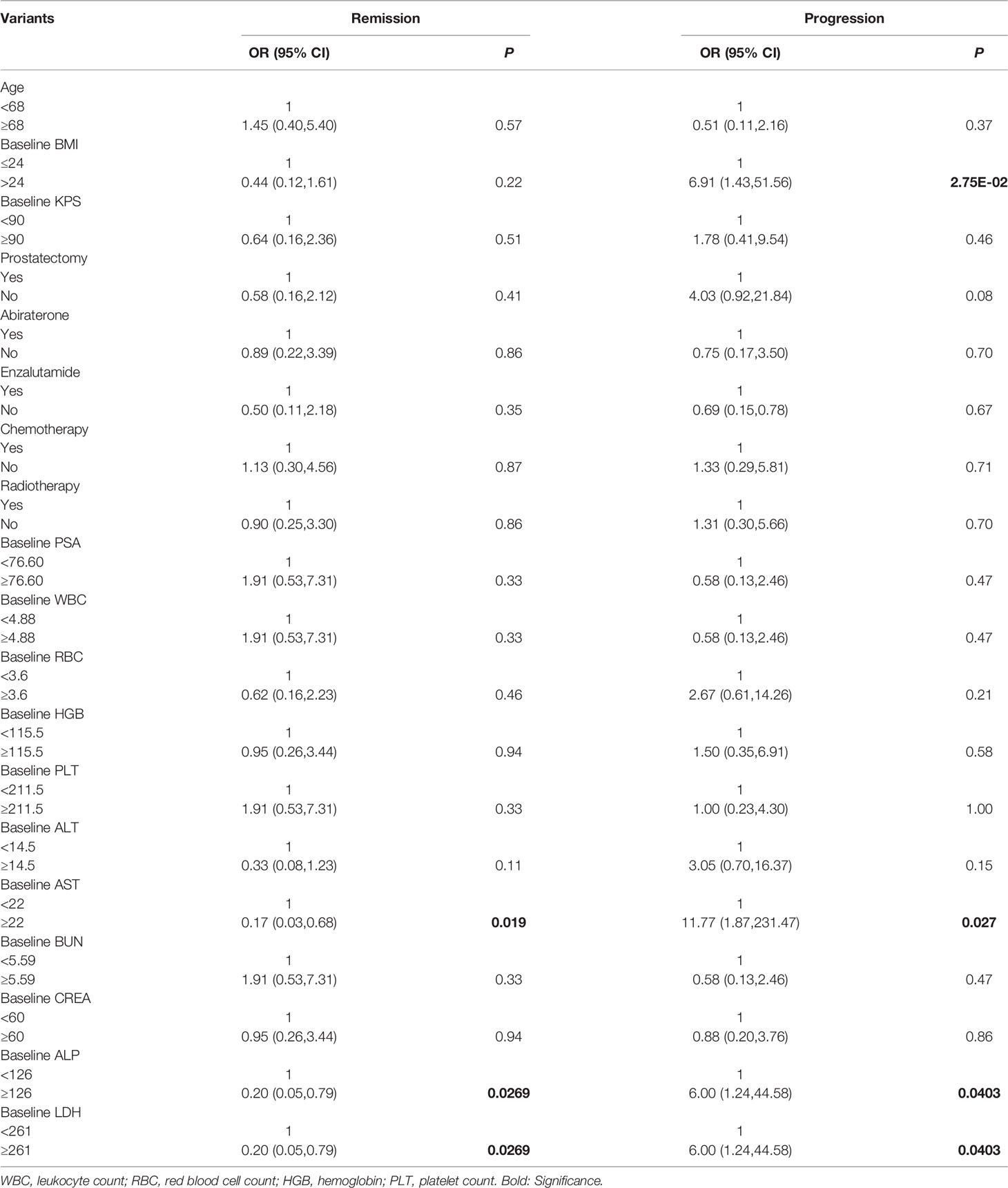
Table 4 Univariate logistic regression analysis on basic factors associated with therapeutic effect.
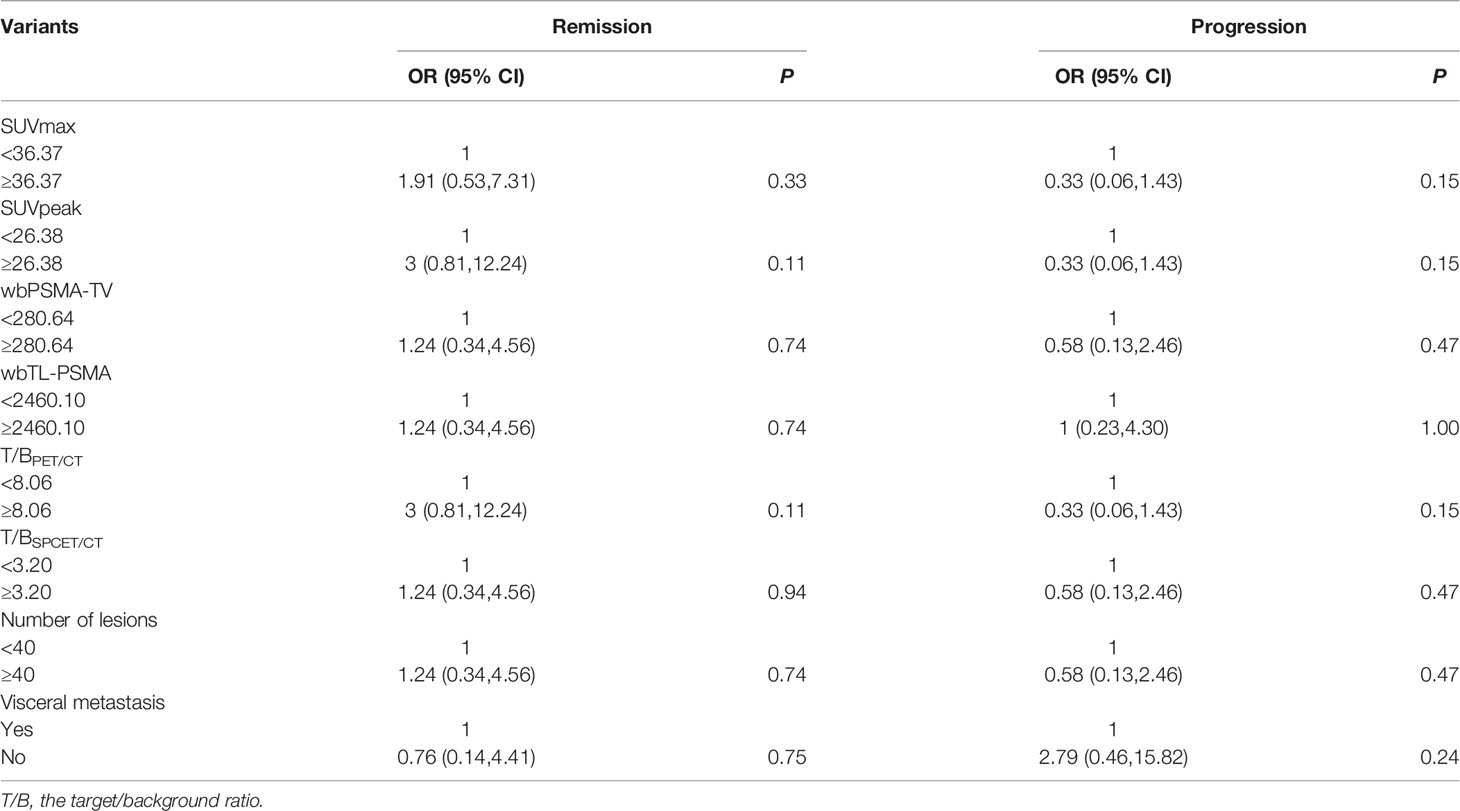
Table 5 Univariate logistic regression analysis on basic imaging factors associated with therapeutic effect.
We assessed the relation among AST, ALP, and LDH with multivariate logistic regression analysis (Figure 7). Only AST and BMI were therefore included in the multivariate logistic regression analysis, and both were independent predictors of progression after RLT (Table 6).
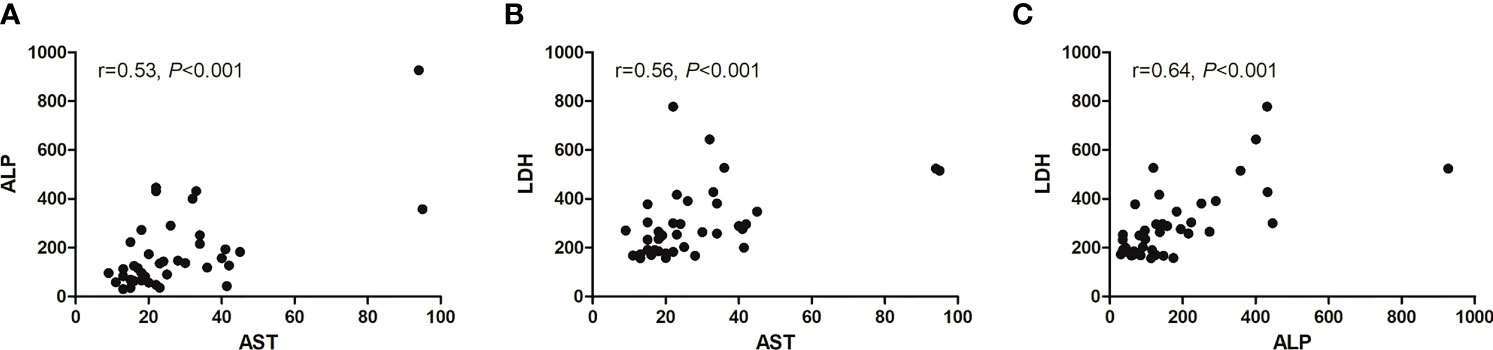
Figure 7 Scatter diagram showing correlation between three baseline serum biomarkers. (A) AST and ALP, (B) AST and LDH, and (C) ALP and LDH.
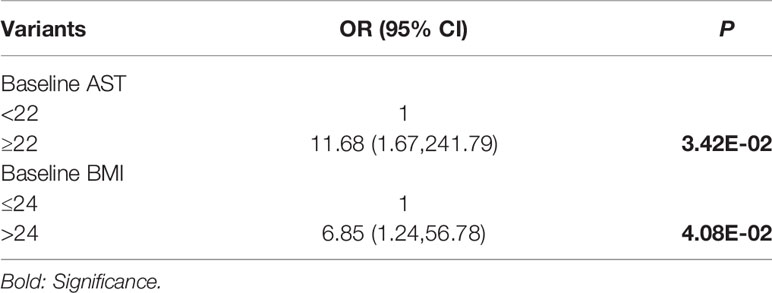
Table 6 Multivariate logistic regression analysis on factors associated with disease progression after PRLT.
The Vision Clinical trial showed that PRLT significantly improved treatment outcomes compared with standard medical treatment, and that 177Lu-PSMA-617 might be a new horizon for the treatment of mCRPC (20, 21). PSMA-I&T and PSMA-617 are the most widely studied PSMA inhibitory ligands in the field of prostate cancer treatment. There was no significant difference in curative effects and toxic reactions between the two radioactive ligands, but the average absorbed dose of 177Lu-PSMA-I&T is lower than that of 177Lu-PSMA-617 in lacrimal gland, which rarely causes symptoms of lacrimal gland dysfunction such as dry eyes and blurred vision (19). However, there are limited sporadic clinical studies on 177Lu-PSMA-I&T (19, 22). To date, PSMA ligand radionuclide therapy for prostate cancer has not been fully established for its widespread use in Asia. This study was to explore the clinical manifestations of 177Lu-PSMA-I&T in the East Asians.
The fixed activity of 177Lu-PSMA is usually used for PRLT, which has some merits for decrease the toxicity of the regimen, but might have some impact on treatment efficacy (15, 22). Herein, we speculated that higher activities per cycle are probably feasible and may increase the response rates and/or duration of response. Gaertner FC et al. reported that individual adaptations of therapy protocols based on diagnostic PSMA PET imaging before therapy might help to further promote efficacy and decrease the toxicity of RLT (23). For patients with large PSMA-positive tumor volumes, higher activities can be safely administered to maximize tumor biologically effective doses (BEDs) without exceeding the tolerable BED to the organs at risk, and for patients with severe tumor burden, the optimal activity can reach 14.84GBq (24). Thus, in this study, we conducted individualized administration based on pre-treatment PET and/or dosimetry studies.
In a large meta-analysis of 145 mCRPC patients who received PSMA RLT in Germany, 72% had PSA declines after the second cycle, which is similar to the current results (16). In this study, the therapeutic effects of 177Lu-PSMA-I&T on East Asian populations were systematically implemented. The results showed that 65% of patients had a PSA decline after the first cycle, and PSA continued to decrease during subsequent treatment. After the last cycle, 70% had a PSA decline. Probably because of differences in tumor staging among treated patients and differences in disease characteristics among ethnic groups, this considerable finding is slightly superior to results of another clinical experience with 177Lu-PSMA-I&T in Germany in PSA response. The percentage of patients achieving maximum PSA declines of 30%, 50%, and 90% were 47%, 38%, 11% in the previous study and 62.5%, 55%, 10% in this study, respectively (22).
68Ga-PSMA PET/CT is of great value for evaluating treatment response (14, 15, 25, 26). Based on baseline images and SUVmax of target lesions, four patients progressed with extensive metastases after two cycles, 8 patients had SD and 11 patients showed better response, with a significant reduction in tumour size and SUVmax. Furthermore, 68Ga-PSMA-PET/CT provides useful details about the target lesions, such as tumour size reduction and SUV decrease and the remain lesions. In short, 177Lu-PSMA-I&T RLT can be applied in patients with mCRPC, who fail to prior therapies.
In current study, significant physical and psychological improvements were observed after therapy. In addition to the changes in biochemical parameters and tumour size in images, VAS, KPS, and QOL were all enhanced after therapy. Notably, some patients with PD still showed pain relief and improved QOL, which was not consistent with a poor PSA response. We supposed that this could be due to a large tumour burden and visceral metastasis, and combined systematic treatment might further improve the outcome.
Treatment-emergent adverse events under RLT with 177Lu-PSMA-I&T were mild in East Asians, and no treatment was stopped due to side effects, which is comparable with other studies [16,18,22]. Salivary glands are radiation-sensitive organs due to significant PSMA expression (27). Previous and current studies found high physiological uptake in the salivary glands of patients undergoing 68Ga-PSMA PET-CT and post-therapy scintigraphy, which could cause xerostomia and impair QOL (28, 29). However, only 15% patients in the current study experienced mild to moderate xerostomia after relatively high-dose treatment. Oral potassium perchlorate and vitamin C reduced uptake by the salivary glands and promoted clearance, and these medications may thus have reduced the incidence of xerostomia. Preclinical study is currently being conducted to confirm this assumption. No other short or long-term side effects (nausea, vomiting, diarrhea, etc.) have been observed in any patients.
In the current study, 70% of patients developed grade 1–4 haematotoxicity, and only 20% worsened to grade 3–4 haematotoxicity. Adverse events were slightly higher than in other mCRPC cohorts (30); however, this may be because some patients had late-stage disease and failed to other treatment strategies. Progressive mCRPC is prone to bone marrow invasion, leading to extensive damage to granulocytes, red blood cells, and platelets. The included patients had already undergone comprehensive treatment and eventually developed mCRPC, and 19 patients had already developed haematological toxicity, including two with grade 3 haematotoxicity prior to PSMA RLT, suggesting that this transient haematological toxicity remains controllable. No significant liver or kidney toxicity was reported in our study.
Tumour burden on whole-body PSMA-TV and TL-PSMA has been reported to be consistent with tumour response (16, 26). In this study, no significant correlation was found between PSMA PET (SUVmax, SUVpeak, T/B, PSMA-TV, and TL-PSMA) and tumour response to PRLT, this phenomenon might be related with the relatively small population in this study. However, 68Ga-PSMA PET/CT was valuable for identifying biomedical recurrence and the remaining tumour, which is helpful for the selection of further treatment. In addition, previous treatment had no impact on tumor response, which was consistent with other study (16).
The impact of baseline laboratory results on PSA response was also assessed. We found that increased AST, ALP, and LDH were negative predictors of a PSA response. It was reported that elevated LDH increased the risk of progression under PSMA RLT (31). In malignant tumours, increased LDH reflects the tumour load and tumour progression, making it valuable for risk stratification in prognostic models and for guiding clinical treatment. In this study, univariate logistic regression analysis showed that mCRPC patients with LDH >261 U/L had a relatively poor PSA response during treatment compared with patients with an LDH <261 U/L, while abnormally elevated LDH during PRLT also indicated a relatively poor treatment outcome. Several researchers have reported that high ALP and LDH were associated with poorer survival (32, 33). Moreover, this study also reflected that overweight (BMI>24) is correlated positively with disease progression after RLT. This is the first time that the correlation between BMI and treatment response was observed, and larger studies are needed to validate the finding. Herein, patients with relevant risk factors (overweight and elevated AST, ALP, and LDH) should be closely monitored to allow their therapy to be adjusted in case of disease progression.
This study had some limitations. First, the study was carried out at a single institution in a relatively small population, which might lead to some bias. Second, the primary endpoint for efficacy was based on PSA level and imaging response, but not all patients underwent 68Ga-PSMA-11 PET/CT after each cycle. Third, personalized dosages were used, which might affect treatment efficacy. However, this real-world data showed that most patients benefited from 177Lu-PSMA RLT, with improved QOL. In addition, the recruited patients were still being followed-up at the time of analysis and survival data were not available. Further studies are warranted to clarify the optimal dose and cycle interval to maximize the benefits of PRLT.
This prospective single-arm clinical trial demonstrated that 177Lu-PSMA-I&T RLT achieve favourable response and significantly improved QOL in East Asian patients with mCRPC with well tolerance, which associates with significant PSA decline and tumor remission including adenopathy and bone metastasis. Furthermore, a subgroup analysis validated increased BMI, AST, ALP, and LDH were independent predictors of progression after RLT outcome. 177Lu-PSMA-I&T RLT might thus be an alternative treatment for mCRPC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
This prospective study was approved by the local ethics committee at Nanjing Medical University (KY20171208-03, Nanjing, China). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This research was supported by grants from Jiangsu Provincial Key Research and Development Program (BE2021605), National Natural Science Foundation of China (92049111,82003532,11805104 and 81570613), Jiangsu Provincial Frontier Grant (BE2017612), Nanjing Science and Technology Development Project (201911042) and Nanjing Medical Foundation (ZKX17027). All the funding supported equally in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all participants and staff from the Nanjing First Hospital for their contribution to the study. We also thank Susan Furness, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, et al. Epidemiology and Genomics of Prostate Cancer in Asian Men. Nat Rev Urol (2021) 18:282–301. doi: 10.1038/s41585-021-00442-8
4. Lorente D, Castro E, Lozano R, Puente J, Romero-Laorden N, Rodríguez-Vida A, et al. Association Between Second Progression-Free Survival (PFS2) and Overall Survival in Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2020) 77:763–6. doi: 10.1016/j.eururo.2020.02.025
5. Shore N, Heidenreich A, Saad F. Predicting Response and Recognizing Resistance: Improving Outcomes in Patients With Castration-Resistant Prostate Cancer. Urology (2017) 109:6–18. doi: 10.1016/j.urology.2017.04.062
6. Rowe SP, Drzezga A, Neumaier B, Dietlein M, Gorin MA, Zalutsky MR, et al. Prostate-Specific Membrane Antigen-Targeted Radiohalogenated PET and Therapeutic Agents for Prostate Cancer. J Nucl Med (2016) 57:90–6. doi: 10.2967/jnumed.115.170175
7. Inubushi M, Miura H, Kuji I, Ito K, Minamimoto R. Current Status of Radioligand Therapy and Positron-Emission Tomography With Prostate-Specific Membrane Antigen. Ann Nucl Med (2020) 34:879–83. doi: 10.1007/s12149-020-01549-5
8. Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, et al. PSMA Ligands for PET Imaging of Prostate Cancer. J Nucl Med (2017) 58:1545–52. doi: 10.2967/jnumed.117.191031
9. Zang S, Shao G, Cui C, Li TN, Huang Y, Yao X, et al. 68Ga-PSMA-11 PET/CT for Prostate Cancer Staging and Risk Stratification in Chinese Patients. Oncotarget (2017) 8:12247–58. doi: 10.18632/oncotarget.14691
10. Gao J, Zhang C, Zhang Q, Fu Y, Zhao X, Chen M, et al. Diagnostic Performance of 68Ga-PSMA PET/CT for Identification of Aggressive Cribriform Morphology in Prostate Cancer With Whole-Mount Sections. Eur J Nucl Med Mol Imaging (2019) 46:1531–41. doi: 10.1007/s00259-019-04320-9
11. Zhang Q, Zang SM, Zhang C, Fu Y, Lv X, Zhang Q, et al. Comparison of 68Ga−PSMA−11 PET−CT With mpMRI for Preoperative Lymph Node Staging in Patients With Intermediate to High−Risk Prostate Cancer. J Transl Med (2017) 15:230–8. doi: 10.1186/s12967-017-1333-2
12. Wang B, Gao J, Zhang Q, Fu Y, Liu G, Shi J, et al. Diagnostic Value of 68Ga-PSMA PET/CT for Detection of PTEN Expression in Prostate Cancer: A Pilot Study. J Nucl Med (2019) 61:873–80. doi: 10.2967/jnumed.119.236059
13. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
14. Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester HJ, et al. Early Experience of Rechallenge Lu-PSMA Radioligand Therapy After an Initial Good Response in Patients With Advanced Prostate Cancer. J Nucl Med (2019) 60(5):644–8. doi: 10.2967/jnumed.118.215715
15. Gafita A, Heck MM, Rauscher I, Tauber R, Cala L, Franz C, et al. Early Prostate-Specific Antigen Changes and Clinical Outcome After Lu-PSMA Radionuclide Treatment in Patients With Metastatic Castration-Resistant Prostate Cancer. J Nucl Med (2020) 61:1476–83. doi: 10.2967/jnumed.119.240242
16. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German Multicenter Study Investigating 177lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med (2017) 58:85–90. doi: 10.2967/jnumed.116.183194
17. Kim YJ, Kim YI. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-Analysis. Clin Nucl Med (2018) 43:728–34. doi: 10.1097/RLU.0000000000002210
18. Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J Nucl Med (2016) 57:97–104. doi: 10.2967/jnumed.115.170167
19. Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic Radioligand Therapy With (177) Lu Labeled Prostate Specific Membrane Antigen Ligand for Imaging and Therapy in Patients With Metastatic Castration Resistant Prostate Cancer. J Urol (2016) 196:382–91. doi: 10.1016/j.juro.2016.02.2969
20. Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand Therapy With Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol (2019) 213(2):275–85. doi: 10.2214/AJR.18.20845
21. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [Lu]-PSMA-617 Radionuclide Treatment in Patients With Metastatic Castration-Resistant Prostate Cancer (LuPSMA Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol (2018) 19(6):825–33. doi: 10.1016/S1470-2045(18)30198-0
22. Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy With Lu-PSMA-I&T in Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2019) 75:920–6. doi: 10.1016/j.eururo.2018.11.016
23. Gaertner FC, Halabi K, Ahmadzadehfar H, Kürpig S, Eppard E, Kotsikopoulos C, et al. Uptake of PSMA-Ligands in Normal Tissues is Dependent on Tumor Load in Patients With Prostate Cancer. Oncotarget (2017) 8:55094–103. doi: 10.18632/oncotarget.19049.23
24. Begum NJ, Thieme A, Eberhardt N, Tauber R, D’Alessandria C, Beer AJ, et al. The Effect of Total Tumor Volume on the Biologically Effective Dose to Tumor and Kidneys for 177Lu-Labeled PSMA Peptides. J Nucl Med (2018) 59:929–33. doi: 10.2967/jnumed.117.203505.24
25. Kessel K, Seifert R, Weckesser M, Roll W, Humberg V, Schlack K, et al. Molecular Analysis of Circulating Tumor Cells of Metastatic Castration-Resistant Prostate Cancer Patients Receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics (2020) 10:7645–55. doi: 10.7150/thno.44556
26. Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester HJ, et al. Experience With Volumetric Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients With Prostate Cancer. J Nucl Med (2017) 58:1962–8. doi: 10.2967/jnumed.117.193581
27. Tönnesmann R, Meyer PT, Eder M, Baranski AC. In Vitro [Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative Autoradiography. Pharmaceut (Basel) (2019) 12:18. doi: 10.3390/ph12010018
28. Taïeb D, Foletti JM, Bardiès M, Rocchi P, Hicks RJ, Haberkorn U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does it Matter? J Nucl Med (2018) 59:747–8. doi: 10.2967/jnumed.118.207993
29. Langbein T, Chaussé G, Baum RP. Salivary Gland Toxicity of PSMA Radioligand Therapy: Relevance and Preventive Strategies. J Nucl Med (2018) 598:1172–3. doi: 10.2967/jnumed.118.214379
30. Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early Side Effects and First Results of Radioligand Therapy With (177) Lu-DKFZ-617 PSMA of Castrate-Resistant Metastatic Prostate Cancer: A Two-Centre Study. EJNMMI Res (2015) 5:114. doi: 10.1186/s13550-015-0114-2
31. Rathke H, Holland-Letz T, Mier W, Flechsig P, Mavriopoulou E, Röhrich M, et al. Response Prediction of Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J Nucl Med (2020) 61(5):689–95. doi: 10.2967/jnumed.119.231431
32. Ferdinandus J, Violet J, Sandhu S, Hicks RJ, Ravi Kumar AS, Iravani A, et al. Prognostic Biomarkers in Men With Metastatic Castration-Resistant Prostate Cancer Receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging (2020) 47(10):2322–7. doi: 10.1007/s00259-020-04723-z
Keywords: 177Lu, prostate cancer, metastatic castration-resistant prostate cancer (mCRPC), prostate-specific membrane antigen (PSMA), radioligand therapy (RLT)
Citation: Bu T, Zhang L, Yu F, Yao X, Wu W, Zhang P, Shi L, Zang S, Meng Q, Ni Y, Shao G, Qiu X, Ai S, Jia R, Guo H and Wang F (2022) 177Lu-PSMA-I&T Radioligand Therapy for Treating Metastatic Castration-Resistant Prostate Cancer: A Single-Centre Study in East Asians. Front. Oncol. 12:835956. doi: 10.3389/fonc.2022.835956
Received: 15 December 2021; Accepted: 28 February 2022;
Published: 24 March 2022.
Edited by:
Benyi Li, University of Kansas Medical Center, United StatesReviewed by:
Marcus Unterrainer, Ludwig Maximilian University of Munich, GermanyCopyright © 2022 Bu, Zhang, Yu, Yao, Wu, Zhang, Shi, Zang, Meng, Ni, Shao, Qiu, Ai, Jia, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, ZmVuZ3dhbmdjbkBob3RtYWlsLmNvbQ==; Ruipeng Jia, cnVpcGVuZ2pAMTYzLmNvbQ==; Hongqian Guo, ZHIuZ2hxQG5qdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.