- Department of Thoracic Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, China
Describing the changes in surgical procedures and factors affecting the surgical outcome of patients who have undergone complete resection of giant mediastinal tumors (GMTs, diameter ≥ 10 centimeters) could improve preoperative decision-making and prognostic evaluations. We accessed data from three sources, which are case reports on surgical treatment of GMTs from PubMed, Web of Science, and EMBASE until June 1, 2019; patients with resected GMT from the Surveillance, Epidemiology, and End Results (SEER) database; and retrospective review of medical records in our institution from 2000 to 2019. The worldwide distribution, clinicopathological characteristics, symptom profile, prognosis of patients with GMT resection, and nomogram for surgical outcome prediction are reported. A total of 242 rare GMT cases from four continents (Asia, North America, South America, and Europe) were included. The median age of the patients was 40 (IQR: 27, range: 13–83) years, and the male-to-female ratio was 1.57:1. Dyspnea, shortness of breath, cough, and chest pain or discomfort were the major symptoms at presentation. The prognosis of benign and low-grade malignant GMTs was superior to that of high-grade malignant GMTs. Tumor malignancy played the most critical role in predicting postoperative survival, followed by longest tumor diameter and a posterior mediastinum location. The findings of this study suggest that the number of successful GMT surgeries has increased over the last decade and describe clinical features of GMTs. Physicians should prioritize tumor malignancy as a leading factor in predicting outcome rather than tumor size.
Introduction
Giant mediastinal tumors (GMTs) with a diameter greater than or equal to 10 centimeters rarely occur but often impose challenges in the surgical and anesthesia community (1, 2). The clinical presentation and pathological diagnosis vary from case to case (3, 4). Despite these tumors having variable malignant potential, complete resection is considered the first-line treatment in appropriate cases. Although the clinicopathological characteristics of GMTs should be mainly consistent with smaller mediastinal tumors, there is no systemic description of their worldwide demographic characteristics and clinical symptoms or overviews of their mediastinal origin, etiologies, or trends in surgical approaches.
While a better understanding of GMT survival patterns is essential to improving preoperative decision-making and postoperative follow-up schedules, the surgical outcomes are not clear due to the rarity of these tumors. Interestingly, although a large tumor size is a poor prognostic factor in cancer medicine, patients with GMTs tend to have low-grade tumors, and tumor size does not seem to be the most critical prognostic factor. We hypothesized that this entity would have different survival risk factors from smaller tumors due to its slow growing nature.
Given this context, the purpose of this study was to provide the general characteristics of GMTs and survival risk factors by analyzing existing data over the last two decades. The worldwide distribution, clinicopathological characteristics, symptom profile, and prognosis of patients who underwent GMT resection are reported. Additionally, a risk prediction model of GMTs with up to 10-year survival rates was constructed, which may help thoracic surgeons screen high-risk patients and provide better preoperative management.
Materials and Methods
Data Sources and Eligibility
The data in this study are from three sources, including from a manual search of the literature on GMTs in PubMed and the Surveillance, Epidemiology, and End Results (SEER, 1975–2016) database and a retrospective retrieval of all data from patients who underwent GMT resection from January 2000 to December 2019 in our center. For the retrospective data in our institute, the requirement for written informed consent for publication was waived given the use of deidentified data.
In this study, GMTs were defined as those that originated from the mediastinum with a diameter greater than or equal to 10 centimeters. From the SEER database, only surgery codes for total surgical removal of the primary site (5) and radical surgery (6) were included. For the literature search, the exclusion criteria were as follows: (I) studies reporting tumors that did not originate from that mediastinum; and (II) potential duplicate reports (the report with the most data was included); (III) studies with incomplete tumor size data (except for apparent size over 10 cm in CT scan); and (IV) reports of incomplete resection. In the current report, patients younger than ten years old were not included.
Data Collection
For patient demographics, tumor location and diagnosis were collected from the three databases and merged. Because not all tumor grade, disease-free survival (DFS), and overall survival (OS) information were obtained for every patient in the literature, only those with clear descriptions were included. Perioperative data and symptoms were only available from the literature and Nanfang cohort, and the patients were considered symptom-free if no specific symptoms were reported. The extent of tumor invasion, lymph node involvement, and distant metastasis was not considered in this study due to a lack of information.
Statistical Analysis and Constructing the Risk Prediction Model
Statistical analysis and survival modeling were performed using R (R version 3.5.1). Normally distributed continuous data are presented as the mean ± standard deviation (range), whereas continuous data with a skewed distribution are presented as the median [interquartile range (IQR), range]. The former was compared with Student’s t-test, and the latter was compared with the Mann–Whitney U-test. Chi-squared analysis or Fisher’s exact test was used to compare categorical variables when appropriate. DFS and OS were defined as previously described (7). Kaplan-Meier survival analyses were conducted. Survival curves were plotted using the Kaplan–Meier method, and differences in survival were assessed with log-rank analysis. The risk prediction model was developed and validated according to the guidelines proposed by Grant et al. (8), and a calibration curve was constructed (9). P-values less than 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics of GMTs
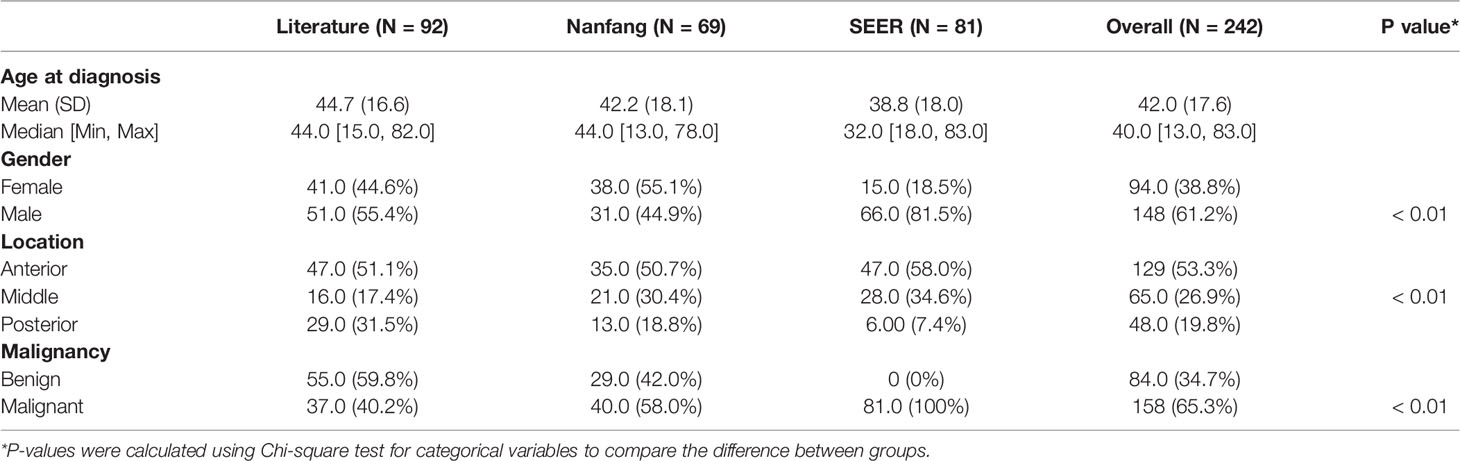
A total of 242 GMT cases from three cohorts representing four continents (Asia, North America, South America, and Europe) were included (Figure S1). The baseline characteristics were different among the 3 groups (Table 1), revealing the clinical diversity of the reported GMT cases. Most of the cases were predominantly from the USA (90) and China (81), followed by Japan (16), Turkey (15), and Italy (11) (Figure 1A). After 2020, GMT resection was more frequently documented, possibly due to advancements in surgical techniques (Figure 1B). The median age at GMT diagnosis was 40 (IQR: 27, range: 13–83), with two peaks at 25–30 and 45–55 years old (Figure 1C). The male-to-female ratio in this study was 1.57:1, with a sex-specific predominance for malignant GMTs (p-value <0.01, Pearson’s chi-squared test with Yates’ continuity correction) (Figure 1D). Similar to primary mediastinal tumors in adults (10, 11), the GMTs were also located predominantly in the anterior mediastinum (53%), followed by the posterior (31%), and middle (16%) mediastinum (Figure 1E). Although minimally invasive thoracoscopic surgery was performed in some cases, the most common surgical procedures for GMTs were thoracotomy and sternotomy (82%) in the Nanfang and literature cohorts (Figure 1F). The average longest diameter in the three cohorts was 15.5 (IQR: 7.5, range: 10–40 cm). The most common etiologies of GMT were germ cell tumors (33%), liposarcoma (10%), and thymoma (10%) (Figure 1G).
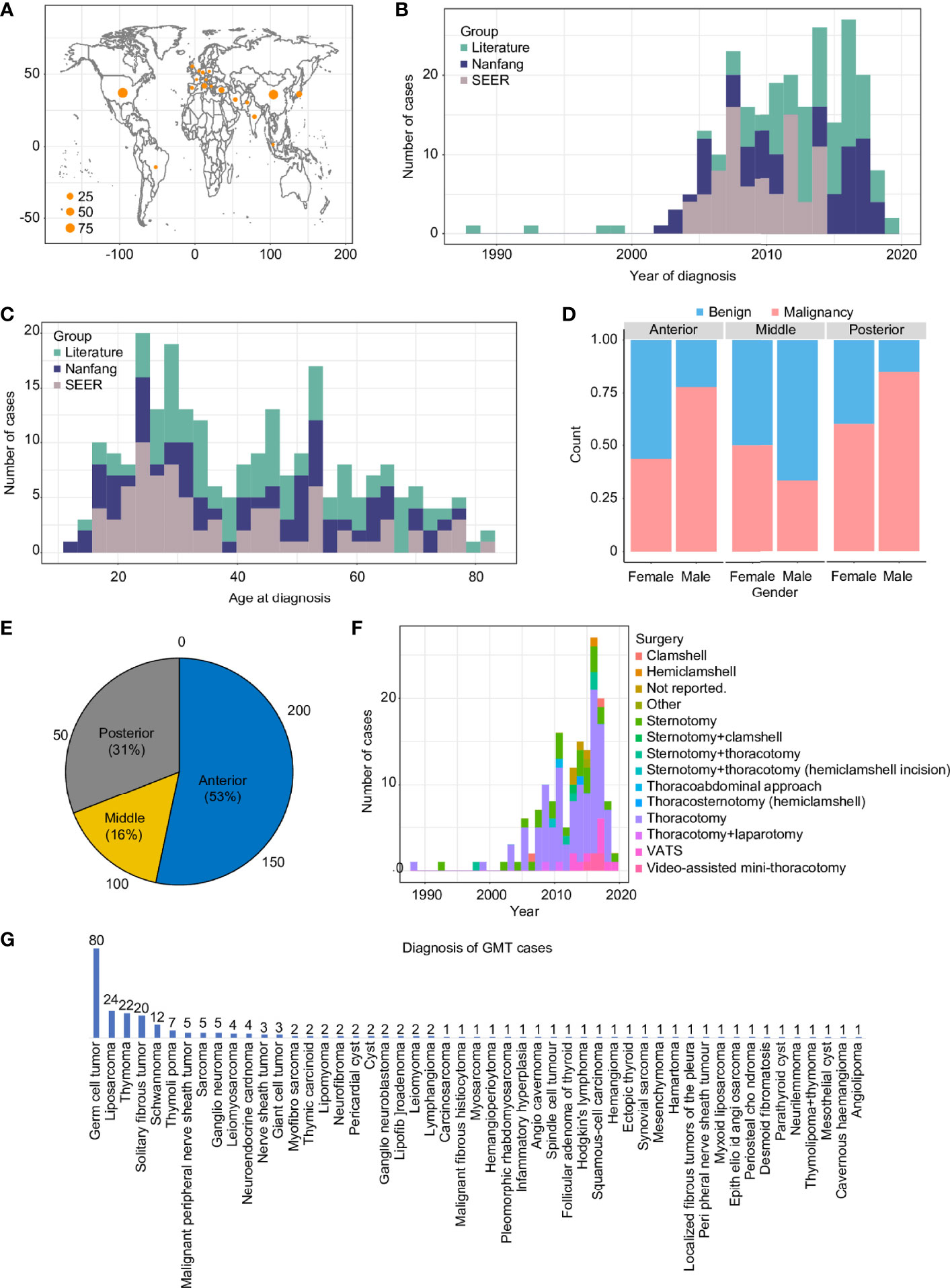
Figure 1 Overview of GMT resections from 1988 to 2019 in three cohorts. GMT distribution in countries around the world (A), in reports from 1988 to 2019 (B), by age (C), by sex (D), by mediastinum location (E), by surgical approach (F), and by pathological diagnosis (G).
Symptomatic Profile of GMTs
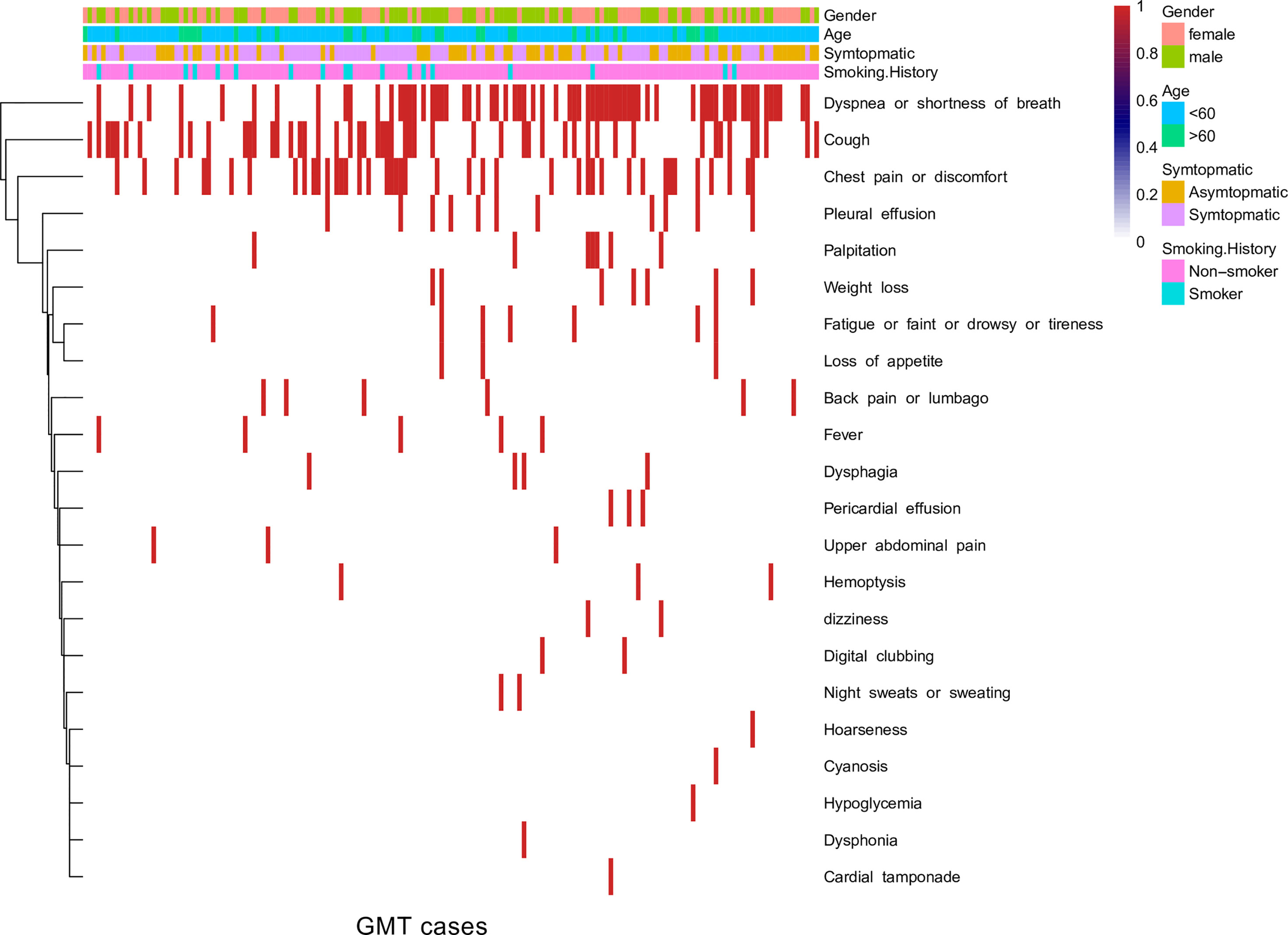
The symptoms of GMTs varied from case to case. In the Nanfang and literature cohorts, the ratio of symptomatic-to-asymptomatic presentations was 5:1. Over the study period, dyspnea, shortness of breath, cough, and chest pain or discomfort were the primary symptoms at presentation (Figure 2). The presenting symptom was not associated with age at diagnosis (p value = 0.9, Pearson’s chi-squared test) or sex (p value = 0.1, Pearson’s chi-squared test). The presence of symptoms at presentation was not associated with a survival of less than one year (p value = 0.9, chi-squared test), and the number of presenting symptoms was not correlated with survival duration (R2 = 0.2).
Perioperative Outcomes
Approximately 5% of patients underwent piecemeal surgery; otherwise, en bloc resection was performed. The mean operation times in the literature and Nanfang cohort were 230 ± 149 (range: 105–508) and 150 (IQR: 91.25, range: 42–450) min, respectively, and the mean intraoperative blood loss was 823 ± 675 (range: 90–1774) and 200 (IQR: 500, range: 20–4000) ml, respectively. The mean intraoperative transfusion volume was 304 ± 584 (range: 0–3000 ml), with a rate of 66.7%. The Mann-Whitney U-test revealed no significant difference in the length of postoperative stay (days) between the two cohorts, although a skewed distribution was observed (median: 7 vs. 15 days, P = 1). The overall postoperative complication rate was 9%, and the mortality rate within one week was 1%. Of note, the deaths in both cohorts were occurred in cases of acute onset or emergent cases.
Oncological Outcomes
Consistent with pathological trends, we provided evidence that benign GMTs had an improved recurrence-free survival compared with malignant GMTs (Figure 3A) and low-grade GMTs (Figure 3B) (P < 0.001). Additionally, better OS was observed for benign GMTs than for malignant GMTs (Figure 3C) and low-grade GMTs (Figure 3D) (P < 0.001).
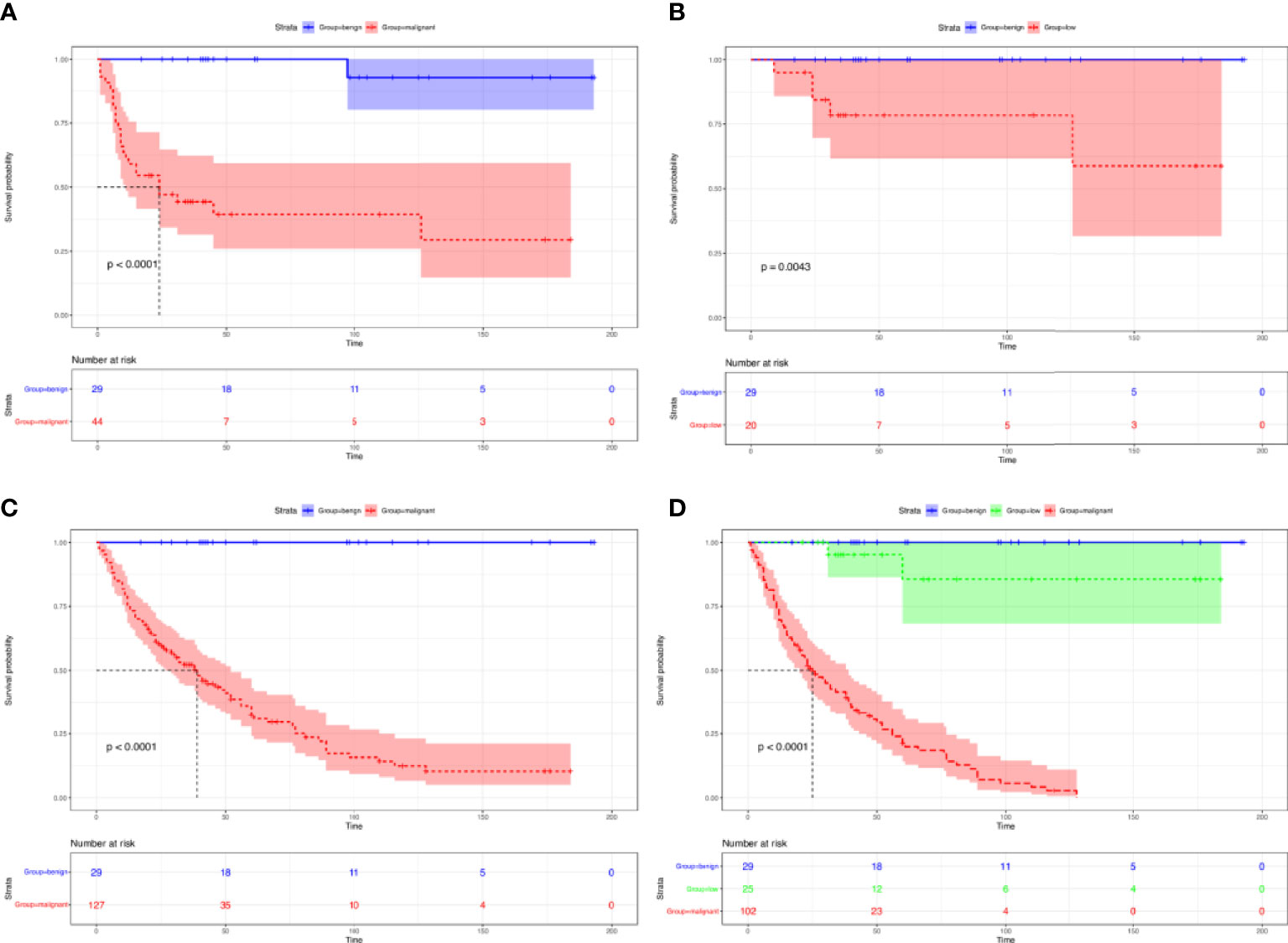
Figure 3 Survival of patients with GMTs who underwent complete resection. Recurrence-free survival in patients with GMTs who underwent complete resection: benign GMTs versus malignant GMTs (A), and benign GMTs versus low-grade malignant GMTs (B). Overall survival in patients with GMTs >10 cm who underwent complete resection: benign GMTs versus malignant GMTs (C), and among benign GMTs and low-grade malignant and malignant GMTs (D).
The prediction model included patient sex, tumor malignancy, longest diameter, and GMT location (Figure 4A). The calibration curve suggested that the model had a good predictive performance (Figure 4B), and it is widely applicable because it only uses tumor malignancy, longest diameter, location, and sex (Figure 4C). We found that tumor malignancy played the most critical role in predicting postoperative survival, followed by tumor longest diameter and posterior mediastinum location.
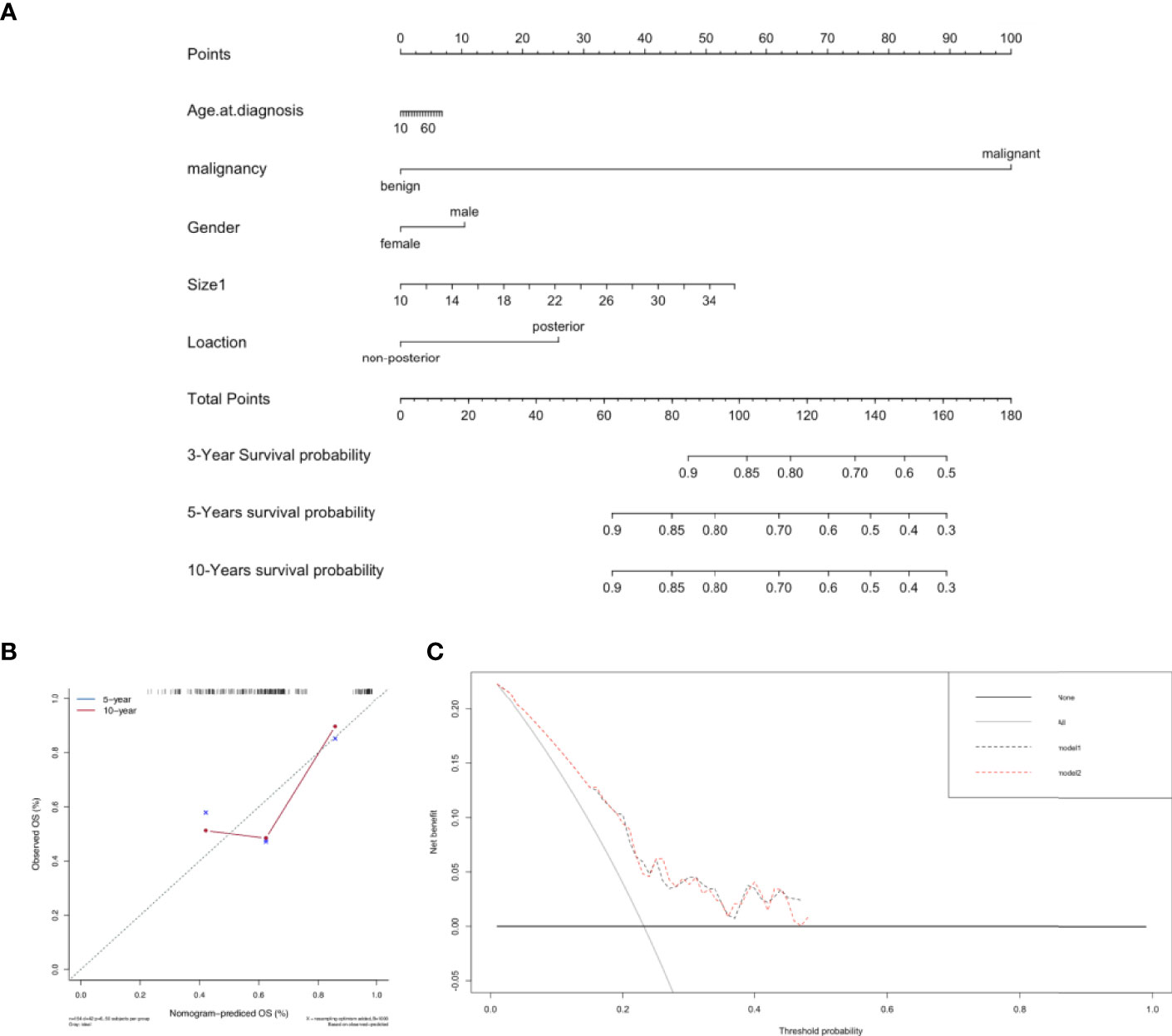
Figure 4 Predictive model for postoperative survival in patients with GMTs who underwent complete resection. (A) Postoperative prognostic nomogram for patients with resected GMTs. (B) Calibration curves for predicting 5-year and 10-year survival. (C) Evaluation of the prediction model by decision-curve analysis.
Discussion
In recent years, GMTs with a diameter greater than or equal to 10 centimeters have been reported in rare case reports (Table S1) (2, 5, 6, 12–91). It is believed that GMTs should follow the basic oncologic principle, and primary surgical resection aided with multimodal therapy is recommended when appropriate. However, direct descriptions of their clinicopathological nature, surgical trends and surgical outcomes and tools for predicting the outcomes of GMTs have yet to be published. To the best of our knowledge, the cases presented in this study are the most extensive report of GMT resection, representing four continents (Asia, North America, South America, and Europe) over the past two decades. Most of the cases were predominantly from the top 3 countries globally by population, namely, the USA (n= 90) and China (n= 81). An increasing trend of successful surgeries was observed, possibly due to the advancement of surgical and anesthetic techniques worldwide.
We first described the features of GMTs in relation to mediastinal tumors, namely, in terms of tumor origin, malignancy, and presenting symptoms. Consistent with a previous report of anterior mediastinal tumors accounting for 50% of all mediastinal masses in children in one report (92) and 44.2% in another (93), 53.3% of the GMTs in this study originated from the anterior mediastinum, confirming the susceptibility of the portion of the mediastinum to tumors. In contrast to the approximately 25% malignancy rate of all mediastinal tumors in both adults and children (92), 65.3% of the GMTs in this study were malignant, accounting for close to 72% of the malignant mediastinal tumors in only children (93). This indirectly reflects that GMTs could have unique malignant characteristics.
In general, malignant lesions are more likely to be symptomatic, and the presenting symptoms were cough, chest pain, fever or chills, and dyspnea (94). Wright and Mathisen (95) reported that more than one-third of mediastinal tumors produce symptoms. In this analysis, 76.4% of the patients with GMTs presented with symptoms, similar to the 70% with symptomatic malignant mediastinal tumors previously reported (10). Based on the first symptomatic heatmap for GMTs, dyspnea, shortness of breath, cough, and chest pain are the major clinical symptoms at presentation and mainly result from tumor compression. Of note, the association of increased GMT risk with the male sex needs further study. Although GMTs are a heterogeneous group of benign and malignant neoplasms, we observed that the predominant diagnosis of GMTs is germ cell tumors.
This study has inherent limitations due to its retrospective design, miscellaneous GMTs all together and use of inconsistent case report data and registry data. First, the Nanfang cohort was from a single-center retrospective study. Not only is the level of evidence not comparable to that from a randomized controlled trial, the potential recall bias during follow-up, surgeon preferences for certain procedures, and updates to instruments could all be confounders. Second, the literature cohort was missing information due to inconsistent report styles, which limited our ability to obtain detailed perioperative and survival data; this warrants further development of a global GMT database. Third, for the SEER cohort, selection bias for malignant disease was obvious. Fourth, the predominant germ cell tumor of GMT in this study based on current data could affect the survival analysis of this entity. We should perform subgroup analysis in the future as more data become available. Fifth, although there is consensus in the society of thoracic surgeons that multidisciplinary treatment and complete surgical resection could improve the prognosis of GMTs, current incomplete data of adjuvant or neoadjuvant treatment, resection status prevent further subgroup analysis for how these factors affect GMTs survival. As such, the development of a consistent report form or a database for GMTs may minimize the amount of missing data and contribute to better understanding of this entity in the mediastinum. Nonetheless, we provided insight based on our collection of GMT cases from across the world in the past two decades.
In conclusion, the surgical treatment and outcome estimations of GMTs is mainly determined by preoperative radiologic images, pathological findings from biopsy, physical status, and the invasion of surrounding anatomical structures. According to this first report of evidence, low-grade GMTs are associated with better survival than high-grade malignant GMTs, suggesting that surgical resection should be recommended for low-grade GMTs. Another proof-of-concept finding is that GMT size is not the most important factor affecting surgical outcome, especially for giant tumors with a slow-glowing nature and unknown pathology. Despite the rareness and surgical challenges of GMTs, the findings of this study provide an oncologic description of GMTs and their associated survival factors. Continuing efforts for consistent reports of such rare cases are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Nanfang Hospital. Written informed consent to participate in this study was provided by the patients or participants’ legal guardian/next of kin.
Author Contributions
KC designed and supervised the study. XS collected and analyzed the data and wrote the manuscript. XD collected the data and revised the manuscript. XL and HW interpreted the results and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The China Scholarship Council supports the Ph.D. scholarship of XS. The database development work was partially supported by the Research Initiative Fund of Southern Hospital 2018 (C1051325), the Major Science and Technology Planning Project of Guangdong Province (2017B020226005), and the Science and Technology Program of Guangzhou (201903010003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Prof. Wujun Wang, Prof. Xiyao Yang, Prof. Ruijun Cai, Prof. Haofei Wang, Prof. Gang Xiong, Prof. Zhiyong Huang and Dr. Di Lu for contributing to the successful surgical treatment of these rare cases in the last two decades. We thank Miss. Wen Dong for building the database of GMTs in our institution.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.820720/full#supplementary-material
References
1. Hartigan PM, Ng JM, Gill RR. Anesthesia in a Patient With a Large Mediastinal Mass. N Engl J Med (2018) 379(6):587–8. doi: 10.1056/NEJMc1804797
2. Gonzalez-Rivas D, Wu CF, de la Torre M. Uniportal Video-Assisted Thoracoscopic Thymectomy and Resection of a Giant Thymoma in a Patient Witness of Jehova. J Thorac Dis (2017) 9(6):E556–9. doi: 10.21037/jtd.2017.05.06
3. Qureshi B, Albany C. Images in Clinical Medicine. Posterior Mediastinal Mass. N Engl J Med (2014) 371(8):e10. doi: 10.1056/NEJMicm1314944
4. Siemienowicz M, Molan M, Doolan L. Massive Anterior Mediastinal Mass Causing Cardiac Compression. J Am Coll Cardiol (2010) 56(24):e47. doi: 10.1016/j.jacc.2010.04.070
5. Ludhani PM, Anathakrishnan R, Muthubaskaran V, Chandrasekar P, Muralidharan S. Giant Solitary Fibrous Tumor of the Pleura. Asian Cardiovasc Thorac Ann (2015) 23(1):72–4. doi: 10.1177/0218492313513591
6. Hayat J, Ahmed R, Alizai S, Awan MU. Giant Ganglioneuroma of the Posterior Mediastinum. Interact Cardiovasc Thorac Surg (2011) 13(3):344–5. doi: 10.1510/icvts.2011.267393
7. Fang W, Filosso PL, Roden AC, Gu Z, Liu Y, Agzarian J, et al. Clinicopathological Features and Current Treatment Outcomes of Neuroendocrine Thymic Tumours. Eur J Cardiothorac Surg (2021) 59(5):1004–13. doi: 10.1093/ejcts/ezaa453
8. Grant SW, Collins GS, Nashef SAM. Statistical Primer: Developing and Validating a Risk Prediction Model. Eur J Cardiothorac Surg (2018) 54(2):203–8. doi: 10.1093/ejcts/ezy180
9. Mehralivand S, Shih JH, Rais-Bahrami S, Oto A, Bednarova S, Nix JW, et al. A Magnetic Resonance Imaging-Based Prediction Model for Prostate Biopsy Risk Stratification. JAMA Oncol (2018) 4(5):678–85. doi: 10.1001/jamaoncol.2017.5667
10. Macchiarini P, Ostertag H. Uncommon Primary Mediastinal Tumours. Lancet Oncol (2004) 5(2):107–18. doi: 10.1016/S1470-2045(04)01385-3
11. Azarow KS, Pearl RH, Zurcher R, Edwards FH, Cohen AJ. Primary Mediastinal Masses. A Comparison of Adult and Pediatric Populations. J Thorac Cardiovasc Surg (1993) 106(1):67–72. doi: 10.1016/S0022-5223(19)33741-9
12. Huang WL, Tseng YL. Rare Presentation of Giant Isolated Enteric Cyst in Anterior Mediastinum. Ann Thorac Surg (2019) 107(6):e417–9. doi: 10.1016/j.athoracsur.2018.09.061
13. Kondov G, Kondov B, Srceva MJ, Damjanovski G, Ferati I, Karapetrov I, et al. Giant Mediastinal Thymic Cyst. Pril (Makedon Akad Nauk Umet Odd Med Nauki) (2017) 38(2):139–45. doi: 10.1515/prilozi-2017-0032
14. Galli A, Giordano L, Muriana P, Bandiera A, Negri G, Zannini P, et al. Multidisciplinary Management of a Giant Cervico-Mediastinal Liposarcoma: A Case Report and Literature Review. Ear Nose Throat J (2017) 96(12):E10–3. doi: 10.1177/014556131709601213
15. Sugiura Y, Hashizume T, Fujimoto H, Nemoto E. A Giant Mediastinal Liposarcoma Weighing 3500g Resected With Clam Shell Approach, A Case Report With Review of Literature. Int J Surg Case Rep (2017) 41:292–5. doi: 10.1016/j.ijscr.2017.10.055
16. Aghajanzadeh M, Asgary MR, Mesbah A, Hemmati H, Delshad MSE, Samidoust P, et al. Giant Thymolipoma of Mediastinum and Neck - Initially Misdiagnosed as Liposarcoma by Core Needle Biopsy. J Family Med Prim Care (2018) 7(5):1079–82. doi: 10.4103/jfmpc.jfmpc_228_17
17. Sakaguchi Y, Komatsu T, Takubo Y, Terada Y. Resected Case of Giant Cystic Thymoma With Spontaneous Intracystic Hemorrhage. Surg Case Rep (2019) 5(1):30. doi: 10.1186/s40792-019-0586-9
18. Loftus TJ, Pipkin M, Machuca T, Oduntan O. Angiographic Embolization Followed by Piecemeal Resection of Giant Posterior Mediastinal Schwannoma: Case Report and Concise Review. Int J Surg Case Rep (2018) 53:250–3. doi: 10.1016/j.ijscr.2018.10.055
19. Yang YS, Bai CY, Li ZC, Li WJ, Li Y. Giant Primary Liposarcoma of the Anterior Mediastinum: A Case Report. Med (Baltimore) (2018) 97(42):e12873. doi: 10.1097/MD.0000000000012873
20. Zhou H, Zhong C, Fu Q, Tang S, Luo Q, Yu L, et al. Thoracoscopic Resection of a Huge Mediastinal Cystic Lymphangioma. J Thorac Dis (2017) 9(10):E887–9. doi: 10.21037/jtd.2017.09.97
21. Mubashir M, Salam A, Sonawalla A, Fatima H, Fatimi SH. Rare Presentation of a Posterior Mediastinal Cystic Schwannoma as a Large Pleural Effusion. Cureus (2017) 9(8):e1558. doi: 10.7759/cureus.1558
22. Raafat E, Karunasiri D, Kamangar N. Solitary Fibrous Tumour of the Pleura Presenting as a Giant Intrathoracic Mass. BMJ Case Rep (2017) 2017:bcr-2017-220695. doi: 10.1136/bcr-2017-220695
23. Huang W, Jiang GN. Resection of Giant Mediastinal Liposarcoma via ’ Dash, Vertical Shape’ Incision. J Surg Case Rep (2017) 2017(1):rjw219. doi: 10.1093/jscr/rjw219
24. Fujita K, Hayashi K, Motoishi M, Sawai S, Terashima T, Mio T. Giant Mature Teratoma in the Mediastinum Presenting With Rapid Growth. Oxf Med Case Rep (2016) 2016(12):omw093. doi: 10.1093/omcr/omw093
25. Liu P, Che WC, Ji HJ, Jiang ZM. A Giant Infiltrating Angiolipoma of the Mediastinum: A Case Report. J Cardiothorac Surg (2016) 11(1):164. doi: 10.1186/s13019-016-0560-6
26. Haratake N, Shoji F, Kozuma Y, Okamoto T, Maehara Y. Giant Leiomyoma Arising From the Mediastinal Pleura: A Case Report. Ann Thorac Cardiovasc Surg (2017) 23(3):153–6. doi: 10.5761/atcs.cr.16-00137
27. Yendamuri S. Resection of a Giant Mediastinal Teratoma. Ann Thorac Surg (2016) 102(5):e401–e2. doi: 10.1016/j.athoracsur.2016.04.041
28. Zhao W, Fang W. Giant Thymoma Successfully Resected via Hemiclamshell Thoracotomy: A Case Report. J Thorac Dis (2016) 8(8):E677–80. doi: 10.21037/jtd.2016.06.41
29. Wu Y, Zhang J, Chai Y. Giant Mediastinal Schwannoma Located in the Lower Right Side of the Chest. Niger J Clin Pract (2016) 19(5):678–80. doi: 10.4103/1119-3077.188701
30. Angelopoulos E, Eleftheriou K, Kyriakopoulos G, Athanassiadi K, Rontogianni D, Routsi C. A Giant Intrathoracic Malignant Schwannoma Causing Respiratory Failure in a Patient Without Von Recklinghausen’s Disease. Case Rep Med (2016) 2016:2541290. doi: 10.1155/2016/2541290
31. Crnjac A, Veingerl B, Vidovic D, Kavalar R, Hojski A. Giant Solitary Fibrous Tumour of the Pleura. Case Report and Review of the Literature. Radiol Oncol (2015) 49(4):395–401. doi: 10.2478/raon-2014-0036
32. Kaya SO, Samancilar O, Usluer O, Acar T, Yener AG. Giant Cavernous Haemangioma of the Anterior Mediastinum. Eurasian J Med (2015) 47(3):216–7. doi: 10.5152/eurasianjmed.2015.0055
33. Pei G, Han Y, Zhou S, Liu Z. Giant Mediastinal Thymolipoma in a Patient With Gardner’s Syndrome. Thorac Cancer (2015) 6(6):808–11. doi: 10.1111/1759-7714.12231
34. Omachi N, Kawaguchi T, Shimizu S, Okuma T, Kitaichi M, Atagi S, et al. Life-Threatening and Rapidly Growing Teratoma in the Anterior Mediastinum. Intern Med (2015) 54(19):2487–9. doi: 10.2169/internalmedicine.54.4622
35. Makdisi G, Roden AC, Shen KR. Successful Resection of Giant Mediastinal Lipofibroadenoma of the Thymus by Video-Assisted Thoracoscopic Surgery. Ann Thorac Surg (2015) 100(2):698–700. doi: 10.1016/j.athoracsur.2014.09.069
36. Ma J, Zhang HM, Zhang LW, Zheng MW, Yu M. Primary Mediastinal Giant Liposarcoma With Smooth Muscle and Neural Differentiation: A Case Report. Oncol Lett (2015) 9(6):2667–9. doi: 10.3892/ol.2015.3103
37. Goswami A, Baruah AR. Giant Thymolipoma: A Rare Case Presentation. Asian Cardiovasc Thorac Ann (2017) 25(2):143–5. doi: 10.1177/0218492315599689
38. Arraras-Martinez MJ, Rieger-Reyes C, Panadero-Paz C, Landa-Oviedo HS, Garcia-Tirado J. Giant Primary Mediastinal Liposarcoma: A Rare Cause of Atrial Flutter. Asian Cardiovasc Thorac Ann (2015) 23(9):1121–4. doi: 10.1177/0218492315589201
39. Sokouti M, Rostambeigi N, Halimi M, Rasihashemi SZ. A Huge Lymphangioma Mimicking Pleural Effusion With Extension to Both Chest Cavities: A Case Report and Review of Literature. Iran J Med Sci (2015) 40(2):181–4.
40. Rena O, Davoli F, Pia F, Roncon A, Papalia E, Casadio C. Giant Cervico-Mediastinal Well-Differentiated Liposarcoma. Heart Lung Circ (2015) 24(7):e112–4. doi: 10.1016/j.hlc.2015.02.015
41. Kirschbaum A, Ritz R, Pehl A, Bartsch D. Giant Intrathoracic Left-Sided Vagal Schwannoma. Thorac Cardiovasc Surg Rep (2013) 2(1):19–22. doi: 10.1055/s-0033-1337368
42. Bouma W, Klinkenberg TJ, Van De Wauwer C, Timens W, Mariani MA. Removal of a Giant Intrathoracic Cyst From the Anterior Mediastinum. J Cardiothorac Surg (2014) 9:152. doi: 10.1186/s13019-014-0152-2
43. Jain D, Arava S, Mishra B, Sharma S, Sharma R, Parshad R. Soft Tissue Giant Cell Tumor of Low Malignant Potential of Mediastinum: A Rare Case Report. Int J Surg Pathol (2015) 23(1):71–4. doi: 10.1177/1066896914540937
44. Kaplan T, Han S, Han U, Atac GK, Yanik S. Thymoma Type B1 Arising in a Giant Supradiaphragmatic Thymolipoma. Asian Cardiovasc Thorac Ann (2014) 22(9):1109–11. doi: 10.1177/0218492313504576
45. Karangelis D, Nikolaidis N, Roubelakis A, Weeden DF. Giant Thoracoabdominal Ganglioneuroblastoma in a 17-Year-Old Patient. Asian Cardiovasc Thorac Ann (2014) 22(6):739–41. doi: 10.1177/0218492313490410
46. Cuce F, Kara K, Asker M, Sonmez G. Giant Dumbbell-Shaped Schwannoma But Not Transforaminal: Transdiaphragmatic. BMJ Case Rep (2014) 2014:bcr2013203487. doi: 10.1136/bcr-2013-203487
47. Miyauchi Y, Matsubara H, Uchida T, Matsuoka H, Ichihara T, Matsumoto M. Successful Thoracoscopic Removal of a Giant Teratoma Following Extraction of Cystic Conponents: A Case Report. Asian J Endosc Surg (2014) 7(1):79–81. doi: 10.1111/ases.12067
48. Dalal U, Jora MS, Dalal AK, Attri AK, Singal R, Gupta S. Primary Germ Cell Tumor of the Mediastinum - Presenting as a Huge Mass. Int J Prev Med (2014) 5(2):230–2.
49. Guimaraes MD, Benveniste MF, Bitencourt AG, Andrade VP, Souza LP, Gross JL, et al. Thymoma Originating in a Giant Thymolipoma: A Rare Intrathoracic Lesion. Ann Thorac Surg (2013) 96(3):1083–5. doi: 10.1016/j.athoracsur.2013.01.031
50. De Luca G, Luciano A, Benincasa G, Sessa R, Petteruti F. Giant Malignant Granular Cell Tumor (GCT) of the Posterior Mediastinum. J Thorac Oncol (2013) 8(8):1107–8. doi: 10.1097/JTO.0b013e318299ad62
51. Aydemir B, Celik S, Okay T, Dogusoy I. Intrathoracic Giant Solitary Fibrous Tumor. Am J Case Rep (2013) 14:91–3. doi: 10.12659/AJCR.883867
52. Rothermel L, Gilkeson R, Markowitz AH, Schroder C. Thoracoscopic Resection of a Giant Teratoma Compressing the Right Heart. Interact Cardiovasc Thorac Surg (2013) 17(3):594–7. doi: 10.1093/icvts/ivt225
53. Wang J, Yan J, Ren S, Guo Y, Gao Y, Zhou L. Giant Neurogenic Tumors of Mediastinum: Report of Two Cases and Literature Review. Chin J Cancer Res (2013) 25(2):259–62. doi: 10.3978/j.issn.1000-9604.2013.03.09
54. Dell’Amore A, Asadi N, Bartalena T, Bini A, Stella F. Thoracoscopic Resection of a Giant Mediastinal Parathyroid Cyst. Gen Thorac Cardiovasc Surg (2014) 62(7):444–50. doi: 10.1007/s11748-013-0256-y
55. Bouchikh M, Arame A, Riquet M, Le Pimpec-Barthes F. Cardiac Failure Due to a Giant Desmoid Tumour of the Posterior Mediastinum. Eur J Cardiothorac Surg (2013) 44(6):1137–9. doi: 10.1093/ejcts/ezt214
56. Matsuo T, Takamori S, Hayabuchi N, Fumihiko M, Kashihara M, Yoshiyama K, et al. Giant Liposarcoma Occupying Most of the Hemi-Thorax and Resected in the Supine Position: Report of a Rare Case. Kurume Med J (2011) 58(2):63–5. doi: 10.2739/kurumemedj.58.63
57. Fazliogullari O, Atalan N, Gurer O, Akgun S, Arsan S. Cardiac Tamponade From a Giant Thymoma: Case Report. J Cardiothorac Surg (2012) 7:14. doi: 10.1186/1749-8090-7-14
58. Takenaka T, Ishida T, Handa Y, Tsutsui S, Matsuda H. Ectopic Thymoma Presenting as a Giant Intrathoracic Mass: A Case Report. J Cardiothorac Surg (2012) 7:68. doi: 10.1186/1749-8090-7-68
59. Zhao XG, Chen G, Chen XF. Giant Periosteal Chondroma of the Rib Associated With Mediastinum Deviation. Ann Thorac Surg (2012) 94(3):1014. doi: 10.1016/j.athoracsur.2012.02.048
60. Filosso PL, Delsedime L, Cristofori RC, Sandri A. Ectopic Pleural Thymoma Mimicking a Giant Solitary Fibrous Tumour of the Pleura. Interact Cardiovasc Thorac Surg (2012) 15(5):930–2. doi: 10.1093/icvts/ivs360
61. Aydin Y, Sipal S, Celik M, Araz O, Ulas AB, Alper F, et al. A Rare Thymoma Type Presenting as a Giant Intrathoracic Tumor: Lipofibroadenoma. Eurasian J Med (2012) 44(3):176–8. doi: 10.5152/eajm.2012.41
62. Thakkar RG, Shah S, Dumbre A, Ramadwar MA, Mistry RC, Pramesh CS. Giant Solitary Fibrous Tumour of Pleura -An Uncommon Intrathoracic Entity- a Case Report and Review of the Literature. Ann Thorac Cardiovasc Surg (2011) 17(4):400–3. doi: 10.5761/atcs.cr.10.01589
63. Tane S, Tanaka Y, Tauchi S, Uchino K, Nakai R, Yoshimura M. Radically Resected Epithelioid Angiosarcoma That Originated in the Mediastinum. Gen Thorac Cardiovasc Surg (2011) 59(7):503–6. doi: 10.1007/s11748-010-0710-z
64. Limmer S, Merz H, Kujath P. Giant Thymoma in the Anterior-Inferior Mediastinum. Interact Cardiovasc Thorac Surg (2010) 10(3):451–3. doi: 10.1510/icvts.2009.225557
65. Liu LG, Wei X, Pan TC. A Giant Primary Myxoid Liposarcoma of the Posterior Mediastinum. Chin Med J (Engl) (2010) 123(13):1818–20. doi: 10.3760/cma.j.issn.0366-6999.2010.13.041
66. Alar T, Ozcelik C, Kilnc N. Giant Mediastinal Mass: Thymolipoma. South Med J (2011) 104(5):353–4. doi: 10.1097/SMJ.0b013e3182142df6
67. Asteriou C, Barbetakis N, Kleontas A, Konstantinou D. Giant Mediastinal Teratoma Presenting With Paroxysmal Atrial Fibrillation. Interact Cardiovasc Thorac Surg (2011) 12(2):308–10. doi: 10.1510/icvts.2010.253039
68. Okuno M, Kawashima M, Miura K, Kadota E, Goto S, Kato M. Resection of Giant Mediastinal Liposarcoma Using the Hemiclamshell Incision. Gen Thorac Cardiovasc Surg (2010) 58(12):654–6. doi: 10.1007/s11748-010-0584-0
69. Taki K, Watanabe M, Iwagami S, Nagai Y, Iwatsuki M, Ishimoto T, et al. Giant Liposarcoma of the Posterior Mediastinum and Retroperitoneum. BMJ Case Rep (2011) 2011:bcr0620114341. doi: 10.1136/bcr.06.2011.4341
70. Shoji T, Sonobe M, Okubo K, Wada H, Bando T, Date H. Giant Primary Liposarcoma of the Chest. Gen Thorac Cardiovasc Surg (2009) 57(3):159–61. doi: 10.1007/s11748-008-0329-5
71. Su JW, Chua YL, Ong BH, Lim CH. Resection of a Giant Malignant Mediastinal Peripheral Nerve Sheath Tumour Under Cardiopulmonary Bypass. Singapore Med J (2009) 50(6):e199–200.
72. De Raet J, Sacre R, Hoorens A, Fletcher C, Lamote J. Malignant Giant Solitary Fibrous Tumor of the Mediastinum. J Thorac Oncol (2008) 3(9):1068–70. doi: 10.1097/JTO.0b013e318183af5d
73. D’Andrilli A, Andreetti C, Ibrahim M, Rendina EA. The Evolution of a Pleural Nodule Into a Giant Fibrous Tumor Associated With Hypoglycemic Coma. Eur J Cardiothorac Surg (2007) 31(5):955–7. doi: 10.1016/j.ejcts.2007.01.039
74. Santoprete S, Ragusa M, Urbani M, Puma F. Shock Induced by Spontaneous Rupture of a Giant Thymoma. Ann Thorac Surg (2007) 83(4):1526–8. doi: 10.1016/j.athoracsur.2006.10.016
75. Marulli G, Rea F, Feltracco P, Calabrese F, Giacometti C, Rizzardi G, et al. Successful Resection of a Giant Primary Liposarcoma of the Posterior Mediastinum. J Thorac Oncol (2007) 2(5):453–5. doi: 10.1097/01.JTO.0000268681.10367.cf
76. Ganti S, Milton R, Davidson L, Anikin V. Giant Pulmonary Hamartoma. J Cardiothorac Surg (2006) 1:19. doi: 10.1186/1749-8090-1-19
77. Di Giammarco G, Di Mauro M, Pano M, Cirmeni S, Contini M, Di Lorenzo C, et al. Giant Metastatic Myxoid Liposarcoma of the Mediastinum: A Case Report. J Thorac Cardiovasc Surg (2005) 129(6):1440–2. doi: 10.1016/j.jtcvs.2005.01.010
78. Uno A, Sakurai M, Onuma K, Yamane Y, Kurita K, Hayashi I, et al. A Case of Giant Mediastinal Leiomyoma With Long-Term Survival. Tohoku J Exp Med (1988) 156(1):1–6. doi: 10.1620/tjem.156.1
79. Gasiorowski L, Dyszkiewicz W, Piwkowski CT. An Unusual Case of Giant Primary Mediastinal Liposarcoma. Thorac Cardiovasc Surg (2009) 57(4):247–8. doi: 10.1055/s-2008-1039056
80. Ohara T, Fukushima K, Hasegawa T, Kitamura S, Kawai T. Giant Benign Mesenchymoma of the Mediastinum Causing Superior Vena Cava Syndrome: Report of a Case. Surg Today (1993) 23(10):917–9. doi: 10.1007/BF00311372
81. Al Refai M, Brunelli A, Fianchini A. Giant Schwannoma of the Posterior Mediastinum. Chest (1999) 115(3):907–8. doi: 10.1378/chest.115.3.907
82. Ershadi R, Rahim M, Abbasi M, Erfanian R. Giant Solitary Fibrous Tumor of the Pleura. J Surg Case Rep (2018) 2018(11):rjy270. doi: 10.1093/jscr/rjy270
83. Quartey B, Lenert J, Deb SJ, Henry LR. Giant Posterior Mediastinal Ancient Schwannoma Requiring Thoracoabdominal Resection: A Case Report and Literature Review. World J Oncol (2011) 2(4):191–4. doi: 10.4021/wjon348w
84. Aghajanzadeh M, Alavi A, Pourrasouli Z, Aghajanzadeh G, Massahnia S. Giant Mediastinal Thymolipoma in 35-Year-Old Women. J Cardiovasc Thorac Res (2011) 3(2):67–70. doi: 10.5681/jcvtr.2011.014
85. Balieiro MA, Lopes AJ, Costa BP, Veras GP, Perelson PS, Acatauassu Nunes R, et al. The Surprising Outcome of a Giant Primary Mediastinal Synovial Sarcoma Treated With Neoadjuvant Chemotherapy. J Thorac Dis (2013) 5(1):94–6. doi: 10.3978/j.issn.2072-1439.2012.12.05
86. Hekmat M, Ghaderi H, Tatari H, Arjmand Shabestari A, Mirjafari SA. Giant Pericardial Cyst: A Case Report and Review of Literature. Iran J Radiol (2016) 13(1):e21921. doi: 10.5812/iranjradiol.21921
87. Mani VR, Ofikwu G, Safavi A. Surgical Resection of a Giant Primary Liposarcoma of the Anterior Mediastinum. J Surg Case Rep (2015) 2015(9):rjv126. doi: 10.1093/jscr/rjv126
88. Vaziri M, Rad K. Progressive Dyspnea in a 40-Year-Old Man Caused by Giant Mediastinal Thymolipoma. Case Rep Surg (2016) 2016:3469395. doi: 10.1155/2016/3469395
89. Sunam GS, Oncel M, Ceran S, Odev K, Yildiran H. Giant Benign Mediastinal Masses Extending Into the Pleural Cavity. Surg J (N-Y) (2016) 2(2):e46–50. doi: 10.1055/s-0036-1584519
90. Goto T, Sato S, Koike T, Tsuchida M. Surgical Resection of a Giant Polycystic Seminoma of the Mediastinum. J Thorac Dis (2018) 10(6):E438–41. doi: 10.21037/jtd.2018.05.79
91. Virgilio E, Ciccone AM, Maurizi G, Vanni C, Ziparo V, Rendina EA. Giant Malignant Melanocytic Schwannoma of the Posterior Mediastinum. Am Surg (2013) 79(1):E22–4. doi: 10.1177/000313481307900112
92. Strollo DC, De Christenson MLR, Jett JR. Primary Mediastinal Tumors. Part 1: Tumors of the Anterior Mediastinum. Chest (1997) 112(2):511–22. doi: 10.1378/chest.112.2.511
93. Grosfeld JL, Skinner MA, Rescorla FJ, West KW, Scherer LR. Mediastinal Tumors in Children: Experience With 196 Cases. Ann Surg Oncol (1994) 1(2):121–7. doi: 10.1007/BF02303555
94. Juanpere S, Canete N, Ortuno P, Martinez S, Sanchez G, Bernado L. A Diagnostic Approach to the Mediastinal Masses. Insights Imaging (2013) 4(1):29–52. doi: 10.1007/s13244-012-0201-0
Keywords: giant mediastinal tumor, mediastinal malignancy, risk prediction model, surgical therapy, global survey
Citation: Shi X, Liu X, Dong X, Wu H and Cai K (2022) Trends, Symptoms, and Outcomes of Resectable Giant Mediastinal Tumors. Front. Oncol. 12:820720. doi: 10.3389/fonc.2022.820720
Received: 23 November 2021; Accepted: 17 January 2022;
Published: 04 February 2022.
Edited by:
Luciano Mutti, Temple University, United StatesReviewed by:
Niccolò Daddi, University of Bologna, ItalyMichael Ried, University Hospital Regensburg, Germany
Copyright © 2022 Shi, Liu, Dong, Wu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaican Cai, ZG9jX2NhaUAxNjMuY29t
 Xiaoshun Shi
Xiaoshun Shi Xiguang Liu
Xiguang Liu Kaican Cai
Kaican Cai