
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 April 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.822983
This article is part of the Research Topic Advances and Novel Technologies in Surgical Instruments for the Treatment of Cancer View all 19 articles
Background and Objectives: After diagnosing a primary bone tumor involving the forearm, various excision strategies and reconstruction methods must be considered. This study explored the oncological and functional outcomes of limb salvage surgery for primary malignant bone tumors in the forearm.
Methods: Patients with primary forearm bone tumors (n = 369) were retrospectively analyzed between 2000 and 2017. There were 266 patients with radial tumors, and 46 (17.3%) were malignant, whereas 103 patients had ulnar lesions and 22 (21.4%) were malignant tumors. The oncological results, prognostic factors, and functional results after limb salvage surgery of forearm malignancies were analyzed.
Results: The follow-up averaged 72.1 (7–192, median 62.5) months. Fifty-six patients who received limb salvage surgery were included in the final evaluation. Radius resection was performed in 38 patients, and distal radius (25 patients) was most frequent. Ulnar resection was performed in 18 patients, and the proximal ulna (13 patients) was most frequent. The surgical margins obtained were intralesional in 3 patients, marginal in 8 patients and wide in 45 patients. Local recurrence occurred in 11 patients (19.6%), and distant metastasis occurred in 14 patients (25%). The 5-year recurrence-free survival rate was 79.8%. Unplanned excision, ulnar involvement, proximal forearm location and inadequate surgical margins were associated with recurrence. The overall 5-year and 10-year survival rates were 83.5 and 71.7%, respectively. Distant metastasis was a poor prognostic factor for the survival rate. Forty-two patients were evaluated by MSTS score with an average of 27.9 ± 1.5.
Conclusions: The incidence of radial malignant tumors is higher than that of ulnar lesions. The distal radius and the proximal ulna are the most frequently involved sites. Unplanned excisions, ulnar tumors, proximal forearm tumors, and inadequate surgical margin are the risk factors for local recurrence. Distant metastasis is an independent poor prognostic factor of death. The oncology control and functional results of limb salvage surgery were satisfactory.
Primary bone tumors arising from the ulna and radius are rare compared with soft tissue tumors (1). Benign bone tumors accounted for most of the forearm tumors. Therefore, according to the general definition a disease is considered rare when it affects fewer than 1 in 2,000 people (2), the location of forearm accounted for 1–2% in all primary malignant bone tumors and surgical treatment is more challenging (3). Many tendons in the forearm are responsible for fine movement of the hand, and tumors often involve essential structures in this narrow space. As a result, the hand function will be significantly reduced after wide resection of the tumors.
Muramatsu (4) suggested the key for local control with forearm tumors was the safe surgical margin. A surgical margin of 5 cm in other sites is easily achieved, but it is challenging in the forearm. The reconstruction following tumor resection is also controversial, with three main problems: (1) some sarcomas are difficult to remove safely; (2) the defects and methods of reconstruction are varied, requiring individual design, and (3) the oncological evaluation and functional assessment need long-term follow up. How could we draw the appropriate surgical treatment strategies, it is urgently necessary to accumulate evidence-based evidence for these rare tumors.
This study included forearm primary malignant bone tumors to clarify (1) the epidemiological characteristics of primary malignant bone tumors in the forearm; (2) the oncological results and related risk factors; and (3) reconstruction methods and functional results after tumor resection.
With institutional review board (I.R.B.) approval, all patients in this study underwent limb salvage surgery for primary sarcoma of the forearm. Inclusion criteria were (1) primary malignant tumor of radius/ulna; (2) limb salvage surgery with resection of the tumor; (3) complete imaging (X-ray, CT, and MRI) and clinical data; (4) oncology results and complications can be evaluated; (5) follow-up time was more than 12 months, or oncological events (local recurrence, distant metastasis, or death) occurred within 12 months. The exclusion criteria were (1) bone defect and reconstruction were not involved; (2) amputation; (3) no surgical treatment or rejection of treatment; (4) incomplete imaging and follow-up data.
Patients with primary bone tumors (n = 369) of the forearm at the Beijing Jishuitan Hospital were analyzed retrospectively. There were 266 radial tumors, and 46 patients (17.3%) had malignant lesions. Forty of these 46 patients underwent limb salvage surgery and were thus eligible for inclusion in this study. There were 103 ulnar tumors, and 22 patients (21.4%) had malignant lesions. Twenty of these 22 patients underwent limb salvage surgery and were thus eligible for inclusion. Fifty-six of these 60 eligible patients followed up for more than 12 months and enrolled in the final study (Figure 1).
The local evaluation included X-ray, CT, and MRI of the forearm in all patients. Staging evaluation included chest CT and bone scans. A preoperative biopsy was performed for tumors suspected of malignancy. The surgical strategy for tumor resection was based on preoperative imaging. Preoperative chemotherapy was recommended for patients younger than 55 y with high-grade sarcoma involvement.
The collected data included
1. Surgical procedure: All these surgical strategies were decided by the Jishuitan sarcoma multidisciplinary team with the same theory and techniques, and all the surgeons were all in our musculoskeletal tumor team. Margin was defined as follows: Intralesional: Piecemeal debulking or curettage, which may leave macroscopic disease; Marginal: Shell out en bloc through pseudocapsule or reactive zone, which may leave either “satellite” or “skip” lesions; Wide: Intracompartmental en bloc with a cuff of normal tissue, which may leave “skip” lesions, Radical: Extracompartmental en bloc entire compartment with no tumor residual (5). We elaborate on the location of the lesion in the long bone, the proportion of resection in the whole bone and the reconstructive method recorded.
High-grade malignant bone tumors contained osteosarcoma, Ewing’s sarcoma, and undifferentiated pleomorphic sarcoma received preoperative chemotherapy, which facilitates to protect the vascular nerve tract, reduced reaction zone, and is conducive to limb salvage procedure. Otherwise, limb salvage will not be performed if the response to chemotherapy is poor or if blood vessels are involved.
2. Oncological concerns: local relapse and recurrence-free interval, distant metastasis, and death were noted and documented in this study.
3. Functional parameters: the complications and MSTS (musculoskeletal tumor society) scores (6) were included in the final evaluation.
Follow-up time was calculated from the date of operation to the last follow-up or death date. Comparison between subgroups was made using chi-square and t-tests. Wilcoxon method was used for correlation comparison of abnormal distribution grade data, with Mann–Whitney for independent samples. Local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS) were calculated using the Kaplan–Meier method. Univariate analysis for prognostic factors was performed using the log-rank test. Multivariate analyses of factors predicting outcome were performed using Cox regression. A P-value of 0.05 or less for two-sided comparisons was considered statistically significant. All analyses were carried out using the SPSS 21.0 software package (IBM, USA).
There were forty-six patients with radial malignant tumors, accounting for 17.3% of 266 total radial tumors, and twenty-two patients with ulnar malignant tumors accounting for 21.4% of 103 total ulnar tumors.
Of the 46 patients with primary malignant bone tumor of the radius, limb salvage surgery was performed in 40 patients and amputation in 6 patients. In 22 patients with malignancy of ulna, limb salvage surgery was performed in 20 patients and amputation in 2 patients. Fifty-six patients followed up for more than 12 months, or progression within 12 months were included in the final evaluation (Table 1). There were 34 men (60.7%) and 22 women (39.3%) with a mean age of 27.8 (5–73, median 20.0) years. The follow-up averaged 72.1 (7–192, median 62.5) months.
Based on the pathological diagnosis, osteosarcoma was reported in 17 patients (30.4%), Ewing’s sarcoma in 10 patients (17.9%), undifferentiated pleomorphic sarcoma in 7 patients (12.5%), low-grade central osteosarcoma in 6 patients (10.7%), chondrosarcoma in 6 patients (10.7%), bone angiosarcoma in 2 patients (3.6%), epithelioid sarcoma in 2 patients (3.6%), parosteal osteosarcoma, low-grade mixed tumor, low-grade myofibroblastic sarcoma, malignant giant cell tumor of bone, spindle cell sarcoma and clear cell sarcoma in 1 patient (1.8%), respectively. There were 17 cases (30.4%) of low-grade sarcoma and 39 cases (69.6%) of high-grade sarcoma based on histology (7, 8).
Of 56 limb salvage procedures in this study, 15 patients (15/56, 26.8%) were recurrent cases following unplanned excision (UE) in another hospital and were referred to our center with reoperation (Group 1), meanwhile, 41 patients (41/56, 73.2%) underwent initial surgery in our hospital (Group 2). In all patients of this study, local recurrence eventually occurred in 11 patients (11/56, 19.6%; see Table 2) at the end of follow-up after our surgery. Six patients in Group 1 (6/15, 40%) had recurrences after re-operation done at other hospitals. This is higher than the recurrence rate if the initial surgery was performed in our center (Group 2) (5/41, 12.2%) (P = 0.02) (Figure 2). The median recurrence-free time for these 11 recurrent cases was 12 (2–38) months, and 90% of the recurrences occurred within three years (10/11). There were 4 cases who eventually had to undergo amputations in these 11 recurrent cases (4/11, 36.4%). The local resection was performed in 7 cases (63.6%), and one case had a second recurrence. The 3-year and 5-year recurrence-free survival rates were 81.9 and 79.8%, respectively. The recurrence rate with inadequate (marginal or intralesional) margins was significantly higher than adequate (wide) resections. Univariate analysis (Table 3) shows the history of UE (P = 0.015), ulnar tumor (P = 0.016), tumor located in the proximal forearm (P = 0.021), and inadequate surgical margin (P <0.001) were associated with recurrence (Figure 3).
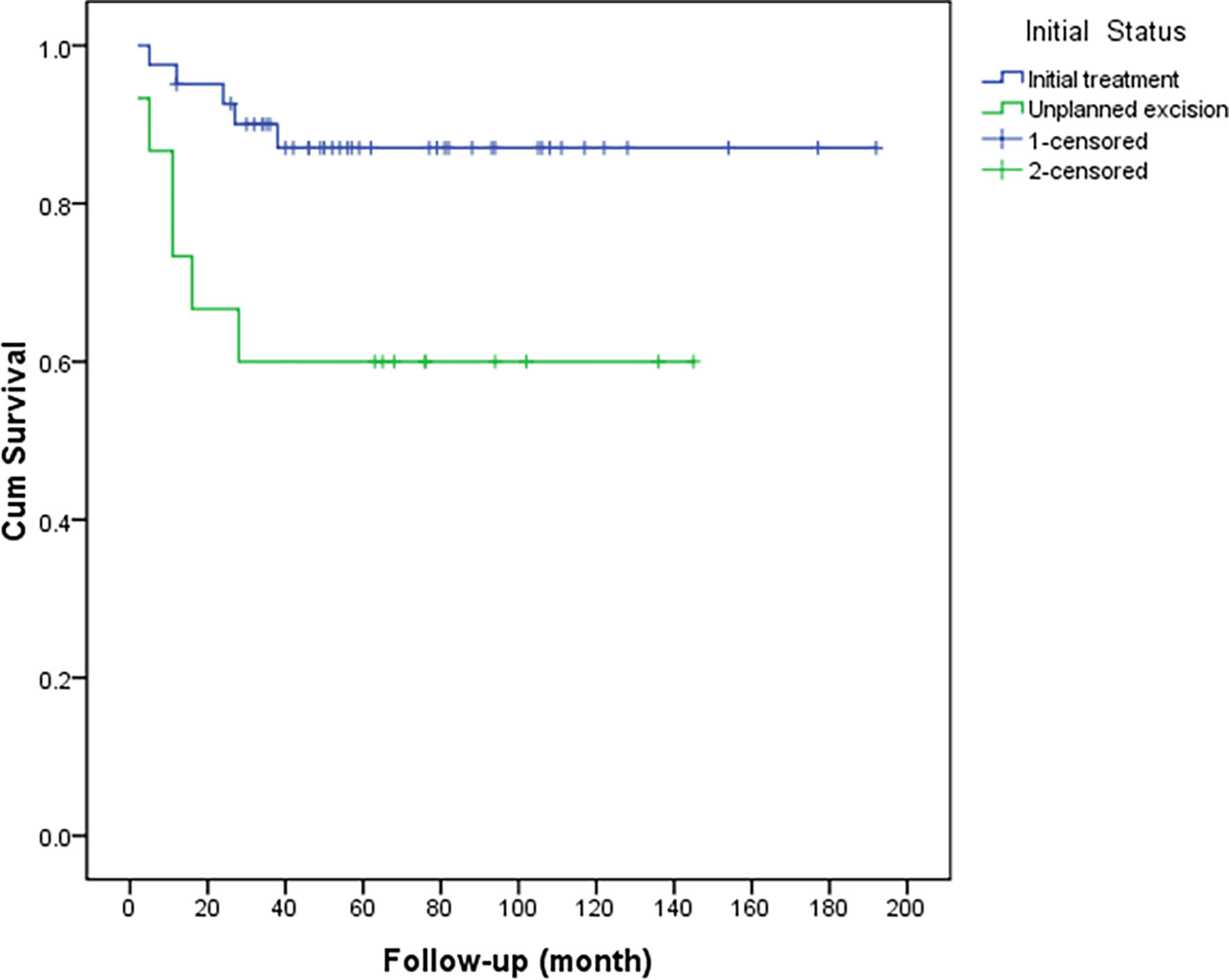
Figure 2 Comparison of recurrence-free survival between patients with recurrence after unplanned excision and those with initial treatment (P = 0.015).
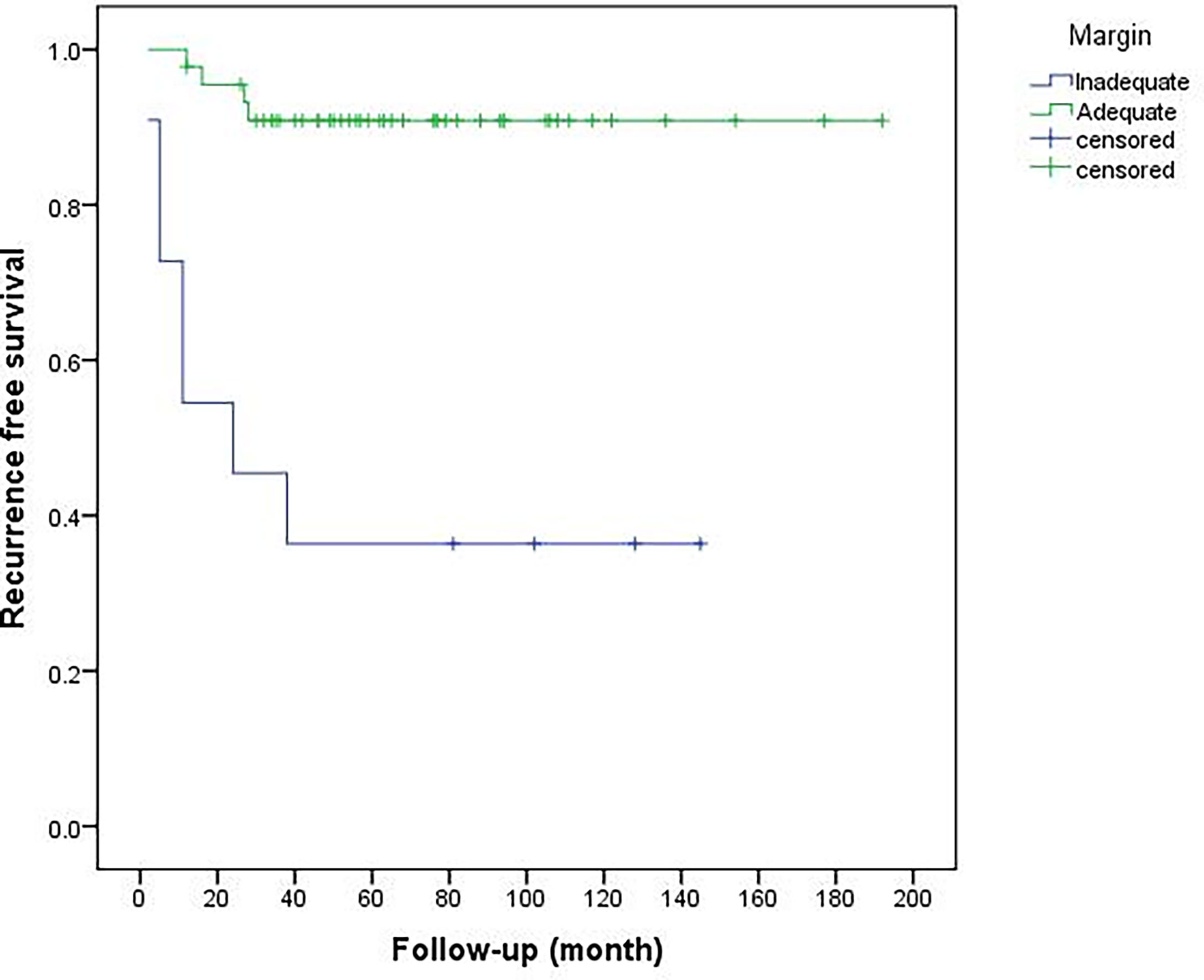
Figure 3 Comparison of recurrence-free survival between inadequate and adequate surgical margins (P < 0.001).
The bone defects after radial tumor resection were divided into proximal 1/3, distal 1/3, and more than 1/3 defect. The proximal 1/3 defect did not receive reconstruction. The distal 1/3 defect received an autogenous iliac bone graft and wrist joint fusion with internal fixation (Figure 4). The more than 1/3 defect from distal to proximal radius received the following procedures: (1) ulna osteotomy and fixation with the end of radius, (2) ulna centralization and wrist arthrodesis with internal fixation (Figure 5), (3) long segment fibula autograft and fixation (less than 1/2 defect), and (4) ipsilateral ulnar osteotomy to replace the radial defect (Figure 6)
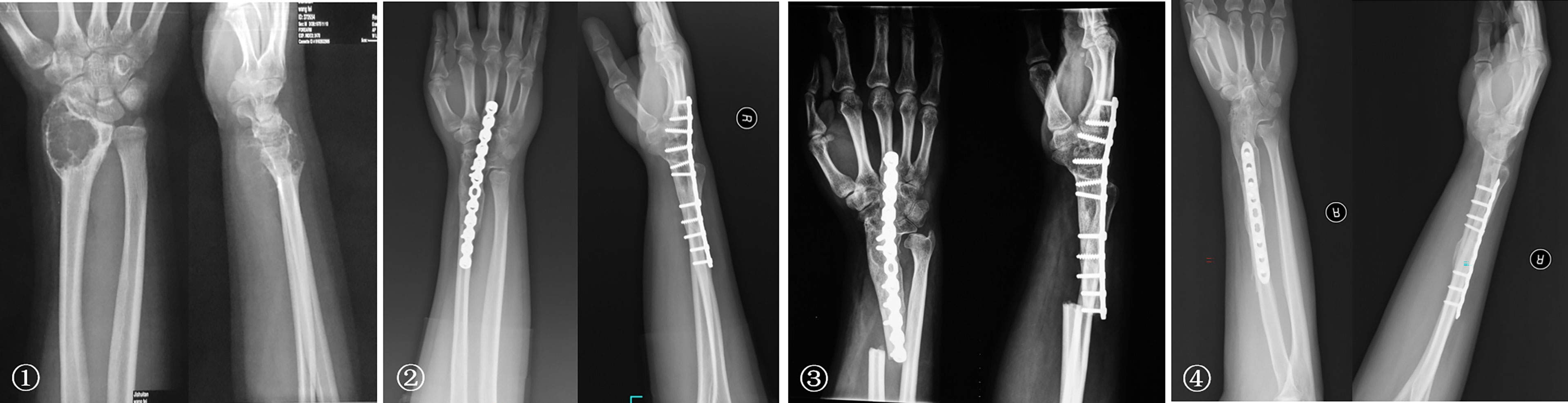
Figure 4 ① The preoperative radiographs of a 29-year-old man with chondrosarcoma. ② Treatment included a distal radius resection and autogenous iliac bone graft with wrist joint fusion. ③ Fracture was caused by trauma eight years after surgery, and internal fixation was performed again. ④ Rotation function of the forearm is shown 192 months postoperatively.
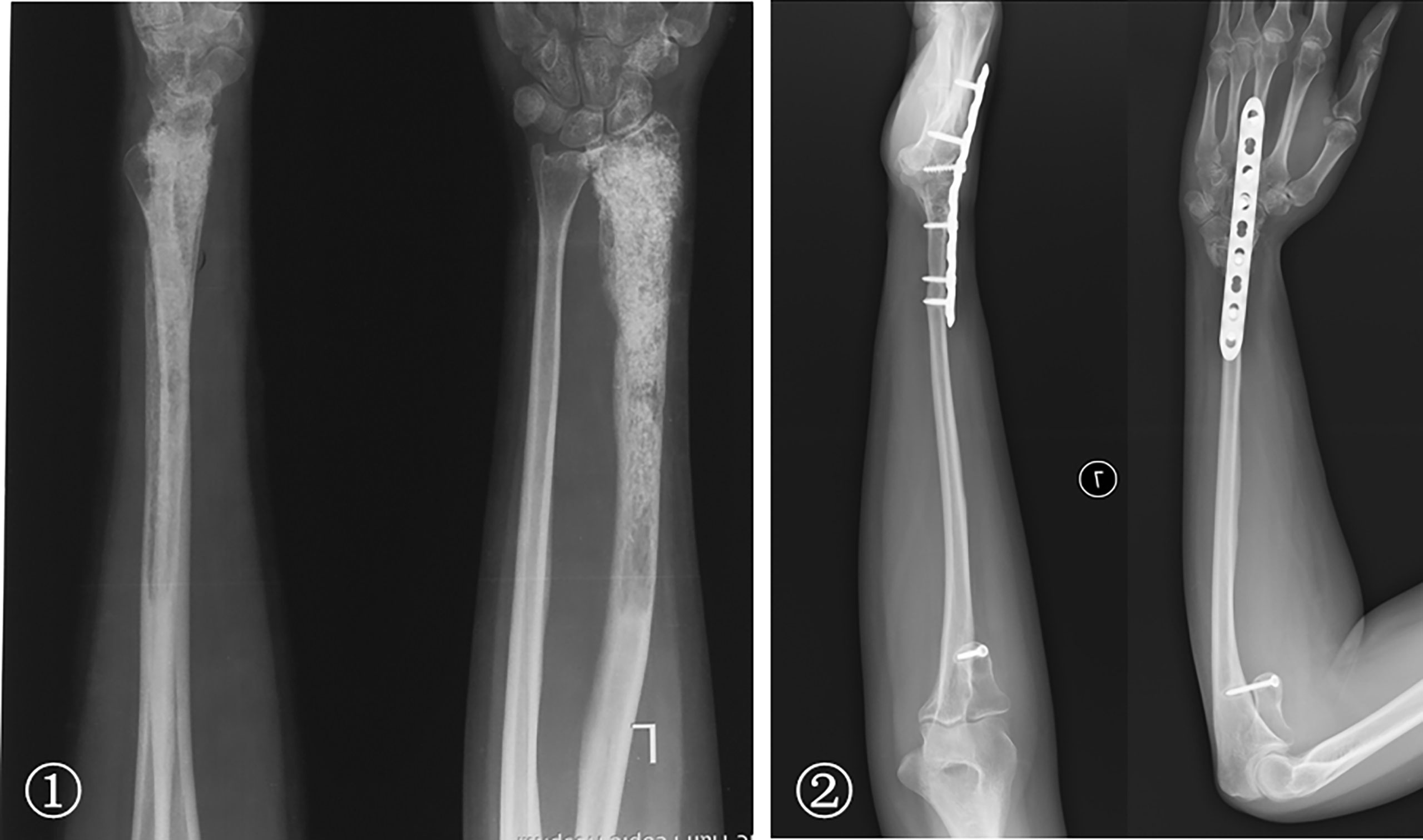
Figure 5 ① A 29-year-old woman with low-grade central osteosarcoma of the middle and distal radius underwent unplanned excision and tumor recurrence. ② Radius resection and ulna centralization with wrist joint fusion was performed; satisfactory bone healing but a loss of forearm rotation is shown 65 months postoperatively.
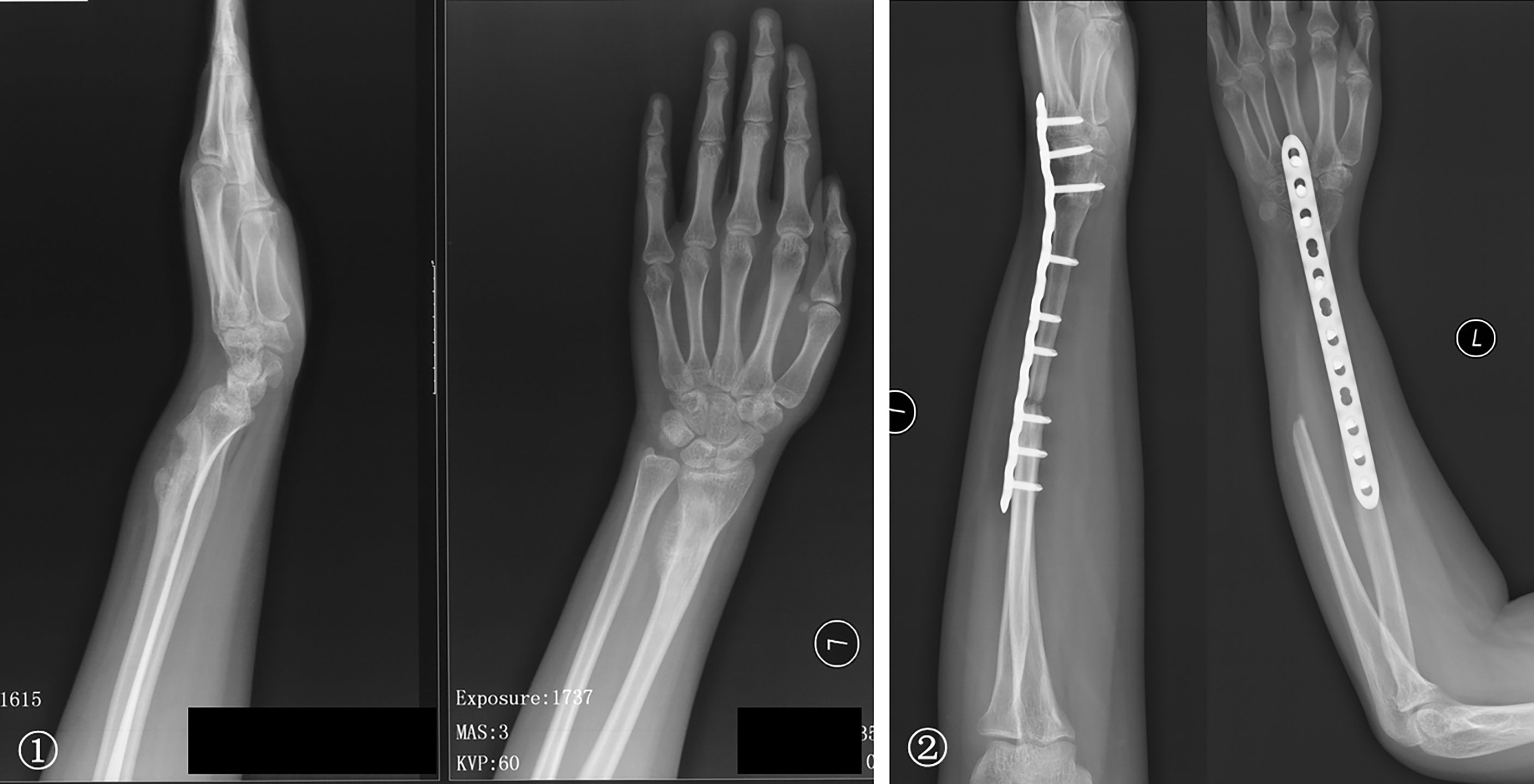
Figure 6 ① The preoperative radiographs of a 19-year-old woman with low-grade central osteosarcoma of the distal radius. ② Treatments included a distal radius resection; ipsilateral ulnar osteotomy to replace the radial defect. Wrist joint fusion was performed; the satisfactory bone healing of the forearm and rotation function is shown 50 months postoperatively.
After resecting the ulnar tumor, the proximal 1/3 defect was treated with (1) elbow prosthesis replacement and (2) inactivated replantation. More than 2/3 defect of the middle segment was treated with (1) elbow prosthesis combined with free vascularized fibula grafting and (2) brachioradialis elbow arthroplasty (Figure 7). The distal 1/3 defect did not receive reconstruction. Although we have performed different methods in reconstruction, the majority is biological reconstruction, which determined relatively few subsequent complications.
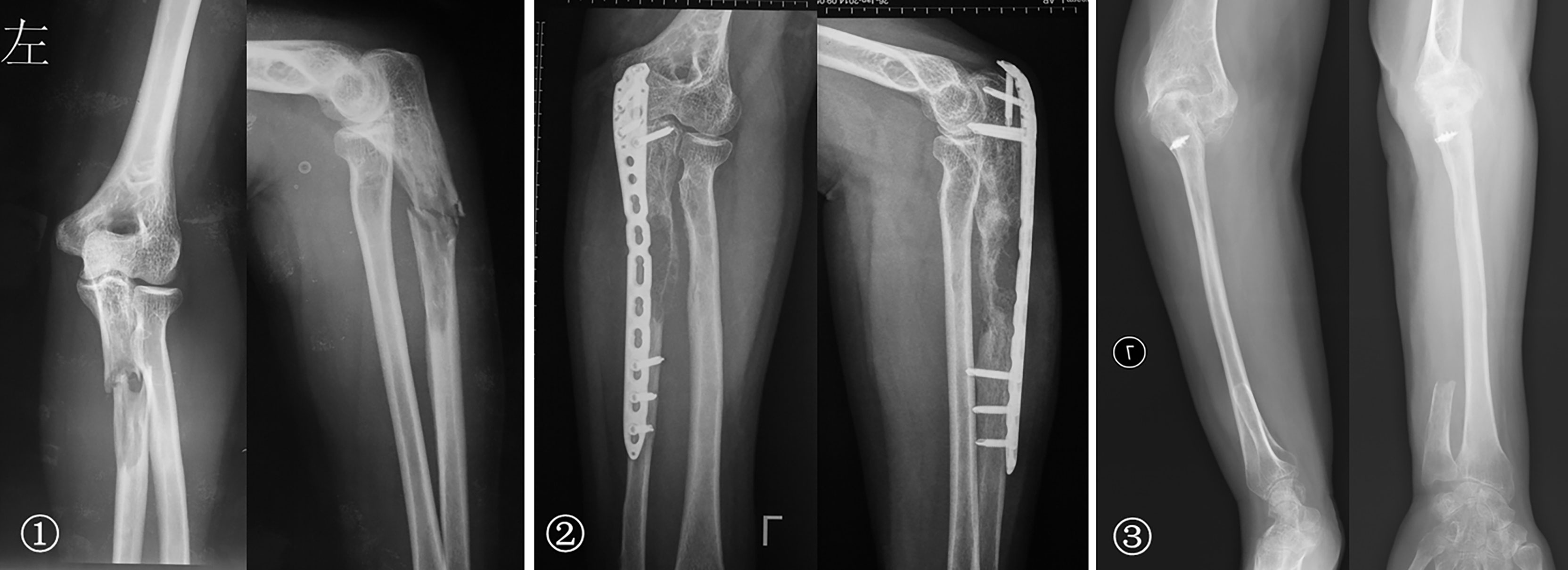
Figure 7 ① The preoperative radiographs of a 51-year-old woman with osteosarcoma of the proximal ulna. ② An unplanned excision and tumor recurrence occurred. ③ The radial head was displaced and inserted into the intercondylar of the humerus after proximal ulna resection; the rotation function of the forearm is shown 76 months postoperatively.
Ten patients (10/56, 17.8%) developed postoperative complications: internal fixation failure in 5 patients, limb shortening deformity, wrist silver fork deformity, prosthetic aseptic loosening, inactivated bone graft joint subluxation, and bone graft nonunion in 1 patient, respectively. Seven patients (7/10, 70%) underwent revision: 5 patients with fixation failure received re-fixation, one patient with nonunion received iliac graft again, and one patient with limb shortening deformity received limb extension by external fixator. The other three patients underwent routine observations without revision.
Twenty-two patients with ulna centralization lost rotational function, but flexion/extension and other fine movements were not significantly limited. At the final follow-up, functional scores were analyzed for both survivor and final limb salvage patients, because 12 patients died and 4 patients underwent amputation due to recurrence (2 patients were repeated), so 42 patients were included in the final functional evaluation. The MSTS score with an average of 27.9 ± 1.5. The function of patients with limb salvage was satisfactory, and the final limb salvage rate was 92.9% (52/56).
The follow-up averaged 72.1 (7–192, median 62.5) months. None of the patients had metastatic disease at presentation and distant metastasis was observed in 14 patients (14/56, 25%) during the follow-up, there were seven osteosarcomas, three Ewing sarcomas, two undifferentiated pleomorphic sarcomas, and two low-grade central osteosarcomas developed metastatic disease, 12 (12/14, 85.7%) of them had high-grade sarcomas. The median time from surgery to the development of distant metastasis was 15 (2–64) months, with 6 (42.9%) metastases occurring within 1 year and 12 cases (85.7%) within two years. The median time from the development of distant metastases to death was 11 (1–84) months. Eleven cases (78.6%) involved only lung metastases, 3 cases (21.4%) involved multiple sites of lung and bone metastases (one scapula, one thoracic vertebra, and one femoral shaft).
The 2-year and 5-year metastasis-free survival rates were 78.6 and 76.0%, respectively. The metastasis-free survival rates with adequate (wide) margins and inadequate (marginal or intralesional) margins were 80.4 and 43.6%, respectively (P = 0.008). The 5-year survival rates of high-grade and low-grade tumors were 81.7 and 88.2%, respectively (P = 0.427).
At the end of follow-up in Oct 2021, forty-two patients survived without tumor, two patients survived with metastatic disease, and twelve cases died of metastasis. The median survival time of dead patients was 29 (7–92) months. The overall 5-year and 10-year survival rates were 83.5 and 71.7%, respectively (Figure 8). Univariate analysis showed inadequate surgical margins (P = 0.048), local recurrences (P <0.001) and distant metastases (P <0.001) were associated with death. Multivariate analysis of the risk ratio model showed only distant metastases were significant independent poor prognostic factors of overall survival (P <0.001) (Table 4).
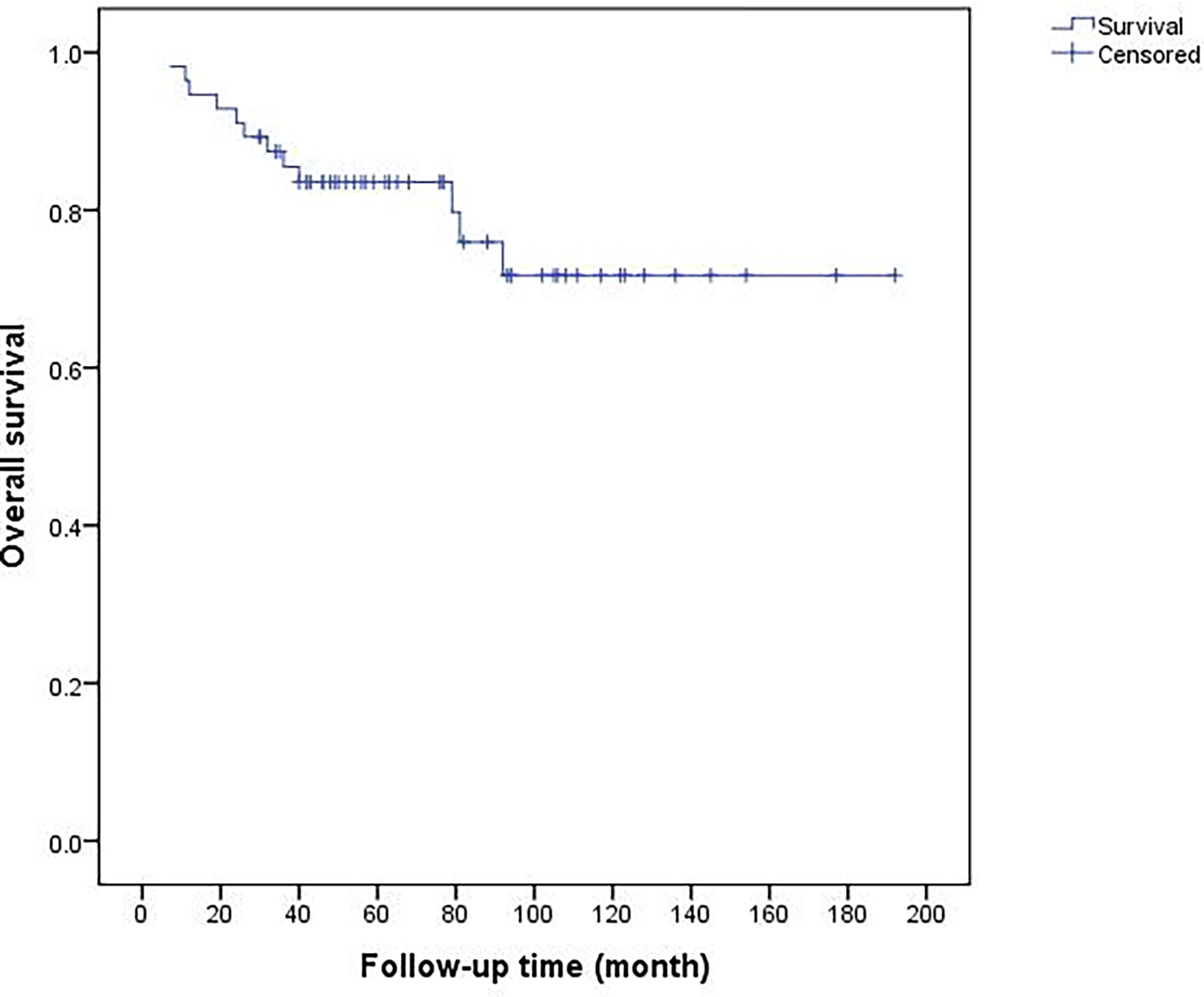
Figure 8 Overall 5-year and 10-year survival rates of 56 patients were 83.5 and 71.7%, respectively.
The incidence of primary malignant bone tumors of the forearm is low. Limited previous studies describe a large case series of bone tumors in the forearm, most of which are soft tissue tumors (9–11). The complex anatomy in the narrow forearm space leads to difficulties of limb salvage surgery and poor function after limb salvage surgery for treating bone sarcoma. In the forearm tumors treated in our center at the past 18 years, more benign tumors were found than malignant tumors, and more soft tissue sarcoma was found than primary bone malignant tumors (1). Many reports on soft tissue sarcoma in the forearm have been published (12), while only some case reports on bone sarcoma have been found (13, 14). The primary malignant tumors in the forearm only occupied 18.4% (68/369) of all primary bone tumors in this study. Although the number of malignant cases in the radius is much greater than that in the ulna, the proportion of malignant ulnar tumors is higher. Therefore, tumors in the ulna are much more likely to be malignant, although the number of malignant tumors in the radius is dominant. This distribution characteristic has not been described previously (15, 16).
In this study, eleven cases (11/56, 19.6%) had local recurrence in the final follow-up. Six of the 15 patients (6/15, 40%) underwent UE before recurrence developed. These factors may be relevant with the high recurrence rate: improper surgical approach, surgical field contamination, and compartment barrier destruction resulted in the spread of the tumors; the biological behavior of recurrent tumors was more aggressive (17, 18). A significant advantage in recurrence-free survival for primary tumors was observed, and their imaging findings were “milder” than those of UE tumors. Because of the high risk of recurrence, radical resection and even amputation should be considered.
Following univariate analysis, tumors located in the ulna and proximal forearm showed a significantly higher risk of local recurrence, the above characteristics were not found in previous studies (12, 19, 20). Compared with bone sarcomas, soft tissue sarcomas of the forearm have predominantly been previously reported, and which was focused on tumor size associated with recurrence (21). Bosma et al. (22) analyzed the different recurrence risks of sarcoma at different sites, and Pradhan et al. (23) compared forearm sarcoma with other sites. However, the different recurrence rates between different sites in the forearm had not previously been analyzed due to the small sample sizes of the studies.
With less soft tissue attached, the coverage is more difficult for limb salvage in the ulna. The proximal anatomical structure is more complex than the distal forearm. The radial nerve, brachial artery, attachment of muscles at the proximal forearm, and juxtaposition of the elbow joint may lead to inadequate resection margins due to the necessary preservation of essential structures. All these factors may contribute to the increase in the ulnar recurrence rate. For ulna malignancies, especially proximal involvement, the implementation of limb salvage needs to be repeatedly evaluated.
The influence of surgical margins on local recurrence has been investigated in many studies (12). Most researchers define adequate margins as wide or extra-compartmental resections. Muramatsu et al. (4) used a 2-cm margin for high-grade sarcomas and a 1-cm margin for low-grade sarcomas, achieving a satisfactory local recurrence rate of 11%. In this study, the inadequate surgical margin increased the recurrence rate significantly. Intralesional and marginal resection was 63% (7/11), while the recurrence rate of adequate margins was 8.9% (4/45). We planned the surgical strategy according to preoperative imaging, and we used the postoperative specimen and pathological slides to evaluate the surgical margin. This was consistent in most cases. Sometimes the postoperative evaluation does not reach the ideal-planned margin. Such outcomes suggest that limb salvage surgery needs to be re-evaluated if it is difficult to achieve a safe margin.
Daecke et al. (19) reported that the metastasis rate of high-grade bone sarcoma in the forearm was 24%, and the 5-year survival rate was 86.2%, which was better than that in other sites. The current study showed that 14 patients (14/56, 25%) had metastases, and the 5-year survival rate of high-grade sarcoma was 81.7%, slightly lower than that of low-grade sarcoma but without statistical significance. The data suggest that (1) lower tumor load in the forearm leads to a lower risk of metastasis than other anatomical locations are unknown, many other variables that contribute to the risk of metastasis (2), perioperative chemotherapy was performed in most high-grade sarcomas, which reduced metastatic risk.
Whether recurrence affects metastases and survival is controversial (24, 25), some studies suggested that safe margins only affect recurrence, which does not increase metastases and reduce survival (26–28). However, more studies have demonstrated the contrary result (9, 29, 30). The current study showed that margins and recurrence were significantly associated with metastasis and survival following univariate analysis. But only metastasis was an independent risk factor for death from the multivariate analysis. Perhaps recurrence causes repeated operations and prolongs tumor-bearing time, which potentially changes of tumor biological behavior and increases the risk of metastases. The overall 5-year survival rates were 83.5%, the 5-year survival rates of high-grade and low-grade tumors were 81.7 and 88.2% respectively in our study. It was better than the 5-year survival rate of 67% and similar to survival at 5-year following limb salvage surgery of 86% in other reports (19, 23). The results validated the concept of safe margins—local control—reduction of metastases—improvement of survival need more evidence to back up.
The premise of function is oncological safety. The anatomical features of two bones in the forearm have extensive influence on rotation and hand function. Since there is no weight-bearing, it is important to ensure flexibility for the forearm and hand. Defects of the distal ulna and proximal radius have little effect on function, and reconstruction is unnecessary. More challenges result from 2/3 or more defects in the middle and distal radius and 2/3 or more defects in the middle and proximal ulna. For the treatment strategy of the distal radius, wrist arthrodesis with structural iliac crest bone graft was chosen (ICBG) for the defect within 7 cm, and good results were achieved (31). For defects over 7 cm, the ulna is directly displaced to centralization. This method is practical and straightforward, but the loss of rotation is not negligible. A segment autogenous fibula transplantation or translocating the ipsilateral ulna as a vascularized autograft to reconstruct the distal radius defects were adopted (32, 33) to maintain rotation. This relatively complex method showed better function and preferred wrist arthrodesis to obtain a stable joint (31). Compared to a few joint replacement options for the distal radius (34), prosthesis replacement is a routine and applicable method for proximal ulna defects. Brachioradialis elbow arthroplasty between the proximal radius and humeral condyle was designed, yielding satisfactory function. It is challenging to cover skin defects due to extensive resections in recurrent cases. Instead, it is preferred to execute microsurgery and flap technology (16, 35). In this study, three patients received flap coverage, and two patients underwent free vascularized fibula grafting with satisfactory postoperative results.
This study has some limitations. Firstly, this is a retrospective analysis spanning 18 years, and there were homogeneity differences in the choice of chemotherapy and surgical techniques. Secondly, this is a single institution report, which lacks multiple center coordination to correct the bias in the enrollment of patients and treatment methods. Finally, this study only included limb salvage cases, which did not compare with the outcomes of amputation. Thus, the selection bias of tumor load and site led to overestimating the survival rate of patients with forearm malignancy.
This study is the most extensive, single-institution case analysis of limb salvage treatment for primary malignant bone tumors in the forearm. A history of unplanned surgery, tumors located in the ulna, proximal forearm, and inadequate surgical margin are important factors leading to local recurrence. To improve local control, limb salvage should be used with caution in patients who underwent unplanned excision. Amputation may be a better choice for high-risk patients with proximally located soft tissue masses adjacent to vascular and nerve tracts. Metastasis is an independent poor prognostic factor of survival. Multidisciplinary collaboration for the systematic treatment of metastatic patients is a potentially effective way to reduce the mortality of these malignant tumors. Limb salvage surgery for malignant bone tumors of the forearm showed a high overall survival rate and relatively satisfactory functional recovery.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Beijing Jishuitan Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
WL and XN conceived and designed the study. WL,YY, TJ, YS, YL, LH and QZ undertook the study. WL and YY analyzed and interpreted the clinical data. WL drafted the manuscript. WL, YY and XN reviewed and edited the manuscript. All authors listed have made a substantial,direct, and intellectual contribution to the work and approved it for publication.
This research was supported by the National Key R&D Program of China (Grant No. 2021YFC2400500), Beijing Municipal Administration of Hospitals Incubating Program (PX2021015), Beijing Natural Science Foundation (L212042), Beijing Jishuitan Hospital Elite Young Scholar Programme (XKGG202105), Chinese Society of Clinical Oncology (CSCO) Research Foundation (Y-2019GCTB-002, Y-young2019-070), National Natural Science Foundation of China (51973021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Chung Ming Chan and G. Douglas Letson who reviewed the manuscript, and Tao Wang, Fajun Yang, Feng Yu, and Hairong Xu, for their contribution to the data collection of this study.
CT, Computed Tomography; MRI, Magnetic Resonance Imaging; MSTS, Musculoskeletal Tumor Society; UE, Unplanned Excision; LRFS, Local Recurrence Free Survival; DMFS, Distant Metastasis Free Survival; OS, overall survival; ICBG, Iliac Crest Bone Graft.
1. Niu X, Xu H, Inwards CY, Li Y, Ding Y, Letson GD, et al. Primary Bone Tumors: Epidemiologic Comparison of 9200 Patients Treated at Beijing Ji Shui Tan Hospital, Beijing, China, With 10 165 Patients at Mayo Clinic, Rochester, Minnesota. Arch Pathol Lab Med (2015) 139(9):1149–55. doi: 10.5858/arpa.2014-0432-OA
2. Haendel M, Vasilevsky N, Unni D, Bologa C, Harris N, Rehm H, et al. McMurry J Et Al: How Many Rare Diseases are There? Nat Rev Drug Discov (2020) 19(2):77–8. doi: 10.1038/d41573-019-00180-y
3. Picci P, Manfrini M, Fabbri N, Gambarotti M, Vanel D. Atlas of Musculoskeletal Tumors and Tumorlike Lesions, The Rizzoli Case Archive. Switzerland: Springer (2014).
4. Muramatsu K, Ihara K, Yoshida K, Tominaga Y, Hashimoto T, Taguchi T. Musculoskeletal Sarcomas in the Forearm and Hand: Standard Treatment and Microsurgical Reconstruction for Limb Salvage. Anticancer Res (2013) 33(10):4175–82.
5. Enneking WF, Spanier SS, Goodman MA. A System for the Surgical Staging of Musculoskeletal Sarcoma. Clin Orthop Relat Res (1980) 153):106–20. doi: 10.1097/00003086-198011000-00013
6. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A System for the Functional Evaluation of Reconstructive Procedures After Surgical Treatment of Tumors of the Musculoskeletal System. Clin Orthop Relat Res (1993) 286):241–6. doi: 10.1097/00003086-199301000-00035
7. Coindre JM. Grading of Soft Tissue Sarcomas: Review and Update. Arch Pathol Lab Med (2006) 130(10):1448–53. doi: 10.5858/2006-130-1448-GOSTSR
8. Christopher DM, Fletcher JAB, Pancras CW, Hogendoorn FM. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer (2013).
9. Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the Prognostic Significance of Microscopic Margins in 2,084 Localized Primary Adult Soft Tissue Sarcomas. Ann Surg (2002) 235(3):424–34. doi: 10.1097/00000658-200203000-00015
10. Popov P, Tukiainen E, Asko-Seljavaara S, Huuhtanen R, Virolainen M, Virkkunen P, et al. Soft-Tissue Sarcomas of the Upper Extremity: Surgical Treatment and Outcome. Plast Reconstr Surg (2004) 113(1):222–30; discussion 231-22. doi: 10.1097/01.PRS.0000095946.90511.1D
11. Bansal K, Prasad A, Shahi P, Sehgal A, Kamal S. Extraosseus Ewing's Sarcoma of the Forearm. Cureus (2020) 12(7):e9051. doi: 10.7759/cureus.9051
12. Baroudi MR, Ferguson PC, Wunder JS, Isler MH, Mottard S, Werier JA, et al. Forearm Soft Tissue Sarcoma: Tumors Characteristics and Oncologic Outcomes Following Limb Salvage Surgery. J Surg Oncol (2014) 110(6):676–81. doi: 10.1002/jso.23686
13. Puri A, Gulia A, Byregowda S, Ramanujan V. Reconstruction of the Elbow and Forearm for Ewing Sarcoma of the Ulna: A New Biological Technique. Int J Shoulder Surg (2016) 10(2):85–8. doi: 10.4103/0973-6042.180721
14. Cahayadi SD, Antoro A, Swandika B. A Giant Cell-Rich Osteosarcoma of the Proximal Ulnar Bone Treated by Elbow Arthroplasty: A Case Report. Int J Surg Case Rep (2019) 58:157–61. doi: 10.1016/j.ijscr.2019.04.017
15. KK U. Dahlin's Bone Tumors-General Aspects and Data on 11,087 Cases. 5th Edition. Philadelphia: Lippincott-Raven Publishers (1996).
16. Rosenberg AE. WHO Classification of Soft Tissue and Bone, Fourth Edition: Summary and Commentary. Curr Opin Oncol (2013) 25(5):571–3. doi: 10.1097/01.cco.0000432522.16734.2d
17. Yang Z, Niu N, Tang J, Wu L, He J, Shi J. Reconstruction of Forearm Support With Ulnar Translocation After Resection of Chondrosarcoma in the Proximal Radius. Orthopade (2020) 49(11):1006–12. doi: 10.1007/s00132-020-03903-x
18. Bilgeri A, Klein A, Lindner LH, Nachbichler S, Knösel T, Birkenmaier C, et al. The Effect of Resection Margin on Local Recurrence and Survival in High Grade Soft Tissue Sarcoma of the Extremities: How Far Is Far Enough? Cancers (Basel) (2020) 12(9):2560. doi: 10.3390/cancers12092560
19. Daecke W, Bielack S, Martini AK, Ewerbeck V, Jurgens H, Kotz R, et al. Osteosarcoma of the Hand and Forearm: Experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol (2005) 12(4):322–31. doi: 10.1245/ASO.2005.06.002
20. Landau MJ, Badash I, Yin C, Alluri RK, Patel KM. Free Vascularized Fibula Grafting in the Operative Treatment of Malignant Bone Tumors of the Upper Extremity: A Systematic Review of Outcomes and Complications. J Surg Oncol (2018) 117(7):1432–9. doi: 10.1002/jso.25032
21. England P, Hong Z, Rhea L, Hirbe A, McDonald D, Cipriano C, et al. Does Advanced Imaging Have a Role in Detecting Local Recurrence of Soft-Tissue Sarcoma? Clin Orthop Relat Res (2020) 478(12):2812–20. doi: 10.1097/CORR.0000000000001351
22. Bosma SE, Rueten-Budde AJ, Lancia C, Ranft A, Dirksen U, Krol AD, et al. Individual Risk Evaluation for Local Recurrence and Distant Metastasis in Ewing Sarcoma: A Multistate Model: A Multistate Model for Ewing Sarcoma. Pediatr Blood Cancer (2019) 66(11):e27943. doi: 10.1002/pbc.27943
23. Pradhan A, Reddy KI, Grimer RJ, Abudu A, Tillman RM, Carter SR, et al. Osteosarcomas in the Upper Distal Extremities: Are Their Oncological Outcomes Similar to Other Sites? Eur J Surg Oncol (2015) 41(3):407–12. doi: 10.1016/j.ejso.2014.11.038
24. Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of Prognostic Factors in 1,041 Patients With Localized Soft Tissue Sarcomas of the Extremities. J Clin Oncol (1996) 14(5):1679–89. doi: 10.1200/JCO.1996.14.5.1679
25. Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized Prospective Study of the Benefit of Adjuvant Radiation Therapy in the Treatment of Soft Tissue Sarcomas of the Extremity. J Clin Oncol (1998) 16(1):197–203. doi: 10.1200/JCO.1998.16.1.197
26. Tanabe KK, Pollock RE, Ellis LM, Murphy A, Sherman N, Romsdahl MM. Influence of Surgical Margins on Outcome in Patients With Preoperatively Irradiated Extremity Soft Tissue Sarcomas. Cancer (1994) 73(6):1652–9. doi: 10.1002/1097-0142(19940315)73:6<1652::AID-CNCR2820730617>3.0.CO;2-X
27. Heinemann M, Ranft A, Langer T, Jurgens H, Kreyer J, Vieth V, et al. Recurrence of Ewing Sarcoma: Is Detection by Imaging Follow-Up Protocol Associated With Survival Advantage? Pediatr Blood Cancer (2018) 65(7):e27011. doi: 10.1002/pbc.27011
28. Spraker-Perlman HL, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Doski J, et al. Factors Influencing Survival After Recurrence in Osteosarcoma: A Report From the Children's Oncology Group. Pediatr Blood Cancer (2019) 66(1):e27444. doi: 10.1002/pbc.27444
29. Zhao R, Yu X, Feng Y, Yang Z, Chen X, Wand J, et al. Local Recurrence Is Correlated With Decreased Overall Survival in Patients With Intermediate High-Grade Localized Primary Soft Tissue Sarcoma of Extremity and Abdominothoracic Wall. Asia Pac J Clin Oncol (2018) 14(2):e109-15. doi: 10.1111/ajco.12807
30. Dickinson IC, Whitwell DJ, Battistuta D, Thompson B, Strobel N, Duggal A, et al. Surgical Margin and its Influence on Survival in Soft Tissue Sarcoma. ANZ J Surg (2006) 76(3):104–9. doi: 10.1111/j.1445-2197.2006.03615.x
31. Wang T, Chan CM, Yu F, Li Y, Niu X. Does Wrist Arthrodesis With Structural Iliac Crest Bone Graft After Wide Resection of Distal Radius Giant Cell Tumor Result in Satisfactory Function and Local Control? Clin Orthop Relat Res (2017) 475(3):767–75. doi: 10.1007/s11999-015-4678-y
32. Gundavda MK, Agarwal MG, Reddy R, Katariya A, Bhadiyadra R. Does a Modified Technique to Achieve Arthrodesis of the Wrist After Resection of the Distal Radius and Translocating the Ipsilateral Ulna as a Vascularized Graft to Reconstruct the Defect Improve Grip Strength and Outcomes Scores? Clin Orthop Relat Res (2021) 479(6):1285–93. doi: 10.1097/CORR.0000000000001604
33. McLean JM, Clayer M, Stevenson AW, Samson AJ. A Modified Ulnar Translocation Reconstruction Technique for Campanacci Grade 3 Giant Cell Tumors of the Distal Radius Using a Clover Leaf Plate. Tech Handb Up Extrem Surg (2014) 18(3):135–42. doi: 10.1097/BTH.0000000000000053
34. Guzik G. The Use of a Custom-Made Prosthesis in the Treatment of Chondrosarcoma of Distal Radius. Ortop Traumatol Rehabil (2016) 18(1):65–72. doi: 10.5604/15093492.1198866
Keywords: forearm, sarcoma, limb salvage, recurrence, metastasis, prognosis
Citation: Liu W, Yang Y, Jin T, Sun Y, Li Y, Hao L, Zhang Q and Niu X (2022) What Are the Results of Limb Salvage Surgery for Primary Malignant Bone Tumor in the Forearm? Front. Oncol. 12:822983. doi: 10.3389/fonc.2022.822983
Received: 26 November 2021; Accepted: 21 March 2022;
Published: 28 April 2022.
Edited by:
Patrick J. Schuler, Ulm University Medical Center, GermanyReviewed by:
Johanna Patricia Adevoso Canal, University of the Philippines Manila, PhilippinesCopyright © 2022 Liu, Yang, Jin, Sun, Li, Hao, Zhang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Liu, bGl1d2VpZmVuZ2pzdEAxMjYuY29t; Xiaohui Niu, bml1eGlhb2h1aUAyNjMubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.