- 1Department of Biomedicine and Prevention, Tor Vergata University, Rome, Italy
- 2Division of Hematology, Cardarelli Hospital, Naples, Italy
- 3Department of Clinical and Experimental Medicine, Section of Hematology, University of Pisa, Pisa, Italy
- 4Department of Oncology-Hematology, University of Milan, Milan, Italy
- 5Azienda Socio Sanitaria Territoriale Papa Giovanni XXIII, Bergamo, Italy
Acute myeloid leukemia (AML) is a heterogeneous disease with a wide variety of clinical presentations, morphological features, and immunophenotypes. The diagnostic approaches to AML that are adopted in Italy have been explored using an online Delphi-based process to expand the global discussion on mandatory tests for the correct diagnosis and, consequently, for optimal management of AML in clinical practice. The final results of the panel of Italian hematologists involved in this work highlight the importance of genetic evaluation for classification and risk stratification and firmly establish that karyotyping, fluorescence in situ hybridization in cases with non-evaluable karyotype, and molecular tests must be performed in every case of AML, regardless of age. Obtaining clinically relevant genetic data at diagnosis is the basis for the success of patient-tailored therapy. The Italian specialists also confirm the role of multidisciplinary diagnostics for AML, now mandatory and expected to become more important in the future context of “precision” medicine.
Introduction
Acute myeloid leukemia (AML) is an uncontrolled clonal proliferation derived from progenitor/precursor hematopoietic cells. Abnormal differentiation of myeloid cells results in high levels of immature malignant cells (blasts) and fewer normally differentiated red blood cells, white blood cells and platelets (1, 2). From a genetic point of view, the definition of AML includes heterogeneous entities, with a wide variety of clinical presentations, morphological features, immunophenotypes. Genetic abnormalities, including chromosomal abnormalities and mutations in proliferation/survival mechanisms (FLT3) and differentiation/apoptosis pathways (CEBPA, RUNX1, and NPM1), play a pathogenetic role in AML, provide prognostic criteria, and can guide therapy (3–5). In recent years, relevant progress has been made not only in understanding the pathogenesis of the disease but also in the development of diagnostic tools and novel therapies (6).
The most recent World Health Organization (WHO) classification, published in 2016, compared with that published in 2008 (7), included new AML entities with different cytogenetic and molecular characteristics (8). Moreover, the prognostic and diagnostic relevance of the new molecular features were reviewed and integrated into existing sets of criteria. Six categories of AML were identified: (1) AML with recurrent genetic abnormalities, (2) AML with myelodysplasia-related changes (AML-MRC), (3) therapy-related myeloid neoplasms, (4) AML not otherwise specified (AML-NOS), (5) myeloid sarcoma, and (6) myeloid proliferations related to Down syndrome (8).
Several organizations, including the National Comprehensive Cancer Network (NCCN), the European Leukemia Net (ELN), and the European Society for Medical Oncology (ESMO), recently developed guidelines and risk score systems to help physicians to design ab initio the best “patient-tailored” therapeutic strategy (9–11). Interestingly, all these risk-stratification systems and guidelines are based on chromosomal abnormalities and gene mutations.
The diagnostic process of a heterogeneous disease such as AML is complex and requires high levels of expertise. A panel of Italian hematologists explored the diagnostic approaches that are adopted across the national territory through a survey that involved a representative sample of the Italian Centers of Hematology.
Information on how Italian hematologists manage the diagnostic workup of AML may contribute to expand the global discussion on mandatory tests for the correct diagnostic approach and, consequently, for optimal management of AML in clinical practice. The diagnosis of the different genetic subtypes of AML is a subject of intense debate within the international scientific community because it has relevant prognostic and therapeutic implications.
Methods
To gather expert opinion on the implementation of accurate diagnosis of AML subtypes, we used an online Delphi-based process (Estimate-Talk-Estimate). This is a group-facilitative method designed to verify the convergence of opinion of a panel of experts in a given area of uncertainty within health-related research (12, 13).
The process was developed over nearly 7 months using the following steps: (1) establishment of a scientific steering committee of five experts who were in charge of reviewing the literature and developing the survey items; (2) selection of a panel of AML specialists; (3) administration of online survey to the panel of AML specialists; (4) collection and analysis of the results; (5) final meeting.
Scientific Steering Committee
Five experts from Italian hematology institutions were identified as representatives of the specialists involved in the medical care of patients with AML. The scientific steering committee defined 26 questions covering four main topics:
Topic 1: application of the WHO 2016 classification (8 items)
Topic 2: confirmatory diagnostic tests (4 items)
Topic 3: implementation of the guidelines (7 items)
Topic 4: therapeutic implications (7 items)
The possible answers to each question were chosen from multiple options, all possible and applicable in clinical practice.
Panel of AML Specialists
Forty-eight specialists (hematologists, cytogeneticists, and molecular biologists) selected from 42 centers among the Italian hematology institutions, were invited to participate in the project during a virtual meeting where the project objectives and methodology were shared.
Administration of the Online Survey
The survey elaborated by the steering committee was delivered to the panel of AML specialists: 34 participants from 31 sites (29 hematologists and 5 cytogeneticists/molecular biologists) expressed an opinion on each of the 26 questions, independently and blindly. All 34 specialists were affiliated to centers with availability of a diagnostic lab, and with an allogeneic or autologous stem cell transplantation unit in 27 and 7 cases, respectively. The survey was performed online on a secure website using an online platform.
Final Meeting
The scientific steering committee collected and analyzed the results, then discussed the real-life AML diagnostic approach adopted in Italy in the light of the international recommendations.
Results
The panel of specialists answered each of the 26 questions based on their own clinical practice, experience, and facilities available (Table 1).
Topic 1: Application of the WHO 2016 Classification
In recent decades, the diagnostic workup for AML, initially based solely on morphological examination, has integrated more and more disciplines and methodologies. The WHO 2016 classification adopted such fundamental tools as cytomorphology, cytochemistry, immunophenotyping, cytogenetics, and molecular genetics. The integration of all these techniques allows for a comprehensive and complementary characterization of each patient with AML, which is a prerequisite for optimal diagnosis and management.
The aim of the questions in the first topic of the Delphi questionnaire was to investigate the specialists’ attitude in applying the WHO diagnostic criteria in clinical practice, focusing on which tests they would consider crucial for the correct identification of AML subtypes (Table 1).
Panelists’ View and Behavior in AML Clinical Practice
Almost all experts participating in the survey stated that they always refer to the WHO 2016 classification (Table 2) for the diagnosis of AML. Generally, despite differences in terms of technological equipment among the centers, there was stringent observance of the WHO 2016 recommendations. However, some differences also emerged; for example, 26% of the specialists perform morphology, immunophenotyping, conventional cytogenetics, and mutational analysis of NPM1, CEBPA, and RUNX1 genes only in young patients. However, the specialists who provided comments in the open field stated that the use of tests is driven more by fitness to undergo intensive chemotherapy than by age. On the other hand, 35% of the specialists perform all of the above diagnostic tests, regardless of age. Finally, 39% of specialists stated that assessment for CEBPA and RUNX1 is performed in selected cases only.
When collecting anamnestic information, most of the specialists (81%) declared that they carefully seek information about previous exposure to chemo-/radiotherapy to exclude therapy-related myeloid neoplasms. An accurate anamnesis, also asking about history of a previous transfusion support or a diagnosis of antecedent myelodysplastic syndrome (MDS), was considered a fundamental step for the identification of AML-MRC.
The turnaround time to obtain information on cytogenetics can be as long as 7–15 days, whereas it can be no longer than 72 h for molecular testing. The specialists were asked to state which molecular changes they considered critical for the diagnosis of AML with recurrent genetic abnormalities. There was no convergence on which molecular assay to request. About one third (36%) of the panelists claimed to request only qualitative polymerase chain reaction (PCR) for the main fusion genes (RUNX1-RUNX1T1, CBFB-MYH11, PML-RARalpha, BCR-ABL1). The remaining two thirds were equally divided between those who request all possible PCR assays (32%) and those who urgently require only qualitative PCR for PML-RARalpha (32%) to exclude a diagnosis of acute promyelocytic leukemia.
Sanger sequencing (29%) and next-generation sequencing (NGS) (29%) are the most frequently used techniques for detecting AML with biallelic CEBPA mutation (Figure 1). According to the panelists, the execution of the molecular assays is mandatory. Sixty-five percent the panelists stated that the use of flow cytometry, even with CD7 assessment, is not reliable enough for the identification of biallelic CEBPA mutations (14).
Over 70% of panelists believed that morphological assessment alone is not sufficient to support the diagnosis of AML-MRC. Morphology assessment should always be associated with clinical history, cytogenetics NPM1 and biallelic CEBPA mutational analysis, in line with WHO recommendations. Sixty percent of the specialists mentioned fluorescence in situ hybridization (FISH) as a valid substitute for karyotyping to clarify difficult diagnosis such as AML-MRC as soon as possible. Such an approach is deemed reliable in situations of punctio sicca, insufficient number of metaphases, or when the turnaround time to obtain information about the karyotyping is too long.
Comments From the Scientific Steering Committee
The outcomes from the first set of answers demonstrated the specialists’ attitude to applying the 2016 WHO AML classification and the recommended diagnostic workup. Nevertheless, such an intention is affected by the technological heterogeneity among the different centers and the use or not of reference laboratories (such as GIMEMA LabNet AML, https://www.gimema.it/ricerca/labnet/) for centralized activities.
It is also evident that the prognostic/therapeutic implications are strong drivers for the specialists to decide which molecular changes should be tested. It may be important to reinforce the existing differences between the WHO 2016 classification, which defines specific AML subtypes by focusing on cytogenetic and molecular features, and the ELN scoring system, which uses the same disease-specific biological aspects to distinguish patients with a “favorable”, “intermediate” or “adverse” risk (6, 8) prognosis.
Several areas of debate emerged from the survey. Medical history appears to be a fundamental starting point in patients’ assessment. In addition to previous hematologic diseases (even undiagnosed) and cytotoxic treatments, there is increasing evidence of a frequent association between bone marrow dysplastic features and autoimmune disorders (15). Therefore, it may be important to consider this aspect in the patient’s history as immediately suspicious for AML-MRC.
With regard to age, the participants stated that it is not an absolute discriminating factor. The impact of age should be evaluated in the light of co-existing co-morbidities, with the aim of selecting patients eligible for intensive chemotherapy and, if needed, allogeneic transplantation. Nevertheless, most new targeted agents are also indicated for the treatment of older patients with AML (16–18), therefore accurate molecular characterization of the disease is desirable regardless of age (19).
With regard to the diagnosis of AML with recurrent genetic abnormalities, molecular assays covering the spectrum of the most relevant gene rearrangements are valuable tools to assist the diagnostic process while waiting for the cytogenetic results. Furthermore, some gene rearrangements such as RUNX1-RUNX1T1, CBFB-MYH11, PML-RARalpha, BCR-ABL1 are also clinically relevant because they serve to track measurable residual disease (MRD) during and after treatment (20–22).
For AML with recurrent genetic abnormalities, the WHO 2016 classification includes cases with biallelic CEBPA mutations. There is a need to identify these mutations because they could have a diagnostic or prognostic role (8, 23, 24). From a diagnostic point of view, the presence of mutations in the CEBPA gene should make clinicians consider a condition of inherited predisposition to AML and prompt screening of family members. Such information is also crucial for the choice of donor in cases of hematopoietic stem cell transplantation (25). From a prognostic standpoint, it is necessary to establish the mutational pattern of CEBPA in order to adopt an appropriate post-induction strategy (26, 27). CEBPA mutation confers a favorable prognosis if present in the biallelic pattern.
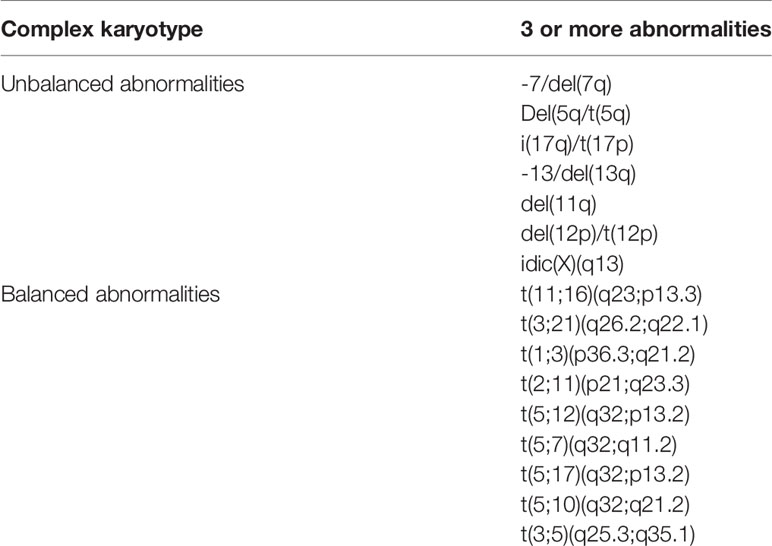
Morphology remains a fundamental diagnostic step and contributes to the identification of subtypes of AML. Nevertheless, classification of AML-MRC depends on a multidisciplinary diagnostic approach. In addition to morphological assessment, necessary for evaluating the presence of bone marrow dysplastic features, a correct diagnosis of AML-MRC requires cytogenetics to identify cases of de novo AML with MDS-related cytogenetic abnormalities (Table 3), and exclude recurrent cytogenetic abnormalities (Table 2), anamnestic investigation, and molecular analysis to exclude NPM1 and biallelic CEBPA mutations (8, 28).
Even though the specialists agree that conventional karyotyping is the reference technique and every effort should be made to obtain its results, they often use FISH analysis in cases of unknown karyotype or to bypass a long turnaround time. FISH provides a targeted and fast approach to obtain diagnostic and prognostic information, especially regarding chromosomes 3, 5 and 7 (29).
Topic 2: Confirmatory Diagnostic Tests
The intrinsic complexity of the diagnostic workup of AML entails some critical issues, such as the availability of adequate technical resources and facilities and the turnaround time to obtain the results. In addition to affecting the diagnostic process, these factors have a relevant impact on the therapeutic decisions. Questions asked in the second topic of the survey focused on the time necessary to complete a full laboratory assessment and on the identification of potential solutions (Table 1).
Panelists’ View and Behavior in AML Clinical Practice
Sixty-five percent of the panelists agreed that, in categories identified by specific genetic abnormalities, the assessment of bone marrow blast count is still relevant, whereas 35% of the AML specialists considered cytomorphology, in the presence of specific genetic lesions, not mandatory or vicariable by flow cytometry.
The turnaround time for the cytogenetic reports was variable. Results are shown in Figure 2A. Information about PML-RARalpha is achieved within 24 hours in 97% of the Italian laboratories (Figure 2B). The turnaround time for the other common genetic abnormalities (BCR-ABL1, FLT3, NPM1, RUNX1-RUNX1T1, CBFB-MYH11) is highly variable (Figure 2C), ranging from 72 hours (39% of the panelists) to 1 week for non-NGS reports (45% of the panelists), up to 3 weeks for NGS reports (16%).
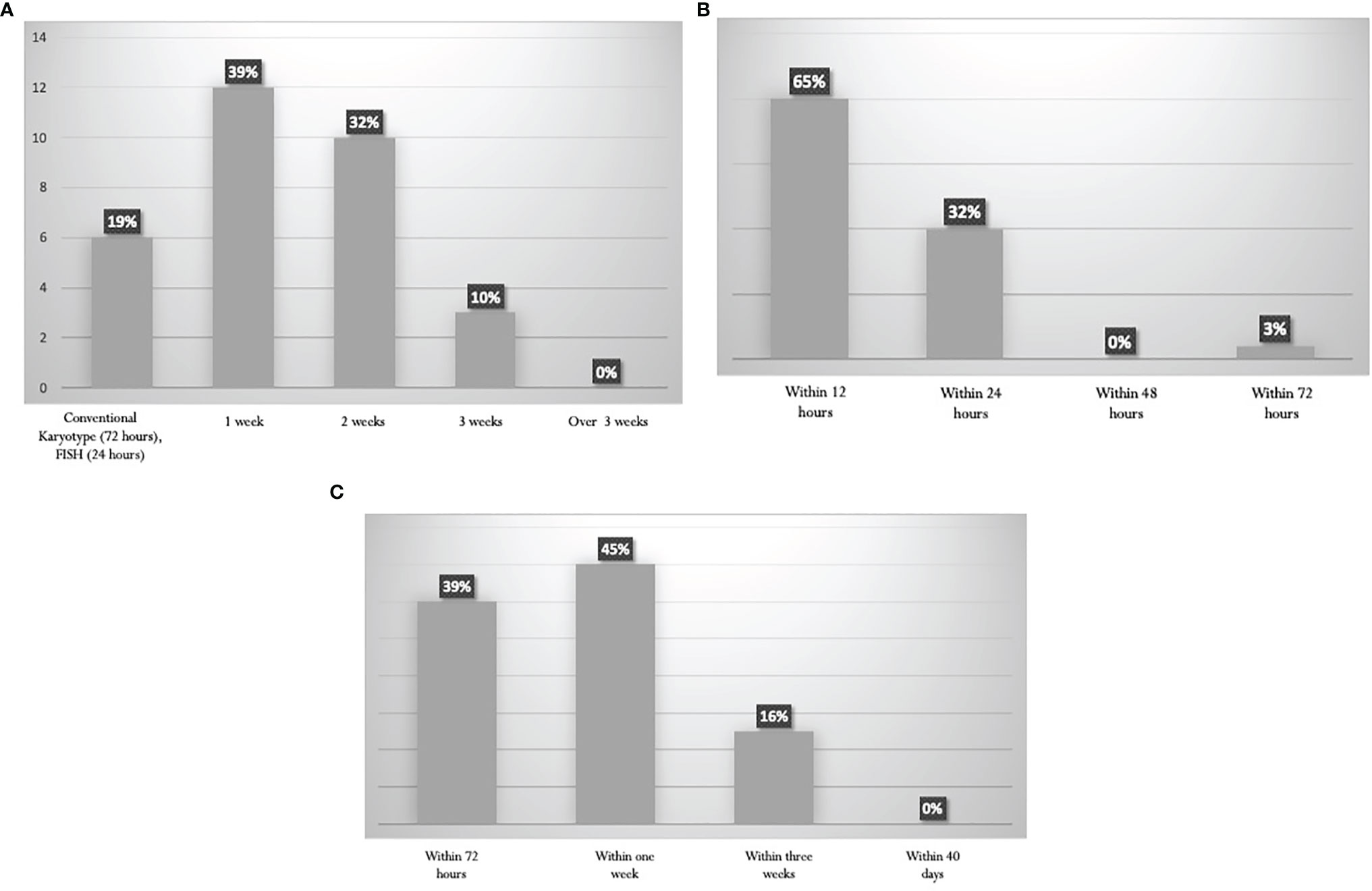
Figure 2 Turnaround times for the diagnostics procedures. (A) Karyotype. (B) PML-RARalpha rearrangement by RT-PCR. (C) Other Recurrent genetic abnormalities (BCR-ABL1, FLT3, NPM1, RUNX1-RUNX1T1, CBFB-MYH11).
Comments From the Scientific Steering Committee
The scientific steering committee emphasized the importance of bone marrow (BM) blast count. In patients with specific genetic alterations, cytomorphological examination of BM, and accurate evaluation of blast infiltration, according to the current guidelines, is of paramount importance to guide diagnosis and assess response to treatment (6). The committee also emphasizes that in the case of t(15;17), t(8;21), inv(16), or t(16;16) and their respective fusion genes, the diagnosis of AML can be made even if the BM blast percentage is less than 20%.
The ELN recommends that the turnaround time for reporting NPM1 and FLT3 mutation should be 48–72 hours, 96 hours for further gene rearrangements, 24 hours for molecular reporting of PML-RARalpha, and 5–7 days for cytogenetics (11). These turnaround times are generally met by most centers (Figure 2).
The committee believes that there is still room for improvement and a strategy to reduce variability among sites should consider the use of laboratory networks, such as the GIMEMA LabNet AML network, to assist in the diagnostic process of AML by centers not fully equipped for complex molecular tests. Finally, the committee agree that genetic assessment for AML should be repeated in cases of disease relapse to demonstrate clonal evolution of the disease, including acquisition of chromosomal abnormalities, the disappearance or appearance of somatic mutations, including FLT3 mutations, where the emergence of mutated clones paves the way for the delivery of new FLT3 inhibitors given as single agents, as gilteritinib.
Topic 3: Implementation of the Guidelines
The questions for the third topic explored how the specialists apply cytogenetic and molecular analyses to risk-stratify cases of AML (Table 1) and to what extent they rely on current recommendations.
Panelists’ View and Behavior in AML Clinical Practice
The search for recurrent translocations with rapid techniques, karyotype assessment, study of the mutational state of NPM1 and calculation of the FLT3-ITD allelic ratio (AR) are fundamental for prognostic stratification. According to the 2017 ELN guidelines (6), a high percentage (74%) of AML experts perform molecular analyses of RUNX1, ASXL1, TP53 genes for a proper definition of the “adverse risk” category. This percentage includes 32% of experts who always perform these analyses and 42% who carry out them specifically to establish eligibility for allogeneic transplantation (Figure 3A). Only 29% of the specialists stated that NGS data are available at diagnosis, and can classify patients within ELN categories before starting treatment (Figure 3B). On the contrary, mutation status by NGS becomes available in most cases at the end of induction, and is used to choose post-consolidation strategies, including allogeneic stem cell transplantation.
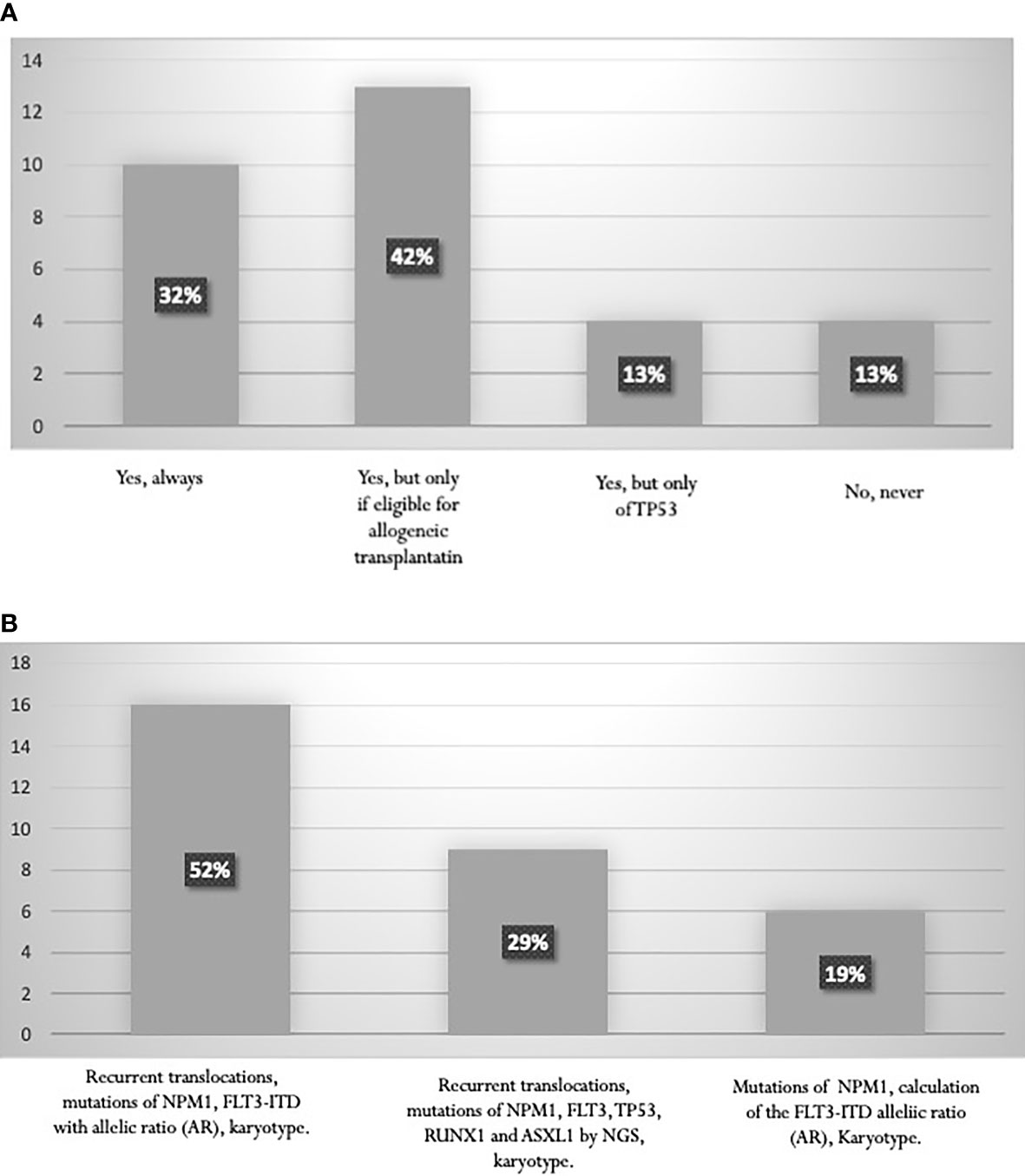
Figure 3 (A) Proportion of laboratories performing RUNX1, ASXL1, TP53 mutation studies. (B) Tests performed for AML stratification according to ELN 2017.
Almost all panelists comply with the indications of the ESMO 2020 guidelines and perform analysis for FLT3 mutations using fragment analysis for FLT3-ITD, and digestion with the ECO-RV restriction enzyme for FLT3-TKD. In NPM1-mutated AML, 71% of the panelists apply the ELN recommendations, indicating that the presence of FLT3-ITD mutation with an AR <0.5 is a favorable prognostic factor. The ESMO 2020 guidelines also recommends testing IDH1 and IDH2 mutations. Across the laboratories involved in this survey, the assay is mainly performed by Sanger sequencing or NGS. The amplification-refractory mutation system PCR is used in 16% of the centers (Figure 4).

Figure 4 Proportion of Laboratories assessing IDH1/IDH2 mutation status according to ESMO 2020 guidelines.
Comments From the Scientific Steering Committee
The widespread use of NGS, although not homogeneously distributed across the national territory, has a relevant impact on the laboratory activities of the centers, because this method entails considerable complexity with report accuracy and data interpretation. The implementation of NGS in clinical practice will certainly contribute to expand the body of information necessary for a proper application of the ELN risk stratification. However, cytogenetics and molecular biology testing should not be omitted; rather, they should be complemented by NGS.
The committee agree that assessment of IDH1 and IDH2 mutation, although useful for a comprehensive genetic characterization of AML (11), is not urgent at the time of diagnosis. This data does not have a therapeutic impact due to the current unavailability of IDH1/IDH2 inhibitors in Europe. Therefore, even though guidelines present decisive drivers for diagnosis, prognosis, and treatment management, they should be adapted to the national clinical practice and the real likelihood that the patients can have access to new agents, based on the National Health System regulations in force. At present, IDH mutational analysis can however help physicians to select for specific treatment combinations, in particular including hypomethylating agents and venetoclax, since these mutations have been associated with improved response and survival (30).
Topic 4: Therapeutic Implications
The last section of the survey focused on the impact that international guidelines and identification of AML subtypes might have on the therapeutic strategy. Considering that, in recent years, robust evidence of the prognostic role of MRD has emerged, specific questions on this issue were asked to understand to what extent MRD-driven therapeutic strategies are adopted among the centers (Table 1).
Panelists’ View and Behavior in AML Clinical Practice
Sixty-one percent of the specialists involved in the Delphi project rely on internal guidelines for AML management (PDTA, diagnosis, therapy, and assistance pathway), and 36% and 3% stated that they refer to the ESMO (11) and NCCN guidelines (9), respectively.
The specialists almost unanimously confirmed that the identification of disease genetic subtypes, such as those characterized by the rearrangement of core binding factors (AML-CBF), APL and AML-MRC, has a significant impact on clinical decisions. For patients considered eligible for intensive chemotherapy, with a diagnosis of AML-MRC and concomitant FLT3 mutation, 80% of the clinicians opted for targeted induction treatment (“7+3” regimen plus midostaurin) (31) rather than an AML-MRC-specific treatment such as CPX-351 (32).
The panelists unanimously stated that FLT3 re-testing is critical upon relapse to offer FLT3 inhibitor treatment if the mutation occurs (33, 34). In addition, all the experts agreed that MRD assessment should be mandatory in clinical practice because it could affect the therapeutic management of AML. Seventy-four percent of the respondents believed that the technique to use for MRD assessment depends on the presence of a molecular marker; 16% of the specialists believed that flow cytometry still has a role, especially in selected situations, and 10% stated that they prefer to use molecular analysis when applicable (35).
The experts agreed on the potential predictive role of hyper-expression of Wilms tumor gene (WT1) (36), even if it is not included in the lists of genes that can be adopted for MRD assessment.
Comments From the Scientific Steering Committee
As observed across the survey, the 2016 WHO classification and identification of AML genetic subtypes seem to play a fundamental role in therapeutic decisions in all centers. Nevertheless, the choice of a therapy for a specific patient with AML who would be eligible for multiple treatments is still a complex process. On the one hand, the presence of a specific mutation or a specific immunophenotype, may indicate the use of targeted agents, as f.i. TKi or Mylotarg in CD33+ AML. On the other hand, the complex genetic background should be also considered, especially in the light of the patient’s clinical history, and in AML-MRC. In conclusion, it would be desirable to carry out a complete “biological” characterization of patients in order to choose the most appropriate treatment among those available. For example, for FLT3-mutated cases, both midostaurin and CPX-351, despite their diverse mechanisms of action, are effective (32, 37). Midostaurin has been developed specifically for FLT3-mutated AML but data concerning MRC- or therapy-related subtypes are still inconclusive (36). CPX-351, indicated for MRC- and therapy-related AML (32), also seems to be effective in FLT3-mutated cases, as reported by the French (38) and Italian (39) groups.
With regard to MRD, at diagnosis, AMLs are characterized by genotypical and phenotypical features that allow leukemic cells to be distinguished from their normal counterparts (40). The leukemia-associated immunophenotype “ (LAIP) identified by multi-color flow cytometry or the “different from normal” pattern can then be used to monitor MRD (41, 42). Moreover, in selected cases, such as those negative for recurrent rearrangements or NPM1 mutations, overexpression of WT1 can be considered for MRD monitoring (43–48). However, WT1 is not completely adequate for MRD, because a reproducible and validated cut-off to distinguish between normal and increased values is still not available (49), and, due to low sensitivity and specificity of the test, it is not yet recognized as a valuable method, unless no other MRD markers are available (50). Because of the solid evidence that the presence of MRD after cytoreductive therapy does affect patient outcome, it seems reasonable that it should also influence treatment decisions (51, 52). In this respect, increasing evidence suggests that outcomes may be improved using a MRD-driven therapeutic strategy, also including allogeneic stem cell transplantation for patients persistently MRD positive or re-converted from MRD negative to MRD positive (53).
The prospective GIMEMA AML1310 trial proposed for patients eligible for intensive chemotherapy is a risk- and MRD-adapted therapeutic project, integrating the upfront genetic features and the post-consolidation MRD (54). Patients at “favorable risk” (those included in the favorable ELN risk group and those at intermediate risk or MRD-negative at consolidation) received autologous stem cell transplantation, whereas patients at “poor risk” (ELN poor risk and intermediate risk but still MRD-positive at consolidation) underwent allogeneic transplantation. As expected, 2-year overall survival and disease-free survival were significantly different between ELN favorable and poor risk categories (overall survival 74% vs 42%, disease-free survival 61% vs 45%). Concerning the “intermediate” cohort, the ELN risk- and MRD-adapted strategy improved outcomes to rates observed in the “favorable” category (overall survival, 79% for MRD negative and 70% for MRD-positive cases; disease-free survival, 61% and 67%, respectively), thus confirming a role for autologous transplantation in the “favorable” category and for allogeneic transplant in cases at novel “poor” risk (55).
Conclusions
The diagnostic workup of AML resulting from this Delphi project (which mirrors Italian hematologic centers in practice) is the product of an integrated approach, primarily based on international guidelines, including patient history, and morphological, immunophenotyping, and cytogenetics/molecular findings. The opinions of the panelists and experts suggest that only a holistic approach to patients with AML might lead to long-lasting therapeutic success.
The originality of this work lies in the initial choice of Italian experts to develop a project aimed at the definition of the best diagnostic process for patients with AML subtypes starting from the assumption that a change of paradigm is needed by some centers that deal with AML. In particular, awareness is increasing to ensure that complete and “performant” diagnostics are fundamental from the first steps of the decision-making process. Morphologic evaluation of bone marrow and peripheral blood smears still plays a decisive diagnostic role in AML. The importance of genetic evaluation, both for classification and for risk stratification, firmly establishes that karyotyping, FISH, and molecular tests have to be performed in every case of AML, independently of age. Furthermore, genetics is continuously evolving and new information becomes available in the scientific community. It has recently been shown that some cases of clinically defined de novo AML are characterized by a chromatin-spliceosome mutational signature and, from a clinical and prognostic perspective, resemble a secondary more than a de novo entity, despite the absence of specific morphological and cytogenetic abnormalities (54). The updated knowledge of the genetic aspects of AML will determine further improvements in the WHO classification, with the possible inclusion of new molecular entities.
Another important aspect is represented by the interdisciplinary approach and the opportunity to create an efficient network between different players in the AML diagnosis (hematologist, cytogeneticist, pathologist, molecular biologist), to harmonize reports, increase the quality of technologies, and share knowledge. We emphasize the role of multidisciplinary diagnostics in AML, which is mandatory today and will gain even more importance in the future, especially in the context of precision medicine. This approach, together with the identification of centralized laboratories that could help centers not technically fully equipped, will overcome the possible problem of turnaround times, which sometimes vary from some hours to a week in different laboratories. Recent data confirm that it may be a feasible strategy to wait for genetic and other laboratory test results in clinically stable patients to assign them to the best available treatment option (56). Therefore, the possibility of timely informative and clinically relevant genetic and diagnostic results at diagnosis represents the basis for the success of the patient-tailored therapy, made possible today by the introduction of many new targeted therapies in the clinical armamentarium.
Demostrating that the diagnostic approaches in AML are not uniform across the Italian territory, this survey highlights the fundamental role of the GIMEMA LabNet and, in general, of a diffuse lab network to support the clinical Centers which are not technically fully equipped, and to finally ensure an adequate management of AML.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
MV designed the project, selected the literature, wrote and reviewed the manuscript. FF designed the project, selected the literature, wrote and reviewed the manuscript. SG designed the project, selected the literature, wrote and reviewed the manuscript. AR designed the project, selected the literature, wrote and reviewed the manuscript. AV designed the project, selected the literature, wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AR has received travel expenses and fees for consultancies and participation in meetings, boards and symposia sponsored by Jazz, ABBVIE, Amgen, Pfizer, Celgene, Novartis, Incyte, Omeros, Astellas, and Roche. AV has served on an advisory board, been an invited speaker, and taken part in a speakers’ bureau for Novartis, Jazz, Pfizer, Astellas, Gilead, Helsinn, and Amgen. SG has received speaker fees for events supported by Amgen, Jazz, Astellas, and Novartis. MV has served on an advisory board, been an invited speaker, and taken part in a speakers’ bureau for Novartis, Jazz, Astellas, BMS/Celgene.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all the participants of the Panel of AML specialists * Ernesta Audisio (Torino), Massimo Bernardi (Milano), Roberta Bertorelle (Padova), Laura Bonaldi (Padova), Massimo Breccia (Roma), Francesco Buccisano (Roma), Roberto Cairoli (Milano), Alessia Campagna (Rozzano, Milano), Anna Candoni (Udine), Daniela Cilloni (Torino), Laura Cudilllo (Roma), Antonio Cuneo (Ferrara), Antonio Curti (Bologna), Francesco di Raimondo (Palermo), Domenica Divona (Roma), Maria Carla De Simone (Napoli), Luana Fianchi (Roma), Claudia Fozza (Sassari), Marco Frigeni (Bergamo), Pietro Galieni (Ascoli Piceno), Michele Gottardi (Castelfranco Veneto, Treviso), Fabio Guolo (Genova), Carmela Gurrieri (Padova), Maria Martelli (Perugia), Giovanni Martinelli (Meldola), Fabrizio Pane (Napoli), Luigi Rigacci (Roma), Giuseppe Rossi (Brescia), Sergio Siragusa (Palermo), Ilaria Tanasi (Verona), Nicoletta Testoni (Bologna), Elisabetta Todisco (Milano), Eleonora Toffoletti (Udine), Giuseppe Visani (Pesaro) who contributed to the survey. Special thanks also go to Luigia Atorino (PhD, Edra S.p.A, Milan, Italy) for medical writing support and Alessia Scotton (Editorial Project Manager, Edra S.p.A, Milan, Itay) for editorial assistance.
References
1. Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal Evolution in Relapsed Acute Myeloid Leukaemia Revealed by Whole-Genome Sequencing. Nature (2012) 481:506–10. doi: 10.1038/nature10738
2. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
3. Sportoletti P, Varasano E, Rossi R, Mupo A, Tiacci E, Vassiliou G, et al. Mouse Models of NPM1-Mutated Acute Myeloid Leukemia: Biological and Clinical Implications. Leukemia (2015) 29:269–78. doi: 10.1038/leu.2014.257
4. Kishtagari A, Levine RL. @ The Role of Somatic Mutations in Acute Myeloid Leukemia Pathogenesis. Cold Spring Harb Perspect Med (2021) 11:a034975. doi: 10.1101/cshperspect.a034975
5. Handschuh LJ. Not Only Mutations Matter: Molecular Picture of Acute Myeloid Leukemia Emerging From Transcriptome Studies. Oncology (2019) 2019:7239206. doi: 10.1155/2019/7239206
6. Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
7. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 Revision of the World Health Organization (WHO) Classification of Myeloid Neoplasms and Acute Leukemia: Rationale and Important Changes. Blood (2009) 114:937–51. doi: 10.1182/blood-2009-03-209262
8. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasm and Acute Leukaemia. Blood (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
9. Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J Natl Compr Canc Netw (2021) 19:16–27. doi: 10.6004/jnccn.2021.0002
10. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
11. Heuser M, Ofran Y, Boissel N, Brunet Mauri S, Craddock C, Janssen J, et al. Acute Myeloid Leukaemia in Adult Patients: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31:697–712. doi: 10.1016/j.annonc.2020.02.018
12. De Meyrick J. The Delphi Method and Health Research. Health Educ (2003) 103:7–16. doi: 10.1108/09654280310459112
13. Rowe G, Wright G. Expert Opinions in Forecasting: Role of the Delphi Technique. In: Armstrong JS, editor. Principles of Forecasting. Norwell, MA: Kluwer Academic Press (2001). doi: 10.1007/978-0-306-47630-3_7
14. Marcolin R, Guolo F, Minetto P, Clavio M, Manconi L, Ballerini F, et al. A Simple Cytofluorimetric Score may Optimize Testing for Biallelic CEBPA Mutations in Patients With Acute Myeloid Leukemia. Leuk Res (2019) 86:106223. doi: 10.1016/j.leukres.2019.106223
15. Montoro J, Gallur L, Merchán B, Molero A, Roldán E, Martínez-Valle F, et al. Autoimmune Disorders are Common in Myelodysplastic Syndrome Patients and Confer an Adverse Impact on Outcomes. Ann Hematol (2018) 97:1349–56. doi: 10.1007/s00277-018-3302-0
16. Laribi K, Sobh M, Ghez D, Baugier de Materre A. Impact of Age, Functional Status, and Comorbidities on Quality of Life and Outcomes in Elderly Patients With AML: Review. Ann Hematol (2021) 100:1359–76. doi: 10.1007/s00277-020-04375-x
17. Griffiths EA, Carraway HE, Chandhok NS, Prebet T. Advances in non-Intensive Chemotherapy Treatment Options for Adults Diagnosed With Acute Myeloid Leukemia. Leuk Res (2020) 91:106339. doi: 10.1016/j.leukres.2020.106339
18. Luger SM. Acute Myeloid Leukemia: How to Treat the Fit Patient Over Age 75? Best Pract Res Clin Haematol (2019) 32:101105. doi: 10.1016/j.beha.2019.101105
19. Döhner H, Dolnik A, Tang L, Seymour JF, Minden MD, Stone RM, et al. Cytogenetics and Gene Mutations Influence Survival in Older Patients With Acute Myeloid Leukemia Treated With Azacitidine or Conventional Care. Leukemia (2018) 32:2546–57. doi: 10.1038/s41375-018-0257-z
20. Walter RB, Ofran Y, Wierzbowska A, Ravandi F, Hourigan CS, Ngai LL, et al. Measurable Residual Disease as a Biomarker in Acute Myeloid Leukemia: Theoretical and Practical Considerations. Leukemia (2021) 35:1529–38. doi: 10.1038/s41375-021-01230-4
21. Ngai LL, Kelder A, Janssen JJWM, Ossenkoppele GJ, Cloos J. MRD Tailored Therapy in AML: What We Have Learned So Far. Front Oncol (2021) 10:603636. doi: 10.3389/fonc.2020.603636
22. Palmieri R, Buccisano F, Maurillo L, Del Principe MI, Paterno G, Venditti A, et al. Current Strategies for Detection and Approach to Measurable Residual Disease in Acute Myeloid Leukemia. Minerva Med (2020) 111:386–94. doi: 10.23736/S0026-4806.20.07016-0
23. Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, et al. Acute Myeloid Leukaemia With Biallelic CEBPA Gene Mutations and Normal Karyotype Represents a Distinct Genetic Entity Associated With a Favourable Clinical Outcome. J Clin Oncol (2010) 28:570–7. doi: 10.1200/JCO.2008.21.6010
24. Taskesen E, Bullinger L, Corbacioglu A, Sanders MA, Erpelinck CA, Wouters BJ, et al. Prognostic Impact, Concurrent Genetic Mutations, and Gene Expression Features of AML With CEBPA Mutations in a Cohort of 1182 Cytogenetically Normal AML Patients: Further Evidence for CEBPA Double Mutant AML as a Distinctive Disease Entity. Blood (2011) 117:2469–75. doi: 10.1182/blood-2010-09-307280
25. Tawana K, Rio-Machin A, Preudhomme C, Fitzgibbon J. Familial CEBPA-Mutated Acute Myeloid Leukemia. Semin Hematol (2017) 54:87–93. doi: 10.1053/j.seminhematol.2017.04.001
26. Wouters BJ, Löwenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA Mutations, But Not Single CEBPA Mutations, Define a Subgroup of Acute Myeloid Leukemia With a Distinctive Gene Expression Profile That is Uniquely Associated With a Favorable Outcome. Blood (2009) 113:3088–91. doi: 10.1182/blood-2008-09-179895
27. Pabst T, Eyholzer M, Fos J, Mueller BU. Heterogeneity Within AML With CEBPA Mutations; Only CEBPA Double Mutations, But Not Single CEBPA Mutations are Associated With Favourable Prognosis. Br J Cancer (2009) 100:1343–6. doi: 10.1038/sj.bjc.6604977
28. Arber DA, Borowitz MJ, Cessna M, Etzell J, Foucar K, Hasserjian RP, et al. Initial Diagnostic Workup of Acute Leukemia: Guideline From the College of American Pathologists and the American Society of Hematology. Arch Pathol Lab Med (2017) 141:1342–93. doi: 10.5858/arpa.2016-0504-CP
29. McMahon CM, Nelson N, Ganetsky A, Mangan JK, Frey NV, Perl AE, et al. Limited FISH Testing for MDS-Defining Cytogenetic Abnormalities Rapidly Identifies Patients With Newly Diagnosed AML Eligible for CPX-351. Blood (2018) 132(suppl 1):4785. doi: 10.1182/blood-2018-99-116415
30. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
31. Larson RA, Mandrekar SJ, Huebner LJ, Sanford BL, Laumann K, Geyer S, et al. Midostaurin Reduces Relapse in FLT3-Mutant Acute Myeloid Leukemia: The Alliance CALGB 10603/RATIFY Trial. Leukemia (2021) 35:2539–51. doi: 10.1038/s41375-021-01179-4
32. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol (2018) 36:2684–92. doi: 10.1200/JCO.2017.77.6112
33. Schmalbrock LK, Dolnik A, Cocciardi S, Sträng E, Theis F, Jahn N, et al. Clonal Evolution of Acute Myeloid Leukemia With FLT3-ITD Mutation Under Treatment With Midostaurin. Blood (2021) 137:3093–104. doi: 10.1182/blood.2020007626
34. Travaglini S, Angelini DF, Alfonso V, Guerrera G, Lavorgna S, Divona M, et al. Characterization of FLT3-ITDmut Acute Myeloid Leukemia: Molecular Profiling of Leukemic Precursor Cells. Blood Cancer J (2020) 10:85. doi: 10.1038/s41408-020-00352-9
35. Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. Update on MRD in Acute Myeloid Leukemia: A Consensus Document From the European LeukemiaNet MRD Working Party. Blood (2021) 138(26):2753–67. doi: 10.1182/blood.2021013626
36. Rossi G, Minervini MM, Carella AM, Melillo L, Cascavilla N. Wilms’ Tumor Gene (Wt1) Expression And Minimal Residual Disease In Acute Myeloid Leukemia. In: van den Heuvel-Eibrink MM, editor. Wilms Tumor. Brisbane: Codon Publications (2016). Chapter 16.
37. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin Plus Chemotherapy for Acute Myeloid Leukemia With a FLT3 Mutation. N Engl J Med (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
38. Chiche E, Rahmé R, Bertoli S, Dumas PY, Micol JB, Hicheri Y, et al. Real-Life Experience With CPX-351 and Impact on the Outcome of High-Risk AML Patients: A Multicentric French Cohort. Blood Adv (2021) 5:176–84. doi: 10.1182/bloodadvances.2020003159
39. Guolo F, Fianchi L, Minetto P, Clavio M, Gottardi M, Galimberti S, et al. CPX-351 Treatment in Secondary Acute Myeloblastic Leukemia is Effective and Improves the Feasibility of Allogeneic Stem Cell Transplantation: Results of the Italian Compassionate Use Program. Blood Cancer J (2020) 10:96. doi: 10.1038/s41408-020-00361-8
40. Szczepanski T, Orfao A, van der Velden VH, San Miguel JF, van Dongen JJ. Minimal Residual Disease in Leukaemia Patients. Lancet Oncol (2001) 2:409–17. doi: 10.1016/S1470-2045(00)00418-641
41. Buccisano F, Palmieri R, Piciocchi A, Maurillo L, Del Principe MI, Paterno G, et al. Use of Measurable Residual Disease to Evolve Transplant Policy in Acute Myeloid Leukemia: A 20-Year Monocentric Observation. Cancers (Basel) (2021) 13:1083. doi: 10.3390/cancers13051083
42. Dix C, Lo TH, Clark G, Abadir E. Measurable Residual Disease in Acute Myeloid Leukemia Using Flow Cytometry: A Review of Where We are and Where We are Going. J Clin Med (2020) 9:1714. doi: 10.3390/jcm9061714
43. Ehinger M, Pettersson L. Measurable Residual Disease Testing for Personalized Treatment of Acute Myeloid Leukemia. APMIS (2019) 127:337–51. doi: 10.1111/apm.12926
44. Salipante SJ, Fromm JR, Shendure J, Wood BL, Wu D. Detection of Minimal Residual Disease in NPM1-Mutated Acute Myeloid Leukemia by Next-Generation Sequencing. Mod Pathol (2014) 27:1438–46. doi: 10.1038/modpathol.2014.57
45. Forghieri F, Comoli P, Marasca R, Potenza L, Luppi M. Minimal/measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives. Int J Mol Sci (2018) 19:3492. doi: 10.3390/ijms19113492
46. Hantel A, Stock W, Kosuri S. Molecular Minimal Residual Disease Testing in Acute Myeloid Leukemia: A Review for the Practicing Clinician. Clin Lymph Myeloma Leuk (2018) 18:636–47. doi: 10.1016/j.clml.2018.06.017
47. Cilloni D, Gottardi E, Messa F, Fava M, Scaravaglio P, Bertini M, et al. Significant Correlation Between the Degree of WT1 Expression and the International Prognostic Scoring System Score in Patients With Myelodysplastic Syndromes. J Clin Oncol (2003) 21:1988–95. doi: 10.1200/JCO.2003.10.503
48. Ueda Y, Mizutani C, Nannya Y, Kurokawa M, Kobayashi S, Takeuchi J, et al. Clinical Evaluation of WT1 mRNA Expression Levels in Peripheral Blood and Bone Marrow in Patients With Myelodysplastic Syndromes. Leuk Lymph (2013) 54:1450–8. doi: 10.3109/10428194.2012.745074
49. Voso MT, Ottone T, Lavorgna S, Venditti A, Maurillo L, Lo-Coco F, et al. MRD in AML: The Role of New Techniques. Front Oncol (2019) 9:655. doi: 10.3389/fonc.2019.00655
50. Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable Residual Disease in AML: A Consensus Document From the European LeukemiaNet MRD Working Party. Blood (2018) 131:1275–91. doi: 10.1182/blood-2017-09-801498
51. Vidriales MB, Perez-Lopez E, Pegenaute C, Castellanos M, Perez JJ, Chandia M, et al. Minimal Residual Disease Evaluation by Flow Cytometry is a Complementary Tool to Cytogenetics for Treatment Decisions in Acute Myeloid Leukaemia. Leuk Res (2016) 40:1–9. doi: 10.1016/j.leukres.2015.10.002
52. Parkin B, Londono-Joshi A, Kang Q, Tewari M, Rhim AD, Malek SN. Ultrasensitive Mutation Detection Identifies Rare Residual Cells Causing Acute Myelogenous Leukemia Relapse. J Clin Invest (2017) 127:3484–95. doi: 10.1172/JCI91964
53. Anthias C, Dignan FL, Morilla R, Morilla A, Ethell ME, Potter MN, et al. Pre-Transplant MRD Predicts Outcome Following Reduced-Intensity and Myeloablative Allogeneic Hemopoietic SCT in AML. Bone Marrow Transplant (2014) 49:679–83. doi: 10.1038/bmt.2014.9
54. Caprioli C, Lussana F, Salmoiraghi S, Cavagna R, Buklijas K, Elidi L, et al. Clinical Significance of Chromatin-Spliceosome Acute Myeloid Leukemia: A Report From the Northern Italy Leukemia Group (NILG) Randomized Trial 02/06. Haematologica (2020) 106:2578–87. doi: 10.3324/haematol.2020.252825
55. Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R, et al. GIMEMA AML1310 Trial of Risk-Adapted, MRD-Directed Therapy for Young Adults With Newly Diagnosed Acute Myeloid Leukemia. Blood (2019) 134:935–45. doi: 10.1182/blood.2018886960
Keywords: acute myeloid leukemia, molecular genetics, AML diagnostics, genetic subtypes, multidisciplinary diagnostics, morphology, mutational analysis
Citation: Voso MT, Ferrara F, Galimberti S, Rambaldi A and Venditti A (2022) Diagnostic Workup of Acute Myeloid Leukemia: What Is Really Necessary? An Italian Survey. Front. Oncol. 12:828072. doi: 10.3389/fonc.2022.828072
Received: 02 December 2021; Accepted: 26 January 2022;
Published: 17 February 2022.
Edited by:
Robert Ohgami, University of California, San Francisco, United StatesReviewed by:
Marianna Rossi, San Matteo Hospital Foundation (IRCCS), ItalyLorenzo Brunetti, University Hospital of Ancona, Italy
Copyright © 2022 Voso, Ferrara, Galimberti, Rambaldi and Venditti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Teresa Voso, dm9zb0BtZWQudW5pcm9tYTIuaXQ=
 Maria Teresa Voso
Maria Teresa Voso Felicetto Ferrara
Felicetto Ferrara Sara Galimberti
Sara Galimberti Alessandro Rambaldi4,5
Alessandro Rambaldi4,5 Adriano Venditti
Adriano Venditti