- Department of Urology, Peking University First Hospital, Beijing, China
Background: Numerous studies have investigated the risk factors of intravesical recurrence (IVR) after radical nephroureterectomy (RNU) in patients with upper urinary tract urothelial carcinoma (UTUC). However, few studies explore the predictors for unfavorable pathological types of IVR following RNU.
Methods: We retrospectively reviewed 155 patients diagnosed with bladder cancer (BC) following RNU. Binary logistic regression was used for the univariable and multivariable analyses. Nomograms were developed based on the multivariable analysis. The concordance index (C-index) was used to assess the performance of the nomograms. We performed internal validation by generating calibration plots.
Results: Muscle-invasive BC (MIBC) was significantly correlated with operation interval (p = 0.004) and UTUC T-stage (p = 0.016). Operation interval (p = 0.002) and UTUC T-stage (p = 0.028) were also risk factors for BC > 3 cm. UTUC grade (p = 0.015), operation interval (p = 0.003), and hydronephrosis (p = 0.049) were independent predictors for high-grade BC (HGBC). MIBC (p = 0.018) and surgical approach (p = 0.003) were associated with multifocal IVR. Besides, MIBC and HGBC were associated with UTUC grade (p = 0.009), operation interval (p = 0.001), and hydronephrosis (p = 0.023). Moreover, only operation interval (p = 0.036) was a predictor for BC with at least one unfavorable pathological type. We developed nomograms for MIBC, HGBC, BC > 3 cm, and MIBC and/or HGBC. The calibration curves of the nomograms showed good agreement between the observation and prediction cases. The C-indexes of the nomograms were 0.820 (95% CI, 0.747–0.894), 0.728 (95% CI, 0.649–0.809), 0.770 (95% CI, 0.679–0.861), and 0.749 (95% CI, 0.671–0.827), respectively.
Conclusions: The current study found that operation interval, UTUC T-stage, UTUC grade, surgical approach, and hydronephrosis are independent predictors for unfavorable pathological types of IVR following RNU. Nomograms based on these predictors were developed and internally validated to assess the risk of developing unfavorable pathological types of IVR. Furthermore, patients at high risk of developing unfavorable pathological types BC may benefit from more active follow-up 1 year after RNU by early detection of IVR.
Introduction
Upper urinary tract urothelial carcinoma (UTUC) is a relatively uncommon disease and accounts for 5%–10% of urothelial carcinomas (1). About 60% of UTUCs are invasive at primary diagnosis. Radical nephroureterectomy (RNU) with the removal of bladder cuff excision is the gold standard treatment. Approximately 22%–47% of UTUC patients develop an intravesical recurrence (IVR) after RNU (2–4). Therefore, IVR monitoring following RNU is of great importance.
Although a few studies have looked into the risk factors for IVR after RNU for UTUC, few have tried to look into predictors for the malignant degree of secondary bladder cancer (BC) (5–7). Presently, IVR after RNU is managed according to the treatment guidelines for primary BC. Transurethral resection of the bladder tumor is recommended for favorable pathological types of IVR, while radical cystectomy (RC) is a curative treatment for most unfavorable pathological types of IVR (8, 9). Considering that advanced IVR after RNU predicts a worse prognosis and necessitates more aggressive treatment, it is critical to find predictors for unfavorable pathological types BC after RNU, such as muscle-invasive, high-grade, >3-cm-diameter, and multifocal IVR.
This study aimed to identify the independent risk factors and develop prediction models based on the predictors for unfavorable pathological types of IVR after RNU.
Materials and Methods
Patients
Our institutional review board approved this study. A review of the archived medical records of our hospital identified 169 patients who had IVR after undergoing RNU with curative intent for primary UTUC between January 2002 and September 2021 in Peaking University First Hospital, Beijing, China. We excluded three patients who had previous bladder tumors, two patients with a history of renal transplantation, and nine patients with incomplete follow-up data. Thus, this study included a total of 155 patients. Routine postoperative follow-up and cystoscopy were performed, and no bladder instillation treatment and adjuvant therapy were performed after RNU. Urologic ultrasound, CT, MRI, and ureteroscopy with or without biopsy were used to diagnose all the cases. Before surgery, cystoscopy and chest X-ray were performed to exclude concomitant bladder tumor and metastasis. Further, surgical methods include open and laparoscopic RNU. The distal ureter and bladder cuff excision was performed using the open extravesical technique and the intramural portion within the bladder wall through an open Gibson incision. Postoperative bladder instillation was not adapted after surgery.
Clinical Variables and Tumor-Related Variables
The collected clinical data included age, sex, body mass index (BMI), smoking and drinking status, history of taking aristolochic acid (AA), preoperative ureteroscopic biopsy, hydronephrosis, surgical approach, neutrophil–lymphocyte ratio (NLR), operation interval (time between RNU and management of BC), and pathological features (multifocality, location, stage, grade, and maximum diameter). All tumors were staged by the Union for International Cancer Control TNM classification of malignant tumors in 2002 and graded according to the WHO classification of 2004. Besides, patients were grouped based on muscle-invasive BC (MIBC) and/or high-grade BC (HGBC) after RNU since patients must receive cystectomy based on the guideline. We also grouped patients by BC with at least one of the above four unfavorable pathological types of IVR to identify predictors for unfavorable pathological types of BC following RNU without dissecting around the kidney. The definition of smoking status was not homogeneous in different studies (10). Our study divided smoking status into three groups, non-smoker, former smoker, and current smoker, based on our data. All medical records were reviewed by two researchers independently.
Statistical Analysis
All analyses were performed using R version 3.6.1 (The R Foundation) and SPSS (version 24.0, IBM, Armonk, NY, USA). Univariate binary logistic regression was performed for each variable to explore the independent predictors for unfavorable pathological types of IVR. Factors with a p-value <0.2 were included for stepwise multivariate binary logistic analysis. The calculation was done based on odds ratios (ORs) and 95% CI, and a value of p < 0.05 was considered statistically significant. Based on the multivariate binary logistic regression analysis results, nomograms were developed using R’s rms package version 3.0. The performance of the prediction model was measured using the concordance index (C-index). We conducted internal validation by constructing calibration plots and validated them with 500 bootstrap samples to reduce bias.
Results
Patients and Tumor Characteristics
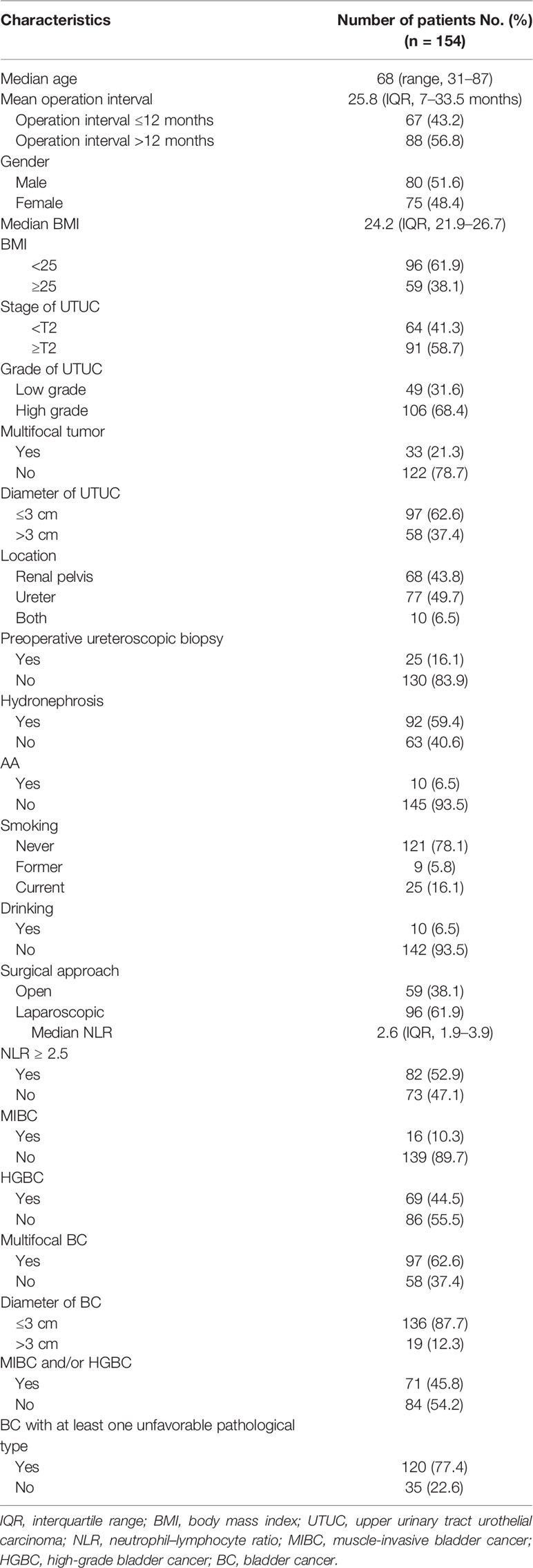
Table 1 summarizes the demographic information of included patients. The median patient age was 66 years (range: 31–87 years), and the median operation interval was 25.8 months (interquartile range (IQR), 7–33.5 months). Within 1 year of the primary procedure, 67 patients out of 155 (43.2%) were diagnosed with BC.
Independent Risk Factors for Unfavorable Pathological Types of Intravesical Recurrence
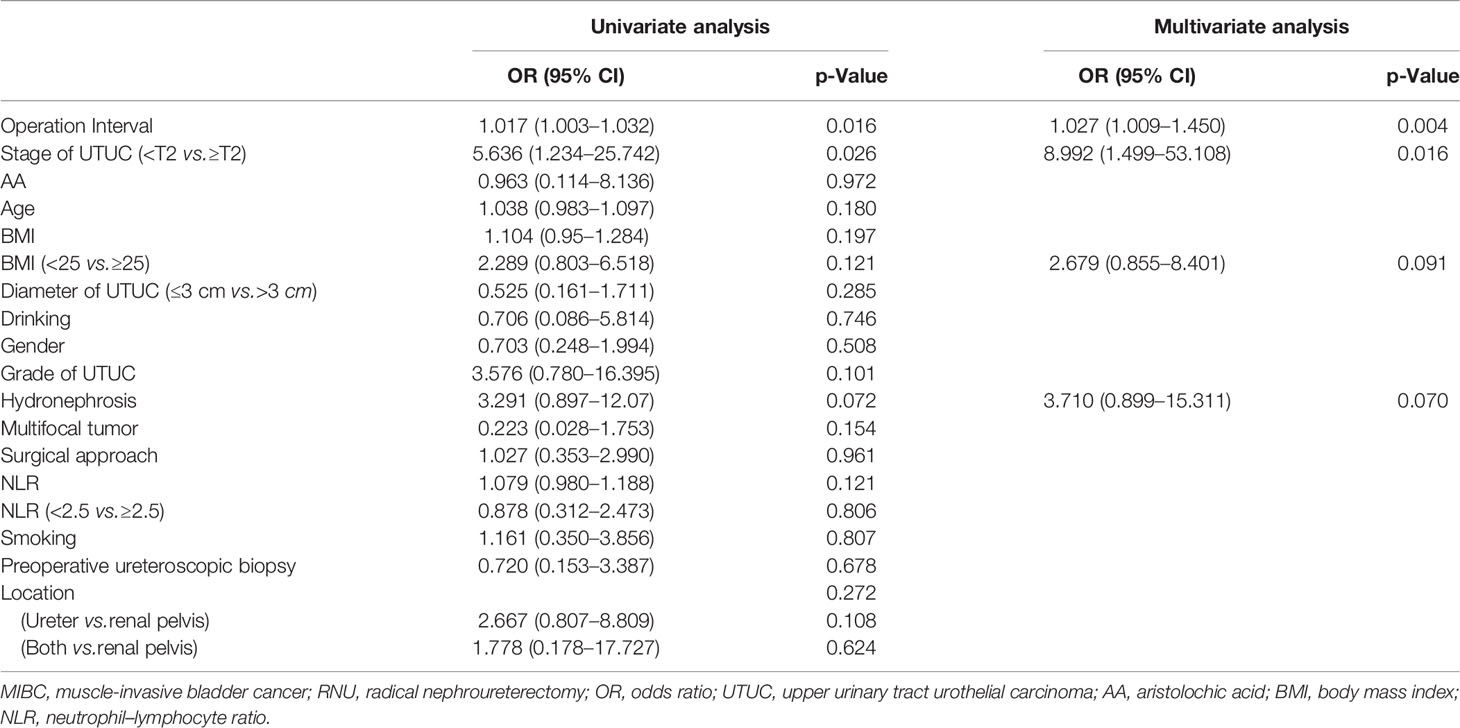
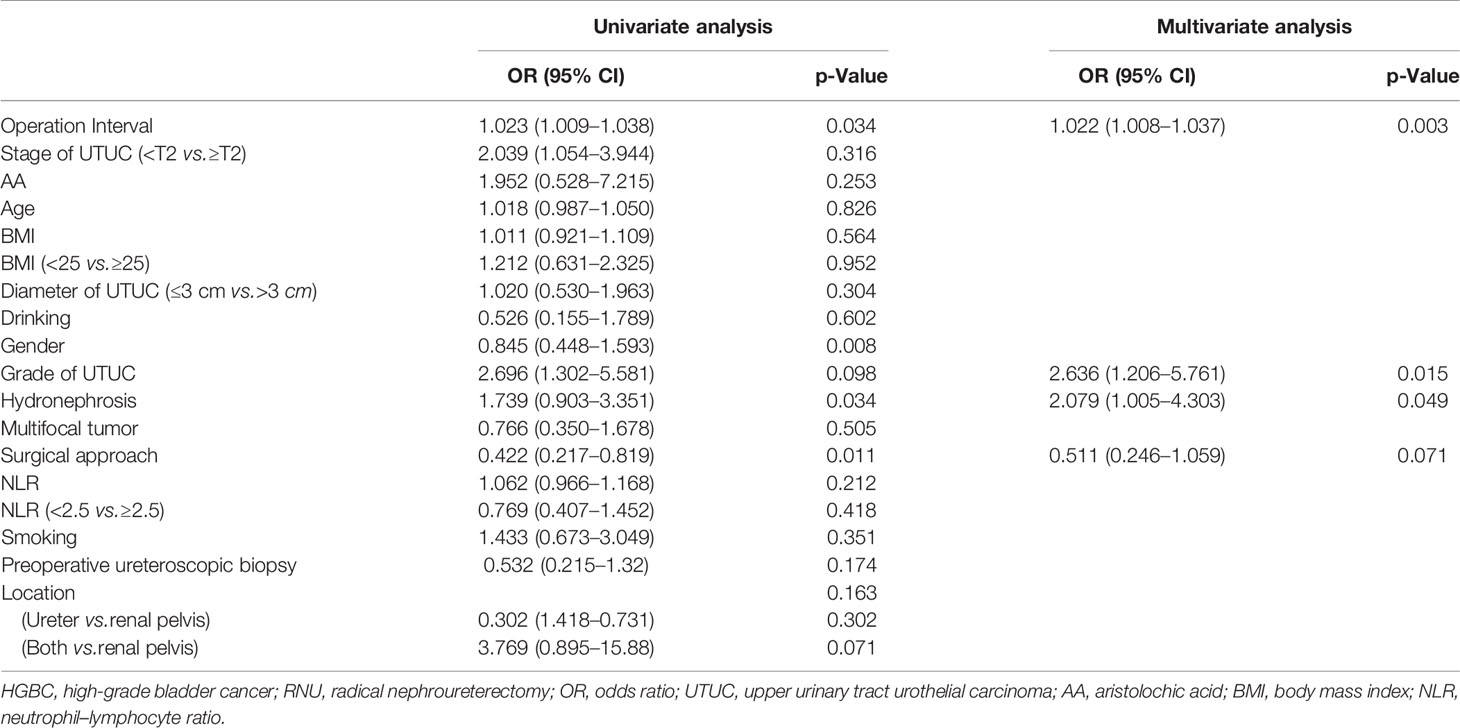
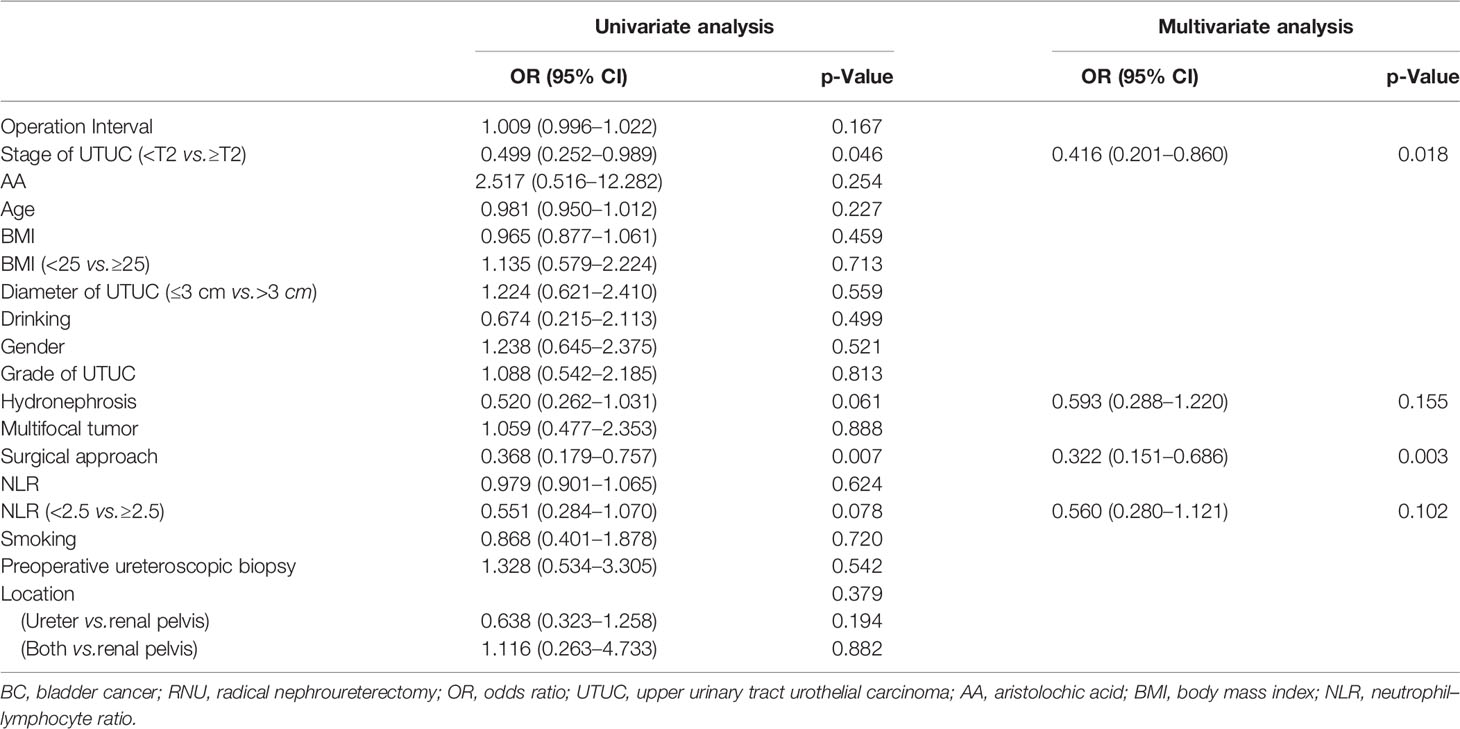
Tables 2, 3, 4, 5, 6, 7 present the results of univariate and multivariate analyses. For multivariate analysis, risk factors with a p-value of less than 0.2 were included. The operation interval (p = 0.004) and stage of primary UTUC (p = 0.016) were significantly correlated with MIBC after RNU. BC > 3 cm after RNU was also associated with operation interval (p = 0.002) and stage of primary UTUC (p = 0.028). High-grade IVR was predicted independently by UTUC grade (p = 0.015), operation interval (p = 0.003) and hydronephrosis (p = 0.049). MIBC (p = 0.018) and surgical approach (p = 0.003) were associated with multifocal IVR. Besides, following RNU, MIBC and HG-BC were linked to primary tumor grade (p = 0.009), operation interval (p = 0.001), and hydronephrosis (p = 0.023). Furthermore, only the operation interval (p = 0.036) was a predictor of BC with at least one unfavorable pathological type. Finally, we divided our research group into two groups based on the time between operations, with a 1-year cutoff. Operation interval was associated with all unfavorable pathological types of IVR except for the multifocal type in the more than 1 year group, while it was only correlated with MIBC in the other group (Supplementary Tables 1–6).
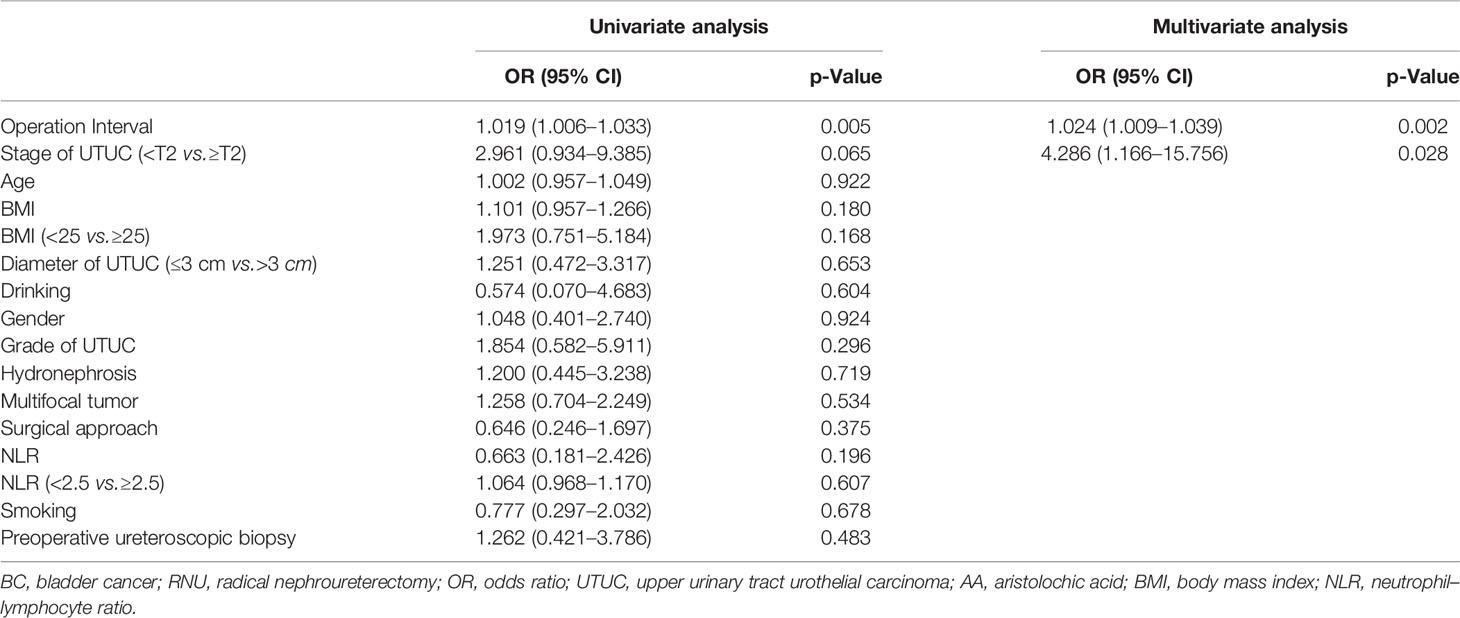
Table 5 Univariate and multivariate analyses for factors associated with BC greater than 3 cm after RNU.

Table 6 Univariate and multivariate analyses for factors associated with MIBC and/or HGBC after RNU.
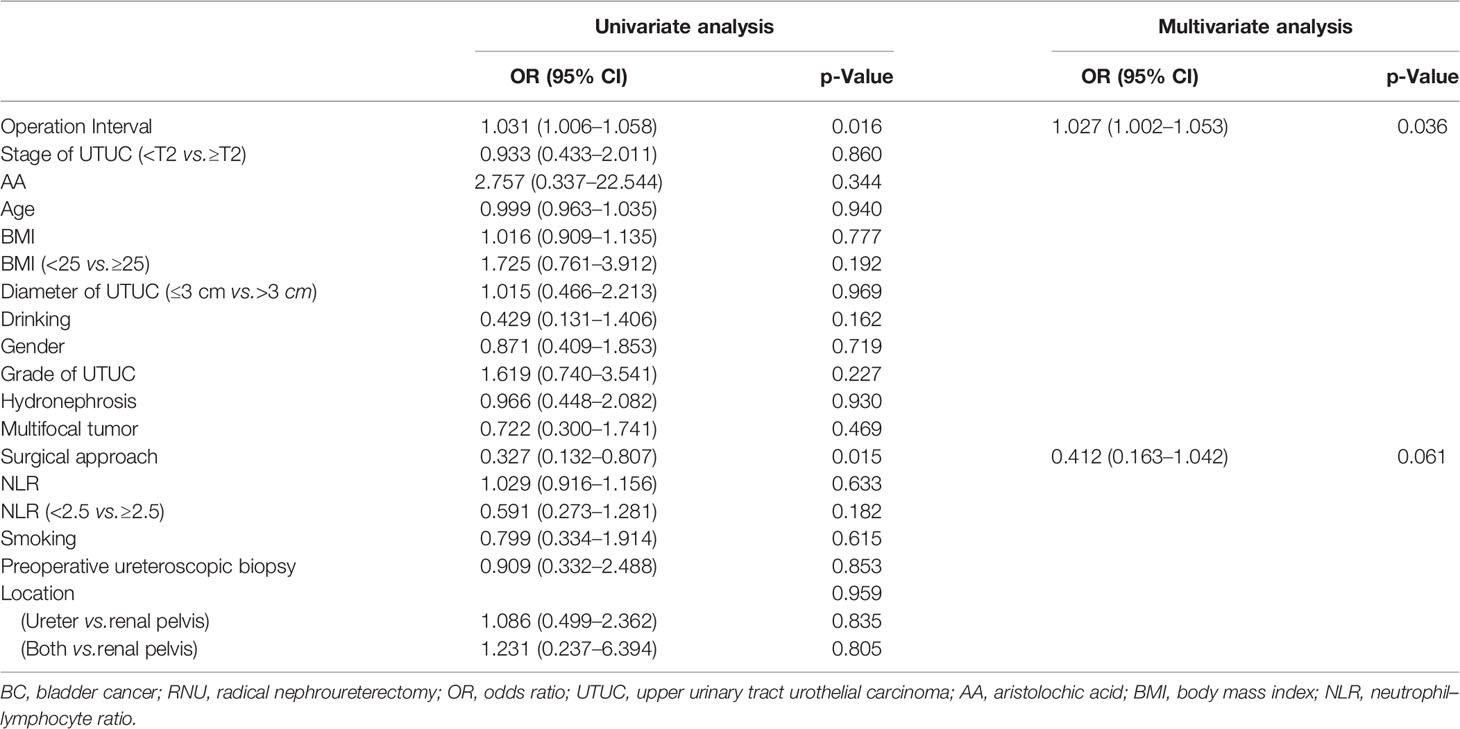
Table 7 Univariate and multivariate analyses for factors associated with BC with at least one unfavorable pathological type after RNU.
Prediction Models for Unfavorable Pathological Types of Intravesical Recurrence
These independent risk factors were pooled to develop the prediction models. We developed nomograms for MIBC, HGBC, BC > 3 cm, and MIBC and/or HGBC. The nomograms are presented in Figure 1. The calibration curves of the prediction models demonstrated good agreement between the observation and prediction cases in our cohort (Figure 2). Moreover, the C-indexes of the above nomograms were 0.820 (95% CI, 0.747–0.894), 0.728 (95% CI, 0.649–0.809), 0.770 (95% CI, 0.679–0.861), and 0.749 (95% CI, 0.671–0.827).
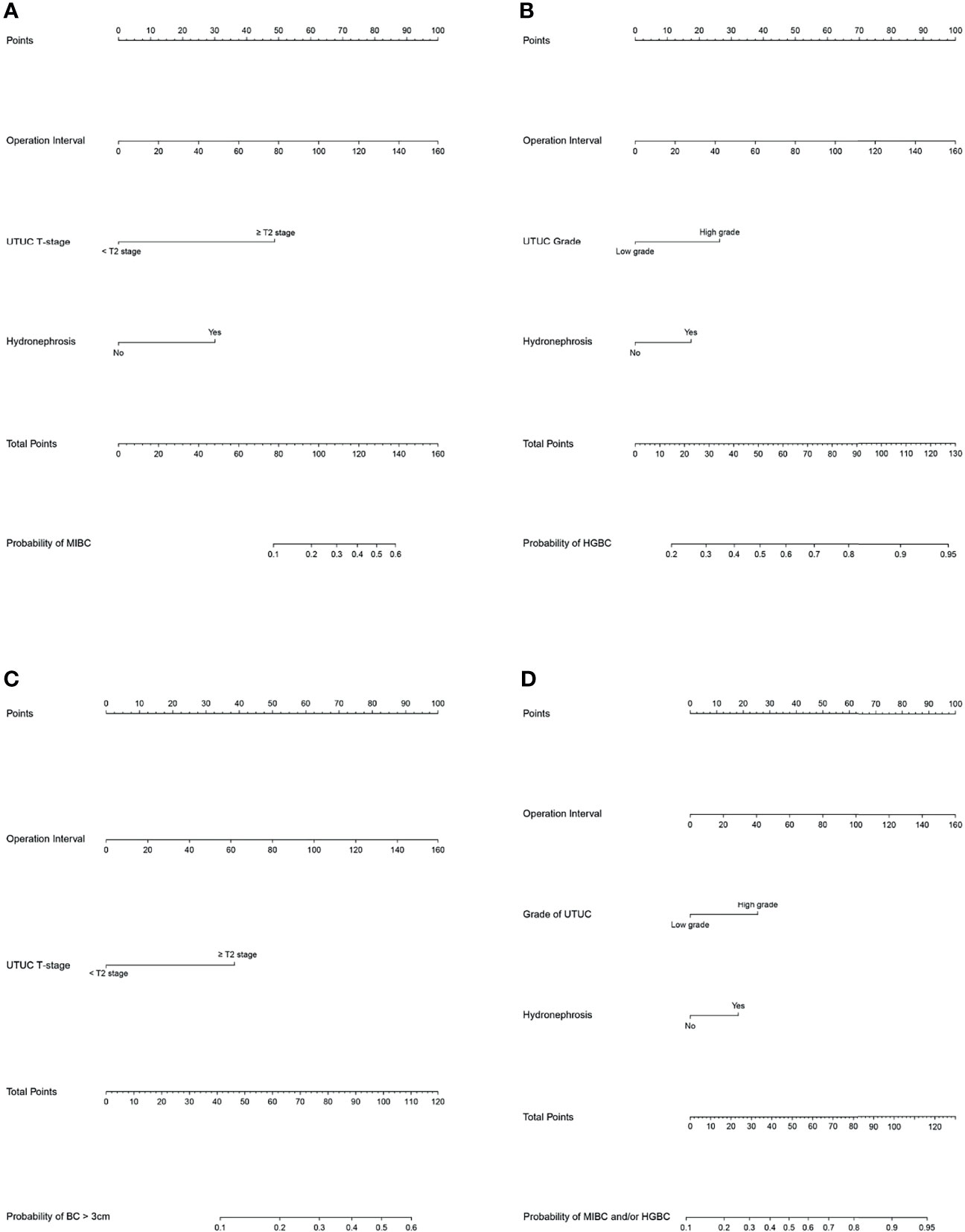
Figure 1 Nomograms for the prediction of unfavorable pathological types intravesical recurrence (IVR) following radical nephroureterectomy (RNU). (A) Nomogram to predict the risk of muscle-invasive bladder cancer (MIBC) after RNU. (A) Bomogram to predict the risk of high-grade bladder cancer (HGBC) after RNU. (C) Nomogram to predict the risk of bladder cancer >3 cm after RNU. (D) Nomogram to predict the risk of MIBC and/or HGBC after RNU. To use the nomogram, first, assign the points of each variable of the patient by drawing a line straight up to the top line labeled “Points”. Sum up the points of the risk factors as total points. Then draw a vertical line down from the axis labeled “Total Points” to the bottom line to get the predicted possibility of unfavorable pathological types intravesical recurrence (IVR) following RNU.
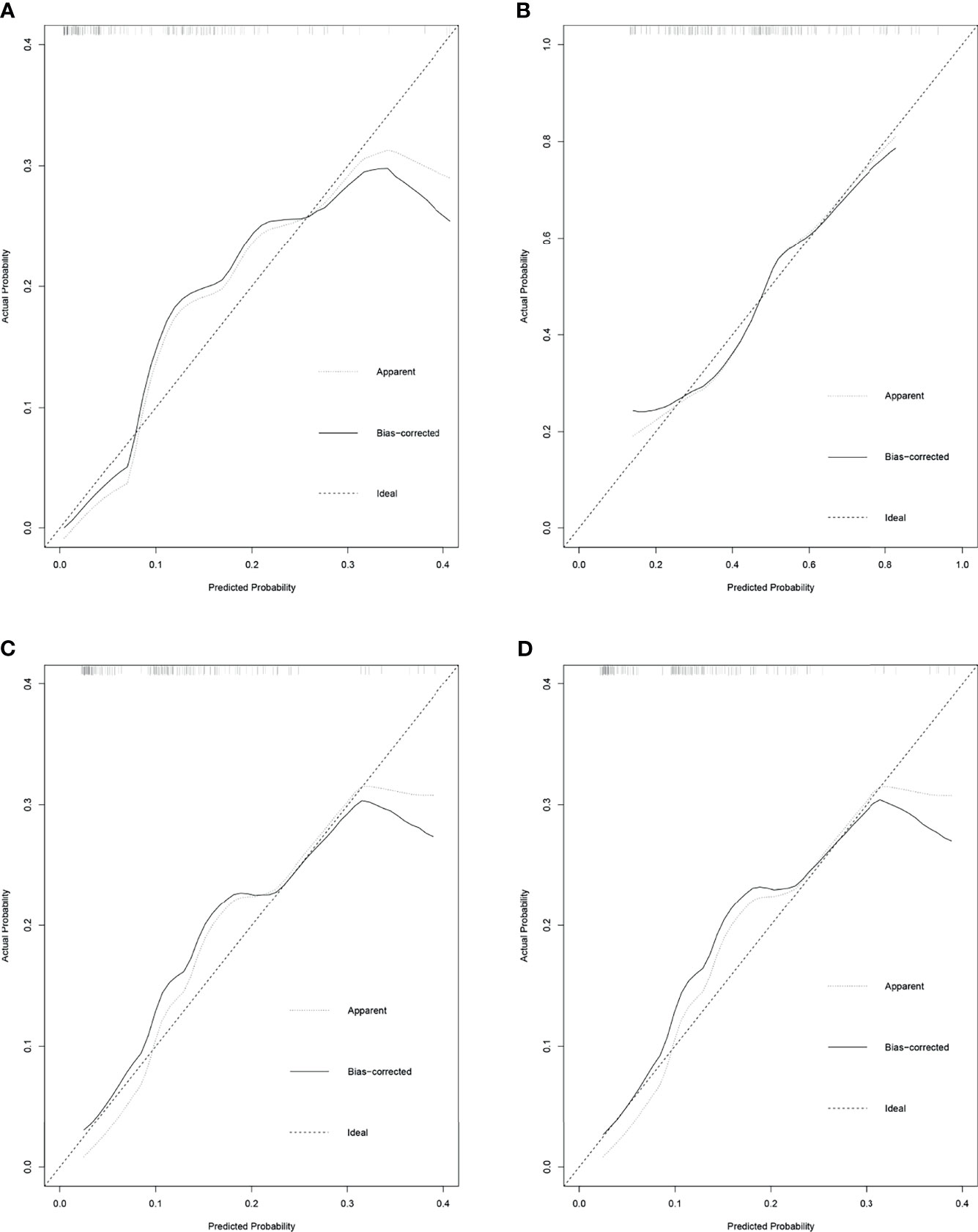
Figure 2 The calibration curves for the prediction nomograms of unfavorable pathological types intravesical recurrence following radical nephroureterectomy (RNU). (A) Calibration curves for the prediction nomograms of muscle-invasive bladder cancer (MIBC) after RNU. (B) Calibration curves for the prediction nomograms of high-grade bladder cancer (HGBC) after RNU. (C) Calibration curves for the prediction nomograms of bladder cancer >3 cm after RNU. (D) Calibration curves for the prediction nomograms of MIBC and/or HGBC after RNU.
Discussion
UTUC is a relatively rare malignancy, accounting for 5%–10% of urothelial carcinomas. Despite significant advances in diagnosis and management, 22%–47% of patients still develop IVR after RNU (3, 11). Many studies have identified risk factors for IVR following RNU, including T-stage, multifocality, diagnostic preoperative ureteroscopic biopsy, male sex, previous BC, smoking, chronic kidney disease, necrosis, tumor location, transurethral resection of the bladder cuff, positive resection margin, and surgical approach (7, 12–16). However, limited data are available about unfavorable pathological types of BC that develop after RNU. Our study explores the predictors and establishes prediction models for unfavorable pathological types of IVR following RNU.
The present study showed that operation interval and T-stage of primary UTUC were risk factors for MIBC and BC > 3 cm after RNU, consistent with Kim’s results (17). We first found that grade of UTUC (p = 0.015), hydronephrosis (p = 0.049), and operation interval (p = 0.003) were independent risk factors for HGBC. Besides, MIBC and HGBC following RNU were associated with UTUC grade (p = 0.009), operation interval (p = 0.001), and hydronephrosis (p = 0.023). These findings support the intraluminal seeding hypothesis as a predominant mechanism of IVR following RNU (7, 18). Moreover, MIBC (p = 0.018) and surgical approach (p = 0.003) were associated with multifocal BC after RNU. Open surgery is a risk factor for multifocal IVR, which may cause a poor prognosis, consistent with a randomized prospective study (15). Only operation interval (p = 0.036) was a predictor for BC with at least one unfavorable pathological type, suggesting that follow-up after RNU is of great importance. Smoking at the time of RC is significantly correlated with increased risk for postoperative infections, complications, and perioperative mortality.
We chose the time between two procedures as an indicator to explore the relationship between time after RNU and the malignant degree of IVR, considering it is an objective variable. Operation interval was a predictor for all unfavorable pathological types of IVR except multifocal type. We further investigated the influence of operation interval on the malignant degree of IVR by dividing the cohort into two groups with a cutoff of 1 year. Operation interval was correlated with MIBC, HGBC, BC > 3 cm, and MIBC and/or HGBC in the more than 1 year group. However, operation interval was only associated with MIBC in the other group. There are currently different opinions on an optimal surveillance regimen in UTUC patients after RNU (19–21). It is widely accepted that patients with low-risk tumors are recommended to receive cystoscopy at 3 months. If negative, perform cystoscopy after 9 months and then yearly for 5 years. For patients with high-risk tumors, both cystoscopy and urinary cytology should be performed at 3 months. If negative, perform repeat cystoscopy and cytology every 3 months for 2 years, every 6 months after that until 5 years, and then yearly. Further, CT urography and chest CT should be performed every 6 months for 2 years, and then once a year. Most studies took 2 years as a cutoff when exploring the follow-up protocol (4, 22–25). However, operation interval was an independent risk factor for unfavorable pathological types of IVR except for the multifocal type in the more than 1 year group, while only associated with MIBC in the other group. Moreover, previous studies have shown that the risk of recurrence and death increases during the follow-up (26). Hence, patients with a high risk of developing unfavorable pathological types BC may benefit from more active follow-up 1 year after RNU by early detection of recurrence. We also established prediction models for unfavorable pathological types BC, which can help identify patients with a high risk of developing unfavorable pathological types of IVR.
Notably, the present study has several limitations because it was a retrospective study that may have caused inherent selection bias and was conducted on a single hospital. Besides, the sample size was relatively small. Moreover, the predictive models were internally validated to exhibit good performance; thus, external validation by long-term follow-up in multiple centers is still required to verify the utility of the nomograms.
Conclusion
To summarize, the current study identified independent risk factors for unfavorable pathological types of BC developing after RNU, such as operation interval, UTUC T-stage, UTUC grade, surgical approach, and hydronephrosis. To assess the risk of developing unfavorable pathological types BC after RNU, accurate prediction models based on these predictors were developed and internally validated. Furthermore, patients at high risk of developing unfavorable pathological types BC may benefit from more active follow-up 1 year after RNU by early diagnosis of IVR.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Peking University First Hospital Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GX and ZZ conceived the study. JZ and XZ extracted all the data and performed the analyses. LZ, ZH, XL, and WY supervised the project and provided guidance throughout the preparation of this manuscript. JZ and XZ prepared the tables and figures. JZ wrote the manuscript. GX provided funding. All authors contributed to and revised the final manuscript.
Funding
This work was supported by funds from the Capital’s Funds for Health Improvement and Research (2020–4–40712) and the National Natural Science Foundation of China (81902871).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank JX and PZ for their help in the process of collecting data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.834692/full#supplementary-material
Abbreviations
UTUC, upper urinary tract urothelial carcinoma; RNU, radical nephroureterectomy; IVR, intravesical recurrence; BC, bladder cancer; BMI, body mass index; AA, aristolochic acid; NLR, neutrophil–lymphocyte ratio; MIBC, muscle-invasive bladder cancer; HGBC, high-grade bladder cancer; OR, odds ratio; C-index, concordance index; IQR, interquartile range.
References
1. Xylinas EN, Kluth LA, Scherr DS, Novara G, Comploj E, Pycha A, et al. Prediction of Intravesical Recurrence After Radical Nephroureterectomy: Development of a Clinical Decision-Making Tool. Eur Urol Suppl (2013) 12(1):e573-e4. doi: 10.1016/s1569-9056(13)61056-8
2. Kates M, Badalato GM, Gupta M, McKiernan JM. Secondary Bladder Cancer After Upper Tract Urothelial Carcinoma in the US Population. BJU Int (2012) 110(9):1325–9. doi: 10.1111/j.1464-410X.2012.11108.x
3. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol (2018) 73(1):111–22. doi: 10.1016/j.eururo.2017.07.036
4. Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF, et al. Multifocal Carcinoma in Situ of the Upper Tract is Associated With High Risk of Bladder Cancer Recurrence. Eur Urol (2012) 61(5):1069–70. doi: 10.1016/j.eururo.2012.02.042
5. Jiang Y, Yao Z, Zhu X, Wu B, Bai S. Risk Factors and Oncological Outcome for Intravesical Recurrence in Organ-Confined Upper Urinary Tract Urothelial Carcinoma Patients After Radical Nephroureterectomy: A Propensity Score-Matched Case Control Study. Int J Surg (2020) 76:28–34. doi: 10.1016/j.ijsu.2020.02.015
6. Sato G, Yoshida T, Yanishi M, Saito R, Murota T, Kawa G, et al. Preoperative Pyuria Predicts for Intravesical Recurrence in Patients With Urothelial Carcinoma of the Upper Urinary Tract After Radical Nephroureterectomy Without a History of Bladder Cancer. Clin Genitourin Cancer (2020) 18(2):e167-e73. doi: 10.1016/j.clgc.2019.09.017
7. Seisen T, Granger B, Colin P, Leon P, Utard G, Renard-Penna R, et al. A Systematic Review and Meta-Analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol (2015) 67(6):1122–33. doi: 10.1016/j.eururo.2014.11.035
8. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol (2019) 76(5):639–57. doi: 10.1016/j.eururo.2019.08.016
9. Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol (2021) 79(1):82–104. doi: 10.1016/j.eururo.2020.03.055
10. Tellini R, Mari A, Muto G, Cacciamani GE, Ferro M, Stangl-Kremser J, et al. Impact of Smoking Habit on Perioperative Morbidity in Patients Treated With Radical Cystectomy for Urothelial Bladder Cancer: A Systematic Review and Meta-Analysis. Eur Urol Oncol (2021) 4(4):580–93. doi: 10.1016/j.euo.2020.10.006
11. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
12. Ishioka J, Saito K, Kijima T, Nakanishi Y, Yoshida S, Yokoyama M, et al. Risk Stratification for Bladder Recurrence of Upper Urinary Tract Urothelial Carcinoma After Radical Nephroureterectomy. BJU Int (2015) 115(5):705–12. doi: 10.1111/bju.12707
13. Katims AB, Say R, Derweesh I, Uzzo R, Minervini A, Wu Z, et al. Risk Factors for Intravesical Recurrence After Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Cancer (ROBUUST Collaboration). J Urol (2021) 206(3):568–76. doi: 10.1097/JU.0000000000001786
14. Marchioni M, Primiceri G, Cindolo L, Hampton LJ, Grob MB, Guruli G, et al. Impact of Diagnostic Ureteroscopy on Intravesical Recurrence in Patients Undergoing Radical Nephroureterectomy for Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. BJU Int (2017) 120(3):313–9. doi: 10.1111/bju.13935
15. Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic Versus Open Nephroureterectomy: Perioperative and Oncologic Outcomes From a Randomised Prospective Study. Eur Urol (2009) 56(3):520–6. doi: 10.1016/j.eururo.2009.06.013
16. Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, et al. The Predictive Value of Positive Urine Cytology for Outcomes Following Radical Nephroureterectomy in Patients With Primary Upper Tract Urothelial Carcinoma: A Multi-Institutional Study. Urol Oncol (2014) 32(1):48.e19–26. doi: 10.1016/j.urolonc.2013.07.003
17. Kim KH, You D, Jeong IG, Hong JH, Ahn H, Kim CS. Muscle-Invasive Bladder Cancer Developing After Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma. Urol Oncol (2013) 31(8):1643–9. doi: 10.1016/j.urolonc.2012.04.014
18. Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, et al. Independent Predictors for Bladder Outcomes After Treatment of Intravesical Recurrence Following Radical Nephroureterectomy in Patients With Primary Upper Tract Urothelial Carcinoma. Ann Surg Oncol (2014) 21(9):3151–8. doi: 10.1245/s10434-014-3657-y
19. Oya M, Kikuchi E, Committee for Establishment of Clinical Practice Guideline for Management of Upper Tract Urothelial C, Japanese Urological A. Evidenced-Based Clinical Practice Guideline for Upper Tract Urothelial Carcinoma (Summary–Japanese Urological Association, 2014 Edition). Int J Urol (2015) 22(1):3–13. doi: 10.1111/iju.12630
20. Petrelli F, Yasser Hussein MI, Vavassori I, Barni S. Prognostic Factors of Overall Survival in Upper Urinary Tract Carcinoma: A Systematic Review and Meta-Analysis. Urology (2017) 100:9–15. doi: 10.1016/j.urology.2016.07.036
21. Seisen T, Colin P, Hupertan V, Yates DR, Xylinas E, Nison L, et al. Postoperative Nomogram to Predict Cancer-Specific Survival After Radical Nephroureterectomy in Patients With Localised and/or Locally Advanced Upper Tract Urothelial Carcinoma Without Metastasis. BJU Int (2014) 114(5):733–40. doi: 10.1111/bju.12631
22. Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper Urinary Tract Urothelial Cell Carcinoma: Location as a Predictive Factor for Concomitant Bladder Carcinoma. World J Urol (2013) 31(1):141–5. doi: 10.1007/s00345-012-0877-2
23. Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, et al. Oncologic Outcomes Following Three Different Approaches to the Distal Ureter and Bladder Cuff in Nephroureterectomy for Primary Upper Urinary Tract Urothelial Carcinoma. Eur Urol (2010) 57(6):963–9. doi: 10.1016/j.eururo.2009.12.032
24. Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol (2021) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
25. Seisen T, Colin P, Roupret M. Risk-Adapted Strategy for the Kidney-Sparing Management of Upper Tract Tumours. Nat Rev Urol (2015) 12(3):155–66. doi: 10.1038/nrurol.2015.24
Keywords: upper urinary tract, urothelial carcinoma, nephroureterectomy, bladder recurrence, risk factors
Citation: Zhu J, Zhang X, Yu W, Li X, He Z, Zhou L, Zhang Z and Xiong G (2022) Risk Factors for Unfavorable Pathological Types of Intravesical Recurrence in Patients With Upper Urinary Tract Urothelial Carcinoma Following Radical Nephroureterectomy. Front. Oncol. 12:834692. doi: 10.3389/fonc.2022.834692
Received: 13 December 2021; Accepted: 15 February 2022;
Published: 13 April 2022.
Edited by:
Yige Bao, Sichuan University, ChinaReviewed by:
Riccardo Tellini, Careggi University Hospital, ItalyGiuseppe Simone, Regina Elena National Cancer Institute (IRCCS), Italy
Copyright © 2022 Zhu, Zhang, Yu, Li, He, Zhou, Zhang and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyuan Zhang, MTU4MTA1MzEyMjhAeWVhaC5uZXQ=; Gengyan Xiong, eGd5NjIwNUBnbWFpbC5jb20=
 Jun Zhu
Jun Zhu Wei Yu
Wei Yu Xuesong Li
Xuesong Li Gengyan Xiong
Gengyan Xiong