
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 June 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.826778
This article is part of the Research Topic Global Excellence in Oncology: North America 2021 View all 9 articles
A correction has been applied to this article in:
Corrigendum: Germline mutations in patients with early-onset prostate cancer
 Tang Tang1†
Tang Tang1† Xintao Tan1†
Xintao Tan1† Ze Wang1
Ze Wang1 Shuo Wang1
Shuo Wang1 Yapeng Wang1
Yapeng Wang1 Jing Xu1
Jing Xu1 Xiajie Wei2
Xiajie Wei2 Dianzheng Zhang3
Dianzheng Zhang3 Qiuli Liu1*
Qiuli Liu1* Jun Jiang1*
Jun Jiang1*Objective: To investigate the inherited mutations and their association with clinical features and treatment response in young-onset prostate cancer patients.
Method: Targeted gene sequencing on 139 tumor susceptibility genes was conducted with a total of 24 patients diagnosed with PCa under the age of 63 years old. Meanwhile, the related clinical information of those patients is collected and analyzed.
Results: Sixty-two germline mutations in 45 genes were verified in 22 patients. BRCA2 (20.8%) and GJB2 (20.8%) were found to be the most frequently mutated, followed by CHEK2, BRCA1, PALB2, CDKN2A, HOXB13, PPM1D, and RECQL (8.3% of each, 2/24). Of note, 58.3% (14/24) patients carry germline mutations in DNA repair genes (DRGs). Four families with HRR (homologous recombination repair)-related gene mutations were described and analyzed in detail. Two patients with BRCA2 mutation responded well to the combined treatment of androgen deprivation therapy (ADT) and radiotherapy/chemotherapy.
Conclusion: Mutations in DRGs are more prevalent in early-onset PCa with advanced clinical stages, and these patients had shorter progression-free survival. ADT Combined with either radiotherapy or chemotherapy may be effective in treating PCa caused by HRR-related gene mutations.
Prostate cancer (PCa) is the second most common cancer in men worldwide (1). More recent data indicate that genetic mutations predispose the patients to PCa (2, 3), evidenced by more than 60 loci linked to approximately 30% of familial PCa (4). It has been estimated that about 42% PCa risks, especially for the early-onset PCa, are attributable to genetic alterations (5). Mutations in DRGs especially HRR-related genes such as BRCA1 and BRCA2 are the well-established risk factors for PCa (6), with approximately 10% PCa patients harboring deleterious mutations in DRGs (7). Furthermore, compared with those carrying somatic mutations in DRGs, patients with hereditary germline mutations have an earlier onset, a higher propensity to metastasis, and shorter progression-free survival (8).
HRR is the most efficient repair tool for DNA double-strand breaks (DSB) with high fidelity (9). BRCA1/2, together with their functional-related factors such as ATM, ATR, and PALB2, form HRR complexes and play pivotal roles in maintaining genome stability (9). Cells with mutations in any of these genes are more vulnerable to DNA damage and cancer development, especially when they are exposed to either endogenous or exogenous attacks (10, 11). Therefore, mutations in HRR-related genes have been frequently observed in different cancer types, including breast, ovarian, pancreatic, stomach, laryngeal, and PCa (12). The BRCA1/2 mutations are predominantly observed in breast and ovarian cancer in women (13) and PCa in men (14). Germline BRCA1/2 mutations are also associated with higher Gleason scores (≥8), a more aggressive phenotype with a higher probability of nodal involvement, distant metastasis, and shorter overall survival (15, 16). Germline BRCA2 and BRCA1 mutations present in 1.2% and 0.44% PCa (17), respectively. The frequency of BRCA2 mutation in early-onset PCa patients (<65 years old) could be as high as 2.2% (18). Based on these data, we hypothesized that germline mutations could be one of the important underlying mechanisms for PCa early onset. To test this hypothesis, we investigated the frequency of genetic mutations of 139 tumor-susceptibility genes in 24 young-onset PCa patients. We found that mutations in DRGs are not only more prevalent in early-onset PCa, patients with these mutations also have much shorter progression-free survivals.
Initially, 239 PCa patients admitted to Daping hospital between September 2016 and December 2020 were screened for this study with the protocol approved by the ethics committee of the Third Affiliated Hospital of the PLA Army Medical University (ethics number: 2018 No. 28). The following criteria were used for inclusion:
1) Patients that had been pathologically diagnosed with prostate cancer
2) Age at diagnosis is younger than 65
3) Informed consent: volunteered to participate in this study
Most individuals were excluded due to the following criteria:
1) Limited sample availability or unqualified samples
2) Unwillingness to participate in this study
3) Unavailability of following-up data
A total of 24 PCa patients were included in the study with their clinical data, including ages, PSA levels at diagnosis, biopsy Gleason score, pathological types from needle biopsy and surgical specimens, metastasis status, clinical stages, androgen deprivation therapy (ADT) strategies, and gene sequencing results. Additionally, the levels of testosterone and PSA during ADT were monitored. The diagnosis of castration-resistant prostate cancer was made during follow-up based on the American AUA guidelines (2018 edition): (1) Serum testosterone <50ng/dL (<1.7nmol/L); (2) Elevated PSA: a PSA increase of >2ng/ml from the lowest level at a minimum of 3 weeks interval or an increase of 25% from the lowest level at the second measurement. Two or more new lesions detected in a bone scan are indicative of disease progression as well.
Peripheral blood was collected from the elbow vein into EDTA-containing tubes and stored at 4°C for further examination. Total DNA is extracted from the mononuclear cells. AllPrep DNA/RNA Mini Kit (Qiagen 80204) was used for gDNA extraction. The size, quality, and quantity of the purified gDNA were estimated using the 2200 Bioanalyzer (Agilent Technologies). The gDNA library was constructed using a KAPA Hyper Prep kit according to the manufacturer’s protocols (Kapa Biosystems). The quantities of the library were estimated using Qubit 3 (Thermo Fisher).
A panel of 139 genes was used in our research (supplementary file), and the gDNA library was enriched for regions of this custom-designed probe manufactured by Agilent. The panel is designed according to the genes that have been reported to be associated with hereditary tumors based on NCCN (National Comprehensive Cancer Network) guideline, HGMD (The Human Gene Mutation Database) and TCGA (The Cancer Genome Atlas) database, as well as previously reported inherit susceptibility genes. About 750ng of library DNA was hybridized with two hybridization reagents and blocking agents of the SureSelectXT Target Enrichment System (Agilent Technologies). The enriched libraries were amplified with the P5/P7 primer. After being qualified by the 2200 Bioanalyzer, Qubit3, and a qPCR NGS library quantification kit (Agilent Technologies), the libraries were sequenced on a Hiseq X10 platform (Illumina, San Diego, CA). 18 DRGs (ATM, ATR, BRCA1, BRCA2, BRIP1, CHEK2, FAM175A, FANCA, GEN1, MLH1, MRE11, MSH2, MSH6, NBN, PALB2, PMS2, RAD51C, RAD51D) are grouped (1).
Primary processing of NGS data for blood samples was performed using Trimmomatic (0.36), including demultiplexing and masking of dual-index adapter sequences. Sequences were aligned against the human reference genome (GRCh37/hg19) using BWA (version 0.7.10). IGV was used to filter alignment and sequence artifacts. Point mutations, small insertions, and deletions were identified by Samtools (version 1.3.1) and Spindel (version 0.2.5b8, 20151210). A mutation was filltered if the depth was smaller than 5, the mutational frequency was less than 20%, or was recurrently found in healthy individuals according to multiple database including NHLBI (National Heart Lung and Blood Institute) Exome Sequencing Project, 1000 Genomes Project, Exome Aggregation Consortium and gnomAD. The effects of variants were annotated using Oncotator (https://software.broadinstitute.org/cancer/cga/oncotator) and Variant Effect Predictor (https://grch37.ensembl.org/info/docs/tools/vep/index.html), as well as an in-house database (GenentronDB). The pathogenicity of germline mutations was analyzed according to the ACMG Guidelines (2015 edition), only mutations that are pathogenic/likely pathogenic and VUS (variant of uncertain significance) are investigated. All the raw data of genetic sequencing have been deposited in public GSA database (https://ngdc.cncb.ac.cn/gsa-human/, accession number: HRA001716).
SPSS 22.0 (IBM, USA) was used for statistical analysis. Mean age at diagnosis, TNM staging, Gleason score, and PSA level were used as classification criteria. The t-test was used for continuous variables, and the chi-square test or Fisher exact test was used for classification variables. For data that did not fit a normal distribution, nonparametric statistical tests were used. P < 0.05 was considered statistically significant.
Six of the 14 patients with germline mutations in DRGs were assembled to further investigate genetic characters in their pedigrees. Four patients and their families agreed to participate in the continued study. Peripheral blood was drawn from the elbow vein of the first/second-degree relatives of these four patients; then Sanger sequencing was conducted to evaluate the hereditary feature.
Twenty-four patients were diagnosed with PCa by prostate needle biopsy or surgical specimens. Among these patients, eight and four have first-degree and none-first-degree relatives with a history of cancer, respectively. The remaining patients have no family history of cancer. At diagnosis, the average age is 54.8 (47~62 years old), and the average PSA value is 87.5 ng/mL (minimum 5.45 ng/mL, maximum 550 ng/mL). Thirteen patients have a Gleason score of 8 or higher. At the time of diagnosis, there were 14 patients with stage T2, 6 with stage T3, and 4 with stage T4. There are six patients diagnosed with bone metastasis by isotopic bone scanning; of note 2 patients with oligometastic prostate cancer received radical resection. Four patients were diagnosed with lymph node metastasis. Nineteen patients received radical resection, and 3 received local radiotherapy. Eight patients received ADT, and 2 received ADT combined with local radiotherapy after radical resection. By MAY 1, 2021, 5 patients showed biochemical recurrence, and one developed into CRPC (Table 1).
A total of 62 germline mutations in 45 genes were detected in 24 peripheral venous blood samples (Figure 1). Missense mutation is the most common type, followed by stop-gained mutation and in-frame mutation. The detection rate of a missense mutation in all samples accounted for 82.3% (51/62). 16.1% (10/62) of the patients have either frameshift (3 frameshifts in FANCD2, BRCA2, and PALB2 gene), non-frameshift (2 non-frameshift in TGFBR1 and APC genes), or stop-gained mutations (5 stop-gained mutations in BRCA2, CDKN2A, and VEGFA genes), which potentially affect the functions of the encoded proteins. Interestingly, we found that fourteen patients (14/24, 58.3%) have one or more germline mutations in above-mentioned 18 DRGs. The most frequently mutant genes were BRCA2 (5/24) and GJB2 (5/24), followed by CDKN2A, CHEK2, EGFR, HOXB13, PPM1D, and RECQL (2/24). Mutation sites of the main genes are listed below (Figure 2).
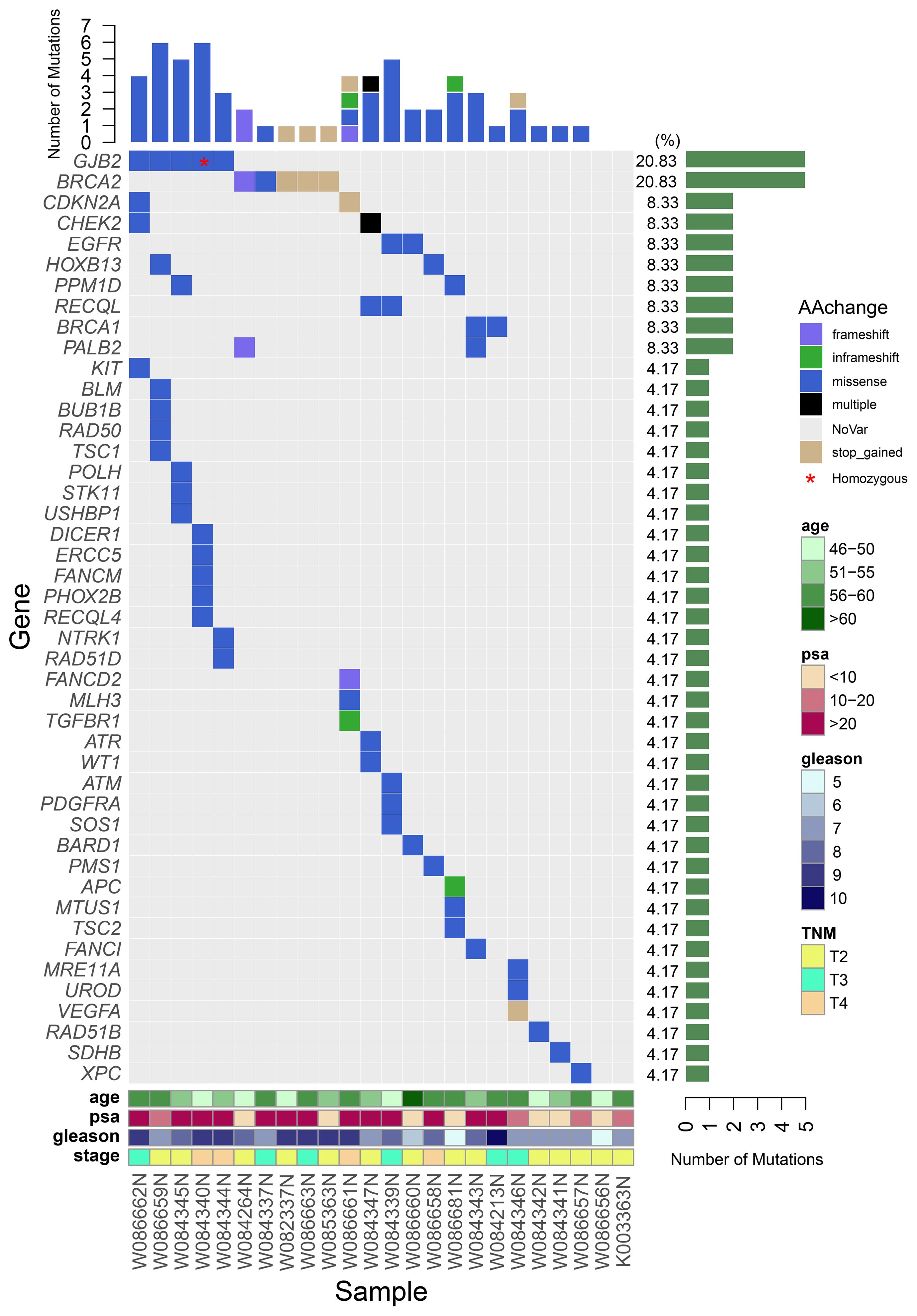
Figure 1 A waterfall plot shows the mutation ratio, mutation type of the mutated genes, and the clinical features of the 24 patients. The top panel shows the number of the mutations in each PCa sample; the left panel shows the frequently mutated genes; the right panel shows the detection ratio of each mutated gene; the bottom panel shows the sample number and the clinical features of the corresponding patient.
Compared to those with non-DRGs mutation carriers, the 14 patients with DRGs’ mutations have a tendency of higher overall Gleason score, higher PSA level at diagnosis, and younger ages (54 years vs 55.9 years, P=0.36). 64% (9/14 patients) of the patients with DRGs’ mutations have a Gleason score of 8 or higher compared to 40% (4/10 patients, P =0.41) of the non-carriers. The average PSA level of the DRGs mutation carriers is 120ng/mL compared to that 41.2 ng/mL (P =0.15) of the non-DRGs mutation carriers. In addition, at the time of diagnosis, 50% (7/14 patients) of patients with DRGs’ mutations have their tumor staged at T3/T4 comparing to 30% (3/10 patients, P=0.42) of the non-DRGs mutation carriers with a similar staging. Of the five patients with GJB2 mutations, two patients (one of them has homozygous GJB2 mutation) were at the T4 stage at their diagnosis with a Gleason score of 9; 1 patient at stage T3 (Gleason score of 9); 2 patients at stage T2 (Gleason score of 7 and 8, respectively). In addition, four of the five patients have a high level of PSA (>100ng/mL). Three CHEK2 mutations were detected in 2 patients at stage T2 and T3, respectively, at their diagnoses.
Among the 24 patients, the data of 4 families of HRR-related mutation carriers are further analyzed. In these four pedigrees, 2 with germline mutation of BRCA2 (Patient A and B), 1 with germline mutation of BRCA1, 1 with germline mutations of BRCA2 and PALB2 (Table 2). Of note, patient B with novel BRCA2 germline mutation responded well to chemotherapy combined with ADT treatment in our previous report (2). In addition, we recently reported the other patient D with BRCA2 and PALB2 mutations, who responded well to radiotherapy and chemotherapy combined with ADT (3). For the other two patients, Patient A was diagnosed with PCa by needle biopsy at 52 years old, with a low PSA level of 7.95ng/dL, Gleason scores 4 + 5, imaging examination revealed no metastasis (T2bN0M0). This patient received radical resection of the prostate, and the PSA level closed to 0 during the follow-up. Results from Sanger sequencing indicate that his healthy brother shares the same germline mutation of BRCA2 (c.1799_1804del, p.Tyr600_Gly602delinsTer), and his father died of bladder cancer at the age of 60. Patient C was diagnosed with PCa at 60 years old through needle biopsy with his PSA level of more than 550 ng/dL, Gleason scores 5 + 5, imaging examination revealed multiple bone metastasis (T3NxM1B). He received ADT combined with radiotherapy. However, his PSA reached the lowest level (0.51 ng/dL) after the radiotherapy before raising again. This patient was diagnosed as CRPC on Aug 28, 2020, then given abiraterone combined with docetaxel and nedaplatin, and PSA level slowly increased to 70 ng/dL in April of 2021. The patient and his father carry the germline mutation of BRCA1 (c.4166G>A, p.S1389N), with no cancer history of other family members. The pedigrees of patient A and patient C are shown in Figure 3.
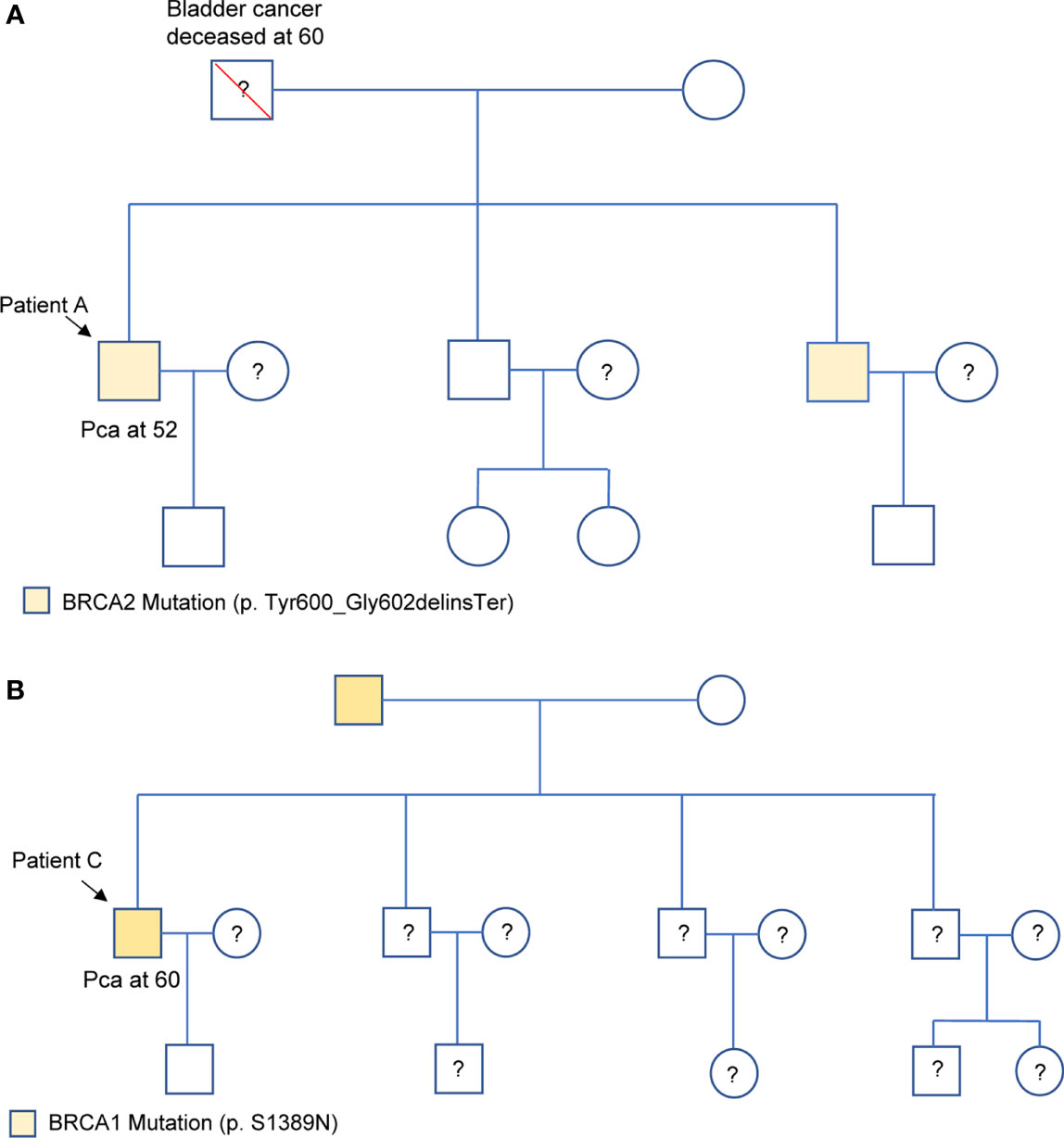
Figure 3 Family pedigrees of Patient A (A) and Patient C (B), respectively. “□,○” indicate normal males and females, red “\” indicates that individual is deceased, dashes between symbols “□—○” indicate a couple, “?” indicate the mutation status is unknown, and “↘” indicate the probands. PCa represents prostate cancer.
In our study, we investigated the germline mutations of 24 patients with early-onset PCa by using targeted gene sequencing of a panel of 139 genes. We found that 58.3% of the early-onset PCa patients carried DRGs’ mutations, including pathogenic/likely pathogenic and VUS. More importantly, we found that in early-onset cohorts patients with DRGs’ mutations have a tendency of higher Gleason score, higher PSA level at diagnosis, and younger ages. At last, by analyzing the case series and their pedigrees in details, we also found that patients with mutations of HRR-related genes might benefit from ADT combined with radiotherapy or platinum-based chemotherapy.
PCa is one of the most heritable cancers (4), and germline mutations in DRGs are more accountable for PCa inheritability (5), with about 22% of PCa patients harbor DRGs’ somatic mutations (6). As for germline mutations, DRGs’ mutations present approximately 4.6% in localized PCa, and 11.8% to 16.2% in metastatic disease in Caucasian population (7, 8). Whereas in Chinese population, Ye et al. have found that 9.8% PCa patients have germline mutations in their DNA repair pathway, and 6.3% of them are BRCA2 mutations with 0.63% of either BRCA1 or ATM (1). Consistently, our findings in this study show that mutations in DRGs are highly associated with PCa incidence and the early-onset of PCa. Dissimilarly, in the early-onset population of our study, we found that the frequency of PCa patients with germline mutations in DRGs was 58.3% (14/24), much higher than that in PCa patients with undefined ages (the patients diagnosed with prostate cancer at any ages, not restricted to the early-onset ages). Consistent with a recent report (18), we found that patients with DRGs’ germline mutations have higher Gleason scores, more advanced clinical stages, and more metastasis. More importantly, compared with another PCa cohort with undefined average ages in Ye et al.’s Chinese population (1), we found that early-onset PCa patients with DRGs’ mutations had a relatively younger ages (54 vs 60 years) and higher PSA levels at diagnosis (120 vs 100 ng/ml), but lower Gleason Scores (≥8, 64.3% vs 84%) and fewer metastasis (28.6% vs 71%). The discrepancy might be due to the limited sample size and that most of the mutations were VUS. Thus the results should be substantiated in a larger size of samples.
HRR pathway plays a vital role in maintaining the stability of the genome, along with multiple repair mechanisms, including base excision repair (BER) of DNA single-strand breaks, mismatch repair (MMR), and non-homologous end joining (NHEJ) (9, 10). In fact, HRR is the most efficient repair tool for DNA double-strand breaks (DSB) with high fidelity, and BRCA1/2 plays an indispensable role in this process (11, 12). Therefore, mutations in BRCA1/2 are closely related to various cancers, especially breast and ovarian cancer in women and PCa in men (13–15). Previous study has shown that BRCA1/2 mutations drive carcinogenesis through somatic inactivating the second wild-type allele, the “two-hit hypothesis”. Due to the combination of germline and somatic mutations, the failure of the BRCA-related HRR mechanism favour the activation of alternative and less effective DNA repair pathways, and finally result in tumor development (19, 20). Our previous report had also revealed that patient D had loss-of-heterozygosity of BRCA2 in his prostate tumors (3). In addition to DNA repair pathways, BRCA1/2 also could repress the progression of prostate cancer by inhibiting PI3K/AKT and MAP/ERK pathways, as well as MMP9 and AR signaling (21). It has been reported that PCa patients with BRCA1/2 mutations can be treated with radiotherapy, cisplatin, anthracyclines, or poly (ADP-ribose) polymerase inhibitors (22, 23). For metastatic castration-resistant PCa with bi-allelic inactivation of BRCA2, chemotherapy with platinum agents has been recommended (24). In our study, the three PCa patients carrying heterozygous BRCA2 germline mutations were treated with ADT combined with radiotherapy or chemotherapy, both primary lesions and metastases shrunk significantly, and their PSA levels were well controlled. In addition, mutations of other HRR-related factors such as ATM, ATR, BRIP1, CDK12, and CHEK2 also play vital roles in tumorigenesis and progression (16, 17). We also found that two young patients with germline mutations have relatively low PSA levels (<4ng/dL). Since the PSA level is not sensitive enough to screen PCa for these kinds of patients with DRGs’ mutations, an imaging examination should be conducted for these patients when encountering PCa-related symptoms at a young age (25).
In addition to DRGs’ mutations, mutations in HOXB13 and GJB2 genes have also been associated with PCa in previous studies. HOX is a highly conserved gene encoding homeodomain-containing transcription factors playing a vital role in body axis patterning and cell differentiation of developing embryos (26). Aberrations in HOX gene expression have been reported in abnormal development and malignancy, indicating that altered expression of HOX could be essential for both tumorigenesis and tumor suppression (27). Ewing et al. firstly found that a rare germline mutation c.252G–>A (p.G84E, rs138213197) in the first exon of HOXB13 is associated with an increased risk of non-aggressive PCa at a young-onset (28). Soon afterward, HOXB13 mutation (c.G216C and c.R229G) was detected in PCa patients with African and Asian ancestry as well (29). A carrier frequency of G84E mutation among European-Americans with familial young-onset PCa is 3.1%, but its frequency is threefold higher (8.4%) for both the Finnish and Swedish populations (30). Although uncommon, this mutation accounts for an eightfold increased risk of PCa diagnosed at 55 or younger (31). This mutation is more associated with hereditary PCa (OR: 6.6; 95% CI 3.3 to 12.0) (32). It has been reported that in a Finnish population that HOXB13 G84E mutation is associated with familial young-onset (<55 years) PCa with elevated PSA (20ng/mL or higher) at the time of diagnosis. Other than the G84E mutation in HOXB13 is mainly reported in the Western population, a similar mutation of G135E in HOXB13 reported by LIN X et al. (33) has been associated with an increased risk of PCa in the Chinese population. However, there is no evidence showing that these mutations are associated with other clinical aggressiveness, such as higher Gleason score, progression, or recurrence. In this study, the two patients carrying the V278L germline mutation of the HOXB13 gene were diagnosised at 50 and 55 years old, respectively. However, due to the small number of the total subjects, there is not enough relevant evidence to reveal the connection between mutations status and clinical features. Previous study also revealed that HOXB13, together with FOXA1 and GATA2, interacts with androgen receptor (AR) to promote the development and differentiation of prostate and PCa (34), while the exact mechanism of HOXB13 in carcinogenesis remains unclear.
GJB2 encodes a gap junction protein and is a putative tumor suppressor. However, more recent studies also showed that the expression level of GJB2 in metastatic tumor lesions is significantly higher than that in the primary tumor, suggesting that GJB2 may promote tumor metastasis (35). Mutations in GJB2 gene are usually associated with non-syndromic hearing loss (NSHL) (36). It has been reported that a 17.3% biallelic mutation of the GJB2 gene in the Chinese population (37). Since GJB2 and its isoforms express at different levels in PCa tissues, it is suggested that GJB2 may play a role in PCa tumorigenesis (38, 39). Our findings show that the rate of germline GJB2 mutation in PCa patients is 20.8% (5/24), higher than the rate in the general populations, indicating that GJB2 mutations might be associated with early-onset PCa. However, the role of GJB2 on PCa need to be further substantiated and the underlying mechanism remains to be unclear.
The major limitation of the present study is the small sample size, which might due to the specific particular cohort, early-onset prostate cancer. We have defined patients at ages under 65 years old as early-onset ones in our cohort according to previous studies (40–43). We noted that the current definition of early-onset prostate cancer is indeed not the classical or common used one (aged ≤55 years) according to previous report (44). However, we have a few reasons to use the current definition in our cohort. Firstly, previous study has shown that the mean age of prostate cancer patients in Asian population are higher than those in Caucasian population, suggesting a delay in prostate cancer development in Asian men (45). Secondly, the tumour latency period, the time when the tumour exists but is not detected (starts at tumour onset and ends at diagnosis by screening or clinical symptoms), might be longer in China; which might be due to the lack of routine PSA screening to certain extent. Thus most of the patients were not admitted to hospital until significant clinical symptoms appeared, evidenced by that most of the patients have advanced stages of tumors at diagnosis (46). At last, there are only a few patients with prostate cancer aged ≤55 years, who fulfilled all the inclusion criteria. Therefore, we used the age under 65 years old as early-onset in our cohort. Of note, half of the patients were diagnosed as prostate cancer at ages ≤ 55 years, only two patients were diagnosed at ages over 60 years old. Another limitation is that most of the mutations in our report are VUS. The effects of the mutations need to be further revealed.
The incidence of early-onset prostate cancer increased since PSA screening was introduced. The biological differences exist between early-onset prostate cancer and late-onset disease. In our study, we found that germline mutation in DRGs is one of the major risk factors for early-onset and a more aggressive PCa. Since the PSA level in early-onset PCa with DRGs’ mutations could be relatively normal, more aggressive imaging examination is suggested than PSA screening for these kinds of patients. HRR-related mutation carriers might benefit from ADT combined with chemo/radiotherapy. Therefore, genetic testing is rather important to guide precise therapy for the patients with early-onset PCa and to direct their family members to clinical genetic counseling.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/gsa-human/browse/HRA001716.
The studies involving human participants were reviewed and approved by The ethics committee of the Third Affiliated Hospital of the PLA Army Medical University (ethics number: 2018 No. 28). The patients/participants provided their written informed consent to participate in this study.
Conception/design: JJ and QL. Provision of study material or patients: TT and XT. Collection and/or assembly of data: TT, XT, ZW, YW, and JX. Data analysis and interpretation: DZ, XW, and JJ. Manuscript writing and revising: TT, XT, QL, DZ, and JJ. All authors contributed to the article and approved the submitted version.
This study is supported by the National Natural Science Foundation of China (81802558) and the University Research Project of Army Medical University (2017XYY07, 2018XLC1014 and 2019CXLCB006).
Author XW was employed by Genetron Health (Beijing) Co. Changping Distric.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the patients who generously donated blood samples that made this research possible.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.826778/full#supplementary-material
1. Wei Y, Wu J, Gu W, Qin X, Dai B, Lin G, et al. Germline DNA Repair Gene Mutation Landscape in Chinese Prostate Cancer Patients. Eur Urol (2019) 76:280–3. doi: 10.1016/j.eururo.2019.06.004
2. Liu Q, Tong D, Liu G, Yi Y, Xu J, Yang X, et al. A Novel BRCA2 Mutation in Prostate Cancer Sensitive to Combined Radiotherapy and Androgen Deprivation Therapy. Cancer Biol Ther (2018) 19:669–75. doi: 10.1080/15384047.2018.1451278
3. Tang T, Wang LA, Wang P, Tong D, Liu G, Zhang J, et al. Case Report: Co-Existence of BRCA2 and PALB2 Germline Mutations in Familial Prostate Cancer With Solitary Lung Metastasis. Front Oncol (2020) 10:564694. doi: 10.3389/fonc.2020.564694
4. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and Heritable Factors in the Causation of Cancer–Analyses of Cohorts of Twins From Sweden, Denmark, and Finland. N Engl J Med (2000) 343:78–85. doi: 10.1056/NEJM200007133430201
5. Cheng HH, Sokolova AO, Schaeffer EM, Small EJ, Higano CS. Germline and Somatic Mutations in Prostate Cancer for the Clinician. J Natl Compr Canc Netw (2019) 17:515–21. doi: 10.6004/jnccn.2019.7307
6. Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol (2017) 2017. doi: 10.1200/PO.17.00029
7. Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men With Metastatic Prostate Cancer. N Engl J Med (2016) 375:443–53. doi: 10.1056/NEJMoa1603144
8. Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol (2019) 37:490–503. doi: 10.1200/JCO.18.00358
9. Ceccaldi R, Rondinelli B, D'Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol (2016) 26:52–64. doi: 10.1016/j.tcb.2015.07.009
10. Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat Rev Mol Cell Biol (2017) 18:495–506. doi: 10.1038/nrm.2017.48
11. Turan V, Oktay K. BRCA-Related ATM-Mediated DNA Double-Strand Break Repair and Ovarian Aging. Hum Reprod Update (2020) 26:43–57. doi: 10.1093/humupd/dmz043
12. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA Repair Defect in BRCA Mutant Cells as a Therapeutic Strategy. Nature (2005) 434:917–21. doi: 10.1038/nature03445
13. Ledermann JA, Drew Y, Kristeleit RS. Homologous Recombination Deficiency and Ovarian Cancer. Eur J Cancer (2016) 60:49–58. doi: 10.1016/j.ejca.2016.03.005
14. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in Patients With Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med (2018) 379:753–63. doi: 10.1056/NEJMoa1802905
15. Wong-Brown MW, Meldrum CJ, Carpenter JE, Clarke CL, Narod SA, Jakubowska A, et al. Prevalence of BRCA1 and BRCA2 Germline Mutations in Patients With Triple-Negative Breast Cancer. Breast Cancer Res Treat (2015) 150:71–80. doi: 10.1007/s10549-015-3293-7
16. Shao N, Zhu Y, Ye DW. Germline DNA-Repair Gene Mutations and Efficacy of Abiraterone or Enzalutamide in Patients With Metastatic Castration-Resistant Prostate Cancer. Eur Urol Focus (2019) 5:745–7. doi: 10.1016/j.euf.2019.02.011
17. Zhao EY, Shen Y, Pleasance E, Kasaian K, Leelakumari S, Jones M, et al. Homologous Recombination Deficiency and Platinum-Based Therapy Outcomes in Advanced Breast Cancer. Clin Cancer Res (2017) 23:7521–30. doi: 10.1158/1078-0432.CCR-17-1941
18. Liu J, Near A, Chiarappa JA, Wada K, Tse J, Burudpakdee C, et al. Clinical Outcomes Associated With Pathogenic Genomic Instability Mutations in Prostate Cancer: A Retrospective Analysis of US Pharmacy and Medical Claims Data. J Med Econ (2019) 22:1080–7. doi: 10.1080/13696998.2019.1649267
19. Roy R, Chun J, Powell SN. BRCA1 and BRCA2: Different Roles in a Common Pathway of Genome Protection. Nat Rev Cancer (2011) 12:68–78. doi: 10.1038/nrc3181
20. Crocetto F, Barone B, Caputo VF, Fontana M, de Cobelli O, Ferro M. BRCA Germline Mutations in Prostate Cancer: The Future Is Tailored. Diagnostics (2021) 11(5):908. doi: 10.3390/diagnostics11050908
21. Moro L, Arbini AA, Yao JL, di Sant'Agnese PA, Marra E, Greco M. Loss of BRCA2 Promotes Prostate Cancer Cell Invasion Through Up-Regulation of Matrix Metalloproteinase-9. Cancer Sci (2008) 99:553–63. doi: 10.1111/j.1349-7006.2007.00719.x
22. Rimar KJ, Tran PT, Matulewicz RS, Hussain M, Meeks JJ. The Emerging Role of Homologous Recombination Repair and PARP Inhibitors in Genitourinary Malignancies. Cancer (2017) 123:1912–24. doi: 10.1002/cncr.30631
23. Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, et al. Olaparib in Patients With Metastatic Castration-Resistant Prostate Cancer With DNA Repair Gene Aberrations (TOPARP-B): A Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol (2020) 21:162–74. doi: 10.1016/S1470-2045(19)30684-9
24. Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-Sensitive Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2016) 69:992–5. doi: 10.1016/j.eururo.2015.11.022
25. Segal N, Ber Y, Benjaminov O, Tamir S, Yakimov M, Kedar I, et al. Imaging-Based Prostate Cancer Screening Among BRCA Mutation Carriers-Results From the First Round of Screening. Ann Oncol (2020) 31:1545–52. doi: 10.1016/j.annonc.2020.06.025
26. Mallo M, Alonso CR. The Regulation of Hox Gene Expression During Animal Development. Development (2013) 140:3951–63. doi: 10.1242/dev.068346
27. Shah N, Sukumar S. The Hox Genes and Their Roles in Oncogenesis. Nat Rev Cancer (2010) 10:361–71. doi: 10.1038/nrc2826
28. Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, et al. Germline Mutations in HOXB13 and Prostate-Cancer Risk. N Engl J Med (2012) 366:141–9. doi: 10.1056/NEJMoa1110000
29. Brechka H, Bhanvadia RR, VanOpstall C, Vander Griend DJ. HOXB13 Mutations and Binding Partners in Prostate Development and Cancer: Function, Clinical Significance, and Future Directions. Genes Dis (2017) 4:75–87. doi: 10.1016/j.gendis.2017.01.003
30. Laitinen VH, Wahlfors T, Saaristo L, Rantapero T, Pelttari LM, Kilpivaara O, et al. HOXB13 G84E Mutation in Finland: Population-Based Analysis of Prostate, Breast, and Colorectal Cancer Risk. Cancer Epidemiol Biomarkers Prev (2013) 22:452–60. doi: 10.1158/1055-9965.EPI-12-1000-T
31. Karlsson R, Aly M, Clements M, Zheng L, Adolfsson J, Xu J, et al. A Population-Based Assessment of Germline HOXB13 G84E Mutation and Prostate Cancer Risk. Eur Urol (2014) 65:169–76. doi: 10.1016/j.eururo.2012.07.027
32. Smith SC, Palanisamy N, Zuhlke KA, Johnson AM, Siddiqui J, Chinnaiyan AM, et al. HOXB13 G84E-Related Familial Prostate Cancers: A Clinical, Histologic, and Molecular Survey. Am J Surg Pathol (2014) 38:615–26. doi: 10.1097/PAS.0000000000000090
33. Hankey W, Chen Z, Wang Q. Shaping Chromatin States in Prostate Cancer by Pioneer Transcription Factors. Cancer Res (2020) 80:2427–36. doi: 10.1158/0008-5472.CAN-19-3447
34. Lin X, Qu L, Chen Z, Xu C, Ye D, Shao Q, et al. A Novel Germline Mutation in HOXB13 is Associated With Prostate Cancer Risk in Chinese Men. Prostate (2013) 73:169–75. doi: 10.1002/pros.22552
35. Wu JI, Wang LH. Emerging Roles of Gap Junction Proteins Connexins in Cancer Metastasis, Chemoresistance and Clinical Application. J BioMed Sci (2019) 26:8. doi: 10.1186/s12929-019-0497-x
36. Mishra S, Pandey H, Srivastava P, Mandal K, Phadke SR. Connexin 26 (GJB2) Mutations Associated With Non-Syndromic Hearing Loss (NSHL). Indian J Pediatr (2018) 85:1061–6. doi: 10.1007/s12098-018-2654-8
37. Liu XW, Wang JC, Wang SY, Li SJ, Zhu YM, Ding WJ, et al. The Mutation Frequencies of GJB2, GJB3, SLC26A4 and MT-RNR1 of Patients With Severe to Profound Sensorineural Hearing Loss in Northwest China. Int J Pediatr Otorhinolaryngol (2020) 136:110143. doi: 10.1016/j.ijporl.2020.110143
38. Hu LP, Liu ZX, Bai ZM, Tan S. Expressions of Cx26, Cx32 and Cx43 in Prostate Cancer and Their Implications. Zhonghua Nan Ke Xue (2014) 20:23–9.
39. Tate AW, Lung T, Radhakrishnan A, Lim SD, Lin X, Edlund M. Changes in Gap Junctional Connexin Isoforms During Prostate Cancer Progression. Prostate (2006) 66:19–31. doi: 10.1002/pros.20317
40. Sacco E, Prayer-Galetti T, Pinto F, Ciaccia M, Fracalanza S, Betto G, et al. Familial and Hereditary Prostate Cancer by Definition in an Italian Surgical Series: Clinical Features and Outcome. Eur Urol (2005) 47:761–8. doi: 10.1016/j.eururo.2005.01.016
41. Koutros S, Schumacher FR, Hayes RB, Ma J, Huang WY, Albanes D, et al. Pooled Analysis of Phosphatidylinositol 3-Kinase Pathway Variants and Risk of Prostate Cancer. Cancer Res (2010) 70:2389–96. doi: 10.1158/0008-5472.CAN-09-3575
42. Zheng SL, Augustsson-Balter K, Chang B, Hedelin M, Li L, Adami HO, et al. Sequence Variants of Toll-Like Receptor 4 are Associated With Prostate Cancer Risk: Results From the CAncer Prostate in Sweden Study. Cancer Res (2004) 64:2918–22. doi: 10.1158/0008-5472.CAN-03-3280
43. Chen YC, Page JH, Chen R, Giovannucci E. Family History of Prostate and Breast Cancer and the Risk of Prostate Cancer in the PSA Era. Prostate (2008) 68:1582–91. doi: 10.1002/pros.20825
44. Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate Cancer in Young Men: An Important Clinical Entity. Nat Rev Urol (2014) 11:317–23. doi: 10.1038/nrurol.2014.91
45. Zlotta AR, Kuk C. Further Evidence of Differences in Prostate Cancer Biomarkers Between Caucasian and Asian Men. Eur Urol (2019) 75:562–3. doi: 10.1016/j.eururo.2018.12.038
Keywords: prostate cancer, early-onset, next-generation sequencing, germline mutations, homologous recombination associated genes
Citation: Tang T, Tan X, Wang Z, Wang S, Wang Y, Xu J, Wei X, Zhang D, Liu Q and Jiang J (2022) Germline Mutations in Patients With Early-Onset Prostate Cancer. Front. Oncol. 12:826778. doi: 10.3389/fonc.2022.826778
Received: 01 December 2021; Accepted: 09 May 2022;
Published: 06 June 2022.
Edited by:
Francesca Sanguedolce, University of Foggia, ItalyReviewed by:
Patrick Pilie, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2022 Tang, Tan, Wang, Wang, Wang, Xu, Wei, Zhang, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuli Liu, bGl1cWl1bGk5MDA4MjdAMTYzLmNvbQ==; Jun Jiang, amlhbmdqdW5fNjRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.