
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Oncol., 24 February 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.830506
This article is a correction to:
Doxycycline Inhibits Cancer Stem Cell-Like Properties via PAR1/FAK/PI3K/AKT Pathway in Pancreatic Cancer
 Huijuan Liu1,2,3*†
Huijuan Liu1,2,3*† Honglian Tao1,2†
Honglian Tao1,2† Hongqi Wang1,2†
Hongqi Wang1,2† Yuyan Yang1,2
Yuyan Yang1,2 Ru Yang1,2
Ru Yang1,2 Xintong Dai1,2
Xintong Dai1,2 Xiujuan Ding1,2
Xiujuan Ding1,2 Haidong Wu2
Haidong Wu2 Shuang Chen2
Shuang Chen2 Tao Sun1,2,4*
Tao Sun1,2,4*A Corrigendum on
Doxycycline Inhibits Cancer Stem Cell-Like Properties via PAR1/FAK/PI3K/AKT Pathway in Pancreatic Cancer
By: Liu H, Tao H, Wang H, Yang Y, Yang R, Dai X, Ding X, Wu H, Chen S and Sun T (2021) Front. Oncol. 10:619317. doi 10.3389/fonc.2020.619317
In the original article, there was a mistake in Figures 2 and 5 as published. The presented figure of siPAR1 (0 h) in Figure 2A and the presented figure of Group 30 μM (cells treated with 30 μM of doxycycline) in Figure 5E were wrongly presented in the original article. Furthermore, the annotation in the ordinate axis of the statistical figure in Figures 2B and 5E should be “invasion cells per field” not the “passed cells per field.” The corrected Figures 2 and 5 appear below.
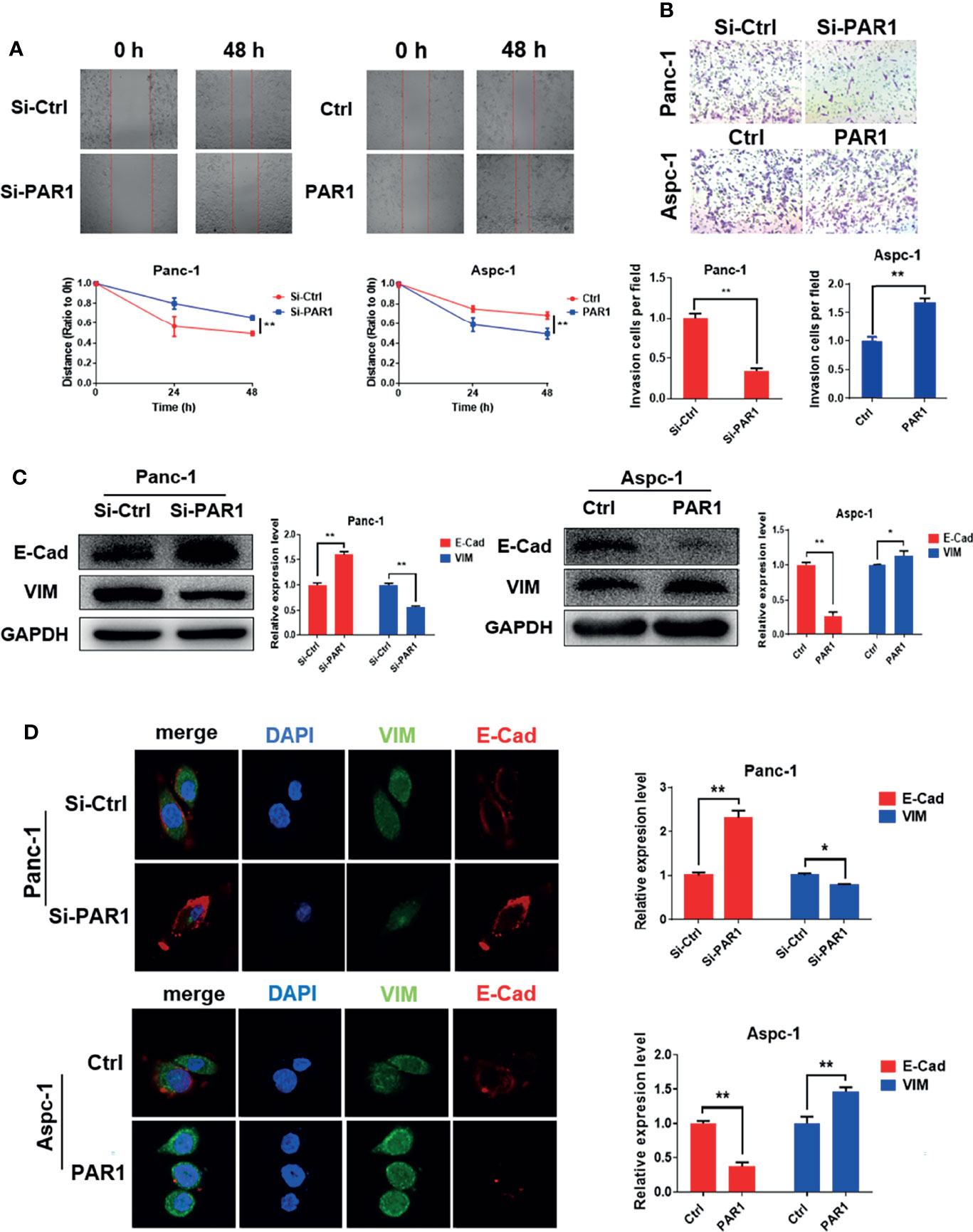
Figure 2 PAR1 promotes EMT progression of pancreatic cancer cells. (A) Effect of PAR1 on Panc-1 and Aspc-1 cell migration potential detected using the wound healing assay. (B) Effect of PAR1 on pancreatic cancer cell invasion potential by using matrigel coated transwell assay. (C) Effect of PAR1 on the E-Cad and VIM expression detected by Western blot analysis. (D) Effect of PAR1 on the E-Cad and VIM expression detected by immunofluorescence. Data are shown as the mean ± SD (*P < 0.05, **P < 0.01).
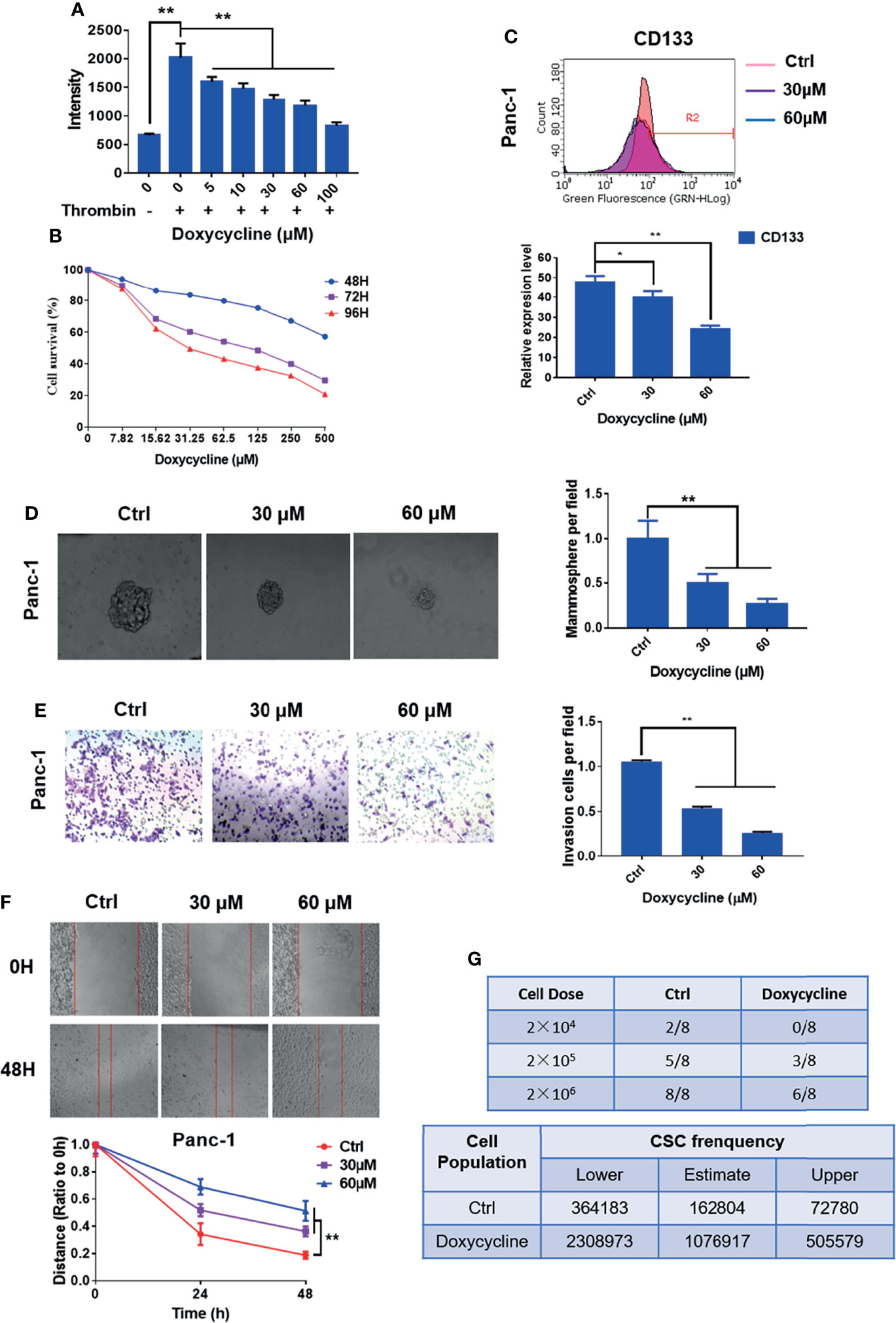
Figure 5 Doxycycline inhibits the cancer stem cell-like properties of pancreatic cancer cells. (A) Effect of doxycycline on PAR1 activation stimulated by thrombin in pancreatic cancer cells detected by the Ca2+-mobilization assay. (B) Effect of doxycycline on pancreatic cancer cell viability after 48, 72, and 96 h treatment. (C) Effect of doxycycline on pancreatic cancer stem cell marker CD133 in Panc-1 cells. (D) Effect of doxycycline on the mammosphere formation of Panc-1 cells. (E) Effect of doxycycline on pancreatic cancer cell invasion ability. (F) Effect of doxycycline on pancreatic cancer cells migration ability. (G) Limiting dilution assay of pancreatic cancer stem cell from Panc-1 cells after treatment with doxycycline in node mice (n = 8). Cancer stem cell frequency was determined by ELDA. Data are shown as the mean ± SD (*P < 0.05, **P < 0.01).
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: protease activation receptor 1, focal adhesion kinase, doxycycline, epithelial–mesenchymal transformation, pancreatic cancer stem cells
Citation: Liu H, Tao H, Wang H, Yang Y, Yang R, Dai X, Ding X, Wu H, Chen S and Sun T (2022) Corrigendum: Doxycycline Inhibits Cancer Stem Cell-Like Properties via PAR1/FAK/PI3K/AKT Pathway in Pancreatic Cancer. Front. Oncol. 12:830506. doi: 10.3389/fonc.2022.830506
Received: 07 December 2021; Accepted: 24 January 2022;
Published: 24 February 2022.
Edited and reviewed by:
Zhi-xiang Yuan, Southwest Minzu University, ChinaCopyright © 2022 Liu, Tao, Wang, Yang, Yang, Dai, Ding, Wu, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Sun, dGFvLnN1bkBuYW5rYWkuZWR1LmNu; Huijuan Liu, bGl1aHVpanVhbnh5ekAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.