
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 26 May 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.811687
This article is part of the Research TopicWomen in Cancer Molecular Targets and Therapeutics, Volume II: 2022View all 23 articles
Objective: Anlotinib, a novel multitarget kinase inhibitor of VEGFR, FGFR, PDGFR and c-Kit, has proven to be effective and safe for refractory soft tissue sarcoma patients, but has not been examined in recurrent or metastatic primary malignant bone tumors in a clinical trial setting.
Methods: This is a multicenter single-arm trial. Patients with pathologically proven recurrent or metastatic primary malignant bone tumors were eligible. Anlotinib was administered orally at 12 mg per day. Each cycle consisted of 2 weeks of treatment followed by 1-week off-treatment. The primary endpoint was progression-free survival (PFS), as assessed in the intention-to-treat (ITT) population. Secondary endpoints included objective response rate (ORR), disease control rate (DCR) and overall survival (OS). Adverse events (AEs) were assessed per NCI CTCAE version 4.03.
Results: A total of 42 patients were enrolled. Median PFS was 5.3 months (95% CI 3.5-8.4 months) in the overall analysis, 4.8 months (95%CI 3.5-7.1 months) in osteosarcoma patients and 2.8 months [95%CI 1.3 months to not reached (NR)] in chondrosarcoma patients. The median OS was 11.4 months (95% CI 10.1 months to NR) in the overall analysis, not reached (95% CI, NR, NR) in osteosarcoma patients and 11.4 months (95% CI 1.8 to 21.1 months) in chondrosarcoma patients. The ORR was 9.52% and DCR was 78.57%. Grade 3 or above AEs occurred in 54.76% of the patients, and included hypertension (19.05%), hypertriglyceridemia (9.52%) and pustulosis palmaris et plantaris (7.14%). No treatment-related death was reported.
Conclusion: Anlotinib demonstrated promising antitumor activities in recurrent or metastatic primary malignant bone tumors with manageable AEs.
Osteosarcoma, chondrosarcoma, Ewing’s sarcoma are common subtypes of malignant bone tumors. Osteosarcoma most commonly affects children, adolescents, and young adults, accounting for 1–2% of all adult cancers (1). Primary therapy for osteosarcoma typically consists of surgical resection and neoadjuvant and adjuvant chemotherapy with doxorubicin, cisplatin, ifosfamide and methotrexate. These treatments have dramatically improved the prognosis of patients with localized osteosarcoma, with a long-term survival rate of 65% to 70% (2). In the absence of targeted therapy, salvage treatment options such as with ifosfamide or gemcitabine plus docetaxel have yielded a rather dismal clinical outcome in osteosarcoma patients who are refractory to chemotherapy or who experience relapse with metastatic disease, with a 4-month progression-free survival (PFS) rate of as low as 12% (3, 4). Genetically, osteosarcoma is characterized by few recurrent genetic alterations and carries mainly somatic copy number alterations (5). The lack of targetable recurrent gene mutations renders molecularly targeted therapy challenging and so far largely disappointing (6). In addition, immune checkpoint inhibitors (ICIs) including those targeting programmed cell death protein-1 (PD-1)/PD-ligand 1 (PD-L1) have demonstrated scant activity in osteosarcoma (7).
Ewing sarcoma, an aggressive sarcoma of bone and soft tissue, may occur at any age with a peak incidence in adolescents and young adults. Primary therapy for Ewing sarcoma mainly consists of surgery, intensive neoadjuvant and adjuvant chemotherapies and/or radiotherapy, with a 5-year overall survival (OS) of 65% to 75% for patients with localized Ewing sarcoma. Despite extensive surgical resection, aggressive chemotherapy, and radiotherapy, the 5-year OS of recurrent or metastatic Ewing sarcoma is less than 30% (8). Chondrosarcoma is inherently resistant to both chemotherapy and radiotherapy and the 5-year survival stands at merely 5% after recurrence (9). Therefore, novel effective therapeutic agents or regimens are urgently needed.
Accumulating evidence suggests that multitarget tyrosine kinase inhibitors (TKIs) have antitumor activities in osteosarcoma. TKIs have been recommended by the National Comprehensive Cancer Network (NCCN) guidelines as second-line therapy for advanced osteosarcoma that progresses after chemotherapy (10). In phase II trials, sorafenib achieved a 4-month PFS rate of 46% in advanced osteosarcoma patients who had failed standard therapy while apatinib achieved a 4-month PFS rate of 56.76% in advanced osteosarcoma patients who had failed chemotherapy (11, 12). In SARC024, a randomized, placebo-controlled phase II trial in patients with progressive metastatic osteosarcoma, regorafenib prolonged median PFS from 1.7 months [95% confidence interval (95% CI): 12-1.8] to 3.6 months (95%CI: 2.0-7.6). The hazard ratio (HR) was 0.42 (95%CI: 0.21-0.85) (13). Though these studies suggest that multitarget TKIs could offer a promising treatment for recurrent metastatic primary malignant bone tumors, improvement in objective response rate (ORR), disease control rate (DCR) or PFS is modest and there is an urgent need for more effective, novel multikinase inhibitors (MKIs) for advanced osteosarcoma.
Anlotinib is a novel MKI that exerts its antitumor activities by inhibiting angiogenesis and suppressing tumor cell proliferation via blocking vascular endothelial growth factor receptors (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and c-Kit (14). Anlotinib has demonstrated stronger in vitro and in vivo anti-angiogenesis activities than other antiangiogenic agents such as lenvatinib and sorafenib, with an IC50 of 0.2, 0.7 and 14.8 nmol/L for VEGFR-2, VEGFR-3 and KIT, respectively, vs. 4, 5.2 and 100 nmol/L for lenvatinib and 90, 20 and 68 nmol/L KIT for sorafenib (15–19). The efficacy and safety of anlotinib have been established in several tumor types including NSCLC and advanced soft tissue sarcoma (20, 21). A phase II trial of 166 refractory soft tissue sarcoma patients demonstrated that anlotinib achieved an ORR of 13% (95%CI 7.6%-18%), a median PFS of 5.6 months and OS of 12 months with acceptable toxicities (22).
The efficacy and safety of anlotinib have not been examined in recurrent metastatic primary malignant bone tumors in a clinical trial setting. Given the promising antitumor activities of anlotinib in soft tissue sarcoma and other tumor types, we hypothesized that anlotinib could be effective for recurrent or metastatic primary malignant bone tumors.
This multicenter, single-arm trial enrolled patients (14 ~70 years of age) with pathologically confirmed primary malignant bone tumors including osteosarcoma, chondrosarcoma, undifferentiated pleomorphic sarcoma of bone/malignant fibrous histiocytoma (MFH) of the bone, giant cell tumor of the bone and Ewing sarcoma/primitive neuroectodermal tumor (PNET). Patients were diagnosed as having recurrent or metastatic disease after surgery and adjuvant therapy. Other inclusion criteria were: at least one measurable lesion according to RECIST 1.1; Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 (except for amputees); treatment failure after anthracyclines-based chemotherapy, or patients could not tolerate chemotherapy (patients with chondrosarcoma or giant cell tumor of the bone were not required to have received first line chemotherapy). We excluded patients who had participated in clinical trials of anlotinib therapy or who had received VEGF inhibitors. Patients with symptomatic brain metastasis or brain metastasis controlled for less than 2 months were also ineligible. Additional eligibility criteria are provided in Supplementary Methods.
The trial was conducted in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice and approved by the institutional review board of Shanghai Sixth People’s Hospital (China). Written informed consent was obtained before enrollment from patients and parents of pediatric patients or their legal surrogates or guardians. The trial is registered with http://www.clinicaltrials.gov (NCT03527888). The study protocol adhered to the SPIRIT statement and the reporting of the study adhered to the CONSORT statement (23, 24).
Anlotinib (12 mg/d) was administered orally. Each cycle consisted of 2 weeks of treatment followed by 1 week off treatment (25). Treatment was continued until disease progression per RECIST 1.1, intolerable toxicity or at the discretion of the investigator. Dose adjustment of anlotinib was allowed at the discretion of the investigator upon drug-related adverse events (AEs) per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (NCI CTC AE 4.03). Two dose reductions were allowed: from 12 mg/d to 10 mg/d and from 10 mg/d to 8 mg/d.
Best supportive care was provided. Patients were allowed to receive bisphosphonates for bone metastasis and, psychological therapy, or other symptomatic treatment. In addition, palliative radiotherapy for the non-target metastatic lesions at bone was allowed to control bone metastasis-associated pain with irradiation field confined to less than 5% of the bone marrow (26). Drugs that could interfere with the study medications were not allowed.
Responses were evaluated by an independent review committee according to RECIST v1.1 using computed tomography (CT) or magnetic resonance imaging (MRI) within 2 weeks of study entry, at 6 weeks post treatment and once every two cycles thereafter. CR, PR, and SD had to be confirmed with a repeat scan after at least 6 weeks.
The primary endpoint was PFS, defined as the time from the date of administration of the first dose of anlotinib to the date of disease progression or death of all causes. Secondary endpoints included ORR, which was the proportion of all evaluable patients who achieved CR or PR per RECIST v1.1, DCR, which was the proportion of patients who achieved CR, PR, or SD lasting for at least 4 weeks, and OS, which was calculated from the date of administration of the first dose of anlotinib to the date of death from any cause.
Data for AEs were recorded from the first date of drug administration until one month after treatment termination, and graded according to NCI CTCAE version 4.03. Safety assessments were based mainly on the occurrence, frequency, severity of AEs and the correlation with study drug.
The median PFS of patients with relapsed osteosarcoma who had received chemotherapy was between 2 to 3 months (3, 27, 28). We hypothesized that anlotinib treatment can prolong the median PFS from 2.5 months to 4.2 months. A total of 37 patients were required to detect a 41% decrease in HR for progression based on 80.2% power and one-sided test at a significance level of 0.025. Assuming a dropout rate of 10%, we planned to enroll 42 patients.
All statistical analysis was undertaken using SAS version 9.2 (SAS Institute Inc., Carry, North Carolina, United States). Statistical analyses were prespecified and followed the intention-to-treat (ITT) principle.
Quantitative variables were expressed in mean and standard deviation or median (range or interquartile range, IQR). Changes from baseline within a group were analyzed by Student’s t test for paired data. Categorical variables were described in frequency and percentage and changes from baseline within a group were analyzed by chi-square (χ2) test or Fisher’s exact test. PFS and OS were estimated by the Kaplan-Meier method and expressed in median (95%CI). The safety set included all patients who received at least one dose of the study drug and had safety assessment.
All tests were two-tailed with a level of significance set at α = 0.05.
Forty-nine patients were screened for eligibility between September 1, 2018 and April 30, 2019; 42 patients were enrolled (Figure 1). Their median age was 28.0 years (IQR: 18.0, 36.0) and 59.5% of them were male. Most patients were diagnosed with osteosarcoma (n=29; 69.05%) or chondrosarcoma (n=9; 21.43%). Furthermore, 25 (59.52%) and 15 (35.71%) patients had stage IVA and IVB disease, respectively, and 39 (92.86%) patients had lung metastasis. All patients received chemotherapy in the adjuvant setting and 7 (16.67%) patients received at least 2 prior lines of chemotherapy. Patient demographic and baseline characteristics are shown in Table 1.
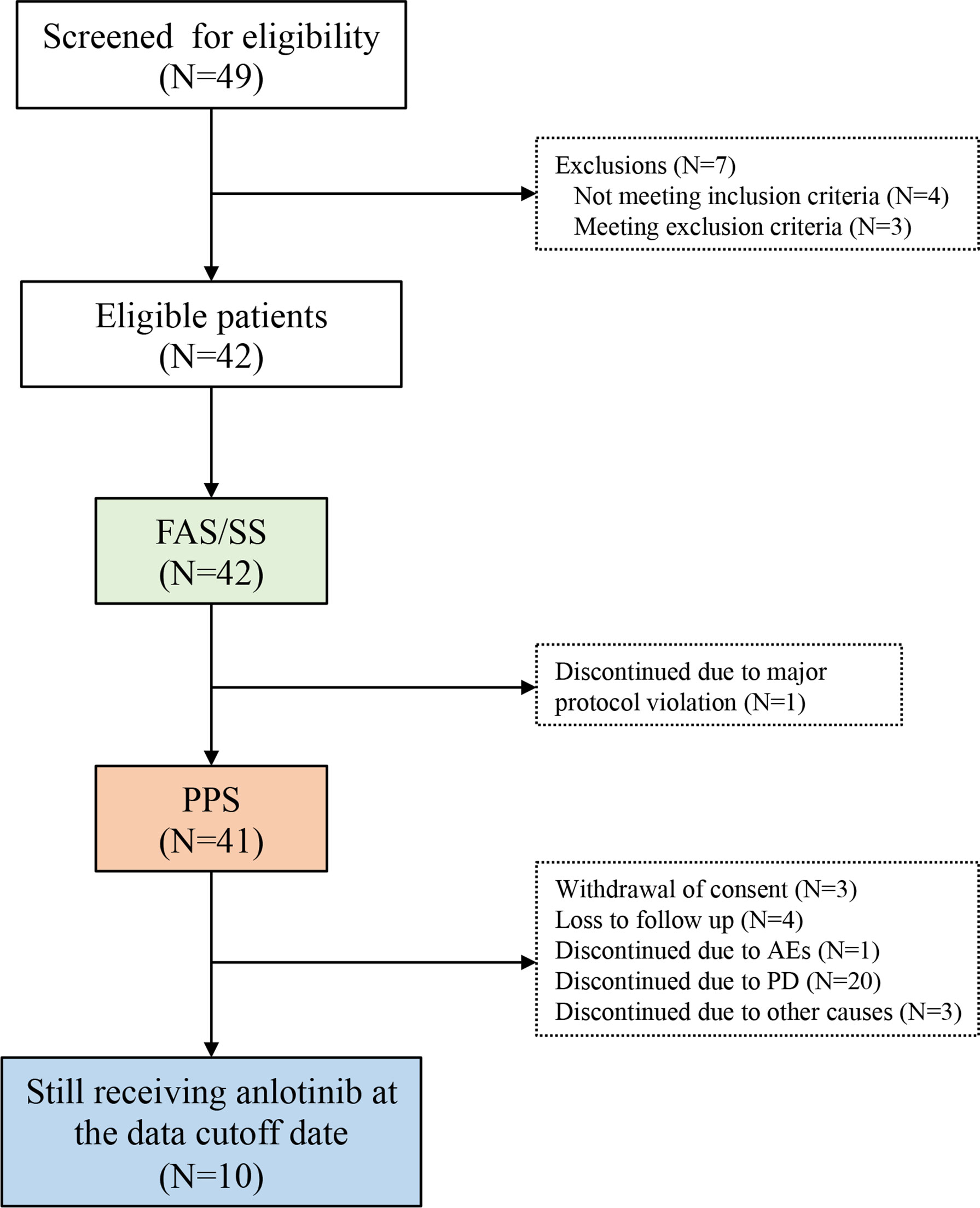
Figure 1 The study flowchart. AE, adverse event; FAS, full analysis set; ITT, intention-to-treat; PPS, per-protocol set; SS, safety set.
The median duration of follow up was 9.6 months (95%CI 8.4-10.8). The data cutoff date was January 1, 2020. PFS events occurred in 28 (66.7%) patients. The median PFS was 5.3 months (95%CI 3.5-8.4 months) and the 3-month PFS rate was 71.27% (Figure 2A). In patients with osteosarcoma, the median PFS was 4.8 months (95%CI 3.5-7.1 months) and the 3-month PFS rate was 75.86% (Figure 2B). In patients with chondrosarcoma, the median PFS was 2.8 months [95%CI 1.3 months to not reached (NR)] and the 3-month PFS rate was 44.44% (Figure 2C). Only 3 patients with Ewing sarcoma/PNET were enrolled. The PFS was 8.3, 8.4 and 11.1 months, respectively. OS events occurred in 14 (28.6%) patients. The median OS was 11.4 months (95% CI 10.1 months to NR) (Figure 2D). The median OS was not reached for osteosarcoma (95% CI, NR, NR) and was 11.4 months (95% CI 1.8 to 21.1 months) for chondrosarcoma.
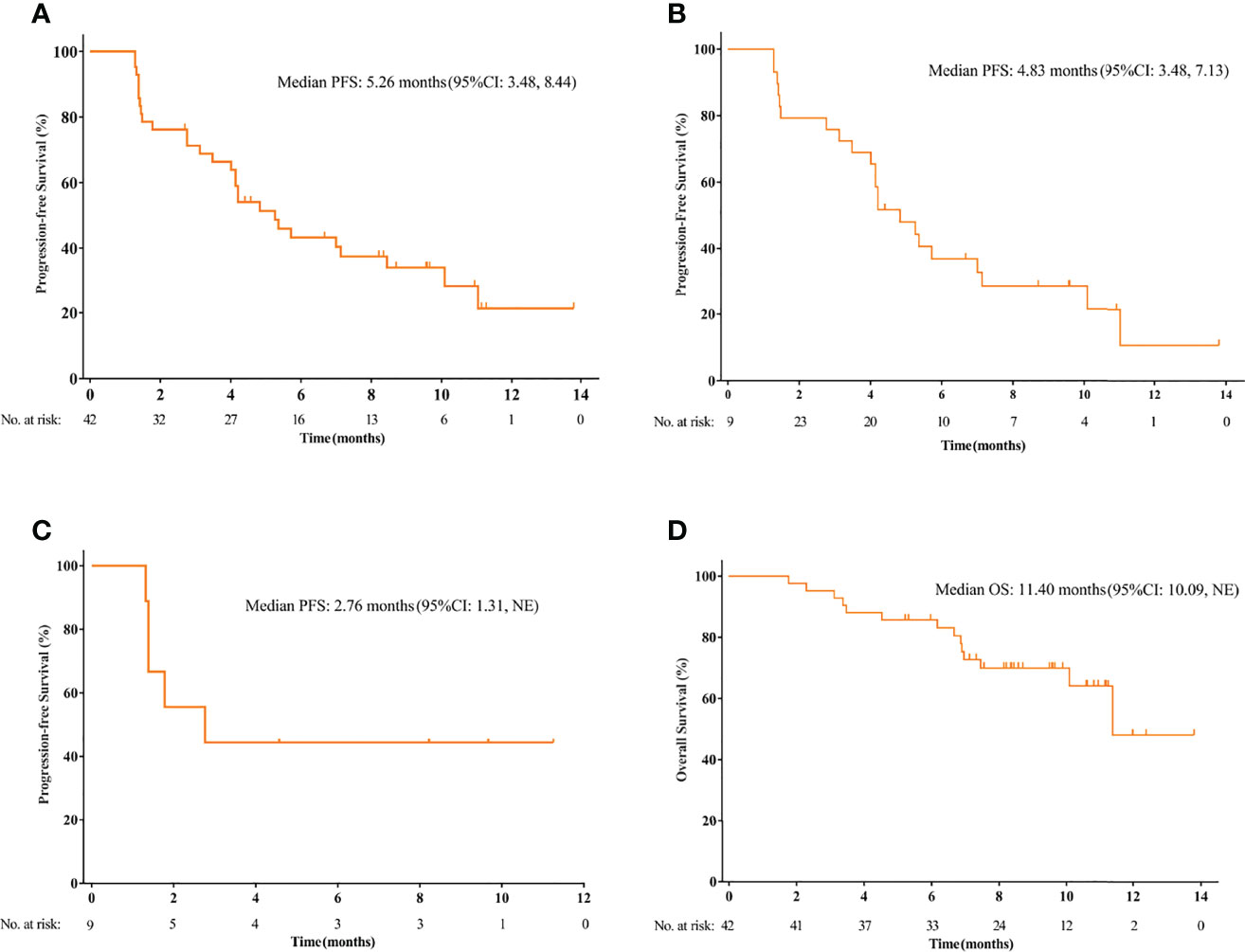
Figure 2 The Kaplan-Meier curve of progression-free survival (PFS) in the intention-to treat (ITT) population (A), and the subsets of osteosarcoma patients (B) and chondrosarcoma patients (C). (D) The Kaplan-Meier curve of overall survival (OS) in the ITT population.
No patient achieved CR. Notably, 4 patients, including 2 osteosarcoma patients achieved PR (Figure 3). Tumor response was observed in 4 patients and the ORR was 9.52% for the overall population. Two patients with osteosarcoma achieved PR and the ORR was 6.90%. In addition, 29 patients had SD and the DCR was 78.57% for the whole population. The DCR for patients with osteosarcoma was 75.86%. Among the 3 patients with Ewing sarcoma/PNET, 2 achieved PR and 1 had SD. The ORR was 66.67% and the DCR was 100% (Table 2).
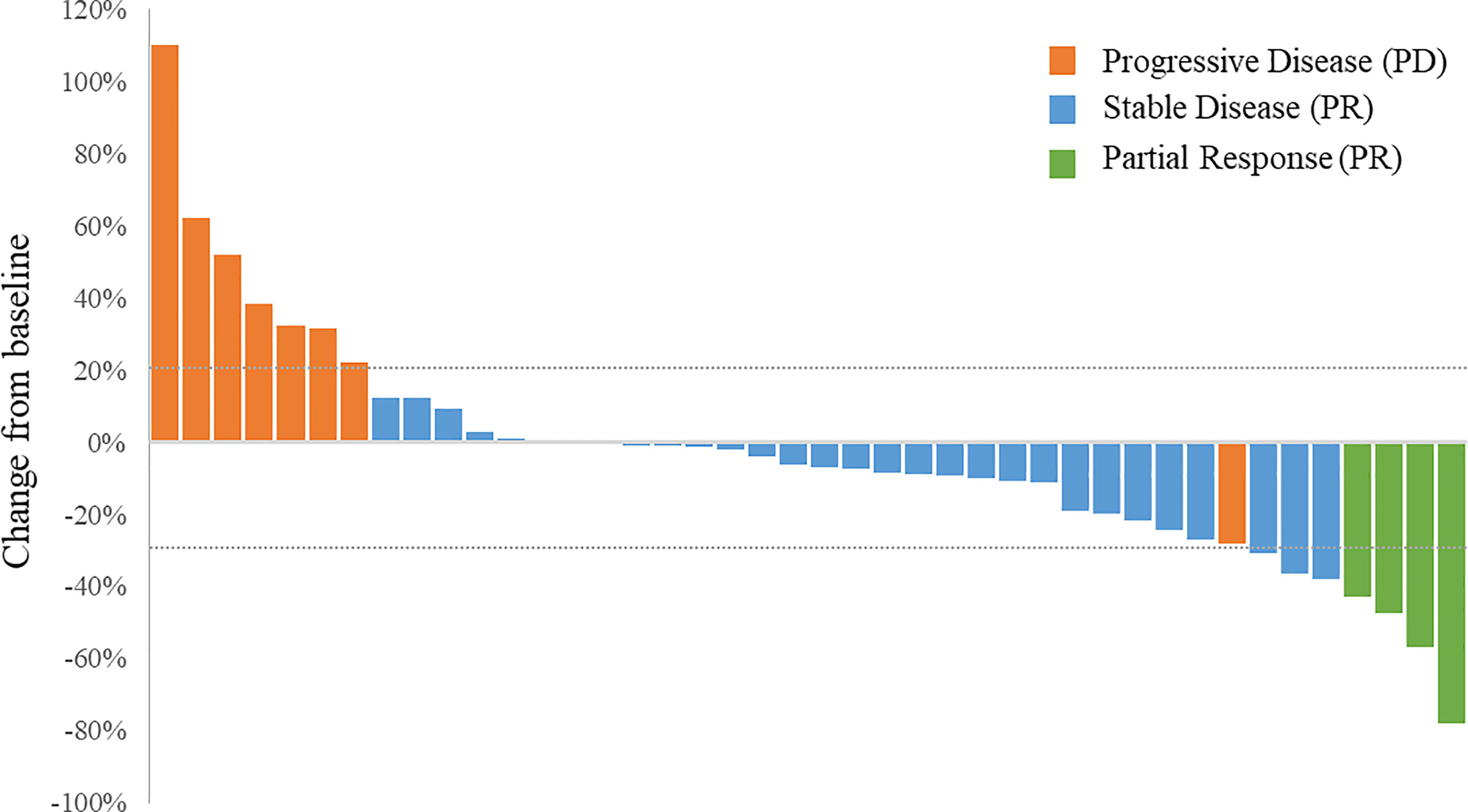
Figure 3 Waterfall plot of the best percentage changes for the sum of target lesion diameters after anlotinib treatment are shown for individual patients with best objective response per RECIST version 1.1 as indicated by the color codes. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
The median treatment cycles was 7 (range 2 to 22). Seven (16.67%) patients underwent dose reductions from 12 mg/d to 10 mg/d. One of them was still intolerable to anlotinib at the reduced dose and permanently discontinued treatment eventually due to AE. Dose modifications are detailed in Supplementary Table 1. Twenty patients discontinued treatment due to PD. As of the data cutoff date, 10 patients were still receiving anlotinib. All (100%) patients experienced at least one AE (Table 3). The most frequent AEs were hypertension (66.67%), hypothyroidism (54.76%), palmar-plantar erythrodysesthesia syndrome (40.48%), proteinuria (40.48%), and diarrhea (40.48%). Grade 3 and above AEs occurred in 23 (54.76%) patients, and included hypertension (19.05%), hypertriglyceridemia (9.52%) and pustulosis palmaris et plantaris (7.14%). In addition, SAEs occurred in 15 (35.71%) patients, of which 7 (16.67%) were treatment-related. Four (9.52%) deaths occurred, including 1 case each of brain metastasis, respiratory difficulty, pleural effusion and sudden death, all of which were not treatment-related.
The current study demonstrated encouraging antitumor activities of anlotinib as second or later line of therapy for recurrent or metastatic primary malignant bone tumors. The 4-month PFS in patients with refractory or relapsed osteosarcoma patients with metastatic disease in the absence of targeted therapy has been reported to be 12% (3, 27, 28). In the current study, anlotinib achieved a 3-month PFS rate of 75.86% and a median PFS of 4.8 months in previously heavily treated advanced osteosarcoma patients, meeting the study primary endpoint. The PFS was comparable to that in the phase II trial for regorafenib (3.6 months, 95% CI, 2.0-7.6 months) and sorafenib (4 months, 95% CI 2-5 months) for advanced osteosarcoma (11, 13). The 3-month PFS rate of 75.86% achieved with anlotinib was also remarkable in comparison with the 4-month PFS rate of 46% achieved with sorafenib therapy (11). Despite notable improvement in PFS with anlotinib and, to a lesser extent, with regorafenib and sorafenib versus chemotherapy, the ORR remains disappointingly low with all three agents (6.89%, 14% and 13.6% for anlotinib, sorafenib and regorafenib, respectively), indicating limited tumor response with MKI monotherapy and highlighting the need for combination therapy such as with chemotherapy.
The DCR with anlotinib was 75.86% for advanced osteosarcoma in this trial, in contrast to 49% with sorafenib as reported earlier. All patients in the current study received adjuvant chemotherapy after surgery compared to 50% in the phase II trial on regorafenib. In a phase II study of refractory or metastatic soft tissue sarcoma, anlotinib achieved a median PFS of 5.6 months and an ORR of 13% (95% CI, 7.6–17) and a DCR of 74% (95% CI, 66–80) (22). Specifically, the median PFS was 5.6 and 11 months, respectively, for liposarcoma and leiomyosarcoma and the ORR was 7.7% in patients with both liposarcoma and leiomyosarcoma. In contrast, regorafenib achieved a median PFS of 1.1 and 3.7 months, respectively, for liposarcoma and leiomyosarcoma and no patients attained CR or PR in the REGOSARC trial, whereas sorafenib achieved a median PFS of 4.2 months for advanced soft tissue sarcoma (4.9 months for liposarcoma), with an ORR of 14.5% and a DCR of 47.4% (29, 30). Although different studies could not be compared directly, these findings suggested that anlotinib attained a numerically higher DCR with osteosarcoma and soft tissue sarcoma than regorafenib and sorafenib; however, randomized studies would be necessary to validate the superiority of anlotinib over other MKIs.
Though immune monotherapy has not demonstrated efficacy in osteosarcoma, the prospect of combining immune therapy and anti-angiogenesis MKI therapy appears promising. In a phase II trial in patients with advanced osteosarcoma progressing after chemotherapy, a combination of apatinib with camrelizumab, a humanized IgG4 κ monoclonal antibody against PD-1, achieved a median PFS of 6.2 months (95% CI 4.0-6.9 months), which is notably longer than 4 months with apatinib (31). Identifying patients with osteosarcoma who could benefit from immunotherapy with biomarkers such as PD-L1 or anti-angiogenesis MKI therapy could lead to further gains in patient survival.
Refractory or relapsed osteosarcoma patients fare very poorly due to lack of effective therapies (2). The unique bone microenvironment also renders immunotherapies largely ineffectual (32). Furthermore, osteosarcoma lacks targetable recurrent gene mutations and small molecules or therapeutic antibodies aiming at a single target may fail to deliver clinical benefits (6). The unique intrinsic properties of recurrent metastatic primary malignant bone tumors indicate that a multitarget inhibitor such as anlotinib or regorafenib, or a combinational approach with diverse mechanisms of actions is required for this patient population. In the current trial, anlotinib achieved a median PFS of 4.83 months (95%CI 3.48 to 7.13) for osteosarcoma. This compares favorably with other analogous agents (median PFS: apatinib, 4.5 months; lenvatinib, 3.4 months; regorafenib, 3.6 months) (11, 13, 33).
Recurrent or metastatic Ewing sarcoma has a very dismal outcome despite the best available therapeutic regimen including surgical resection and chemoradiotherapy (8). No (0/13) Ewing sarcoma in the SARC024 trial responded to regorafenib (13). In the current study, 2 out of the 3 patients with Ewing sarcoma/PNET achieved PR and 1 had SD, suggesting that anlotinib should be further explored as monotherapy or in combination with other agents for the treatment of Ewing sarcoma. The efficacy of anlotinib in Ewing sarcoma/PNET patients is comparable to that of anlotinib plus irinotecan for recurrent or refractory unresectable Ewing sarcoma (34). A small proportion of Ewing sarcoma patients are refractory to chemotherapy and anlotinib could offer an effective treatment for this subset of patients.
The incidence of grade 3 and above elevated triglycerides and hypertriglyceridemia was high (11.9%) in this trial. This is in line with previous studies of anlotinib for refractory solid tumors and refractory metastatic soft tissue sarcomas (22, 25). The rate of grade 3 and above hypertension (19.05%) in this trial is higher than that (4.2%) in a phase II study of 116 patients with refractory metastatic soft tissue sarcomas and that (10%) of phase I of 35 refractory solid tumors (22, 25). The rate of grade 3 and above pustulosis palmaris et plantaris (7.14%) is consistent with that of previous studies (22, 25). Notably, no treatment-related hematologic toxicities or bleeding events occurred. Grade 3 anemia (6%), leucopenia (3%) and bleeding (3%) were reported in osteosarcoma patients treated with sorafenib (11). Frequent hematologic toxicities were also reported for regorafenib, including grade 1 anemia (24%), lymphopenia (14%) and thrombocytopenia (10%). In addition, grade 3 hypophosphatemia and hypokalemia each occurred in 7% osteosarcoma patients treated with regorafenib. No new safety concerns emerged in this trial. Overall, the toxicity profile of anlotinib in this trial is consistent with the prior reports of anlotinib in other clinical studies and seems to be better than that of other oral anti-VEGFR TKIs (35).
This study has several limitations. First, this is a single arm study without a control group. In addition, the small sample size does not permit meaningful subgroup analyses to define the subset of patients who will truly benefit from anlotinib therapy. Furthermore, the study only included Han Chinese and the efficacy and safety of anlotinib in primary malignant bone tumor patients of other ethnicities remain yet to be defined.
In conclusion, anlotinib demonstrated promising antitumor activities and manageable safety profile in patients with recurrent or metastatic primary malignant bone tumors. The findings from this study support the use of anlotinib in patients with advanced primary malignant bone tumors who have progressed after chemotherapy, but further studies are needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The trial was approved by the institutional review board of Shanghai Sixth People’s Hospital (China). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conceptualization: YY. Data curation: LT, XN, ZW, QC, CT, ZF and YY. Formal analysis: LT. Investigation: LT, XN, ZW, QC, CT, ZF and YY. Methodology: LT, YY. Project administration: YY. Resources: YY. Supervision: YY. Visualization: LT. Roles/writing - original draft: LT. Writing - review and editing: LT, XN, ZW, QC, CT, ZF and YY. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QW declared a shared affiliation, with the author QC to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the patients for participation in this study. Chia Tai Tianqing Pharmaceutical Group provided anlotinib.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.811687/full#supplementary-material
1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma Incidence and Survival Rates From 1973 to 2004: Data From the Surveillance, Epidemiology, and End Results Program. Cancer (2000) 115:1531–43. doi: 10.1002/cncr.24121
2. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J Clin Oncol (2015) 33:3029–35. doi: 10.1200/JCO.2014.59.4895
3. Palmerini E, Jones RL, Marchesi E, Paioli A, Cesari M, Longhi A, et al. Gemcitabine and Docetaxel in Relapsed and Unresectable High-Grade Osteosarcoma and Spindle Cell Sarcoma of Bone. BMC Cancer (2016) 16:280. doi: 10.1186/s12885-016-2312-3
4. Fan XL, Cai GP, Zhu LL, Ding GM. Efficacy and Safety of Ifosfamide-based Chemotherapy for Osteosarcoma: A Meta-Analysis. Drug Des Devel Ther (2015) 9:5925–32. doi: 10.2147/DDDT.S91217
5. Wu CC, Livingston JA. Genomics and the Immune Landscape of Osteosarcoma. Adv Exp Med Biol (2020) 1258:21–36. doi: 10.1007/978-3-030-43085-6_2
6. Sayles LC, Breese MR, Koehne AL, Leung SG, Lee AG, Liu HY, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov (2019) 9:46–63. doi: 10.1158/2159-8290.CD-17-1152
7. Wu CC, Beird HC, Andrew Livingston J, Advani S, Mitra A, Cao S, et al. Immuno-Genomic Landscape of Osteosarcoma. Nat Commun (2020) 11:1008. doi: 10.1038/s41467-020-14646-w
8. Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol (2015) 33:3036–46. doi: 10.1200/JCO.2014.59.5256
9. Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, Duan Z. Advances in Immune Checkpoint Inhibitors for Bone Sarcoma Therapy. J Bone Oncol (2019) 15:100221. doi: 10.1016/j.jbo.2019.100221
10. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. Nccn Guidelines Insights: Bone Cancer, Version 2. 2017. J Natl Compr Canc Netw (2017) 15:155–67. doi: 10.6004/jnccn.2017.0017
11. Grignani G, Palmerini E, Dileo P, Asaftei SD, D'Ambrosio L, Pignochino Y, et al. A Phase II Trial of Sorafenib in Relapsed and Unresectable High-Grade Osteosarcoma After Failure of Standard Multimodal Therapy: An Italian Sarcoma Group Study. Ann Oncol (2012) 23:508–16. doi: 10.1093/annonc/mdr151
12. Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Apatinib for Advanced Osteosarcoma After Failure of Standard Multimodal Therapy: An Open Label Phase Ii Clinical Trial. Oncologist (2019) 24:e542–50. doi: 10.1634/theoncologist.2018-0542
13. Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J Clin Oncol (2019) 37(16):1424–31. doi: 10.1200/JCO.18.02374
14. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: A Novel Multi-Targeting Tyrosine Kinase Inhibitor in Clinical Development. J Hematol Oncol (2018) 11:120. doi: 10.1186/s13045-018-0664-7
15. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib Inhibits Angiogenesis Via Suppressing the Activation of VEGFR2, Pdgfrbeta and FGFR1. Gene (2018) 654:77–86. doi: 10.1016/j.gene.2018.02.026
16. Grande E, Díez JJ, Zafon C, Capdevila J. Thyroid Cancer: Molecular Aspects and New Therapeutic Strategies. J Thyroid Res (2012) 2012:847108. doi: 10.1155/2012/847108
17. Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, et al. A Novel Inhibitor That Targets Multiple Kinases, has Potent Antitumor Activities Against Stem Cell Factor Producing Human Small Cell Lung Cancer H146, Based on Angiogenesis Inhibition. Int J Cancer (2008) 122:664–71. doi: 10.1002/ijc.23131
18. Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 Is a Novel and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor-2 Tyrosine Kinase With Potent Activity In Vitro and In Vivo. Cancer Sci (2011) 102:1374–80. doi: 10.1111/j.1349-7006.2011.01939.x
19. Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, et al. Preclinical Characterization of Anlotinib, a Highly Potent and Selective Vascular Endothelial Growth Factor Receptor-2 Inhibitor. Cancer Sci (2018) 109:1207–19. doi: 10.1111/cas.13536
20. Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, et al. Anlotinib as a Third-line Therapy in Patients With Refractory Advanced Non-Small-Cell Lung Cancer: A Multicentre, Randomised Phase II Trial (Alter0302). Br J Cancer (2018) 118:654–61. doi: 10.1038/bjc.2017.478
21. Li S. Anlotinib: A Novel Targeted Drug for Bone and Soft Tissue Sarcoma. Front Oncol (2021) 11:664853. doi: 10.3389/fonc.2021.664853
22. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients With Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res (2018) 24:5233–8. doi: 10.1158/1078-0432.CCR-17-3766
23. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. Spirit 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
24. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ (2010) 340:c332. doi: 10.1136/bmj.c332
25. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, Pharmacokinetics, and Antitumor Properties of Anlotinib, an Oral Multi-Target Tyrosine Kinase Inhibitor, in Patients With Advanced Refractory Solid Tumors. J Hematol Oncol (2016) 9:105. doi: 10.1186/s13045-016-0332-8
26. Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, et al. Lenvatinib With Etoposide Plus Ifosfamide in Patients With Refractory or Relapsed Osteosarcoma (ITCC-050): A Multicentre, Open-Label, Multicohort, Phase 1/2 Study. Lancet Oncol (2021) 22:1312–21. doi: 10.1016/S1470-2045(21)00387-9
27. Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary Metastatic Osteosarcoma: Presentation and Outcome of Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J Clin Oncol (2003) 21:2011–8. doi: 10.1200/JCO.2003.08.132
28. Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O 3rd, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol (2016) 34:3031–8. doi: 10.1200/JCO.2015.65.5381
29. Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and Efficacy of Regorafenib in Patients With Advanced Soft Tissue Sarcoma (REGOSARC): A Randomised, Double-Blind, Placebo-controlled, Phase 2 Trial. Lancet Oncol (2016) 17:1732–42. doi: 10.1016/S1470-2045(16)30507-1
30. Santoro A, Comandone A, Basso U, Soto Parra H, De Sanctis R, Stroppa E, et al. Phase II Prospective Study With Sorafenib in Advanced Soft Tissue Sarcomas After Anthracycline-based Therapy. Ann Oncol (2013) 24:1093–8. doi: 10.1093/annonc/mds607
31. Xie L, Xu J, Sun X, Guo W, Gu J, Liu K, et al. Apatinib Plus Camrelizumab (Anti-Pd1 Therapy, Shr-1210) for Advanced Osteosarcoma (Apfao) Progressing After Chemotherapy: A Single-arm, Open-Label, Phase 2 Trial. J Immunother Cancer (2020) 8:e000798. doi: 10.1136/jitc-2020-000798
32. Le Cesne A, Marec-Berard P, Blay JY, Gaspar N, Bertucci F, Penel N, et al. Programmed Cell Death 1 (Pd-1) Targeting in Patients With Advanced Osteosarcomas: Results From the PEMBROSARC Study. Eur J Cancer (2019) 119:151–7. doi: 10.1016/j.ejca.2019.07.018
33. Gaspar N, Casanova M, Sirvent F, Venkatramani R, Morland B, Gambart M, et al. Single-Agent Expansion Cohort of Lenvatinib (LEN) and Combination Dose-Finding Cohort of LEN + Etoposide (ETP) +Ifosfamide (IFM) in Patients (Pts) Aged 2 to # 25 Years With Relapsed/refractory Osteosarcoma (Os). J Clin Oncol (2018) 36: 15_suppl:11527. doi: 10.1200/JCO.2018.36.15_suppl.11527
34. Xu J, Xie L, Sun X, Liu K, Tang X, Yan T, et al. Anlotinib, Vincristine, and Irinotecan for Advanced Ewing Sarcoma After Failure of Standard Multimodal Therapy: A Two-Cohort, Phase Ib/II Trial. Oncologist (2021) 26:e1256–62. doi: 10.1002/onco.13726
35. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and Safety of Regorafenib in Adult Patients With Metastatic Osteosarcoma: A Non-Comparative, Randomised, Double-blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol (2019) 20:120–33. doi: 10.1016/S1470-2045(18)30742-3
Shanghai 6th People’s Hospital, Shanghai 200233, China.
Beijing Jishuitan Hospital, Beijing 100035, China.
The First Affiliated Hospital, The Air Force Medical University, Xi’an 710032, Shaanxi Province, China.
Henan Cancer Hospital, Zhengzhou 450003, Henan Province, China.
West China Hospital, Sichuan University, Chengdu 610044, Sichuan Province, China.
Beijing Cancer Hospital, Beijing 100142, China.
Keywords: osteosarcoma, chondrosarcoma, multitarget tyrosine kinase inhibitor, angiogenesis, anlotinib, progress-free survival
Citation: Tang L, Niu X, Wang Z, Cai Q, Tu C, Fan Z and Yao Y (2022) Anlotinib for Recurrent or Metastatic Primary Malignant Bone Tumor: A Multicenter, Single-Arm Trial. Front. Oncol. 12:811687. doi: 10.3389/fonc.2022.811687
Received: 09 November 2021; Accepted: 26 April 2022;
Published: 26 May 2022.
Edited by:
Massimo Broggini, Mario Negri Pharmacological Research Institute (IRCCS), ItalyReviewed by:
Anthony Paul Conley, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2022 Tang, Niu, Wang, Cai, Tu, Fan and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Yao, eWFuZ3lhb182QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.