- 1Department of Radiation Oncology, Fujian Medical University Union Hospital, Fuzhou, China
- 2The Graduate School, Fujian Medical University, Fuzhou, China
- 3Department of Radiation Oncology, Fujian Branch of Shanghai Children’s Medical Center Affiliated to Shanghai Jiaotong University School of Medicine, Fuzhou, China
- 4Department of Radiation Oncology, Fujian Children’s Hospital, Fuzhou, China
- 5Department of Medical Imaging Technology, College of Medical Technology and Engineering, Fujian Medical University, Fuzhou, China
- 6Union Clinical Medicine College, Fujian Medical University, Fuzhou, China
- 7School of Clinical Medicine, Fujian Medical University, Fuzhou, China
Objective: The purpose of this study was to investigate the relationship between lymph node harvest and the prognosis in locally advanced rectal cancer (LARC) patients after neoadjuvant chemoradiotherapy (nCRT).
Methods: Patients who were diagnosed with clinical LARC and treated with nCRT and radical surgery between June 2008 and July 2017 were included in this study. The relationship between lymph node retrieval and prognosis was analyzed. Other lymph node-related indicators were explored.
Results: A total of 837 patients with a median follow-up of 61 (7-139) months were included in the study. The five-year DFS and OS rates of all patients were 74.9% and 82.3%, respectively. Multivariate survival analysis suggested that dissection of ≥ 12 lymph nodes did not improve OS or DFS. 7 was selected as the best cutoff value for the total number of lymph nodes retrieved by Cox multivariate analysis (χ2 = 10.072, HR: 0.503, P=0.002). Dissection of ≥ 5 positive lymph nodes (PLNs) was an independent prognostic factor for poorer DFS (HR: 2.104, P=0.004) and OS (HR: 3.471, p<0.001). A positive lymph node ratio (LNR) of more than 0.29 was also an independent prognostic factor for poorer DFS (HR: 1.951, P=0.002) and OS (HR: 2.434, p<0.001).
Conclusion: The recommends that at least 7 harvested lymph nodes may be more appropriate for LARC patients with nCRT. PLN and LNR may be prognostic factors for LARC patients with ypN+ after nCRT.
Introduction
Globally, the incidence of colorectal cancer ranks third among all malignant tumors, accounting for 8.0% to 9.0% of all new cases, and it is the third leading cause of cancer deaths worldwide (1). Neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) is recommended as a standard treatment mode for locally advanced rectal cancer (LARC) in the National Comprehensive Cancer Network’s (NCCN) Clinical Practice Guidelines (2). Regional lymph node status is an important factor affecting overall survival (OS) and disease-free survival (DFS) in patients with nonmetastatic rectal cancer (RC) (3).
In the current TNM staging system, rectal cancer with regional lymph node metastasis is classified into stage III (4). According to the current guidelines of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), at least 12 lymph nodes retrieved during surgery are required to determine the presence or absence of lymph node metastasis and improve the accuracy of postoperative staging of rectal cancer (4–6). However, these conclusions are derived from patients with rectal cancer who have not received neoadjuvant treatment and have undergone radical surgical resection as the initial treatment. Many previous studies have proposed that the harvest rate of lymph nodes in rectal cancer patients receiving neoadjuvant therapy is significantly lower than that in patients undergoing surgical resection as the initial treatment (7–9). The study of Baxter et al. (10) showed that less than 20% of patients with stage II rectal cancer who received preoperative radiotherapy had more than 12 lymph nodes detected during the operation. For these patients, it is unclear whether the reduction in the number of lymph nodes detected will lead to insufficient staging or affect the prognosis. At present, the medical community has not reached a consensus on the requirements for the total number of lymph nodes retrieved from patients undergoing neoadjuvant therapy.
In this study, we retrospectively analyzed the clinical data of 837 LARC patients who underwent nCRT in our center from June 2008 to July 2017 and explored the relationship between lymph node detection and prognosis after nCRT combined with TME.
Materials and Methods
Study Population
The data of 1083 LARC patients who underwent neoadjuvant chemoradiotherapy in our center from June 2008 to July 2017 were collected according to the following inclusion criteria (see below). A total of 246 patients were excluded from the study based on the exclusion criteria (see below). A total of 837 patients were finally included. All cases had records of imaging data: 808 patients underwent MRI, 811 patients underwent endoscopic US and 790 patients underwent both. All cases were followed up regularly through outpatient clinics or by telephone until 2020.04.23.
The inclusion criteria were as follows: 1. Patients who were pathologically diagnosed with rectal cancer by colonoscopy and received treatment for the first time; 2. Complete medical history data; 3. According to the 7th edition of the AJCC/UICC TNM staging system (patients diagnosed before 2010 were restaged according to the 7th edition staging system), patients were clearly diagnosed with clinical stage II/III LARC after completing the preoperative imaging examinations; 4. According to preoperative MR, colonoscopy and digital rectal examination, the lower edge of the tumor was ≤12 cm from the anal edge; 5. Patients who received neoadjuvant therapy and laparoscopic radical surgery for rectal cancer.
The exclusion criteria were as follows: 1. Patients with a history of other organ tumors or those diagnosed with simultaneous/metachronous multisource cancer; 2. Patients who accepted neoadjuvant radiotherapy/chemotherapy alone or received sequential chemotherapy; 3. Distant metastasis was detected before or during the operation; 4. A previous history of pelvic radiotherapy or surgery; 5. Patients received open surgery.
Treatment
Neoadjuvant Chemoradiotherapy
Radiotherapy was administered with 3-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) techniques. Radiotherapy treatments were planned using the Varian Eclipse treatment planning system, version 13.5. The delineation of the target and the prescription dose followed the 62 and 83 International Commission on Radiation Units and Measurements (ICRU) reports. The definition of the target regions was as follows: gross tumor volume (GTV) was defined as the volume of the primary tumor (GTV-T) and metastatic lymph nodes (GTV-N) determined by CT imaging before treatment. The clinical target volume (CTV) was defined as GTV plus all mesorectum, presacral soft tissue, internal iliac and obturator lymphatic drainage regions. The planning target volume (PTV) was defined as the GTV or CTV with uniform margins of 5 mm. All patients received a long-term radiotherapy regimen: a total dose of 50 Gy/25 F (2.0 Gy/F) was delivered to PTV-GTV, and a total dose of 45 Gy/25 F (1.8 Gy/F) was delivered to PTV-CTV. The doses to the normal tissues were limited according to the RTOG pelvic normal tissue contouring guidelines. Irradiation was performed continuously for 5 days a week with weekends off. The total duration of treatment was 5 weeks.
All patients received 5-fluorouracil (5-FU)-based concurrent chemotherapy during neoadjuvant radiotherapy. There were two main concurrent chemotherapy regimens: 1. regimens containing oxaliplatin, including the XELOX, FOLFOX4, mFOLFOX6 and FOLFOX6 regimens; and 2. regimens not containing oxaliplatin, including a single agent capecitabine regimen and a De Gramont regimen.
Surgery and Pathology
Radical resection surgery was performed approximately 8–12 weeks after completion of nCRT. All operations were conducted following the principles of TME. The surgeon decides the surgical procedure, surgical extent and anus preservation according to the size, location and extent of the tumor, as shown in the relevant preoperative imaging examinations. The criteria of lymph node collection and counting were as follows: 1. To meet the requirements of lymph node dissection in TME surgery, at least 3 sites of lymph nodes, including pararectal lymph nodes, the lymph nodes adjacent to the superior rectal artery and the lymph nodes around the root of the inferior mesenteric artery, were removed during the operation; 2. The tissue samples obtained during the operation were manually dissected by the surgeons to find and collect the lymph nodes; 3. Samples were immersed in 10% formalin after resection and sent to the pathology department on the same day. Pathological examinations and diagnoses of the samples were performed independently by 2 experienced pathologists. In addition to the lymph nodes removed by the surgeons, the pathologists tried to collect residual lymph nodes from the other samples. Pathological TNM stage classification was evaluated based on the 7th edition of the UICC/AJCC TNM staging system. To assess the response to treatments, tumor regression grade (TRG) is used to evaluate histologic tumor regression after CRT. AJCC TRG classification system (grade 0, no sign of tumor cells; grade 1, single cell or small groups of cancer cells; grade 2, residual cancer with a desmoplastic response; grade 3, no regression) was used in this study (11). Positive lymph node (PLN) was defined as the number of positive lymph nodes after surgery. The positive lymph node ratio (LNR) was calculated as the quotient between the number of tumor-infiltrated lymph nodes and total number of retrieved lymph nodes.
Follow-Up
Patients were reviewed once every 3 months within the first 2 years after surgery, then every 6 months for the next 3 years and annually thereafter. The re-examinations included a physical examination (particularly a digital rectal examination), serum carbohydrate antigen 199 (CA199), carcinoembryonic antigen (CEA), chest CT scanning, liver plus pelvic MRI, transrectal color Doppler ultrasound, colonoscopy and other necessary examinations. The last follow-up date was April 23, 2020. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of disease recurrence (locoregional or distant recurrence) or death from any cause. Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause.
Statistical Analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 23.0) and Stata (StataCorp 2013, Stata Statistical Software for Windows, Release 13). X-tile (Yale University, X-tile Bioinformatics Software for Windows, Version 3.6.1) was used to select the best cutoff value for survival analysis. For comparisons between groups, the chi-square test was used for categorical variables, while the Mann–Whitney rank sum test or Kruskal–Wallis rank sum test was used for nonnormally distributed continuous variables. Survival curves were drawn by the Kaplan–Meier method and compared using log-rank tests. The independent prognostic factors for rectal cancer were identified by the Cox proportional hazard regression model. Before Cox analysis, it was necessary to perform collinearity diagnosis and test proportional hazards (PH) assumptions for the included analysis factors. For the variables with violations of the PH assumptions, time-dependent Cox regression was applied. The independent variable was directly included in the Cox analysis if it did not meet the time-dependent characteristics; otherwise, it was used as a stratifying variable for stratified Cox analysis. The most significant statistical cutoff point for the total number of lymph nodes retrieved was determined by a cutoff point survival analysis. The likelihood ratio chi-square was calculated with Cox regression to measure homogeneity, and a higher likelihood ratio chi-square score indicates better homogeneity. P < 0.05 was considered statistically significant unless specified otherwise.
Results
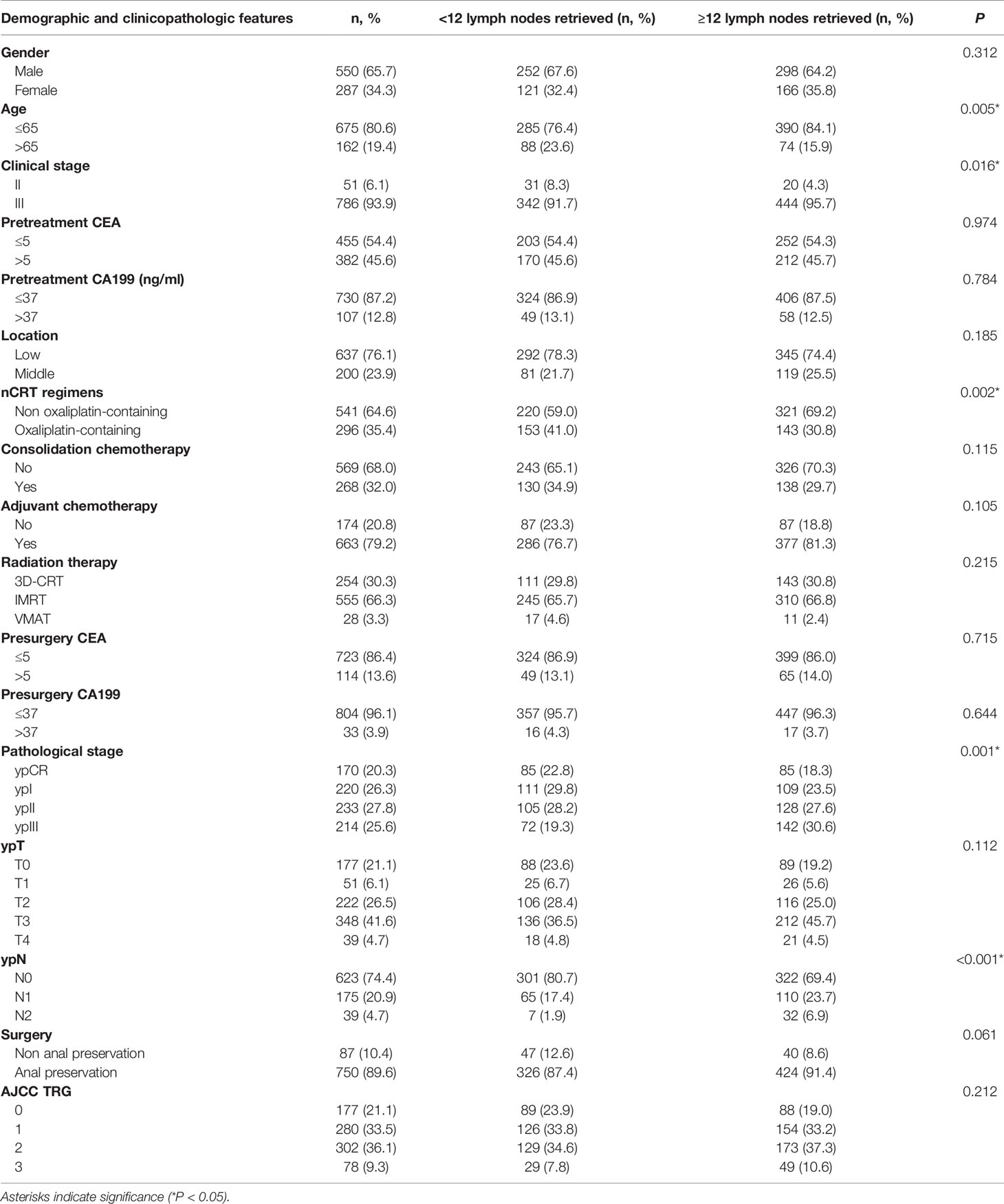
A total of 837 patients with stage II/III rectal cancer who underwent nCRT and TME in our center from June 2008 to July 2017 were included in this study. The median follow-up was 61 (7~139) months. The clinical and pathological data of the patients are shown in Table 1. A total of 93.9% of patients were clinical stage III before treatment. The median number of lymph nodes detected was 12 (0~42). A total of 373 (44.6%) patients had fewer than 12 lymph nodes harvested, and 464 (55.4%) patients had 12 or more lymph nodes retrieved.
The Response to nCRT and Its Involvement With the Total Number of Lymph Nodes Retrieved
Median total number of lymph nodes retrieved in the four TRG0-3 groups was 11 (0~31), 12 (0~37), 13 (1~42), and 13 (3~41) (P=0.011, Figure 1A). A cohort of patients with TRG0-1 was considered a good treatment response group, and another with TRG2-3 was considered a poor treatment response group. The total number of lymph nodes retrieved in the good response group was significantly lower than that in the poor response group, and the median total numbers were 12 (0~37) and 13 (1~42), respectively (P=0.001).
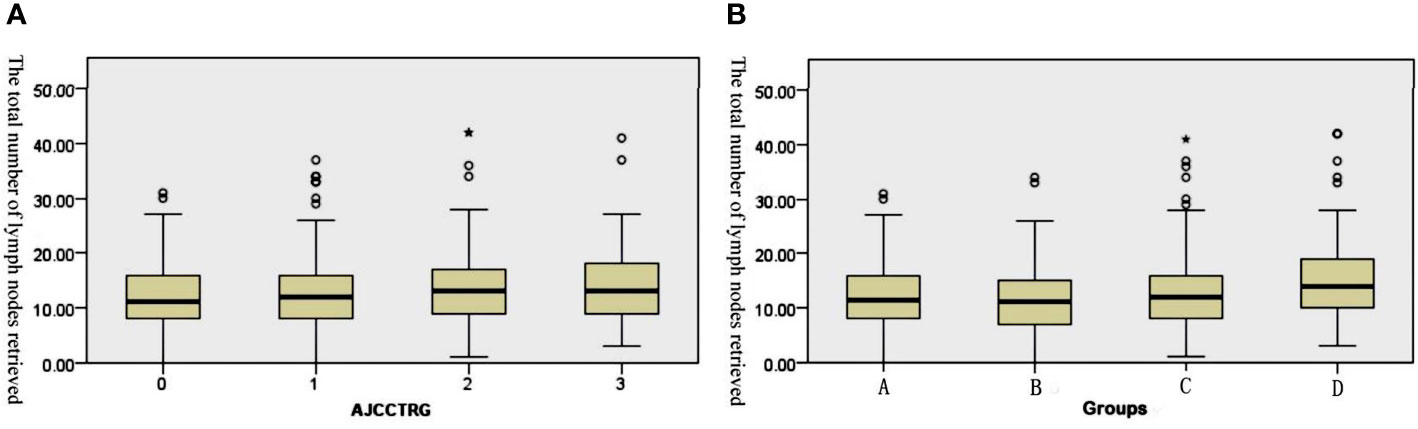
Figure 1 Box plots of the number of retrieved lymph nodes. (A) The retrieved lymph nodes in the four stage groups after the operation. (B) The retrieved lymph nodes in the different TRG groups after the operation.
Based on the postoperative staging, all patients were divided into four groups: Group A (ypCR), Group B (ypT1-2N0), Group C (ypT3-4N0) and Group D (ypT1-4N+). The median total number of lymph nodes retrieved in the four groups was 11.5 (0~31), 11 (0~34), 12 (1~41) and 14 (3~42). The results of the Kruskal–Wallis tests revealed that there were significant differences in the total number of lymph nodes among the four subgroups (H=27.158, p<0.001). After pairwise comparison, the total number of lymph nodes retrieved in Group D was significantly higher than that in the other three groups (the adjusted P values were p<0.001, p<0.001 and P=0.017, respectively, Figure 1B). Further analysis showed that 612 (73.1%) patients achieved tumor downstaging after nCRT, while 225 (26.9%) patients did not achieve tumor downstaging. The median total number of lymph nodes retrieved in the two groups was 12 (0~41) and 13 (1~42), and the difference between them was statistically significant (U=55239.0, p<0.001).
Survival Analysis
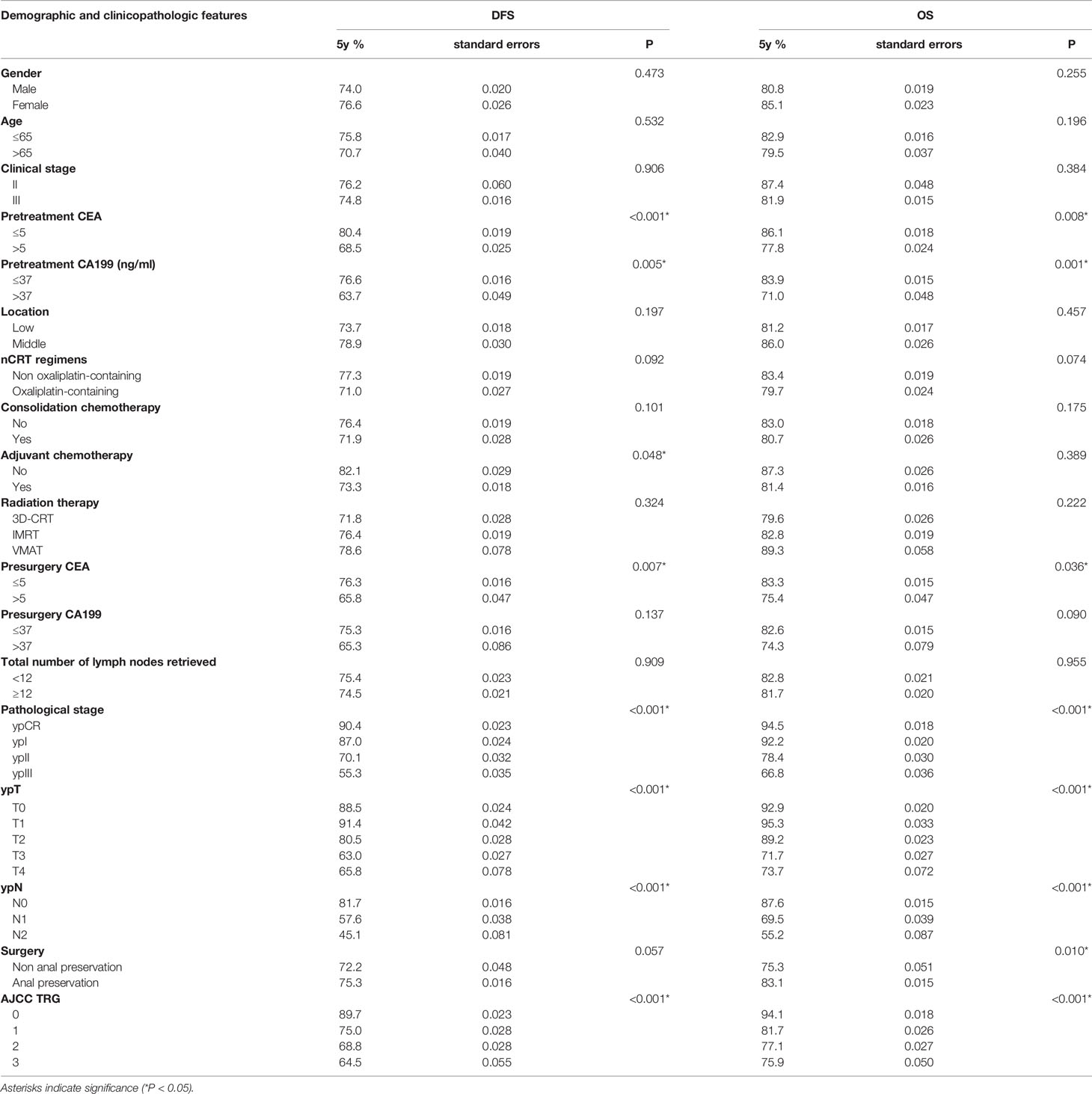
The 5-year DFS was 74.9%, and the 5-year OS was 82.3% for all patients in our cohort. Kaplan–Meier survival analysis showed that dissecting fewer than 12 lymph nodes was not significantly related to a poor DFS and OS (P=0.909 and 0.955, respectively). The results are detailed in Table 2.
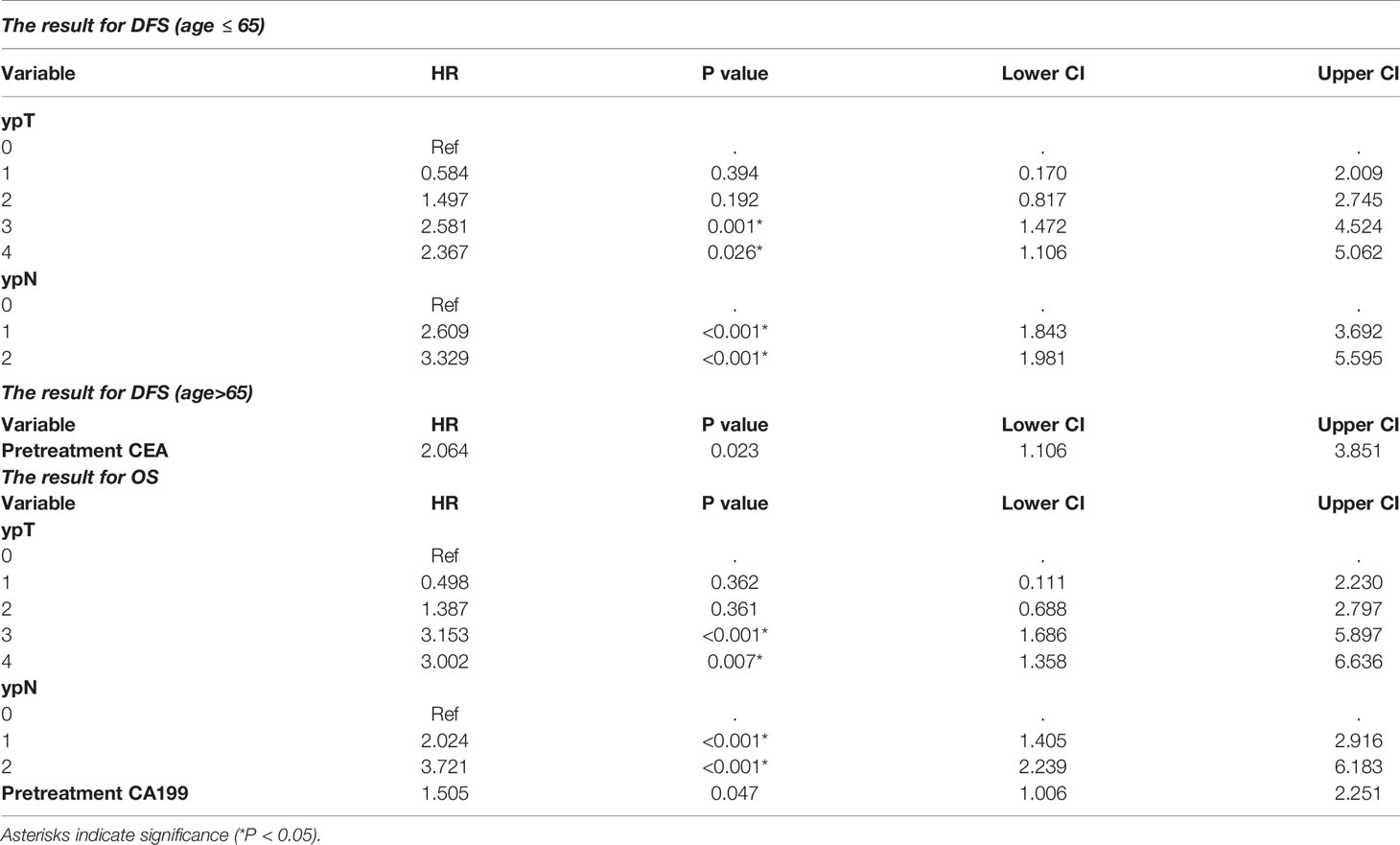
The stepwise multivariate Cox analysis for DFS showed that dissection of ≥ 12 lymph nodes was not an independent prognostic factor for DFS in the ≤ 65 age group (P=0.413 in the ≤ 65 age group and P=0.390 in the other group). For OS, the results remained nonsignificant in all patients (P=0.154). The results are detailed in Table 3.
Subgroup survival analyses were conducted in the TRG0~1 and TRG2~3 groups separately. Univariate and multivariate survival analyses showed that dissection of ≥ 12 lymph nodes was not significantly associated with DFS (P=0.839 and P=0.330, respectively) or OS (P=0.601 and P=0.199, respectively) in the TRG0~1 group or in the TRG2~3 group (P=0.945 and P=0.552 for DFS, respectively; P=0.991 and P=0.641 for OS, respectively).
New Lymph Node Related Indicators
The Best Cutoff Value of the Total Number of Lymph Nodes Retrieved
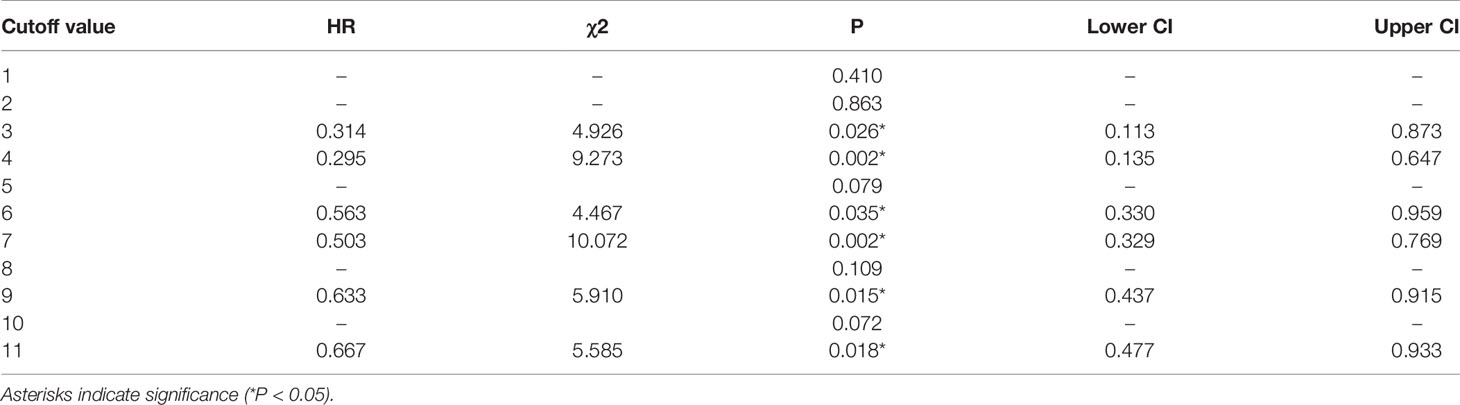
Cox multivariate analysis was adopted, and 1 to 11 were used as the cutoff values of the total number of lymph nodes retrieved in the model. The results indicated that there were significant differences in OS when the cutoff values were 3, 4, 6, 7, 9 and 11 (see Table 4). The highest chi-square value was deemed the cutoff point. Therefore, 7 was selected as the best cutoff value for the total number of lymph nodes retrieved. Survival curves based on multivariate Cox regression are shown in Figure 2.
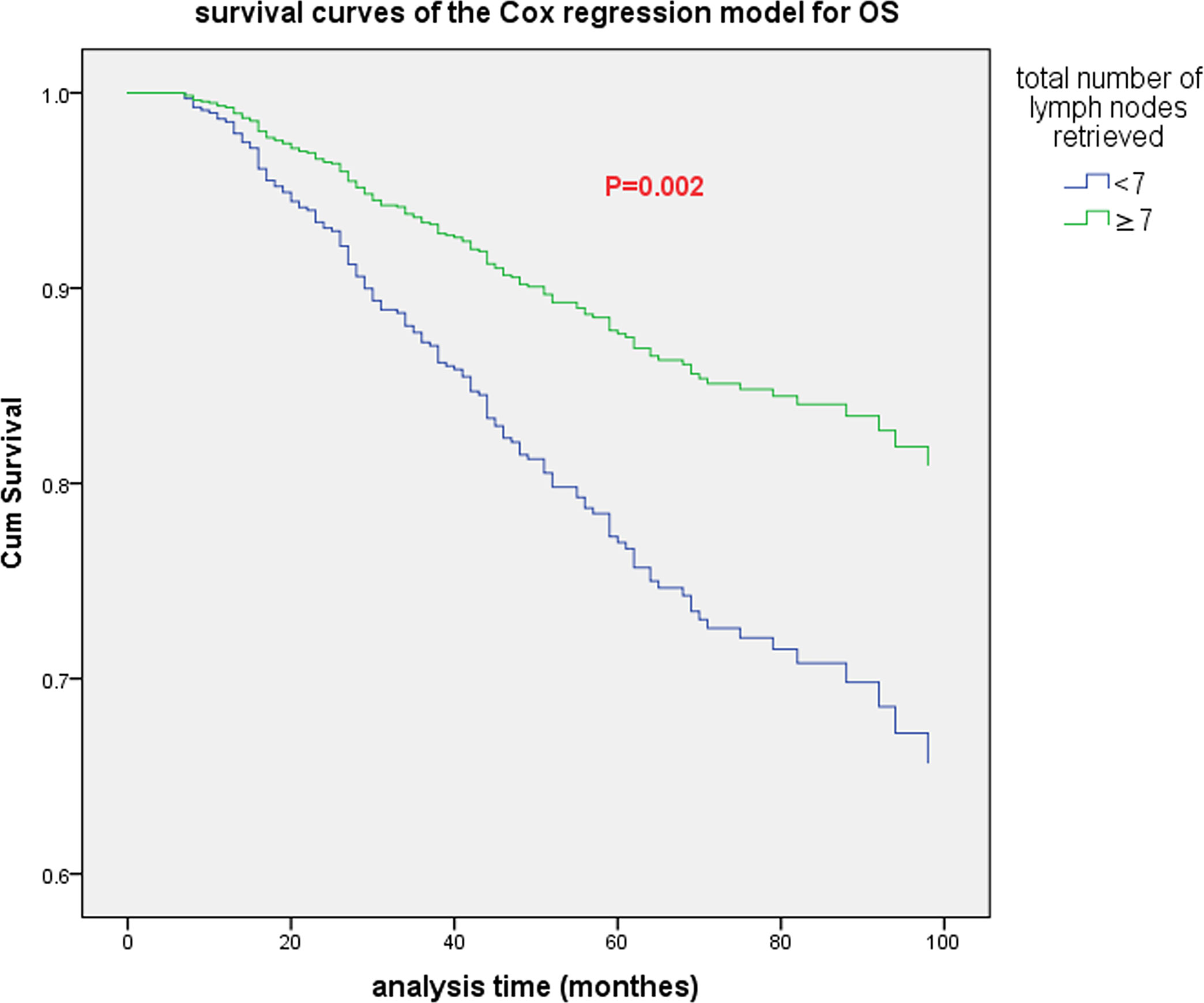
Figure 2 The survival curve (based on multivariate Cox regression) of all patients when the optimal cutoff value of the total number of lymph nodes retrieved was 7.
Positive Lymph Node
The optimal cutoff value for PLNs was confirmed to be 5 by using X-tile software. The results are detailed in the supplementary materials (Supplementary Figure S1). Univariate analysis showed that PLN (5 as the cutoff value) was significantly associated with DFS (5-year DFS: 58.4% vs. 31.5%, P=0.040) and OS (5-year OS: 70.3% vs. 40.4%, p<0.001). Cox multivariate analysis showed that patients with PLN ≤ 5 had significantly better DFS (HR: 2.104, 95% CI: 1.261-3.512, P=0.004) and OS (HR: 3.471, 95% CI: 1.999-6.030, p<0.001) than those with PLN>5. The patient survival curves are shown in Figure 3.
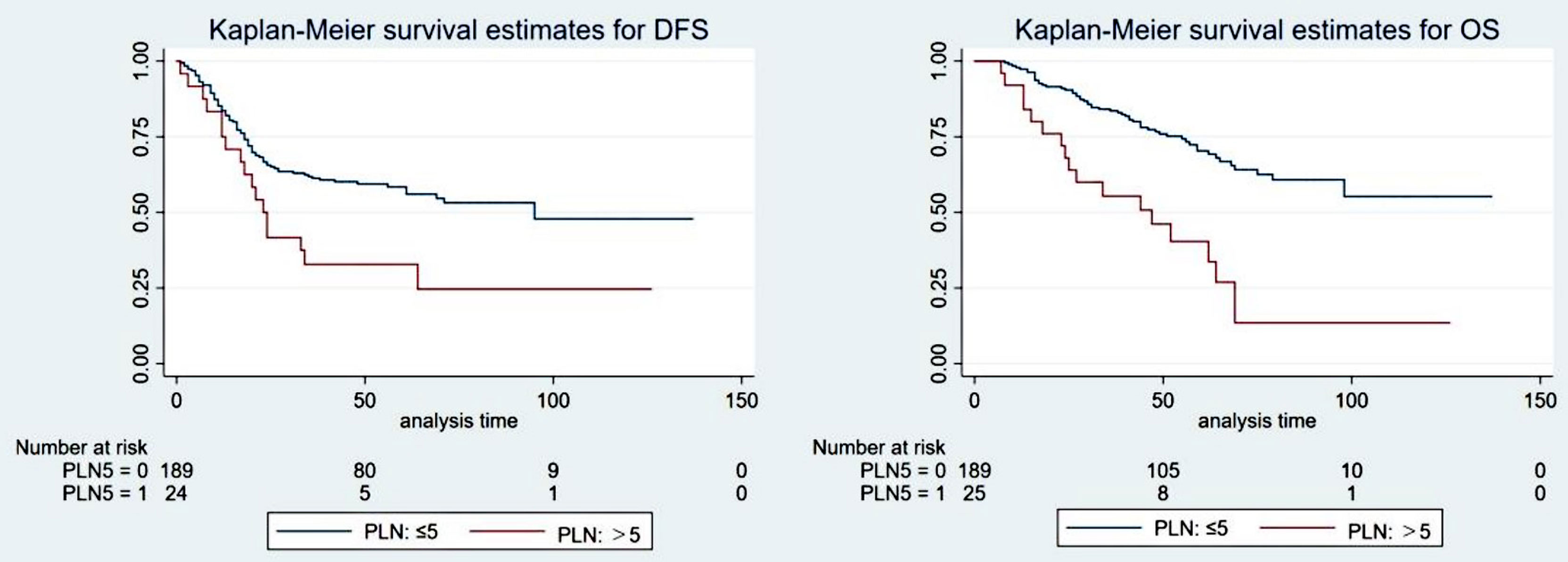
Figure 3 The survival curve of the ypT1-4N+ group when the optimal cutoff value of the positive lymph nodes (PLNs) was 5.
Positive Lymph Node Ratio
The optimal cutoff value for the LNR was also confirmed by X-tile software. Detailed descriptions are provided in the supplementary materials (Supplementary Figure S2). Univariate analysis showed that the LNR (0.29 as the cutoff value) was significantly associated with DFS (5-year DFS: 60.7% vs. 37.5%, P=0.001) and OS (5-year OS: 72.3% vs. 49.0%, p<0.001). Cox multivariate analysis showed that LNR ≥0.29 was an independent prognostic factor for poorer DFS (HR: 1.951, 95% CI: 1.289-2.955, P=0.002) and OS (HR: 2.434, 95% CI: 1.519-3.899, p<0.001). The patient survival curves are shown in Figure 4.
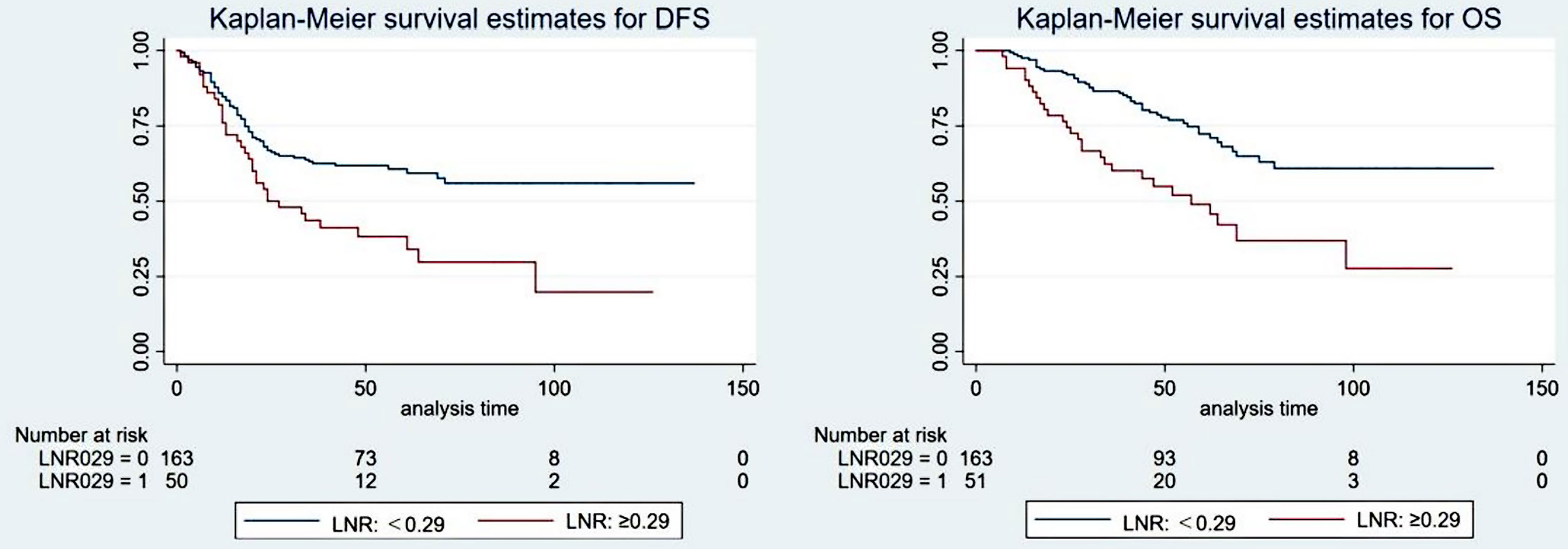
Figure 4 The survival curve of the ypT1-4N+ group when the optimal cutoff value of the positive lymph node ratio (LNR) was 0.29.
Discussion
An accurate evaluation of lymph nodes has important reference significance for postoperative pathological staging after TME surgery and follow-up treatments, and it is a powerful prognostic factor as confirmed by several studies (12–15). The AJCC recommends that at least 12 lymph nodes should be examined for accurate tumor staging (16). However, the number of lymph nodes retrieved varies greatly under the influence of many factors (17), and approximately 30-50% of patients have fewer than 12 lymph nodes retrieved (15, 18–21). Insufficient lymph node dissection can lead to tumor understaging and poor subsequent treatment selection and ultimately result in a poor prognosis (12, 14, 15, 22–24). Unfortunately, due to controllable and uncontrollable factors, the goal of examining a sufficient number of lymph nodes is generally difficult to achieve. These factors may include the experience of the surgeon, the experience and work attitudes of the pathologist, the patient’s condition (age, sex, degree of obesity, etc.) and the characteristics of the disease (stage, tumor location and distance from the anus) (12). Previous studies have revealed that neoadjuvant therapy can lead to a significantly decreased number of lymph nodes detected (7–9). The mechanism behind this phenomenon could be radiation‐induced fibrosis, lymphocyte depletion, tissue contraction, adipocyte replacement and interstitial atrophy, making it more difficult to detect lymph nodes during surgery or pathological examination (25–27). In addition, a greater time interval to TME operations leads to an increased incidence of interstitial fibrosis, which further reduces the number of lymph nodes retrieved. The previous criteria for dissecting at least 12 lymph nodes might not be suitable for patients after nCRT.
The comparison of the different postoperative stage groups in this study showed that the total number of lymph nodes retrieved in ypT1-4N+ patients whose prognoses were poor was significantly greater than that in other groups. The reason for the increase in the total number of lymph nodes detected in this group might be as follows: the patients in this group had a poor response to nCRT, which showed poor tumor regression and the presence of positive lymph nodes, significantly reducing the difficulty of retrieving lymph nodes during surgery when compared with the other three groups. Due to the poor response of these groups to nCRT, this phenomenon mostly indicated that the patients were not sensitive to subsequent systemic treatments, their recurrence rate was high and they had worse long-term survival in spite of the increased number of lymph nodes retrieved (28, 29).
A previous study showed that TRG is significantly related to the survival of rectal cancer patients. Patients who achieved a complete remission had a significant survival benefit compared to those who did not (30). Some scholars compared the survival after neoadjuvant treatment for rectal cancer in the two groups using 12 as the cutoff point (31). The results showed that a reduction in the total number of lymph nodes retrieved did not have an adverse impact on the prognosis, suggesting that the tumor responded well to neoadjuvant therapy and had better local control. Gurawalia et al. (22) analyzed 364 rectal cancer patients diagnosed during 2010-2014, 91 of whom received neoadjuvant therapy, and the results showed that patients with fewer than 12 lymph nodes retrieved were more likely to achieve pCR (40% vs. 26%, p<0.05) and a lower TRG classification. Wang et al. (32) compared the total number of lymph nodes retrieved between the TRG0-1 group and TRG2-3 group. A subgroup analysis of the TRG0-1 group showed that the prognosis between the group with < 12 lymph nodes retrieved and the group with ≥ 12 lymph nodes retrieved was similar in terms of OS, DFS, LRFS and DMFS. However, patients with ≥ 12 lymph nodes retrieved had better OS, DMFS and DFS in the TRG2-3 group. Therefore, the author believed that retrieving sufficient lymph nodes was still necessary, especially for patients with a potentially poor tumor response. However, our study reported different outcomes. Survival analysis showed that whether the goal of 12 lymph node dissections was achieved had no difference in the long-term survival of patients regardless of the TRG group. This difference in results could be due to the differences in the treatment regimens in the study design. A total of 64.4% of the patients received interval chemotherapy in the study of Wang et al., while 0.0% did so in our study. It was noted that though the results of our study showed that the total number of lymph nodes retrieved in the TRG2-3 group was significantly more than that in the TRG0-1 group (P=0.001), the absolute difference observed was too small to be clinically significance. The same consideration was observed in the analysis of the correlation between the number of retrieved nodes and downstaging after nCRT. Large randomized control trials are necessary to test these hypotheses.
At present, the influence of the total number of lymph nodes retrieved after neoadjuvant treatment on the prognosis of patients with rectal cancer is still controversial. Several studies found that dissection of < 12 lymph nodes did not adversely affect survival (7, 33, 34). In contrast, Narayanan et al. (35) extracted data from patients diagnosed with rectal cancer during 2004-2014 from the NCDB. They found that retrieving ≥ 12 lymph nodes in patients who did not reach ypCR after neoadjuvant therapy can improve OS but not in patients who achieved ypCR. In our study, there was no statistically significant difference in DFS and OS between the group with < 12 lymph nodes and the group with ≥ 12 lymph nodes. Subsequent Cox multivariate analysis showed that 7 may be the best cutoff value for the total number of lymph nodes retrieved after nCRT. Other more sensitive lymph node-related parameters should be further evaluated, such as PLN and LNR. PLN and LNR have been considered important prognostic factors for RC (36–39).
Several studies have shown that the number of positive lymph nodes is significantly reduced or even disappears after neoadjuvant treatment, achieving the goal of downgrading and indicating a better prognosis. In a meta-analysis including 7 studies, the number of positive lymph nodes decreased significantly after neoadjuvant therapy (40). Another meta-analysis indicated that nCRT could reduce the number of positive lymph nodes by an average of 0.7 (14). The results of our study showed that when 5 was taken as the best cutoff value for PLN, the difference in survival between the two groups was statistically significant.
The current 8th edition of the NCCN guidelines does not formulate new postoperative staging guidelines for patients who underwent neoadjuvant therapy. The guidelines use 4 as the cutoff value for N1 and N2 staging, while the results of our study show that 5 was the best cutoff value for N staging in patients who received neoadjuvant therapy. The cutoff value indicated that neoadjuvant therapy might have the potential to posteriorly shift the cutoff value of N staging. If neoadjuvant therapy can be incorporated into the AJCC staging system, it will be more conducive to accurately assessing the prognosis of patients and guiding follow-up treatments.
In various recent studies, the LNR has been proven to be an independent prognostic indicator, and a lower LNR indicates a better survival rate (41–43). In a study of 605 patients with rectal cancer, Dekker et al. (44) found that LNR ≥ 0.6 was significantly related to OS and LRFS, while N stage was not. Other studies have shown that the LNR has prognostic value in patients with ypN+ (stage III) rectal cancer regardless of the total number of lymph nodes retrieved (12, 45). In our study, there were significant differences in survival when 0.29 was used as the best cutoff value for the LNR. If it is difficult to retrieve the recommended number of lymph nodes from patients due to difficulty in lymph node dissection during surgery, the LNR can be used as a prognostic factor for these patients.
This study is a retrospective analysis. Although the sample size was large, selection bias was still unavoidable. Pathological factors have a great influence on the detection of lymph nodes, but the analysis of pathological techniques and other factors was absent in this study. The time span was 9 years in this study, and the prognosis was affected by the development of new radiotherapy techniques, chemotherapy regimens and other treatment methods. However, the above factors were included in the multivariate analysis, which offset the deviation caused by the development of treatments to a certain extent.
Conclusion
For LARC patients undergoing nCRT, the recommendation of dissection of ≥ 12 lymph nodes may not be appropriate. The recommends that at least 7 harvested lymph nodes may be more appropriate. New lymph node-related parameters, such as PLN and LNR, provide major new insights into predicting the outcomes of LARC patients undergoing nCRT. 5 positive lymph nodes represents a stronger independent prognostic indicator and can be treated as the best cutoff value for N1 and N2 staging when neoadjuvant therapy can be incorporated into the AJCC staging system. In the future, further prospective controlled studies are required to verify this finding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Fujian Medical University Union Hospital Ethic Review Board (reference number: 2021KY171). The requirement for consent was waived by the ethics committee due to the retrospective nature of the study.
Author Contributions
Conception and design: ZL, AL, and BX. Administrative support: JS, AL, and BX. Provision of study materials or patients: XL, RZ, and CC. Collection and assembly of data: ZL, JS, and AL. Data analysis and interpretation: ZL, JS, and AL. Writing – original draft: ZL. Writing – review and editing: AL. All authors contributed to the article and approved the submitted version.
Funding
Benhua Xu, Joint Funds for the innovation of science and Technology, Fujian province (2017Y9061) and Fujian provincial health technology project (2021CXA011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the volunteers for their participation in these studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.816485/full#supplementary-material
Supplementary Figure 1 | The optimal cutoff value of positive lymph nodes (PLNs) by X-tile software. The optimal cutoff value is shown on a histogram (left panel) and a summarized results table (right panel). of the entire cohort. The blue and gray bars/characters designate low-risk and high-risk subsets, respectively. The optimal cutoff value of PLNs is 5 based on the lowest p values (0.0005) and the maximum χ2 (19.0055) of log-rank tests.
Supplementary Figure 2 | The optimal cutoff value of the positive lymph node ratio (LNR) by X-tile software. The optimal cutoff value is shown on a histogram (left panel) and a summarized results table (right panel). The blue and gray bars/characters designate low-risk and high-risk subsets, respectively. The optimal cutoff value of LNR is 0.29 based on the lowest p values (0.0047) and the maximum χ2 (14.5450) of log-rank tests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw (2020) 18(7):806–15. doi: 10.6004/jnccn.2020.0032
3. Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN Categorization for Colon Cancer Based on National Survival Outcomes Data. J Clin Oncol (2010) 28(2):264–71. doi: 10.1200/JCO.2009.24.0952
4. Jin M, Frankel WL. Lymph Node Metastasis in Colorectal Cancer. Surg Oncol Clin N Am (2018) 27(2):401–12. doi: 10.1016/j.soc.2017.11.011
5. Sloothaak DA, Sahami S, van der Zaag-Loonen HJ, van der Zaag ES, Tanis PJ, Bemelman WA, et al. The Prognostic Value of Micrometastases and Isolated Tumour Cells in Histologically Negative Lymph Nodes of Patients With Colorectal Cancer: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2014) 40(3):263–9. doi: 10.1016/j.ejso.2013.12.002
6. Awwad GE, Tou SI, Rieger NA. Prognostic Significance of Lymph Node Yield After Long-Course Preoperative Radiotherapy in Patients With Rectal Cancer: A Systematic Review. Colorectal Dis (2013) 15(4):394–403. doi: 10.1111/codi.12011
7. Ha YH, Jeong SY, Lim SB, Choi HS, Hong YS, Chang HJ, et al. Influence of Preoperative Chemoradiotherapy on the Number of Lymph Nodes Retrieved in Rectal Cancer. Ann Surg (2010) 252(2):336–40. doi: 10.1097/SLA.0b013e3181e61e33
8. de la Fuente SG, Manson RJ, Ludwig KA, Mantyh CR. Neoadjuvant Chemoradiation for Rectal Cancer Reduces Lymph Node Harvest in Proctectomy Specimens. J Gastrointest Surg (2009) 13(2):269–74. doi: 10.1007/s11605-008-0717-2
9. Wichmann MW, Muller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, et al. Effect of Preoperative Radiochemotherapy on Lymph Node Retrieval After Resection of Rectal Cancer. Arch Surg (2002) 137(2):206–10. doi: 10.1001/archsurg.137.2.206
10. Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of Preoperative Radiation for Rectal Cancer on Subsequent Lymph Node Evaluation: A Population-Based Analysis. Int J Radiat Oncol Biol Phys (2005) 61(2):426–31. doi: 10.1016/j.ijrobp.2004.06.259
11. Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG, et al. Comparison of Tumor Regression Grade Systems for Locally Advanced Rectal Cancer After Multimodality Treatment. J Natl Cancer Inst (2014) 106(10):dju248. doi: 10.1093/jnci/dju248
12. Li Destri G, Di Carlo I, Scilletta R, Scilletta B, Puleo S. Colorectal Cancer and Lymph Nodes: The Obsession With the Number 12. World J Gastroenterol (2014) 20(8):1951–60. doi: 10.3748/wjg.v20.i8.1951
13. Koo T, Song C, Kim JS, Kim K, Chie EK, Kang SB, et al. Impact of Lymph Node Ratio on Oncologic Outcomes in Ypstage III Rectal Cancer Patients Treated With Neoadjuvant Chemoradiotherapy Followed by Total Mesorectal Excision, and Postoperative Adjuvant Chemotherapy. PloS One (2015) 10(9):e0138728. doi: 10.1371/journal.pone.0138728
14. Mechera R, Schuster T, Rosenberg R, Speich B. Lymph Node Yield After Rectal Resection in Patients Treated With Neoadjuvant Radiation for Rectal Cancer: A Systematic Review and Meta-Analysis. Eur J Cancer (2017) 72:84–94. doi: 10.1016/j.ejca.2016.10.031
15. Bustamante-Lopez L, Nahas CS, Nahas SC, Ribeiro U Jr., Marques CF, Cotti G, et al. Understanding the Factors Associated With Reduction in the Number of Lymph Nodes in Rectal Cancer Patients Treated by Neoadjuvant Treatment. Int J Colorectal Dis (2017) 32(6):925–7. doi: 10.1007/s00384-016-2747-7
16. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
17. Hanley JA, McNeil BJ. The Meaning and Use of the Area Under a Receiver Operating Characteristic (ROC) Curve. Radiology (1982) 143(1):29–36. doi: 10.1148/radiology.143.1.7063747
18. Scarinci A, Di Cesare T, Cavaniglia D, Neri T, Colletti M, Cosenza G, et al. The Impact of Log Odds of Positive Lymph Nodes (LODDS) in Colon and Rectal Cancer Patient Stratification: A Single-Center Analysis of 323 Patients. Updates Surg (2018) 70(1):23–31. doi: 10.1007/s13304-018-0519-3
19. Wu Z, Qin G, Zhao N, Jia H, Zheng X. A Statistical Tool for Risk Assessment as a Function of the Number of Lymph Nodes Retrieved From Rectal Cancer Patients. Colorectal Dis (2018) 20(8):O199–206. doi: 10.1111/codi.14264
20. Bhatti ABH, Akbar A, Hafeez A, Syed AA, Khattak S, Jamshed A, et al. Impact of Lymph Node Ratio and Number on Survival in Patients With Rectal Adenocarcinoma After Preoperative Chemo Radiation. Int J Surg (2015) 13:65–70. doi: 10.1016/j.ijsu.2014.11.038
21. Hall MD, Schultheiss TE, Smith DD, Fakih MG, Kim J, Wong JY, et al. Impact of Total Lymph Node Count on Staging and Survival After Neoadjuvant Chemoradiation Therapy for Rectal Cancer. Ann Surg Oncol (2015) 22 Suppl 3:S580–7. doi: 10.1245/s10434-015-4585-1
22. Gurawalia J, Dev K, Nayak SP, Kurpad V, Pandey A. Less Than 12 Lymph Nodes in the Surgical Specimen After Neoadjuvant Chemo-Radiotherapy: An Indicator of Tumor Regression in Locally Advanced Rectal Cancer? J Gastrointest Oncol (2016) 7(6):946–57. doi: 10.21037/jgo.2016.09.03
23. Lee CHA, Wilkins S, Oliva K, Staples MP, McMurrick PJ. Role of Lymph Node Yield and Lymph Node Ratio in Predicting Outcomes in non-Metastatic Colorectal Cancer. BJS Open (2019) 3(1):95–105. doi: 10.1002/bjs5.96
24. Ceelen W, Willaert W, Varewyck M, Libbrecht S, Goetghebeur E, Pattyn P, et al. Effect of Neoadjuvant Radiation Dose and Schedule on Nodal Count and Its Prognostic Impact in Stage II-III Rectal Cancer. Ann Surg Oncol (2016) 23(12):3899–906. doi: 10.1245/s10434-016-5363-4
25. Damin DC, Rosito MA, Contu PC, Tarta C, Ferreira PR, Kliemann LM, et al. Lymph Node Retrieval After Preoperative Chemoradiotherapy for Rectal Cancer. J Gastrointest Surg (2012) 16(8):1573–80. doi: 10.1007/s11605-012-1916-4
26. Fajardo LF. Effects of Ionizing Radiation on Lymph Nodes. A Review. Front Radiat Ther Oncol (1994) 28:37–45. doi: 10.1159/000423371
27. Shvero J, Koren R, Marshak G, Sadov R, Hadar T, Yaniv E, et al. Histological Changes in the Cervical Lymph Nodes After Radiotherapy. Oncol Rep (2001) 8(4):909–11. doi: 10.3892/or.8.4.909
28. de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Kalady MF, Geisler D, Dietz D, et al. Downstaging Without Complete Pathologic Response After Neoadjuvant Treatment Improves Cancer Outcomes for cIII But Not cII Rectal Cancers. Ann Surg Oncol (2010) 17(7):1758–66. doi: 10.1245/s10434-010-0924-4
29. Voss RK, Lin JC, Roper MT, Al-Temimi MH, Ruan JH, Tseng WH, et al. Adjuvant Chemotherapy Does Not Improve Recurrence-Free Survival in Patients With Stage 2 or Stage 3 Rectal Cancer After Neoadjuvant Chemoradiotherapy and Total Mesorectal Excision. Dis Colon Rectum (2020) 63(4):427–40. doi: 10.1097/DCR.0000000000001558
30. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor Regression Grading After Preoperative Chemoradiotherapy for Locally Advanced Rectal Carcinoma Revisited: Updated Results of the CAO/ARO/AIO-94 Trial. J Clin Oncol (2014) 32(15):1554–62. doi: 10.1200/JCO.2013.54.3769
31. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological Response Following Long-Course Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Histopathology (2005) 47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x
32. Wang Y, Zhou M, Yang J, Sun X, Zou W, Zhang Z, et al. Increased Lymph Node Yield Indicates Improved Survival in Locally Advanced Rectal Cancer Treated With Neoadjuvant Chemoradiotherapy. Cancer Med (2019) 8(10):4615–25. doi: 10.1002/cam4.2372
33. Doll D, Gertler R, Maak M, Friederichs J, Becker K, Geinitz H, et al. Reduced Lymph Node Yield in Rectal Carcinoma Specimen After Neoadjuvant Radiochemotherapy has No Prognostic Relevance. World J Surg (2009) 33(2):340–7. doi: 10.1007/s00268-008-9838-8
34. Klos CL, Shellito PC, Rattner DW, Hodin RA, Cusack JC, Bordeianou L, et al. The Effect of Neoadjuvant Chemoradiation Therapy on the Prognostic Value of Lymph Nodes After Rectal Cancer Surgery. Am J Surg (2010) 200(4):440–5. doi: 10.1016/j.amjsurg.2010.03.013
35. Narayanan S, Attwood K, Gabriel E, Nurkin S. Pathologic Complete Response Despite Nodal Yield Has Best Survival in Locally Advanced Rectal Cancer. J Surg Res (2020) 251:220–7. doi: 10.1016/j.jss.2020.01.019
36. Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph Node Evaluation and Survival After Curative Resection of Colon Cancer: Systematic Review. J Natl Cancer Inst (2007) 99(6):433–41. doi: 10.1093/jnci/djk092
37. Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph Node Status After Neoadjuvant Radiotherapy for Rectal Cancer Is a Biologic Predictor of Outcome. Cancer (2009) 115(23):5432–40. doi: 10.1002/cncr.24622
38. Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph Node Ratio Versus Number of Affected Lymph Nodes as Predictors of Survival for Resected Pancreatic Adenocarcinoma. World J Surg (2010) 34(4):768–75. doi: 10.1007/s00268-009-0336-4
39. Wu SG, Li Q, Zhou J, Sun JY, Li FY, Lin Q, et al. Using the Lymph Node Ratio to Evaluate the Prognosis of Stage II/III Breast Cancer Patients Who Received Neoadjuvant Chemotherapy and Mastectomy. Cancer Res Treat (2015) 47(4):757–64. doi: 10.4143/crt.2014.039
40. Miller ED, Robb BW, Cummings OW, Johnstone PA. The Effects of Preoperative Chemoradiotherapy on Lymph Node Sampling in Rectal Cancer. Dis Colon Rectum (2012) 55(9):1002–7. doi: 10.1097/DCR.0b013e3182536d70
41. Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon Cancer Survival Is Associated With Decreasing Ratio of Metastatic to Examined Lymph Nodes. J Clin Oncol (2005) 23(34):8706–12. doi: 10.1200/JCO.2005.02.8852
42. Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, et al. Prognostic Significance of Metastatic Lymph Node Ratio in Node-Positive Colon Carcinoma. Ann Surg Oncol (2007) 14(5):1712–7. doi: 10.1245/s10434-006-9322-3
43. Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, et al. The Prognostic Value of Lymph Node Ratio in a Population-Based Collective of Colorectal Cancer Patients. Ann Surg (2010) 251(6):1070–8. doi: 10.1097/SLA.0b013e3181d7789d
44. Dekker JW, Peeters KC, Putter H, Vahrmeijer AL, van de Velde CJ. Metastatic Lymph Node Ratio in Stage III Rectal Cancer; Prognostic Significance in Addition to the 7th Edition of the TNM Classification. Eur J Surg Oncol (2010) 36(12):1180–6. doi: 10.1016/j.ejso.2010.09.007
45. Li Destri G, Barchitta M, Pesce A, Latteri S, Bosco D, Di Cataldo A, et al. Predictive Value of the Number of Harvested Lymph Nodes and Cut-Off for Lymph Node Ratio in the Prognosis of Stage II and III Colorectal Cancer Patients. J Invest Surg (2019) 32(1):1–7. doi: 10.1080/08941939.2017.1369605
Keywords: locally advanced rectal cancer, neoadjuvant chemoradiotherapy, lymph nodes retrieved, prognosis, PLN, LNR
Citation: Lin Z, Li X, Song J, Zheng R, Chen C, Li A and Xu B (2022) The Effect of Lymph Node Harvest on Prognosis in Locally Advanced Middle-Low Rectal Cancer After Neoadjuvant Chemoradiotherapy. Front. Oncol. 12:816485. doi: 10.3389/fonc.2022.816485
Received: 16 November 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
Alessandro Ottaiano, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Gaetano Luglio, University of Naples Federico II, ItalyDario Baratti, Fondazione Istituto Nazionale Tumori (IRCCS), Italy
Copyright © 2022 Lin, Li, Song, Zheng, Chen, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benhua Xu, YmVuaHVheHVAc2luYS5jb20=; Anchuan Li, bGlhbmNodWFuQG91dGxvb2suY29t
†These authors have contributed equally to this work and share corresponding authorship
 Zhuangbin Lin
Zhuangbin Lin Xiaobo Li1,5,6
Xiaobo Li1,5,6 Rong Zheng
Rong Zheng Benhua Xu
Benhua Xu