
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 May 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.816915
This article is part of the Research Topic Evidence and Emerging Option in Diagnosis and Management of Upper Tract Urothelial Carcinomas View all 13 articles
 Xiang Dai
Xiang Dai Fei Wang
Fei Wang Yiqing Du
Yiqing Du Caipeng Qin
Caipeng Qin Shicong Lai
Shicong Lai Yuxuan Song
Yuxuan Song Zixiong Huang
Zixiong Huang Songchen Han
Songchen Han Xiaopeng Zhang
Xiaopeng Zhang Tao Xu*
Tao Xu*Purpose: To evaluate the prognostic value of metabolic syndrome (MetS) in upper tract urothelial carcinoma (UTUC) patients based on propensity score matching (PSM) analysis.
Patients and Methods: A total of 573 patients with UTUC after radical nephroureterectomy were included at Peking University People’s Hospital from January 2007 to April 2021. MetS was diagnosed according to the criteria of Chinese Diabetes Society and was defined as the presence of 3 or more of the following 4 conditions (obesity, hyperglycemia, hypertension, high triglycerides and/or low high-density lipoprotein-cholesterol). Patients were divided into two groups based on whether they had MetS, whose variables were adjusted using 1:1 PSM analysis with a caliber of 0.02 to minimize selection bias. Univariate and multivariate Cox regression analysis were used to evaluate the association of MetS and its components with pathological outcomes after adjusting preoperative confounders by propensity score matching. The Kaplan-Meier method was used to estimate overall survival (OS), cancer-specific survival (CSS), and intravesical recurrence-free survival (IVRFS) after surgery.
Results: MetS was significantly correlated with older age, a history of coronary heart disease, high Charlson Comorbidity Index, low estimated Glomerular filtration rate, and low aspartate/alanine aminotransferase ratio (all P<0.05). Multivariate Cox regression analysis and Kaplan-Meier curves demonstrated that MetS showed no statistical correlation with lower OS or IVRFS and approaching significance with lower CSS (P=0.063) before PSM. After PSM, the 5-year OS, CSS, and IVRFS were 64.1%, 74.7%, and 77.2%, respectively, in the MetS group, compared with 67.4%, 78.8%, and 77.2%, respectively, in non-MetS group. Univariate Cox regression analyses showed that MetS and its components were not associated with decreased OS, CSS, or IVRFS (all P>0.05).
Conclusion: In our study, no statistical difference was found between MetS and survival outcomes in UTUC, except a marginal association with lower CSS. Further studies are needed to evaluate the role of MetS and its each single component on UTUC.
There is a high incidence of urothelial carcinoma and it ranks among the top ten malignant tumors worldwide. Bladder urothelial carcinoma accounts for its vast majority. Upper tract urothelial carcinoma (UTUC) accounts for only 5-10% of the total urothelial carcinoma but the proportion is higher in the Asian population, at about 9.3-29.9%, with an average of 17.9% (1). Therefore, UTUC may have different pathogenesis and clinical characteristics in the Asian population.
Metabolic Syndrome (MetS) is defined as a group of clinical manifestations including obesity, hyperglycemia, dyslipidemia (hypertriglyceridemia and/or hypo-high-density-lipoproteinemia), and hypertension. The components mentioned above seriously affected the health and showed aggregation at the onset. There is now increasing evidence of an association between MetS and tumor development and prognosis, such as colorectal cancer (2), breast cancer (3) and urinary cancer including prostate cancer (4) and bladder cancer (5). Few studies have investigated the link between MetS and UTUC, including one cohort study and two studies based on Surveillance, Epidemiology and End Results (SEER)-Medicare linked database (6–8). Although their studies showed that the patients with MetS had inferior survival outcomes, they may not be able to select the most suitable diagnostic criteria of MetS for the Chinese population. In 2004, the Chinese Diabetes Society (CDS) released MetS’s diagnostic criteria for the Chinese population (9), and reaffirmed the criteria in the latest consensus in 2019. In the determination of obesity, CDS adopts the same index as the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) but with a lower cut-off (BMI≥25kg/m2 vs BMI≥28kg/m2). This is different from International Diabetes Federation (IDF), which selects waist circumference as the evaluation criteria of centripetal obesity. At the same time, the CDS combined hypertriglyceridemia and hypo-high-density-lipoproteinemia. In general, the CDS takes into account the baseline characteristics of the lower BMI in the Asian population and imposes stricter requirements on the diversity of metabolic abnormalities. The aim of our study was to evaluate the prognostic value of MetS in a large Chinese cohort based on the diagnostic criteria from CDS using propensity score matching (PSM) analysis.
This study received the approval from the Internal Ethics Review Board of the Peking University People’s Hospital. We retrospectively collected the clinical and pathological records of 652 patients diagnosed with UTUC. We excluded 79 patients because they had non-urothelial carcinoma (n=48); were treated with ureteroscopic management (n=14); or did not show at the follow-up appointment (n=17). In total, 573 patients were included for further study (Figure 1). They were treated with radical nephroureterectomy (RNU) from January 2007 to April 2021. The type of bladder cuff removal included transvesical, extravesical, and endoscopic.
Lymph node dissection (LND) was performed when invasive UTUC was suspected or suspicious lymph node metastasis was found by preoperative imaging. The pathological staging was assessed according to the 2002 Union for International Cancer Control (UICC) Tumor-node-metastasis (TNM) classification system. Tumor grade was determined according to the World Health Organization/International Society of Urologic Pathology 2004 classification (WHO/ISUP) grading system. Other pathological features were simultaneously retrieved from the pathological reports.
MetS was diagnosed according to the criteria of CDS and was defined as the presence of 3 or more of the following 4 conditions: (1) obesity: body mass index (BMI)≥25kg/m2; (2) hyperglycemia: fasting plasma glucose (FPG) ≥ 6.1mmol/L and/or 2-hour postprandial blood glucose (2hPG) ≥ 7.8mmol/L or drug treatment for any type of diabetes mellitus, (3) high blood pressure: systolic blood pressure≥140 mmHg or diastolic blood pressure≥90 mmHg or antihypertensive drug treatment, or (4) high triglycerides (defined as ≥1.7mmol/L) and/or low high-density lipoprotein-cholesterol (defined as <0.9mmol/L in males and <1.0mmol/L in females).
Before the operation, blood and urinary samples were routinely obtained. For patients who were followed, cystoscopy was done every 3 months for the first 2 years after RNU and once a year thereafter. Computed tomography or magnetic resonance imaging, blood and urinary laboratory tests, and other evaluations were also performed. Overall survival (OS) was evaluated from the date of surgery to the date of death from all causes. Cancer-specific survival (CSS) was defined as the interval between surgery and cancer-specific death. Intravesical recurrence-free survival (IVRFS) was defined as the interval between surgery and identification of a subsequent bladder tumor during cystoscopy confirmed by pathological evaluation.
Differences in clinical and pathological characteristics among MetS and non-MetS groups were compared using Chi-square test for categorical variables and Student’s t-test or Mann-Whitney U test for continuous variables. Continuous variables with normal distribution were present as mean ± standard deviation (SD) and non-normal variables were reported as median (interquartile range). Univariate and multivariate Cox regression analysis were used to evaluate the association of MetS and its components with pathological outcomes after adjusting preoperative confounders by propensity score matching. All factors with p-value<0.1 in univariate Cox regression analysis were included in multivariate analysis. Patients were divided into two groups based on whether they had MetS, or whether variables were adjusted using 1:1 PSM analysis with a caliber of 0.02 to minimize selection bias. Standardized differences were used to compared the balance of baseline characteristics and covariates between MetS and non-MetS patients before and after matching, expressed as absolute standardized difference. The Kaplan-Meier method was used to estimate OS, CSS, and IVRFS after surgery. Statistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA) and Stata version 15 (StataCorp LLC, Texas, USA). A two-sided P value <0.05 was considered statistically significant.
Of the 573 UTUC patients in the entire cohort, 186 (32.5%) were diagnosed with MetS. The baseline characteristics of patients before PSM are demonstrated in Table 1. Median age of patients at surgery was 67.27 ± 9.97 year, with a median follow-up duration of 32.8 months (range from 1 to 179 months). Low-grade and high-grade UTUC were seen in 106 patients (18.5%) and 456 patients (79.6%), respectively, and 11 patients (1.9%) were in unclear classification. Non-muscle-invasive UTUC (pTa-Tis-T1) was seen in 145 (25.3%) of patients, 125 (21.8%) showed pT2, 274 (47.8%) showed pT3 or pT4, and 29 (5.1%) showed unclear results. A total of 230 patients (40.1%) were diagnosed with obesity, 176 patients (30.7%) with hyperglycemia, 371 patients (64.7%) with hypertension, and 313 patients (54.6%) with hyperlipidemia. Patients with MetS were significantly older (P=0.04), more likely to have a history of coronary heart disease (CHD) (P=0.002); had a higher Charlson Comorbidity Index (CCI) (P<0.001), a lower estimated Glomerular filtration rate(eGFR) (P=0.014), and lower AST/ALT ratio (P<0.001). Each component of MetS was also significantly higher in the MetS group, compared with patients in the non-MetS group. Although differences of these baseline characteristics and laboratory tests were statistically significant, there was no statistical difference in pathological characteristics including tumor stage and grade.
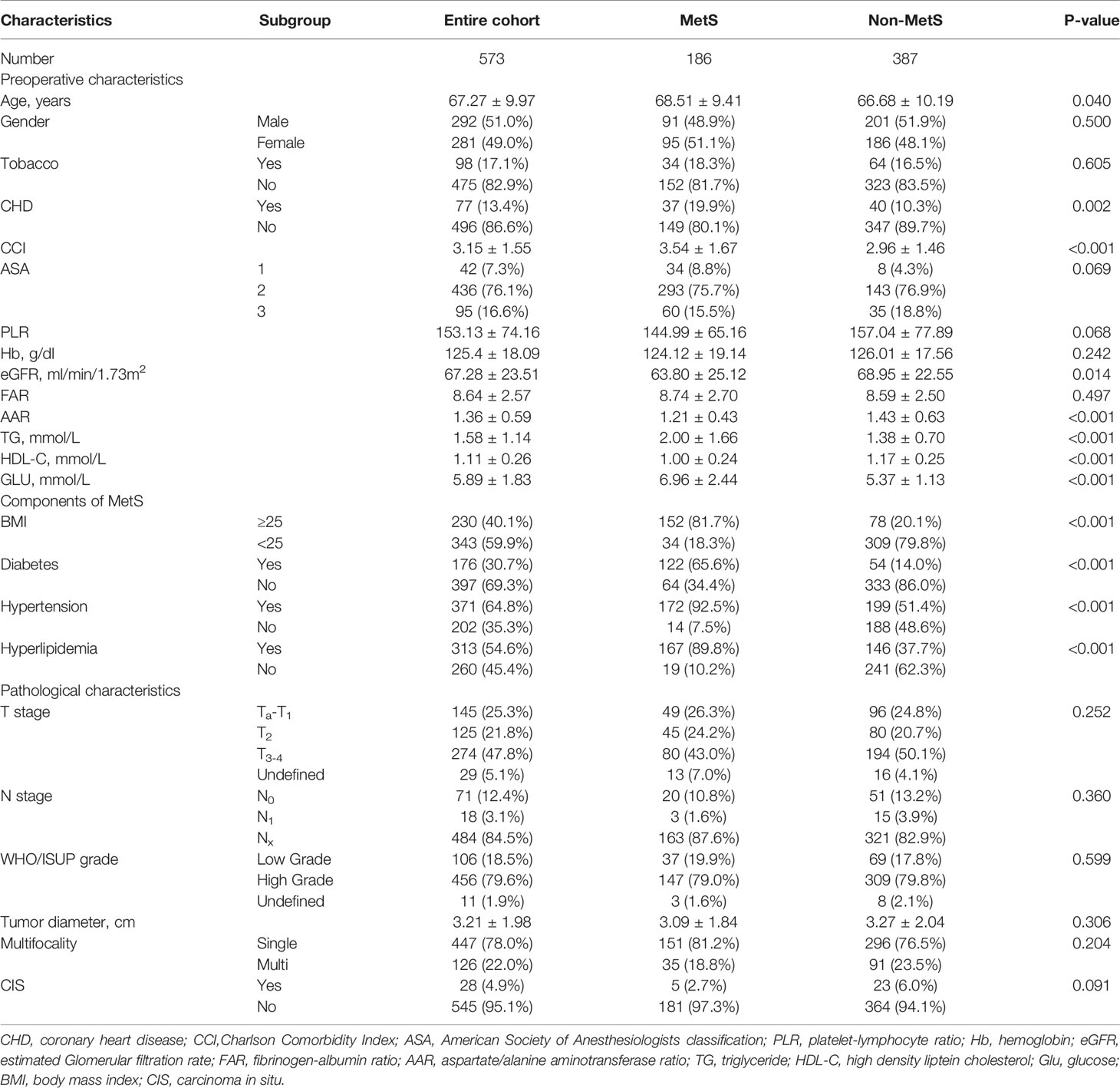
Table 1 Clinicopathological characteristics of the entire cohort and subgroups according to MetS before propensity score matching.
Before PSM, with a median follow-up of 32.8 months, there were 169 (29.5%) overall deaths after a median (interquartile range [IQR]) of 20 (9.8-40.0) months and 121 (21.1%) cancer-specific deaths after a median (IQR) of 15.1 (8.4-27.5) months, postoperatively. Also, 79 patients (13.8%) experienced intravesical recurrence (IVR) after a median (IQR) of 10.5 (6.4-28.5) months. After controlling for clinicopathological characteristics, there was a borderline effect in which members of the MetS group demonstrated a somewhat lower CSS compared with patients without MetS (95% confidence interval [CI] 0.978-2.351, P=0.063) based on multivariate Cox regression analyses, as shown in Table 2. Moreover, patient gender, pathologic T stage, tumor grade, and tumor size were revealed as significant co-predictors of CSS. However, MetS was not found to be an independent predictor for OS (95%CI 0.683-1.300, p=0.716) and IVRFS (95%CI 0.590-1.122, p=0.361). For OS, patient gender (P=0.029), pathologic T stage (P=0.010), tumor grade (P=0.002), and tumor size (P=0.001) were also significant co-predictors. Patient gender (P=0.009), PLR (P=0.001) and tumor multifocality (P=0.002) were revealed as independent predictors for IVRFS. Kaplan-Meier curves demonstrated that MetS showed no statistical correlation with lower OS and IVRFS and a marginal association with lower CSS (P=0.06) than those without MetS (Figures 2A–C).
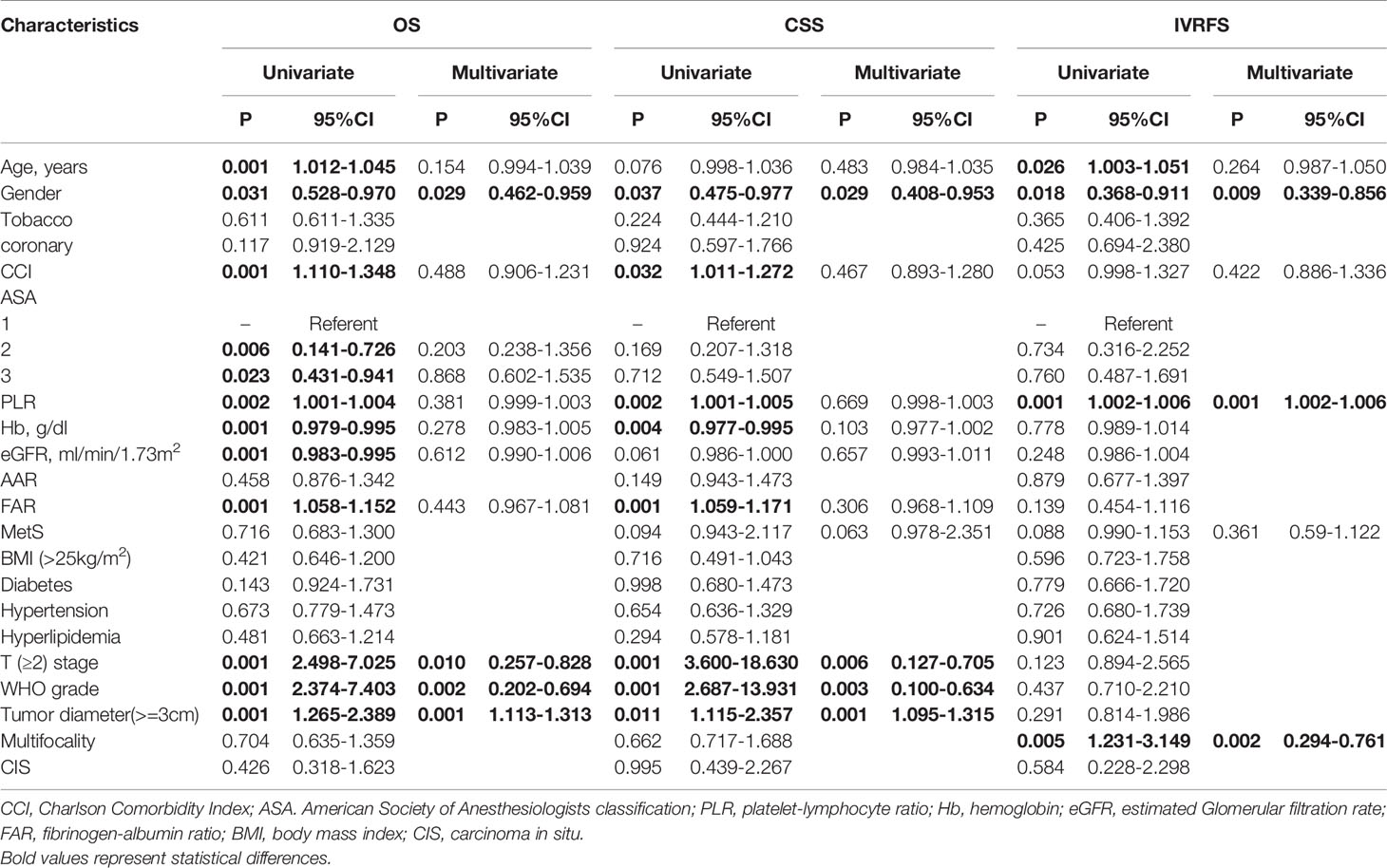
Table 2 Univariate and multivariate cox regression analyses for OS, CSS and IVRFS before propensity score matching.
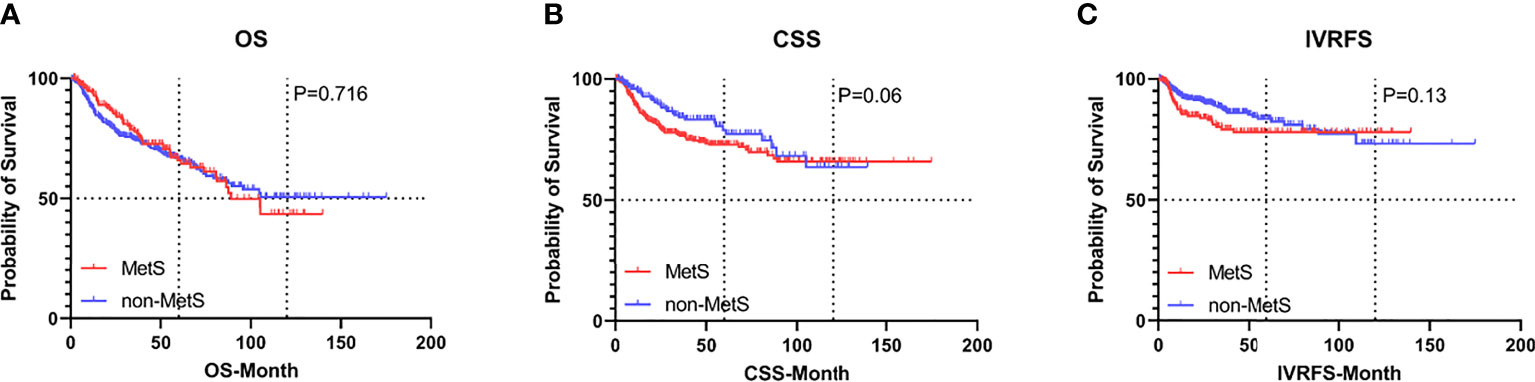
Figure 2 Kaplan-Meier curves for survival outcomes in UTUC patients according to the presence of MetS before propensity score matching. (A) OS, (B) CSS, and (C) IVRFS. OS, overall survival; CSS, cancer-specific survival; IVRFS, intravesical recurrence-free survival.
After PSM, the distributions of baseline and clinicopathological characteristics between MetS and non-MetS groups are summarized Table 3. Absolute standardized differences for all observed covariates were below 15%, suggesting an acceptable improvement in covariate balance. Only the standard difference of CHD, which is closely associated with MetS, declined less than other covariates. Age, gender, CCI, ASA classification, PLR, eGFR, AST/ALT ratio, and T stage were controlled in matching with a caliber of 0.02 (Figures 3–5). As shown in Table 3, nearly all clinicopathological characteristics did not have any statistical difference between MetS and non-MetS group, except each component of MetS diagnosis (all P<0.05).
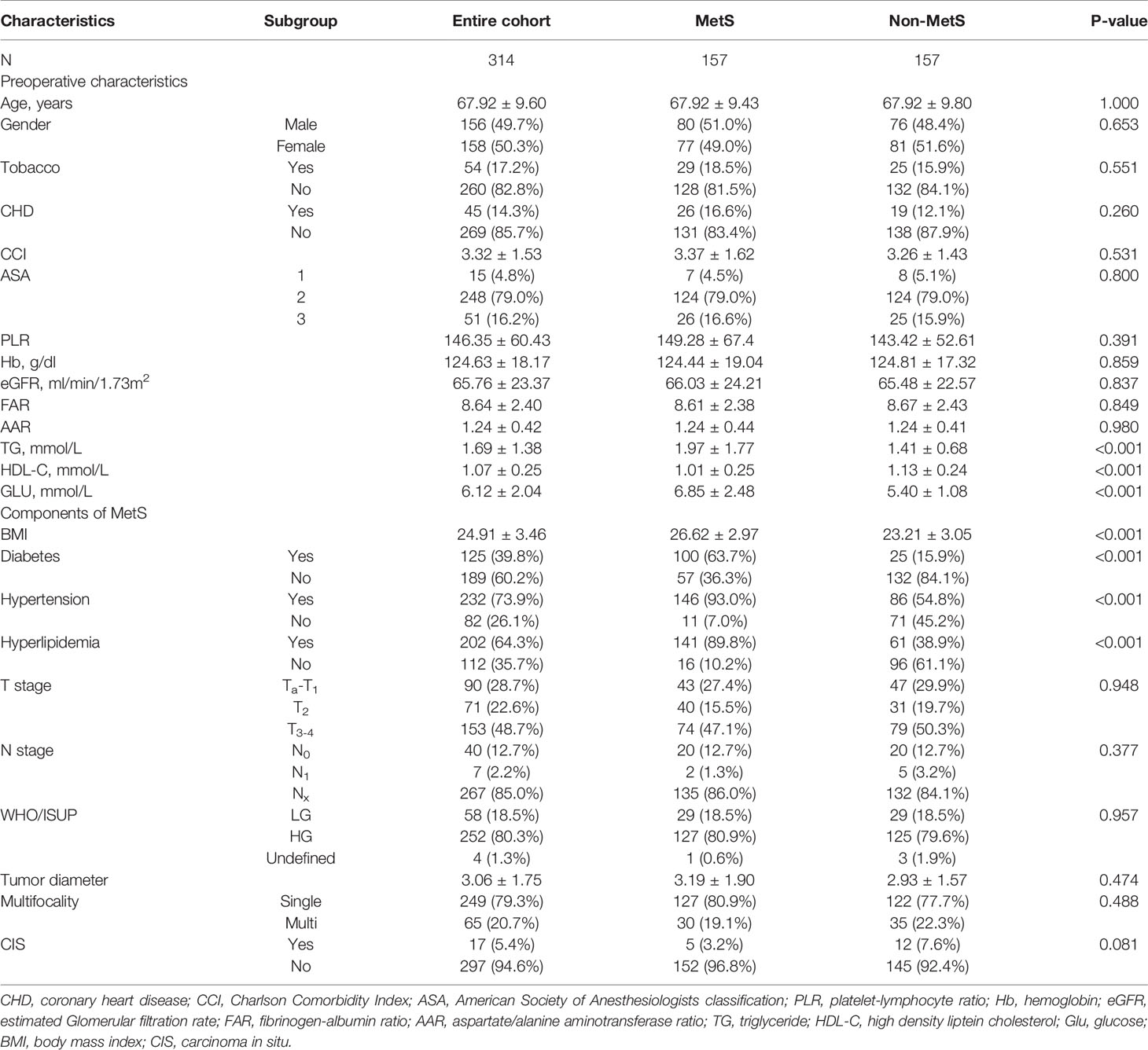
Table 3 Clinicopathological characteristics of the entire cohort and subgroups according to MetS after propensity score matching.
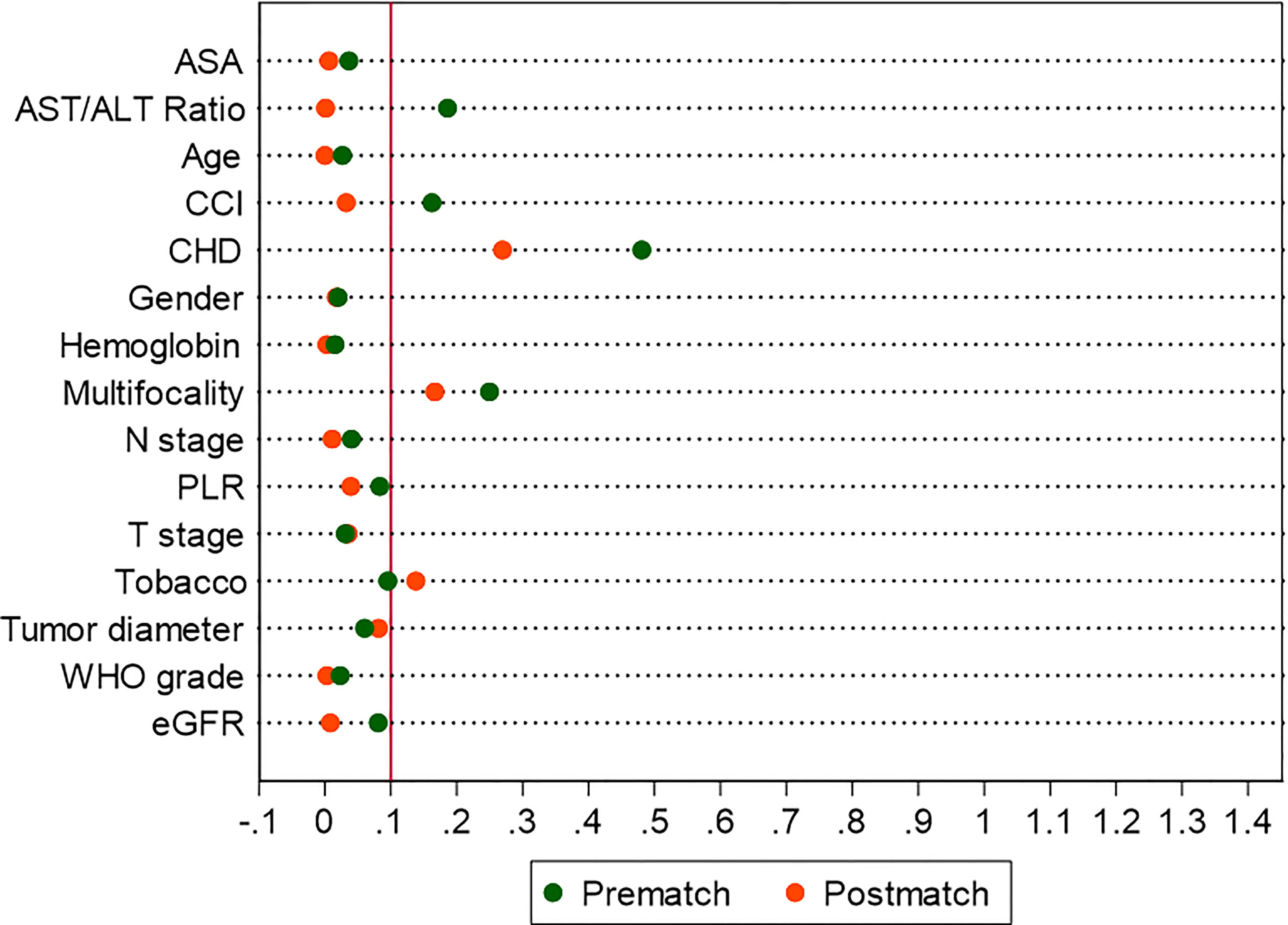
Figure 4 Absolute standardized differences in all characteristics between MetS and non-MetS group, before and after propensity score matching.
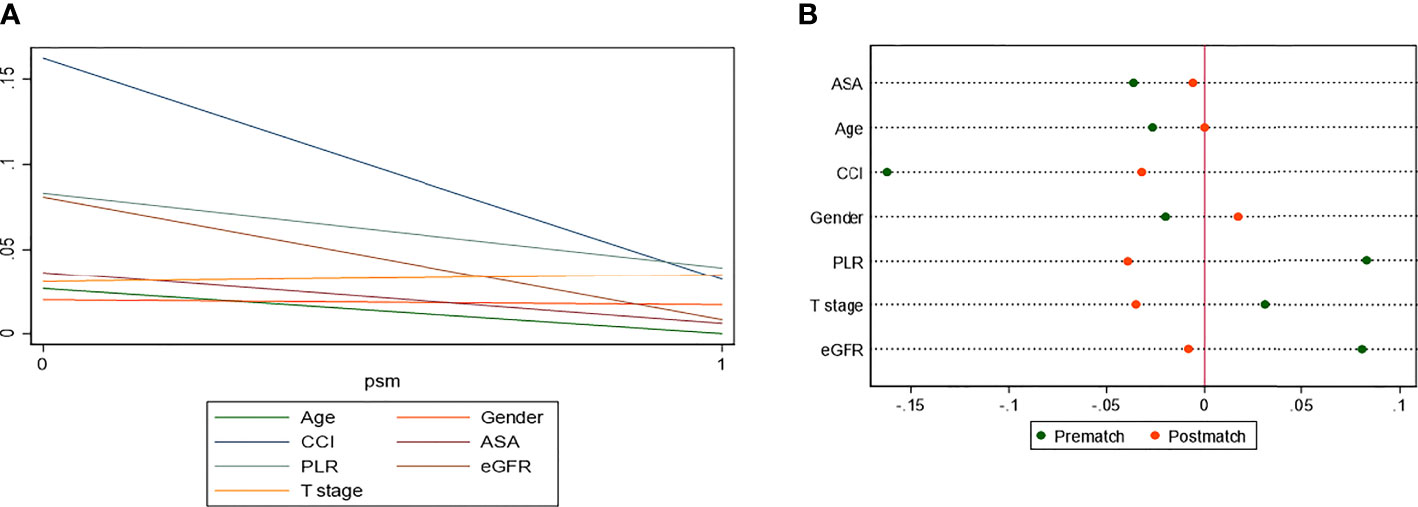
Figure 5 Standardized differences in covariates between MetS and non-MetS group, prematch and postmatch: (A) Line-plot; (B) Dot-plot.
With a median (IQR) follow-up duration of 33.1 (14.0-58.6) months postoperatively, there were 90 (28.7%) overall deaths after a median (IQR) of 32.6 (13.9-68.1) months and 58 (18.5%) cancer-specific deaths after a median (IQR) of 17.7 (9.7-39.1) months. Also, 50 patients (15.9%) experienced IVR after a median (IQR) of 10.1 (6.4-23.5) months. The 5-year OS, CSS, and IVRFS were 64.1%, 74.7%, and 77.2%, respectively, in the MetS group, as compared with 67.4%, 78.8%, and 77.2%, respectively, in non-MetS group. Kaplan-Meier curves demonstrated that MetS patients had almost the same CSS, RFS, and OS as those without MetS (all P>0.05; Figure 6). Univariate Cox proportional hazards regression analyses showed that MetS and its components were not associated with decreased OS, CSS, and IVRFS (all P>0.05; Table 4). After adjusting clinical confounders, multivariate Cox regression analysis showed that age, pathological T stage, tumor grade, and tumor size were significant co-predictors of OS (all P<0.05). T stage and tumor grade were also significant co-predictors of CSS (both P<0.05). Age and patient gender were significant co-predictors of IVRFS (both P<0.05) (Table 4).
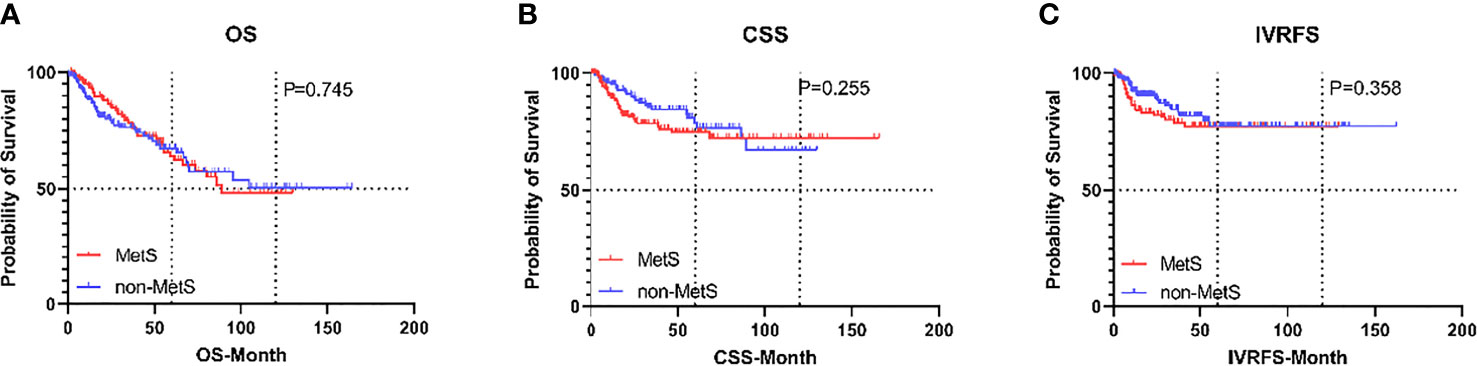
Figure 6 Kaplan-Meier curves for survival outcomes in UTUC patients according to the presence of MetS after propensity score matching. (A) OS, (B) CSS, and (C) IVRFS. OS, overall survival; CSS, cancer-specific survival; IVRFS, intravesical recurrence-free survival.
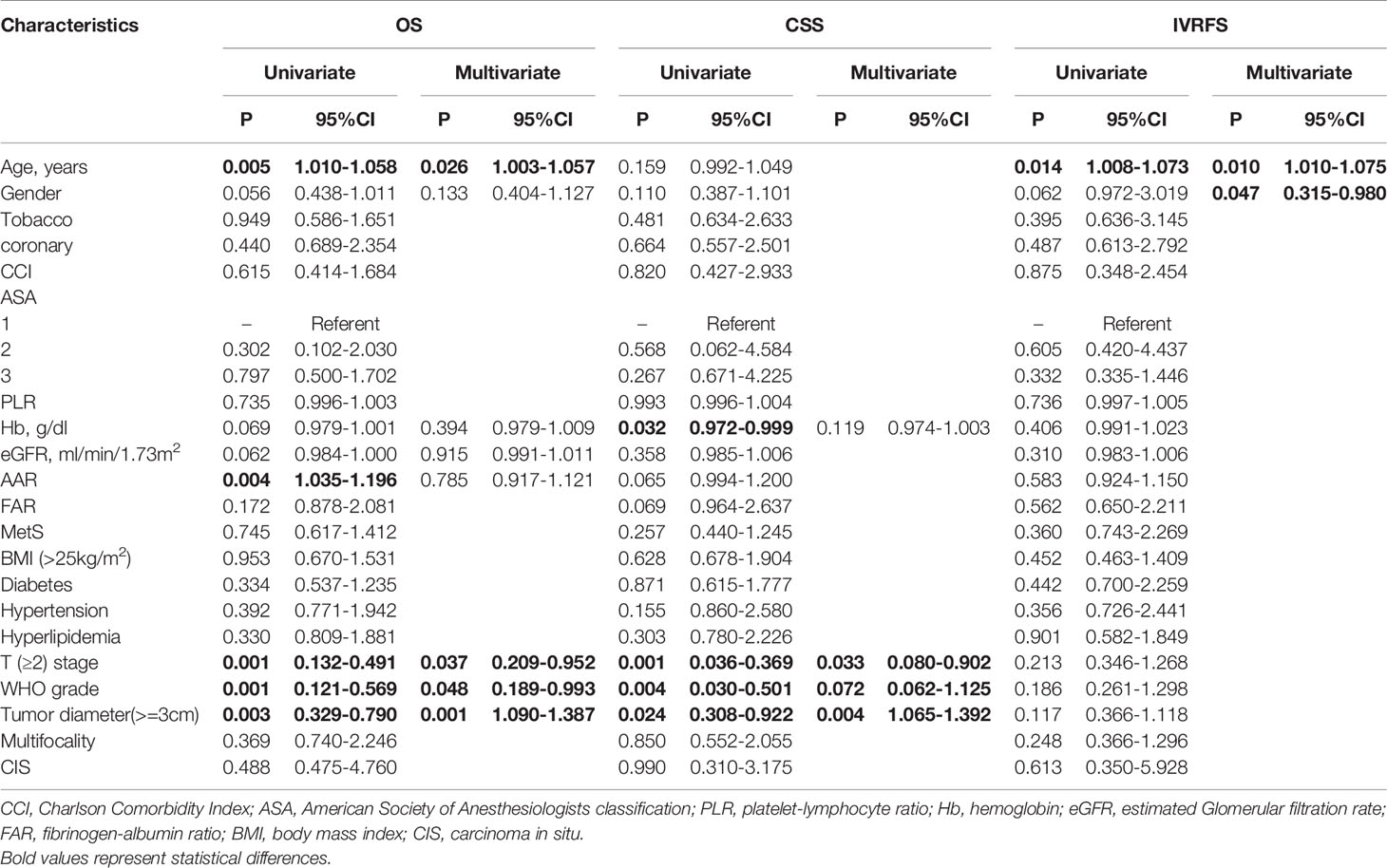
Table 4 Univariate and multivariate cox regression analyses for OS, CSS and IVRFS after propensity score matching.
In our present single-center study, 573 patients with UTUC treated with RNU were included and we observed whether MetS had negative impact on survival outcomes. Moreover, we re-evaluated the association between MetS and outcomes of UTUC after adjusting preoperative confounders by propensity score matching with a sample size of 314 patients. The results showed that the existence of MetS was not an independent factor for worse pathological outcomes and survival outcomes including death or IVR. However, it is worth noting that there presented a correlation which was of marginal significance between MetS and lower CSS in both multivariate Cox regression analysis and Kaplan-Meier survival analysis, indicating that MetS may be associated with increased risk of UTUC.
Evidence based on several current studies showed that patients diagnosed with MetS were more likely to have worse survival outcomes of several types of malignant tumors (10), such as breast cancer (3) and bladder cancer (5). But the inverse relationship between MetS and outcomes were presented in patients with ovarian (11) or renal cancer (12). Even within the same cancer, the results remain controversial. The results of evaluating prognostic value of MetS in localized clear cell renal cell carcinoma (ccRCC) (12, 13) were diametrically opposed. As for UTUC, a recent study based on the Chinese population demonstrated that MetS was a negative prognostic factor of CSS in UTUC and the trend was particularly persisted in patients with non-muscle-invasive UTUC, high-grade disease, and large tumor size (8). As described by another study based on Surveillance, Epidemiology and End Results-Medicare-Linked Database (SEER), MetS and its components were significant risk factors for UTUC among people aged over 65 (7). Whether MetS can be a predictor of UTUC still remains a topic of concern.
MetS, as a cluster of metabolic abnormalities, has complex clinical manifestations and diagnostic criteria. Diagnostic criteria suitable for western populations, which were mostly from large international or European and American institutions, such as the American Heart Association (AHA), the National Heart, Lung, and Blood Institute (NHLBI), and the International Diabetes Federation (IDF). Although some researchers have modified the diagnostic criteria to accommodate local populations, it could also be one of important reasons for differences in results. We adopted the latest version of diagnostic criteria released by CDS, making our best efforts to ensure that the diagnostic criteria was appropriate for the Chinese population. We also collected the use of therapeutic drug for MetS during follow-up according to diagnostic criteria to ensure that MetS could be diagnosed accurately when patients were admitted with normal blood pressure or blood glucose level. MetS may be more easily diagnosed with CDS criteria than others, due to its lower cut-off of BMI. In our study, patients diagnosed with MetS accounted for 32.5% of the total patients, which was higher than 24.4% based on IDF criteria in Xu’s research (8) and 17.1% based on National Cholesterol Education Program-Adult Treatment Panel III (ATPIII) criteria in Lu’s research (7). Difference of diagnostic criteria is expected to affect the level of baseline and clinicopathological characteristics but whether it will result in inconsistency in the prognostic value of MetS on UTUC prognosis still needs further research.
Obesity, as a major component of MetS, has been determined in many studies to be associated with poor prognosis of renal cancer (14) or other cancers. In IDF and ATPIII diagnostic criteria, obesity was defined using waist circumference as the indicator and recent studies have also shown that waist circumference could reflect centripetal obesity more directly than BMI. But the cut-off value of waist circumference was determined from data obtained from the American population. Studies showed that there was significant heterogeneity in waist circumference and BMI among different population and the baseline BMI of the Chinese population was significantly lower than that of western population. A study including 971 Chinese patients showed that BMI was a better predictor of cardiovascular events than waist circumference (9). Unfortunately, the cut-off values of waist circumference or waist-to-hip ratio in Chinese population have not been determined yet, which requires further collaborative research.
A meta-analysis concerning the impact of BMI on urothelial carcinoma provided conclusions that being obese and underweight were predictors for predicted worse survival outcomes, while being overweight was a protective factor (15). But in UTUC patients, previous studies revealed contradictory results. Ehdaie et al. (16) and Dabi et al. (17) showed that increased BMI impacts oncological outcomes in western patients with UTUC, but Kang et al. (18) Liu et al. (19) and Inamoto et al. (20) demonstrated that a preoperative decreased BMI was an independent predictor for OS and CSS after analyzing data from Korean, Chinese, and Japanese populations. Given the large differences in baseline BMI between Asian, and Western populations, collaborative international studies are needed to explained the controversy after rigorous matching. A meta-analysis including 10 studies showed that diabetes increased the risk of IVR in UTUC patients (21), and an international retrospective study discovered that hypertension was a significant risk factor for IVR based on data set from 17 centers worldwide (22). However, the effect of diabetes and hypertension on overall or cancer-specific survival has not been proven. An earlier study showed a weak inverse association between HDL-cholesterol and progress of bladder urothelial carcinoma (23). Xu et al. (8) discovered that patients with hypertriglyceridemia or low HDL-cholesterol were more likely to have adverse pathological features and lower OS, CSS, and RFS of UTUC in univariable Cox regression analyses, but not confirmed in multivariate Cox regression analyses. According to our data and analysis, all components found no statistically significant association with survival outcomes. Further studies are needed to confirm the impact of MetS and its components on progress or prognosis of UTUC.
There is increasing evidence to indicate that there is a certain relationship between MetS and survival prognosis in certain types of cancers. However, numerous mechanisms of how MetS affected pathological features and survival outcomes have been proposed but fail to elaborate at the molecular level, involving insulin-like growth factor (IGF) axis, pro-inflammatory cytokines, circulating factors, angiogenesis, and other important aspects (10). Meanwhile, a network containing these factors and a variety of complex signaling pathways regulates the relation between MetS and tumor progress. The insulin-like growth factor (IGF) system, composed of different subtypes with their receptors and binding proteins, plays an important role on tumor formation, differentiation, and progression. Increased IGF strongly correlates with insulin resistance and obesity, promoting proliferation and migration of pathological cell and overexpression of IGF-1R (24) and IGFBP-5 (24) in UTUC which was proved by in vitro experiment. Central fat distribution and low HDL-cholesterol is also associated with production of many pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and elevated level of reactive oxygen species (ROS). In addition, MetS patients tend to have high level of adipose tissue, which correlated with an elevated level of serum leptin and reduction of adiponectin. All these factors have been demonstrated to stimulate angiogenesis, which promotes epithelial cell proliferation (10).
Abundant evidence has described the closed association between each single component of MetS with tumor, and combining all the components of MetS as a single condition may not be appropriate. But at the same time, considering MetS and its components as independent factors for UTUC may reveal justification for pre-operative use of MetS-specific drugs including as statin and metformin.
In our study, some potential predicting factors were not included in the multiple Cox analysis, such as N stage, type of bladder cuff management and lymphovascular invasion (LVI). LND is not currently included in UTUC’s standard procedure and is performed only when lymph node metastasis is suspected. Only 89 patients (15.5%) underwent LND as shown in Tables 1, 3, which may result in potential sampling error. Common types of bladder cuff removal included transvesical, extravesical, and endoscopic approaches. The result of a study comparing different methods of bladder cuff management showed that there was no difference in terms of OS, CSS, and RFS among the three distal ureteral management, but patients who underwent the endoscopic approach had a higher risk of IVR (25). Meanwhile, other researchers have also reached different conclusions (26) and the impact of methods of bladder cuff management on oncological outcome is still controversial. The diagnosis of some pathological features including LVI are extremely dependent on pathological reports and sometimes it is difficult to obtain accurate information on some pathological features due to non-standard reporting formats. Smoking, as a factor closely associated with dyslipidemia, is known to increase the risk of experiencing adverse events and IVR in urothelial carcinoma patients (27, 28) but the relation and biological basis between smoking and MetS remains unclear. A recent annual cross-sectional survey showed that life-course cigarette smoking is associated with increased odds of MetS and low high-density lipoprotein-cholesterol (29), which may portend an association between MetS and tumor recurrence.
Our study is not devoid of limitations. First, limited by retrospective data, we could not detail the duration of each component and use of a corresponding drug. Also, small sample size prevented further subgroup analysis based on medication status, such as statin (n=23) or metformin (n=24). Thus, although we adjusted preoperative confounders by propensity score matching with a small caliber of 0.02, some confounders which may affect survival outcomes, like tobacco consumption and some pathological features, were not included. Finally, the duration, dose, and regimen of adjuvant chemotherapy were incomplete, so we were not able to evaluate its potential mitigation effect on survival outcomes.
In our study, no statistical difference was found between MetS and survival outcomes in UTUC, except a marginal association with lower CSS. Further studies are needed to evaluate the role of MetS and its each single components on UTUC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committee of Peking University People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XD carried out the study, participated in the data analysis and drafted the manuscript. XD, YD, FW, SH, and ZH collected the data. XD, YD, CQ, SL, YS, and TX participated in designing the study. XD, YD, XZ, and TX revised the manuscript for important intellectual content. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
This study was funded by the National Key Research and Development Program of China (No. 2018YFA0902802), Natural science foundation of Beijing, China (No. 7202219), National natural science foundation of China (No. 81802533), and Beijing Municipal Science & Technology Commission (No. Z191100006619010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fang D, Li YX, Jiang YH, Zhang J, Liu ZH, Xiong GY, et al. Characteristics and Regional Difference of Chinese Upper Tract Urothelial Carcinoma Patients: A Multi-Center Study by CUDA-UTUC Collaborative Group. Chin J Urol (2017) 38(12):885–90. doi: 10.3760/cma.j.issn.1000-6702.2017.12.002
2. Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, et al. Metabolic Syndrome, Metabolic Comorbid Conditions and Risk of Early-Onset Colorectal Cancer. Gut (2021) 70(6):1147–54. doi: 10.1136/gutjnl-2020-321661
3. Dibaba DT, Ogunsina K, Braithwaite D, Akinyemiju T. Metabolic Syndrome and Risk of Breast Cancer Mortality by Menopause, Obesity, and Subtype. Breast Cancer Res Treat (2019) 174(1):209–18. doi: 10.1007/s10549-018-5056-8
4. Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, et al. Meta-Analysis of Metabolic Syndrome and Prostate Cancer. Prostate Cancer Prostat Dis (2017) 20(2):146–55. doi: 10.1038/pcan.2017.1
5. Sha N, Xu H, Chen T, Tian D, Xie W, Xie L, et al. The Evaluation of the Association Between the Metabolic Syndrome and Tumor Grade and Stage of Bladder Cancer in a Chinese Population. Onco Targets Ther (2016) 9:1175–9. doi: 10.2147/OTT.S102424
6. Lee HY, Tang JH, Chen YH, Wu WJ, Juan YS, Li WM, et al. The Metabolic Syndrome is Associated With the Risk of Urothelial Carcinoma From a Health Examination Database. Int J Clin Oncol (2021) 26(3):569–77. doi: 10.1007/s10147-020-01834-3
7. Lu Y, Zhang W, Fan S, Liang Z, Li Z, Tian J, et al. Metabolic Syndrome and Risk of Upper Tract Urothelial Carcinoma: A Case-Control Study From Surveillance, Epidemiology and End Results-Medicare-Linked Database. Front Oncol (2020) 10:613366. doi: 10.3389/fonc.2020.613366
8. Xu H, Tan P, Zheng X, Ai J, Lin T, Jin X, et al. Metabolic Syndrome and Upper Tract Urothelial Carcinoma: A Retrospective Analysis From a Large Chinese Cohort. Urol Oncol (2019) 37(4):291.e19–e28. doi: 10.1016/j.urolonc.2018.12.005
9. group, C.D.S.M.S.R.C. Recommendations of Chinese Diabetes Society on Metabolic Syndrome. Chin J Diabetes (2004) 12:151–61. doi: 10.3321/j.issn:1006-6187.2004.03.002
10. Uzunlulu M, Telci Caklili O, Oguz A. Association Between Metabolic Syndrome and Cancer. Ann Nutr Metab (2016) 68(3):173–9. doi: 10.1159/000443743
11. Chen Y, Zhang L, Liu W, Wang K, et al. Case-Control Study of Metabolic Syndrome and Ovarian Cancer in Chinese Population. Nutr Metab (Lond) (2017) 14:21. doi: 10.1186/s12986-017-0176-4
12. Liu Z, Wang H, Zhang L, Li S, Fan Y, Meng Y, et al. Metabolic Syndrome is Associated With Improved Cancer-Specific Survival in Patients With Localized Clear Cell Renal Cell Carcinoma. Transl Androl Urol (2019) 8(5):507–18. doi: 10.21037/tau.2019.10.04
13. Kriegmair MC, Mandel P, Porubsky S, Dürr J, Huck N, Nuhn P, et al. Metabolic Syndrome Negatively Impacts the Outcome of Localized Renal Cell Carcinoma. Horm Cancer (2017) 8(2):127–34. doi: 10.1007/s12672-017-0289-2
14. Zhang GM, Zhu Y, Ye DW. Metabolic Syndrome and Renal Cell Carcinoma. World J Surg Oncol (2014) 12:236. doi: 10.1186/1477-7819-12-236
15. Yang Z, Bai Y, Hu X, Wang X, Han P. The Prognostic Value of Body Mass Index in Patients With Urothelial Carcinoma After Surgery: A Systematic Review and Meta-Analysis. Dose Response (2020) 18(4):1559325820979247. doi: 10.1177/1559325820979247
16. Ehdaie B, Chromecki TF, Lee RK, Lotan Y, Margulis V, Karakiewicz PI, et al. Obesity Adversely Impacts Disease Specific Outcomes in Patients With Upper Tract Urothelial Carcinoma. J Urol (2011) 186(1):66–72. doi: 10.1016/j.juro.2011.03.031
17. Dabi Y, El Mrini M, Duquesnes I, Delongchamps NB, Sibony M, Zerbib M, et al. Impact of Body Mass Index on the Oncological Outcomes of Patients Treated With Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. World J Urol (2018) 36(1):65–71. doi: 10.1007/s00345-017-2095-4
18. Kang HW, Jung HD, Ha YS, Kim TH, Kwon TG, Byun SS, et al. Preoperative Underweight Patients With Upper Tract Urothelial Carcinoma Survive Less After Radical Nephroureterectomy. J Korean Med Sci (2015) 30(10):1483–9. doi: 10.3346/jkms.2015.30.10.1483
19. Liu JY, Li YH, Liu ZW, Zhang ZL, Ye YL, Yao K, et al. Influence of Body Mass Index on Oncological Outcomes in Patients With Upper Urinary Tract Urothelial Carcinoma Treated With Radical Nephroureterectomy. Int J Urol (2014) 21(2):136–42. doi: 10.1111/iju.12208
20. Inamoto T, Komura K, Watsuji T, Azuma H. Specific Body Mass Index Cut-Off Value in Relation to Survival of Patients With Upper Urinary Tract Urothelial Carcinomas. Int J Clin Oncol (2012) 17(3):256–62. doi: 10.1007/s10147-011-0284-5
21. Gao X, Zhou L, Ai J, Wang W, Di X, Peng L, et al. The Impact of Diabetes on the Prognosis of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Front Oncol (2021) 11:741145. doi: 10.3389/fonc.2021.741145
22. Katims AB, Say R, Derweesh I, Uzzo R, Minervini A, Wu Z, et al. Risk Factors for Intravesical Recurrence After Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Cancer (Robuust Collaboration). J Urol (2021) 206(3):568–76. doi: 10.1097/JU.0000000000001786
23. Sha N, Xu H, Chen T, Tian DW, Xie WQ, Xie LG, et al. The Evaluation of the Association Between the Metabolic Syndrome and Tumor Grade and Stage of Bladder Cancer in a Chinese Population. Onco Targets Ther (2016) 9:1175–9. doi: 10.2147/OTT.S102424
24. Liang PI, Wang YH, Wu TF, Wu WR, Liao AC, Shen KH, et al. IGFBP-5 Overexpression as a Poor Prognostic Factor in Patients With Urothelial Carcinomas of Upper Urinary Tracts and Urinary Bladder. J Clin Pathol (2013) 66(7):573–82. doi: 10.1136/jclinpath-2012-201278
25. Xylinas E, Rink M, Cha EK, Clozel T, Lee RK, Fajkovic H, et al. Impact of Distal Ureter Management on Oncologic Outcomes Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Eur Urol (2014) 65(1):210–7. doi: 10.1016/j.eururo.2012.04.052
26. Lai SC, Wu PJ, Liu JY, Seery S, Wang JY, et al. Oncological Impact of Different Distal Ureter Managements During Radical Nephroureterectomy for Primary Upper Urinary Tract Urothelial Carcinoma. World J Clin Cases (2020) 8(21):5104–15. doi: 10.12998/wjcc.v8.i21.5104
27. Tellini R, Mari A, Muto G, Cacciamani GE, Moschini M. Impact of Smoking Habit on Perioperative Morbidity in Patients Treated With Radical Cystectomy for Urothelial Bladder Cancer: A Systematic Review and Meta-Analysis. Eur Urol Oncol (2021) 4(4):580–93. doi: 10.1016/j.euo.2020.10.006
28. Xylinas E, Kluth LA, Rieken M, Lee RK, Elghouayel M, Ficarra V, et al. Impact of Smoking Status and Cumulative Exposure on Intravesical Recurrence of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy. BJU Int (2014) 114(1):56–61. doi: 10.1111/bju.12400
Keywords: metabolic syndrome, upper tract urothelial carcinoma, propensity score matching, prognosis, survival
Citation: Dai X, Wang F, Du Y, Qin C, Lai S, Song Y, Huang Z, Han S, Zhang X and Xu T (2022) Could Metabolic Syndrome Be a Predictor of Survival Outcomes in Upper Tract Urothelial Carcinoma? A Propensity Score Matching Study in a Large Chinese Center. Front. Oncol. 12:816915. doi: 10.3389/fonc.2022.816915
Received: 17 November 2021; Accepted: 25 April 2022;
Published: 26 May 2022.
Edited by:
Chengfei Liu, University of California, Davis, United StatesReviewed by:
Emily Jane Gallagher, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2022 Dai, Wang, Du, Qin, Lai, Song, Huang, Han, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Xu, eHV0YW9AcGt1cGguZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.