- 1Department of Oncology, Hospital of Chinese Medicine of Changxing County, Huzhou, China
- 2Department of Thoracic Radiation Oncology, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
- 3Institute of Cancer and Basic Medicine, Chinese Academy of Sciences, Hangzhou, China
Immune checkpoint inhibitor (ICI) treatment has dramatically revolutionized the landscape of therapeutic approaches in multiple cancers, particularly, non-small-cell lung cancer (NSCLC). With the increasing use of programmed death-1 (PD-1) inhibitors in the clinic, the emerging toxicity profile presents a novel learning curve for clinicians. Here we report the first case of an NSCLC patient displaying sarcoid/granulomatous-like reaction (SLR, also known as GLR) in the liver during an anti-PD-1 therapy which showed efficacious response of complete regression. Also, this is the first report describing the SLR induced by toripalimab, a novel PD-1 inhibitor. Given this kind of hepatic findings can be easily mistaken as metastasis, even resulting in premature use of second-line treatments. In particular, we briefly review the clinical features of all those cases reporting sarcoidosis and SLRs manifested on different organs during anti-PD-(L)1 therapy. We anticipate that these clinical cases would help to alert the attention of clinicians that SLRs, as a rare immune-related adverse event (irAE), is manageable and that histopathological analysis is necessary before interpreting it as disease progression.
Introduction
Lung cancer represents the most commonly diagnosed neoplasm and the leading cause of cancer-related death worldwide, and approximately 85% of the patients are histologically categorized as NSCLC (1). Although the introduction of targeted therapies has largely improved outcomes of NSCLC patients, heterogeneity of responses and resistance to these agents are noted. Given the fact that most of the patients have advanced disease on presentation, the 5-year survival among all NSCLC patients remains less than 18% (2, 3). Recently, ICI treatment has become a groundbreaking change in cancer management, as it may achieve enormous long-term survival benefits in several cancers, particularly, NSCLC. Based on the marvelous results achieved in a series of clinical trials, pembrolizumab (an anti-PD-1 antibody) and atezolizumab (an anti-PD-L1 antibody) have already gained global approval as first-line options for eligible patients with metastatic NSCLC either as monotherapy or combined with chemotherapy (4–9). In fact, more and more novel agents targeting PD-(L)1 are continually under investigation. Toripalimab is such a selective recombinant, humanized immunoglobulin G4 monoclonal antibody against PD-1. After having received its global approval in China for use in the unresectable or metastatic melanoma that has failed previous systemic treatment, several clinical trials of toripalimab in other advanced/metastatic cancers are underway (10). Most recently, a phase I trial reported that toripalimab displayed encouraging antitumor activity and manageable safety profiles in NSCLC patients, which is comparable to other PD-1 antagonists such as nivolumab and pembrolizumab (11).
However, along with the robust antitumor activity displayed, ICI treatment also causes non-specific systemic consequences in the setting of immune activation. Specifically, toxicities manifest as a wide spectrum of immune-related adverse events (irAEs), namely, dermatitis, endocrinopathies, autoimmune colitis, pneumonia, hepatitis, and neuropathies (12). Sarcoidosis or SLR is a granulomatous (like) disease which has been sporadically reported and recognized as a rare, but important, kind of irAE secondary to ICI treatment (13). In this present article, we report the first case of an NSCLC patient who got a CR on toripalimab treatment and displayed the hepatic SLR mimicking disease progression. In addition, we also summarize the recent pieces of literature that describe sarcoidosis and SLR induced by ICI therapy in various cancers.
We present the following case in accordance with the CARE reporting checklist.
Case Presentation
A 69-year-old Chinese man was diagnosed with squamous cell lung carcinoma in February 2020, complaining of stimulating dry cough for 10 months and tachypnea for 2 weeks. Chest and abdomen contrast enhanced computed tomography (CT) scan showed two masses in the bilateral lower lung lobe (right: 32 mm, left: 31 mm) and the appearance of left hilar lymphadenopathy. A CT-guided percutaneous intrathoracic lung biopsy was conducted to the mass on the left lower lung lobe and a histopathological analysis confirmed it as squamous cell lung carcinoma. In addition, both of the pulmonary masses and left hilar lymphadenopathy were confirmed as hypermetabolic on [18F]2-fluoro-2-deoxy-D-glucose–positron emission tomography/CT (FDG–PET/CT) (Figure 1A). Brain magnetic resonance imaging (MRI), bone scan, and abdomen B-ultrasound were performed and showed no metastatic findings. Given these clinical contents, he had a final diagnosis of metastatic squamous cell lung carcinoma with stage IVA (cT2N1M1a) which is unresectable. The immunohistochemistry (IHC) result of the biopsy from the tumor tissue showed the positive expression of PD-L1 protein (PD-L1 >1%, TPS, 22C3). After multidiscipline discussion and communication with the patient, he received PC chemotherapy regimens (paclitaxel 300 mg and carboplatin 400 mg), along with toripalimab (240 mg) at day 1 every 3 weeks. The response of the patient was remarkable: a routine CT scan after two cycles of this combination treatment showed about 80% of tumor shrinkage of the known tumoral masses and hilar lymphadenopathy; a repeated CT scan after 4 cycles showed that all the lesions have almost disappeared and the amount of FDG uptake were lost from the PET/CT scan (Figure 1B), with an evaluation of clinical CR by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
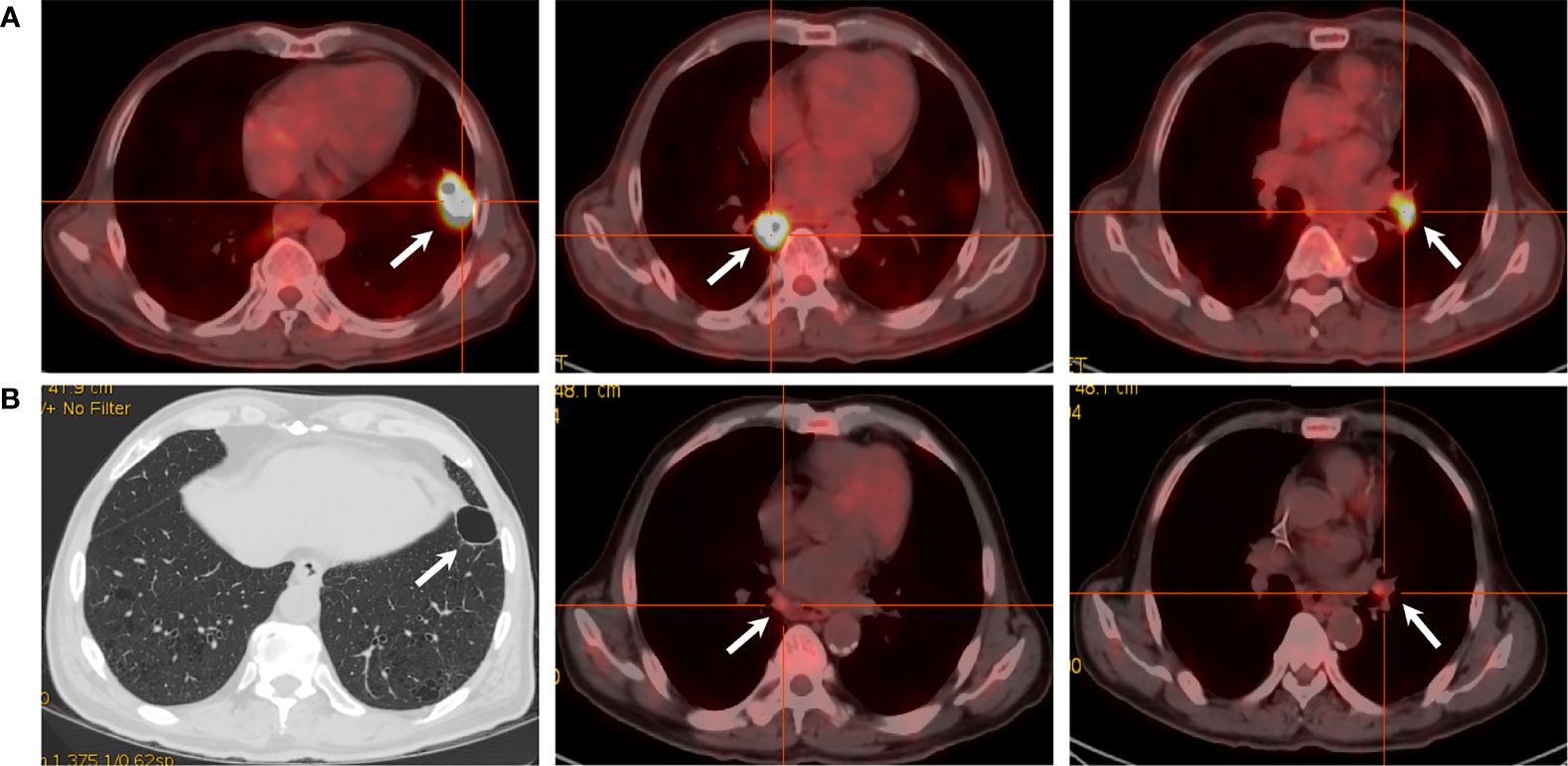
Figure 1 PET/CT scans of the target lesions. (A) Before toripalimab and chemotherapy administration in February 2020, PET/CT showed three lesions with intense hypermetabolic activity. (B) After 4 cycles of the combination treatment in June 2020, the solid portion of the left lower lobe lung mass disappeared with a cavity left. Also note the dramatic decrease in the size and metabolism of the other two masses.
Given the favorable tumor regression, chemotherapy of PC regimens were discontinued after 4 cycles and toripalimab as a single drug treatment was continued. Overall, toripalimab was well tolerated except that of a cutaneous diffuse maculopapular rash with mild itching which was improved dramatically and kept on a good control with the treatment of oral antihistamine agents plus topical compound flumetasone ointment. However, a CT scan indicated a 10.0 × 6.3 mm hypodense nodule in the liver quadrate lobe after 6 cycles, which was considered as disease progression with live metastasis in support of the radiology examination, namely, abdominal contrast enhanced CT, hepatic ultrasound, and hepatic contrast enhanced MRI (Figures 2A–C). A radiofrequency ablation of liver metastasis and a secondary systemic chemotherapy had even been placed on the agenda. The clinical presentation of the patient, however, seemed to be in healthy status without any symptoms or liver enzyme elevation. Specifically, acid-fast and periodic acid-Schiff-diastase staining was negative for atypical fungal and mycobacterial infection. The patient denied any preexisting granulomatous disease and a thorough evaluation of virus, mycobacterium tuberculosis, autoantibodies were all negative. Laboratory tests of aspartate aminotransferase (AST), alanine transaminase (ALT), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (AKP), bilirubin, and serum calcium were within normal range. Considering the paradox that remote metastasis occurs while primary tumors are still kept in good control, and the discrepancy between the radiology findings and clinical presentation, an ultrasound-guided biopsy of the liver nodule was conducted to ensure a correct diagnosis. Histopathological analysis revealed a noncaseating granulomatous pattern with a mixed cellular infiltrate of lymphocytes, macrophages, and fibrocytes, but no malignant cells (Figures 3A, B). Taken together, the patient was referred as a granulomatous disease induced by immunotherapy, and hepatic metastasis was ruled out. Given no other clinical features of multi-organ involvement, such as mediastinal lymph node enlargement, were observed in this case, a final diagnosis of SLR was made for this patient. As the patient was systemically in excellent health, he was continued with the immunotherapy and no immunosuppressive treatment towards the hepatic SLR was administered. After 8 cycles of toripalimab treatment, radiographic evaluations showed that the hepatic nodule had almost disappeared spontaneously (Figures 2D–F) and the primary malignancies were still in CR. Up until October 25, 2021, this patient had received 23 cycles of toripalimab treatment (4 cycles of PC chemotherapy along with toripalimab and 19 cycles of toripalimab alone maintenance immunotherapy), and he is well-tolerated in good health with sustained CR of the primary intrathoracic lesions.
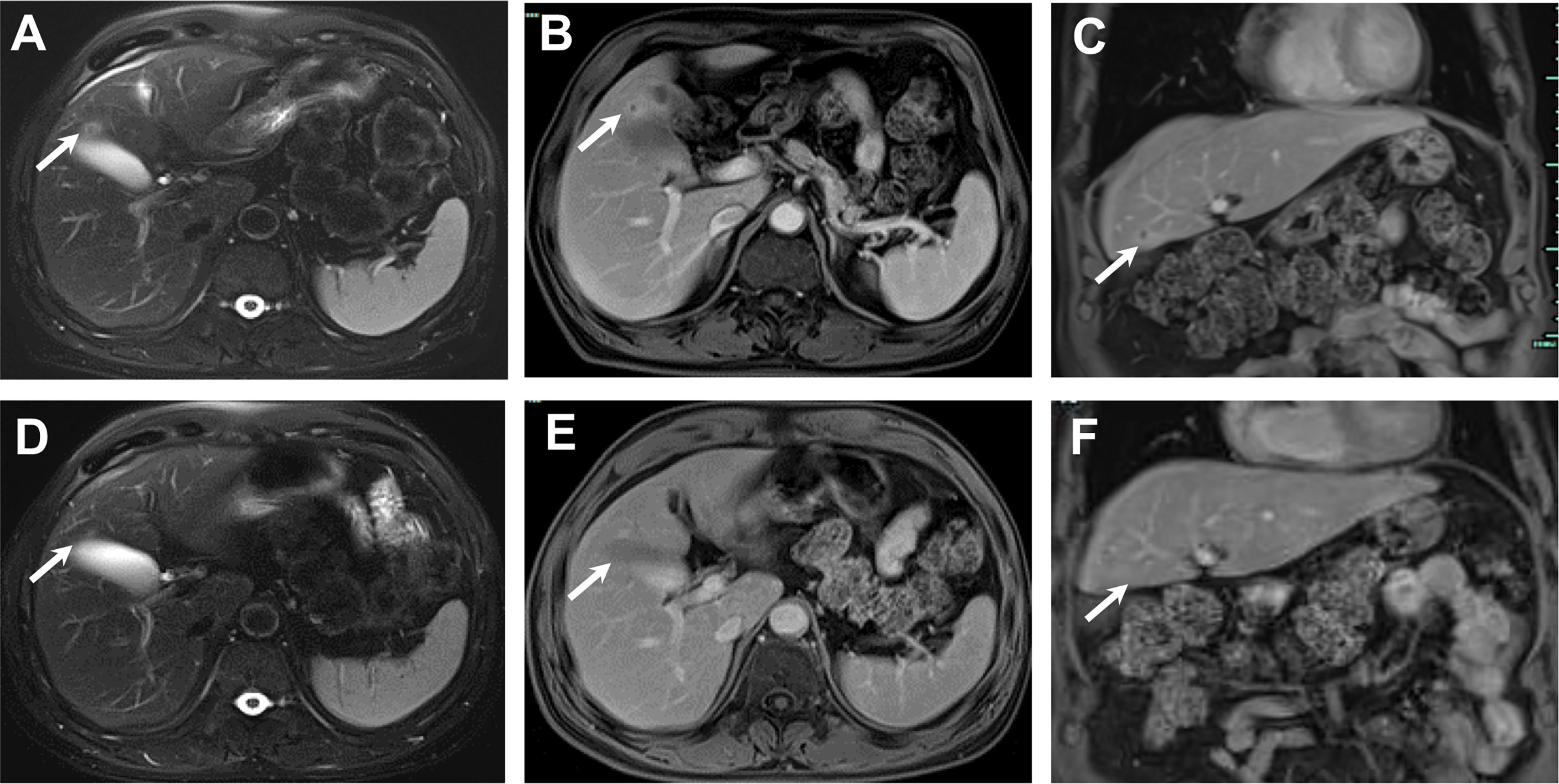
Figure 2 The MRI course of the SLR in liver. November 2020, 13 mm sized nodule in liver quadrate lobe showed circular hyperintensity on axial unenhanced T2-weighted image (A) and circular hyperenhancement in portal venous phase with axial image (B) and coronal image (C). January 2021, post 2 more doses of toripalimab, the nodule in liver quadrate lobe shrunk to 6 mm in diameter, axial unenhanced T2-weighted image (D) and axial enhanced T1-weighted image in portal venous phase with axial image (E) and coronal image (F).
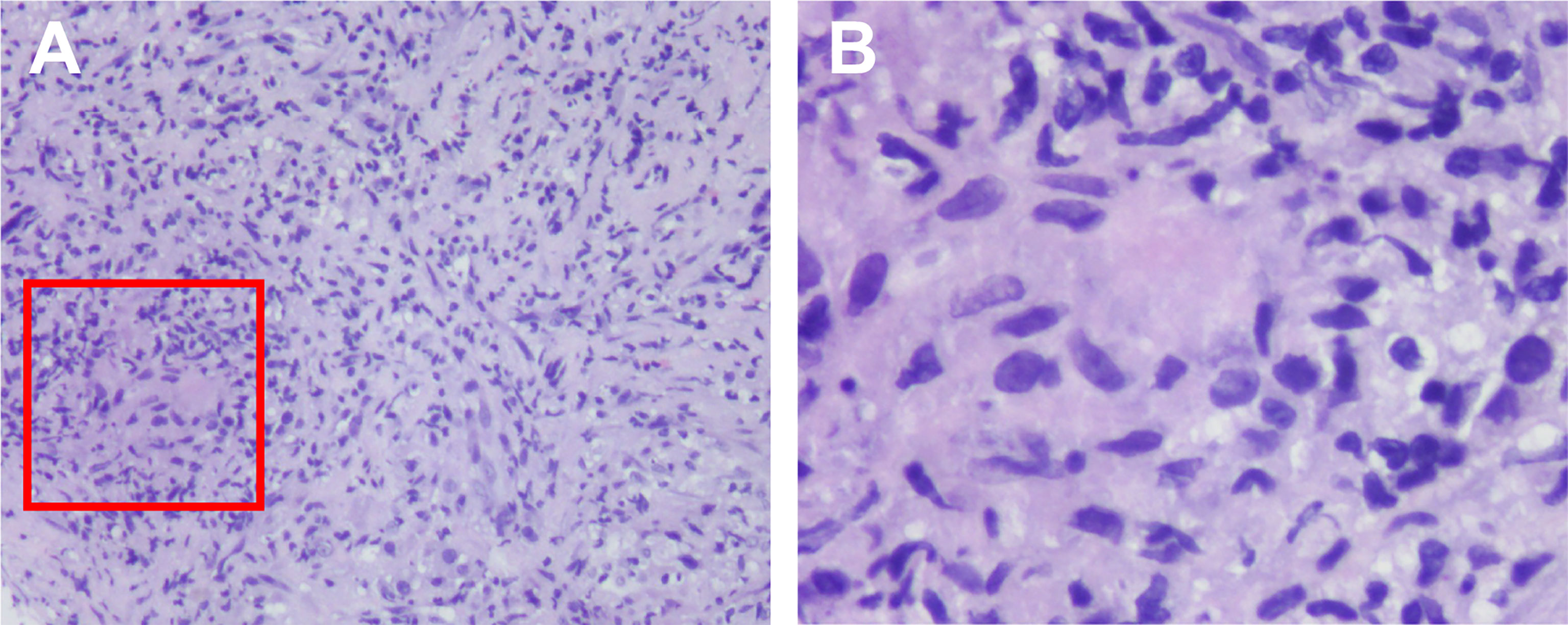
Figure 3 Histological findings of the SLR in liver. Histologic examination of the biopsy tissue showing noncaseating epithelioid granulomatous reaction (within the red box) by hematoxylin–eosin stain, with a mixed cellular infiltrate of lymphocytes, macrophages and fibrocytes. The magnification were ×10 (A) and ×40 (B).
Overview of the Clinical Features of Sarcoidosis and SLRs Associated to ICIs Treatment
SLRs refer to the atypical response patterns of localized non-caseating granulomatous inflammation without fulfilling the systemic sarcoidosis criteria. Unlike the typical sarcoidosis which is obscure in etiology, SLR is majorly described in the setting of active cancer or after the delivery of immunotherapy (14). It was reported that PD-1 or PD-L1 expression was increased both in sarcoidosis and in the peripheral blood upon PD-1 blockade treatment, which may contribute to the pathogenesis of SLR (15–17). Although the exact underlying mechanism remains elusive, a line of cell factors such as interleukin-2, interleukin-12, interferon-γ, and tumor-necrosis factor-α is thought to be involved in the formation of granulomatous inflammation (14, 18, 19). To better understand the clinical presentation of this rare irAE, we briefly summarize all the searchable studies reporting sarcoidosis and SLRs related to ICIs therapy published in the English literature up to February 2021. We identified a total of 53 cases developing sarcoidosis or SLRs, which were summarized in Table 1, with the content of demographic features, primary malignancy, ICI treatment, sites, onset and follow-up.
Demographic and Epidemiologic Features
Based on these records, the median age at the onset of SLRs was 60 years (range, 31–83 years), with a comparable male to female ratio (34/63, 54%). The most common primary tumor was melanoma (49/63, 78%), followed by NSCLC (7/63, 11%), and others. Most cases were documented with treatment of PD-1 inhibitors nivolumab (18/63, 29%) or pembrolizumab (22/63, 36%), followed by CTLA4 inhibitor ipilimumab (12/63, 19%), the combination of CTLA4 and PD-1 inhibitor therapy (7/63, 11%), and anti-PD-L1 antibodies atezolizumab/durvalumab (2/63, 3%). Toripalimab, however, has not been reported so far. A total of 52 cases (83%) occurred in the setting of palliative therapy for metastatic diseases, and 11 cases (18%) in neoadjuvant therapy. The onset time ranges from less than one month to 43 months after immunotherapy initiation, with a median interval of 3 months.
Clinical Phenotypes, Symptoms and Diagnosis
In regards with the clinical phenotypes, multi-system involvement occurred more frequently than single organ involvement (58.0% vs 42.0%). The thorax (intrapulmonary, hilar and mediastinal lesions) and skin (subcutaneous and cutaneous lesions) were two most commonly affected sites, with 84.1 and 42.9% respectively. Other organ involvement, such as extra-thoracic lymphadenopathy, spleen, central nervous system, eyes and bone were relatively rare.
As for the accompanied symptoms, subcutaneous and cutaneous reactions usually presented as cutaneous erythema, subcutaneous nodules with itching or pain. A few cases manifested with nonspecific flu-like symptoms such as arthralgia, myalgia, fatigue, fever, sweat, chill, nausea, and vomiting. When central neural system (CNS) was implicated, symptoms of headache, aphasia, visual field deficits, and seizure would develop. These symptoms were always in a mild to moderate extent. But life-threatening ones occasionally occurred with a relative higher incidence among patients with neural and intrapulmonary implications.
The diagnosis of sarcoidosis and SLRs is usually challenging, given a variety of cases were asymptomatic and the nodular lesion was only revealed by radiological evaluations. It is difficult to distinguish between metastatic and granulomatous lesions by both contrast enhanced CT and MRI (27). Neither can FDG-PET/CT, since FDG uptake is associated with enhanced cellular metabolism but irrespective of the nature of the cells. Therefore, it is predisposed to draw a false positive report for a malignancy (66), and differential diagnosis is crucial. Misdiagnosing SLRs as progressive diseases is apt to misdirect the course of cancer management. For example, in the case reported by Swathi B. Reddy, a melanoma patient developed mediastinal/hilar lymphadenopathy and several subcutaneous nodules after 8 cycles of ipilimumab monotherapy. Those newly occurred lesions were firstly referred as disease progression and secondary pembrolizumab was initiated. Her condition worsened dramatically after one dose of pembrolizumab with fever, dyspnea, nausea, vomiting, and transaminitis. A chest CT scan showed emerging bilateral pulmonary consolidations. A biopsy was taken and a histopathology examination revealed non-necrotizing granulomatous inflammation consistent with sarcoidosis. Subsequently, pembrolizumab was withheld, corticosteroid therapy was initiated and this patient generally recovered from this irAE (30). A similar scenario was also seen in other cases (37, 46). In particular, a special 68Ga-DOTA-NOC/TATE PET/CT, adopted by Anne-Leen Deleu and her colleagues, was reported to be capable of distinguishing atezolizumab-induced thoracic SLRs from malignancies in a triple-negative breast carcinoma patient (61). However, further exploration for diagnosis is absolutely warranted, and in this case, tissue biopsy is still the gold standard for diagnosis nowadays.
Treatments
The managements to this adverse side effect were quite personalized. Watchful waiting was chosen in 11 cases without any medical intervention because of limited organ affection and mild symptoms. Corticosteroids were the mainstay of immunosuppressive therapy when necessary. The cessation of immunotherapy and the admission of corticosteroids were adopted in 68% (23/40) and 49% (25/51) of cases, respectively. Appropriate corticosteroid treatments were efficient for symptom relief and may not attenuate anti-tumor efficacy of immunotherapy. Tropical, oral or intravenous corticosteroids were prescribed according to the manifestations, which worked well in most cases (51). In a few refractory cases, infliximab, methotrexate, mycophenolate, and hydroxychloroquine were also considered (15, 53, 54). Overall, ICI-induced sarcoidosis and SLRs were manageable with benign outcomes. Either resolution or improvement was noted in 89% (47/53) of reported cases, and the rest of them are stable. After good control of sarcoidosis and SLRs, the reintroduction of immunotherapy was also considered in some cases. Interestingly, it seems like patients with this kind of documented irAEs preferred better clinical benefits on immunotherapy. Clinical response rate was documented for 54% (25/41) of metastatic patients, followed by stable disease with 22% (9/41), while only 17% (7/41) of patients experienced tumor progression, highlighting the potential role of this reaction as a predictive parameter to ICI therapies (28, 47).
Discussion and Conclusion
Immunotherapy, especially the checkpoint inhibitor-based immunotherapy, has been a major breakthrough in the field of oncology for providing an efficacious and durable therapeutic option for patients with advanced-stage cancer. PD-1 antagonists selectively block the PD-1 receptor and attenuate its interaction with the ligands, PD-L1, and PD-L2 (67). Disruption of the PD-(L)1 pathway helps induce immune tolerance and reinvigorates the innate antitumor capabilities of the immune system by upregulating T-cell activation and proliferation (68, 69). While the triggered auto-immunity exerts potent anti-tumor activity, it opens the door to a novel class of irAEs as well. IrAEs may manifest in any organ system but most frequently in the lung, skin, gut, endocrine glands, and liver (12). In the past few years, sarcoidosis and SLRs have been increasingly reported as a kind of rare irAE secondary to ICI treatment. Herein, we report the first case of hepatic SLR mimicking malignant metastasis in a NSCLC patient treated with the anti-PD-1 antibody toripalimab.
Our case adds to the growing bodies of literature of the world about ICIs-induced SLRs for malignancies. To the best of our knowledge, isolated hepatic granulomatous involvement has never been previously reported. It is noteworthy that liver injury has actually been recognized as a complication of ICI treatment (12), which manifests as hepatitis with liver enzymes or bilirubin elevation. But most of these patients were mild in severity without any radiologic findings or histological features except hepatic injury (70). However, in our case, the patient was asymptomatic and his laboratory tests were normal, the only clinical manifestation is the SLR found in liver radiologically presented as a single low-attenuation nodular lesion with dynamic contrast enhancement. He was firstly referred as a disease progression with liver metastasis, which was then correctly diagnosed as SLR depended on the histopathological findings. This is also the first reported case of granuloma formation induced by toripalimab, which showed efficiency and safety profiles comparable with other anti-PD-1 antibodies (11). The low incidence may probably be due to the limited use of this agent only for clinical trial. Based on the PC chemotherapy regimens along with toripalimab, our patient exhibited favorable therapeutic response at the presence of SLR, which also supports the positive relationship between SLRs and favorable therapeutic outcome. Similar with many previous cases reported, the SLR developed in this patient was gradually improved spontaneously. However, it is worth noting that although toripalimab, as the first domestic anti-PD-1 monoclonal antibody developed in China, has received conditional approvals for the treatments of melanoma, nasopharyngeal carcinoma and urothelial carcinoma in China, it is still restrictedly applied for clinical trials only in NSCLC patients all over the world. Though most of these preclinical studies in NSCLC are ongoing in China, we anticipate that further more international multicentric studies of toripalimab may start to provide more solid evidences for supporting its clinical use in the future.
In summary, we report a first case displaying isolated hepatic SLR in an NSCLC patient treated with toripalimab, and then we perform a brief review about the clinical features of sarcoidosis and SLRs associated to ICI treatment. With the increasing use of ICIs, we will see more similar cases in the near future. Misinterpretation of sarcoidosis and SLRs as disease progression would cause unnecessary second-line cancer treatments. It deserves the attention of clinicians on this kind of rare irAE, and histopathological evaluation of suspicious lesions developing upon immunotherapy, particularly in the setting of mixed responses, where it is necessary and critical to make sure and correct clinical decisions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhejiang Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HYL and BCW had the idea for the article and provided the final approval of the version to be published. YXL performed the literature search, data analysis, and drafted the manuscript. WZ were involved in revising the manuscript critically for important scientific content. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (82102947 to HL), and the Zhejiang Provincial Natural Science Foundation of China (LQ22H160046 to HL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-Small-Cell Lung Cancer. Nat Rev Dis Primers (2015) 1:15009. doi: 10.1038/nrdp.2015.9
3. Zappa C, Mousa SA. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Trans Lung Cancer Res (2016) 5:288–300. doi: 10.21037/tlcr.2016.06.07
4. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
5. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet Oncol (2016) 17:1497–508. doi: 10.1016/S1470-2045(16)30498-3
6. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (London England) (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
7. Nomura S, Goto Y, Mizutani T, Kataoka T, Kawai S, Okuma Y, et al. A Randomized Phase III Study Comparing Continuation and Discontinuation of PD-1 Pathway Inhibitors for Patients With Advanced Non-Small-Cell Lung Cancer (JCOG1701, SAVE Study). Jpn J Clin Oncol (2020) 50:821–5. doi: 10.1093/jjco/hyaa054
8. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
9. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in Combination With Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared With Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
10. Shui L, Cheng K, Li X, Shui P, Li S, Peng Y, et al. Durable Response and Good Tolerance to the Triple Combination of Toripalimab, Gemcitabine, and Nab-Paclitaxel in a Patient With Metastatic Pancreatic Ductal Adenocarcinoma. Front Immunol (2020) 11:1127. doi: 10.3389/fimmu.2020.01127
11. Wang Z, Ying J, Xu J, Yuan P, Duan J, Bai H, et al. Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. JAMA Netw Open (2020) 3:e2013770. doi: 10.1001/jamanetworkopen.2020.13770
12. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (Oxford England: 1990) (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
13. Gkiozos I, Kopitopoulou A, Kalkanis A, Vamvakaris IN, Judson MA, Syrigos KN. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J Thorac Oncol (2018) 13:1076–82. doi: 10.1016/j.jtho.2018.04.031
14. Al-Dliw M, Megri M, Shahoub I, Sahay G, Limjoco TI, Shweihat Y. Pembrolizumab Reactivates Pulmonary Granulomatosis. Respir Med Case Rep (2017) 22:126–9. doi: 10.1016/j.rmcr.2017.07.010
15. Birnbaum MR, Ma MW, Fleisig S, Packer S, Amin BD, Jacobson M, et al. Nivolumab-Related Cutaneous Sarcoidosis in a Patient With Lung Adenocarcinoma. JAAD Case Rep (2017) 3:208–11. doi: 10.1016/j.jdcr.2017.02.015
16. Reuss JE, Kunk PR, Stowman AM, Gru AA, Slingluff CL Jr., Gaughan EM. Sarcoidosis in the Setting of Combination Ipilimumab and Nivolumab Immunotherapy: A Case Report & Review of the Literature. J Immunother Cancer (2016) 4:94. doi: 10.1186/s40425-016-0199-9
17. Paolini L, Poli C, Blanchard S, Urban T, Croué A, Rousselet MC, et al. Thoracic and Cutaneous Sarcoid-Like Reaction Associated With Anti-PD-1 Therapy: Longitudinal Monitoring of PD-1 and PD-L1 Expression After Stopping Treatment. J Immunother Cancer (2018) 6:52. doi: 10.1186/s40425-018-0372-4
18. Frohlich M, Wang H, Sakr L. Sarcoid-Like Reaction Discovered on EBUS-TBNA of Intrathoracic Lymph Nodes During Immunotherapy for Metastatic Melanoma. J Immunother (Hagerstown Md: 1997) (2020) 43:75–8. doi: 10.1097/CJI.0000000000000298
19. Rambhia PH, Reichert B, Scott JF, Feneran AN, Kazakov JA, Honda K, et al. Immune Checkpoint Inhibitor-Induced Sarcoidosis-Like Granulomas. Int J Clin Oncol (2019) 24:1171–81. doi: 10.1007/s10147-019-01490-2
20. Andersen R, Nørgaard P, Al-Jailawi MK, Svane IM. Late Development of Splenic Sarcoidosis-Like Lesions in a Patient With Metastatic Melanoma and Long-Lasting Clinical Response to Ipilimumab. Oncoimmunology (2014) 3:e954506. doi: 10.4161/21624011.2014.954506
21. Murphy KP, Kennedy MP, Barry JE, O’Regan KN, Power DG. New-Onset Mediastinal and Central Nervous System Sarcoidosis in a Patient With Metastatic Melanoma Undergoing CTLA4 Monoclonal Antibody Treatment. Oncol Res Treat (2014) 37:351–3. doi: 10.1159/000362614
22. Martínez Leboráns L, Esteve Martínez A, Victoria Martínez AM, Alegre de Miquel V, Berrocal Jaime A. Cutaneous Sarcoidosis in a Melanoma Patient Under Ipilimumab Therapy. Dermatol Ther (2016) 29:306–8. doi: 10.1111/dth.12380
23. Wilgenhof S, Morlion V, Seghers AC, Du Four S, Vanderlinden E, Hanon S, et al. Sarcoidosis in a Patient With Metastatic Melanoma Sequentially Treated With Anti-CTLA-4 Monoclonal Antibody and Selective BRAF Inhibitor. Anticancer Res (2012) 32:1355–9.
24. Reule RB, North JP. Cutaneous and Pulmonary Sarcoidosis-Like Reaction Associated With Ipilimumab. J Am Acad Dermatol (2013) 69:e272–3. doi: 10.1016/j.jaad.2013.07.028
25. Rodriguez EF, Lipson E, Suresh K, Cappelli LC, Monaco SE, Maleki Z. Immune Checkpoint Blocker-Related Sarcoid-Like Granulomatous Inflammation: A Rare Adverse Event Detected in Lymph Node Aspiration Cytology of Patients Treated for Advanced Malignant Melanoma. Hum Pathol (2019) 91:69–76. doi: 10.1016/j.humpath.2019.07.001
26. Vogel WV, Guislain A, Kvistborg P, Schumacher TN, Haanen JB, Blank CU. Ipilimumab-Induced Sarcoidosis in a Patient With Metastatic Melanoma Undergoing Complete Remission. J Clin Oncol: Off J Am Soc Clin Oncol (2012) 30:e7–e10. doi: 10.1200/JCO.2011.37.9693
27. Garanzini EM, Scaramuzza D, Spadarella G, Di Guardo L, Marchianò A. Sarcoidosis-Like Disease Mimicking Metastases During Adjuvant Ipilimumab Therapy in Advanced Melanoma Patient: CT Scan and MRI Help in Managing Difficult Clinical Decision. BJR Case Rep (2020) 6:20190065. doi: 10.1259/bjrcr.20190065
28. Tetzlaff MT, Nelson KC, Diab A, Staerkel GA, Nagarajan P, Torres-Cabala CA, et al. Granulomatous/sarcoid-Like Lesions Associated With Checkpoint Inhibitors: A Marker of Therapy Response in a Subset of Melanoma Patients. J Immunother Cancer (2018) 6:14. doi: 10.1186/s40425-018-0323-0
29. Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-Like Syndrome and Lymphadenopathy Due to Checkpoint Inhibitors. J Oncol Pharm Pract: Off Publ Int Soc Oncol Pharm Pract (2017) 23:620–4. doi: 10.1177/1078155216667635
30. Reddy SB, Possick JD, Kluger HM, Galan A, Han D. Sarcoidosis Following Anti-PD-1 and Anti-CTLA-4 Therapy for Metastatic Melanoma. J Immunother (Hagerstown Md: 1997) (2017) 40:307–11. doi: 10.1097/CJI.0000000000000181
31. Cheshire SC, Board RE, Lewis AR, Gudur LD, Dobson MJ. Pembrolizumab-Induced Sarcoid-Like Reactions During Treatment of Metastatic Melanoma. Radiology (2018) 289:564–7. doi: 10.1148/radiol.2018180572
32. van Willigen WW, Gerritsen WR, Aarntzen E. 18f-FDG PET/CT of Multiorgan Sarcoid-Like Reaction During Anti-PD-1 Treatment for Melanoma. Clin Nucl Med (2019) 44:905–6. doi: 10.1097/RLU.0000000000002779
33. McKenna MC, Molloy K, Crowther S, Feeney J, Gillis A, Connolly M, et al. Pembrolizumab-Related Sarcoid-Like Reaction Presenting as Reactivation of Quiescent Scars. J Oncol Pract (2018) 14:200–1. doi: 10.1200/JOP.2017.027383
34. Jespersen H, Bjursten S, Ny L, Levin M. Checkpoint Inhibitor-Induced Sarcoid Reaction Mimicking Bone Metastases. Lancet Oncol (2018) 19:e327. doi: 10.1016/S1470-2045(18)30252-3
35. Keukeleire S, Schwarze J, Awada G, Everaert H, Van Binst AM, Cras L, et al. An Atypical Sarcoid-Like Reaction During Anti-Protein Death 1 Treatment in a Patient With Metastatic Melanoma. Melanoma Res (2020) 30:524–7. doi: 10.1097/CMR.0000000000000680
36. Woodbeck R, Metelitsa AI, Naert KA. Granulomatous Tumoral Melanosis Associated With Pembrolizumab Therapy: A Mimicker of Disease Progression in Metastatic Melanoma. Am J Dermatopathol (2018) 40:523–6. doi: 10.1097/DAD.0000000000001066
37. Lomax AJ, McGuire HM, McNeil C, Choi CJ, Hersey P, Karikios D, et al. Immunotherapy-Induced Sarcoidosis in Patients With Melanoma Treated With PD-1 Checkpoint Inhibitors: Case Series and Immunophenotypic Analysis. Int J Rheum Dis (2017) 20:1277–85. doi: 10.1111/1756-185X.13076
38. Marcoval J, Bauer-Alonso A, Fornons-Servent R, Jiménez-Colomo L, Sabaté-Llobera A, Penín RM. Subcutaneous Sarcoidosis Induced by Pembrolizumab in a Melanoma Patient Mimicking Subcutaneous Metastasis at 18F-FDG PET/Ct. Rev Esp Med Nucl Imagen Mol (2020) 40(4):255–6. doi: 10.1016/j.remnie.2020.09.004
39. Yatim N, Mateus C, Charles P. Sarcoidosis Post-Anti-PD-1 Therapy, Mimicking Relapse of Metastatic Melanoma in a Patient Undergoing Complete Remission. La Rev Med Interne (2018) 39:130–3. doi: 10.1016/j.revmed.2017.11.008
40. Lise Q-K, Audrey A-G. Multifocal Choroiditis as the First Sign of Systemic Sarcoidosis Associated With Pembrolizumab. Am J Ophthalmol Case Rep (2016) 5:92–3. doi: 10.1016/j.ajoc.2016.12.014
41. Lu Y. FDG PET/CT Course of Pembrolizumab-Associated Multiorgan Sarcoidosis. Clin Nucl Med (2019) 44:167–8. doi: 10.1097/RLU.0000000000002408
42. Wang LL, Patel G, Chiesa-Fuxench ZC, McGettigan S, Schuchter L, Mitchell TC, et al. Timing of Onset of Adverse Cutaneous Reactions Associated With Programmed Cell Death Protein 1 Inhibitor Therapy. JAMA Dermatol (2018) 154:1057–61. doi: 10.1001/jamadermatol.2018.1912
43. Ogawa T, Ishitsuka Y, Iwamoto K, Koguchi-Yoshioka H, Tanaka R, Watanabe R, et al. Programmed Cell Death 1 Blockade-Induced Cutaneous Sarcoid-Like Epithelioid Granulomas in Advanced Melanoma: A Case Report. J Eur Acad Dermatol Venereol (2018) 32:e260–1. doi: 10.1111/jdv.14781
44. Laroche A, Alarcon Chinchilla E, Bourgeault E, Doré MA. Erythema Nodosum as the Initial Presentation of Nivolumab-Induced Sarcoidosis-Like Reaction. J Cutan Med Surg (2018) 22:627–9. doi: 10.1177/1203475418776934
45. Danlos FX, Pagès C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, et al. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest (2016) 149:e133–6. doi: 10.1016/j.chest.2015.10.082
46. Fukuchi K, Hikawa M, Sano Y, Kasuya A, Aoshima M, Tatsuno K, et al. Sarcoid-Like Reaction and Vitiligo Occurring After Nivolumab Therapy in a Patient With Metastatic Melanoma. J Dermatol (2019) 46:e359–60. doi: 10.1111/1346-8138.14887
47. Hiraki T, Hatanaka M, Arimura A, Kawahira H, Kirishima M, Kitazono I, et al. Granulomatous/sarcoid-Like Reactions in the Setting of Programmed Cell Death-1 Inhibition: A Potential Mimic of Disease Recurrence. J Cutan Pathol (2020) 47:154–60. doi: 10.1111/cup.13569
48. Ung C, Gragoudas E. Checkpoint Inhibitor-Induced Sarcoid Choroidal Granulomas. Am J Ophthalmol Case Rep (2020) 18:100652. doi: 10.1016/j.ajoc.2020.100652
49. Montaudié H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary Sarcoid-Like Granulomatosis Induced by Nivolumab. Br J Dermatol (2017) 176:1060–3. doi: 10.1111/bjd.14808
50. Tulbah RI, Rowe SP, Solnes LB, Javadi MS. Nivolumab-Associated Pulmonary and Bone Sarcoidosis in a Patient With Melanoma of Unknown Primary. Clin Nucl Med (2019) 44:e519–21. doi: 10.1097/RLU.0000000000002724
51. Urrego-Callejas T, Sandoval-Álvarez S, Gómez-Wolff R, Vásquez G. Cutaneous and Pulmonary Sarcoid-Like Reaction Induced by Nivolumab: Case Report and Brief Literature Review. J Clin Rheumatol: Pract Rep Rheum Musculoskeletal Dis (2019) 27(8S):S460–64. doi: 10.1097/RHU.0000000000001227
52. Apalla Z, Kemanetzi C, Papageorgiou C, Bobos M, Manoli M, Fotiadou C, et al. Challenges in Sarcoidosis and Sarcoid-Like Reactions Associated to Immune Checkpoint Inhibitors: A Narrative Review Apropos of a Case. Dermatol Ther (2021) 34(1):e14618. doi: 10.1111/dth.14618
53. Dunn-Pirio AM, Shah S, Eckstein C. Neurosarcoidosis Following Immune Checkpoint Inhibition. Case Rep Oncol (2018) 11:521–6. doi: 10.1159/000491599
54. Tan I, Malinzak M, Salama AKS. Delayed Onset of Neurosarcoidosis After Concurrent Ipilimumab/Nivolumab Therapy. J Immunother Cancer (2018) 6:77. doi: 10.1186/s40425-018-0390-2
55. Fakhri G, Akel R, Salem Z, Tawil A, Tfayli A. Pulmonary Sarcoidosis Activation Following Neoadjuvant Pembrolizumab Plus Chemotherapy Combination Therapy in a Patient With Non-Small Cell Lung Cancer: A Case Report. Case Rep Oncol (2017) 10:1070–5. doi: 10.1159/000484596
56. Grosse A, Grosse C. Diagnostic Yield of Broncho-Alveolar Lavage for Pembrolizumab Induced Sarcoid-Like Reaction of the Lung. Cytopathol: Off J Br Soc Clin Cytol (2019) 30:686–7. doi: 10.1111/cyt.12740
57. Lainez S, Tissot C, Cottier M, Vergnon JM. EBUS-TBNA Can Distinguish Sarcoid-Like Side Effect of Nivolumab Treatment From Tumor Progression in Non-Small Cell Lung Cancer. Respiration Int Rev Thorac Dis (2017) 94:518–21. doi: 10.1159/000480155
58. Beer L, Hochmair M, Kifjak D, Haug AR, Prayer F, Mayerhoefer ME, et al. Particular Findings on Lung CT in Patients Undergoing Immunotherapy for Bronchogenic Carcinoma. Wiener Klin Wochenschr (2020) 132:467–74. doi: 10.1007/s00508-020-01667-0
59. Noguchi S, Kawachi H, Yoshida H, Fukao A, Terashita S, Ikeue T, et al. Sarcoid-Like Granulomatosis Induced by Nivolumab Treatment in a Lung Cancer Patient. Case Rep Oncol (2018) 11:562–6. doi: 10.1159/000492383
60. Sanderson E, Wimaleswaran H, Senko C, White S, McDonald CF. Durvalumab Induced Sarcoid-Like Pulmonary Lymphadenopathy. Respirol Case Rep (2020) 8:e00542. doi: 10.1002/rcr2.542
61. Deleu AL, Hanssens M, Maes A, Van de Wiele C. 68ga-DOTA-TATE PET/CT Imaging for Differentiating a Sarcoid-Like Reaction From Progression Following Immunotherapy in a Triple-Negative Breast Carcinoma Patient. Eur J Nucl Med Mol Imaging (2021) 48(3):945–6. doi: 10.1007/s00259-020-04993-7
62. Lafon M, Blaye C, Kind M, Bechade D, Chassaigne F, Italiano A, et al. Sarcoidosis-Like Reaction in Metastatic Triple Negative Breast Cancer Treated by Anti-PD-L1. Breast J (2019) 25:971–3. doi: 10.1111/tbj.13386
63. Kim C, Gao J, Shannon VR, Siefker-Radtke A. Systemic Sarcoidosis First Manifesting in a Tattoo in the Setting of Immune Checkpoint Inhibition. BMJ Case Rep (2016) 1757–790X. doi: 10.1136/bcr-2016-216217
64. Zhang M, Schembri G. Nivolumab-Induced Development of Pulmonary Sarcoidosis in Renal Cell Carcinoma. Clin Nucl Med (2017) 42:728–9. doi: 10.1097/RLU.0000000000001758
65. Cousin S, Toulmonde M, Kind M, Cazeau AL, Bechade D, Coindre JM, et al. Pulmonary Sarcoidosis Induced by the Anti-PD1 Monoclonal Antibody Pembrolizumab. Ann Oncol: Off J Eur Soc Med Oncol (2016) 27:1178–9. doi: 10.1093/annonc/mdw125
66. Willis H, Heilbrun M, Dechet C. Co-Existing Sarcoidosis Confounds the Staging of Bilateral Renal Cell Carcinoma. J Radiol Case Rep (2011) 5:18–27. doi: 10.3941/jrcr.v5i1.553
67. Xu J, Sun HH, Fletcher CD, Hornick JL, Morgan EA, Freeman GJ, et al. Expression of Programmed Cell Death 1 Ligands (PD-L1 and PD-L2) in Histiocytic and Dendritic Cell Disorders. Am J Surg Pathol (2016) 40:443–53. doi: 10.1097/PAS.0000000000000590
68. Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatol (Baltimore Md) (2020) 72:315–29. doi: 10.1002/hep.31227
69. Suozzi KC, Stahl M, Ko CJ, Chiang A, Gettinger SN, Siegel MD, et al. Immune-Related Sarcoidosis Observed in Combination Ipilimumab and Nivolumab Therapy. JAAD Case Rep (2016) 2:264–8. doi: 10.1016/j.jdcr.2016.05.002
Keywords: Sarcoid-like reaction, immune checkpoint inhibitor, Toripalimab, PD-1, case report
Citation: Lin Y, Zhu W, Wu B and Lan H (2022) Case Report: Hepatic Sarcoid-Like Reaction Associated With Checkpoint Inhibition in a NSCLC Patient and a Literature Review. Front. Oncol. 12:824308. doi: 10.3389/fonc.2022.824308
Received: 29 November 2021; Accepted: 15 February 2022;
Published: 10 March 2022.
Edited by:
Cyril Corbet, Fonds National de la Recherche Scientifique (FNRS), BelgiumReviewed by:
Roberta Poli, San Luigi Gonzaga University Hospital, ItalyJii Bum Lee, Yonsei University College of Medicine, South Korea
Copyright © 2022 Lin, Zhu, Wu and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiyin Lan, bGFuaHVpeWluQHpqdS5lZHUuY24=; Bingchen Wu, d3ViYzA1NzJAMTYzLmNvbQ==
 Yuxin Lin1
Yuxin Lin1 Huiyin Lan
Huiyin Lan