
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 03 March 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.811247
This article is part of the Research Topic Development of small molecule inhibitors and antibodies targeting AXL for tumor therapy and infectious disease control View all 5 articles
AXL, along with MER and TYRO3, is a receptor tyrosine kinase from the TAM family. Although AXL itself is not thought to be a potent oncogenic driver, overexpression of AXL is known to trigger tumor cell growth, survival, invasion, metastasis, angiogenesis, epithelial to mesenchymal transition, and immune suppression. Overexpression of AXL is associated with therapy resistance and poor prognosis. Therefore, it is being studied as a marker of prognosis in cancer treatment or as a target in various cancer types. Recently, many preclinical and clinical studies on agents with various mechanisms targeting AXL have been actively conducted. They include small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates. This article reviewed the fundamental role of AXL in solid tumors, and the development in research of AXL inhibitors in recent years. Emphasis was placed on the function of AXL in acquired therapy resistance in patients with non-small cell lung cancer (NSCLC). Since clinical needs increase in NSCLC patients with acquired resistance after initial therapy, recent research efforts have focused on a combination treatment with AXL inhibitors and tyrosine kinase inhibitors or immunotherapy to overcome resistance. Lastly, we deal with challenges and limitations encountered in the development of AXL inhibitors.
AXL, first reported by Bryan at al. in 1991, is a protein coding gene separated from human myeloid leukemia cells (1). AXL, a receptor tyrosine kinase, consists of a transmembrane: an intracellular and an extracellular domain. Two fibronectin type-III and two immunoglobulin-like motifs make up the AXL extracellular domain (2). The kinase domain is phosphorylated by dimerization when growth arrest-specific 6 (Gas6), an AXL ligand, binds to the receptor. Then, a cross-phosphorylation method activates downstream proteins and signaling cascades are triggered (3). As a ligand that activates the TAM receptors, there is Protein S along with Gas6 (4). Since Gas6 has the highest affinity with AXL among the TAM family, AXL/Gas6 pathway is mainly being studied in cancer (5).
Unlike other TAM receptors, AXL is involved in many cellular functions in variuos cell types (6). In cancer cells, AXL signaling changes tissue specifically through AXL dysregulation (7) (Figure 1). Activated AXL promotes phosphorylation of the growth factor receptor-bound 2 protein and mediates cell proliferation by activating the mitogen-activated protein kinase signaling pathway (8). AXL/Gas6 signaling is also involved in cell survival through the PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase B) pathway (9) and activates pro-survival proteins including mouse double minute 2 homolog, inhibitor of NF-KB, and mammalian target of rapamycin (10). Steroid receptor coactivator (SRC) phosphorylated by AXL activates focal adhesion kinase (FAK) and induces the migration of cells (11). Migration is also conducted through p38 (3). Suppression of the SRC/FAK and p38 is related to decrease cell viability in the drug-resistant mesenchymal cells (11). Matrix metalloproteinase 9 is a required factor for AXL-mediated tumor invasion (12). Upregulated AXL increases the expression of the suppressor of cytokine signaling 1/3 through the Janus kinase/signal transducer and activator of transcription pathway, and down-regulation of Toll-like receptor signaling leads to immune suppression (13). AXL is involved in angiogenesis through Dickkopf-related protein 3 (14).
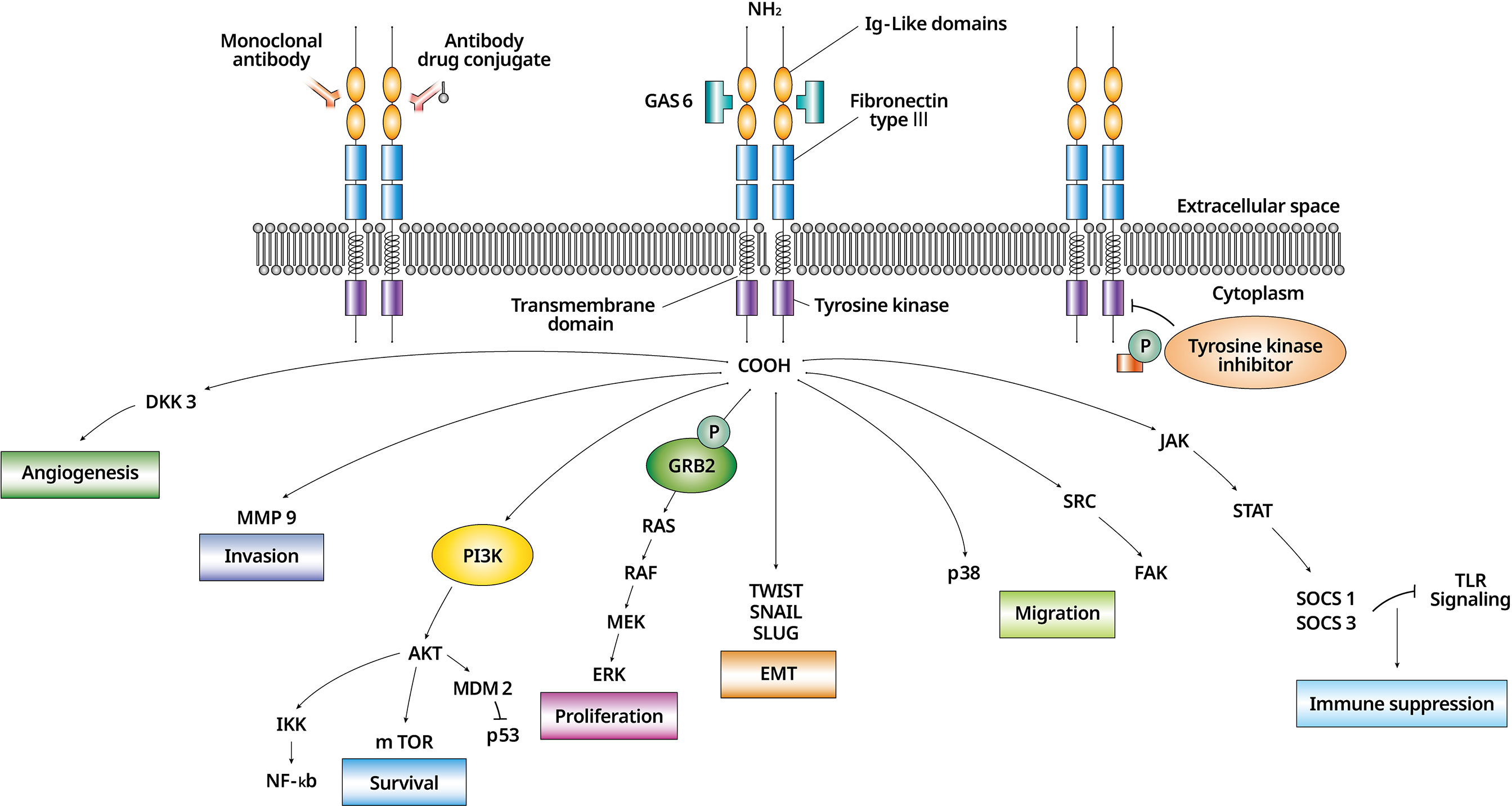
Figure 1 The biology of AXL/Gas6 signaling activation and downstream effectors in malignant cell. AXL/Gas6 activation and downstream signaling is shown. AXL promotes cell survival, proliferation, invasion, migration, angiogenesis, EMT, and immune suppression. An AXL inhibitor is expressed as a monoclonal antibody, an antibody-drug conjugate, and a tyrosine kinase inhibitor according to three different mechanisms. EMT, epithelial to mesenchymal transition; Gas6, growth arrest-specific 6.
It has been shown that AXL is overexpressed in various carcinomas like NSCLC, breast cancer, gastric and colorectal cancer, and prostate cancer (15). Overexpression of AXL is known to be related to drug resistance by many PI3K-, ERK-, or epidermal growth factor receptor (EGFR)- inhibiting target agents (16). In NSCLC, higher AXL expression was seen in mesenchymal cancer cells than in epithelial cancer cells (17). AXL overexpression was dependent on the expression of vimentin, a marker for epithelial to mesenchymal transition (EMT), indicating that EMT may contribute to resistance acquisition (18, 19). Some studies showed that down-regulation of AXL can inhibit EMT progression and can induce a more increased response to TKI (20).
In order to overcome the occurrence of cancer and drug resistance, many studies on AXL inhibitors are being conducted. Many AXL inhibitors exist in the form of tyrosine kinase inhibitors; they are also being developed in the form of antibody-drug conjugate and monoclonal antibodies. AXL tyrosine kinase inhibitors can be categorized into two types depending on whether they bind to the active site of the DFG motif (type I) of AXL or not (type II) (21). As for AXL inhibitors, apart from single targets, dual or multi-targets like MET (mesenchymal-epithelial transition factor), VEGFR (vascular endothelial growth factor) are being developed (15). Monoclonal antibodies (mAbs) downregulate receptor expression or activation and downstream signaling by blocking the ligand of AXL (Gas6) (22). It has been reported that anti-AXL mAb promote the therapeutic effects of small molecule inhibitors against other targets such as EGFR, or VEGF, and also chemotherapy (23). An antibody-drug conjugate (ADC) is dependent on AXL expression but acts independently of activation of AXL/Gas6 signaling (24). Recently AXL-107- monomethyl auristatin E (MMAE) (24) and AXL-specific chimeric antigen receptor (CAR) (25) have also shown promising results in preclinical studies.
In lung cancer, AXL expression varies from ~33.0% to ~93.2% depending on known reports (26). In some reports, differences in AXL expression did not correlate with clinical outcomes (27). But many studies revealed that patients with NSCLC who exhibited high AXL mRNA expression showed poorer prognosis than patients exhibiting low AXL mRNA expression (28–30). And after using targeted therapy like EGFR inhibitor, increased AXL expression level was shown (31, 32). Considering that AXL’s genetic mutation and amplification are low in overall carcinoma (15), various studies are being conducted to find good biomarkers, such as phosphorylated AXL level (33).
Preclinical studies showed that the Gas6–AXL axis induced acquired resistance to the EGFR-tyrosine kinase inhibitors (TKI) erlotinib or osimertinib in NSCLC cells with EGFR-mutation positivity, and that AXL inhibitors combined with other drugs could overcome this resistance (34–36). Some clinical studies combining AXL inhibitors and immune checkpoint inhibitors (ICI) are ongoing in NSCLC patients who have failed therapy with ICI (37).
In this review, we introduced the progress of AXL inhibitors in recent years and updated the understanding of AXL inhibitors with a focus on lung cancer.
We categorized the mechanisms of action into four groups: 1) antibody-drug conjugates (ADCs), 2) TKIs, and 3) mAbs, 4) Soluble receptors (Gas6 target). A list of selected AXL inhibitors according to the three categories are summarized in Table 1.
Enapotamab vedotin is an AXL-targeted ADC created by combining human AXL-targeted immunoglobulin G1 with MMAE, the microtubule-destroying agent. In the preclinical study, enapotamab vedotin (2 and 4mg/kg) exhibited anti-tumor effects in osimertinib-resistant patient-derived xenograft (PDX) models (38). The study evaluating the safety of enapotamab vedotin (HuMax-AXL-ADC) treatment is an ongoing phase I/II study of solid tumors, including NSCLC (NCT02988817).
CAB-AXL-ADC binds selectively to human and cyno AXL expressing cells in the tumor microenvironment but has reduced binding under normal tissue conditions. CAB-AXL-ADC demonstrated the ability to induce cytotoxicity of human tumor cell lines expressing AXL in vitro, and inhibit tumor growth in lung, prostate, pancreatic human tumor xenografts, and in selected gemcitabine-resistant pancreatic cancer-derived xenograft models, in vivo (39). A phase I trial using CAB-AXL-ADC alone in advanced solid tumor patients is ongoing, as well as a phase II trial combining CAB-AXL-ADC with PD-1 (programmed cell death protein 1) inhibitors (NCT03425279). In addition, a separate phase II study is being conducted only for NSCLC participants (NCT04681131). These studies are yet to report interim results.
BGB324 is a small molecule AXL TKI. BGB324 revealed AXL inhibitory activity (EC50/IC50, 14 nmol/L) in both an in vitro biochemical kinase assay and a cell-based assay in HeLa cells reflecting AXL signaling (40). BGB324 inhibited the appearance of EMT-related EGFR inhibitor resistance in NSCLC xenografts in a preclinical study (41).
In another study, BGB324 inhibited the aggressiveness of PDA cells (pancreatic cancer) in vitro and enhanced gemcitabine efficacy in vivo (42). Phase I and II clinical studies of BGB324 are ongoing in non-small cell carcinoma, in which combinations with other agents such as docetaxel (NCT02922777), erlotinib (NCT02424617), and pembrolizumab (NCT03184571) are also being investigated.
A small molecule inhibitor, SLC-391 has potent efficacy against many cancer cell lines, by inhibiting AXL/PI3K/AKT-dependent cell proliferation and survival (50 mg/kg p.o, CT-26 murine colon carcinoma cell line). In addition, a synergistic anti-tumor effect was observed when a high PD-1 expression CT-26 syngeneic model was treated with a combination of SLC-391 and a PD-1 inhibitor, and the overall survival rate of the combination group was dramatically prolonged, in comparison with the vehicle control group (43). A phase I study of SLC-391 in solid tumors is ongoing (NCT03990454).
A novel AXL inhibitor, DS-1205c is another sulfate hydrate, with similar stoichiometries to DS-1205b. In a preclinical study, DS-1205b intensely suppressed hGas6-dependent cell migration in vitro and showed potent anti-tumor effects in AXL-overexpressing NSCLC xenograft cells in vivo (IC50, 1.3 nM) (44). A phase I clinical study of DS-1205c plus EGFR TKI (gefitinib) is ongoing (NCT03599518). The study of DS-1205c plus EGFR TKI (osimertinib) was terminated based on a business decision by the sponsor (NCT03255083).
ASP2215 is a highly selective, dual AXL/FLT3 (FMS-like tyrosine kinase 3) inhibitor that showed anti-leukemic activity in relapsed or refractory acute myeloid leukemia (AML) patients. In the preclinical study, ASP2215 regressed the tumor size and decreased proliferation in FLT3 mutated cells and AML xenograft models (IC50, 0.29 nM and 0.73 nM, respectively (45). A study combining ASP2215 and erlotinib in EGFR-positive NSCLC patients after EGFR inhibitor use was terminated due to adverse events related to the combination therapy (NCT02495233). Reported SAEs (serious adverse events) were alanine aminotransferase increase (n=4), aspartate aminotransferase increase (n=3), renal failure-acute (n=1), and pleural effusion (n=1) in 10 subjects.
A dual AXL/c-Met inhibitor, BPI-9016M potently regressed tumor size in NSCLC PDX models. The effect was greater in high c-Met expression tumors (IC50 ranging from 5.3 μM to 27.1 μM) (46). BPI-9016M showed favorable safety and pharmacokinetic profiles in a phase I clinical trial involving 20 Chinese NSCLC patients. Grade 3 or higher treatment-related adverse events (TRAEs) reported during treatment included hypertension (15%), laryngitis (5%), and pulmonary thromboembolism (PTE) (5%). Dose-limiting toxicity (DLT) was not detected, and the maximum tolerated dose was not found. Among 19 patients, one showed a partial response (PR) and 10 showed stable disease (SD) (NCT02478866). A phase I clinical trial of BPI-9016M in c-Met dysregulated NSCLC patients is also underway (NCT02929290).
INCB081776 is a novel AXL/MER inhibitor that increases anti-tumor immune activity. In preclinical data, INCB081776 potently inhibited the recombinant AXL/MER enzymes (IC50, 0.61 nM and 3.17 nM against AXL/MER, respectively) (47). A phase I study of INCB081776 in advanced solid tumors is ongoing (melanoma, NSCLC, squamous head & neck cancer, soft tissue sarcoma) (NCT03522142).
PF-07265807 is an AXL/MER inhibitor that enhances the function of dendritic cells to cross-prime CD8+ T cells. In preclinical assays, PF-07265807 alone revealed anti-tumor effects and showed increased cure rates in tumor models when combined with PD-1 inhibitor (48). A phase I clinical trial of PF-07265807 in advanced solid malignancies is ongoing (NCT 04458259).
Q702 is an AXL, MER and CSF1R kinase inhibitor. A study group presented the potential of Q702 leading to tumor regression through immune stimulating activity by decreasing T-reg cells, M2 macrophages, and myeloid-derived suppressor cells and promoting antigen presentation and direct cytotoxic activity in syn-tumor models including AML and EGFR TKI resistant NSCLC (49). The same group presented the anti-tumor efficacy and immune mechanism of Q702, in combination with anti-PD-1, in the various syngeneic models in a recent report (50). A phase I clinical trial of Q702 in advanced solid malignancies is underway (NCT04648254).
RXDX-106 is a pan TAM RTK (receptor tyrosine kinase) family inhibitor (IC50 of TYRO3, 3.50 nM; AXL, 0.69 nM, MER: 1.89 nM). In wild-type mice, RXDX-106 treatment leads to tumor growth inhibition, but not in immuno-deficient mice. RXDX-106 also increases the effects of ICIs, resulting in enhanced anti-tumor efficacy and survival (51). A clinical trial of RXDX-106 in advanced solid tumors for which standard therapy was not effective was terminated by the sponsor (NCT03454243).
TP-0903 is an AXL inhibitor (IC50, 27 nM in vitro) (52, 53). TP-0903 inhibits AXL phosphorylation and modifies EMT. TP-0903 reduces anti-apoptotic proteins like BCL-2, MCL-1, and XIAP, and enhances dose-dependent chronic lymphocytic leukemia (CLL) cell death (54, 55). A phase I study of TP-0903 in advanced solid malignancies, including EGFR-mutation NSCLC patients is ongoing (NCT02729298).
MGCD265 is a multi-target inhibitor, suppressing tumor cell growth, survival, and angiogenesis (56). In an in vitro study, MGCD265 showed potent inhibition of c-Met/VEGFR/Tie-2/Ron action, with IC50s in the nano-molar range (57). A phase II study of MGCD265 in Met-altered NSCLC patients was conducted, although efficacy data were not statistically significant in the results. Dehydration (6/68), pneumonia (5/68), and myocardial infarction (2/68) were shown in the SAE report (NCT02544633).
MGCD516 is a small molecule inhibitor targeting multiple RTKs. In a preclinical study, MGCD516 showed potent anti-tumor effects and had better efficacy compared to imatinib and crizotinib in different sarcoma models (56). A phase III trial combining MGCD516 and nivolumab in advanced NSCLC patients is currently underway (NCT03906071).
YW327.6S2 is a mAb for AXL, which inhibits receptor activation and downstream signaling. In a preclinical study, YW327.6S2 decreased tumor size and increased the anti-VEGF treatment efficacy in NSCLC and breast cancer xenograft models. YW327.6S2 elevated the anti-tumor activity of erlotinib and chemotherapy in NSCLC models. The study group showed that AXL mAbs affect tumor cells and stroma by modulating tumor-associated vasculature and immune cell functions (23). Research on this drug is in the preclinical stage.
D9 and E8 are selective anti-AXL mAbs. They inhibit Gas6-induced phosphorylation of AXL and downstream signaling. D9 and E8 induced the suppression of Gas6-mediated phosphorylation of AXL and conducted via receptor down-regulation and internalization irrespective of Gas6 in pancreatic cancer cells and xenograft models (58). Research on this drug is in the preclinical stage.
MAb173 inhibited Kaposi sarcoma cell invasion by inducing receptor degradation in vitro (59). In a preclinical study, irrespective of Kaposi sarcoma associated herpes virus infection, MAb173 reduced tumor size and down-regulated AXL protein levels in Kaposi sarcoma tumor cells. These results showed that AXL has an important role in Kaposi sarcoma pathogenesis (59). In another in vivo study, MAb173 potently increased Renal cell carcinoma (RCC) cell apoptosis and decreased RCC tumor size by 78% (60). Research on this drug is in the preclinical stage.
An AXL decoy receptor, AVB-S6-500 is a soluble receptor to bind Gas6 ligand. In a preclinical study of ovarian cancer and renal cell carcinoma, AVB-S6-500 reduced Gas6-induced AXL phosphorylation and tumor growth (61, 62). Many clinical studies of AVB-S6-500 in ovarian cancer (NCT03639246, NCT04019288, NCT04729608), renal cell carcinoma (NCT04300140) and pancreatic cancer (NCT04983407) are ongoing.
In this section, we will review ongoing clinical trials using AXL inhibitors in lung cancer as shown in Table 2. AXL inhibitors are being investigated in two settings. The first is the setting of acquired resistance after EGFR inhibitor use. The EGFR T790M mutation is the most frequently cause of resistance after initial 1st or 2nd generation EGFR TKI use in metastatic EGFR-mutant NSCLC patients. Up-regulation of the bypass signaling pathway is also an important resistance mechanism. Up-regulation of AXL expression levels has been also shown in EGFR-mutant NSCLC patients who developed resistance to erlotinib treatment. Some xenograft studies of AXL inhibitors combined with EGFR TKI showed meaningful results in overcoming such resistance (44, 63). AXL overexpression was observed with an EMT-like feature in transcriptomic analyses of NSCLC cell lines (17). This also implies that targeting AXL could overcome acquired resistance after EGFR TKI use related to EMT. Second, AXL inhibitors are being explored in combination with ICIs due to their immune-modulatory effects. Although ICIs have emerged as promising anti-tumor agents, there still exists a need to overcome the resistance developed after using them and to find treatment combinations with synergistic mechanisms of action. AXL is also related to resistance to cytotoxic T-cell mediated cell death (64). In a previous preclinical study, an AXL inhibitor made the cells more sensitive to T-cell-mediated killing (65). In various types of solid tumors, overexpression of AXL has been shown after PD-1 inhibitor failure (15). In preclinical models of NSCLC, AXL inhibition has shown a synergistic effect in combinations with ICIs (37).
The results of a BGB324 clinical trial in advanced NSCLC patients were recently reported (NCT02424617). In phase I, BGB324 alone and BGB324 plus erlotinib (in patients who previously progressing on erlotinib, arm A) were evaluated. In phase II, patients who previously had progression after any EGFR inhibitor use (arm B) or who were responding/stable on erlotinib treatment in the first line setting (arm C) were treated with BGB324 plus erlotinib. In the run-in arm, two-eights of the patients achieved SD for 1 year, including 19% tumor shrinkage in one patient. In arm A, one-eighth of the patients achieved a tumor shrinkage of 38%, with treatment duration of 2 years until progression. Five patients additionally reported SD. In arm B, one achieved a PR and one a SD on the combination; durations of treatment were 1 year, and 6 months, respectively. None of these 2 patients had EGFR T790M mutation. Median progression free survival (mPFS) was 1.4 months. In arm C, 11/13 patients were evaluable for efficacy. One PR was reported with 47% tumor shrinkage; duration of treatment was 315 days. Nine other patients achieved SD; mPFS is currently 12.2 months. Treatment was tolerable. Common TRAEs (> 20% of patients) were diarrhea (70%; Grade 3, 20%), nausea (50%; Grade 3, 0%), QTc prolongation (35%; Grade 3, 3%), vomiting (35%; Grade 3, 0%), and fatigue (25%; Grade 3 5%). One unrelated Grade 4 and no treatment-related deaths were reported. Bemcentinib with erlotinib combination is feasible and tolerable in NSCLC patients. A benefit was seen in a subset of patients who showed progression after EGFR TKI use or were taking erlotinib concurrently in remission, in the first line (66).
A phase I study of the AXL inhibitor DS-1205c plus gefitinib in advanced EGFR-mutant NSCLC patients is currently being conducted (NCT03599518). Patients who showed progression on EGFR TKI and did not have mutation were eligible. A dose-escalation cohort (200-1200 mg twice a day, BID) was completed in April 2020. One patient receiving 800 mg BID had DLT, so the recommended dose for expansion was 800 mg BID. SAEs caused by DS-1205c were not observed. One patient continued with SD for more than three months (67).
A phase I study of the AXL inhibitor DS-1205c plus osimertinib in advanced EGFR-mutant NSCLC patients was initiated April 10, 2019, but has been terminated based on a business decision by the sponsor (NCT03255083). According to their interim report, of a total 13 enrolled patients, nine patients were SD, three patients were PD and one patient was not evaluable. Frequent TEAEs included elevated liver enzyme, vomiting, and fatigue.
According to the final report of Astellas (ISN 2215-CL-5101, no publications based on the results of this study), 10 patients were enrolled in the study of phase Ib/II combining ASP2215 and erlotinib in EGFR-positive advanced NSCLC patients who showed progression after EGFR inhibitor use. Since only two patients received more than two cycles of study treatment, there was not enough data to allow for meaningful results regarding the efficacy of this combination regimen. This study demonstrated that ASP2215 in combination with erlotinib at the dose levels evaluated did not show an acceptable safety profile due to increased hepatic enzymes.
In a preclinical study, ONO-7475 (AXL/MER inhibitor) plus osimertinib overtly reduced tumor size and suppressed tumor growth compared to osimertinib alone, or combination therapy after acquisition of osimertinib resistance in AXL-overexpressing EGFR-mutated NSCLC xenograft models (68). In their study, cell line-derived xenograft (CDX) mice models and cell line–based analysis showed that the combination of ONO-7475 and osimertinib was more effective at the initial phase than at the osimertinib-acquired resistance phase in high-AXL–expressing EGFR-mutated NSCLC cells (68). Clinical trials are not yet in progress.
In a preclinical study, BGB324 was shown to increase the efficacy of a PD-1 inhibitor (69). A phase II clinical trial of BGB324 plus pembrolizumab for previously treated and immuno-therapy naive patients with stage IV lung adenocarcinoma is ongoing. According to their interim study report (38 patients; 24 and 14 in stage 1 and 2, respectively), the most frequent TRAEs were increased hepatic enzymes (37%), diarrhea (29%), and asthenia (17%). With the use of corticosteroids, TRAEs were well-managed. According to reports, among 29 evaluable patients, 7 patients (24%) showed PR. In AXL-positive patients, the objective response rate was 40%, mPFS was 5.9 months (mPFS of total patients was 4.0 months), and the median overall survival was not reached (70).
Glesatinib and sitravatinib revealed anti-tumor effects and increased PD-1 inhibition by enhancing an anti-tumor microenvironment (71). A phase II clinical trial in refractory NSCLC patients found that sitravatinib can induce recovery of response to nivolumab. Among six evaluable patients, two patients showed PR, and the treatment was well-tolerated (72).
Overexpression of the AXL gene is involved in resistance to anti-cancer therapeutics in various types of cancer. Therefore, development of AXL inhibitors is ongoing to overcome the acquired resistance that emerges after a favorable response to therapy. In lung cancer, especially NSCLC, EMT and bypass signal activation are known mechanisms of resistance after EGFR TKI use. In order to overcome this resistance, many clinical studies using AXL inhibitors are being conducted. AXL inhibitors are highly anticipated for lung cancer treatment in terms of their ability to inhibit EMT and regulate the tumor microenvironment. However, there are still many hurdles to overcome in order to incorporate AXL inhibitors into clinical practice. Since the effect of AXL inhibitor alone is not yet satisfactory, the bio-chemical understanding of the role of AXL in the treatment of lung cancer needs to be further progressed. Combining AXL inhibitor with EGFR TKI or immunotherapy is also a part of efforts to develop more effective treatment strategy. In terms of toxicity, some studies have reported SAEs such as increased hepatotoxicity, but finding an appropriate combination will be a challenge. Moreover, the expression level of the AXL gene is not yet optimized for use as a predictive biomarker for AXL inhibitors, and it is also important to set the cut-off of the AXL expression level properly for each cancer type. Ongoing clinical trials and future studies are warranted to develop more effective and safe treatment strategies utilizing AXL inhibitors, whether in combination or as a single-agent.
All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2019R1A2C4069993) to SL.
1. O’Bryan JP, Frye R, Cogswell P, Neubauer A, Kitch B, Prokop C, et al. Axl, a Transforming Gene Isolated From Primary Human Myeloid Leukemia Cells, Encodes a Novel Receptor Tyrosine Kinase. Mol Cell Biol (1991) 11(10):5016–31. doi: 10.1128/mcb.11.10.5016-5031.1991
2. Yamagata M, Sanes JR, Weiner JA. Synaptic Adhesion Molecules. Curr Opin Cell Biol (2003) 15(5):621–32. doi: 10.1016/S0955-0674(03)00107-8
3. Korshunov VA. Axl-Dependent Signalling: A Clinical Update. Clin Sci (2012) 122(8):361–8. doi: 10.1042/CS20110411
4. Stitt TN, Conn G, Goret M, Lai C, Bruno J, Radzlejewski C, et al. The Anticoagulation Factor Protein S and its Relative, Gas6, are Ligands for the Tyro 3/Axl Family of Receptor Tyrosine Kinases. Cell (1995) 80(4):661–70. doi: 10.1016/0092-8674(95)90520-0
5. Linger RM, Keating AK, Earp HS, Graham DK, et al. Taking Aim at Mer and Axl Receptor Tyrosine Kinases as Novel Therapeutic Targets in Solid Tumors. Expert Opin Ther Targets (2010) 14(10):1073–90. doi: 10.1517/14728222.2010.515980
6. Axelrod H, Pienta KJ. Axl as a Mediator of Cellular Growth and Survival. Oncotarget (2014) 5(19):8818. doi: 10.18632/oncotarget.2422
7. Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The Receptor Tyrosine Kinase Axl in Cancer: Biological Functions and Therapeutic Implications. Int J Cancer (2014) 134(5):1024–33. doi: 10.1002/ijc.28246
8. Stenhoff J, Dahlbäck B, Hafizi S. Vitamin K-Dependent Gas6 Activates ERK Kinase and Stimulates Growth of Cardiac Fibroblasts. Biochem Biophys Res Commun (2004) 319(3):871–8. doi: 10.1016/j.bbrc.2004.05.070
9. Linger RM, Keating AK, Earp HS, Graham DK. TAM Receptor Tyrosine Kinases: Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer. Adv Cancer Res (2008) 100:35–83. doi: 10.1016/S0065-230X(08)00002-X
10. Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular Signaling Pathways Involved in Gas6-Axl-Mediated Survival of Endothelial Cells. Am J Physiol-Heart Circulatory Physiol (2004) 287(3):H1207–13. doi: 10.1152/ajpheart.00020.2004
11. Wilson C, Nicholes K, Bustos D, Lin E, Song Q, Stephan J-P, et al. Overcoming EMT-Associated Resistance to Anti-Cancer Drugs via Src/FAK Pathway Inhibition. Oncotarget (2014) 5(17):7328. doi: 10.18632/oncotarget.2397
12. Tai K, Shieh Y, Lee C, Shiah S, Wu C-W. Axl Promotes Cell Invasion by Inducing MMP-9 Activity Through Activation of NF-κb and Brg-1. Oncogene (2008) 27(29):4044–55. doi: 10.1038/onc.2008.57
13. Tanaka M, Siemann DW. Gas6/Axl Signaling Pathway in the Tumor Immune Microenvironment. Cancers (2020) 12(7):1850. doi: 10.3390/cancers12071850
14. Li Y, Ye X, Tan C, Hongo J-A, Zha J, Liu J, et al. Axl as a Potential Therapeutic Target in Cancer: Role of Axl in Tumor Growth, Metastasis and Angiogenesis. Oncogene (2009) 28(39):3442–55. doi: 10.1038/onc.2009.212
15. Gay CM, Balaji K, Byers LA. Giving AXL the Axe: Targeting AXL in Human Malignancy. Br J Cancer (2017) 116(4):415–23. doi: 10.1038/bjc.2016.428
16. Zhu C, Wei Y, Wei X. AXL Receptor Tyrosine Kinase as a Promising Anti-Cancer Approach: Functions, Molecular Mechanisms and Clinical Applications. Mol Cancer (2019) 18(1):1–22. doi: 10.1186/s12943-019-1090-3
17. Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An Epithelial–Mesenchymal Transition Gene Signature Predicts Resistance to EGFR and PI3K Inhibitors and Identifies Axl as a Therapeutic Target for Overcoming EGFR Inhibitor Resistance. Clin Cancer Res (2013) 19(1):279–90. doi: 10.1158/1078-0432.CCR-12-1558
18. Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an Essential Epithelial-to-Mesenchymal Transition-Induced Regulator of Breast Cancer Metastasis and Patient Survival. Proc Natl Acad Sci (2010) 107(3):1124–9. 10.1073/pnas.0909333107
19. Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi J, Nevo J, Gjerdrum C, et al. Vimentin Regulates EMT Induction by Slug and Oncogenic H-Ras and Migration by Governing Axl Expression in Breast Cancer. Oncogene (2011) 30(12):1436–48. doi: 10.1038/onc.2010.509
20. Wu F, Li J, Jang C, Wang J, Xiong J. The Role of Axl in Drug Resistance and Epithelial-to-Mesenchymal Transition of non-Small Cell Lung Carcinoma. Int J Clin Exp Pathol (2014) 7(10):6653.
21. Zhao Z, Wu H, Wang L, Liu Y, Knapp S, Liu Q, et al. Exploration of Type II Binding Mode: A Privileged Approach for Kinase Inhibitor Focused Drug Discovery? ACS Chem Biol (2014) 9(6):1230–41. doi: 10.1021/cb500129t
22. Duan Y, Luo L, Qiao C, Li X, Wang J, Liu H, et al. A Novel Human Anti-AXL Monoclonal Antibody Attenuates Tumour Cell Migration. Scandinavian J Immunol (2019) 90(2):e12777. doi: 10.1111/sji.12777
23. Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An Anti-Axl Monoclonal Antibody Attenuates Xenograft Tumor Growth and Enhances the Effect of Multiple Anticancer Therapies. Oncogene (2010) 29(38):5254–64. doi: 10.1038/onc.2010.268
24. Boshuizen J, Koopman LA, Krijgsman O, Shahrabi A, Gresnigt–van den Heuvel E, Ligtenberg MA, et al. Cooperative Targeting of Melanoma Heterogeneity With an AXL Antibody-Drug Conjugate and BRAF/MEK Inhibitors. Nat Med (2018) 24(2):203–12. doi: 10.1038/nm.4472
25. Cho JH, Okuma A, Al-Rubaye D, Intisar E, Junghans RP, Wong WW, et al. Engineering Axl Specific CAR and SynNotch Receptor for Cancer Therapy. Sci Rep (2018) 8(1):1–8. doi: 10.1038/s41598-018-22252-6
26. Zhang G, Wang M, Zhao H, Cui W. Function of Axl Receptor Tyrosine Kinase in non-Small Cell Lung Cancer. Oncol Lett (2018) 15(3):2726–34. doi: 10.3892/ol.2017.7694
27. Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or Axl Receptor Tyrosine Kinase Inhibition Promotes Apoptosis, Blocks Growth and Enhances Chemosensitivity of Human non-Small Cell Lung Cancer. Oncogene (2013) 32(29):3420–31. doi: 10.1038/onc.2012.355
28. Wang Y, Xia H, Zhuang Z, Miao L, Chen X, Cai H. Axl-Altered microRNAs Regulate Tumorigenicity and Gefitinib Resistance in Lung Cancer. Cell Death Dis (2014) 5(5):e1227–7. doi: 10.1038/cddis.2014.186
29. Shinh Y-S, Lai C-Y, Kao Y-R, Shiah S-G, Chu Y-W, Lee H-S, et al. Expression of Axl in Lung Adenocarcinoma and Correlation With Tumor Progression. Neoplasia (2005) 7(12):1058–64. doi: 10.1593/neo.05640
30. Ishikawa M, Sonobe M, Nakayama E, Kobayashi M, Kikuchi R, Kitamura J, et al. Higher Expression of Receptor Tyrosine Kinase Axl, and Differential Expression of its Ligand, Gas6, Predict Poor Survival in Lung Adenocarcinoma Patients. Ann Surg Oncol (2013) 20(3):467–76. doi: 10.1245/s10434-012-2795-3
31. Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL Mediates Resistance to Cetuximab Therapy. Cancer Res (2014) 74(18):5152–64. doi: 10.1158/0008-5472.CAN-14-0294
32. Tian Y, Zhang Z, Miao L, Yang Z, Yang J, Wang Y, et al. Anexelekto (AXL) Increases Resistance to EGFR-TKI and Activation of AKT and ERK1/2 in non-Small Cell Lung Cancer Cells. Oncol Res (2016) 24(5):295. doi: 10.3727/096504016X14648701447814
33. Iida S, Miki Y, Suzuki T, Mori K, Saito M, Niikawa H, et al. Activation of AXL and Antitumor Effects of a Monoclonal Antibody to AXL in Lung Adenocarcinoma. Anticancer Res (2014) 34(4):1821–7.
34. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL Kinase Causes Resistance to EGFR-Targeted Therapy in Lung Cancer. Nat Genet (2012) 44(8):852–60. doi: 10.1038/ng.2330
35. Namba K, Shien K, Takahashi Y, Torigoe H, Sato H, Yoshioka T, et al. Activation of AXL as a Preclinical Acquired Resistance Mechanism Against Osimertinib Treatment in EGFR-Mutant non–Small Cell Lung Cancer Cells. Mol Cancer Res (2019) 17(2):499–507. doi: 10.1158/1541-7786.MCR-18-0628
36. Kim D, Bach D-H, Fan Y-H, Hong J-Y, Park HJ, Lee SK. AXL Degradation in Combination With EGFR-TKI can Delay and Overcome Acquired Resistance in Human non-Small Cell Lung Cancer Cells. Cell Death Dis (2019) 10(5):1–12. doi: 10.1038/s41419-019-1601-6
37. Lorens J, Arce-Lara CE, Arriola E, Brunsvig P, Carcereny Costa E, Domine M, et al. Phase II Open-Label, Multi-Centre Study of Bemcentinib (BGB324), a First-in-Class Selective AXL Inhibitor, in Combination With Pembrolizumab in Patients With Advanced NSCLC. 2018. Am Soc Clin Oncol (2018) 36(15):3078. doi: 10.1200/JCO.2018.36.15_suppl.3078
38. Koopman LA, Terp MG, Zom GG, Janmaat ML, Jacobsen K, Gresnigt-van den Heuvel E, et al. Enapotamab Vedotin, an AXL-Specific Antibody-Drug Conjugate, Shows Preclinical Antitumor Activity in non-Small Cell Lung Cancer. JCI Insight (2019) 4(21):e128199. doi: 10.1172/jci.insight.128199
39. Sharp LL, Chang C, Frey G, Wang J, Liu H, Xing C, et al. Anti-Tumor Efficacy of BA3011, a Novel Conditionally Active Biologic (CAB) Anti-AXL-ADC. AACR (2018) 78(13_Supplement):827. doi: 10.1158/1538-7445.AM2018-827
40. Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a Selective Small Molecule Inhibitor of Axl Kinase, Blocks Tumor Spread and Prolongs Survival in Models of Metastatic Breast Cancer. Cancer Res (2010) 70(4):1544–54. doi: 10.1158/0008-5472.CAN-09-2997
41. Wnuk-Lipinska K, Tiron C, Gausdal G, Sandal T, Frink R, Hinz S, et al. BGB324, a Selective Small Molecule Axl Kinase Inhibitor to Overcome EMT-Associated Drug Resistance in Carcinomas: Therapeutic Rationale and Early Clinical Studies. AACR (2014) 74(19_Supplement):1747. doi: 10.1158/1538-7445.AM2014-1747
42. Ludwig KF, Du W, Sorrelle NB, Wnuk-Lipinska K, Topalovski M, Toombs JE, et al. Small-Molecule Inhibition of Axl Targets Tumor Immune Suppression and Enhances Chemotherapy in Pancreatic Cancer. Cancer Res (2018) 78(1):246–55. doi: 10.1158/0008-5472.CAN-17-1973
43. Lai S, Li R, Raha P, Hu Y, Yan J, Zhang H, et al. Abstract B148: Activity of the TAM Kinase-Targeting Compound, SLC-391, is Mediated by the Engagement of the Immune System in CT-26 Syngeneic Mouse Model. AACR (2018) 17(1_Supplement):B148. doi: 10.1158/1535-7163.TARG-17-B148.
44. Jimbo T, Hatanaka M, Komatsu T, Taira T, Kumazawa K, Maeda N, et al. DS-1205b, a Novel Selective Inhibitor of AXL Kinase, Blocks Resistance to EGFR-Tyrosine Kinase Inhibitors in a non-Small Cell Lung Cancer Xenograft Model. Oncotarget (2019) 10(50):5152. doi: 10.18632/oncotarget.27114
45. Mori M, Kaneko N, Ueno Y, Yamada M, Tanaka R, Saito R, et al. Gilteritinib, a FLT3/AXL Inhibitor, Shows Antileukemic Activity in Mouse Models of FLT3 Mutated Acute Myeloid Leukemia. Investigational New Drugs (2017) 35(5):556–65. doi: 10.1007/s10637-017-0470-z
46. Zhang P, Li S, Lv C, Si J, Xiong Y, Ding L, et al. BPI-9016M, a C-Met Inhibitor, Suppresses Tumor Cell Growth, Migration and Invasion of Lung Adenocarcinoma via Mir203-DKK1. Theranostics (2018) 8(21):5890. doi: 10.7150/thno.27667
47. Favata M, Lasky K, Lo Y, Feldman P, Li J, Chen Y, et al. Characterization of INCB081776, a Potent and Selective Dual AXL/MER Kinase Inhibitor. AACR (2018) 78(13_Supplement):3759. doi: 10.1158/1538-7445.AM2018-3759
48. Wong J, Harrison J, Bouhana K, Nguyen N, Shaabani N, Vartabedian VF, et al. The Potent and Selective MERTK/AXL Inhibitor PF-5807/ARRY-067 Activates Dendritic Cells to Cross-Prime CD8+ T Cells for Anti-Tumor Activity. AACR (2021) 81(13_Supplement):1735. doi: 10.1158/1538-7445.AM2021-1735
49. Yang Y-I, Kang H, Park D, Jeon Y, Kim J, Choi B, et al. Q702, Selective Axl, Mer and CSF1R Triple Kinase Inhibitor With Dual Potentials Leading to Tumor Regression: Immuno-Oncology Therapy and Targeted Cancer Therapy. AACR (2019) 79(13_Supplement):4139. doi: 10.1158/1538-7445.AM2019-4139
50. Yang Y-I, Kang H, Park D, Jeon Y, Kim J, Choi B, et al. Q702, Selective Axl/Mer/CSF1R Triple Kinase Inhibitor Enhance the Activity of Immune Checkpoint Inhibitor by Alteration of Immunosuppressive Tumor Microenvironment. AACR (2020) 80(16_Supplement):4974. doi: 10.1158/1538-7445.AM2020-4974
51. Yokoyama Y, Lew ED, Seelige R, Tindall EA, Walsh C, Fagan PC, et al. Immuno-Oncological Efficacy of RXDX-106, a Novel TAM (TYRO3, AXL, MER) Family Small-Molecule Kinase Inhibitor. Cancer Res (2019) 79(8):1996–2008. doi: 10.1158/0008-5472.CAN-18-2022
52. Mollard A, Warner SL, Call LT, Wade ML, Bearss JJ, Verma A, et al. Design, Synthesis, and Biological Evaluation of a Series of Novel AXL Kinase Inhibitors. ACS Med Chem Lett (2011) 2(12):907–12. doi: 10.1021/ml200198x
53. Jimenez L, Wang J, Morrison MA, Whatcott C, Soh KK, Warner S, et al. Phenotypic Chemical Screening Using a Zebrafish Neural Crest EMT Reporter Identifies Retinoic Acid as an Inhibitor of Epithelial Morphogenesis. Dis Models Mech (2016) 9(4):389–400. doi: 10.1242/dmm.021790
54. Sinha S, Boysen J, Nelson M, Secreto C, Warner SL, Bearss DJ, et al. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination With BTK Inhibitors. Clin Cancer Res (2015) 21(9):2115–26. doi: 10.1158/1078-0432.CCR-14-1892
55. Patel V, Keating MJ, Wierda WG, Gandhi V, et al. Preclinical Combination of TP-0903, an AXL Inhibitor and B-PAC-1, a Procaspase-Activating Compound With Ibrutinib in Chronic Lymphocytic Leukemia. Leukemia Lymphoma (2016) 57(6):1494–7. doi: 10.3109/10428194.2015.1102243
56. Patwardhan PP, Ivy KS, Musi E, de Stanchina E, Schwartz GK. Significant Blockade of Multiple Receptor Tyrosine Kinases by MGCD516 (Sitravatinib), a Novel Small Molecule Inhibitor, Shows Potent Anti-Tumor Activity in Preclinical Models of Sarcoma. Oncotarget (2016) 7(4):4093. doi: 10.18632/oncotarget.6547
57. Beaulieu N, Beaulieu C, Dupont I, Nguyen H, Chute I, Gravel S, et al. Preclinical Development of MGCD265, a Potent Orally Active C-Met/VEGFR Multi-Target Kinase Inhibitor. AACR (2008) 68(9_Supplement):4838. doi: 10.1016/S1359-6349(08)72013-5
58. Leconet W, Larbouret C, Chardès T, Thomas G, Neiveyans M, Busson M, et al. Preclinical Validation of AXL Receptor as a Target for Antibody-Based Pancreatic Cancer Immunotherapy. Oncogene (2014) 33(47):5405–14. doi: 10.1038/onc.2013.487
59. Liu R, Gong M, Li X, Zhou Y, Gao W, Tulpule A, et al. Induction, Regulation, and Biologic Function of Axl Receptor Tyrosine Kinase in Kaposi Sarcoma. Blood J Am Soc Hematol (2010) 116(2):297–305. doi: 10.1182/blood-2009-12-257154
60. Yu H, Liu R, Ma B, Li X, Yen H, Zhou Y, et al. Axl Receptor Tyrosine Kinase Is a Potential Therapeutic Target in Renal Cell Carcinoma. Br J Cancer (2015) 113(4):616–25. doi: 10.1038/bjc.2015.237
61. Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, et al. AXL Is an Essential Factor and Therapeutic Target for Metastatic Ovarian Cancer. Cancer Res (2010) 70(19):7570–9. doi: 10.1158/0008-5472.CAN-10-1267
62. Xiao Y, Zhao H, Tian L, Nolley R, Diep AN, Ernst A, et al. S100A10 Is a Critical Mediator of GAS6/AXL–Induced Angiogenesis in Renal Cell Carcinoma. Cancer Res (2019) 79(22):5758–68. doi: 10.1158/0008-5472.CAN-19-1366
63. Planchard D, Janne P, Yu H, Moro-Sibilot D, Goldberg T, Gu X, et al. Phase I Study of the AXL Inhibitor DS-1205c in Combination With Osimertinib in Subjects With Metastatic or Unresectable EGFR-Mutant NSCLC. Ann Oncol (2018) 29:viii545–viii546. doi: 10.1093/annonc/mdy292.127
64. Terry S, et al. AXL Targeting Overcomes Human Lung Cancer Cell Resistance to NK-And CTL-Mediated Cytotoxicity. Cancer Immunol Res (2019) 7(11):1789–802. doi: 10.1158/2326-6066.CIR-18-0903
65. Aguilera TA, Giaccia AJ. Molecular Pathways: Oncologic Pathways and Their Role in T-Cell Exclusion and Immune Evasion—a New Role for the AXL Receptor Tyrosine Kinase. Clin Cancer Res (2017) 23(12):2928–33. doi: 10.1158/1078-0432.CCR-17-0189
66. Byers LA, Gold KA, Peguero JA, Johnson ML, Nieva JJ, Harb WA, et al. Ph I/II Study of Oral Selective AXL Inhibitor Bemcentinib (BGB324) in Combination With Erlotinib in Patients With Advanced EGFRm NSCLC: End of Trial Update. Wolters Kluwer Health (2021). doi: 10.1200/JCO.2021.39.15_suppl.9110
67. Nishio M, Okamoto I, Murakami H, Horinouchi H, Toyozawa R, Takeda M, et al. 570p A First-in-Human Phase I Study of the AXL Inhibitor DS-1205c in Combination With Gefitinib in Subjects With EGFR-Mutant NSCLC. Ann Oncol (2020) 31:S488. doi: 10.1016/j.annonc.2020.08.684
68. Okura N, Nishioka N, Yamada T, Taniguchi H, Tanimura K, Katayama Y, et al. ONO-7475, a Novel AXL Inhibitor, Suppresses the Adaptive Resistance to Initial EGFR-TKI Treatment in EGFR-Mutated Non–Small Cell Lung Cancer. Clin Cancer Res (2020) 26(9):2244–56. doi: 10.1158/1078-0432.CCR-19-2321
69. Gausdal G, Davidsen K, Wnuk-Lipinska K, Wiertel K, Kang J, Engelsen A, et al. BGB324, a Selective Small Molecule Inhibitor of the Receptor Tyrosine Kinase AXL, Enhances Immune Checkpoint Inhibitor Efficacy. Cancer Res (2016) 76(14_Supplement):566. doi: 10.1158/1538-7445.AM2016-566
70. Felip E, Brunsvig P, Vinolas N, Ponce Aix S, Carcereny Costa E, Dómine Gomez M, et al. A Phase II Study of Bemcentinib (BGB324), a First-in-Class Highly Selective AXL Inhibitor, With Pembrolizumab in Pts With Advanced NSCLC: OS for Stage I and Preliminary Stage II Efficacy. Am Soc Clin Oncol (2019) 35(15_suppl):9098. doi: 10.1200/JCO.2019.37.15_suppl.9098
71. Du W, Huang H, Sorrelle N, Brekken RA. Sitravatinib Potentiates Immune Checkpoint Blockade in Refractory Cancer Models. JCI Insight (2018) 3(21). doi: 10.1172/jci.insight.124184
Keywords: AXL, AXL inhibitor, resistance, targeted therapy, immunotherapy
Citation: Sang YB, Kim J-H, Kim C-G, Hong MH, Kim HR, Cho BC and Lim SM (2022) The Development of AXL Inhibitors in Lung Cancer: Recent Progress and Challenges. Front. Oncol. 12:811247. doi: 10.3389/fonc.2022.811247
Received: 08 November 2021; Accepted: 08 February 2022;
Published: 03 March 2022.
Edited by:
S. M. Mazidur Rahman, International Centre for Diarrhoeal Disease Research (ICDDR), BangladeshReviewed by:
Derek Brandon Oien, Mayo Clinic, United StatesCopyright © 2022 Sang, Kim, Kim, Hong, Kim, Cho and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sun Min Lim, bGltbG92ZTIwMDhAeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.