- Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Although PD-1 blockade therapy has been promising in cancer treatment, only 4% (pancreatic cancer) to 70% (melanoma) of patients have a positive response to this blockade therapy, which is one of its important disadvantages. Therefore, it is important to seek out new targets for cancer immunotherapy to improve the overall response rate in patients. Lymphocyte activation gene-3 (LAG-3), an immune checkpoint receptor, is mainly expressed in activated immune cells. LAG-3 maintains the body’s immune homeostasis under physiological conditions while mediating tumour immune escape. Several preclinical and clinical examinations have shown that LAG-3 blockade effectively alleviates the patient’s tolerance to PD-1 immune checkpoint inhibitors. Moreover, the combination of LAG-3 and PD-1 blockade has good clinical efficacy in cancers. Hence, synchronous LAG-3 and PD-1 inhibition may be a potential new strategy for tumour immunotherapy.
Introduction
Tumour cells can evade the recognition and killing of the immune system of the body with the help of immune checkpoint receptors (1). Thus, blocking immune checkpoint receptors might be a widely effective method of tumor immunotherapy. Currently, although anti-PD-1/PD-L1 antibodies (2) are relatively mature, similar to anti-CTLA4 antibodies, the overall response rate is low in patients because of drug resistance (3, 4). Therefore, finding new tumour immunotherapy targets is urgent. As a new immune checkpoint receptor, LAG-3 plays a vital role in immune homeostasis maintenance and tumour immune escape and is widely present in various activated immune cells (5, 6). The combination of PD-1 and LAG-3 blockade may be a new treatment for drug-resistant patients.
LAG-3 Expression and Ligand
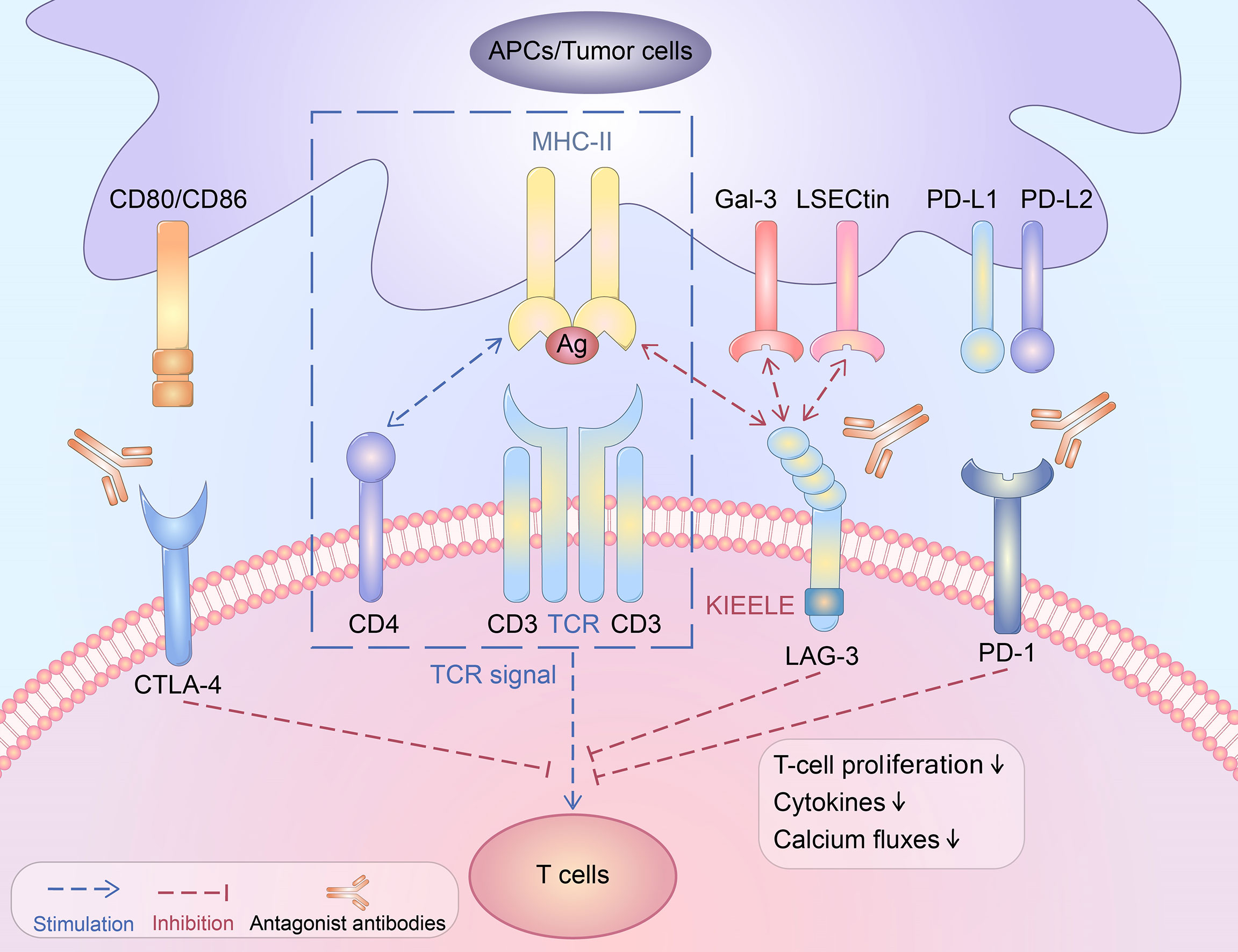
As a type I transmembrane protein, the LAG-3 molecule contains three domains: a transmembrane domain, an extracellular domain, and an intracellular domain. Four immunoglobulin superfamily domains constitute the extracellular domain of the LAG-3 molecule, which binds to its ligand. There are three parts of the transmembrane domain and intracellular domain: the highly conserved KIEELE motif (7), the potential serine phosphorylation site S454, and the glutamate-proline-rich sequence. The glutamate-proline rich sequence is also known as the EP repeated sequence and is closely related to the signal transduction of the intracellular region (8).
LAG-3 is expressed widely on different cells: (i) T-cell subpopulations including activated CD4+ T helper cells (Th) and cytotoxic CD8+ T cells (CTL) (9–12). T-cell activation is a necessary condition for LAG-3 expression on T cell subpopulations (13). The LAG-3 level is closely associated with the expression levels of IL-2, IL-7, and IL-12 (14). Additionally, activated CD4+ effector T cells have been shown to express LAG-3, especially on activated natural regulatory T cells (nTregs) and inducible T-regulatory cells (iTregs) (15). (ii) Natural killer (NK) cells and invariant NKT cells (16). (iii) LAG-3+CD138hi plasma cells or regulatory B cells (Bregs), which suppress the immune system through upregulation of IL-10 expression (17). (iv) Other cells, such as plasmacytoid dendritic cells (pDC) and neuronal cells, which do not belong to the lymphocyte lineage (18).
As a typical ligand of LAG-3, MHC-II interacts with LAG-3 through the domain of D1 (19). LAG-3 has a higher affinity for MHC-II than CD4 molecules (20). Significantly, LAG-3 may be cleaved by metalloproteases and release a soluble form of LAG-3(sLAG-3) (21). It remains unclear whether sLAG-3 has the same high affinity for MHC-II as LAG-3. A previous study showed that MHC-II signalling induced specifically by sLAG-3 severely impaired the differentiation of monocytes (22). In contrast, another study showed that naturally cleaved sLAG-3 does not specifically bind with MHC-II, and only the cell surface LAG-3 dimer or the dimeric LAG-3:Ig fusion protein possesses a high affinity for MHC-II (21). The function and mechanism of sLAG-3 remain to be further explored. LAG-3 negatively modulates T-cell activation and the production of related cytokines by transmitting blocking signals via its cytoplasmic domain (23). Studies have shown that the combination of LAG-3 and MHC-II contributes to avoiding apoptosis of tumour cells and promoting tumour-specific CD4+ T-cell recruitment but reduces the response of CD8+ T cells (7, 13, 23–27). As the second ligand of LAG-3, galectin-3 (Gal-3) shows high expression in multiple cancer cells and activated T lymphocytes and modulates T-cell activation. The cell toxicity of CD8+ T cells can be inhibited by binding to LAG-3 (28, 29). Additionally, fibrinogen-like protein 1 (FGL1), produced by the liver, is a recently identified ligand of LAG-3 (30). As one member of the fibrinogen family, FGL1 muffles antigen-specific T cells by binding to LAG-3 (31). The expression of FGL1 is related to IL-6 via JAK2/STAT3 signalling (32). Studies have shown that FGL1 is abundantly secreted in multiple cancers, including lung cancer, prostate cancer, melanoma, and colorectal cancer (33).
The Immunosuppressive Function of LAG-3
The interplay between MHC-II and LAG-3 can lower the growth capacity of CD4+ T cells and the secretion of cytokines (13). Moreover, the addition of LAG-3 antibodies can reinstate the activity of CD4+ T cells (34). LAG-3 does not bind to all MHC-II molecules but selectively recognizes and combines antigen peptide-MHC-II complexes (pMHC-II) and controls the pMHC-II-mediated CD4+ T-cell response (35). It has been proven that LAG-3 is an independent negative regulator and that there is no competitive relationship with other regulatory molecules, such as CD4 (35). Experiments have shown that LAG-3 restrains CD4+ T- cell functions by directly transmitting inhibitory signals through the intracellular region but does not block the interaction between CD4-MHC-II or TCR-MHC-II (8, 23, 35).
The activity of CD8+ T cells was higher in LAG-3 knockout (KO) mice than in wild-type mice. Low-level LAG-3 expression was noted in the initial activation of CD8+ T cells, and LAG-3 expression increased after tumour antigen stimulation. Additionally, LAG-3 could significantly inhibit the cytotoxicity of CD8+ T cells (36). In addition, a previous study showed that LAG-3 directly suppressed CD8+ T cells through signal transduction but did not rely on the interaction between CD4+ T cells and MHC-II (35).
Treg cells, including nTreg and iTreg cells, negatively regulate immunity and can decrease the activity of T cells. LAG-3 was capable of inducing the activation of Treg cells and activating their immunosuppressive function (15, 37). LAG-3 is expressed on both activated natural Tregs (nTregs) and induced CD4+ FoxP3+ Treg (iTreg) cells (15). The addition of LAG-3 antibodies can significantly control the activity of Treg cells. Compared with wild-type mice, the negative regulatory function of nTreg cells was significantly downregulated in LAG-3 KO mice (15, 38, 39).
Although the LAG-3 inhibition-induced signalling pathway remains unclear, several studies have discussed this issue. Experiments have shown that LAG-3 is highly correlated with CD3 and that the cross-linking of these two molecules can block the proliferation of T cells, cytokine production, and calcium production (Figure 1). LAG-3 may downregulate the immune response by interfering with TCR signal-transduction (40). Furthermore, the inhibition of LAG-3 in effector CD4+ T cells occurs in a KIEELE motif-dependent manner (23). LAG-3 may transduce two independent inhibitory signals through the KIEELE motif and the FSAL motif in the EP repeat sequence. Both the FSAL motif and KIEELE motif are key points in LAG-3-induced signalling pathways (8). However, how these motifs regulate the TCR response and downstream molecules is still unclear.
LAG-3 and Tumour Immunity
MHC-II and FGL-1, as ligands of LAG-3, are related to LAG-3-mediated tumour immune escape (19, 20, 41). MHC-II recruits CD4+ T cells and enhances antitumour immunity in the early stage of tumorigenesis. The activity and expression level of the LAG-3 molecule increases on the surface of TIL cells with the development of tumours. After binding to MHC-II, LAG-3-induced antitumour immunity turns into immunosuppression. For example, MHC-II-expressing melanoma cells block the functions of tumour-infiltrating CD4+ T cells, thus evading the recognition and killing of the immune system (42). FGL-1 is secreted by the liver (30). Under normal circumstances, the expression of FGL-1 is low in hepatocytes, and its expression markedly increases when cancers occur (31, 33).
Generally, the LAG-3 molecule detected on T cells is regarded as a marker of aggressive progression of cancers. Under the stimulation of tumour antigens, lymphocytes highly express LAG-3 (43, 44). Furthermore, the LAG-3 expression level is obviously related to the survival rate and prognosis of inpatients (45). For example, the number of CTLA-4+LAG-3+ T-cell subsets significantly increased in AML patients, and the production level of LAG-3 was closely related to the classification of patients with AML (46). LAG-3 presented increased expression on the TILs of NSCLC patients (47, 48), and high LAG-3 was also found in nonadenocarcinoma tissues (47). LAG-3 expression in T cells from the peripheral blood of soft tissue sarcoma patients was higher than that in healthy people. LAG-3 is mainly produced and localizes to CD8+ TILs in individuals with soft tissue sarcoma. High LAG-3 expression is related to late-stage disease, high pathological grade, and low survival rates (49). The phenomenon is most obvious in CD8+ TILs in which LAG-3 is highly expressed and combined with its ligand and is detected on the cancer cell surface, leading to the decline or exhaustion of T-cell function and thereby promoting tumour immune escape (36). For example, the overexpression of LAG-3 was detected on CD8+ TILs in ovarian cancer tumour tissues, and cytokine secretion was strongly decreased (50, 51). Although the number of endogenous CD8+ T cells was increased in Hodgkin’s lymphoma patients, their response was low. Moreover, their function was negatively associated with LAG-3 expression on TILs (52–54). The gene expression profile data showed that LAG-3 caused T-cell exhaustion in the tumour microenvironment by cooperating with a variety of inhibitory receptors in patients with melanoma (6, 55, 56).
Chemotherapy affected the expression levels of LAG-3 and PD-1, which ultimately mediated tumour immune escape. The proportion of CD8+LAG3+PD-1+ T cells was significantly higher in patients receiving preoperative paclitaxel plus platinum chemotherapy than in patients receiving surgery alone (57).
Combined Anti-LAG-3 and Anti-PD-1 Blocking
Immune homeostasis is maintained by the balance between costimulatory and inhibitory signals. Increased checkpoint receptors can alleviate antigen-specific T-cell activation, bringing about a proinflammatory response lower than normal circumstances (6). After long-term activation of tumour antigens, checkpoint receptor expression is maintained, which causes effector T cells to enter an “exhaustion” state. Exhausted T cells show a gradual decrease in proliferation capacity and loss of function, including the production of inflammatory cytokines and degranulation (58, 59). Multiple clinical trials have shown that blocking the immune checkpoint PD-1 can significantly improve the clinical treatment effect in malignant tumours by restoring the function of effector T cells. Unfortunately, only a few patients benefit from this therapy because of the development of drug resistance mechanisms within the tumour microenvironment (60–63). The latest data showed that LAG-3 might also be vital in the development of resistance to the curing of PD-1 or PD-L1 by inhibiting the activity and proliferation of CD8+ T cells and increasing the inhibitory activity of Tregs (64–66). LAG-3 can regulate the activity of PD-1+ cells (67). LAG-3 and PD-1 synergistically regulate T-cell function. Combined anti-LAG-3 and anti-PD-1 antibody treatment has shown a strong antitumour effect in mice resistant to single-antibody treatment. Excitingly, there was no obvious evidence of autoimmunity, thus suggesting the possibility of clinical efficacy and safety by combining anti-LAG-3 and anti-PD-1 antibody treatment (36).
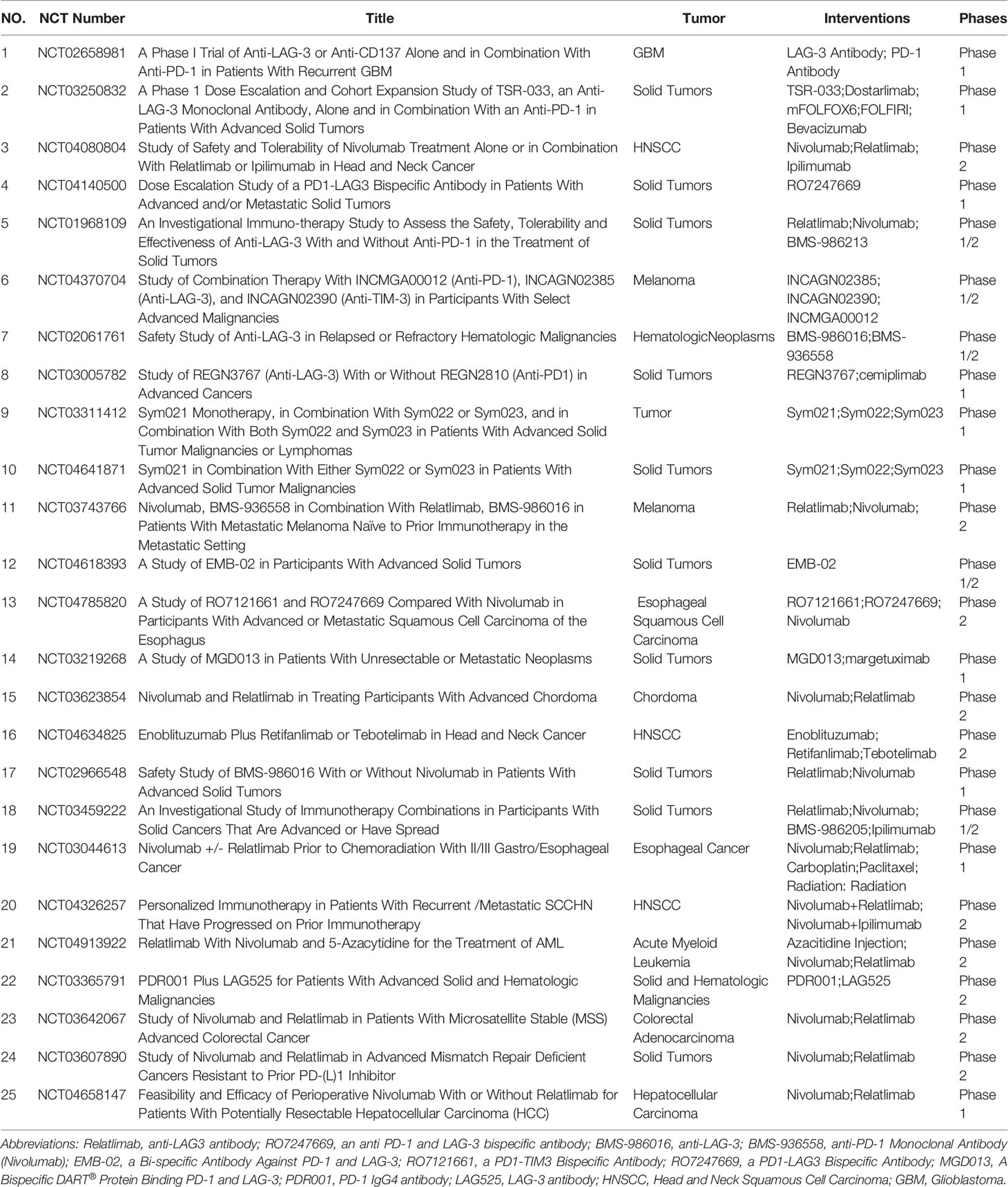
Currently, several molecules targeting LAG-3 are in clinical development (Table 1). Although these molecules were well tolerated, the effect of single-molecule-based therapy was limited. Some animal experiments have implied that LAG-3 might be a potential target in combination with anti-PD-1. (i) Both LAG-3 and PD-1 were observed on T cells in the tumour microenvironment in animal models of mouse MC38 colorectal adenocarcinoma and SalN fibroma. Furthermore, the antitumour efficacy of combined immunotherapy far exceeded that of any single immunotherapy (36). (ii) In a mouse model of ovarian cancer, tumour-infiltrating T cells coexpressed LAG-3 and PD-1. Moreover, LAG-3 and PD-1 blockade upregulated effector T-cell activity, thereby inhibiting tumour growth (51). (iii) Both LAG-3 and PD-1 blockade caused IFN-γ secretion and CD8+ T-cell cytotoxicity upregulation in melanoma (68). (iv) Another study on mouse prostate cancer showed that compared with inhibition of a single target, the combined suppression of PD-1 and LAG-3 markedly improved the antitumour effect of antitumour vaccines (69). (v) Recently, a new fully human anti-LAG-3 therapeutic IgG4 antibody was developed (REGN3767). Furthermore, the combination of REGN3767 and PD-1 antibody showed higher antitumour efficacy and accelerated the production of proinflammatory cytokines in tumour-specific T cells from mouse tumour models established using humanized PD-1xLAG-3 mice. REGN3767 had good pharmacokinetics and toxicology in nonhuman primates, showing good clinical application prospects (70). (vi) In a humanized mouse non-small cell lung cancer model, the LAG-3 antibody TSR-033 enhanced the efficacy of anti-PD-1 monotherapy and increased immune system activation (71). (vii) FS118, a bispecific antibody against PD-L1 and LAG-3, has been confirmed to enhance the activation of T cells in mouse tumour models, bringing about potent antitumour activity (72).
As an IgG4κ bispecific DART® molecule that combines LAG-3 and PD-1, tebotelimab could disrupt nonredundant blocking pathways and further restore the function of exhausted T cells. In an open-label, randomized, phase II/III MAHOGANY trial (NCT04082364), tebotelimab reversed PD-1- and LAG-3-mediated inhibitory effects by controlling the interaction with PD-L1/PD-L2 or MHC-II molecules, thereby restoring exhausted T-cell function and enhancing antitumour immunity. Additionally, dual inhibition of PD-1 and LAG-3 improved the effectiveness of HER2 antibodies by increasing the innate and adaptive immune response against HER2-overexpressing cancer cells (73).
Eftilagimod alpha (efti, IMP321 or LAG-3Ig), a soluble LAG-3 protein and MHC-II agonist, activates APC, causing CD8+ T-cell activation. An open-label, multicentre, dose-escalation study in phase I advanced melanoma invalids was performed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of the combined efti and PD-1 antibody. Twenty-four melanoma patients received pembrolizumab and subcutaneous efti injections at doses of one mg, six mg, or thirty mg biweekly. The main adverse event of efti was a reaction at the injection site. Dose-limiting toxicity was not reported. The count of activated CD8+ T cells and CD4+ T cells and the IFN-γ expression levels were increased after subcutaneous injection of efti. More importantly, a 33% overall response rate (ORR) was still observed in some patients who were resistant to pembrolizumab. The clinical trial showed that the combination of efti and pembrolizumab is well tolerated and has encouraging antitumour activity (68).
Conclusion and Outlook
Although PD-1 blockade has undergone a paradigm shift in multiple malignant cancers, most tumours show a high rate of primary resistance to this drug. Multiple preclinical and clinical data showed that the double-checkpoint inhibition of LAG-3 and PD-1 can be an application for overcoming ICB resistance. Therefore, combined PD-1 and LAG-3 inhibition may be a promising immunotherapy program for cancers. However, the synergistic effects based on anti-PD-1 and anti-LAG-3 need to be further confirmed in different tumours through more clinical trial data.
Author Contributions
YW and ZL contributed to the conception of the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Education Department of Henan Province, Key Scientific Research Project plan of Colleges and Universities in Henan Province, Project No.21A320027.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen DS, Irving BA, Hodi FS. Molecular Pathways: Next-Generation Immunotherapy–Inhibiting Programmed Death-Ligand 1 and Programmed Death-1. Clin Cancer Res (2012) 18(24):6580–7. doi: 10.1158/1078-0432.CCR-12-1362
2. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
3. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative Ipilimumab Plus Nivolumab in Locoregionally Advanced Urothelial Cancer: The NABUCCO Trial. Nat Med (2020) 26(12):1839–44. doi: 10.1038/s41591-020-1085-z
4. Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab Plus Sargramostim vs Ipilimumab Alone for Treatment of Metastatic Melanoma: A Randomized Clinical Trial. JAMA (2014) 312(17):1744–53. doi: 10.1001/jama.2014.13943
5. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-Inhibitory Receptors With Specialized Functions in Immune Regulation. Immunity (2016) 44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001
6. Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, et al. Induction and Transcriptional Regulation of the Co-Inhibitory Gene Module in T Cells. Nature (2018) 558(7710):454–9. doi: 10.1038/s41586-018-0206-z
7. Workman CJ, Vignali DAA. The CD4-Related Molecule, LAG-3 (CD223), Regulates the Expansion of Activated T Cells. Eur J Immunol (2003) 33(4):970–9. doi: 10.1002/eji.200323382
8. Maeda TK, Sugiura D, Okazaki I-M, Maruhashi T, Okazaki T. Atypical Motifs in the Cytoplasmic Region of the Inhibitory Immune Co-Receptor LAG-3 Inhibit T Cell Activation. J Biol Chem (2019) 294(15):6017–26. doi: 10.1074/jbc.RA119.007455
9. Wang R, Xu A, Zhang X, Wu J, Freywald A, Xu J, et al. Novel Exosome-Targeted T-Cell-Based Vaccine Counteracts T-Cell Anergy and Converts CTL Exhaustion in Chronic Infection via CD40L Signaling Through the Mtorc1 Pathway. Cell Mol Immunol (2017) 14(6):529–45. doi: 10.1038/cmi.2016.23
10. Bae J, Hideshima T, Zhang GL, Zhou J, Keskin DB, Munshi NC, et al. Identification and Characterization of HLA-A24-Specific XBP1, CD138 (Syndecan-1) and CS1 (SLAMF7) Peptides Inducing Antigens-Specific Memory Cytotoxic T Lymphocytes Targeting Multiple Myeloma. Leukemia (2018) 32(3):752–64. doi: 10.1038/leu.2017.316
11. Jahan N, Talat H, Curry WT. Agonist OX40 Immunotherapy Improves Survival in Glioma-Bearing Mice and Is Complementary With Vaccination With Irradiated GM-CSF-Expressing Tumor Cells. Neuro Oncol (2018) 20(1):44–54. doi: 10.1093/neuonc/nox125
12. Dong Y, Li X, Zhang L, Zhu Q, Chen C, Bao J, et al. CD4 T Cell Exhaustion Revealed by High PD-1 and LAG-3 Expression and the Loss of Helper T Cell Function in Chronic Hepatitis B. BMC Immunol (2019) 20(1):27. doi: 10.1186/s12865-019-0309-9
13. Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DAA. Phenotypic Analysis of the Murine CD4-Related Glycoprotein, CD223 (LAG-3). Eur J Immunol (2002) 32(8):2255–63. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A
14. Sun H, Sun C, Xiao W. Expression Regulation of Co-Inhibitory Molecules on Human Natural Killer Cells in Response to Cytokine Stimulations. Cytokine (2014) 65(1):33–41. doi: 10.1016/j.cyto.2013.09.016
15. Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in Regulatory T Cells. Immunity (2004) 21(4):503–13. doi: 10.1016/j.immuni.2004.08.010
16. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a Novel Lymphocyte Activation Gene Closely Related to CD4. J Exp Med (1990) 171(5):1393–405. doi: 10.1084/jem.171.5.1393
17. Lino AC, Dang VD, Lampropoulou V, Welle A, Joedicke J, Pohar J, et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity (2018) 49(1):120–33.e9. doi: 10.1016/j.immuni.2018.06.007
18. Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 Regulates Plasmacytoid Dendritic Cell Homeostasis. J Immunol (2009) 182(4):1885–91. doi: 10.4049/jimmunol.0800185
19. Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, et al. Characterization of the Major Histocompatibility Complex Class II Binding Site on LAG-3 Protein. Proc Natl Acad Sci USA (1997) 94(11):5744–9. doi: 10.1073/pnas.94.11.5744
20. Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major Histocompatibility Complex Class II Interaction Analyzed With CD4- and Lymphocyte Activation Gene-3 (LAG-3)-Ig Fusion Proteins. Eur J Immunol (1995) 25(9):2718–21. doi: 10.1002/eji.1830250949
21. Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, et al. Metalloproteases Regulate T-Cell Proliferation and Effector Function via LAG-3. EMBO J (2007) 26(2):494–504. doi: 10.1038/sj.emboj.7601520
22. Buisson S, Triebel F. LAG-3 (CD223) Reduces Macrophage and Dendritic Cell Differentiation From Monocyte Precursors. Immunology (2005) 114(3):369–74. doi: 10.1111/j.1365-2567.2004.02087.x
23. Workman CJ, Dugger KJ, Vignali DAA. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J Immunol (2002) 169(10):5392–5. doi: 10.4049/jimmunol.169.10.5392
24. Zhai W, Zhou X, Wang H, Li W, Chen G, Sui X, et al. A Novel Cyclic Peptide Targeting LAG-3 for Cancer Immunotherapy by Activating Antigen-Specific CD8 T Cell Responses. Acta Pharm Sin B (2020) 10(6):1047–60. doi: 10.1016/j.apsb.2020.01.005
25. Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-Activation Gene 3/Major Histocompatibility Complex Class II Interaction Modulates the Antigenic Response of CD4+ T Lymphocytes. Eur J Immunol (1994) 24(12):3216–21. doi: 10.1002/eji.1830241246
26. Workman CJ, Cauley LS, Kim I-J, Blackman MA, Woodland DL, Vignali DAA. Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. J Immunol (2004) 172(9):5450–5. doi: 10.4049/jimmunol.172.9.5450
27. Workman CJ, Vignali DAA. Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223). J Immunol (2005) 174(2):688–95. doi: 10.4049/jimmunol.174.2.688
28. Newlaczyl AU, Yu L-G. Galectin-3–a Jack-of-All-Trades in Cancer. Cancer Lett (2011) 313(2):123–8. doi: 10.1016/j.canlet.2011.09.003
29. Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, et al. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol Res (2015) 3(4):412–23. doi: 10.1158/2326-6066.CIR-14-0150
30. Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-Like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell (2019) 176(1-2):334–47.e12. doi: 10.1016/j.cell.2018.11.010
31. Guo M, Yuan F, Qi F, Sun J, Rao Q, Zhao Z, et al. Expression and Clinical Significance of LAG-3, FGL1, PD-L1 and CD8T Cells in Hepatocellular Carcinoma Using Multiplex Quantitative Analysis. J Transl Med (2020) 18(1):306. doi: 10.1186/s12967-020-02469-8
32. Wang J, Wei W, Tang Q, Lu L, Luo Z, Li W, et al. Oxysophocarpine Suppresses Hepatocellular Carcinoma Growth and Sensitizes the Therapeutic Blockade of Anti-Lag-3 via Reducing FGL1 Expression. Cancer Med (2020) 9(19):7125–36. doi: 10.1002/cam4.3151
33. Sun C, Gao W, Liu J, Cheng H, Hao J. FGL1 Regulates Acquired Resistance to Gefitinib by Inhibiting Apoptosis in Non-Small Cell Lung Cancer. Respir Res (2020) 21(1):210. doi: 10.1186/s12931-020-01477-y
34. Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology (2017) 153(4):1107–19.e10. doi: 10.1053/j.gastro.2017.06.017
35. Maruhashi T, Okazaki I-M, Sugiura D, Takahashi S, Maeda TK, Shimizu K, et al. LAG-3 Inhibits the Activation of CD4 T Cells That Recognize Stable pMHCII Through its Conformation-Dependent Recognition of pMHCII. Nat Immunol (2018) 19(12):1415–26. doi: 10.1038/s41590-018-0217-9
36. Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res (2012) 72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620
37. Zhang Q, Chikina M, Szymczak-Workman AL, Horne W, Kolls JK, Vignali KM, et al. LAG3 Limits Regulatory T Cell Proliferation and Function in Autoimmune Diabetes. Sci Immunol (2017) 2(9):eaah4569. doi: 10.1126/sciimmunol.aah4569
38. Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, et al. Lymphocyte Activation Gene 3 (LAG-3) Modulates the Ability of CD4 T-Cells to be Suppressed In Vivo. PloS One (2014) 9(11):e109080. doi: 10.1371/journal.pone.0109080
39. Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of Lymphocyte Activation Gene-3 (Lag-3) in Conventional and Regulatory T Cell Function in Allogeneic Transplantation. PloS One (2014) 9(1):e86551. doi: 10.1371/journal.pone.0086551
40. Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR Complex-Associated Lymphocyte Activation Gene-3 Molecules Inhibit CD3/TCR Signaling. J Immunol (1998) 161(8):4058–65.
41. Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, et al. Characterization of the Lymphocyte Activation Gene 3-Encoded Protein. A New Ligand for Human Leukocyte Antigen Class II Antigens. J Exp Med (1992) 176(2):327–37. doi: 10.1084/jem.176.2.327
42. Marty Pyke R, Thompson WK, Salem RM, Font-Burgada J, Zanetti M, Carter H. Evolutionary Pressure Against MHC Class II Binding Cancer Mutations. Cell (2018) 175(2):416–28.e13. doi: 10.1016/j.cell.2018.08.048
43. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discovery (2015) 5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863
44. Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 Targets LAG-3 and 4-1BB Describe and Regulate Dysfunctional Antigen-Specific CD8+ T Cells in the Tumor Microenvironment. J Exp Med (2017) 214(2):381–400. doi: 10.1084/jem.20160485
45. Shen R, Postow MA, Adamow M, Arora A, Hannum M, Maher C, et al. LAG-3 Expression on Peripheral Blood Cells Identifies Patients With Poorer Outcomes After Immune Checkpoint Blockade. Sci Trans Med (2021) 13(608):eabf5107. doi: 10.1126/scitranslmed.abf5107
46. Chen C, Liang C, Wang S, Chio CL, Zhang Y, Zeng C, et al. Expression Patterns of Immune Checkpoints in Acute Myeloid Leukemia. J Hematol Oncol (2020) 13(1):28. doi: 10.1186/s13045-020-00853-x
47. He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship With PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J Thorac Oncol (2017) 12(5):814–23. doi: 10.1016/j.jtho.2017.01.019
48. Datar I, Sanmamed MF, Wang J, Henick BS, Choi J, Badri T, et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin Cancer Res (2019) 25(15):4663–73. doi: 10.1158/1078-0432.CCR-18-4142
49. Que Y, Fang Z, Guan Y, Xiao W, Xu B, Zhao J, et al. LAG-3 Expression on Tumor-Infiltrating T Cells in Soft Tissue Sarcoma Correlates With Poor Survival. Cancer Biol Med (2019) 16(2):331–40. doi: 10.20892/j.issn.2095-3941.2018.0306
50. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-Infiltrating NY-ESO-1-Specific CD8+ T Cells Are Negatively Regulated by LAG-3 and PD-1 in Human Ovarian Cancer. Proc Natl Acad Sci USA (2010) 107(17):7875–80. doi: 10.1073/pnas.1003345107
51. Huang R-Y, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 Co-Inhibitory Molecules Collaborate to Limit CD8+ T Cell Signaling and Dampen Antitumor Immunity in a Murine Ovarian Cancer Model. Oncotarget (2015) 6(29):27359–77. doi: 10.18632/oncotarget.4751
52. Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, et al. Expression of LAG-3 by Tumor-Infiltrating Lymphocytes is Coincident With the Suppression of Latent Membrane Antigen-Specific CD8+ T-Cell Function in Hodgkin Lymphoma Patients. Blood (2006) 108(7):2280–9. doi: 10.1182/blood-2006-04-015164
53. El Halabi L, Adam J, Gravelle P, Marty V, Danu A, Lazarovici J, et al. Expression of the Immune Checkpoint Regulators LAG-3 and TIM-3 in Classical Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk (2021) 21(4):257–66.e3. doi: 10.1016/j.clml.2020.11.009
54. el Halabi L, Adam J, Marty V, Bosq J, Lazarovici J, Danu A, et al. Strong Expression of the Immune Checkpoint Regulators LAG3 and Tim3 in Hodgkin Lymphoma. Blood (2016) 128(22):2952–. doi: 10.1182/blood.V128.22.2952.2952
55. Karlsson J, Nilsson LM, Mitra S, Alsén S, Shelke GV, Sah VR, et al. Molecular Profiling of Driver Events in Metastatic Uveal Melanoma. Nat Commun (2020) 11(1):1894. doi: 10.1038/s41467-020-15606-0
56. Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103 Tumor-Resident CD8 T Cells Are Associated With Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin Cancer Res (2018) 24(13):3036–45. doi: 10.1158/1078-0432.CCR-17-2257
57. Chen Z, Huang Y, Hu Z, Zhao M, Bian Y, Chen Z, et al. Dissecting the Single-Cell Transcriptome Network in Patients With Esophageal Squamous Cell Carcinoma Receiving Operative Paclitaxel Plus Platinum Chemotherapy. Oncogenesis (2021) 10(10):71. doi: 10.1038/s41389-021-00359-2
58. Yang Z-Z, Kim HJ, Villasboas JC, Chen Y-P, Price-Troska T, Jalali S, et al. Expression of LAG-3 Defines Exhaustion of Intratumoral PD-1 T Cells and Correlates With Poor Outcome in Follicular Lymphoma. Oncotarget (2017) 8(37):61425–39. doi: 10.18632/oncotarget.18251
59. Thommen DS, Schreiner J, Müller P, Herzig P, Roller A, Belousov A, et al. Progression of Lung Cancer Is Associated With Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol Res (2015) 3(12):1344–55. doi: 10.1158/2326-6066.CIR-15-0097
60. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J Clin Oncol (2010) 28(19):3167–75. doi: 10.1200/JCO.2009.26.7609
61. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
62. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
63. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733
64. Andrews LP, Somasundaram A, Moskovitz JM, Szymczak-Workman AL, Liu C, Cillo AR, et al. Resistance to PD1 Blockade in the Absence of Metalloprotease-Mediated LAG3 Shedding. Sci Immunol (2020) 5(49):eabc2728. doi: 10.1126/sciimmunol.abc2728
65. Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential Expression of Immune-Regulatory Genes Associated With PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res (2015) 21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244
66. Johnson DB, Nixon MJ, Wang Y, Wang DY, Castellanos E, Estrada MV, et al. Tumor-Specific MHC-II Expression Drives a Unique Pattern of Resistance to Immunotherapy via LAG-3/FCRL6 Engagement. JCI Insight (2018) 3(24):e120360. doi: 10.1172/jci.insight.120360
67. Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen H-R, Pyle KJ, et al. Functionally Distinct LAG-3 and PD-1 Subsets on Activated and Chronically Stimulated CD8 T Cells. J Immunol (2009) 182(11):6659–69. doi: 10.4049/jimmunol.0804211
68. Atkinson V, Khattak A, Haydon A, Eastgate M, Roy A, Prithviraj P, et al. Eftilagimod Alpha, a Soluble Lymphocyte Activation Gene-3 (LAG-3) Protein Plus Pembrolizumab in Patients With Metastatic Melanoma. J Immunother Cancer (2020) 8(2):e001681. doi: 10.1136/jitc-2020-001681
69. Zahm CD, Moseman JE, Delmastro LE, G Mcneel D. PD-1 and LAG-3 Blockade Improve Anti-Tumor Vaccine Efficacy. Oncoimmunology (2021) 10(1):1912892. doi: 10.1080/2162402X.2021.1912892
70. Burova E, Hermann A, Dai J, Ullman E, Halasz G, Potocky T, et al. Preclinical Development of the Anti-LAG-3 Antibody REGN3767: Characterization and Activity in Combination With the Anti-PD-1 Antibody Cemiplimab in Human -Knockin Mice. Mol Cancer Ther (2019) 18(11):2051–62. doi: 10.1158/1535-7163.MCT-18-1376
71. Ghosh S, Sharma G, Travers J, Kumar S, Choi J, Jun HT, et al. TSR-033, a Novel Therapeutic Antibody Targeting LAG-3, Enhances T-Cell Function and the Activity of PD-1 Blockade and. Mol Cancer Ther (2019) 18(3):632–41. doi: 10.1158/1535-7163.MCT-18-0836
72. Kraman M, Faroudi M, Allen NL, Kmiecik K, Gliddon D, Seal C, et al. FS118, a Bispecific Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin Cancer Res (2020) 26(13):3333–44. doi: 10.1158/1078-0432.CCR-19-3548
Keywords: Lymphocyte Activation Gene-3 (LAG-3), immune checkpoint, drug resistance, Programmed Cell Death 1 (PD-1), immunotherapy
Citation: Wei Y and Li Z (2022) LAG3-PD-1 Combo Overcome the Disadvantage of Drug Resistance. Front. Oncol. 12:831407. doi: 10.3389/fonc.2022.831407
Received: 08 December 2021; Accepted: 22 March 2022;
Published: 14 April 2022.
Edited by:
Eyad Elkord, University of Salford, United KingdomReviewed by:
Amir Baghbanzadeh, Tabriz University of Medical Sciences, IranCopyright © 2022 Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoming Li, bGFiXzIwMTRAMTYzLmNvbQ==
 Yiming Wei
Yiming Wei Zhaoming Li
Zhaoming Li