- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Linkou, Taiwan
- 3Department of Radiation Oncology, Chang Gung Memorial Hospital, Chiayi, Taiwan
- 4Department of Radiology, Chang Gung Memorial Hospital, Chiayi, Taiwan
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Lo Sheng Sanatorium and Hospital Ministry of Health and Welfare, Taoyuan, Taiwan
Aim: We probed the prognostic value of the preoperative high-sensitivity modified Glasgow prognostic score (HS-mGPS), neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) for patients with oral cavity squamous cell carcinoma (OSCC) to identify patients with the highest risk of having poor survival outcomes.
Materials and Methods: We executed a retrospective assessment of the records of 303 patients with OSCC who had been subjected to curative surgery between January 2008 and December 2017. The HS-mGPS was categorized using C-reactive protein and albumin thresholds of 3 mg/L and 35 g/L, respectively. Moreover, receiver operating characteristic curve analyses were executed to find out the optimal PLR and NLR cutoffs. We plotted survival curves and compared them through the use of the Kaplan–Meier method and log-rank test, respectively. Through a Cox proportional hazard model, we identified prognostic variables. We also plotted a nomogram comprising the HS-mGPS and clinicopathological factors and assessed its performance with the concordance index.
Results: The PLR and NLR cutoffs were 119.34 and 4.51, respectively. We noted an HS-mGPS of 1−2 to be associated with a shorter median overall survival (OS) and disease-fee survival (DFS) compared with an HS-mGPS of 0. Multivariate analysis revealed that an HS-mGPS of 1−2 and an NLR of ≥4.51 were independent risk factors related to poor OS and DFS. The HS-mGPS appeared to have better prognostic effect than did the PLR and NLR, and the combination of the HS-mGPS and NLR appeared to exhibit optimal discriminative ability for OS prognostication. The nomogram based on the HS-mGPS and NLR yielded accurate OS prediction (concordance index = 0.803).
Conclusion: Our findings suggest that preoperative HS-mGPS is a promising prognostic biomarker of OSCC, and the nomogram comprising the HS-mGPS and NLR provided accurate individualized OSCC survival predictions.
Introduction
Oral cavity cancer is the commonest malignancy in the head and neck region. Of malignant tumors in the oral cavity, 90% are categorized as oral squamous cell carcinoma (OSCC) (1). The global OSCC incidence is increasing, with over 300,000 cases diagnosed in 2020 (2). For OSCC, the current mainstay of treatment entails curative surgery that is executed along with or without adjuvant therapy. Despite the application of advanced diagnostic modalities and multidisciplinary management, the OSCC prognosis remains unsatisfactory, and approximately 40% of patients experience locoregional recurrence and distant metastasis (3). Therefore, the identification of practical biomarkers for OSCC prognosis would be of clinical value. Studies have reported that several molecular biomarkers related to cancer cell differentiation and proliferation, metastasis, and angiogenesis could be applied to enhance OSCC survival estimations (4). However, the molecular biomarkers and costly laboratory techniques employed may not be widely applicable in clinical practice.
Increasing evidence demonstrates that cancer-related inflammation in the tumor microenvironment has a crucial role in various cancer development stages; such inflammation affects the proliferation and migration of tumor cells, induces angiogenesis and distant metastasis, and diminishes treatment responses to anticancer therapies (5). Furthermore, tumor-specific immunity is important for cancer surveillance and elimination (6). Several hematologic and biochemical immune inflammation indices have prognostic value for patients with OSCC; examples of these indices include serum albumin and C-reactive protein (CRP) levels, the neutrophil/lymphocyte ratio (NLR), and the platelet/lymphocyte ratio (PLR) (7–9). The modified Glasgow prognostic score (mGPS), which incorporates both serum CRP (cutoff: 10 mg/L) and albumin (cutoff: 35 g/L) levels, is an independent overall survival (OS) and cancer-specific survival predictor in OSCC (10). Based on a stricter serum CRP level (3 mg/L) cutoff, the high-sensitivity mGPS (HS-mGPS) has been reported to be a better prognostic indicator than conventional mGPS for various malignancies (11–13), and this superiority was also indicated in a study on head and neck cancer (14). However, the aforementioned study enrolled only 35 (27.1%) patients with OSCC (14), and the authors did not examine several crucial OSCC risk factors in their survival analysis, such as extranodal extension (ENE) (15) and depth of invasion (DOI) (16).
Attempting to fill the aforementioned gap, we executed the current study to probe the prognostic effect of nutrition-inflammation-based indices, including the HS-mGPS, NLR and PLR in patients with OSCC receiving curative surgery. We also investigated whether a prognostic model based on the combination of these indices would be useful for screening out patients with the highest risk of having a poor prognosis. We hypothesized that HS-mGPS and OSCC prognosis would have a significant association given that HS-mGPS reflects both cancer-related inflammation and host nutrition status. A confirmation of our hypothesis would indicate that preoperative HS-mGPS could be used for the early detection of high-risk patients and for treatment optimization, ultimately improving OSCC prognosis.
Materials and Methods
Study Design and Populations
We executed the current retrospective single institute study to probe the clinical outcomes of patients who had a new OSCC diagnosis and then underwent primary surgery between January 1, 2007, and December 31, 2018, at our hospital. In total, 336 patients with newly diagnosed OSCC and who satisfied the following inclusion criteria were identified from medical records: (1) having pathologically diagnosed OSCC; (2) undergoing treatment with curative surgery with or without adjuvant therapy; (3) being aged more than 18 years old at time of diagnosis. The exclusion criteria were as follows: (1) having incomplete preoperative laboratory data and follow-up records (n = 11); (2) having a diagnosis of a hematologic or autoimmune disease (n = 2); (3) having a history of an active infectious or inflammatory disorder within 1 month prior to OSCC surgery (n = 2); (4) having a cancer history (n = 8); (5) having metastasis or unresectable disease at diagnosis (n = 7); or (6) having undergone neoadjuvant therapy before curative surgery (n = 3). Ultimately, 303 patients were eligible for inclusion, and they constituted our study population. This study’s protocol adhered to the Declaration of Helsinki and was ratified by our institute’s institutional review board.
Clinical Data Collection
Medical staff collected relevant medical records from the hospital’s electronic resources and patient charts. Preoperative laboratory data—including serum CRP and albumin levels and absolute neutrophil, lymphocyte, and platelet counts—were retrospectively collected within 2 weeks before operation. As prognostic factors, certain clinicopathological features of each patient were reviewed and evaluated, namely surgical margins, cancer cell differentiation, overall cancer stage, age, perineural invasion (PNI), ENE, sex, and DOI. The pathological tumor, node, metastasis (TNM) stage of all patients was recorded on the basis of the latest staging manual set forth by the American Joint Committee on Cancer (AJCC) (8th Edition, 2018). For defining and documenting the presence of underlying comorbidities, we applied the Charlson Comorbidity Index (CCI) score (17). Through the review of patient interview and clinical data and records, we gleaned data on betel nut chewing, cigarette smoking, and alcohol consumption history. A cigarette smoker was construed as someone who smoked ≥10 cigarettes/day for ≥1 year (18). Moreover, a betel nut chewer was construed as someone who chewed betel nuts ≥2 times a day for ≥1 year. An alcohol drinker was construed as a person who consumed >1 alcoholic beverages per week for >6 months (19). Patients with none, one, or at least two of the aforementioned habits were categorized into corresponding exposure groups.
Calculation of Hematologic and Biochemical Indexes
During the study period, serum CRP (reference value: <5 mg/L) values as well as albumin (reference value: 35–55 g/L) values were measured through an automated analyzer (Roche Hitachi Cobas 8000, Rotkreuz, Switzerland), and hemoglobin, lymphocyte, platelet, and neutrophil counts were determined through a hematology analyzer (Sysmex SE-9000, Kobe, Japan). Preoperative HS-mGPS of 2, 1, and 0 were assigned to patients identified as having both hypoalbuminemia (<35 g/L) and a high CRP level (>3 mg/L), having either hypoalbuminemia or a high CRP level, and having neither hypoalbuminemia nor a high CRP level, respectively (12). To calculate the NLR, the neutrophil count/lymphocyte count ratio was derived; to calculate the PLR, the platelet count/lymphocyte count ratio was derived.
Treatment Protocol
Each of the included patients underwent routine preoperative workups that involved lab tests, physical exams, medical history taking, computed tomography (CT) or magnetic resonance imaging (MRI) executed on the head and neck, bone scintigraphy, hepatic ultrasonography, and chest radiography. Furthermore, if metastasis was suspected after such workups, chest or abdomen CT scanning was conducted. All patients underwent curative surgery with concomitant unilateral or bilateral neck dissection. For immediately reconstructing surgical defects, plastic surgeons used pedicle, free, or local flaps. Postoperative adjuvant treatment planning was determined by the consensus of the multidisciplinary tumor board of our hospital. Lin et al. (20) provided the detailed adju4vant treatment guidelines; briefly speaking, those identified as having multiple metastatic lymph nodes, ENE, or positive surgical margins were given adjuvant chemoradiotherapy (CRT), and patients with a solitary metastatic neck lymph node and pathologic T4 disease were administered adjuvant radiotherapy (RT). The intensity-modulated radiotherapy dose per fraction was 2 Gy for 5 days a week, and the total doses were 60−66 and 66 Gy for adjuvant RT and adjuvant CRT, respectively. In accordance with patient preferences and physicians’ judgments, the adjuvant chemotherapy regimen was 100 mg/m2 intravenous cisplatin once every three weeks or 40 mg/m2 intravenous cisplatin once a week.
Follow-Up
All patients had routine outpatient follow-up every two months, at 3-month intervals, and at 6-month intervals during the first year, during the second year, and after the second year, respectively. They had head and neck MRI or CT at 6-month intervals for a period of 2 years at 12-month intervals after that. Physical examination and flexible fiberoptic examinations were performed during every follow-up session. We derived OS to be the interval spanning from the surgery date to the date of death from any cause, the date of censoring, or December 31, 2019 (the study’s final follow-up date). We also derived disease-free survival (DFS) to be the interval spanning from the curative surgery date to that of treatment failure (according to clinical evidence such as locoregional recurrence or distant metastasis), that of censoring, or that of death.
Statistical Analysis
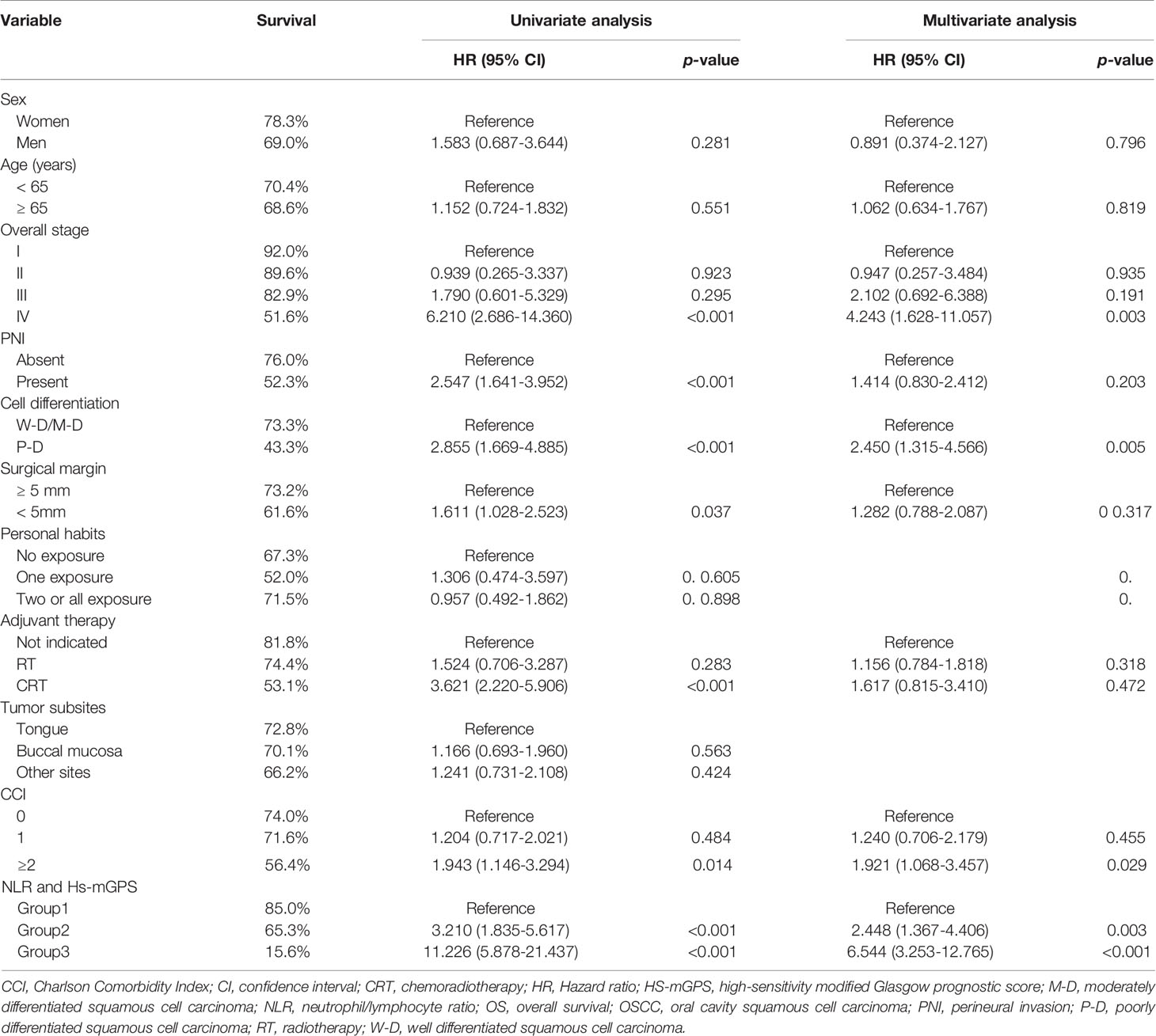
Data normality was investigated using the Kolmogorov–Smirnov test. Numbers and percentages were employed to represent how categorical variables were distributed; medians with interquartile ranges and means with standard deviations were applied to express nonnormally and normally distributed continuous variables, respectively. We established optimal NLR and PLR cutoffs through the execution of receiver operating characteristic (ROC) curve analyses and appraised the equivalent areas under the curves (AUCs). Group data had a nonnormal distribution; accordingly, we executed the χ2 test to compare the groups’ clinicopathological features for categorical variables and executed the Mann–Whitney U test to compare these features for continuous variables. Survival outcomes were assessed and survival curves were compared through the use of the Kaplan–Meier method and the log-rank test, respectively. After testing Cox’s proportional hazards assumption of the Cox model, we determined risk factors that were related to poor OS and DFS by employing the Cox proportional hazard model and estimated the equivalent hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) through univariate and multivariate analyses. In the univariate analysis, we evaluated potential risk factors by employing the log-rank test; for the multivariate analysis, we employed only those determined to have reached the level of statistical significance (p < 0.1) in the univariate analysis.
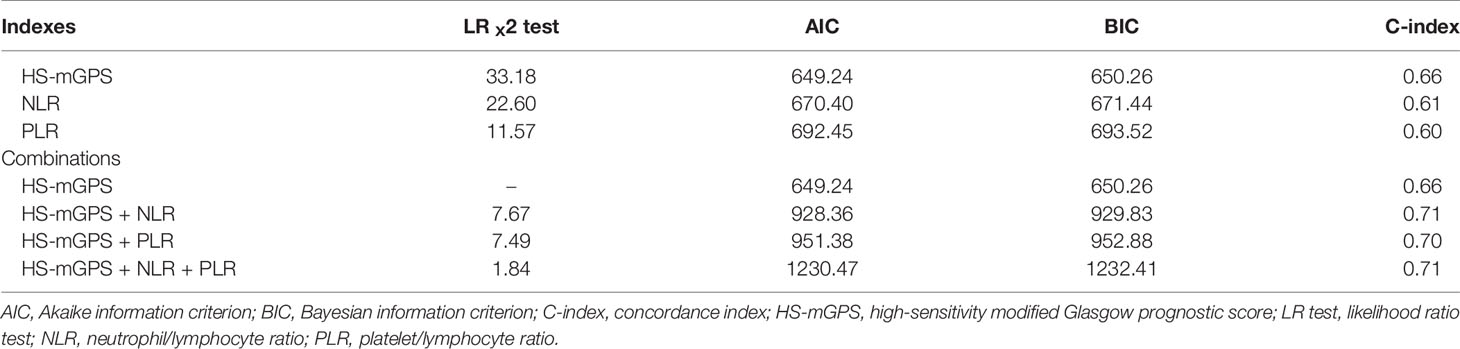
The likelihood ratio χ2 (LRχ2) test was applied to examine the predictive homogeneity of the indices and their combination. Through the application of Harrell’s concordance index (C-index), with values ranging from 0.5 to 1.0 (21), we could derive predictive accuracy; moreover, through the application of the Akaike information criterion (AIC) as well as Bayesian information criterion (BIC), we could examine prognostic discriminative ability (22). Overall, low BIC and AIC values and high C-index and LRχ2 values were associated with more favorable prognostic discriminative ability. The aforementioned C-index values were thought to represent total chance (C-index = 0.5) and perfect predictability (C-index = 1.0) (23). SPSS 21.0 (SPSS Inc., Chicago, IL, USA) constituted the platform on which the aforementioned analyses were executed. Furthermore, we considered statistical significance to be represented by a two-sided p value of <0.05.
On the basis of significant clinicopathological variables of multivariate analysis for OS, a prognostic nomogram was constructed through the ‘rms’ package in R (version 5.1-0; Vanderbilt University, Nashville, Tennessee, USA), with OS at 3 and 5 years used as endpoints (24). To analyze the established nomogram’s OS prediction performance, the C-index was applied for both the proposed nomogram and the traditional TNM system. Additionally, a consistency assessment was conducted between actual survival outcomes and nomogram-predicted OS by constructing calibration plots.
Results
Baseline Characteristics
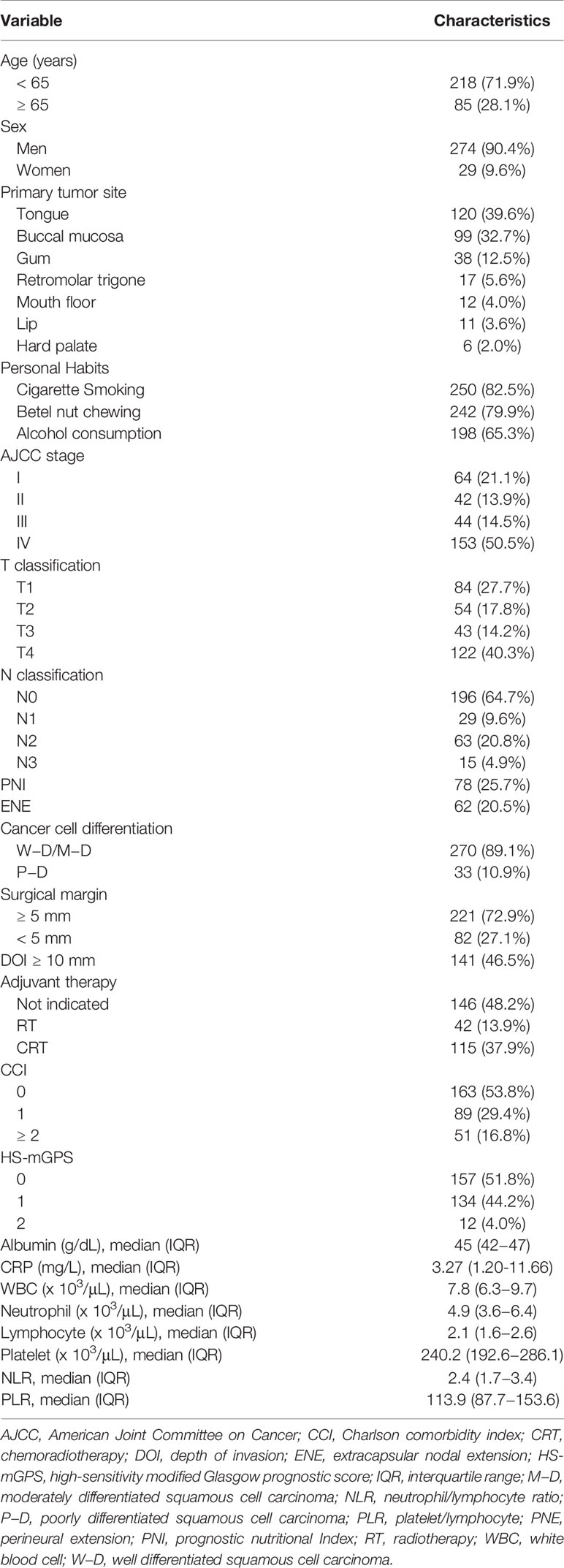
The baseline clinicopathological and demographic characteristics of the enrollees are presented in Table 1. Of the 303 enrollees, 274 (90.4%) were men. The patients’ median age was determined to be 57 (range, 31–86) years, and 218 (71.9%) patients were younger than 65 years. The top three primary tumor locations were the tongue (n = 120, 39.6%), buccal mucosa (n = 99, 32.7%), and gum (n = 38, 12.5%). Of the patients, 82.5% (n = 250) were smokers, 79.9% (n = 242) were betel nut chewers, and 65.3% (n = 198) were alcohol consumers. Approximately half of the patients had a stage IV disease (n = 153, 50.5%); 21.1% (n = 64) had a stage I disease, 14.5% had a stage III disease (n = 44), and 13.9% (n = 42) had a stage II disease. PNI was present in 78 (25.7%) patients; in addition, 107 (35.3%) patients were identified as having neck lymph node metastasis that was pathologically confirmed, and 62 (20.5%) patients had ENE. In total, 270 (89.1%) patients had OSCC that was well differentiated to moderately differentiated, and 33 (10.9%) patients had OSCC that was poorly differentiated. After surgery, 146 (48.2%) patients received no adjuvant treatment, 42 (13.9%) patients received adjuvant RT, and 115 (37.9%) received adjuvant CRT.
Determination of Biomarker Cutoff Values
HS-mGPSs ranged from 0 to 2: 157 (51.8%), 134 (44.2%), and 12 (4.0%) patients had a score of 0, 1, and 2, respectively (Table 1). An HS-mGPS value of ≥1 was determined to be significantly associated with an unfavorable prognosis (25), and only 4.0% of the patients had an HS-mGPS of 2; hence, the patients were categorized as follows for the subsequent analysis: those with an HS-mGPS of 0 (n = 157, 51.8%) and those with a score of 1−2 (n = 146, 48.2%). Additionally, the optimal NLR and PLR cutoffs were 4.51 (p = 0.009) and 119.34 (p = 0.003), respectively, according to an ROC analysis involving the calculation of AUC and Youden’s J-point for balancing specificity and sensitivity (Supplementary File 1).
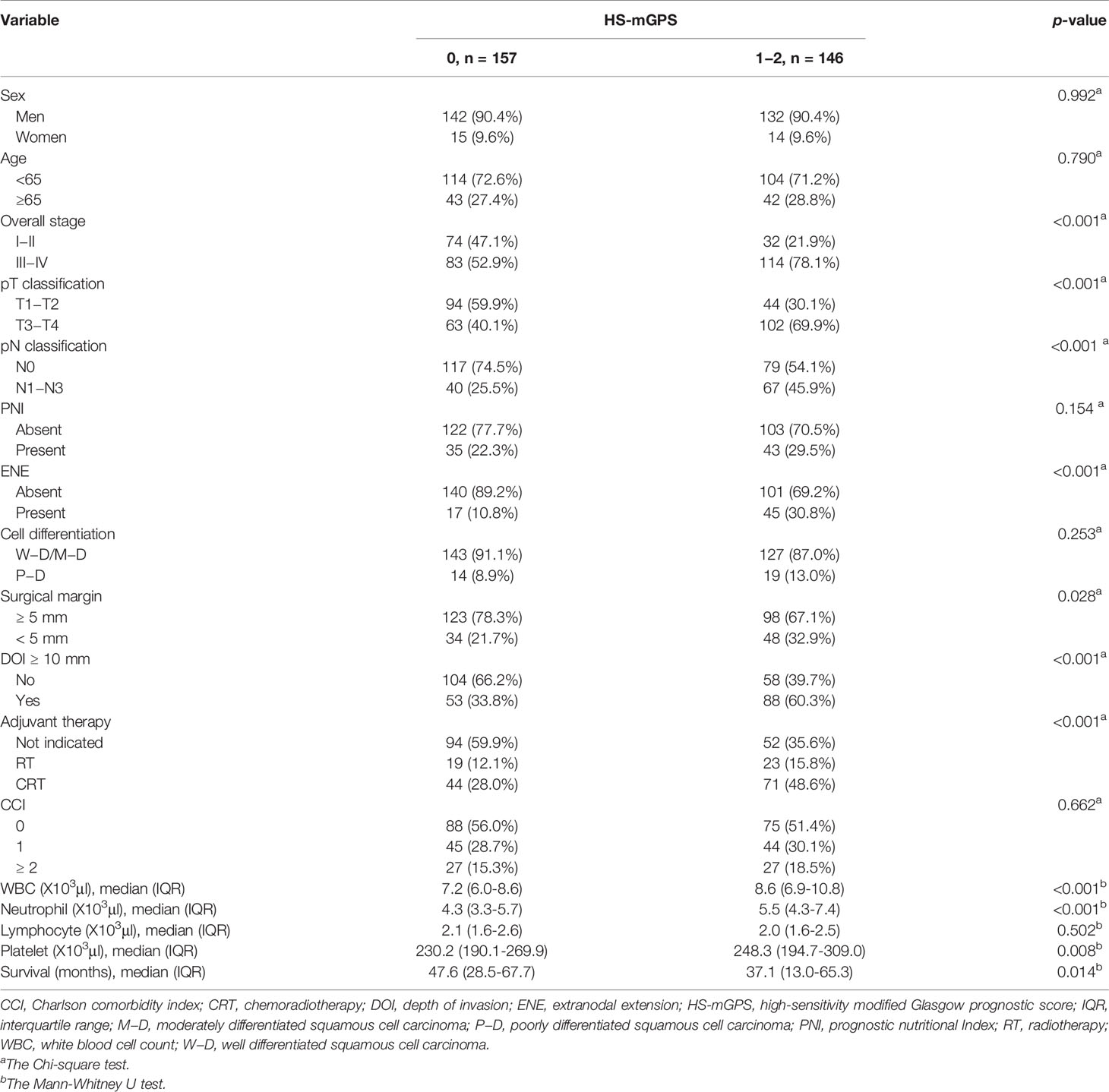
Association Between HS-mGPS and Clinicopathological Features
As shown in Table 2, patients with an HS-mGPS of 1−2 were more likely to have advanced T and N stages (both p < 0.001), a late-stage disease (p < 0.001), ENE (p < 0.001), a DOI of ≥10 mm (p < 0.001), a requirement for adjuvant therapy (p < 0.001), and a short median survival time (p = 0.014). By contrast, an HS-mGPS of 1−2 had no significant association with sex, age, PNI, tumor cell differentiation, or CCI.
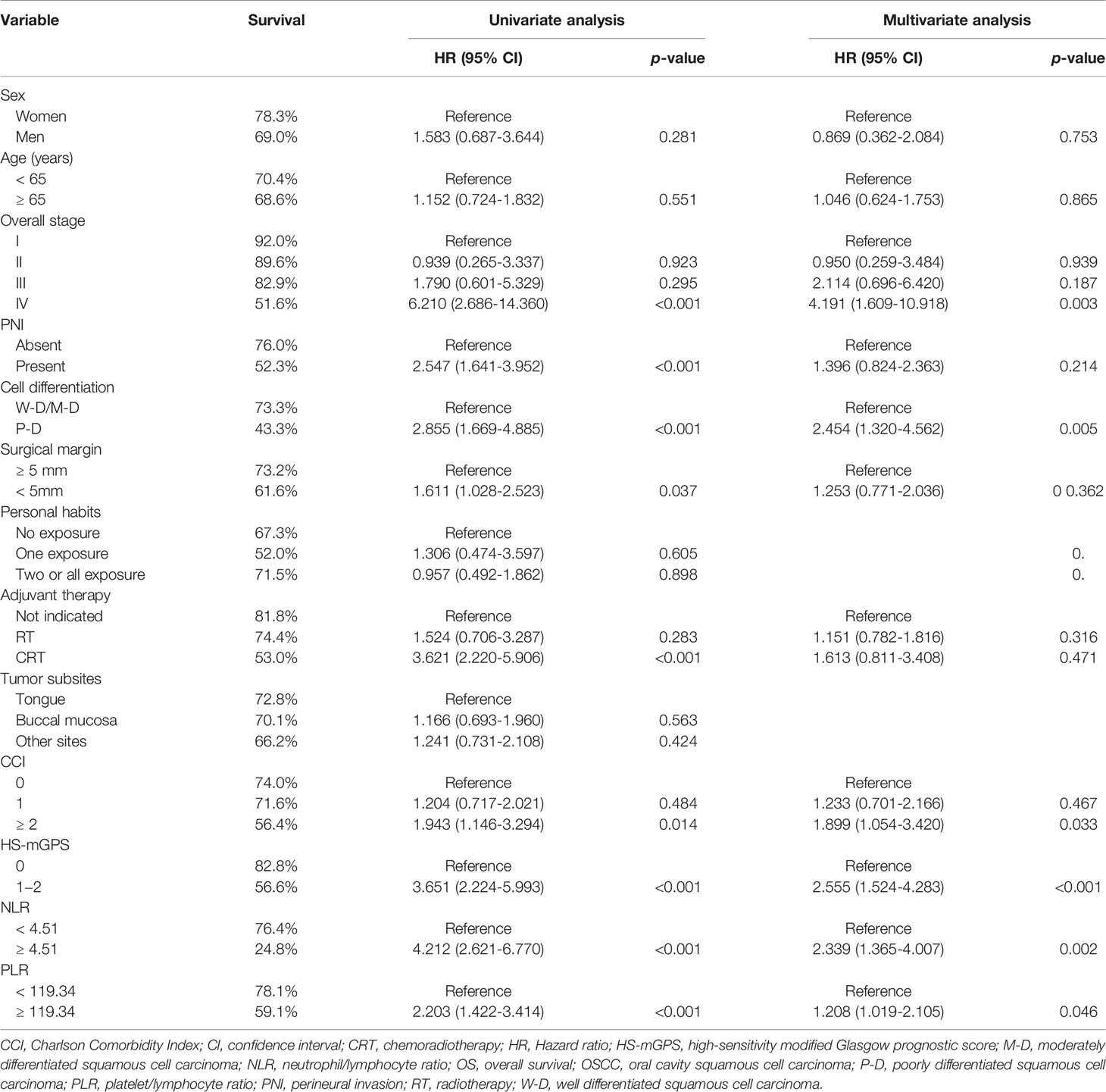
Correlation Between HS-mGPS and OS
Through our OS analysis in which the median (range) follow-up period was 40.9 (1.4−122.7) months, we determined an HS-mGPS of 1−2 to be significantly correlated with a shorter median OS (68.1 v.s. 103.2 months, p < 0.001) compared with an HS-mGPS of 0 (Figure 1A). In our univariate analysis, poor OS indicators were stage IV disease, PNI, poor tumor differentiation, a surgical margin of <5 mm, a need for adjuvant CRT, a CCI of ≥2, an HS-mGPS of 1−2, an NLR of ≥4.51, and a PLR of ≥119.34 (Table 3). We performed a multivariate analysis and demonstrated an NLR of ≥4.51 (p = 0.002), stage IV disease (p = 0.003), poor tumor differentiation (p = 0.005), a CCI of ≥2 (p = 0.033), an HS-mGPS of 1−2 (p < 0.001), and a PLR of ≥119.34 (p = 0.046) to constitute independent predictors of poor OS. We identified an HS-mGPS of 1−2 to be correlated with a 2.555 times higher all-cause mortality risk relative to an HS-mGPS of 0. The risk of mortality was 2.339 times higher among patients with a high (≥ 4.51) NLR compared with those with a low (< 4.51) NLR, the mortality risk was 1.208 times higher among patients with a high PLR (≥119.34) compared with those with a low PLR (<119.34).
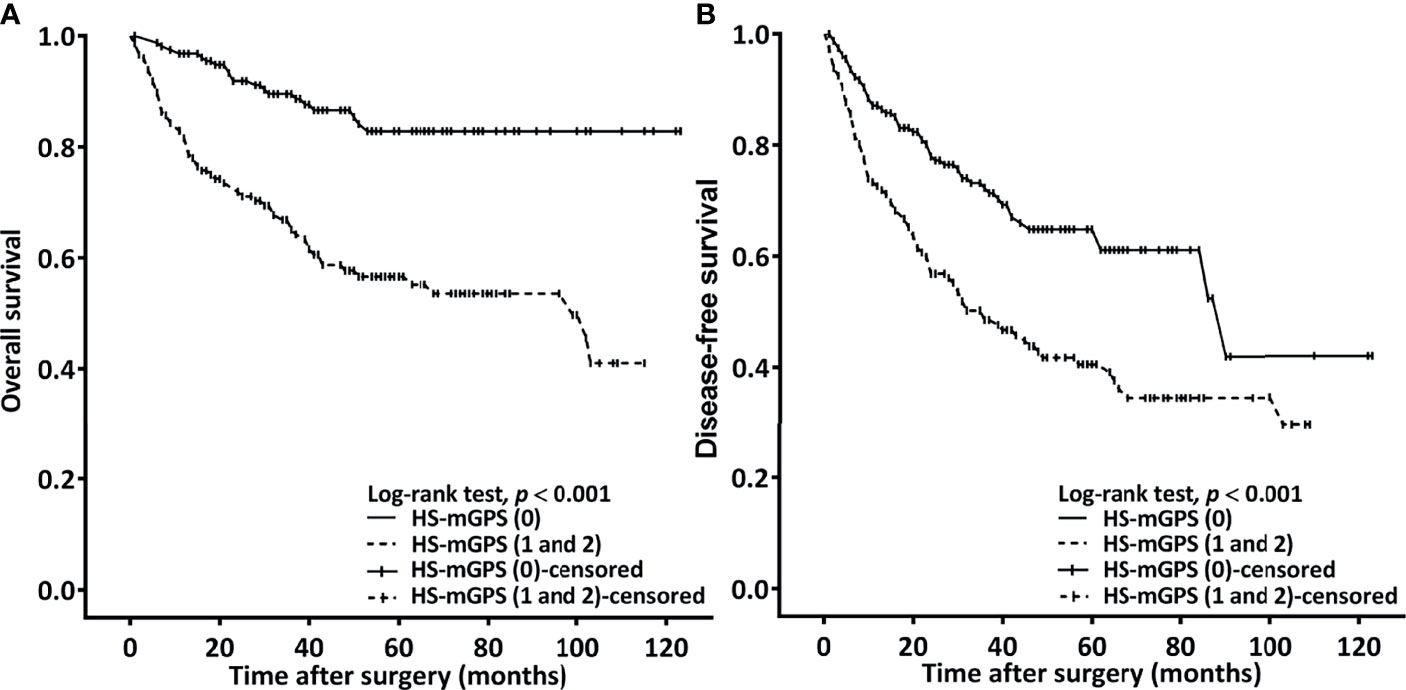
Figure 1 Kaplan–Meier curves plotted to estimate OS (A) and DFS (B) on the basis of patients’ HS-mGPS.
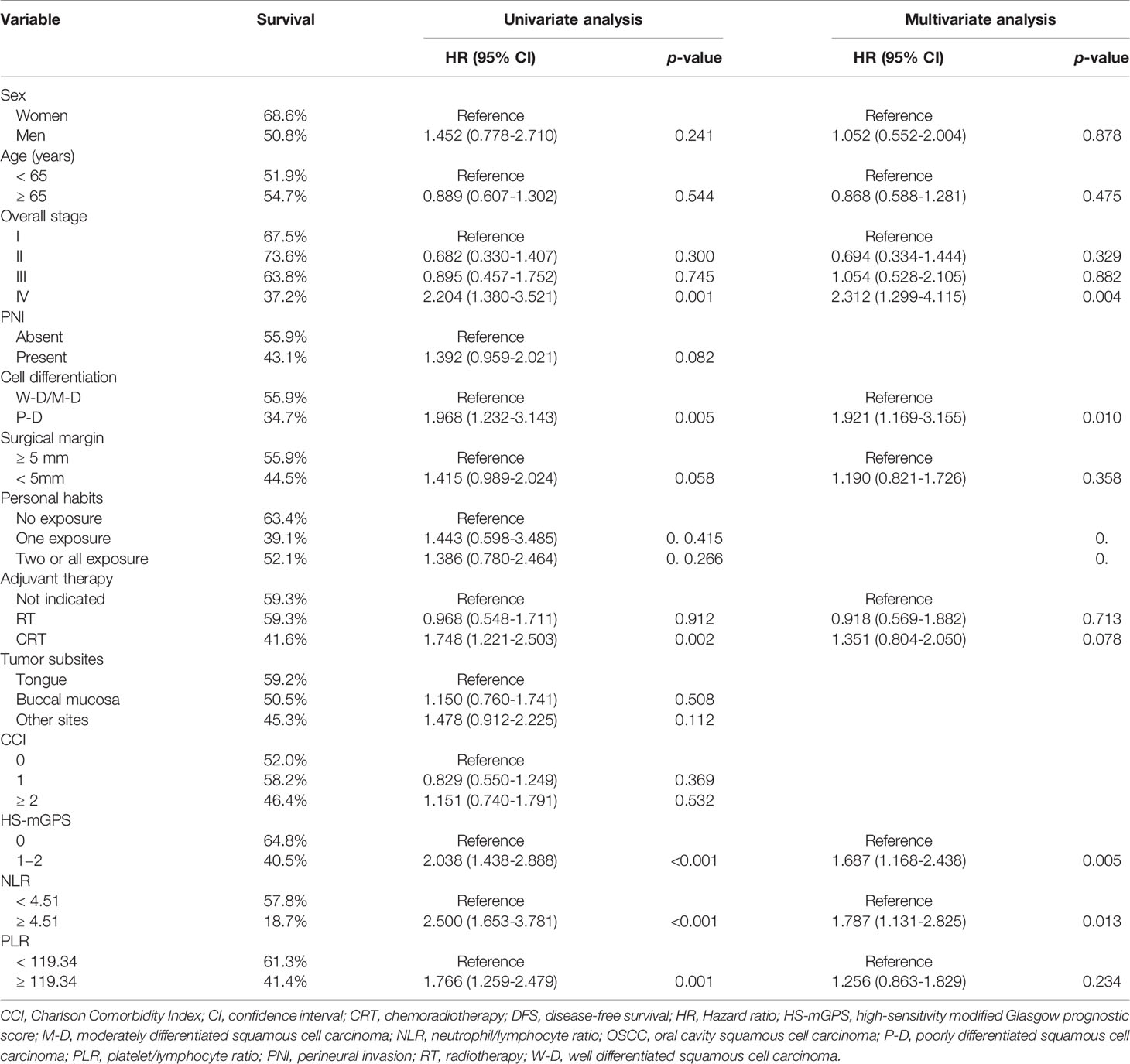
Correlation Between HS-mGPS and DFS
An HS-mGPS of 1−2 was noted to be correlated with a significantly shorter median DFS relative to an HS-mGPS of 0 (36.6 vs. 90.5 months, p < 0.001; Figure 1B). The correlation between clinicopathological variables and DFS is presented in Table 4. In our univariate analysis, we determined an NLR of ≥4.51, poor tumor differentiation, stage IV disease, need for adjuvant CRT, an HS-mGPS of 1 or 2, and a PLR of ≥119.34 to exhibit a significant correlation with poor DFS. We demonstrated through a multivariate analysis that an NLR of ≥4.51 (p = 0.013), stage IV disease (p = 0.004), poor tumor differentiation (p = 0.010), an HS-mGPS of 1−2 (p = 0.005) constituted independent risk factors for poor DFS. However, our results did not indicate a PLR of ≥119.34 to constitute an independent risk factor for unfavorable DFS.
Prognostic Efficacy Estimation
Table 5 shows results derived from comparing the prognostic effect of the indices included, namely the HS-mGPS, NLR, and PLR. Among these indices, the HS-mGPS had the highest LRχ2 and C-index scores and the lowest AIC and BIC scores, suggesting its superior prognostic value for determining OS in our study setting. We further investigated the prognostic effect of the combinations of indices by adding the NLR and PLR, separately, to the HS-mGPS; the results demonstrated that the combination of the HS-mGPS and NLR had the highest predictive accuracy (higher C-index), prognostic stratification performance (lower AIC and BIC values), and predictive homogeneity (higher LRχ2 score).
To identify patients with the highest treatment failure risk, we stratified the patients into the following groups: group 1 (n = 145, 47.9%), comprising those with low NLR (< 4.51) and an HS-mGPS of 0; group 2 (n = 128, 42.2%), comprising those with either an NLR of ≥4.51 or an HS-mGPS of 1−2; group 3 (n =30, 9.9%), comprising those with a high NLR (≥ 4.51) and an HS-mGPS of 1−2. On the basis of Kaplan–Meier curves, we probed the OS (Figure 2A) and DFS (Figure 2B) of the patients in these groups. In groups 1, 2, and 3, the median OS periods were determined to be >103.6, >53.1, and 28.6 (95% CI: 8.6–48.8) months, respectively, and the median DFS periods were derived to be 90.6 (95% CI: 58.2–122.1), 48.4 (95% CI: 19.5–77.6), and 15.3 (95% CI: 4.6–39.2) months, respectively. We noted the groups to differ significantly with respect to OS and DFS, according to the results of a log-rank test (both p < 0.001, Figure 2). In the multivariate Cox regression analysis, we noted group 3 to be independently associated with the poorest OS (HR = 6.544, 95% CI: 3.253–12.765, p < 0.001, Table 6).
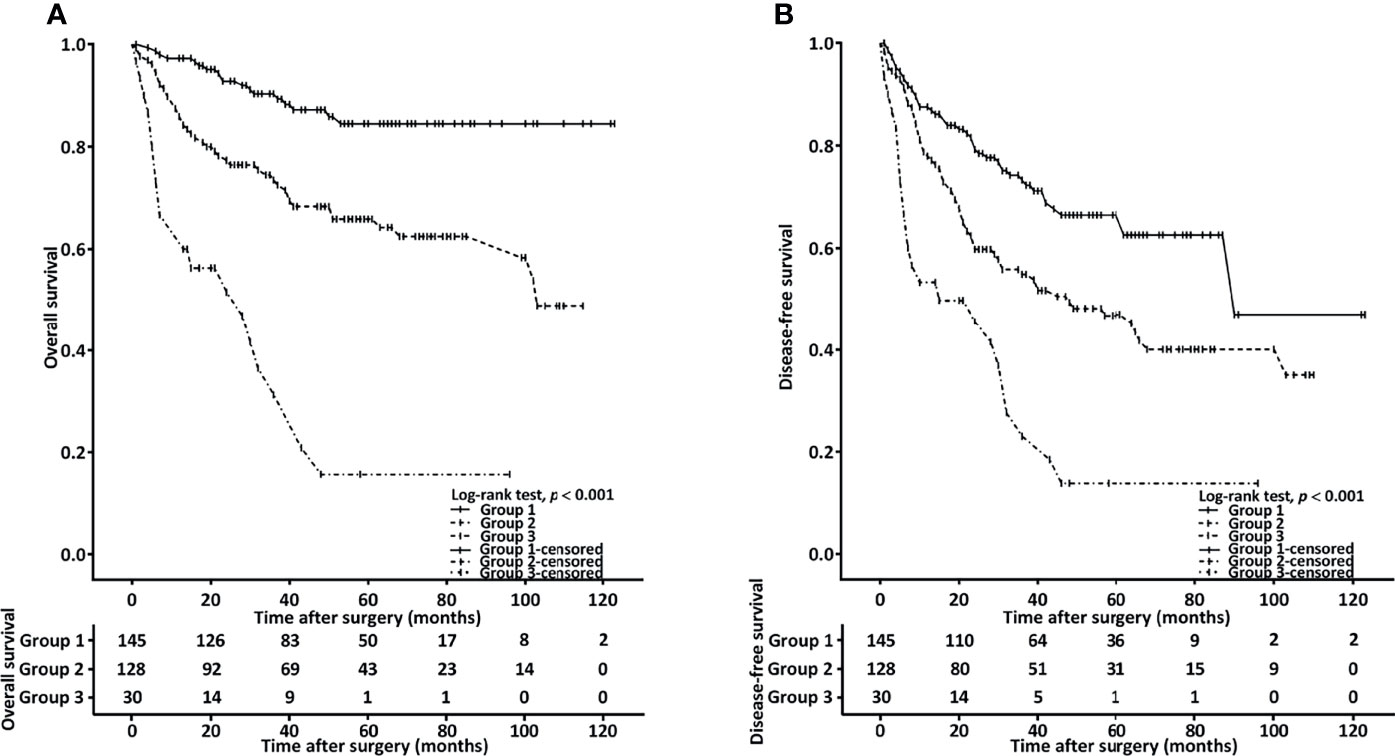
Figure 2 Combined effect of HS-mGPS and NLR on OS (A) and DFS (B) determined using the Kaplan–Meier method. Group 1, patients with a low NLR (< 4.51) and HS-mGPS of 0; group 2, patients with either an NLR of ≥ 4.51 or HS-mGPS of 1−2; group 3, patients with a high NLR (≥ 4.51) and HS-mGPS of 1−2.
Prognostic Nomogram
To provide an accurate estimation of OS based on our study findings, we constructed a prognostic nomogram that comprised the aforementioned groups’ model, overall stage, sex, cancer cell differentiation, and age (Figure 3A). As the nomogram shows, the model combined HS-mGPS and NLR had the strongest effect on OS, followed by TNM stage and cancer cell differentiation. Regarding OS prediction, the derived C-index for the established nomogram was 0.803, exceeding that of the nomogram consisting of TNM staging alone (C-index = 0.695, Supplementary File 2). Additionally, the calibration plots of the nomogram for 3-year (Figure 3B) and 5-year (Figure 3C) OS estimations revealed the predicted and actual survival outcomes to have a satisfactory level of consistency between them.
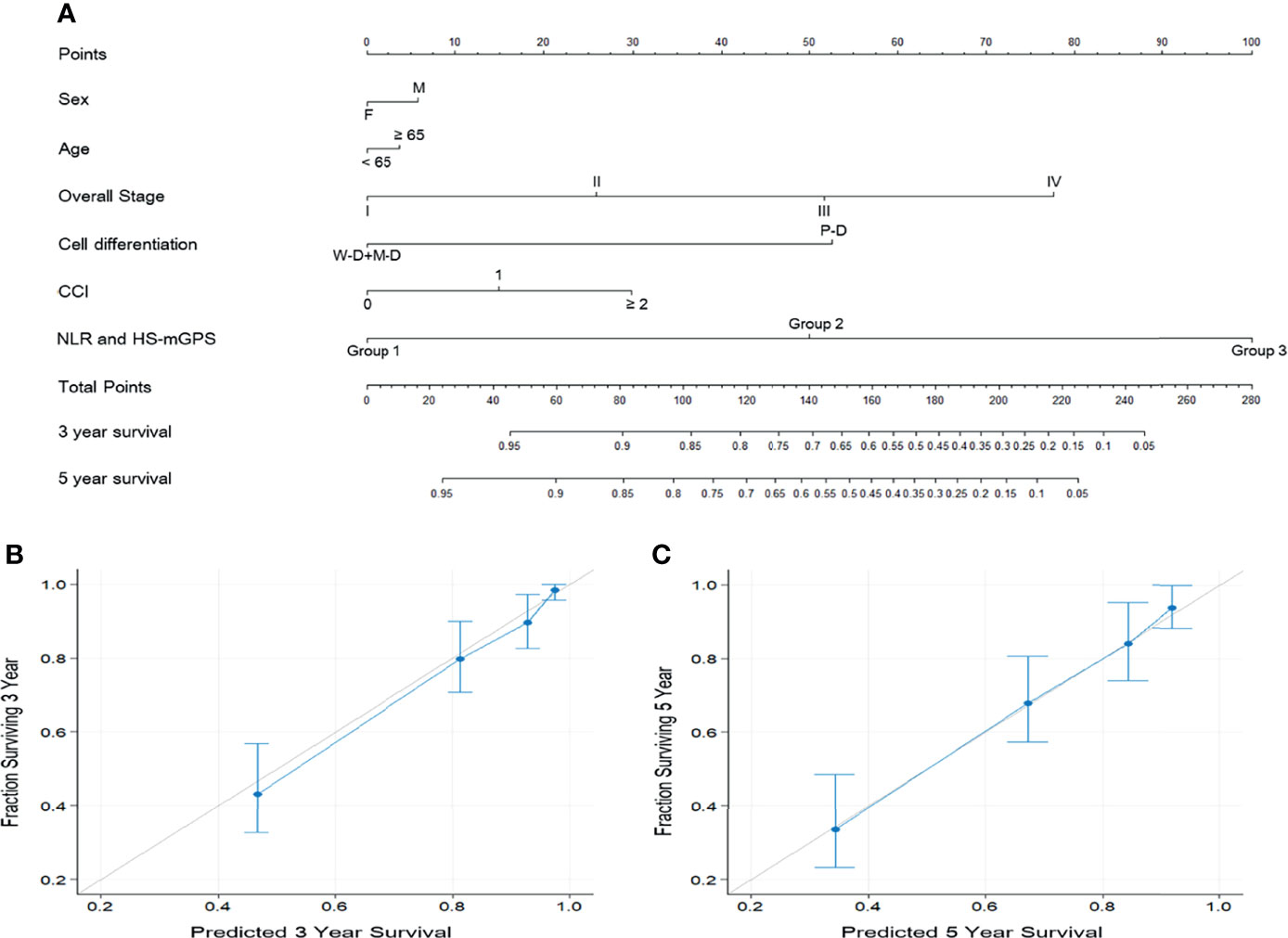
Figure 3 Prognostic nomogram predicting the OS of patients with OSCC (A). The nomogram was constructed and interpreted as follows: A vertical line was drawn from each variable to the uppermost horizontal axis marked as points. The points where the vertical line crosses the axis represent the degree of risk a variable contributes. The points from all variables were added to obtain the summarized points, and a vertical line was drawn from the points on the axis marked as total points to the below axes marked as 3-year survival and 5-year survival to obtain the 3- and 5-year OS predictions of the nomogram. Calibration plots for (B) 3-year OS and (C) 5-year OS predictions revealed high agreement between the OS predictions of the nomogram and actual survival outcomes. The 45° light gray line represents the ideal survival prediction, and the blue line indicates the predicted outcomes. Blue dots with bars represent the performance and 95% CI of the nomogram when applied to cohorts that survived.
Discussion
Effective prognostic markers for better treatment planning and patient stratification related to OSCC are pressing requirements. Evidence demonstrates that systemic inflammation and host nutrition status play key roles in cancer angiogenesis and progression (26, 27), and several inflammation- and nutrition-based indices, such as NLR and PLR, have been proposed as prognostic biomarkers for patients with OSCC (28, 29). Hanai et al. reported that the HS-mGPS but not mGPS was an independent prognostic marker for head and neck cancer; the HS-mGPS may help identify patients with cachexia sensitively in those with an early-stage disease or good performance status, highlighting the prognostic impact of optimal nutritional support (14). However, only 35 participants with oral cavity cancer were enrolled in their study, and the absence of robust evidence for the clinical use of the HS-mGPS for patients with OSCC precludes the formulation of clear recommendations. According to a literature review, our current study represents the first examination of the HS-mGPS’s prognostic value for patients with OSCC. We observed that an HS-mGPS of 1−2 was associated with a late disease stage, a need for adjuvant therapy, advanced T and N stages, ENE, a DOI of ≥10 mm, and a short median survival. Additionally, our multivariate analysis revealed an HS-mGPS of 1−2 to constitute an independent risk factor for poor DFS and OS. We compared the prognostic efficacy of the HS-mGPS, NLR, and PLR as well as that of their combination. When we applied the LRχ2 value, C-index, and AIC and BIC values, the HS-mGPS appeared to have the best prognostic effects, and the HS-mGPS combined with the NLR revealed the optimal prognostic discriminative ability. To identify patients with the highest treatment failure risk, we grouped our patients according to the HS-mGPS (0 or 1−2) and NLR (< or ≥ 4.51). We revealed that patients with a high NLR (≥ 4.51) and an HS-mGPS of 1−2 had the poorest OS, suggesting that an HS-mGPS of 1−2 and NLR of ≥4.51 may indicate some synergism that contributes to a poor OSCC prognosis. Currently, TNM staging system is the most widely used classification system of OSCC for treatment planning and prognosis estimation. The prognostic nomogram that incorporated the TNM staging system, group model, and clinicopathological variables yielded more favorable results compared with the nomogram that was based on TNM staging alone (C-index: 0.803 vs 0.695); therefore, the established nomogram may provide more accurate patient stratification and aid personalized treatment planning. Our study results confirm the HS-mGPS’s prognostic value for patients with OSCC, suggesting the consideration of host nutrition and inflammatory factors as rational adjuncts to the staging of OSCC. Given that the mainstay of treatment for OSCC is ablative surgery, patients who are estimated to have poor prognosis from the proposed nomogram might require more aggressive adjuvant management and close follow up, which warrants further investigation.
The HS-mGPS integrated with both systemic inflammation (CRP) and host nutrition status (albumin level) is a biochemical index established by Proctor et al., who modified the serum CRP threshold from 10 mg/L (conventional mGPS) to 3 mg/L to increase the index’s prognostic importance (30). One of the main advantages of the HS-mGPS is that it can be easily obtained from laboratory tests, and its prognostic superiority over GPS and mGPS has been reported in studies involving various cancers (12, 13, 31). Our study results reveal that the HS-mGPS had better prognostic value than did the NLR and PLR, which is in line with the results of studies examining patients with soft tissue sarcoma (32); this result may be explained by the fast reactivity of CRP and the interaction of host nutrition status and anti-tumor immune response in determining HS-mGPS. Nevertheless, the underlying mechanism connecting the HS-mGPS and OSCC prognosis remained uncertain, and it may be explained as follows. Studies have indicated that OSCC increases the secretion of interleukin 6 and 8 (33), which potentially results in CRP synthesis within the liver and may cause autocrine tumor growth factor activity to induce head and neck cancer progression (34). Extensive tumor invasion and necrosis may also positively upregulate systemic inflammation, and the resultant elevated CRP level is linked to a poor OSCC prognosis (35). A chronic malnutrition indicator, hypoalbuminemia is associated with a poor head and neck cancer prognosis (36). Hwang et al. revealed serum CRP and albumin levels to be negatively associated (37), and this is possibly explained by the decreased hepatic synthesis of albumin due to increased systemic inflammation (38). Cancer-related systemic inflammation and a poor nutrition status may be reflected by a high HS-mGPS, and the resultant sarcopenia has a synergistic effect with systemic inflammation, conferring a poorer prognosis on patients with advanced OSCC (39). The aforementioned studies have suggested the possible mechanism through which a high HS-mGPS negatively influences DFS and OS within OSCC. Further large-scale prospective research should be executed to validate our findings and clarify the underlying mechanism of the association of the HS-mGPS with OSCC prognosis.
PLR and NLR were identified by our study as constituting independent prognostic factors for OS. As suggested by Hasegawa et al., who conducted a study on patients with primary OSCC, preoperative NLR elevation is an independent predictor of poor disease-specific survival as well as poor OS in such patients; this finding is in line with our results (40). However, unlike the study by Chen et al. (41), our study did not reveal an independent role of PLR in predicting DFS. This discrepancy may be explained by the difference between the PLR cutoff values and AJCC manual editions used in the mentioned studies. Lymphocytes, neutrophils, and platelets are involved in carcinogenesis not only in systemic circulation but also in the tumor microenvironment. Studies have reported that platelets downregulate natural killer cell activity through transforming growth factor beta secretion, modulate tumor angiogenesis through the vascular endothelial growth factor pathway, and help circulating tumor cells to adhere to the microvascular endothelium, thereby encouraging tumor cell progression and metastasis (42, 43). Lymphocytes are essential to host antitumor immunity through their induction of direct tumor cell cytotoxicity and cytokine secretion, such as tumor necrosis factor alpha and interferon gamma (44). Therefore, decreased lymphocyte counts may reflect impaired immune surveillance and favorable conditions for tumor progression, which eventually indicated a poor cancer-related prognosis (45). Neutrophils promote tumor-related angiogenesis through cytokine and chemokine secretion and release nitric oxide as well as reactive oxygen species, engendering downregulation of T-cell function and consequently enhancing cancer cell invasion, proliferation, and metastasis (46). A high NLR and PLR may reflect increased cancer-related inflammation and a weakened antitumor immune response, which are associated with a poor prognosis related to various cancers (47). Nevertheless, no consensus has been reached on optimal NLR and PLR cutoffs because tumor location, cancer stage, and age distribution may contribute to variations in cutoffs, rendering it difficult to verify the validity of such cutoffs in different studies. By contrast, the cutoffs of serum CRP and albumin levels used in HS-mGPS calculations have been well validated with regard to various tumors (11–13). Therefore, HS-mGPS could be useful for OSCC patient stratification, surpassing the NLR and PLR in terms of clinical applicability and validity.
Studies have reported that the HS-mGPS is significantly correlated with tumor aggression. For example, patients with prostate cancer and an HS-mGPS of ≥1 had significantly higher prostate-specific antigen and testosterone levels than did those with an HS-mGPS of <1 (48). Another study also identified a significant association between a high HS-mGPS and a larger tumor size and higher grade of soft tissue sarcoma (31). A study on hypopharyngeal cancer detected no significant correlation of the GPS with patients’ clinicopathological characteristics (49). By contrast, we revealed an HS-mGPS of 1−2 to exhibit a significant correlation with advanced-stage disease, more advanced T and N stages, ENE, and a DOI of ≥10 mm, suggesting that the HS-mGPS may have high sensitivity for predicting head and neck cancer aggression. The assumed mechanism underlying these correlations are as follows: (1) A substantial tumor burden, such as greater tumor volume and nodal metastasis, may occur along with high cancer-related systemic inflammation, thus possibly increasing CRP (50) and interleukin (33) levels and thereby engendering a high HS-mGPS (2). Higher cancer-related inflammation increases the depletion of serum albumin and impairs the synthesis of albumin within the liver (51), contributing to a high HS-mGPS. All the aforementioned studies have provided potential insight into how the HS-mGPS is associated with the OSCC aggression, which warrants further verification and investigation.
Our study’s strength is the use of a relatively large data set of patients with OSCC who received curative surgery as a primary treatment. After identifying the prognostic value of the HS-mGPS, we demonstrated that the combination of the biochemical (HS-mGPS) and hematological (NLR) indices enabled comprehensive and refined OSCC prognostication. The established nomogram based on this combined model confirmed the clinical applicability of the HS-mGPS and NLR and provided accurate individualized survival prediction. However, the study was not without limitations. First, the retrospective and single-institution design may have introduced bias. To minimize potential bias, we enrolled a relatively large number of patients with OSCC who were all treated with curative surgery. Second, we did not use an independent data set to confirm our derived results, meaning external validity remained unconfirmed. Furthermore, the HS-mGPS, NLR, and PLR were all measured before surgery. Future research should explore the changes in these indices in response to curative treatment over time and examine how these changes are associated with OSCC prognosis.
Conclusions
Our study indicated the HS-mGPS to be a promising prognostic biomarker for patients with OSCC who have undergone curative surgery. The HS-mGPS could be considered in clinical practice due to the high availability, reproducibility, and low cost of this biomarker-related approach to patient prognosis estimations. The established nomogram comprised the combination of HS-mGPS and NLR and confirmed the clinical applicability of the combined model with accurate individualized survival estimations. Further large-scale prospective research is necessary to validate our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Chang Gung Memorial Hospital’s Institutional Review Board ratified our executed study (board approval number: 202100555B0C601). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-TT and C-WL conceived, designed, and supervised the study. K-HF, C-MH, and C-HL collected the data of patients and followed up. S-WC and EH analyzed the data. M-ST and G-HC provided technical assistance with the data analysis. Y-TT and C-WL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant (CMRPG6L0231) from Chang Gung Memorial Hospital, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Health Information and Epidemiology Laboratory at Chiayi Chang Gung Memorial Hospital for their helpful advice and assistance with data analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.825967/full#supplementary-material
Supplementary File 1 | Receiver operating characteristic curve and AUC analyses of the NLR and PLR. AUC, area under the curve; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Supplementary File 2 | Kaplan–Meier estimates of overall survival (A) and disease-free survival (B) according to the cancer stages.
References
1. Johnson NW, Jayasekara P, Amarasinghe AA. Squamous Cell Carcinoma and Precursor Lesions of the Oral Cavity: Epidemiology and Aetiology. Periodontol 2000 (2011) 57(1):19–37. doi: 10.1111/j.1600-0757.2011.00401.x
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Warnakulasuriya S. Living With Oral Cancer: Epidemiology With Particular Reference to Prevalence and Life-Style Changes That Influence Survival. Oral Oncol (2010) 46(6):407–10. doi: 10.1016/j.oraloncology.2010.02.015
4. Lu Z, Liang J, He Q, Wan Q, Hou J, Lian K, et al. The Serum Biomarker Chemerin Promotes Tumorigenesis and Metastasis in Oral Squamous Cell Carcinoma. Clin Sci (Lond) (2019) 133(5):681–95. doi: 10.1042/CS20181023
5. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis (2009) 30(7):1073–81. doi: 10.1093/carcin/bgp127
6. Zou W. Immunosuppressive Networks in the Tumour Environment and Their Therapeutic Relevance. Nat Rev Cancer (2005) 5(4):263–74. doi: 10.1038/nrc1586
7. Lee S, Kim DW, Kwon S, Kim HJ, Cha IH, Nam W. Prognostic Value of Systemic Inflammatory Markers for Oral Cancer Patients Based on the 8th Edition of Ajcc Staging System. Sci Rep (2020) 10(1):12111. doi: 10.1038/s41598-020-68991-3
8. DEP D, Young CK, Chien HT, Tsao CK, Fok CC, Fan KH, et al. Prognostic Roles of Scc Antigen, Crp and Cyfra 21-1 in Oral Cavity Squamous Cell Carcinoma. Anticancer Res (2019) 39(4):2025–33. doi: 10.21873/anticanres.13313
9. Tsai MH, Chuang HC, Lin YT, Lu H, Chen WC, Fang FM, et al. Clinical Impact of Albumin in Advanced Head and Neck Cancer Patients With Free Flap Reconstruction-A Retrospective Study. PeerJ (2018) 6:e4490. doi: 10.7717/peerj.4490
10. Farhan-Alanie OM, McMahon J, McMillan DC. Systemic Inflammatory Response and Survival in Patients Undergoing Curative Resection of Oral Squamous Cell Carcinoma. Br J Oral Maxillofac Surg (2015) 53(2):126–31. doi: 10.1016/j.bjoms.2014.10.007
11. Chen P, Fang M, Wan Q, Zhang X, Song T, Wu S. High-Sensitivity Modified Glasgow Prognostic Score (Hs-Mgps) Is Superior to the Mgps in Esophageal Cancer Patients Treated With Chemoradiotherapy. Oncotarget (2017) 8(59):99861–70. doi: 10.18632/oncotarget.21734
12. Osugi J, Muto S, Matsumura Y, Higuchi M, Suzuki H, Gotoh M. Prognostic Impact of the High-Sensitivity Modified Glasgow Prognostic Score in Patients With Resectable Non-Small Cell Lung Cancer. J Cancer Res Ther (2016) 12(2):945–51. doi: 10.4103/0973-1482.176168
13. Takeno S, Hashimoto T, Shibata R, Maki K, Shiwaku H, Yamana I, et al. The High-Sensitivity Modified Glasgow Prognostic Score Is Superior to the Modified Glasgow Prognostic Score as a Prognostic Predictor in Patients With Resectable Gastric Cancer. Oncology (2014) 87(4):205–14. doi: 10.1159/000362601
14. Hanai N, Sawabe M, Kimura T, Suzuki H, Ozawa T, Hirakawa H, et al. The High-Sensitivity Modified Glasgow Prognostic Score Is Superior to the Modified Glasgow Prognostic Score as a Prognostic Predictor for Head and Neck Cancer. Oncotarget (2018) 9(97):37008–16. doi: 10.18632/oncotarget.26438
15. Shaw RJ, Lowe D, Woolgar JA, Brown JS, Vaughan ED, Evans C, et al. Extracapsular Spread in Oral Squamous Cell Carcinoma. Head Neck (2010) 32(6):714–22. doi: 10.1002/hed.21244
16. Pentenero M, Gandolfo S, Carrozzo M. Importance of Tumor Thickness and Depth of Invasion in Nodal Involvement and Prognosis of Oral Squamous Cell Carcinoma: A Review of the Literature. Head Neck (2005) 27(12):1080–91. doi: 10.1002/hed.20275
17. D’Hoore W, Sicotte C, Tilquin C. Risk Adjustment in Outcome Assessment: The Charlson Comorbidity Index. Methods Inf Med (1993) 32(5):382–7. doi: 10.1055/s-0038-1634956
18. Husten CG. How Should We Define Light or Intermittent Smoking? Does It Matter? Nicotine Tob Res (2009) 11(2):111–21. doi: 10.1093/ntr/ntp010
19. Kao HK, Lofstrand J, Loh CY, Lao WW, Yi JS, Chang YL, et al. Nomogram Based on Albumin and Neutrophil-To-Lymphocyte Ratio for Predicting the Prognosis of Patients With Oral Cavity Squamous Cell Carcinoma. Sci Rep (2018) 8(1):13081. doi: 10.1038/s41598-018-31498-z
20. Lin CY, Fan KH, Lee LY, Hsueh C, Yang LY, Ng SH, et al. Precision Adjuvant Therapy Based on Detailed Pathologic Risk Factors for Resected Oral Cavity Squamous Cell Carcinoma: Long-Term Outcome Comparison of Cgmh and Nccn Guidelines. Int J Radiat Oncol Biol Phys (2020) 106(5):916–25. doi: 10.1016/j.ijrobp.2019.08.058
21. Brentnall AR, Cuzick J. Use of the Concordance Index for Predictors of Censored Survival Data. Stat Methods Med Res (2018) 27(8):2359–73. doi: 10.1177/0962280216680245
22. Vrieze SI. Model Selection and Psychological Theory: A Discussion of the Differences Between the Akaike Information Criterion (Aic) and the Bayesian Information Criterion (Bic). Psychol Methods (2012) 17(2):228–43. doi: 10.1037/a0027127
23. Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for Predicting Local Recurrence, Distant Metastases, and Overall Survival for Patients With Locally Advanced Rectal Cancer on the Basis of European Randomized Clinical Trials. J Clin Oncol (2011) 29(23):3163–72. doi: 10.1200/JCO.2010.33.1595
24. Harrell FE Jr, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med (1996) 15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
25. Souza Cunha M, Wiegert EVM, Calixto-Lima L, Oliveira LC. Relationship of Nutritional Status and Inflammation With Survival in Patients With Advanced Cancer in Palliative Care. Nutrition (2018) 51-52:98–103. doi: 10.1016/j.nut.2017.12.004
26. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
27. Singh R, Mishra MK, Aggarwal H. Inflammation, Immunity, and Cancer. Mediators Inflamm (2017) 2017:6027305. doi: 10.1155/2017/6027305
28. Takenaka Y, Oya R, Kitamiura T, Ashida N, Shimizu K, Takemura K, et al. Platelet Count and Platelet-Lymphocyte Ratio as Prognostic Markers for Head and Neck Squamous Cell Carcinoma: Meta-Analysis. Head Neck (2018) 40(12):2714–23. doi: 10.1002/hed.25366
29. Yang L, Huang Y, Zhou L, Dai Y, Hu G. High Pretreatment Neutrophil-To-Lymphocyte Ratio as a Predictor of Poor Survival Prognosis in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Head Neck (2019) 41(5):1525–35. doi: 10.1002/hed.25583
30. Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the Systemic Inflammation-Based Glasgow Prognostic Score: A Glasgow Inflammation Outcome Study. Cancer (2013) 119(12):2325–32. doi: 10.1002/cncr.28018
31. Nakamura T, Matsumine A, Asanuma K, Matsubara T, Sudo A. The Value of the High-Sensitivity Modified Glasgow Prognostic Score in Predicting the Survival of Patients With a Soft-Tissue Sarcoma. Bone Joint J (2015) 97-B(6):847–52. doi: 10.1302/0301-620X.97B.35098
32. Hou T, Guo T, Nie R, Hong D, Zhou Z, Zhang X, et al. The Prognostic Role of the Preoperative Systemic Immune-Inflammation Index and High-Sensitivity Modified Glasgow Prognostic Score in Patients After Radical Operation for Soft Tissue Sarcoma. Eur J Surg Oncol (2020) 46(8):1496–502. doi: 10.1016/j.ejso.2020.05.026
33. St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, et al. Interleukin 6 and Interleukin 8 as Potential Biomarkers for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg (2004) 130(8):929–35. doi: 10.1001/archotol.130.8.929
34. Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, et al. Interleukin-6 Predicts Recurrence and Survival Among Head and Neck Cancer Patients. Cancer (2008) 113(4):750–7. doi: 10.1002/cncr.23615
35. Huang SF, Wei FC, Liao CT, Wang HM, Lin CY, Lo S, et al. Risk Stratification in Oral Cavity Squamous Cell Carcinoma by Preoperative Crp and Scc Antigen Levels. Ann Surg Oncol (2012) 19(12):3856–64. doi: 10.1245/s10434-012-2392-5
36. Danan D, Shonka DC Jr, Selman Y, Chow Z, Smolkin ME, Jameson MJ. Prognostic Value of Albumin in Patients With Head and Neck Cancer. Laryngoscope (2016) 126(7):1567–71. doi: 10.1002/lary.25877
37. Hwang JC, Jiang MY, Lu YH, Wang CT. Precedent Fluctuation of Serum Hs-Crp to Albumin Ratios and Mortality Risk of Clinically Stable Hemodialysis Patients. PloS One (2015) 10(3):e0120266. doi: 10.1371/journal.pone.0120266
38. Don BR, Kaysen G. Serum Albumin: Relationship to Inflammation and Nutrition. Semin Dial (2004) 17(6):432–7. doi: 10.1111/j.0894-0959.2004.17603.x
39. Lee J, Liu SH, Dai KY, Huang YM, Li CJ, Chen JC, et al. Sarcopenia and Systemic Inflammation Synergistically Impact Survival in Oral Cavity Cancer. Laryngoscope (2021) 131(5):E1530–8. doi: 10.1002/lary.29221
40. Hasegawa T, Iga T, Takeda D, Amano R, Saito I, Kakei Y, et al. Neutrophil-Lymphocyte Ratio Associated With Poor Prognosis in Oral Cancer: A Retrospective Study. BMC Cancer (2020) 20(1):568. doi: 10.1186/s12885-020-07063-1
41. Chen S, Guo J, Feng C, Ke Z, Chen L, Pan Y. The Preoperative Platelet-Lymphocyte Ratio Versus Neutrophil-Lymphocyte Ratio: Which Is Better as a Prognostic Factor in Oral Squamous Cell Carcinoma? Ther Adv Med Oncol (2016) 8(3):160–7. doi: 10.1177/1758834016638019
42. Nie D, Yang E, Li Z. Pretreatment Thrombocytosis Predict Poor Prognosis in Patients With Endometrial Carcinoma: A Systematic Review and Meta-Analysis. BMC Cancer (2019) 19(1):73. doi: 10.1186/s12885-018-5264-y
43. Belluco C, Forlin M, Delrio P, Rega D, Degiuli M, Sofia S, et al. Elevated Platelet Count Is a Negative Predictive and Prognostic Marker in Locally Advanced Rectal Cancer Undergoing Neoadjuvant Chemoradiation: A Retrospective Multi-Institutional Study on 965 Patients. BMC Cancer (2018) 18(1):1094. doi: 10.1186/s12885-018-5022-1
44. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-Related Inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
45. Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, et al. Lymphocyte Nadir Predicts Tumor Response and Survival in Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiotherapy: Immunologic Relevance. Radiother Oncol (2019) 131:52–9. doi: 10.1016/j.radonc.2018.12.001
46. Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: Important Contributors to Tumor Progression and Metastasis. Cancer Metastasis Rev (2015) 34(4):735–51. doi: 10.1007/s10555-015-9594-9
47. Jiang J, Ma T, Xi W, Yang C, Wu J, Zhou C, et al. Pre-Treatment Inflammatory Biomarkers Predict Early Treatment Response and Favorable Survival in Patients With Metastatic Colorectal Cancer Who Underwent First Line Cetuximab Plus Chemotherapy. Cancer Manag Res (2019) 11:8657–68. doi: 10.2147/CMAR.S211089
48. Ando K, Sakamoto S, Saito S, Maimaiti M, Imamura Y, Sazuka T, et al. Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel. Cancers (Basel) (2021) 13(4):773. doi: 10.3390/cancers13040773
49. Iuchi H, Kyutoku T, Ito K, Matsumoto H, Ohori J, Yamashita M. Impacts of Inflammation-Based Prognostic Scores on Survival in Patients With Hypopharyngeal Squamous Cell Carcinoma. OTO Open (2020) 4(4):2473974X20978137. doi: 10.1177/2473974X20978137
50. Hsu YP, Hsieh CH, Chien HT, Lai CH, Tsao CK, Liao CT, et al. Serum Markers of Cyfra 21-1 and C-Reactive Proteins in Oral Squamous Cell Carcinoma. World J Surg Oncol (2015) 13:253. doi: 10.1186/s12957-015-0656-9
Keywords: high-sensitivity modified Glasgow prognostic score, oral cavity squamous cell carcinoma, overall survival, disease-free survival, nomogram
Citation: Tsai Y-T, Fang K-H, Hsu C-M, Lai C-H, Chang S-W, Huang EI, Tsai M-S, Chang G-H and Luan C-W (2022) Prognostic Role of High-Sensitivity Modified Glasgow Prognostic Score for Patients With Operated Oral Cavity Cancer: A Retrospective Study. Front. Oncol. 12:825967. doi: 10.3389/fonc.2022.825967
Received: 30 November 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
Moran Amit, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Ching-Chieh Yang, Chi Mei Medical Center, TaiwanNaomi Kiyota, Kobe University Hospital, Japan
Copyright © 2022 Tsai, Fang, Hsu, Lai, Chang, Huang, Tsai, Chang and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Wei Luan, a2lsaWtraWxpazEyMTQxNUBnbWFpbC5jb20=
 Yao-Te Tsai1
Yao-Te Tsai1 Ku-Hao Fang
Ku-Hao Fang Cheng-Ming Hsu
Cheng-Ming Hsu Chia-Hsuan Lai
Chia-Hsuan Lai Ming-Shao Tsai
Ming-Shao Tsai Chih-Wei Luan
Chih-Wei Luan